Key Points
Question
Are thyroid function test abnormalities or thyroid autoimmunity a risk factor for preterm birth?
Findings
In this individual participant data meta-analysis of 19 prospective cohort studies including 47 045 women, subclinical hypothyroidism (odds ratio [OR], 1.29), isolated hypothyroxinemia (OR, 1.46), and thyroid peroxidase antibody positivity (OR, 1.33) were each significantly associated with a higher risk of preterm birth.
Meaning
These findings provide evidence that subclinical hypothyroidism, isolated hypothyroxinemia, and thyroid peroxidase antibody positivity in pregnant women are risk factors for preterm birth.
Abstract
Importance
Maternal hypothyroidism and hyperthyroidism are risk factors for preterm birth. Milder thyroid function test abnormalities and thyroid autoimmunity are more prevalent, but it remains controversial if these are associated with preterm birth.
Objective
To study if maternal thyroid function test abnormalities and thyroid autoimmunity are risk factors for preterm birth.
Data Sources and Study Selection
Studies were identified through a search of the Ovid MEDLINE, EMBASE, Web of Science, the Cochrane Central Register of Controlled Trials, and Google Scholar databases from inception to March 18, 2018, and by publishing open invitations in relevant journals. Data sets from published and unpublished prospective cohort studies with data on thyroid function tests (thyrotropin [often referred to as thyroid-stimulating hormone or TSH] and free thyroxine [FT4] concentrations) or thyroid peroxidase (TPO) antibody measurements and gestational age at birth were screened for eligibility by 2 independent reviewers. Studies in which participants received treatment based on abnormal thyroid function tests were excluded.
Data Extraction and Synthesis
The primary authors provided individual participant data that were analyzed using mixed-effects models.
Main Outcomes and Measures
The primary outcome was preterm birth (<37 weeks’ gestational age).
Results
From 2526 published reports, 35 cohorts were invited to participate. After the addition of 5 unpublished data sets, a total of 19 cohorts were included. The study population included 47 045 pregnant women (mean age, 29 years; median gestational age at blood sampling, 12.9 weeks), of whom 1234 (3.1%) had subclinical hypothyroidism (increased thyrotropin concentration with normal FT4 concentration), 904 (2.2%) had isolated hypothyroxinemia (decreased FT4 concentration with normal thyrotropin concentration), and 3043 (7.5%) were TPO antibody positive; 2357 (5.0%) had a preterm birth. The risk of preterm birth was higher for women with subclinical hypothyroidism than euthyroid women (6.1% vs 5.0%, respectively; absolute risk difference, 1.4% [95% CI, 0%-3.2%]; odds ratio [OR], 1.29 [95% CI, 1.01-1.64]). Among women with isolated hypothyroxinemia, the risk of preterm birth was 7.1% vs 5.0% in euthyroid women (absolute risk difference, 2.3% [95% CI, 0.6%-4.5%]; OR, 1.46 [95% CI, 1.12-1.90]). In continuous analyses, each 1-SD higher maternal thyrotropin concentration was associated with a higher risk of preterm birth (absolute risk difference, 0.2% [95% CI, 0%-0.4%] per 1 SD; OR, 1.04 [95% CI, 1.00-1.09] per 1 SD). Thyroid peroxidase antibody–positive women had a higher risk of preterm birth vs TPO antibody–negative women (6.6% vs 4.9%, respectively; absolute risk difference, 1.6% [95% CI, 0.7%-2.8%]; OR, 1.33 [95% CI, 1.15-1.56]).
Conclusions and Relevance
Among pregnant women without overt thyroid disease, subclinical hypothyroidism, isolated hypothyroxinemia, and TPO antibody positivity were significantly associated with higher risk of preterm birth. These results provide insights toward optimizing clinical decision-making strategies that should consider the potential harms and benefits of screening programs and levothyroxine treatment during pregnancy.
This individual participant data meta-analysis pooled data from 19 cohort studies to assess whether maternal thyroid function test abnormalities and thyroid autoimmunity are risk factors for preterm birth among pregnant women without overt thyroid disease.
Introduction
Preterm birth complicates 5% to 15% of births worldwide. It is the most important direct cause of morbidity and mortality in children younger than 5 years,1 and is an important risk factor for psychiatric, metabolic, cardiovascular, and renal disease later in life.2,3 However, in the majority of cases, no known risk factors can be identified.4,5
Thyroid hormone regulates metabolism, growth, and development in most tissues of the human body, including various physiological processes related to preterm birth, such as placental development and function, fetal growth, and expression of neuropeptides involved in the onset of labor.6,7,8,9 Overt hypothyroidism and hyperthyroidism during pregnancy are well-known risk factors for preterm birth and occur in approximately 0.5% and 0.05% of pregnancies, respectively.10,11 During pregnancy, thyroid function test abnormalities (subclinical hypothyroidism and isolated hypothyroxinemia) and thyroid autoimmunity are much more frequent than overt thyroid disease.11 However, it remains to be determined whether such thyroid function test abnormalities are risk factors for preterm birth.
Estimates for the association of subclinical hypothyroidism or isolated hypothyroxinemia with preterm birth in previous observational studies range from odds ratios (ORs) of 0.57 to 3.3.12,13,14,15 The interpretation of these studies is limited by 2 main factors. First, the studies have used different upper limits for thyrotropin (often referred to as thyroid-stimulating hormone or TSH; ranging from >2.5 to >6.0 mIU/L). Second, most are single-center studies and lack statistical power. Furthermore, only a few studies have investigated whether isolated hypothyroxinemia could be a risk factor for adverse pregnancy outcomes.
This study assessed whether thyroid function test abnormalities and thyroid peroxidase (TPO) antibody positivity were associated with preterm birth.
Methods
The Consortium on Thyroid and Pregnancy is a collaboration of prospective birth cohorts that aims to study the association of maternal thyroid function and autoimmunity with adverse pregnancy and child outcomes. For the current study, we followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for Individual Patient Data and preregistered the study protocol (CRD42016043494), which appears in Supplement 1 along with an outline of protocol deviations.
To identify studies for inclusion, we conducted a systematic literature search for articles on the association of thyroid function or autoimmunity with preterm birth published from database inception to March 18, 2018, without language restrictions, using the Ovid MEDLINE, EMBASE, Web of Science, Cochrane Central Register of Controlled Trials, and Google Scholar databases (eMethods in Supplement 2). In addition, unpublished data were identified via open invitations sent to relevant journals,16,17 international conferences, social media, and personal contacts. The search was repeated on June 19, 2019, to identify studies published after March 18, 2018, that would have been eligible for inclusion.
We included prospective cohort studies that included participants consecutively without selection criteria related to health status (such as comorbidities or thyroid disease) and had data on thyrotropin concentration, free thyroxine (FT4) concentration, or TPO antibody measurements and data on gestational age at birth. We excluded studies in which participants received treatment based on abnormal thyroid function tests (predominantly hospital-based cohorts) or studies that only included women with overt thyroid disease.
Possible studies for inclusion were independently assessed for suitability by 2 of the authors (T.I.M.K. and P.N.T.) and any disagreement was resolved by discussion with a third author (R.P.P.). Investigators from each eligible study were invited to join the consortium. Study quality and risk of bias were assessed using the Newcastle-Ottawa scale.
Local institutional review board approval and participant informed consent was obtained for all separate cohorts; no additional approvals were obtained for the current study. After obtaining individual participant data, all participants with data on thyrotropin concentration, FT4 concentration, or TPO antibody concentration, and gestational age at birth were included. We excluded participants who had a miscarriage or perinatal death, preexisting thyroid disease, thyroid-interfering medication use, in vitro fertilization, or multiple pregnancies.
