Abstract
Background:
It is established that antibiotic prophylaxis prevents infection following transrectal ultrasound-guided prostate biopsy. This study compares the infective complications in transrectal prostate biopsy (TRPB) in empirical versus targeted prophylactic antibiotics.
Patients and Methods:
Urine and rectal swabs were obtained prior to TRPB. They were randomized into targeted antibiotic (TA) and empirical antibiotic (EA) groups. TA had prophylactic antibiotics according to rectal swab culture, whereas EA had the standard parenteral ciprofloxacin. They were followed up weekly for 4 weeks. Chi-square or Fisher's exact tests were used to compare categorical variables, Student's “t”-test was used to compare means of numerical variables, and P < 0.05 was considered statistically significant.
Results:
One hundred patients were studied, fifty in each group. The mean age was 66 years, with men aged 60–69 years accounting for 50% of the study population. Providencia stuartii, Escherichia coli, and Citrobacter freundii were the most predominant bacteria identified in the prebiopsy rectal swab culture, with resistance to ciprofloxacin (57%) being much more common than that to levofloxacin (21%). Postbiopsy infection occurred in one (2%) patient in the TA group and five (10%) patients in the EA group. Difference in the infection rate between the two groups was statistically significant (P = 0.042). Three of the patients with postbiopsy infection in the EA group had urosepsis and required hospitalization. Fluoroquinolone-resistant bacteria were responsible for infection in all the six patients. TA reduced the risk of postbiopsy infection by 5.6 folds.
Conclusion:
TA was associated with a decreased risk of infection in TRPB.
Keywords: Antibiotic prophylaxis, biopsy, empirical, fluoroquinolone resistance, prostate, targeted, Prophylaxie aux antibiotiques, prostate, biopsie, ciblée, empirique, résistance à la fluoroquinolone
Résumé
Contexte:
Il est établi que la prophylaxie antibiotique prévient l’infection après une biopsie transrectale guidée par une échographie de la prostate (TRPB). Cette étude compare les complications infectieuses liées au TRPB entre antibiotiques prophylactiques empiriques et ciblés.
Patients et méthodes:
Des écouvillons urinaires et rectaux ont été obtenus avant le TRPB. Ils ont été randomisés en groupes d’antibiotiques ciblés (TA) et d’antibiotiques empiriques (EA). TA avait des antibiotiques prophylactiques selon la culture sur écouvillon rectal, alors que EA avait la ciprofloxacine parentérale standard. Ils ont été suivis chaque semaine pendant quatre semaines. Les tests exacts du chi carré ou de Fischer ont été utilisés pour comparer les variables qualitatives, le test de l’étudiant a été utilisé pour comparer la moyenne des variables numériques et P <-0,05 a été considéré comme statistiquement significatif.
Résultats:
Cent patients ont été étudiés; 50 dans chaque groupe. L’âge moyen était de 66 ans, les hommes de 60 à 69 ans représentant 50% de la population étudiée. Providencia Stuartii, Escherichia Coli et Citrobacter Freundii étaient les bactéries les plus prédominantes identifiées dans la culture du prélèvement rectal avant biopsie, la résistance à la ciprofloxacine (57%) étant beaucoup plus commune qu’à la lévofloxacine (21%). Une infection après la biopsie s’est produite chez 1 patient (2%) du groupe TA et 5 patients (10%) du groupe EA. La différence de taux d’infection entre les deux groupes était statistiquement significative (p = 0,042). Trois des patients présentant une infection post-biopsie dans le groupe EA présentaient une urosepsie et devaient être hospitalisés. Des bactéries résistantes à la fluoroquinolone étaient responsables de l’infection chez les six patients. L’AT réduit le risque d’infection après la biopsie de 5,6 fois.
Conclusion:
L’AT était associée à une diminution du risque d’infection dans le TRPB.
INTRODUCTION
Cancer of the prostate is the second most frequently diagnosed cancer in men worldwide and the most frequently diagnosed noncutaneous cancer.[1,2] Nearly, a million new cases were diagnosed in 2008 worldwide.[1,2] Several other studies [3,4,5,6] have shown the significant prevalence of prostate cancer and possible mortality associated with it, thus procedures for early diagnosis and prompt treatment cannot be overemphasized.
Prostate needle biopsy is the standard procedure for the diagnosis of carcinoma of the prostate, and transrectal ultrasound-guided prostate biopsy (TRUSPB) is the most commonly used approach. However, the procedure may result in infective complications due to the possibility of bacterial translocation from the rectum to the prostate, blood, and other parts of the urogenital tract.
Empirical antibiotic (EA) prophylaxis with a fluoroquinolone is usually given to prevent infective complication. This has been reported to reduce infective complications to 8% compared with placebo which is 25%.[7] Recent studies have demonstrated an increased incidence of infective complications following prostate biopsy using empirical antibiotics (EA).[8,9,10,11] It has also been reported that the rate of infective complications requiring hospitalization is significantly greater in more recent years.[12] These infective complications may be severe leading to urosepsis and death. The reason for the increase in biopsy infective complications appears to be due to the increase in the incidence of fluoroquinolone-resistant bacteria [13,14,15] and the lack of an evidence-based standardized protocol of antibiotic prophylaxis for transrectal prostate biopsy (TRPB).
Targeted antibiotic (TA) prophylaxis based on the rectal swab result has been shown to effectively reduce postbiopsy infective complications, and it is as well cost-effective in the long run.[16] Therefore, the identification of prebiopsy rectal microbes, culture, and antibiotic sensitivity pattern and the choice of the appropriate antibiotics will reduce the incidence of postbiopsy infective complications, hospital admission, and the attendant high cost of treatment. The aim of this study was, therefore, to compare the infective complications following empirical and targeted prophylactic antibiotic use in patients undergoing TRPB.
