Abstract
A taste associated with emetic drugs produces conditioned disgust reactions in rats (predominantly gaping), unlike nonemetic drugs that can still produce conditioned taste avoidance but not conditioned disgust. That difference suggests nausea is a prerequisite for learning disgust reactions to tastes. Depletion of forebrain serotonin (5-HT) by 5,7-dihydroxytryptamine (5,7-DHT) lesions of the dorsal raphe nucleus and median raphe nucleus prevents LiCl-induced conditioned disgust reactions (Limebeer et al., 2004). Here we demonstrate that partial depletion of 5-HT in the insular cortex (IC) prevents LiCl-induced conditioned disgust reactions. Furthermore, a double dissociation occurred in the partial regulation of disgust and taste avoidance by selective 5-HT3 receptor antagonism/agonism in the posterior (granular) region of the IC and the anterior (dorsal agranular) region of the IC, respectively. Intracranial administration of the 5-HT3 receptor antagonist, ondansetron (OND), to the posterior IC impaired the establishment of LiCl-induced conditioned gaping reactions, but not LiCl-induced conditioned taste avoidance (CTA). Likewise, posterior IC administration of the 5-HT3 receptor agonist m-chlorophenylbiguanide (mCPBG) enhanced the establishment of LiCl-induced conditioned gaping and produced conditioned gaping on its own (which was prevented by intracranially administered OND), with no effect on CTA. On the other hand, anterior IC administration of OND partially reduced the establishment of LiCl-induced CTA, and mCPBG produced a weak CTA, both without effect on gaping. These results suggest that activation of 5-HT3 receptors in the posterior IC is important for the production of nausea-induced conditioned disgust reactions, while activation of 5-HT3 receptors in the anterior IC are involved in the production of CTA.
Introduction
Although rats are incapable of vomiting, considerable evidence indicates that conditioned disgust reactions (primarily gaping) elicited by an illness-paired flavor (e.g., Grill and Norgren, 1978; Parker et al., 2008, 2009) or an illness-paired context (Limebeer et al., 2008; Rock et al., 2008) represent a model of nausea-induced behavior in rats. Unlike the traditionally employed measure of conditioned taste avoidance (CTA), conditioned gaping reactions are exclusively elicited by flavors paired with emetic treatments (but not by rewarding drugs; Parker, 1995), and when systemically administered, anti-emetic treatments more effectively attenuate conditioned disgust than CTA (for review, see Parker et al., 2008, 2009). Effective anti-emetic [e.g., ondansetron (OND)] treatments attenuate vomiting (Matsuki et al., 1997) and the establishment of conditioned disgust (Limebeer and Parker, 2000) by reducing serotonin availability at the 5-HT3 receptor (Limebeer and Parker, 2000, 2003). Forebrain serotonin plays a crucial role in the generation of these reactions, because selective 5,7-dihydroxytryptamine (5,7-DHT) lesions of the dorsal raphe nucleus and median raphe nucleus, which reduced forebrain serotonin by >85% (assessed in striatum and hippocampus), prevented LiCl-induced conditioned disgust, although CTA was spared (Limebeer et al., 2004; see also Asin et al., 1980).
The forebrain structure critical for the production of nausea-induced conditioned disgust may be the insular cortex (IC), which is an area involved in the sensation of nausea in humans and other animals (Kaada, 1951; Penfield and Faulk, 1955; Fiol et al., 1988; Contreras et al., 2007; Catenoix et al., 2008). Ablation of the IC prevents LiCl-induced conditioned disgust reactions (Kiefer and Orr, 1992) without modifying unconditioned disgust elicited by quinine. The IC is the site of topographical input of visceral (granular IC; Cechetto and Saper, 1987; Allen et al., 1991; Saper, 2004) and gustatory (dorsal agranular IC; Kosar et al., 1986; Lundy and Norgren, 2004) neural input. Although most gastrointestinal visceral sensory afferents to the insular cortex terminate in the posterior granular field (albeit anterior/dorsal region of this field; Cechetto and Saper, 1987), most efferent projections from the insular cortex to autonomic structures originate in the more anterior agranular field (Allen et al., 1991; Saper, 2004; Bagaev and Aleksandov, 2006). Neurotoxin-induced lesions of the region of the anterior IC consistently interfere with the establishment of LiCl-induced CTAs (Nerad et al., 1996; for review, see Reilly, 2009), but lesions of the more posterior region of primary visceral input to the IC (posterior IC or interoceptive IC; Contreras et al., 2007) do not interfere with LiCl-induced CTA (Mackey et al., 1986; Nerad et al., 1996); yet temporary lesions to the posterior IC do reduce behavioral signs of malaise induced by LiCl (Contreras et al., 2007). Here, in a double dissociation, we demonstrate that 5-HT activity (at the 5-HT3 receptor) in the posterior IC may selectively produce the nausea-induced reactions of conditioned disgust, while activity in the anterior IC may be involved in the production of CTA learning.
Materials and Methods
Subjects
Male Sprague Dawley rats were obtained from Charles River Canada and singly housed in polycarbonate cages (44 × 25 × 21 cm). Subjects were provided with food pellets (Highland Rat Chow) ad libitum. The animal quarters were kept on a reversed 12 h light/dark cycle (lights on from 1900 to 0700 h) and maintained at 22 ± 2°C and 45 ± 20% relative humidity. All animals were handled before testing, and the guidelines set out by the Canadian Council on Animal Care Committee and the Animals for Research Act were followed. The experiments were approved by the University of Guelph Animal Care Committee.
Drugs
LiCl (Sigma) was prepared in a 0.15 m solution with sterile water and was administered intraperitoneally (i.p.) at a volume of 20 ml/kg (127.2 mg/kg). Ondansetron (Sigma) was prepared in sterile saline at both 0.1 μg/μl and 1 μg/μl and intracranially microinfused into either the anterior IC or posterior IC, at a rate of 1 μl/min for 1 min. The drug mCPBG (Research Biochemicals International) was also prepared in sterile saline at 10 μg/μl and 30 μg/μl and intracranially microinfused at a rate of 1 μg/min into either the anterior IC or posterior IC.
Surgical procedures
Bilateral 5,7-DHT insular cortex lesion.
Thirty minutes before surgery, rats were pretreated with desipramine [10 mg/kg mixed in 0.9% (w/v) NaCl and administered at a volume of 1 ml/kg i.p.; Sigma] to inhibit the uptake of 5,7-DHT (5,7-DHT creatinine sulfate, free base; Sigma) by noradrenergic neurons and prevent depletion of norepinephrine (NE) (Baumgarten et al., 1978). The neurotoxin 5,7-DHT is taken up by 5-HT-containing neurons and results in degeneration of axons and terminals containing 5-HT. Rats were anesthetized with isoflurane gas (4–5% induction, 1.5% maintenance in O2), and the skin was prepared by cleaning with soap (Bacti-Stat; Ecolab) and wiping with 70% isopropyl alcohol followed by 7% betadine solution (Purdue Products). The rat was then administered a 5 mg/kg injection (i.p.) of the anti-inflammatory/analgesic drug carprofen (Rimadyl; Pfizer) and a local anesthetic (0.1 ml; s.c.; Marcaine; Hospira) on either side of the skull. Once the rat was stabilized in the flat skull position (according to Paxinos and Watson, 2007) in the stereotaxic frame, a 30 gauge stainless steel cannula was bilaterally lowered to three sites in the IC, and 6 μg/2 μl 5,7-DHT (creatinine sulfate, free base), dissolved in a 0.1% ascorbic acid vehicle and delivered in a total volume of 0.7 μl, was infused over a period of 2 min. The cannula was then left in place for 4 min following infusion before being raised and moved to the next two bilateral IC sites, where the same infusion procedure was followed, after which the cannula was removed. The sham-lesioned rats underwent the same surgical procedure, except that 0.1% ascorbic acid vehicle was infused in place of the neurotoxin. The coordinates relative to bregma were: +1.2 mm anteroposterior (AP), +5.2 mm mediolateral (ML), −4.6 mm dorsoventral (DV) (site 1); 0.0 mm AP, +6.0 mm ML, −4.8 mm DV (site 2); and −1.2 mm AP, +6.2 mm ML, −4.8 mm DV (site 3). Immediately following completion of the surgical procedure and recovery from anesthesia, the rats were returned to their home cages and monitored daily for the duration of the experiment. They were allowed to recover for 1 week before surgical implantation of an intraoral cannula as described by Limebeer et al. (2010).