Primary and Secondary Outcomes
The primary outcome was preterm birth defined as a gestational age at birth of less than 37 weeks. Gestational age was assessed using either ultrasound measurements or time after the last menstrual period. Secondary outcomes were very preterm birth (<32 weeks’ gestational age) and the gestational age at birth.
Exposures
Exposure variables included thyroid function test abnormalities, continuous thyroid function test measurements (thyrotropin and FT4 concentrations), TPO antibody positivity, and thyroglobulin antibody positivity. Thyroid function test abnormalities were defined according to cohort-specific 2.5th and 97.5th population percentiles for thyrotropin and FT4 concentrations after exclusion of TPO antibody–positive women. Subclinical hypothyroidism was defined as a thyrotropin concentration above the 97.5th percentile and a FT4 concentration within the normal range (ie, 2.5th-97.5th percentile). Overt hyperthyroidism was defined as a thyrotropin concentration below the 2.5th percentile and a FT4 concentration above the 97.5th percentile. Subclinical hyperthyroidism was defined as a thyrotropin concentration below the 2.5th percentile and a FT4 concentration within the normal range. Isolated hypothyroxinemia was defined as a FT4 concentration below the 2.5th percentile and a thyrotropin concentration within the normal range. Thyroid peroxidase antibody positivity and thyroglobulin antibody positivity were defined in a cohort-specific manner according to cutoffs recommended by the manufacturer. For analyses with continuous thyrotropin and FT4 concentrations as exposure variables, the concentrations for all cohorts were log-transformed and then standardized to population-specific standard deviation scores after removal of outliers (±4 SD from the mean) to enable comparisons among different cohorts and assays.
Statistical Analyses
Primary Analyses
We studied the association of thyroid function test abnormalities (the reference group was women with normal thyroid), thyrotropin and FT4 concentrations continuously, and TPO and thyroglobulin antibody positivity with preterm birth and very preterm birth using generalized linear mixed models with a random intercept for each cohort. For the same exposures, the association with gestational age at birth was studied using linear mixed models with a random intercept for each cohort.
The primary analyses were repeated with a 2-step approach by using random-effect models according to the DerSimonian and Laird method to pool estimates and the Firth bias reduction method in case of near or complete separation in smaller cohorts. Heterogeneity across studies was assessed using the I2 statistic. To evaluate potential publication bias, funnel plots and Egger tests were used. All analyses were adjusted for maternal age, body mass index (BMI), ethnicity, smoking, parity, gestational age at blood sampling, and fetal sex. We used multilevel multiple imputation18 for missing data on covariates. Five imputed data sets were created and pooled for analyses using Rubin rules.19
Secondary and Sensitivity Analyses
We performed a prespecified sensitivity analysis for which analyses on thyroid function or thyroid function test abnormalities were additionally adjusted for TPO antibody positivity. A post hoc sensitivity analysis assessed whether the association of TPO antibody positivity with preterm birth and very preterm birth differed for each increase in continuous thyrotropin concentration by adding a product interaction term into the models and stratification of analyses according to thyrotropin cutoffs recommended by the guidelines of the American Thyroid Association.20 To assess TPO antibody positivity, a post hoc sensitivity analysis explored different cutoffs based on a recent study showing a dose-dependent effect on thyroid function (alternative cutoffs: 94th population percentile, higher absolute concentrations of >100 and >500 mIU/L, and higher relative concentrations of 2 × and 5 × the assay cutoff).21 In addition, a prespecified analysis explored whether the association of thyrotropin concentration, FT4 concentration, or TPO antibody positivity differed according to differences in gestational age at the time of blood sampling, BMI, or parity.
A 2-sided threshold for statistical significance of <.05 was used. Because no adjustment was made for multiple testing, findings from the secondary analyses and sensitivity analyses should be interpreted as exploratory. All statistical analyses were performed using R version 3.4.4 (R Project for Statistical Computing).
Results
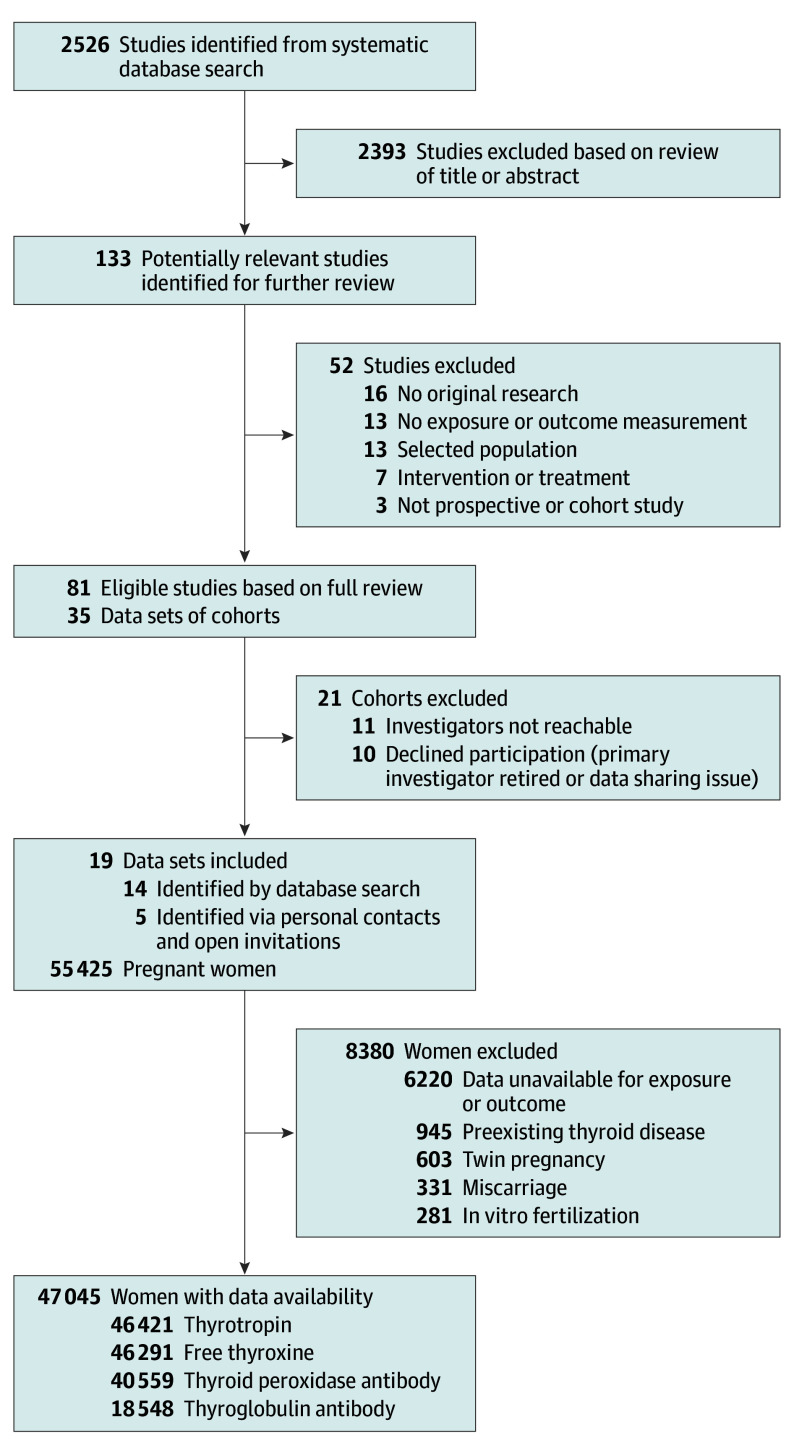
From 2526 published reports, 133 remained eligible for inclusion based on screening of the title and abstract (Figure 1). After reading the full text, a total of 35 cohorts were invited to participate. Five unpublished data sets were added via personal contacts and responses from open invitations. Four studies were published after the index date of our systematic search that otherwise would have been eligible for inclusion. A total of 19 cohorts from Europe, the United States, Chile, Australia, Pakistan, and Japan responded to the invitation and were able to participate.
