Key Points
Question
Among heavy smokers (ie, ≥20 pack-years), what is the association between time since smoking cessation and subsequent risk of cardiovascular disease?
Findings
In this observational cohort study of 8770 participants, former heavy smokers’ risk of cardiovascular disease was significantly lower within 5 years of smoking cessation relative to current smokers (hazard ratio, 0.61) but remained significantly elevated for at least 5 to 10 years and possibly for 25 years after cessation relative to never smokers.
Meaning
Compared with never smokers, former heavy smokers may have significantly elevated cardiovascular disease risk beyond 5 years after cessation.
Abstract
Importance
The time course of cardiovascular disease (CVD) risk after smoking cessation is unclear. Risk calculators consider former smokers to be at risk for only 5 years.
Objective
To evaluate the association between years since quitting smoking and incident CVD.
Design, Setting, and Participants
Retrospective analysis of prospectively collected data from Framingham Heart Study participants without baseline CVD (original cohort: attending their fourth examination in 1954-1958; offspring cohort: attending their first examination in 1971-1975) who were followed up through December 2015.
Exposures
Time-updated self-reported smoking status, years since quitting, and cumulative pack-years.
Main Outcomes and Measures
Incident CVD (myocardial infarction, stroke, heart failure, or cardiovascular death). Primary analyses included both cohorts (pooled) and were restricted to heavy ever smokers (≥20 pack-years).
Results
The study population included 8770 individuals (original cohort: n = 3805; offspring cohort: n = 4965) with a mean age of 42.2 (SD, 11.8) years and 45% male. There were 5308 ever smokers with a median 17.2 (interquartile range, 7-30) baseline pack-years, including 2371 heavy ever smokers (406 [17%] former and 1965 [83%] current). Over 26.4 median follow-up years, 2435 first CVD events occurred (original cohort: n = 1612 [n = 665 among heavy smokers]; offspring cohort: n = 823 [n = 430 among heavy smokers]). In the pooled cohort, compared with current smoking, quitting within 5 years was associated with significantly lower rates of incident CVD (incidence rates per 1000 person-years: current smoking, 11.56 [95% CI, 10.30-12.98]; quitting within 5 years, 6.94 [95% CI, 5.61-8.59]; difference, −4.51 [95% CI, −5.90 to −2.77]) and lower risk of incident CVD (hazard ratio, 0.61; 95% CI, 0.49-0.76). Compared with never smoking, quitting smoking ceased to be significantly associated with greater CVD risk between 10 and 15 years after cessation in the pooled cohort (incidence rates per 1000 person-years: never smoking, 5.09 [95% CI, 4.52-5.74]; quitting within 10 to <15 years, 6.31 [95% CI, 4.93-8.09]; difference, 1.27 [95% CI, −0.10 to 3.05]; hazard ratio, 1.25 [95% CI, 0.98-1.60]).
Conclusions and Relevance
Among heavy smokers, smoking cessation was associated with significantly lower risk of CVD within 5 years relative to current smokers. However, relative to never smokers, former smokers’ CVD risk remained significantly elevated beyond 5 years after smoking cessation.
This cohort study uses Framingham Heart Study data to assess the association between years since smoking cessation and incident cardiovascular disease (CVD), compared with current and never smoking, among participants without baseline CVD over a median follow-up of 26 years.
Introduction
Cigarette smoking is a risk factor for cardiovascular disease (CVD) and is responsible for 20% of CVD deaths in the United States1,2,3,4,5,6; smoking cessation reduces CVD risk.7 However, estimates of the time course of CVD risk reduction among former smokers following cessation have been inconsistent relative to persistent smokers (2-10 years),8,9,10,11,12 and never smokers (2-20 years).8,9,10,11,12
Some clinical CVD risk calculators do not distinguish risk between never smokers and former smokers.13 The Atherosclerotic CVD (ASCVD) Risk Estimator Plus allows clinicians to identify former smokers but considers their CVD risk identical to never smokers after 5 years.14 Uncertainty about the time course of CVD risk reduction following smoking cessation could underestimate CVD risk among former smokers, a group increasing as US smoking prevalence declines.15
Cardiovascular disease risk estimates among former smokers could be improved by frequent, long-term smoking exposure assessment (including status, intensity, abstinence periods, and relapse), objective and time-updated assessment of other CVD risk factors, and continuous CVD incidence surveillance. Data from the Framingham Heart Study (FHS) were analyzed to determine the association between years since smoking cessation and subsequent CVD risk among former smokers relative to persistent smokers and never smokers.
Methods
Samples
This investigation included FHS original cohort members attending their fourth examination cycle (1954-1958) and FHS offspring cohort participants attending their first examination cycle (1971-1975), when smoking data were first reliably collected. The Boston University Medical Center and Vanderbilt University Medical Center institutional review boards approved the study protocol. All participants provided written informed consent.
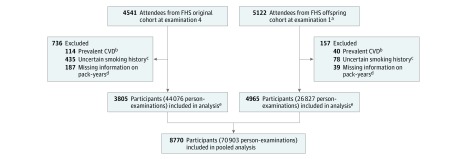
There were 4541 original cohort attendees at examination cycle 4 and 5124 offspring cohort attendees at their first examination cycle. The protocol sequentially excluded individuals whose last contact date was equivalent to their baseline date, those with baseline prevalent CVD (self-reported for the offspring cohort; self-reported [before examination 1] and adjudicated [between examinations 1 and 4] for the original cohort), and those with unclear smoking history or missing baseline pack-year information. Participants presented for subsequent examination cycles approximately every 2 years in the original cohort (median, 11 examinations) or 4 years in the offspring cohort (median, 7 examinations).
Exposure
Methods of quantifying smoking duration and intensity in the FHS have been previously described.16 Self-reported smoking habits at baseline were used to categorize participants as current, former, or never smokers and to calculate years since quitting for former smokers and pack-years of smoking for ever smokers. Smoking information was updated at subsequent FHS examination cycles to calculate cumulative smoking exposure variables.