Bilateral cannulation of the posterior IC or anterior IC.
Rats were implanted with bilateral indwelling guide cannulas into either the posterior IC (Experiments 2a, 3, and 4a) or the anterior IC (Experiment 2b and 4b). The stereotaxic surgery procedure was similar to that of Experiment 1, except that in Experiments 2a, 3, and 4a the guide cannulas (22 gauge, 6 mm below pedestal) were set at a divergent 10° angle and lowered into the posterior IC using the following coordinates from bregma: −0.5 mm AP; +5.0 ML; −4.5 DV (Contreras et al., 2007), and in Experiments 2b and 4b, the stainless steel guide cannulas (22 gauge, 6 mm below pedestal) were lowered into the anterior IC using the following coordinates from bregma: +1.5 mm AP, +5.0 mm ML −4.5 mm DV. All guide cannulas were stabilized by six screws secured in the skull and dental cement. Once the dental cement had hardened, a stainless steel obdurator was inserted in the guide cannula to maintain patency. Immediately following removal from the stereotaxic frame, the rats were surgically implanted with an intraoral cannula (as described in detail by Limebeer et al., 2010).
Behavioral procedures
Experiment 1: effect of bilateral 5,7-DHT IC lesions on LiCl-induced conditioned gaping.
Three days after the implantation of the intraoral cannula, all rats received two adaptation trials to the taste reactivity (TR) procedure separated by 24 h. The rat was placed into a clear Plexiglas TR chamber (22.5 × 26 × 20 cm) sitting on a table with a glass top. The room was dark with two 60 W lights on either side of the chamber. The rat's intraoral cannula was attached to an infusion pump (model KDS100; KD Scientific) via an infusion tube inserted through the opaque lid of the chamber. Three minutes after being placed in the chamber, the rat was infused with water for 3 min at a rate of 1 ml/min. The first conditioning trial occurred 24 h later.
During the two TR conditioning trials (spaced 72 h apart), the rat was placed in the TR chamber and 3 min later was intraorally infused with 0.1% saccharin solution at a rate of 1 ml/min. The orofacial and somatic responses were video recorded (Sony DCR-HC48) from a mirror beneath the chamber placed at a 45° angle that facilitated viewing of the rat's ventral surface. Immediately following the saccharin infusion, the rat was injected with LiCl. The final groups after tissue analysis were sham/LiCl (n = 4) and lesion/LiCl (n = 4). There were no saline control groups in this experiment, because rats do not display conditioned gaping reactions when infused with a saline-paired saccharin solution (Parker, 1988); therefore, there would be no gaping reactions to be reduced by 5,7-DHT lesions. Seventy-two hours following the second conditioning trial, each rat received a TR test trial. The rat was placed in the TR chamber and 3 min later was intraorally infused with the 0.1% saccharin solution for 3 min (1 ml/min), and its orofacial reactions were recorded. Seventy-two hours later, the rats received a quinine TR test to determine whether the lesions modified the rats' reactivity to an unconditionally aversive taste. The rats were individually placed into the TR chambers and infused (1 ml/min) with a 0.05% quinine solution for 2 min. The rats' orofacial and somatic responses were video recorded.
Experiment 2: effect of bilateral intracranial (2a: posterior IC; 2b: anterior IC) administration of OND on establishment of LiCl-induced conditioned gaping and CTA. Experiment 2a (posterior IC) and 2b (anterior IC) were conducted similarly to Experiment 1, except that the rats were administered intracranial microinfusions of the 5-HT3 antagonist OND or vehicle (VEH) immediately before both conditioning trials. Following two adaptation trials separated by 24 h, the rats received two conditioning trials and a test trial, each separated by 72 h. On each of the two conditioning trials, the rats were bilaterally microinfused with VEH or 0.1 μg/μl or 1 μg/μl OND for 1 min into the posterior IC (Experiment 2a) or the anterior IC (Experiment 2b). The injector was then left in place for 1 min following the infusion before being removed. Immediately after the infusions, the rats were conditioned as in Experiment 1; that is, 3 min following placement in the TR chambers, they received a 3 min intraoral infusion of 0.1% saccharin solution, followed immediately by an injection of LiCl. The drug-free TR test was conducted 72 h later. In Experiment 2, the rats also received a 120 min two-bottle consumption test 24 h after the TR test trial, having been water deprived for 16 h. The finals groups (following histology) were: Experiment 2a (posterior IC): VEH (n = 8), 0.1 OND (n = 9), and 1 OND (n = 8); and Experiment 2b (anterior IC): VEH (n = 8), 0.1 OND (n = 9), and 1 OND (n = 8).
Experiment 3: effect of intracranial (posterior IC) administration of mCPBG on the establishment of LiCl-induced conditioned gaping and CTA.
Experiment 3 was conducted similarly to Experiment 2a, with the exception that the rats were microinfused with 30 μg/μl mCPBG (a 5-HT3 agonist) immediately before both conditioning trials. During conditioning, 3 min following placement in the TR chamber, all rats received an intraoral infusion of 0.1% saccharin solution for 3 min, followed immediately by an injection of LiCl. The drug-free TR test was conducted 72 h later. As in Experiment 2, the rats received a 120 min two-bottle consumption test 24 h after the TR test trial, having been water deprived for 16 h. Following histology, the final groups were 30 μg of mCPBG (n = 7) and VEH (n = 8).
Experiment 4: effect of intracranial administration (4a: posterior IC and 4b: anterior IC) of mCPBG following saccharin exposure on the production of conditioned gaping and CTA.
Experiment 4a investigated whether microinfusions of the 5-HT3 agonist, mCPBG, into the posterior IC would induce conditioned gaping. Therefore, after the intraoral saccharin infusion in both conditioning trials, all rats were microinfused with either 0 (VEH), 10, or 30 μg of mCPBG instead of receiving LiCl injections. Three minutes following placement in the TR chambers, all groups received a 3 min intraoral infusion of 0.1% saccharin solution, followed immediately by an microinfusion of either 0 (VEH), 10, or 30 μg of mCPBG, a 5HT3 agonist. In addition, a separate group was pretreated with 0.1 μg of OND immediately before the intraoral saccharin infusion, which was followed by an infusion of 30 μg of mCPBG. A drug-free TR test was conducted 72 h later. As in Experiments 2 and 3, the rats received a 120 min two-bottle consumption test 24 h after the TR test trial, having been water deprived for 16 h. Following histology, the finals groups were: VEH (n = 10), 10 μg of mCPBG (n = 10), 30 μg of mCPBG (n = 9), and OND-30 μg of mCPBG (n = 10).
Experiment 4b was identical to Experiment 4a, except that following the intraoral infusion of saccharin solution, the rats were microinfused into the anterior IC with either VEH or 30 μg of mCPBG. Following histology, the final groups were VEH (n = 8) and 30 μg of mCPBG (n = 8).