Figure 1. Flowchart of the Study and Participant Selections.
After exclusions, the final study population comprised 47 045 participants (Figure 1), of whom 1234 (3.1%) had subclinical hypothyroidism (increased thyrotropin concentration with normal FT4 concentration), 904 (2.2%) had isolated hypothyroxinemia (decreased FT4 concentration with normal thyrotropin concentration), and 3043 (7.5%) were TPO antibody positive. Preterm birth and very preterm birth occurred in 2357 (5.0%) and 349 (0.7%) pregnancies, respectively (Table). Cohort-specific population characteristics, details on data collection, cohort-specific number of participants with available thyroid function and gestational age at birth, data quality assessment by the Newcastle-Ottawa scale (indicating high-quality data for all cohorts), and cohort-specific percentile cutoffs for thyroid function test abnormalities (thyrotropin and FT4 concentrations) appear in eTables 1-4 in Supplement 2.
Table. Characteristics of the Total Study Population (N = 47 045).
| No. of Participants/ Total No. (%)a |
Median (95% Range) | |
|---|---|---|
| Maternal Demographics | ||
| Age, y | 46 893 | 29.0 (5.2)b |
| Gestational age at blood sampling, wk | 46 876 | 12.9 (7.0-39.7) |
| Body mass indexc | 31 728 | 23.9 (4.4)b |
| Parity | ||
| 0 | 24 180/44 336 (54.5) | |
| 1 | 13 217/44 336 (29.8) | |
| 2 | 4435/44 336 (10.0) | |
| ≥3 | 2504/44 336 (5.6) | |
| Smoking status | ||
| Nonsmoker or past smoker | 38 733/43 662 (88.7) | |
| Current smoker | 4929/43 662 (11.3) | |
| Education leveld | ||
| Low | 9677/31 574 (30.6) | |
| Middle | 10 647/31 574 (33.7) | |
| High | 11 250/31 574 (35.6) | |
| Maternal Concentrations | ||
| Thyrotropin, mIU/Le | 46 421 | 1.33 (0.13-4.52) |
| Free thyroxine, pmol/L | 46 291 | 13.0 (7.2-21.9) |
| Thyroid peroxidase antibody positivity | 3043/40 559 (7.5) | |
| Thyroglobulin antibody positivity | 1080/18 548 (5.8) | |
| Pregnancy Characteristics | ||
| Gestational age at birth, wk | 47 045 | 39.9 (35.3-42.0) |
| Sex | ||
| Male | 18 936/37 355 (50.7) | |
| Female | 18 419/37 355 (49.3) | |
| Late preterm birth (gestational age <37 wk)f | 2357/47 045 (5.0) | |
| Early preterm birth (gestational age <32 wk) | 349/47 045 (0.7) | |
SI conversion factor: To convert free thyroxine to ng/dL, divide by 12.871.
The total No. indicates the No. of participants with available data on the thyroid function tests. Cohort-specific descriptive characteristics appear in eTable 1 in Supplement 2.
Expressed as mean (SD).
Calculated as weight in kilograms divided by height in meters squared.
Defined as estimated years of education beyond primary school (low: 0-4 years; middle: 4-8 years; high: >8 years).
Often referred to as thyroid-stimulating hormone or TSH.
Includes very preterm birth (<32 weeks’ gestational age).
Data on specific covariates were missing for up to 33% of the women and from as many as 3 cohorts (total percentage of missing data for maternal age: 0.3% [0 cohorts]; gestational age at the time of blood sampling: 0.4% [0 cohorts]; parity: 5.8% [1 cohort]; smoking status: 7.2% [1 cohort]; sex of the child: 21.4% [2 cohorts]; BMI: 32.9% [3 cohorts]). The women that were not included due to missing data on gestational age at birth had similar mean standardized thyrotropin concentrations in SDs vs those who were included (−0.03 vs 0, respectively; P = .28), but different mean standardized FT4 concentrations in SDs (0.06 vs 0; P = .05) and different proportions with TPO antibody positivity (13.0% vs 7.5%; P < .001) (eTable 5 in Supplement 2).
Preterm and Very Preterm Birth
Thyroid Function Test Abnormalities
Women with subclinical hypothyroidism had a higher risk of preterm birth vs euthyroid women (6.1% vs 5.0%, respectively; absolute risk difference, 1.4% [95% CI, 0% to 3.2%]; OR, 1.29 [95% CI, 1.01 to 1.64]), but not of very preterm birth (0.7% vs 0.8%; absolute risk difference, 0% [95% CI, −0.3% to 0.8%]; OR, 1.03 [95% CI, 0.52 to 1.20]; Figure 2). Women with isolated hypothyroxinemia had a higher risk of preterm birth vs euthyroid women (7.1% vs 5.0%, respectively; absolute risk difference, 2.3% [95% CI, 0.6% to 4.5%]; OR, 1.46 [95% CI, 1.12 to 1.90]) and a higher risk of very preterm birth (1.9% vs 0.8%; absolute risk difference, 1.2% [95% CI, 0.4% to 2.5%]; OR, 2.57 [95% CI, 1.55 to 4.27]). Among women with overt hyperthyroidism, there was no statistically significant difference in the rate of preterm birth vs euthyroid women (4.0% vs 5.0%, respectively; absolute risk difference, −1.2% [95% CI, −2.8% to 1.7%]; OR, 0.76 [95% CI, 0.43 to 1.34]; Figure 2).
Figure 2. Association of Thyroid Function Test Abnormalities With Preterm Birth.