Smoking status (current, former, or never) and years since quitting were modeled as time-varying exposures; each participant contributed person-examinations and person-time to the category reflecting smoking status at each assessment. For individuals who developed CVD, the event contributed to the smoking group in which a participant held membership at the time of CVD diagnosis.
To avoid misclassification bias, participants were censored after 1 missed examination plus an additional year without an update (ie, in the original cohort, 5 years without an update, and in the offspring cohort, 9 years without an update).16 Participants could not reenter the sample after being censored because of uncertainty about smoking patterns during their absence.
Outcome
Surveillance continued through December 2015 for development of CVD outcomes, including myocardial infarction, stroke, heart failure, and CVD death. For suspected CVD events, medical records were obtained with written patient consent; events were adjudicated by 3 study physicians as previously described.17,18,19
Covariates
Covariates were selected a priori and in part to maximize comparability with prior studies.8,9,20,21,22 These included established Framingham CVD risk factors (age, sex, systolic blood pressure, antihypertensive medication use, diabetes mellitus, and total cholesterol)13 as well as body mass index (BMI), alcohol consumption, and educational attainment, which may confound the association between smoking and CVD risk.20,21,22 Examination decade was included to address temporal trends (eAppendix in the Supplement). We did not include diet and exercise as potential confounders because they were infrequently captured in the original cohort.
Missing Data
Ten complete data sets in each cohort were imputed using multiple imputation by chained equation techniques to account for missingness; predictive mean matching23 was used for continuous variables and the discriminant function with a noninformative Jeffrey prior24 was used for categorical variables.
Statistical Analysis
Summary statistics were calculated in each cohort separately and combined and stratified by smoking status and intensity. Data from the original and offspring cohorts were analyzed pooled and separately in Poisson regression models to estimate age-, sex-, and education-adjusted incidence rates of first CVD events per 1000 person-years stratified by time-varying smoking status; current and former smokers were categorized as having more than or less than the median of 20 cumulative pack-years (eAppendix in the Supplement).
After confirming that the proportionality assumption was met by assessing interactions with time, Cox proportional hazards regression was used to estimate age-, sex-, and education-adjusted hazard ratios (HRs) for CVD risk comparing former and current smokers with never smokers. An a priori focus on heavier smokers was based on a prior examination of adverse event risk by smoking status in the FHS.16 In this sample, 71% of CVD events among ever smokers occurred in those with a cumulative smoking history of at least 20 pack-years. Primary analyses focused on these heavy smokers and never smokers in the pooled cohort. Analyses stratified by cohort were considered exploratory.
The time course and magnitude of decreasing CVD risk was analyzed in former heavy smokers vs current heavy smokers in 2 ways using the pooled cohort and original and offspring cohorts separately. First, a categorical variable was created for years since quitting (<5, 5 to <10, 10 to <15, 15 to <25, and ≥25), with current smokers serving as the reference group. Cox proportional hazards regression and Poisson regression were implemented to estimate cause-specific HRs and incidence rates per 1000 person-years, respectively, for CVD risk associated with categorical years since quitting. Second, years since quitting was modeled as a continuous variable (up to 25), assigning current smokers a value of 0. Former smokers with more than 25 years since quitting were assigned a value of 26. In Cox proportional hazards regression, the variable of continuous years since quitting was modeled using restricted cubic splines with 5 knots25 to allow a nonlinear association with the log hazard of CVD, presented graphically.
Analogous methods were used to model the time course and magnitude of lowering CVD risk in former heavy vs never smokers (the reference group in models including categorical years since quitting). In models with continuous years since quitting, never smokers were assigned a value of 50, well above all former smokers. To anchor the HR at years since quitting = 0, a sensitivity analysis explored the influence of including current smokers in the spline comparing former heavy smokers with never smokers.
Cox proportional hazards regression was performed in the pooled cohort (primary analysis) and in the original and offspring cohorts separately (exploratory analyses). A robust sandwich variance estimator was used in all models, and baseline hazards were allowed to differ by cohort in pooled analyses to account for birth cohort effects. Cox and Poisson models were adjusted for all covariates described above. Fourth-order polynomials were placed on age; cubic terms on BMI, total cholesterol, and systolic blood pressure; and quadratic terms on examination decade to allow nonlinear associations between the log hazards of CVD and these continuous predictors. Sensitivity analyses were adjusted for cumulative pack-years when comparing CVD risk among former and current smokers; second-order polynomials were placed on cumulative pack-years. Choice of polynomial terms for continuous covariates is described in the eAppendix in the Supplement. Further sensitivity analyses examined the use of linear terms for continuous predictors instead of polynomial terms. All dynamic variables were updated at each examination and modeled as time-varying covariates. Static variables (sex and education level) remained constant during follow-up.
A sensitivity analysis addressed the influence of observed baseline age differences in the original and offspring cohorts by restricting to offspring person-examinations among participants aged 50 years or older (the mean baseline age of the original cohort) and estimated incidence rates in heavy ever smokers and never smokers by categorical years since quitting. Additional sensitivity analyses included all ever smokers without restriction by pack-years. Other sensitivity analyses included either 2 or 6 smoking data assessments to approximate prior study designs.26
Statistical significance was assessed using a 2-sided P<.05. Statistical analyses used SAS version 9.4. (SAS Institute Inc).