Behavioral measures
The videotapes from the TR conditioning and test trials were scored by observers blind to the experimental conditions using “The Observer” (Noldus) event-recording program. Disgust reactions were defined by the gaping (large openings of the mouth and jaw, with lower incisors exposed), which is the most sensitive and predominant conditioned disgust reaction (Breslin et al., 1992; Parker, 1995) that is reliably scored by raters with an inter-rater reliability greater than r = 0.90. Also, the amount of time that rats spent displaying hedonic reactions [tongue protrusions (extensions of the tongue out of the mouth) and mouth movements (movement of the lower mandible without opening the mouth)] and locomotor reactions (forward locomotion and horizontal rearing) were measured. However, in none of the experiments did our manipulations of interest modify hedonic or locomotor reactions; therefore, they will not be presented in the results or discussed. The amounts of saccharin and water consumed in the 120 min two-bottle CTA tests were converted to saccharin preference ratios (saccharin/[saccharin + water]).
Verification of 5,7-DHT lesions and histology
In Experiment 1, following completion of behavioral testing, rats were decapitated and their brains were removed. The IC, medial prefrontal cortex, hippocampus and striatum were dissected on ice, rapidly frozen over dry ice, and stored at a temperature of −80° until high performance liquid chromatographic assays were conducted, using the Waters 600 multisolvent pump (Waters Corporation), a 250 × 4.6 mm column with ODS2 5 μm packing material (Hichrom), a Coulochem 5100A detector with a 5020 guard cell and a 5011 analytical cell (ESA), a TSP AS3000 refrigerated autosampler (Thermal Separation Products), and a SP4290 integrator (Spectra Physics). The extent of the 5,7-DHT lesions was determined indirectly by measuring 5-HT, 5-hydroxyindole acetic acid (5-HIAA), dopamine (DA), and noradrenaline (NE) levels in each of these brain regions following extraction in 0.1 N perchloric acid containing 2 μm sodium bisulphate as an antioxidant. Biochemical verification of lesions was defined as 5-HT levels <40% of mean sham control levels in the IC (Tran-Nguyen et al., 2001); a total of three rats in the lesioned group did not reach this level and were excluded from the analysis.
In Experiments 2–4, cannula placements into the posterior IC and anterior IC were evaluated by histology. Rats were deeply anesthetized using an 85 mg/kg injection of Euthansol (Intervet Canada) followed by transcardial perfusion with phosphate-buffered saline (0.1 m) and 4% formalin. The brains were removed and stored at 4°C in 4% formalin solution for 24–48 h, after which they were placed in a 20% sucrose solution overnight at room temperature. The brains were then sliced in 60 μm sections using a CM1850 Leica cryostat, and relevant sections were mounted on glass microscope slides. The tissue was stained with cresyl violet 24 h later and examined for accurate cannula placement using a Leica MZ6 stereomicroscope with a Leica DFC420 digital camera and Leica Application Suite software. Rats with improper cannula placements, such as those located outside of the target region or who were not bilateral, were excluded from the behavioral analyses. Accurate bilateral posterior IC placements (Experiments 2a, 3, and 4a) were between −0.24 and −0.72 mm posterior of bregma. Accurate bilateral anterior IC placements (Experiments 2b and 4b) were between 1.68 and 1.20 mm anterior to bregma.
Data analysis
For Experiment 1, independent t tests between lesion and sham groups were conducted on the levels of 5-HT, 5-HIAA, DA, and NE in the IC, dorsal striatum, medial prefrontal cortex, and hippocampus. In each of the experiments, the number of gapes was analyzed as a factorial ANOVA with group as a between-groups factor and trials as a within-group factor. In Experiments 2–4, the saccharin preference ratios from the two-bottle CTA test were analyzed as a single factor ANOVA. Bonferroni post hoc tests were conducted as appropriate. Significance for all analyses was defined as p < 0.05. Nonsignificant F values are not reported.
Results
Experiment 1: effect of bilateral 5,7-DHT IC lesions on LiCl-induced conditioned gaping
Bilateral 5,7-DHT lesions of the IC significantly and exclusively reduced 5-HT and 5-HIAA levels in IC. As seen in Table 1, treatment with 5,7-DHT reduced IC 5-HT levels by 76% compared to shams, (t(6) = 4.1, p = 0.006), an effect that is supported by the significantly lower concentration of the 5-HT metabolite, 5-HIAA, in the same area, (t(6) = 3.2, p = 0.019). The reduction of 5-HT and 5-HIAA was exclusive to the IC, as there were no significant decreases in the dorsal striatum, mPFC, or hippocampus. There was no effect of lesion on the levels of either DA or NE in the IC, dorsal striatum, mPFC, or hippocampus.
Table 1.
Levels (ng/mg wet tissue weight) of 5-HT, 5-HIAA, DA, and NE in the insular cortex, dorsal striatum, medial prefrontal cortex, and hippocampus following 5,7-DHT treatment
| Structure/Group | 5-HT | 5-HIAA | DA | NE |
|---|---|---|---|---|
| Insular cortex | ||||
| Sham | 0.2731 ± 0.0479 | 0.1610 ± 0.0302 | 0.0886 ± 0.0270 | 0.1904 ± 0.0284 |
| Lesion | 0.0617 ± 0.0183 | 0.0639 ± 0.0051 | 0.0536 ± 0.0097 | 0.1869 ± 0.0120 |
| Percent depletion | 76%*** | 60%* | 29% | 2% |
| Dorsal striatum | ||||
| Sham | 0.3213 ± 0.0208 | 0.3270 ± 0.0204 | 4.0486 ± 1.1504 | 0.2463 ± 0.0388 |
| Lesion | 0.2698 ± 0.0222 | 0.3136 ± 0.0230 | 4.9253 ± 1.2689 | 0.1713 ± 0.0317 |
| Percent depletion | 16% | 4% | −17% | 30% |
| mPFC | ||||
| Sham | 0.1228 ± 0.0224 | 0.2181 ± 0.0414 | 0.1006 ± 0.0321 | 0.2286 ± 0.0341 |
| Lesion | 0.1051 ± 0.0160 | 0.2861 ± 0.0429 | 0.0743 ± 0.0069 | 0.2816 ± 0.0228 |
| Percent depletion | 14% | −14% | 26% | 3% |
| Hippocampus | ||||
| Sham | 0.2754 ± 0.0138 | 0.1770 ± 0.0074 | 0.0101 ± 0.0010 | 0.2812 ± 0.0190 |
| Lesion | 0.2472 ± 0.0202 | 0.1846 ± 0.0147 | 0.0104 ± 0.0010 | 0.2467 ± 0.0169 |
| Percent depletion | 11% | −4% | −3% | 12% |
mPFC, Medial prefrontal cortex; 5-HT, serotonin; 5-HIAA, 5-hydroxyindole acetic acid; DA, dopamine; NE, norepinephrine; 5,7-DHT, 5,7-dihydroxytryptamine.
***p < 0.001;
*p < 0.025.
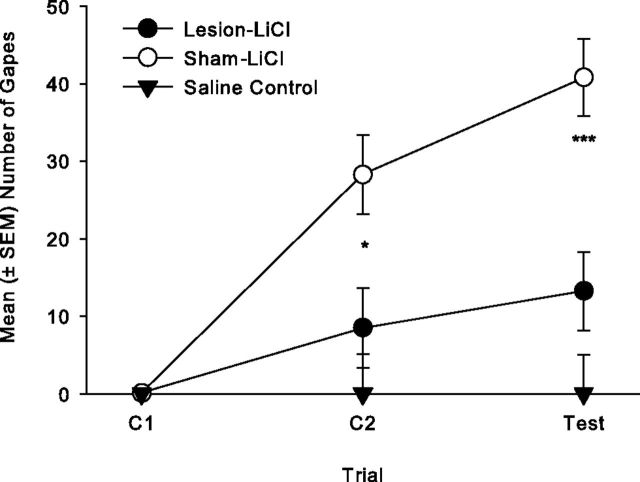
Bilateral 5,7-DHT IC lesions impaired the establishment of LiCl-induced conditioned gaping reactions. Figure 1 presents the mean number of gapes displayed on each trial by the various groups. The 2 × 3 mixed factors ANOVA revealed significant effects of group (F(1,6) = 10.2; p = 0.018), trial (F(2,12) = 33.0, p < 0.001), and group × trial (F(2,12) = 9.1, p < 0.004); on both conditioning trial 2 (p < 0.05) and the test trial (p < 0.01), the sham group displayed significantly more gapes than the lesioned group, indicating that bilateral 5,7-DHT IC lesions impaired the establishment of conditioned gaping. When the number of gapes was evaluated as a single factor repeated-measures ANOVA across trials for each group, both sham/LiCl (F(2,6) = 22.1; p = 0.002) and lesion/LiCl (F(2,6) = 14.5; p = 0.005) displayed a significant effect of trials; both groups displayed more gaping on the test trial than on C1 (p < 0.05), but only group, sham/LiCl, displayed more gaping on C2 than on C1 (p < 0.05). Therefore, the partial lesions did not completely block the establishment of LiCl-induced conditioned gaping reactions.