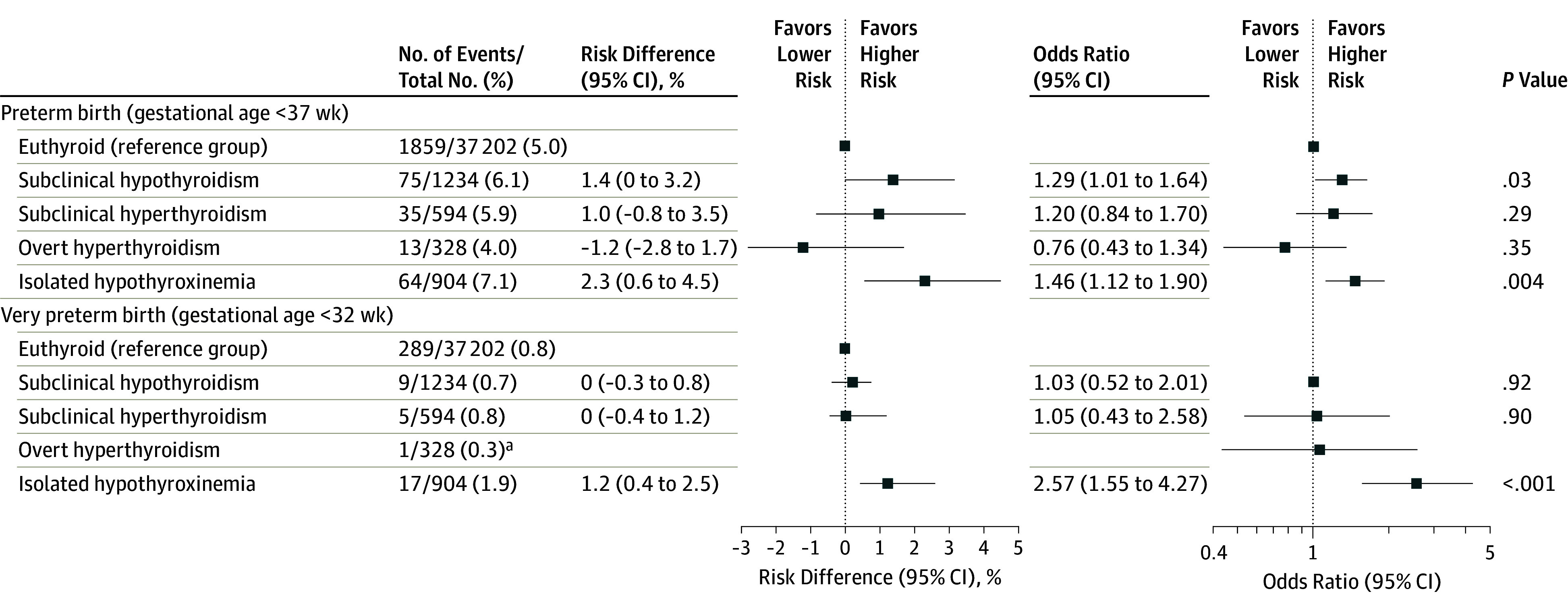
All analyses were adjusted for maternal age, body mass index, ethnicity, smoking, parity, gestational age at blood sampling, and fetal sex. Euthyroid was defined as the 2.5th-97.5th cohort-specific percentile for thyrotropin (often referred to as thyroid-stimulating hormone or TSH) and free thyroxine (FT4) concentrations; subclinical hypothyroidism, increased thyrotropin concentration with a normal FT4 concentration; subclinical hyperthyroidism, decreased thyrotropin concentration with a normal FT4 concentration; overt hyperthyroidism, decreased thyrotropin concentration with an increased FT4 concentration; and isolated hypothyroxinemia, a normal thyrotropin concentration with a decreased FT4 concentration. These clinical entities were calculated for cohorts that had thyrotropin concentration, FT4 concentration, and thyroid peroxidase antibody data available. Absolute differences and corresponding 95% CIs were back-calculated from the results of multivariable models and adjusted for baseline risk imprecision.
aThere were too few samples to conduct a reliable analysis.
Continuous Analyses of Thyroid Function
Continuous analyses showed that each 1-SD higher maternal thyrotropin concentration was significantly associated with a 4% higher relative risk of preterm birth (absolute risk difference, 0.2% [95% CI, 0% to 0.4%] per 1 SD; OR, 1.04 [95% CI, 1.00 to 1.09] per 1 SD), but not very preterm birth (absolute risk difference, 0% [95% CI, −0.05% to 0.10%] per 1 SD; OR, 1.04 [95% CI, 0.93 to 1.16] per 1 SD; Figure 3 and eTable 6 in Supplement 2). Each 1-SD higher maternal FT4 concentration was not associated with preterm birth, but was significantly associated with a 12% lower risk of very preterm birth (absolute risk difference, −0.10% [95% CI, −0.20% to −0.01%] per 1 SD; OR, 0.88 per 1 SD [95% CI, 0.79 to 0.95]; Figure 3 and eTable 6 in Supplement 2).
Figure 3. Association of Thyrotropin and Free Thyroxine (FT4) Concentrations With Preterm Birth.
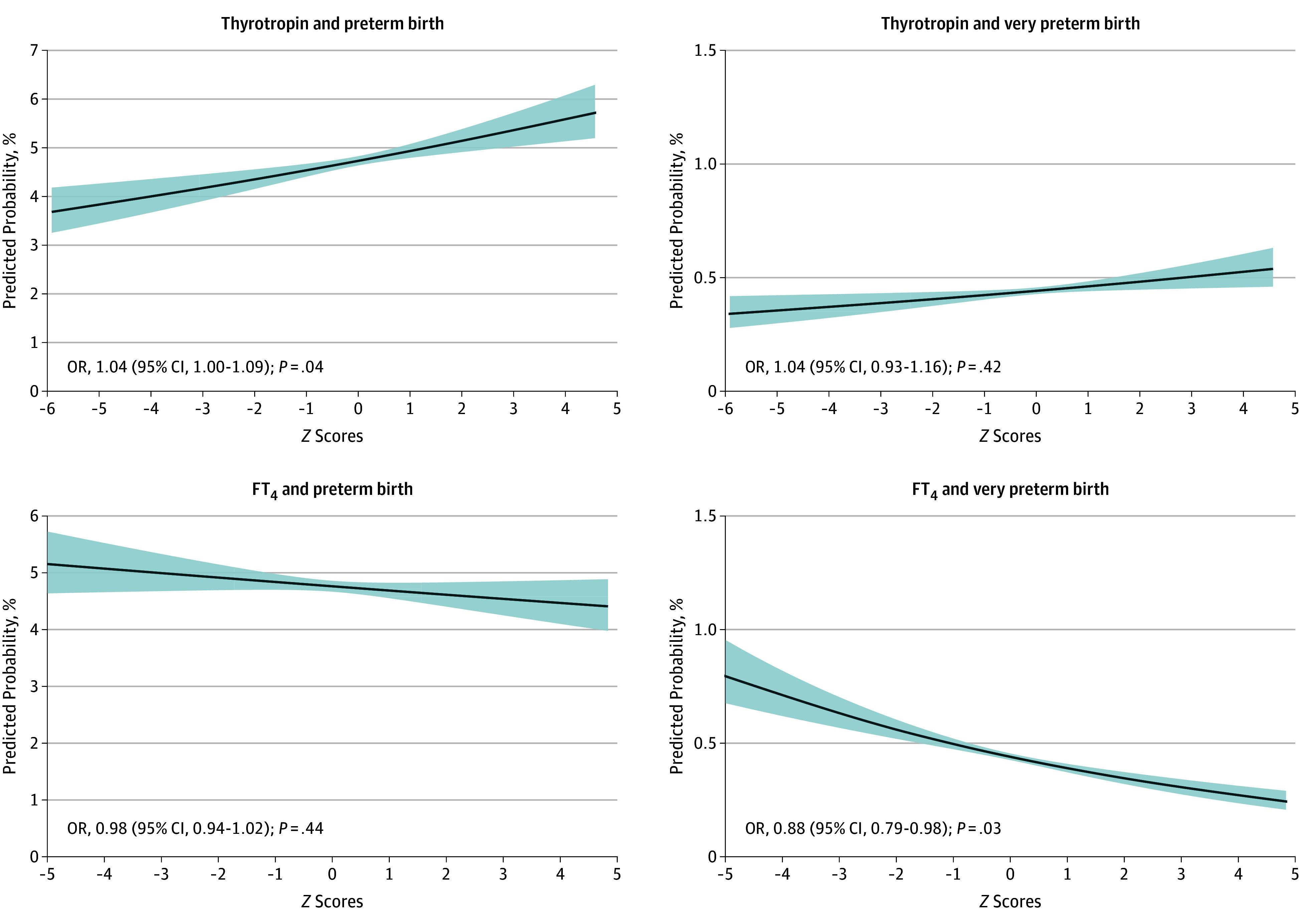
Preterm birth was defined as less than 37 weeks’ gestational age and very preterm birth was defined as less than 32 weeks’ gestational age. The thyrotropin and FT4 concentrations for all cohorts were log transformed and then standardized to population-specific standard deviation scores after removal of outliers (±4 SD from the mean) to enable comparison between different cohorts and assays. All analyses were adjusted for maternal age, body mass index, ethnicity, smoking, parity, gestational age at blood sampling, and fetal sex. OR indicates odds ratio.