Results
There were 8770 participants (3805 original cohort members and 4965 offspring cohort members) meeting inclusion criteria (Figure 1). In the pooled cohort, the mean age was 42.2 years (SD, 11.8 years), 56% were female, and 75% had at least a high school education (Table 1). Only 2% and 27% of the cohort had baseline diabetes and hypertension, respectively, and median BMI (calculated as weight in kilograms divided by height in meters squared) was in the normal range (median, 24.8; interquartile range, 22.4-27.7), but 78% of the cohort consumed alcohol in the past year (current drinkers), and only 40% of the cohort had never smoked. There were 5308 ever smokers with a median 17.2 (interquartile range, 7-30) baseline pack-years, including 2371 heavy ever smokers (406 [17%] former and 1965 [83%] current). In the original and offspring cohorts, heavy former and current smokers (≥20 pack-years) had similar cumulative pack-year distributions (eFigure in the Supplement) and CVD risk factors (eTables 1 and 2 in the Supplement). The proportion of missing data was low; 89% of offspring cohort person-examinations had complete data. However, in the original cohort, only 51% of person-examinations had complete data because information on alcohol consumption and blood glucose levels was not collected at all examinations.
Figure 1. Sample Derivation.
CVD indicates cardiovascular disease; FHS, Framingham Heart Study.
aTwo participants in the FHS offspring cohort were ineligible for sample inclusion because their last follow-up date was equal to their examination 1 date.
bPrevalent CVD defined as definite myocardial infarction, stroke (excluding transient ischemic attack), and heart failure.
cIncludes individuals who at baseline reported being a never smoker but with a greater than 0 pack-year history, had an age at quitting smoking that was older than their baseline age, or were completely missing smoking data.
dTo accurately calculate cumulative pack-years of smoking during the follow-up period, a reliable measure of smoking history at baseline was essential. Individuals excluded were missing pack-years of smoking at baseline because of inability to report age at starting/stopping smoking and/or intensity of smoking (cigarettes per day) prior to baseline.
eOriginal cohort participants were seen approximately every 2 years. After 5 years without an update (effectively 1 missed examination plus an additional year), individuals were censored to avoid carrying values forward for an extended period without reassessment. Similarly, offspring participants were seen approximately every 4 years and were thus censored after 9 years without an update (also corresponding to a single missed examination plus an additional year).
Table 1. Baseline Participant Characteristics.
| Characteristics | Pooled Cohort (n=8770) | Original Cohort (n=3805) | Offspring Cohort (n=4965) | |||
|---|---|---|---|---|---|---|
| No. | Summary | No. | Summary | No. | Summary | |
| Age, mean (SD), y | 8770 | 42.2 (11.8) | 3805 | 50.1 (8.5) | 4965 | 36.1 (10.4) |
| Sex, No. (%) | 8770 | 3805 | 4965 | |||
| Male | 3906 (44.5) | 1528 (40.2) | 2378 (47.9) | |||
| Female | 4864 (55.5) | 2277 (59.8) | 2587 (52.1) | |||
| Education, No. (%) | 7523 | 3760 | 3763 | |||
| Less than high school graduate | 1867 (24.8) | 1564 (41.6) | 303 (8.1) | |||
| High school graduate | 2435 (32.4) | 1144 (30.4) | 1291 (34.3) | |||
| More than high school | 3221 (42.8) | 1052 (28.0) | 2169 (57.6) | |||
| Blood pressure, mean (SD), mm Hg | 8769 | 3805 | 4964 | |||
| Systolic | 126.6 (20.1) | 132.9 (22.6) | 121.8 (16.4) | |||
| Diastolic | 80.6 (11.6) | 83.2 (12.0) | 78.6 (10.9) | |||
| Antihypertensive medication, No. (%) | 8751 | 276 (3.2) | 3797 | 124 (3.3) | 4954 | 152 (3.1) |
| Hypertension, No. (%) | 8761 | 2350 (26.8) | 3803 | 1402 (36.9) | 4958 | 948 (19.1) |
| Body mass index, median (IQR)a | 8759 | 24.8 (22.4-27.7) | 3798 | 25.3 (22.9-28.0) | 4961 | 24.5 (22.0-27.5) |
| Diabetes, No. (%) | 8645 | 163 (1.9) | 3771 | 75 (2.0) | 4874 | 88 (1.8) |
| Total cholesterol, mean (SD), mg/dL | 8699 | 216.3 (46.5) | 3764 | 238.9 (44.1) | 4935 | 199.0 (40.5) |
| Current drinker, No. (%)b | 8684 | 6744 (77.7) | 3764 | 2566 (68.2) | 4920 | 4178 (84.9) |
| Smoking, No. (%) | 8770 | 3805 | 4965 | |||
| Current | 4115 (46.9) | 1906 (50.1) | 2209 (44.5) | |||
| Former | 1193 (13.6) | 268 (7.0) | 925 (18.6) | |||
| Never | 3462 (39.5) | 1631 (42.9) | 1831 (36.9) | |||
| Cigarettes/d, median (IQR)c | 4115 | 20.0 (10.0-30.0) | 1906 | 20.0 (9.0-20.0) | 2209 | 20.0 (15.0-30.0) |
| Cumulative smoking pack-years, median (IQR) | ||||||
| Current smokers | 4115 | 18.8 (8.0-31.0) | 1906 | 21.4 (10.4-32.8) | 2209 | 16.0 (6.8-29.7) |
| Former smokers | 1193 | 12.0 (4.4-25.3) | 268 | 12.0 (3.0-27.6) | 925 | 12.0 (5.0-24.5)) |
| Years since quitting smoking, median (IQR)d | 1193 | 5.9 (3.0-10.0) | 268 | 3.0 (1.9-3.2) | 925 | 6.0 (3.0-10.0) |
Abbreviation: IQR, interquartile range.
SI conversion factor: To convert total cholesterol to millimoles per liter, multiply by 0.0259.
Calculated as weight in kilograms divided by height in meters squared.
Self-reported consumption of at least 1 alcoholic beverage per month.
Among current smokers only.
Among former smokers only.
Of 4115 current smokers at baseline, 1589 (38.6%) quit and never relapsed, while 2117 (51.4%) continued to smoke until they developed CVD or were censored. Most (84.7%) baseline former smokers remained abstinent during follow-up. Among baseline ever smokers, there were 591 smokers who relapsed (ie, began smoking again after reporting abstinence during at least 1 clinic visit). Abstinence periods ranged from 0 to 68 years (median, 3 years; interquartile range, 0-15 years).