Figure 1.
Mean (±SEM) number of gapes elicited by a 3 min intraoral infusion of LiCl-paired 0.1% saccharin solution across trials for groups lesion/LiCl (n = 4) and sham/LiCl (n = 4) in Experiment 1. Group lesion/LiCl displayed fewer gapes than group sham/LiCl on C2 (*p < 0.05) and test (***p < 0.01).
The final quinine TR test revealed that lesioned rats and sham rats did not differ in reactivity to an unconditionally aversive test [mean (± SEM) number of gapes elicited by 0.05% quinine solution: lesion/LiCl = 15.2 ± 4.1; sham/LiCl = 17.5 ± 2.4 (t(6) = 0.5, n.s.)].
Experiment 2: effect of bilateral intracranial (2a: posterior IC, 2b: anterior IC) administration of OND on establishment of LiCl-induced conditioned gaping and CTA
Experiment 2a (posterior IC)
The accurate injector tip placements (circles) in the posterior IC are presented in Figure 2A. The tips of the injectors were bilaterally located in the posterior IC between −0.24 mm and −0.72 mm posterior to bregma for a total of 25 rats. Fifteen rats were excluded from the analysis because these rats either had inaccurate placements that were located outside of the posterior IC or were not bilateral. Microinfusions of the 5-HT3 antagonist, OND, into the posterior IC during conditioning impaired the establishment of conditioned gaping elicited by LiCl-paired saccharin solution but did not affect CTA. The mean number of gapes displayed by all groups across trials is presented in Figure 3A. The 3 × 3 ANOVA revealed a significant effect of group (F(2,22) = 3.3; p = 0.05), trial (F(2,44) = 17.1; p < 0.001), and group × trial (F(4,44) = 2.9; p = 0.03). Single factor ANOVAs for each trial revealed a significant group effect only during the drug-free TR test trial (F(2,24) = 3.7; p = 0.04; on the test trial the rats receiving bilateral microinfusions of either dose of OND to the posterior IC during conditioning gaped significantly less than saline controls (p < 0.05). In addition, the data for each group across trials were assessed as single factor repeated-measures ANOVA. Groups VEH (F(2,14) = 9.3; p = 0.003) and 1.0 OND (F(2,14) = 4.2; p = 0.038) displayed a significant effect of trials, and the trials effect for group 0.1 OND was marginally significant (F(2,16) = 3.4; p = 0.057). Each group displayed more gaping reactions on the test trial than on C1 (p < 0.05), and only group VEH displayed progressively more gaping on each trial in succession (p < 0 0.05). Therefore, although OND delivery to the posterior IC impaired the establishment of LiCl-induced conditioned gaping, it did not prevent this conditioned disgust reaction.
Figure 2.

A, Traces of infusion sites in the posterior IC (circles) for rats (N = 25) in Experiment 2a on drawings of coronal sections. Numbers indicate AP sections relative to bregma. B, Traces of infusion sites in the anterior IC (circles) for the various groups (N = 25) in Experiments 2b on drawings of coronal sections. Numbers indicate AP sections relative to bregma.
Figure 3.
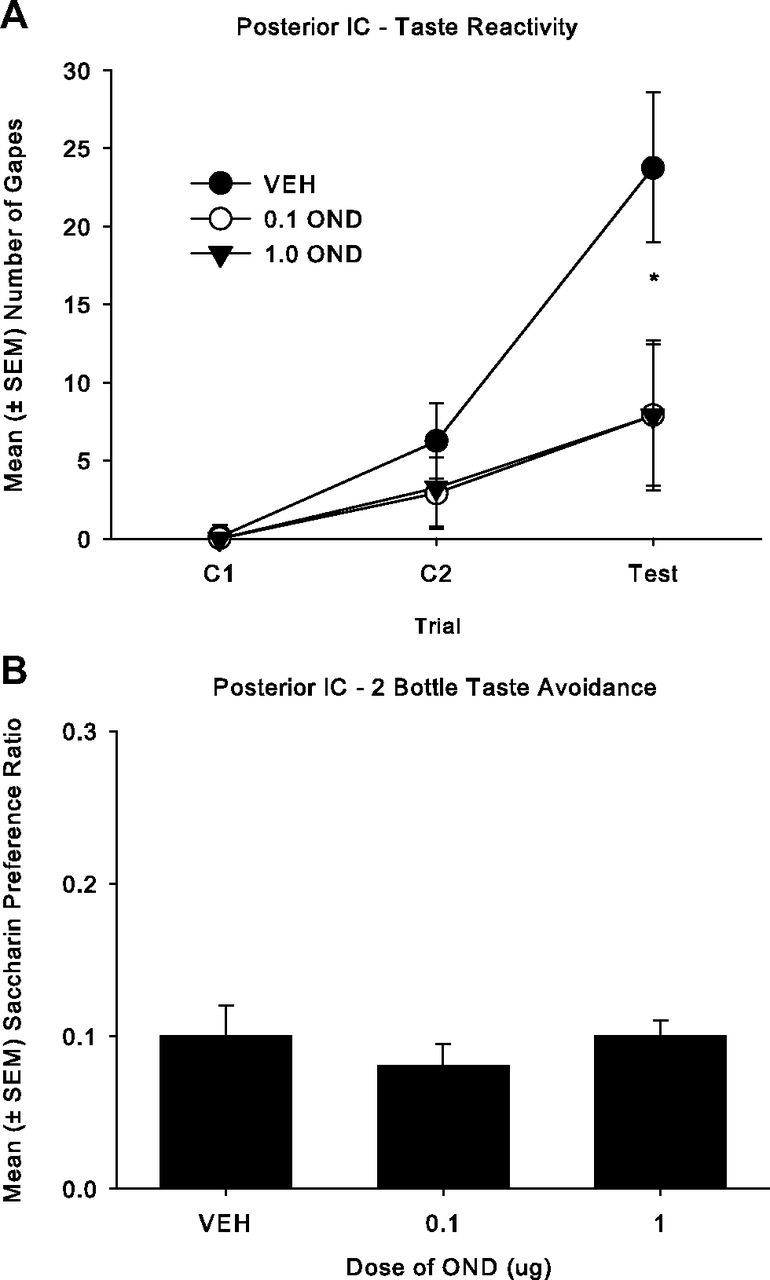
A, Mean (± SEM) number of gapes elicited by a 3 min infusion of a LiCl-paired 0.1% saccharin solution across trials for the posterior IC groups: 1 μg of OND (n = 8), 0.1 μg of OND (n = 9), and VEH (n = 8) in Experiment 2a. Both doses of OND (0.1 and 1 μg) significantly reduced the number of gapes during the drug-free test compared to VEH (*p < 0.05). B, Mean (± SEM) saccharin preference ratio (saccharin solution/[saccharin solution + water]) in a 120 min two-bottle consumption test for the posterior IC groups: 1 μg of OND (n = 8), 0.1 μg of OND (n = 9), and VEH (n = 8) in Experiment 2a. There were no group differences.