TPO Antibody and Thyroglobulin Antibody Positivity
Thyroid peroxidase antibody–positive women had a higher risk of preterm birth vs TPO antibody–negative women (6.6% vs 4.9%, respectively; absolute risk difference, 1.6% [95% CI, 0.7% to 2.8%]; OR, 1.33 [95% CI, 1.15 to 1.56]; Figure 4) and very preterm birth (1.7% vs 0.7%; absolute risk difference, 1.0% [95% CI, 0.6% to 1.7%]; OR, 2.45 [95% CI, 1.81 to 3.32]; Figure 4). There was no significant association of thyroglobulin antibody positivity with preterm birth vs those with thyroglobulin antibody negativity (4.3% vs 4.4%, respectively; absolute risk difference, −0.5% [95% CI, −1.6% to 0.9%]; OR, 0.88 [95% CI, 0.64 to 1.20]; eTable 6 in Supplement 2).
Figure 4. Association of Thyroid Peroxidase (TPO) Antibody Positivity With Preterm Birth.
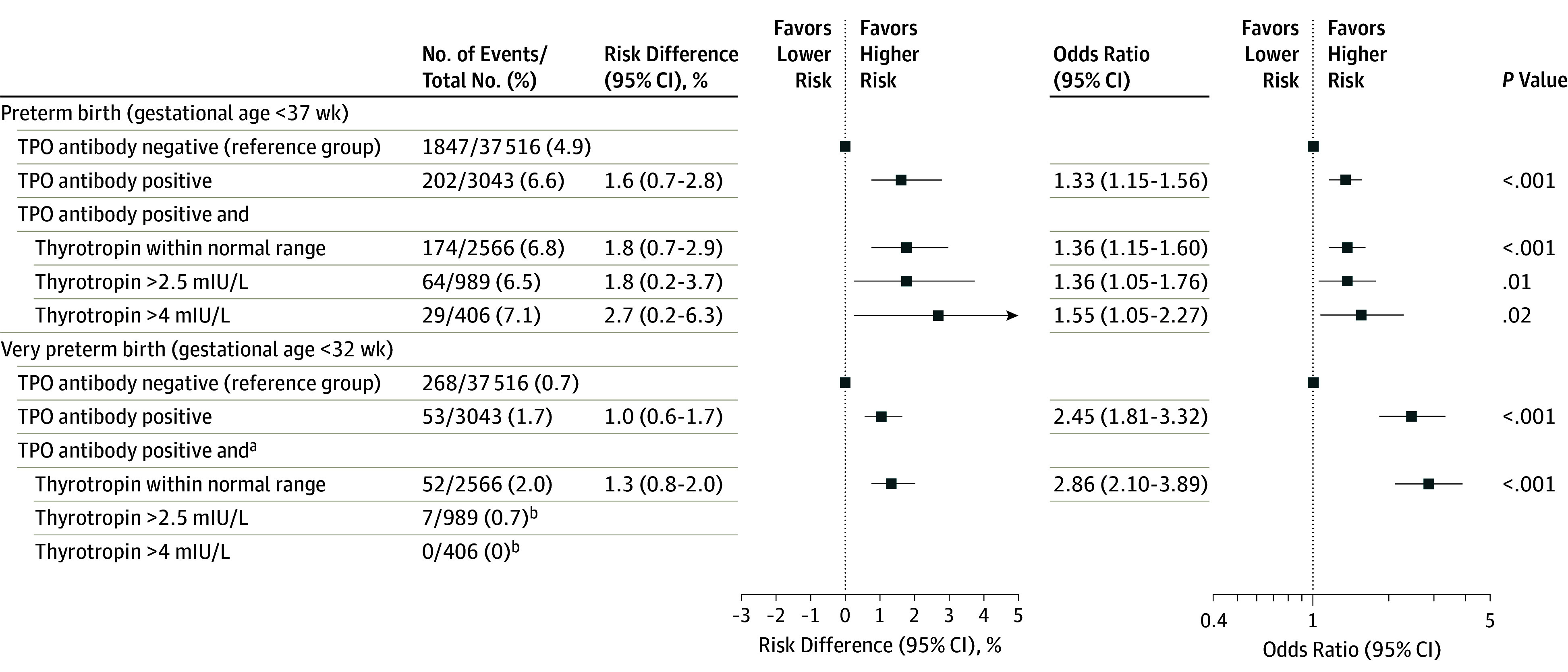
All analyses were adjusted for maternal age, body mass index, ethnicity, smoking, parity, gestational age at blood sampling, and fetal sex. Absolute differences and corresponding 95% CIs were back-calculated from the results of multivariable models and adjusted for baseline risk imprecision.
aThyroid peroxidase antibody–positive women and thyrotropin (often referred to as thyroid-stimulating hormone) concentrations within the normal range or higher than 2.5 mIU/L and 4 mIU/L were compared with TPO antibody–negative women regardless of their thyrotropin concentration.
bThere were too few samples to conduct a reliable analysis.
Gestational Age at Birth
The results relating to the gestational age at birth were similar to those for preterm and very preterm birth (eTable 7 in Supplement 2).
Sensitivity Analyses
In the prespecified sensitivity analysis, the results for thyrotropin concentration, FT4 concentration, and TPO antibody positivity remained similar when all 3 exposures were added to the same model, but the association of subclinical hypothyroidism with preterm birth was no longer statistically significant after additional adjustment for TPO antibody positivity (eTable 8 in Supplement 2).
In the post hoc sensitivity analysis on the association of TPO antibody positivity with preterm and very preterm birth, the P value for interaction for risk of preterm birth with higher thyrotropin concentrations among TPO antibody–positive women was .08; for very preterm birth, P < .001 for interaction; and for gestational age at birth continuously, P = .006 for interaction. Subsequent stratified analyses showed that TPO antibody–positive women and a thyrotropin concentration above 4.0 mIU/L had an excess risk of preterm birth vs TPO antibody–negative women (7.1% vs 4.9%, respectively; absolute risk difference, 2.7% [95% CI, 0.2%-6.3%]; OR, 1.55 [95% CI, 1.05-2.27]). However, stratified analyses could not be performed for very preterm birth (Figure 4).
In a post hoc sensitivity analysis, alternative TPO antibody concentration cutoffs were significantly associated with a higher risk of preterm birth, but the results were similar to those identified for manufacturer-based cutoffs (eTable 9 in Supplement 2). In the prespecified analysis on the association of thyroid function with preterm birth, TPO antibody positivity or thyroglobulin antibody positivity did not differ significantly according to the gestational age at the time of blood sampling, BMI, or parity (P > .07 for interaction for all analyses without meaningful differences after stratification; eTables 10 and 11 in Supplement 2).
The primary analyses were similar using a 2-step approach; however, some cohort-specific analyses with very few cases exhibited inflated ORs (eFigures 1 and 2 in Supplement 2). The funnel plots did not indicate any bias and the I2 values were mostly less than 40%. However, for the analyses of TPO antibody–positive women, the I2 values were 77% for preterm birth and 69% for very preterm birth (eFigure 2 in Supplement 2).