During a 26.4-year median follow-up, participants experienced 2435 first CVD events (1612 in the original cohort and 823 in the offspring cohort) (eTable 3 in the Supplement). In both cohorts pooled and separately, current smoking was associated with significantly higher CVD incidence rates per 1000 person-years vs never smoking (Table 2). Incidence rate differences were 4.68 (95% CI, 3.56-5.99) in the pooled cohort, 5.00 (95% CI, 3.17-7.25) in the original cohort, and 4.50 (95% CI, 3.20-6.10) in the offspring cohort. After further categorizing current smokers by pack-years, the association was attenuated in the original cohort with less than 20 cumulative pack-years (incidence rate difference, 0.61; 95% CI, −1.73 to 3.57) but remained significant for current smokers with 20 or more pack-years in the original cohort (incidence rate difference, 6.62; 95% CI, 4.48-9.27) and all current smokers in both the offspring cohort (incidence rate difference among those with <20 pack-years, 3.03 [95% CI, 1.13-5.83] and among those with ≥20 pack-years, 4.92 [95% CI, 3.48-6.68]) and the pooled cohort (incidence rate difference among those with <20 pack-years, 1.65 [95% CI, 0.07-3.62] and among those with ≥20 pack-years, 6.00 [95% CI, 4.61-7.51]) (Table 2). Former heavy smoking in the pooled cohort and offspring cohort was also associated with increased CVD incidence compared with never smoking (pooled cohort incidence rate difference, 1.12 [95% CI, 0.26-2.04]; offspring cohort incidence rate difference, 1.42 [95% CI, 0.68-2.32]) (Table 2).
Table 2. Adjusted Risk of Incident CVD by Smoking Statusa.
| Smoking Status | Person-Examinations, No. | Incident CVD Cases, No. | Person-Years, No. | Incidence Rate Per 1000 Person-Years (95% CI)b,c | Hazard Ratio (95% CI)b,c | P Valueb |
|---|---|---|---|---|---|---|
| Pooled Cohort | ||||||
| Never | 29 089 | 895 | 91 227 | 6.24 (5.69-6.84) | 1 [Reference] | |
| Former | 20 654 | 874 | 68 921 | 6.47 (5.89-7.12) | 1.06 (0.96-1.16) | .27 |
| <20 Pack-years | 11 102 | 337 | 39 345 | 6.14 (5.41-6.95) | 0.95 (0.84-1.08) | .45 |
| ≥20 Pack-years | 9552 | 537 | 29 576 | 7.56 (6.74-8.47) | 1.17 (1.04-1.31) | .009 |
| Current | 21 111 | 666 | 67 478 | 11.11 (10.13-12.18) | 1.75 (1.57-1.96) | <.001 |
| <20 Pack-years | 7226 | 108 | 25 753 | 8.53 (7.00-10.39) | 1.25 (1.01-1.55) | .04 |
| ≥20 Pack-years | 13 885 | 558 | 41 725 | 12.71 (11.57-13.97) | 1.91 (1.70-2.14) | <.001 |
| Original Cohort | ||||||
| Never | 18 862 | 663 | 39 521 | 10.21 (9.14-11.41) | 1 [Reference] | |
| Former | 10 763 | 509 | 22 556 | 9.89 (8.73-11.22) | 1.00 (0.88-1.13) | .98 |
| <20 Pack-years | 5136 | 207 | 10 825 | 9.86 (8.38-11.58) | 0.99 (0.84-1.16) | .92 |
| ≥20 Pack-years | 5627 | 302 | 11 731 | 10.09 (8.68-11.72) | 1.03 (0.89-1.20) | .66 |
| Current | 14 445 | 440 | 30 313 | 15.34 (13.76-17.11) | 1.49 (1.31-1.71) | <.001 |
| <20 Pack-years | 4718 | 77 | 9862 | 11.13 (8.81-14.06) | 1.06 (0.83-1.35) | .66 |
| ≥20 Pack-years | 9727 | 363 | 20 450 | 16.75 (14.88-18.86) | 1.65 (1.44-1.91) | <.001 |
| Offspring Cohort | ||||||
| Never | 10 227 | 232 | 51 706 | 2.76 (2.32-3.28) | 1 [Reference] | |
| Former | 9891 | 365 | 46 365 | 3.42 (2.91-4.02) | 1.24 (1.05-1.47) | .01 |
| <20 Pack-years | 5966 | 130 | 28 520 | 2.73 (2.22-3.35) | 0.97 (0.79-1.20) | .81 |
| ≥20 Pack-years | 3925 | 235 | 17 845 | 4.28 (3.54-5.17) | 1.50 (1.24-1.82) | <.001 |
| Current | 6666 | 226 | 37 165 | 7.35 (6.30-8.56) | 2.63 (2.16-3.21) | <.001 |
| <20 Pack-years | 2508 | 31 | 15 890 | 5.90 (4.05-8.59) | 2.07 (1.40-3.06) | <.001 |
| ≥20 Pack-years | 4158 | 195 | 21 275 | 7.81 (6.65-9.17) | 2.74 (2.23-3.36) | <.001 |
Abbreviation: CVD, cardiovascular disease.
All data are time-updated; ie, as individuals begin and quit smoking, they contribute person-examinations and person-time to various groups. An individual’s event contributes only to the group he or she was in at the time of the event. Twenty cumulative pack-years was the median cumulative pack-years among former smokers at the time of quitting.
Incidence rates, hazard ratios, and P values include data from all 10 multiple imputations. Other data are based on the first imputation only.
Incidence rates and hazard ratios are adjusted for age, sex, and education.