When delivered to the posterior IC, OND did not modify the establishment of CTA (Fig. 3B). The groups did not significantly differ in their saccharin preference in the 120 min two-bottle conditioned taste avoidance test.
Experiment 2b (anterior IC)
The accurate injector tip placements (circles) in the anterior IC are presented in Figure 2B. The tips of the injectors were bilaterally located in the anterior IC between 1.68 and 1.20 mm anterior to bregma for total of 25 rats. A total of 14 rats either had placements outside of the anterior IC or were not bilateral. In contrast to Experiment 2a, intracranial microinfusions of the 5-HT3 antagonist, OND, into the anterior IC did not interfere with the establishment of conditioned gaping elicited by a LiCl-paired saccharin solution, but did attenuate CTA. The mean number of gapes displayed by all groups across all trials is presented in Figure 4A. The 3 × 3 ANOVA revealed only a significant effect of trial (F(2,44) = 27.8, p < 0.001), with the number of gapes increasing across trials for all groups. However, neither the dose nor the dose × trial interaction was significant.
Figure 4.

A, Mean (± SEM) number of gapes elicited by a 3 min infusion of a LiCl-paired 0.1% saccharin solution across trials for the anterior IC groups 1 μg of OND (n = 8), 0.1 μg of OND (n = 9), and VEH (n = 8) in Experiment 2b. The groups did not differ significantly on any trial. B, Mean (± SEM) saccharin preference ratio (saccharin solution/[saccharin solution + water]) in a 120 min two-bottle consumption test for the anterior IC groups: 1 μg of OND (n = 8), 0.1 μg of OND (n = 9), and VEH (n = 8) in Experiment 2b. Intracranial administration of 1 μg of OND weakly attenuated LiCl-induced CTA relative to infusion of VEH (*p < 0.05).
On the other hand, as seen in Figure 4B, intracranial administration of OND to the anterior IC attenuated LiCl-induced CTA, with a significant effect of group (F(2,22) = 3.4; p = 0.05); subsequent comparison tests revealed that at a dose of 1 μg, but not 0.1 μg, and OND pretreated rats displayed a weaker CTA than VEH pretreated rats (p = 0.05).
Experiment 3: effect of intracranial posterior IC administration of mCPBG on the establishment of LiCl-induced conditioned gaping and CTA
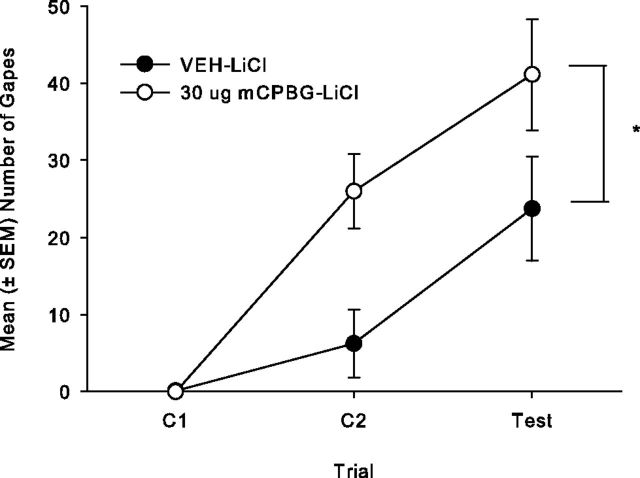
The tips of the injectors were bilaterally located in the posterior IC between −0.24 and −0.72 mm posterior to bregma for total of 15 rats. A total of 10 rats were excluded from the analysis because their placements were either inaccurate or not bilateral. Bilateral intracranial microinfusions of 30 μg of mCPBG into the posterior IC during conditioning trials significantly enhanced conditioned gaping reactions to a LiCl-paired saccharin solution, but that did not affect LiCl-induced CTA. The mean number of gapes displayed by both groups is presented in Figure 5. The 2 × 3 repeated-measures ANOVA revealed only significant main effects of group (F(1,13) = 6.7; p = 0.02) and trial (F(2,26) = 28.9; p < 0.001) with no interaction. Overall, rats pretreated with the 5-HT3 agonist mCPBG displayed significantly more LiCl-induced gaping than controls. When 30 μg of mCPBG was delivered to the posterior IC, it did not modify the strength of the LiCl-induced CTA. Group mCPBG/LiCl (mean ± SEM = 0.07 ± 0.007) did not significantly differ from group VEH/LiCl (mean ± SEM = 0.10 ± 0.03) in preference for LiCl-paired saccharin (t(13) = 0.97; n.s.).
Figure 5.
Mean (± SEM) number of gapes elicited by a 3 min infusion of a LiCl-paired 0.1% saccharin solution across trials for the posterior IC groups: 30 μg of mCPBG (n = 7) and VEH (n = 8) in Experiment 3. Bilateral infusion of mCPBG significantly enhanced the number of gapes elicited by LiCl-paired saccharin solution pooled across trials compared to VEH (*p < 0.05).
Experiment 4: effect of intracranial administration (4a: posterior IC and 4b: anterior IC) of mCPBG following saccharin exposure on the production of conditioned gaping and CTA
Experiment 4a (posterior IC)
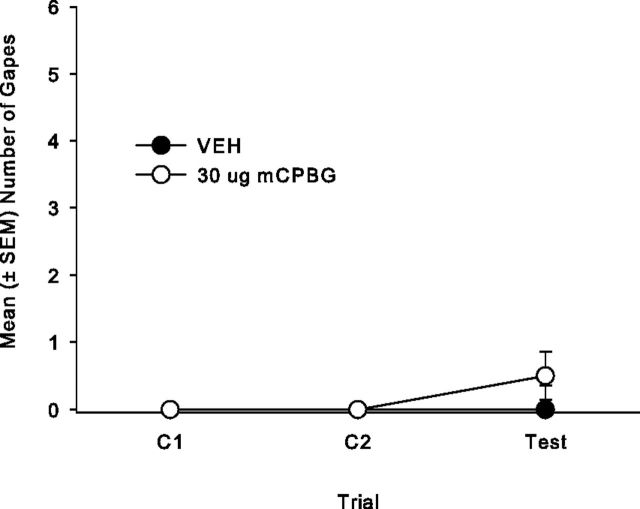
The tips of the injectors were bilaterally located in the posterior IC between −0.11 and −0.83 mm posterior to bregma for total of 39 rats. A total of 15 rats were excluded from the analysis because their placements were either inaccurate or not bilateral. When paired with a saccharin solution, bilateral intracranial microinfusions of 30 μg of mCPBG into the posterior IC produced conditioned gaping reactions, and this effect was blocked by 0.1 μg OND pretreatment, but it did not produce a CTA. The mean number of gapes displayed by all groups is presented in Figure 6A. The 4 × 3 ANOVA revealed a significant effect of group (F(3,35) = 10.0; p < 0.001), trial, (F(2,70) = 6.5; p = 0.003), and group × trial (F(6,70) = 4.8; p < 0.001). Subsequent single factor ANOVAs for each trial revealed that the number of gapes significantly differed among groups on C2 (F(3,35) = 7.0; p = 0.001) and during the drug-free test trial (F(3,35) = 6.9; p = 0.001). Rats receiving bilateral microinfusions of 30 μg of mCPBG into the posterior IC gaped significantly more than all other groups on C2 (p < 0.01) and test (p < 0.01). The single factor ANOVA of saccharin preference ratios (Fig. 6B) among the groups, however, was not significant. When delivered to the posterior IC, mCPBG did not produce a CTA.
Figure 6.