Discussion
Subclinical hypothyroidism, isolated hypothyroxinemia, and TPO antibody positivity were associated with higher risk for preterm birth. Isolated hypothyroxinemia and TPO antibody positivity were associated with higher risk for very preterm birth. The association of TPO antibody positivity with preterm birth did not appear to be related to differences in thyroid function, but was modified by the thyrotropin concentration as exemplified by the higher risk of preterm birth in TPO antibody–positive women and a thyrotropin concentration above 4.0 mIU/L.
Because randomized trials of treatment of thyroid function test abnormalities during pregnancy are scarce, observational studies form the basis of clinical guidelines on thyroid function and pregnancy.20 However, previous observational studies on the association of thyroid function and autoimmunity with preterm birth have used widely different definitions for thyroid function test abnormalities. This variation limits the ability to develop clearly defined recommendations in international guidelines and perform aggregate data meta-analysis. Furthermore, most studies lacked statistical power to study the risk of very preterm birth, a more clinically relevant outcome related to high mortality and morbidity, the demand for specialist neonatal services, and financial burdens.22,23,24 By collecting individual participant data, the current study allowed for standardization of the definition of thyroid function test abnormalities.
To our knowledge, the current study is the first individual participant data meta-analysis that shows isolated hypothyroxinemia is associated with a higher risk of preterm birth and very preterm birth and produced estimates in the same range as well-known risk factors such as adolescent or older maternal age, low BMI, obesity, smoking, and bacterial vaginosis.12,13,25,26,27 These results are in line with those from a previous population-based study, but opposite to those from an aggregate data meta-analysis including 5 studies.15,28 Isolated hypothyroxinemia is a pregnancy-specific disease entity with a multifactorial underlying pathophysiology that has remained relatively understudied.29
Available evidence predominantly links isolated hypothyroxinemia to suboptimal neurocognitive development of the offspring,29,30 but international guidelines do not recommend levothyroxine treatment in women with isolated hypothyroxinemia because randomized clinical trials have not proven a beneficial effect on child IQ.20 In 2 large randomized clinical trials in which women with isolated hypothyroxinemia were treated with levothyroxine during the first half of pregnancy, there was no statistically significant difference in preterm birth with treatment. In 1 trial of pregnant women with either subclinical hypothyroidism or isolated hypothyroxinemia, preterm birth occurred in 5.6% of the levothyroxine group vs 7.9% of the control group.31 In the second trial with pregnant women who had isolated hypothyroxinemia, preterm birth occurred in 12% of the levothyroxine group vs 8% of the placebo group and birth at less than 34 weeks’ gestational age occurred in 4% of the levothyroxine group and 3% of the placebo group.32
Consistent with the results of the current study, women with isolated hypothyroxinemia in the 2 trials31,32 had higher preterm birth rates than euthyroid women did in the current meta-analysis. The results of the current study provide further insights on potential risks of isolated hypothyroxinemia that may help to optimize clinical decision-making strategies, taking into account all potential harms and benefits of screening programs and subsequent levothyroxine treatment. Further studies will be required to elucidate the underlying pathophysiology of isolated hypothyroxinemia and to study if it is also a risk factor for other adverse pregnancy outcomes.
In the current study, TPO antibody positivity was associated with a higher risk of preterm birth, consistent with previous smaller meta-analyses using aggregate data and studies on miscarriage.12,13 Two recent clinical trials showed that preconception treatment of euthyroid TPO antibody–positive women who had either a previous miscarriage or fertility treatment with low-dose levothyroxine neither reduced the rate of preterm birth, nor any other pregnancy or neonatal outcomes.33,34 These studies did not include women with thyroid function test abnormalities.33,34 The results of the current study show a higher risk for TPO antibody–positive women and a thyrotropin concentration higher than 4.0 mIU/L, which confirms the results from a small randomized clinical trial showing that levothyroxine treatment in TPO antibody–positive women lowers preterm birth rates only if the thyrotropin concentration was higher than 4.0 mIU/L.35
Taken together, the results of this study are in alignment with the recommendation of the American Thyroid Association that different thyrotropin cutoffs should be used for TPO antibody–positive women vs TPO antibody–negative women.20 A sensitivity analysis showed that the association of subclinical hypothyroidism with preterm birth was no longer apparent after additional adjustment for TPO antibody positivity, suggesting that it is the TPO antibody positivity that occurs in one-third of women with subclinical hypothyroidism that underlies any associations of subclinical hypothyroidism with preterm birth. However, this study lacked statistical power for a subgroup analysis on the association of TPO antibody positivity in combination with a thyrotropin concentration higher than 2.5 mIU/L for preterm birth or higher than 4.0 mIU/L for very preterm birth.
There remains an important role for observational studies in the evaluation of thyroid function test abnormalities during pregnancy. For example, observational studies have indicated that the interpretation of the results of large randomized trials31,32,33,34 may be limited because of risks related to potential overtreatment, a late start of levothyroxine therapy, or sole inclusion of women with normal thyroid.36,37,38,39 Moreover, observational studies are needed to study detrimental clinical outcomes with a relatively low incidence such as very preterm birth, given the impracticality of randomized trials for such outcomes. For example, to study if levothyroxine therapy could reverse excess preterm birth risks as identified in this study for isolated hypothyroxinemia, TPO antibody positivity, and TPO antibody positivity with a thyrotropin concentration greater than 4.0 mIU/L, a total of 3674, 6090, and 3814 women, respectively, would have to be randomized, which in the case of population screening would translate to the screening of 196 470, 95 530, and 448 706 women (with a 2-sided α level of .05, a power level of 80%, and a lost to follow-up rate or declined participation rate of 15%).
The results of the current study do not change the consideration that there is currently insufficient evidence for a benefit of routine thyroid function screening in pregnant women; the potential harms and benefits of levothyroxine for other clinically meaningful outcomes should be taken into account as well. The results support the concept of a reflex TPO antibody measurement in women with a thyrotropin concentration higher than 4.0 mIU/L, and gestational thyrotropin monitoring for TPO antibody–positive women prior to conception. The current study in itself does not validate a reflex FT4 concentration or TPO antibody measurement for women with a normal thyrotropin concentration until further randomized trials are performed.
The results of the current study add to the limited knowledge on the complicated and multifactorial mechanisms underlying preterm and very preterm birth.40 Because thyroid hormone regulates key processes in placental and fetal growth and development, the associations for FT4 concentration could be mediated via effects on either placental function, fetal growth, or both.8,9,11,40,41 Alternatively, thyroid hormone and TPO antibody positivity could be involved in infectious and inflammatory pathways leading to preterm birth.42,43 Another potential mechanism by which low thyroid hormone availability could be associated with preterm birth is that it may lead to an earlier onset of labor via an increase in oxytocin and vasopressin, but a decrease in progesterone, or through thyroid hormone effects specific for the cervix, endometrium, or fetal membranes.7,44,45,46,47 Further studies on pathways via which thyroid hormone is involved in the pathogenesis of preterm birth are needed to further optimize risk identification.
Limitations
This study has several limitations. First, there was a lack of statistical power to optimally investigate the risk of very preterm birth in specific clinically relevant subgroups. Although very preterm birth is clinically more relevant than late preterm birth, the relatively sparse occurrence of TPO antibody positivity combined with a thyrotropin concentration higher than 2.5 or 4.0 mIU/L necessitates an even larger data set than that available in the current study to examine associations with very preterm birth.
Second, only 5 of the 19 studies had data available on thyroglobulin antibodies. Although this association was studied in a large number of women, data were only available for 40.0% of included women, which may have affected the results.
Third, pregnancy-related changes in thyroid-binding proteins could interfere with FT4 immunoassays. However, the use of assay-specific reference ranges as advocated by the current American Thyroid Association guidelines and standardization of FT4 concentrations per cohort or assay should mostly eliminate the potential between-assay differences in absolute FT4 concentrations.