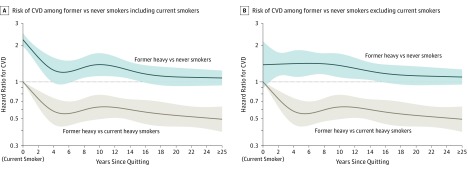
In adjusted models, smoking cessation was associated with a rapid decline in CVD risk vs continued smoking (Figure 2) such that risk was significantly lower within 5 years of cessation in the pooled cohort (HR, 0.61 [95% CI, 0.49-0.76]; incidence rates per 1000 person-years: current smoking, 11.56 [95% CI, 10.30-12.98]; quitting within 5 years, 6.94 [95% CI, 5.61-8.59]; difference, −4.51 [95% CI, −5.90 to −2.77]) (Table 3). In sensitivity analyses adjusting for cumulative pack-years, results were similar (HR, 0.62 [95% CI, 0.50-0.77]; incidence rate difference, −4.29 [95% CI, −5.64 to −2.59]) (eTable 4 in the Supplement). However, former heavy smoking was associated with higher CVD risk compared with never smoking until 10 to 15 years after cessation in the pooled cohort (HR, 1.25 [95% CI, 0.98-1.60]; incidence rates per 1000 person-years: never smoking, 5.09 [95% CI, 4.52-5.74]; quitting within 10 to <15 years, 6.31 [95% CI, 4.93-8.09]; difference, 1.27 [95% CI, −0.10 to 3.05]) (Table 3). As shown in Figure 2, 16 years after cessation was the point at which the 95% confidence interval for CVD risk among former smokers vs never smokers consistently included the null value of 1. Results remained consistent whether current smokers (ie, years since quitting = 0) were included in the analysis (HR, 1.16 [95% CI, 0.98-1.37]; incidence rate difference, 0.94 [95% CI, −0.12 to 2.17]) (Figure 2A) or not included in the analysis (HR, 1.17 [95% CI, 0.99-1.39]; incidence rate difference, 1.00 [95% CI, −0.06 to 2.29]) (Figure 2B). The time course of CVD risk appeared to differ by cohort (Table 3). In the original cohort, former heavy smoking was no longer significantly associated with increased CVD risk compared with never smoking within 5 to 10 years of cessation (HR, 1.28 [95% CI, 0.97-1.69]; incidence rate difference, 1.88 [95% CI, −0.20 to 4.64]), while in the offspring cohort, this did not occur until at least 25 years after cessation (HR, 1.10 [95% CI, 0.79-1.54]; incidence rate difference, 0.26 [95% CI, −0.54 to 1.39]). Sensitivity analyses with linear terms for continuous covariates instead of polynomial terms were comparable (eTable 5 in the Supplement).
Figure 2. Risk of Incident CVD in Heavy Ever and Never Smokers.
Plotted data are limited to never smokers and heavy ever smokers with at least 20 cumulative pack-years and are adjusted for age, sex, education, examination decade, systolic blood pressure, antihypertensive medication use, diabetes mellitus, body mass index, total cholesterol, and alcohol consumption. Dynamic variables are updated. Splines have 5 knots at 1, 4, 9, 15, and 22 years since quitting. Data from 3274 participants over 23 437 person-examinations contributed to the comparison of former vs current smokers in both panels. Shaded areas indicate 95% CIs. Panel A includes current smokers at years since quitting = 0 in the comparison of cardiovascular disease (CVD) risk among former smokers vs never smokers and contains data from 6720 individuals over 52 526 person-examinations. This anchors the hazard ratio at years since quitting = 0 to capture the steep decline in the first 4 years since quitting but creates some instability in the hazard ratio estimate around 4 years since quitting. Panel B excludes current smokers at years since quitting = 0 from the comparison of CVD risk among former smokers vs never smokers and contains data from 5190 individuals over 38 641 person-examinations. This plot cannot reflect the steep decline in the hazard ratio over the first 4 years but provides a more stable estimate of years since quitting.
Table 3. Multivariable-Adjusted Risk of Incident CVD by Smoking Status and Years Since Quitting: Never Smokers and Ever Smokers With at Least 20 Pack-Yearsa.
| Years Since Quitting | Person-Examinations, No. | Incident CVD Cases, No. | Person-Years, No. | Incidence Rate Per 1000 Person-Years (95% CI) | Former vs Current Smokers | Former vs Never Smokers | ||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |||||
| Pooled Cohort | ||||||||
| Current smokers | 13 885 | 558 | 41 725 | 11.56 (10.30-12.98) | 1 [Reference] | |||
| <5 | 2586 | 101 | 7851 | 6.94 (5.61-8.59) | 0.61 (0.49-0.76) | <.001 | 1.40 (1.12-1.73) | .002 |
| 5 to <10 | 2088 | 99 | 6342 | 7.04 (5.66-8.76) | 0.61 (0.49-0.77) | <.001 | 1.42 (1.15-1.77) | .001 |
| 10 to <15 | 1429 | 76 | 4455 | 6.31 (4.93-8.09) | 0.54 (0.42-0.70) | <.001 | 1.25 (0.98-1.60) | .08 |
| 15 to <25 | 2123 | 145 | 6741 | 6.11 (5.01-7.44) | 0.55 (0.45-0.68) | <.001 | 1.22 (1.00-1.47) | .045 |
| ≥25 | 1326 | 116 | 4187 | 5.02 (4.00-6.31) | 0.45 (0.35-0.58) | <.001 | 0.98 (0.78-1.22) | .85 |
| Never smokers | 29 089 | 895 | 91 227 | 5.09 (4.52-5.74) | 1 [Reference] | |||
| Original Cohort | ||||||||
| Current smokers | 9727 | 363 | 20 450 | 13.04 (11.21-15.18) | 1 [Reference] | |||
| <5 | 1711 | 70 | 3613 | 8.62 (6.63-11.20) | 0.68 (0.52-0.88) | .004 | 1.31 (1.01-1.70) | .04 |
| 5 to <10 | 1268 | 63 | 2623 | 8.39 (6.35-11.07) | 0.66 (0.49-0.87) | .004 | 1.28 (0.97-1.69) | .08 |
| 10 to <15 | 850 | 44 | 1757 | 7.45 (5.37-10.32) | 0.59 (0.42-0.83) | .002 | 1.11 (0.80-1.53) | .54 |
| 15 to <25 | 1128 | 63 | 2361 | 5.65 (4.24-7.53) | 0.49 (0.36-0.66) | <.001 | 0.86 (0.65-1.14) | .29 |
| ≥25 | 670 | 62 | 1378 | 5.97 (4.39-8.11) | 0.50 (0.35-0.73) | <.001 | 0.90 (0.66-1.22) | .49 |