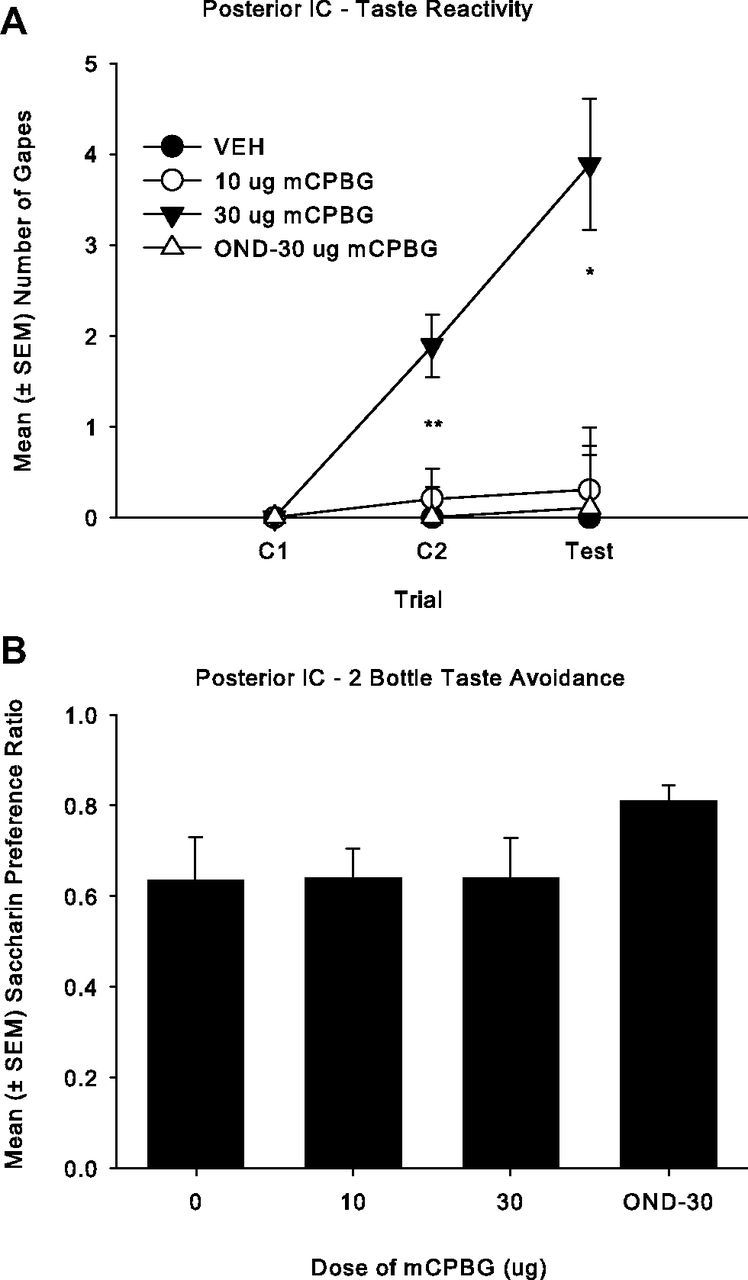
A, Mean (± SEM) number of gapes elicited by a 3 min infusion of a 0.1% saccharin solution that was paired with the posterior IC administration of: VEH (n = 10), 10 μg of mCPBG (n = 10), 30 μg of mCPBG (n = 9), or 0.1 μg of OND–30 μg of mCPBG (n = 10) in Experiment 4a. Bilateral infusion of 30 μg of mCPBG produced more gaping than the 10 μg of mCPBG and VEH on C2 (p < 0.01) and on the test trial (p < 0.01), which was blocked by OND pretreatment. B, Mean (± SEM) saccharin preference ratio (saccharin/[saccharin + water]) in a 120 min two-bottle consumption test for the posterior IC groups: VEH (n = 10), 10 μg of mCPBG (n = 10), 30 μg of mCPBG (n = 9) and 0.1 μg of OND–30 μg of mCPBG (n = 10) in Experiment 4a; the groups did not differ.
Experiment 4b (anterior IC)
The tips of the injectors were bilaterally located in the anterior IC between +1.68 and +1.20 mm anterior to bregma for total of 23 rats. A total of six rats were excluded from the analysis because their placements were either inaccurate or not bilateral. When explicitly paired with saccharin solution, bilateral intracranial microinfusions of 30 μg of mCPBG into the anterior IC produced a very weak CTA relative to group VEH, but not conditioned gaping. The 2 × 3 repeated-measures ANOVA of the gaping reactions revealed no significant effects (Fig. 7). However, rats receiving pairings of a saccharin solution with anterior IC infusions of 30 μg of mCPBG (mean ± SEM = 0.65 ± 0.053) displayed significantly lower saccharin preference ratios (t(14) = 2.3, p = 0.039) than those conditioned with VEH (mean ± SEM = 0.80 ± 0.033).
Figure 7.
Mean (± SEM) number of gapes elicited by a 3 min infusion of a 0.1% saccharin solution that was paired with anterior IC administration of 30 μg of mCPBG (n = 8) and VEH (n = 8) in Experiment 4b. The groups did not differ significantly on any trial.
Discussion
Conditioned disgust reactions are a more selective measure of the nausea-inducing effects of drugs than is conditioned taste avoidance (for review, see Parker et al., 2008). Partial depletion of serotonin in the entire IC impaired (but did not completely prevent) the establishment and/or expression of LiCl-induced conditioned disgust reactions in rats but did not modify unconditioned gaping elicited by bitter quinine. Saline controls were not included in the experiment because rats do not display conditioned gaping when re-exposed to a saline-paired saccharin solution (e.g., Parker, 1988). Application of 5,7-DHT to the IC substantially reduced 5-HT levels in that region. The fact that 5-HT levels were not significantly altered in the striatum, hippocampus, and mPFC suggests that the toxin was successfully confined to the intended target site. The results provide strong evidence that 5-HT projections to the insula are required for the conditioned gaping response, although we cannot rule out the possibility that damage to putative collateral projections of these serotonergic neurons to other brain regions may also contribute to this effect.
Experiments 2–4 evaluated the regional specificity in the establishment of these behavioral reactions. The results of Experiment 2 revealed a regionally specific double dissociation in the effects of the 5-HT3 antagonist OND on LiCl-induced conditioned gaping reactions and on LiCl-induced CTA. That is, when administered to the region of the IC with primary visceral input, the posterior IC, both doses of OND (0.1 and 1 μg) attenuated (but did not completely prevent) the establishment of LiCl-induced conditioned gaping but did not modify CTA. On the other hand, when administered to the region with primary gustatory input, the anterior IC, OND (1 μg but not 0.1 μg) produced a very weak attenuation of the strength of a LiCl-induced CTA but did not modify conditioned gaping. Given that intracranial administration of OND to the posterior IC reduced LiCl-induced conditioned gaping reactions, Experiment 3 evaluated whether infusion of the 5-HT3 agonist mCPBG into the posterior IC would enhance the establishment of these reactions. Indeed, activation of the 5-HT3 receptors enhanced the gaping reactions elicited by LiCl-induced saccharin solution. Since the 5-HT3 antagonist reduced LiCl-induced gaping and the 5-HT3 agonist enhanced LiCl-induced gaping when administered to the posterior IC, it is reasonable to suggest that activation of 5-HT3 receptors is a factor that plays a role in the generation of the sensation of conditioned disgust within this region of the IC (Penfield and Faulk, 1955; Contreras et al., 2007).