Fourth, studies that were published while conducting statistical analyses for the current study could not be included. Fifth, because the included studies were observational, residual or unmeasured confounding cannot be excluded. However, the analysis of individual participant data allowed for adjustment for multiple relevant confounders. Sixth, causality cannot be determined from observational studies.
Conclusions
Among pregnant women without overt thyroid disease, subclinical hypothyroidism, isolated hypothyroxinemia, and TPO antibody positivity were significantly associated with higher risk of preterm birth. These results provide insights toward optimizing clinical decision-making strategies that should consider the potential harms and benefits of screening programs and levothyroxine treatment during pregnancy.
Preregistered study protocol and details on deviations
eMethods. Details on search strategy and systematic search and statistics
eTable 1A. Maternal demographics per cohort
eTable 1B. Maternal thyroid function test results per cohort
eTable 1C. Description of euthyroidism and thyroid function test abnormalities per cohort
eTable 1D. Description of pregnancy characteristics per cohort
eTable 1E. Percentage of missing data of covariates per cohort
eTable 2. Date and place of data collection for the included cohorts
eTable 3. Newcastle - Ottawa Quality Assessment Scale per cohort
eTable 4. Cohort-specific cut-offs of TSH and FT4 for defining thyroid function test abnormalities
eTable 5. Comparison of TSH and FT4 concentrations and TPOAb positivity between women with or without data on gestational age at birth
eTable 6. The association of TSH and FT4 concentrations or TgAb positivity with preterm birth
eTable 7. Association of thyroid function test abnormalities, TSH or FT4 concentrations, TPOAb or TgAb positivity with gestational age at birth (weeks)
eTable 8. Association of TPOAb positivity with mutual adjustments with TSH and FT4 or subclinical hypothyroidism or hypothyroxinemia with preterm birth
eTable 9. Sensitivity analyses for the association of TPOAb cut-offs with preterm birth
eTable 10. P values for the interaction terms between TPOAb, TgAb, TSH or FT4 with BMI, parity or gestational age at the time of blood sampling in association with preterm birth (<37 weeks)
eTable 11. Association of FT4 or TSH with preterm birth (<37 weeks) according to gestational age at the time of blood sampling or BMI or parity
eFigure 1A. Two‐step meta‐analyses and funnel plots for the association of subclinical hypothyroidism with preterm and very preterm birth
eFigure 1B. Two‐step meta‐analyses and funnel plots for the association of subclinical hyperthyroidism with preterm and very preterm birth
eFigure 1C. Two‐step meta‐analyses and funnel plots for the association of hypothyroxinemia with preterm and very preterm birth
eFigure 1D. Two‐step meta‐analyses and funnel plots for the association of overt hyperthyroidism with preterm birth
eFigure 2. Two‐step meta‐analyses and funnel plots for the association of TPOAb positivity with preterm and very preterm birth
Supplemental acknowledgements and grant details
References
- 1.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388(10063):3027-3035. doi: 10.1016/S0140-6736(16)31593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nosarti C, Reichenberg A, Murray RM, et al. Preterm birth and psychiatric disorders in young adult life. Arch Gen Psychiatry. 2012;69(6):E1-E8. doi: 10.1001/archgenpsychiatry.2011.1374 [DOI] [PubMed] [Google Scholar]
- 3.Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol. 2008;19(1):151-157. doi: 10.1681/ASN.2007020252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162-2172. doi: 10.1016/S0140-6736(12)60820-4 [DOI] [PubMed] [Google Scholar]
- 5.Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand. 2008;87(6):590-600. doi: 10.1080/00016340802005126 [DOI] [PubMed] [Google Scholar]
- 6.Ramadurai SM, Nielsen HC, Chen Y, Hatzis D, Sosenko IR. Differential effects in vivo of thyroid hormone on the expression of surfactant phospholipid, surfactant protein mRNA and antioxidant enzyme mRNA in fetal rat lung. Exp Lung Res. 1998;24(5):641-657. doi: 10.3109/01902149809099585 [DOI] [PubMed] [Google Scholar]
- 7.Ciosek J, Drobnik J. Vasopressin and oxytocin release and the thyroid function. J Physiol Pharmacol. 2004;55(2):423-441. [PubMed] [Google Scholar]
- 8.Chen CY, Chen CP, Lin KH. Biological functions of thyroid hormone in placenta. Int J Mol Sci. 2015;16(2):4161-4179. doi: 10.3390/ijms16024161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barjaktarovic M, Korevaar TI, Chaker L, et al. The association of maternal thyroid function with placental hemodynamics. Hum Reprod. 2017;32(3):653-661. [DOI] [PubMed] [Google Scholar]
- 10.Dong AC, Stagnaro-Green A. Differences in diagnostic criteria mask the true prevalence of thyroid disease in pregnancy: a systematic review and meta-analysis. Thyroid. 2019;29(2):278-289. doi: 10.1089/thy.2018.0475 [DOI] [PubMed] [Google Scholar]
- 11.Korevaar TIM, Medici M, Visser TJ, Peeters RP. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat Rev Endocrinol. 2017;13(10):610-622. doi: 10.1038/nrendo.2017.93 [DOI] [PubMed] [Google Scholar]
- 12.Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ. 2011;342:d2616. doi: 10.1136/bmj.d2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He X, Wang P, Wang Z, He X, Xu D, Wang B. Thyroid antibodies and risk of preterm delivery: a meta-analysis of prospective cohort studies. Eur J Endocrinol. 2012;167(4):455-464. doi: 10.1530/EJE-12-0379 [DOI] [PubMed] [Google Scholar]
- 14.Maraka S, Ospina NM, O’Keeffe DT, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. 2016;26(4):580-590. doi: 10.1089/thy.2015.0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheehan PM, Nankervis A, Araujo Júnior E, Da Silva Costa F. Maternal thyroid disease and preterm birth: systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(11):4325-4331. doi: 10.1210/jc.2015-3074 [DOI] [PubMed] [Google Scholar]
- 16.Korevaar TI, Taylor PN, Dayan CM, Peeters RP. An invitation to join the consortium on thyroid and pregnancy. Eur Thyroid J. 2016;5(4):277. doi: 10.1159/000452331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korevaar TI, Taylor PN, Dayan CM, Peeters RP. An invitation to join the consortium on thyroid and pregnancy. Obstet Gynecol. 2016;128(4):913. doi: 10.1097/AOG.0000000000001670 [DOI] [PubMed] [Google Scholar]
- 18.Jolani S, Debray TP, Koffijberg H, van Buuren S, Moons KG. Imputation of systematically missing predictors in an individual participant data meta-analysis: a generalized approach using MICE. Stat Med. 2015;34(11):1841-1863. doi: 10.1002/sim.6451 [DOI] [PubMed] [Google Scholar]
- 19.van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw. 2011;1(3):26. [Google Scholar]
- 20.Alexander EK, Pearce EN, Brent GA, et al. 2017 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315-389. doi: 10.1089/thy.2016.0457 [DOI] [PubMed] [Google Scholar]
- 21.Korevaar TIM, Pop VJ, Chaker L, et al. Dose dependency and a functional cutoff for TPO-antibody positivity during pregnancy. J Clin Endocrinol Metab. 2018;103(2):778-789. doi: 10.1210/jc.2017-01560 [DOI] [PubMed] [Google Scholar]