| Never smokers | 18 862 | 663 | 39 521 | 6.72 (5.77-7.82) | 1 [Reference] | |||
| Offspring Cohort | ||||||||
| Current smokers | 4158 | 195 | 21 275 | 7.71 (6.27-9.48) | 1 [Reference] | |||
| <5 | 875 | 31 | 4239 | 3.83 (2.59-5.65) | 0.51 (0.34-0.75) | <.001 | 1.51 (1.02-2.25) | .04 |
| 5 to <10 | 820 | 36 | 3720 | 4.29 (2.97-6.18) | 0.57 (0.40-0.82) | .002 | 1.68 (1.17-2.41) | .005 |
| 10 to <15 | 579 | 32 | 2698 | 3.86 (2.61-5.72) | 0.50 (0.34-0.74) | <.001 | 1.48 (1.01-2.17) | .046 |
| 15 to <25 | 995 | 82 | 4379 | 4.70 (3.52-6.29) | 0.62 (0.46-0.83) | .002 | 1.78 (1.36-2.33) | <.001 |
| ≥25 | 656 | 54 | 2809 | 3.00 (2.10-4.28) | 0.41 (0.28-0.60) | <.001 | 1.10 (0.79-1.54) | .57 |
| Never smokers | 10 227 | 232 | 51 706 | 2.57 (2.07-3.20) | 1 [Reference] | |||
Abbreviation: CVD, cardiovascular disease.
This analysis is limited to never and ever heavy smokers with at least 20 cumulative pack-years. Incidence rates and hazard ratios are adjusted for age, sex, education, examination decade, systolic blood pressure, antihypertensive medication, diabetes mellitus, body mass index, total cholesterol, and alcohol consumption. All dynamic variables are time-updated.
Incidence rates of CVD among current smokers were higher in the original cohort (13.04; 95% CI, 11.21-15.18) than in the offspring cohort (7.71; 95% CI, 6.27-9.48) (Table 3). These differences were substantially attenuated in sensitivity analyses restricted to offspring person-examinations among heavy ever smokers and never smokers aged 50 years or older (incidence rate among current smokers, 11.50; 95% CI, 8.94-14.78) (eTable 6 in the Supplement). In sensitivity analyses including all ever smokers, results comparing CVD risk in former vs current and never smokers were similar (among former smokers quitting <5 years prior vs current smokers: HR, 0.60 [95% CI, 0.50-0.72]; incidence rate difference, −3.92 [95% CI, −4.91 to −2.75]; among former smokers quitting 10-15 years prior vs never smokers: HR, 1.08 [95% CI, 0.88-1.33]; incidence rate difference, 0.38 [95% CI, −0.57 to 1.56]) (eTable 7 in the Supplement). Compared with primary analyses using all available follow-up time points, sensitivity analyses with 6 smoking status assessments produced estimates of similar magnitude but using fewer smoking status assessments produced risk estimates that did not monotonically decrease with years since quitting (eTables 8 and 9 in the Supplement).
Discussion
This study found that compared with current heavy smoking, smoking cessation among former heavy smokers was associated with lower CVD risk within 5 years of cessation, reaffirming the cardiovascular benefit of smoking cessation demonstrated by others8,10,11,12,27 but also revealing a slow ensuing CVD risk decline over decades (Figure 2). Compared with never smoking, it took 10 to 15 years (pooled cohort) (5 to 10 years in the original cohort and ≥25 years in the offspring cohort) following cessation for former heavy smoking (vs never smoking) to cease being significantly associated with elevated CVD risk.
The upper estimate of this time course is a decade longer than that of the Nurses’ Health Study results for coronary heart disease and cardiovascular death8,28 and more than 20 years longer than in some prior reports for coronary heart disease10,11 and stroke.29 Results from the British Regional Heart Study12 found myocardial infarction risk to persist for more than 20 years after cessation, but findings were limited to men and smoking data were assessed only at baseline. Prior studies primarily examined single CVD components as opposed to composite CVD. In analyses of the full cohort (ie, including those with <20 pack-years), former smoking (vs never smoking) was no longer significantly associated with excess CVD risk within 10 to 15 years of cessation, highlighting the need to stratify by cumulative pack-years. Although the exact amount of time after quitting at which former smokers’ CVD risk ceases to differ significantly from that of never smokers is unknown (and may further depend on cumulative exposure), these findings support a longer time course of risk reduction than was previously thought, yielding implications for CVD risk stratification of former smokers.
Currently, the ASCVD Risk Estimator Plus14 is used by clinicians to help inform patients regarding their 10-year and lifetime CVD risk and to guide behavior changes to reduce CVD risk. Despite limitations,30,31 this model is well calibrated in the population for which it was intended32 and, importantly, improves on prior CVD risk calculators13,33,34,35 by differentiating risk between former and never smokers. However, the tool considers risk in former smokers to be equivalent to that of never smokers after 5 years since quitting. Thus, as the proportion of former smokers in the United States increases with more current smokers quitting, so does the potential to underestimate CVD risk using current tools, especially among heavier smokers. The present investigation does not support the assumption that former smokers achieve the same CVD risk as never smokers within 5 years of quitting. Future studies should investigate the extent to which including comprehensive data on smoking exposure, such as pack-years smoked and years since quitting, would improve the performance of existing CVD risk prediction tools and, by extension, CVD health outcomes.