In Experiment 4, administration of the 5-HT3 agonist mCPBG to the posterior IC or to the anterior IC was explicitly paired with an intraorally infused saccharin solution. Bilateral microinfusions of 30 μg of mCPBG into the posterior IC produced a low level of conditioned gaping (which was blocked by 0.1 μg of OND pretreatment) but did not produce CTA. In contrast, bilateral microinfusions of 30 μg of mCPBG relative to VEH into the anterior IC produced a suppression of saccharin preference but did not produce conditioned gaping reactions. Since conditioned gaping reactions are selectively produced by emetic treatments and are attenuated by systemic administration of anti-emetic drugs (e.g., Parker et al., 2009), the results of the present experiments suggest that the activation of 5-HT3 receptors in the posterior IC by 5-HT may be necessary for the establishment of these nausea-induced behaviors in rats. Indeed, Contreras et al. (2007) reported that temporary lesions of this region of the IC also attenuate the LiCl-induced malaise reaction of lying on the belly (see Parker et al., 1984; Tuerke et al., 2012) in rats. This region of the IC monitors interoceptive input that regulates homeostasis (Craig, 2002; Saper, 2004). Although most gastrointestinal visceral sensory afferents to the IC terminate in the posterior granular field (albeit anterior/dorsal region of this field; Cechetto and Saper, 1987), most efferent projections from the IC to autonomic structures (including the NTS) originate in the more anterior agranluar field (Allen et al., 1991; Saper, 2004; Bagaev and Aleksandov, 2006).
The finding that OND delivered to the anterior IC selectively attenuated CTA is consistent with the literature regarding effects of IC lesions on CTA learning. Damage to the anterior IC has been reported to attenuate (but not eliminate) LiCl-induced CTA (e.g., Braun et al., 1972; Hankins et al., 1974; Lasiter, 1982; Lasiter and Glanzman, 1985; Dunn and Everitt, 1988; Bermúdez-Rattoni and McGaugh, 1991; Nerad et al., 1996; Roman and Reilly, 2007), but damage to the posterior IC has been reported to be ineffective in attenuation of LiCl-induced CTA (Mackey et al., 1986; Nerad et al., 1996). The effects of 5-HT3 antagonism in the anterior IC mirror the effects of NMDA lesions of the basolateral amygdala (BLA) on gaping and CTA (Rana and Parker, 2008); that is, reduced CTA but not gaping. Indeed, the anterior IC and the BLA are directly interconnected (Allen et al., 1991), and they are functionally interrelated in the acquisition and retention of CTA (Burecsová, 1978; Buresová et al., 1979; Yamamoto et al., 1981).
Functional magnetic resonance imaging investigations have shown that the human insula responds to images of disgusting stimuli, including foods, scenes, smells, or facial expressions of disgust that evoke sensations of illness (Phillips et al., 1998; Calder et al., 2001, 2007; Heining et al., 2003; Murphy et al., 2003). Penfield and Faulk (1955) observed that electrical stimulation of the human insula produces sensations of nausea, unpleasant tastes, and sensations in the stomach. Calder et al. (2000) report that a patient, NK, with damage to the insula, showed a marked and selective impairment in recognizing the facial expression and vocalization of disgust, as well as his own responsiveness to disgust-provoking scenarios.
The posterior IC has recently been shown to be essential in drug craving as well as malaise in rats (Contreras et al., 2007; Hollander et al., 2008; Forget et al., 2010), and in humans damage to the insula promotes termination of cigarette smoking (Naqvi et al., 2007) immediately with no relapse (Naqvi et al., 2007). Inactivation of the posterior IC also produces decreased nicotine self-administration under both fixed and progressive ratio schedules without affecting the self-administration of food (Forget et al., 2010). As well, this inactivation prevented reinstatement following extinction of nicotine seeking induced by nicotine-associated cues or by a nicotine prime, but not reinstatement of food seeking. The effect on addiction-related behavior is not specific to nicotine-maintained responding, because Contreras et al. (2007) demonstrated that temporary inactivation of the posterior IC also prevented the urge to seek amphetamine in a place preference task. These authors suggest that this region of the IC may serve as a gauge of deviations from a “well being state” that are present in states of addiction or nausea-inducing treatments. Nausea may therefore be produced by the activation of 5-HT3 receptors in the posterior IC, which produces conditioned gaping reactions in rats that are re-exposed to the flavor with which it is paired. As well, LiCl-induced nausea is reduced by inhibition of 5-HT3 receptors in this region. On the other hand, serotonin activation in the anterior IC appears to be at least partially involved in the production of CTA. Delivery of the 5-HT3 antagonist OND directly to this region resulted in a weak attenuation of a LiCl-induced CTA, and central administration of the 5-HT3 agonist mCPBG to this region produced a weak CTA.
Footnotes
This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Operating Grant (92057) to L.A.P. and an NSERC CGS D Scholarship to K.J.T. We thank Dr. John Chambers, Centre for Addiction and Mental Health, Toronto Ontario, for his kind and skillful assistance in the HPLC analysis of tissue samples in Experiment 1. Since collection of this data, Dr. Chambers passed away in 2011.
References
- Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 1991;311:1–16. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- Asin KE, Wirtshafter D, Kent EW. The effects of electrolytic median raphe lesions on two measures of latent inhibition. Behav Neural Biol. 1980;28:408–417. doi: 10.1016/s0163-1047(80)91734-3. [DOI] [PubMed] [Google Scholar]
- Bagaev V, Aleksandrov V. Visceral-related area in the rat insular cortex. Autonom Neurosci. 2006;125:16–21. doi: 10.1016/j.autneu.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Baumgarten HG, Klemm HP, Lachenmayer L, Björklund A, Lovenberg W, Schlossberger HG. Mode and mechanism of action of neurotoxic indoleamines: a review and a progress report. Ann NY Acad Sci. 1978;305:3–24. doi: 10.1111/j.1749-6632.1978.tb31507.x. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Rattoni F, McGaugh JL. Insular cortex and amygdala differentially effect acquisition on inhibitory avoidance and conditioned taste aversion. Brain Res. 1991;549:165–170. doi: 10.1016/0006-8993(91)90616-4. [DOI] [PubMed] [Google Scholar]
- Braun JJ, Slick TB, Lorden JF. Involvement of gustatory neocortex in learning of taste aversions. Physiol Behav. 1972;9:637–641. doi: 10.1016/0031-9384(72)90023-6. [DOI] [PubMed] [Google Scholar]
- Breslin PA, Spector AC, Grill HJ. A quantitative comparison of taste reactivity behaviors to sucrose before and after lithium chloride pairings: a unidimensional account of palatability. Behav Neurosci. 1992;106:820–836. doi: 10.1037//0735-7044.106.5.820. [DOI] [PubMed] [Google Scholar]
- Buresová O. Neocortico-amygdalar interaction in the conditioned taste aversion in rats. Act Nerv Super (Praha) 1978;20:224–230. [PubMed] [Google Scholar]
- Buresová O, Aleksanyan ZA, Bures J. Electrophysiological analysis of retrieval of conditioned taste aversion in rats. Unit activity changes in critical brain regions. Physiol Bohemoslov. 1979;28:525–536. [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young AW. Impaired recognition and experience of disgust following brain injury. Nat Neurosci. 2000;3:1077–1078. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nat Rev Neurosci. 2001;2:352–363. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Beaver JD, Davis MH, van Ditzhuijzen J, Keane J, Lawrence AD. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur J Neurosci. 2007;25:3422–3428. doi: 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- Catenoix H, Isnard J, Guénot M, Petit J, Remy C, Mauguière F. The role of the anterior insular cortex in ictal vomiting: A stereotactic electroencephalography study. Epilepsy Behav. 2008;13:560–563. doi: 10.1016/j.yebeh.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Dunn LT, Everitt BJ. Double dissociations of the effects of amygdala and insular cortex lesions on conditioned taste-aversion, passive-avoidance, and neophobia in the rat using the excitotoxin ibotenic acid. Behav Neurosci. 1988;102:3–23. doi: 10.1037//0735-7044.102.1.3. [DOI] [PubMed] [Google Scholar]