- 22.Boyle EM, Johnson S, Manktelow B, et al. Neonatal outcomes and delivery of care for infants born late preterm or moderately preterm: a prospective population-based study. Arch Dis Child Fetal Neonatal Ed. 2015;100(6):F479-F485. doi: 10.1136/archdischild-2014-307347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vohr B. Long-term outcomes of moderately preterm, late preterm, and early term infants. Clin Perinatol. 2013;40(4):739-751. doi: 10.1016/j.clp.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 24.Boyle EM, Poulsen G, Field DJ, et al. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. BMJ. 2012;344:e896. doi: 10.1136/bmj.e896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhattacharya S, Raja EA, Mirazo ER, et al. Inherited predisposition to spontaneous preterm delivery. Obstet Gynecol. 2010;115(6):1125-1133. doi: 10.1097/AOG.0b013e3181dffcdb [DOI] [PubMed] [Google Scholar]
- 26.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 2003;189(1):139-147. doi: 10.1067/mob.2003.339 [DOI] [PubMed] [Google Scholar]
- 27.Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am J Obstet Gynecol. 2000;182(2):465-472. doi: 10.1016/S0002-9378(00)70240-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korevaar TIM, Steegers EAP, Chaker L, et al. Thyroid function and premature delivery in TPO antibody-negative women: the added value of hCG. J Clin Endocrinol Metab. 2017;102(9):3360-3367. doi: 10.1210/jc.2017-00846 [DOI] [PubMed] [Google Scholar]
- 29.Dosiou C, Medici M. Management of endocrine disease: isolated maternal hypothyroxinemia during pregnancy: knowns and unknowns. Eur J Endocrinol. 2017;176(1):R21-R38. doi: 10.1530/EJE-16-0354 [DOI] [PubMed] [Google Scholar]
- 30.Korevaar TIM, Tiemeier H, Peeters RP. Clinical associations of maternal thyroid function with foetal brain development: epidemiological interpretation and overview of available evidence. Clin Endocrinol (Oxf). 2018;89(2):129-138. doi: 10.1111/cen.13724 [DOI] [PubMed] [Google Scholar]
- 31.Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(6):493-501. doi: 10.1056/NEJMoa1106104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casey BM, Thom EA, Peaceman AM, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network . Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376(9):815-825. doi: 10.1056/NEJMoa1606205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380(14):1316-1325. doi: 10.1056/NEJMoa1812537 [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318(22):2190-2198. doi: 10.1001/jama.2017.18249 [DOI] [PubMed] [Google Scholar]
- 35.Nazarpour S, Ramezani Tehrani F, Simbar M, Tohidi M, Alavi Majd H, Azizi F. Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. Eur J Endocrinol. 2017;176(2):253-265. doi: 10.1530/EJE-16-0548 [DOI] [PubMed] [Google Scholar]
- 36.Jansen TA, Korevaar TIM, Mulder TA, et al. Maternal thyroid function during pregnancy and child brain morphology: a time window-specific analysis of a prospective cohort [published online June 28, 2019]. Lancet Diabetes Endocrinol. doi: 10.1016/S2213-8587(19)30153-6 [DOI] [PubMed] [Google Scholar]
- 37.Maraka S, Mwangi R, McCoy RG, et al. Thyroid hormone treatment among pregnant women with subclinical hypothyroidism: US national assessment. BMJ. 2017;356:i6865. doi: 10.1136/bmj.i6865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korevaar TI, Muetzel R, Medici M, et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol. 2016;4(1):35-43. doi: 10.1016/S2213-8587(15)00327-7 [DOI] [PubMed] [Google Scholar]
- 39.Korevaar TIM, Chaker L, Peeters RP. Improving the clinical impact of randomised trials in thyroidology. Lancet Diabetes Endocrinol. 2018;6(7):523-525. doi: 10.1016/S2213-8587(17)30316-9 [DOI] [PubMed] [Google Scholar]
- 40.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760-765. doi: 10.1126/science.1251816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75-84. doi: 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500-1507. doi: 10.1056/NEJM200005183422007 [DOI] [PubMed] [Google Scholar]
- 43.De Vito P, Incerpi S, Pedersen JZ, Luly P, Davis FB, Davis PJ. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. 2011;21(8):879-890. doi: 10.1089/thy.2010.0429 [DOI] [PubMed] [Google Scholar]
- 44.Datta M, Roy P, Banerjee J, Bhattacharya S. Thyroid hormone stimulates progesterone release from human luteal cells by generating a proteinaceous factor. J Endocrinol. 1998;158(3):319-325. doi: 10.1677/joe.0.1580319 [DOI] [PubMed] [Google Scholar]
- 45.Maruo T, Matsuo H, Mochizuki M. Thyroid hormone as a biological amplifier of differentiated trophoblast function in early pregnancy. Acta Endocrinol (Copenh). 1991;125(1):58-66. doi: 10.1530/acta.0.1250058 [DOI] [PubMed] [Google Scholar]
- 46.Akerlund M. Vasopressin and oxytocin in normal reproduction and in the pathophysiology of preterm labour and primary dysmenorrhea: development of receptor antagonists for therapeutic use in these conditions. Rocz Akad Med Bialymst. 2004;49:18-21. [PubMed] [Google Scholar]
- 47.Thornton S, Baldwin PJ, Harris PA, et al. The role of arginine vasopressin in human labour: functional studies, fetal production and localisation of V1a receptor mRNA. BJOG. 2002;109(1):57-62. doi: 10.1111/j.1471-0528.2002.01132.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preregistered study protocol and details on deviations
eMethods. Details on search strategy and systematic search and statistics
eTable 1A. Maternal demographics per cohort
eTable 1B. Maternal thyroid function test results per cohort
eTable 1C. Description of euthyroidism and thyroid function test abnormalities per cohort
eTable 1D. Description of pregnancy characteristics per cohort
eTable 1E. Percentage of missing data of covariates per cohort
eTable 2. Date and place of data collection for the included cohorts
eTable 3. Newcastle - Ottawa Quality Assessment Scale per cohort
eTable 4. Cohort-specific cut-offs of TSH and FT4 for defining thyroid function test abnormalities
eTable 5. Comparison of TSH and FT4 concentrations and TPOAb positivity between women with or without data on gestational age at birth
eTable 6. The association of TSH and FT4 concentrations or TgAb positivity with preterm birth
eTable 7. Association of thyroid function test abnormalities, TSH or FT4 concentrations, TPOAb or TgAb positivity with gestational age at birth (weeks)
eTable 8. Association of TPOAb positivity with mutual adjustments with TSH and FT4 or subclinical hypothyroidism or hypothyroxinemia with preterm birth
eTable 9. Sensitivity analyses for the association of TPOAb cut-offs with preterm birth
eTable 10. P values for the interaction terms between TPOAb, TgAb, TSH or FT4 with BMI, parity or gestational age at the time of blood sampling in association with preterm birth (<37 weeks)
eTable 11. Association of FT4 or TSH with preterm birth (<37 weeks) according to gestational age at the time of blood sampling or BMI or parity
eFigure 1A. Two‐step meta‐analyses and funnel plots for the association of subclinical hypothyroidism with preterm and very preterm birth
eFigure 1B. Two‐step meta‐analyses and funnel plots for the association of subclinical hyperthyroidism with preterm and very preterm birth
eFigure 1C. Two‐step meta‐analyses and funnel plots for the association of hypothyroxinemia with preterm and very preterm birth
eFigure 1D. Two‐step meta‐analyses and funnel plots for the association of overt hyperthyroidism with preterm birth
eFigure 2. Two‐step meta‐analyses and funnel plots for the association of TPOAb positivity with preterm and very preterm birth
Supplemental acknowledgements and grant details