In Figure 2A, CVD risk appeared to increase at about 10 years after cessation among former heavy smokers, but only when current smokers were included in the analysis. However, 95% confidence bands include a smooth decline in risk without this artifactual increase, as evidenced by the smooth curve when current smokers are not included (Figure 2B).
A strength of the present analysis is the rich data source including 2 distinct but similar cohorts with a median follow-up of more than 26 years. The FHS features frequent, repeated, in-person assessments of smoking status and intensity over the course of half a century or more, allowing for comprehensive lifetime capture of smoking. In addition, the FHS simultaneously collected data on CVD risk factors, which allows robust adjustment for confounders. Continuous participant follow-up for CVD incidence also allows for accurate and near-complete event capture. This investigation extends prior knowledge by using a self-reported smoking ascertainment method, including prospectively gathered and regularly time-updated data on smoking status and intensity collected during in-person visits. Sensitivity analyses with fewer smoking status assessments led to inconsistent results in which relative risk among former smokers did not decrease in a monotonic fashion with increasing years since quitting.
In lifetime analyses, people develop comorbidities with increasing age, which could influence smoking cessation patterns. To minimize this potential bias from unmeasured confounding, analyses accounted for baseline differences between cohorts (eg, age), differences among current, former, and never smokers, temporal trends (eg, declining smoking rate, increasing BMI), birth cohort effects, and factors that could influence both smoking cessation and CVD risk, including sociodemographic factors and known CVD risk factors, which were time-updated. Thus, the effect of unmeasured confounding on the overall findings is likely modest.
Limitations
This study also has several limitations. First, compared with some prior studies,26 the sample size was smaller, but it was large enough to address the questions of interest and also provided thoroughly captured longitudinal smoking data. The limited sample size also precluded analyses yielding CVD risk estimates among subcategories of lighter smokers (<20 lifetime pack-years); thus, the primary findings are applicable to ever smokers with a cumulative smoking history of at least 20 pack-years. Second, information on environmental tobacco smoke exposure and use of other types of tobacco was not available for most participants and was not included. Third, as with other investigations using data from the FHS original and offspring cohorts, this investigation is composed primarily of white individuals of European ancestry, potentially limiting generalizability of results to individuals of other races/ethnicities.
Conclusions
Among heavy smokers, smoking cessation was associated with significantly lower CVD risk within 5 years relative to current smokers. However, relative to never smokers, former smokers’ risk remained significantly elevated beyond 5 years after smoking cessation.
eTable 1. Baseline Characteristics of Original Cohort by Smoking Status and Intensity
eTable 2. Baseline Characteristics of Offspring Cohort by Smoking Status and Intensity
eTable 3. CVD Event Types by Cohort
eTable 4. Adjusted Risk of Incident CVD in Heavy Former vs. Current Smokers – Additionally Adjusted for Cumulative Pack-Years
eTable 5. Comparison of Adjusted Risk of Incident CVD by Years Since Quitting: Polynomial Versus Linear Terms
eTable 6. Comparison of CVD Incidence Rates by Cohort and Influence of Age
eTable 7. Adjusted Risk of Incident CVD Among all Current and Former Smokers
eTable 8. Adjusted Risk of Incident CVD Among Heavy Ever and Never Smokers Using 6 Time Points
eTable 9. Adjusted Risk of Incident CVD Among Heavy Ever and Never Smokers Using 2 Time Points
eFigure. Cumulative Pack-Years Among Current and Former Smokers With ≥20 Pack-Years
eAppendix. Supplemental Methods
eReferences
References
- 1.Dawber TR. Summary of recent literature regarding cigarette smoking and coronary heart disease. Circulation. 1960;22:164-166. [PubMed] [Google Scholar]
- 2.Doll R, Hill AB. The mortality of doctors in relation to their smoking habits: a preliminary report. Br Med J. 1954;1(4877):1451-1455. doi: 10.1136/bmj.1.4877.1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surgeon General’s Advisory Committee on Smoking and Health Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington, DC: US Government Printing Office; 1964. [Google Scholar]
- 4.Doyle JT, Dawber TR, Kannel WB, Heslin AS, Kahn HA. Cigarette smoking and coronary heart disease: combined experience of the Albany and Framingham studies. N Engl J Med. 1962;266:796-801. doi: 10.1056/NEJM196204192661602 [DOI] [PubMed] [Google Scholar]
- 5.Fenelon A, Preston SH. Estimating smoking-attributable mortality in the United States. Demography. 2012;49(3):797-818. doi: 10.1007/s13524-012-0108-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Department of Health and Human Services The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General Rockville, MD: Dept of Health and Human Services; 2014:1081.
- 7.Department of Health and Human Services The Health Consequences of Smoking: Cardiovascular Disease: A Report of the Surgeon General Rockville, MD: Dept of Health and Human Services; 1983:385.