- Fiol M, Leppik IE, Mireles R, Maxwell R. Ictus emeticus and the insular cortex. Epilepsy Res. 1988;2:127–131. doi: 10.1016/0920-1211(88)90030-7. [DOI] [PubMed] [Google Scholar]
- Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry. 2010;68:265–271. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978;201:267–269. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- Hankins WG, Garcia J, Rusiniak KW. Cortical lesions: flavor illness and noise shock conditioning. Behav Biol. 1974;10:173–181. doi: 10.1016/s0091-6773(74)91767-2. [DOI] [PubMed] [Google Scholar]
- Heining M, Young AW, Ioannou G, Andrew CM, Brammer MJ, Gray JA, Phillips ML. Disgusting smells activate human anterior insula and ventral striatum. Ann NY Acad Sci. 2003;1000:380–384. doi: 10.1196/annals.1280.035. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaada BR. Somato-motor, autonomic and electrocorticographic responses to electrical stimulation of rhinencephalic and other structures in primates, cat, and dog; a study of responses from the limbic, subcallosal, orbito-insular, piriform and temporal cortex, hippocampus-fornix and amygdala. Acta Physiol Scand Suppl. 1951;24:1–262. [PubMed] [Google Scholar]
- Kiefer SW, Orr MR. Taste avoidance, but not aversion, learning in rats lacking gustatory cortex. Behav Neurosci. 1992;106:140–146. doi: 10.1037//0735-7044.106.1.140. [DOI] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Res. 1986;379:329–341. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- Lasiter PS. Cortical substrates of taste aversion learning: direct amygdalocortical projections to the gustatory neocortex do not mediate conditioned taste aversion learning. Physiol Psychol. 1982;10:377–383. [Google Scholar]
- Lasiter PS, Glanzman DL. Cortical substrates of taste aversion earning: involvement of the dorsolateral amygdaloid nuclei and temporal neocortex in taste aversion learning. Behav Neurosci. 1985;99:257–276. doi: 10.1037//0735-7044.99.2.257. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Parker LA. The antiemetic drug ondansetron interferes with lithium-induced conditioned rejection reactions, but not lithium-induced taste avoidance in rats. J Exp Psychol Anim Behav Process. 2000;26:371–384. doi: 10.1037//0097-7403.26.4.371. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Parker LA. The 5-HT1A agonist, 8-OH-DPAT, dose-dependently interferes with the establishment and the expression of lithium-induced conditioned rejection reactions. Psychopharmcology (Berl) 2003;166:120–126. doi: 10.1007/s00213-002-1309-6. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Parker LA, Fletcher PJ. 5,7-Dihydroxytryptamine lesions of the dorsal and median raphe nuclei interfere with lithium-induced conditioned gaping, but not conditioned taste avoidance, in rats. Behav Neurosci. 2004;118:1391–1399. doi: 10.1037/0735-7044.118.6.1391. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Krohn JP, Cross-Mellor S, Litt DE, Ossenkopp KP, Parker LA. Exposure to a context previously associated with nausea elicits conditioned gaping in rats: A model of anticipatory nausea. Behav Brain Res. 2008;187:33–40. doi: 10.1016/j.bbr.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Vemuri VK, Bedard H, Lang ST, Ossenkopp KP, Makriyannis A, Parker LA. Inverse agonism of cannabinoid CB1 receptors potentiates LiCl-induced nausea in the conditioned gaping model in rats. Br J Pharmacol. 2010;161:336–349. doi: 10.1111/j.1476-5381.2010.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy RF, Norgren R. In: Gustatory system: in the rat nervous system. Ed 3. Paxinos G, editor. San Diego: Elsevier; 2004. pp. 890–922. [Google Scholar]
- Mackey WB, Keller J, van der Kooy D. Visceral cortex lesions block conditioned taste aversions induced by morphine. Pharmacol Biochem Behav. 1986;24:71–78. doi: 10.1016/0091-3057(86)90047-x. [DOI] [PubMed] [Google Scholar]
- Matsuki N, Wang CH, Okada F, Tamura M, Ikegaya Y, Lin SC, Hsu YN, Chaung LJ, Chen SJ, Saito H. Male/female differences in drug-induced emesis and motion sickness in Suncus murinus. Pharmacol Biochem Behav. 1997;57:721–725. doi: 10.1016/s0091-3057(96)00389-9. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerad L, Ramírez-Amaya V, Ormsby CD, Bermúdez-Rattoni F. Differential effects of anterior and posterior insular cortex lesions on the acquisition of conditioned taste aversion and spatial learning. Neurobiol Learn Mem. 1996;66:44–50. doi: 10.1006/nlme.1996.0042. [DOI] [PubMed] [Google Scholar]
- Parker LA. A comparison of avoidance and rejection responses elicited by conditionally and unconditionally aversive tasting solutions. Learn Motiv. 1988;19:1–12. [Google Scholar]
- Parker LA. Rewarding drugs produce taste avoidance, but not taste aversion. Neurosci Biobehav Rev. 1995;19:143–157. doi: 10.1016/0149-7634(94)00028-y. [DOI] [PubMed] [Google Scholar]
- Parker LA, Hills K, Jensen K. Behavioral CRs elicited by lithium- or amphetamine-paired contextual cues. Learn Behav. 1984;12:307–315. [Google Scholar]
- Parker LA, Rana SA, Limebeer CL. Conditioned nausea in rats: Assessment by conditioned disgust reactions, rather than conditioned taste avoidance. Can J Exp Psychol. 2008;62:198–209. doi: 10.1037/a0012531. [DOI] [PubMed] [Google Scholar]
- Parker LA, Limebeer CL, Rana SA. Conditioned disgust, but not taste avoidance, may reflect conditioned nausea in rats. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: behavioral and neural processes. New York: Oxford UP; 2009. pp. 92–113. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 5. London: Academic; 2007. [DOI] [PubMed] [Google Scholar]
- Penfield W, Faulk ME., Jr The insula: Further observations on its function. Brain. 1955;78:445–470. doi: 10.1093/brain/78.4.445. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, Williams SC, Bullmore ET, Brammer M, Gray JA. Neural responses to facial and vocal expressions of fear and disgust. Proc Biol Sci. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana SA, Parker LA. Differential effects of neurotoxin-induced lesions of the basolateral amygdala and central nucleus of the amygdala on lithium-induced conditioned disgust reactions and conditioned taste avoidance. Behav Brain Res. 2008;189:284–297. doi: 10.1016/j.bbr.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Reilly S. Central gustatory system lesions and conditioned taste aversion. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: behavioral and neural mechanisms. New York: Oxford UP; 2009. pp. 309–327. [Google Scholar]
- Rock EM, Limebeer CL, Mechoulam R, Piomelli D, Parker LA. The effect of cannabidiol and URB597 on conditioned gaping (a model of nausea) elicited by a lithium-paired context in the rat. Psychopharmcology (Berl) 2008;196:389–395. doi: 10.1007/s00213-007-0970-1. [DOI] [PubMed] [Google Scholar]
- Roman C, Reilly S. Effects of insular cortex lesions on conditioned taste aversion and latent inhibition in the rat. Eur J Neurosci. 2007;26:2627–2632. doi: 10.1111/j.1460-9568.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- Saper CB. Central autonomic systems. In: Paxinos G, editor. The rat nervous system. Ed 3. San Diego: Elsevier; 2004. pp. 761–795. [Google Scholar]
- Tran-Nguyen LT, Bellew JG, Grote KA, Neisewander JL. Serotonin depletion attenuates cocaine seeking but enhances sucrose seeking and the effects of cocaine priming on reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;157:340–348. doi: 10.1007/s002130100822. [DOI] [PubMed] [Google Scholar]
- Tuerke KJ, Winters BD, Parker LA. Ondansetron interferes with unconditioned lying-on belly and acquisition of conditioned gaping induced by LiCl as models of nausea-induced behaviors in rats. Physiol Behav. 2012;105:856–860. doi: 10.1016/j.physbeh.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Azuma S, Kawamura Y. Significance of cortical-amygdalar-hypothamic connections in retention of conditioned taste aversion in rats. Exp Neurol. 1981;74:758–768. doi: 10.1016/0014-4886(81)90249-1. [DOI] [PubMed] [Google Scholar]