- 8.Kawachi I, Colditz GA, Stampfer MJ, et al. Smoking cessation and time course of decreased risks of coronary heart disease in middle-aged women. Arch Intern Med. 1994;154(2):169-175. doi: 10.1001/archinte.1994.00420020075009 [DOI] [PubMed] [Google Scholar]
- 9.Thun MJ, Carter BD, Feskanich D, et al. 50-Year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368(4):351-364. doi: 10.1056/NEJMsa1211127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg L, Kaufman DW, Helmrich SP, Shapiro S. The risk of myocardial infarction after quitting smoking in men under 55 years of age. N Engl J Med. 1985;313(24):1511-1514. doi: 10.1056/NEJM198512123132404 [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg L, Palmer JR, Shapiro S. Decline in the risk of myocardial infarction among women who stop smoking. N Engl J Med. 1990;322(4):213-217. doi: 10.1056/NEJM199001253220401 [DOI] [PubMed] [Google Scholar]
- 12.Cook DG, Shaper AG, Pocock SJ, Kussick SJ. Giving up smoking and the risk of heart attacks: a report from the British Regional Heart Study. Lancet. 1986;2(8520):1376-1380. doi: 10.1016/S0140-6736(86)92017-9 [DOI] [PubMed] [Google Scholar]
- 13.D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. doi: 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 14.Lloyd-Jones DM, Huffman MD, Karmali KN, et al. Estimating longitudinal risks and benefits from cardiovascular preventive therapies among medicare patients: the Million Hearts longitudinal ASCVD risk assessment tool: a special report from the American Heart Association and American College of Cardiology. Circulation. 2017;135(13):e793-e813. doi: 10.1161/CIR.0000000000000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamal A, Phillips E, Gentzke AS, et al. Current cigarette smoking among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(2):53-59. doi: 10.15585/mmwr.mm6702a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tindle HA, Stevenson Duncan M, Greevy RA, et al. Lifetime smoking history and risk of lung cancer: results from the Framingham Heart Study. J Natl Cancer Inst. 2018;110(11):1201-1207. doi: 10.1093/jnci/djy041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doyle JT, Dawber TR, Kannel WB, Kinch SH, Kahn HA. The relationship of cigarette smoking to coronary heart disease: the second report of the combined experience of the Albany, NY and Framingham, Mass studies. JAMA. 1964;190:886-890. doi: 10.1001/jama.1964.03070230022006 [DOI] [PubMed] [Google Scholar]
- 18.Lloyd-Jones DM, Martin DO, Larson MG, Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;129(12):1020-1026. doi: 10.7326/0003-4819-129-12-199812150-00005 [DOI] [PubMed] [Google Scholar]
- 19.Fox CS, Evans JC, Larson MG, et al. A comparison of death certificate out-of-hospital coronary heart disease death with physician-adjudicated sudden cardiac death. Am J Cardiol. 2005;95(7):856-859. doi: 10.1016/j.amjcard.2004.12.011 [DOI] [PubMed] [Google Scholar]
- 20.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342(7795):d671. doi: 10.1136/bmj.d671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925-1932. doi: 10.1016/j.jacc.2008.12.068 [DOI] [PubMed] [Google Scholar]
- 22.Clair C, Rigotti NA, Porneala B, et al. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA. 2013;309(10):1014-1021. doi: 10.1001/jama.2013.1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Little RJA. Missing-data adjustments in large surveys. J Bus Econ Stat. 1988;6(3):287-296. doi: 10.2307/1391878 [DOI] [Google Scholar]
- 24.Schafer J. Analysis of Incomplete Multivariate Data. New York, NY: Chapman & Hall; 1997. doi: 10.1201/9781439821862 [DOI] [Google Scholar]
- 25.Harrell F. Regression Modeling Strategies with Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed New York, NY: Springer; 2015. [Google Scholar]
- 26.Doll R, Peto R, Boreham J, Sutherland I. Mortality from cancer in relation to smoking: 50 years observations on British doctors. Br J Cancer. 2005;92(3):426-429. doi: 10.1038/sj.bjc.6602359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed AA, Patel K, Nyaku MA, et al. Risk of heart failure and death after prolonged smoking cessation: role of amount and duration of prior smoking. Circ Heart Fail. 2015;8(4):694-701. doi: 10.1161/CIRCHEARTFAILURE.114.001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawachi I, Colditz GA, Stampfer MJ, et al. Smoking cessation in relation to total mortality rates in women: a prospective cohort study. Ann Intern Med. 1993;119(10):992-1000. doi: 10.7326/0003-4819-119-10-199311150-00005 [DOI] [PubMed] [Google Scholar]
- 29.Kawachi I, Colditz GA, Stampfer MJ, et al. Smoking cessation and decreased risk of stroke in women. JAMA. 1993;269(2):232-236. doi: 10.1001/jama.1993.03500020066033 [DOI] [PubMed] [Google Scholar]
- 30.Amin NP, Martin SS, Blaha MJ, Nasir K, Blumenthal RS, Michos ED. Headed in the right direction but at risk for miscalculation: a critical appraisal of the 2013 ACC/AHA risk assessment guidelines. J Am Coll Cardiol. 2014;63(25 pt A):2789-2794. doi: 10.1016/j.jacc.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67(18):2118-2130. doi: 10.1016/j.jacc.2016.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311(14):1406-1415. doi: 10.1001/jama.2014.2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421. doi: 10.1161/circ.106.25.3143 [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611-619. doi: 10.1001/jama.297.6.611 [DOI] [PubMed] [Google Scholar]
- 35.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837-1847. doi: 10.1161/01.CIR.97.18.1837 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline Characteristics of Original Cohort by Smoking Status and Intensity
eTable 2. Baseline Characteristics of Offspring Cohort by Smoking Status and Intensity
eTable 3. CVD Event Types by Cohort
eTable 4. Adjusted Risk of Incident CVD in Heavy Former vs. Current Smokers – Additionally Adjusted for Cumulative Pack-Years
eTable 5. Comparison of Adjusted Risk of Incident CVD by Years Since Quitting: Polynomial Versus Linear Terms
eTable 6. Comparison of CVD Incidence Rates by Cohort and Influence of Age
eTable 7. Adjusted Risk of Incident CVD Among all Current and Former Smokers
eTable 8. Adjusted Risk of Incident CVD Among Heavy Ever and Never Smokers Using 6 Time Points
eTable 9. Adjusted Risk of Incident CVD Among Heavy Ever and Never Smokers Using 2 Time Points
eFigure. Cumulative Pack-Years Among Current and Former Smokers With ≥20 Pack-Years
eAppendix. Supplemental Methods
eReferences