PATIENTS AND METHODS
Patients attending the urology clinic of Lagos State University Teaching Hospital and had indications (elevated serum prostate-specific antigen [PSA] and or digital rectal examination suspicious of prostate cancer) for ultrasound-guided transrectal prostate biopsy were randomized into two groups: TAs and EAs. Patients had prebiopsy urinalysis and urine culture. Those with prebiopsy abnormal urinalysis or urinary tract infection, recent antibiotic use in the preceding 3 months, and past history of fluoroquinolone adverse reaction were excluded from the study. Aspirin use was discontinued 10 days before the biopsy.
The rectal swab was collected from all the patients in the clinic 5 days before the biopsy to allow adequate time for results; the collection was done with sterile swab sticks and transported to the laboratory immediately. The swab was inoculated on MacConkey agar and incubated at 35°C–37°C for 18–24 h. Mixed cultures were separated, and pure isolates were kept inside peptone for further identification. Thereafter, Gram staining was done to group the organisms into Gram positive or Gram negative and was further tested for antibiotic sensitivity. Antibiotic susceptibility test was done using Kirby–Baker method on Mueller–Hinton agar. The laboratory investigations were done by the same microbiologist in our laboratory. The TA group had intravenous antibiotics based on the rectal swab culture sensitivity and the EA group had the usual intravenous ciprofloxacin 200 mg within 1 h before the biopsy. TRUSPB was done under caudal anesthesia with CHISON 8300 digital ultrasound diagnostic B/Wsystem (Chison Medical Imaging Limited, China) using a disposable sterile, size 18G, trucut needle (AGUJA semi-automatic needle, Italy) for each patient. Twelve cores were taken from each patient. They were discharged home after the procedure and were followed up weekly for 4 weeks. All the patients also had postbiopsy urinalysis and urine culture. During each visit, the patients were asked if they had dysuria or fever, their vital signs were assessed, and any temperature >38°C was recorded as fever. They also had urinalysis and urine m/c/s weekly for 4 weeks. Blood culture was also done in those whose temperature was >38°C and who had sepsis.
Other clinical data obtained included family history of prostate cancer, serum PSA, comorbidities (hypertension and diabetes), prostate size measured with transrectal ultrasound scan, histopathology of the biopsy specimen, and stage of the disease for those with prostate cancer.
SPSS software version 21 for Windows (SPSS, Inc., Chicago, IL, USA) was used for data analysis. Chi-square test or Fisher's exact test was used to compare categorical variables, whereas Student's t-test was used to compare means of numerical variables. Multiple logistic regression was used to evaluate for the potential risk factor for the development of postbiopsy sepsis. For all statistical tests, confidence interval was set at 95% and statistical tests were considered significant if P < 0.05.
RESULTS
Study population
There were 112 patients who were recruited for the study, but only 100 who made the inclusion criteria were included in the analysis. There were fifty patients in each group (EA vs. TA groups). The patients were aged 44–85 (mean = 66.0) years. Figure 1 shows the age group distribution of the patients, with men of 60–69 years accounting for 50% of the study population.
Figure 1.
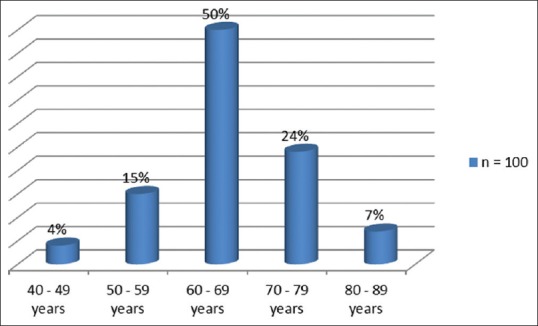
Age distribution of patients
Comparison of the two groups of TA and EA prophylaxis is shown in Table 1. Essentially, there was no significant difference between the two groups with regard to age, serum PSA, prostate size, family history of prostate cancer, history of diabetes, histopathological diagnosis, and stage of the disease.
Table 1.
Comparison of the two groups of patients (empirical and targeted antibiotic groups)
| Variable | Targeted (n=50) | Empirical (n=50) | Total (n=100) | P |
|---|---|---|---|---|
| Age | ||||
| 40-49 | 4 | 1 | 4 | 0557 |
| 50-59 | 15 | 34 | 65 | |
| 60-69 | 50 | 15 | 31 | |
| 70-79 | 24 | |||
| 80-89 | 7 | |||
| PSA | ||||
| <4.0 | 1 | 0 | 1 | 0.662 |
| 4.0-10 | 19 | 18 | 37 | |
| 10-20 | 21 | 23 | 44 | |
| 20-100 | 1 | 3 | 4 | |
| >100 | 8 | 6 | 14 | |
| Prostate size | 0.201 | |||
| <40 | 4 | 1 | 5 | |
| 40-100 | 19 | 26 | 45 | |
| >100 | 27 | 23 | 50 | |
| Histology | ||||
| BPH | 35 | 36 | 71 | 0.662 |
| Adenocarcinoma | 15 | 14 | 29 | |
| LUTS | ||||
| Yes | 41 | 50 | 91 | 0.002 |
| No | 9 | 0 | 9 | |
| Comorbidity (diabetes) | ||||
| Yes | 10 | 12 | 22 | 0.629 |
| No | 40 | 38 | 78 | |
| Metastasis | ||||
| Yes | 4 | 3 | 7 | 0.695 |
| No | 46 | 47 | 93 | |
| Postbiopsy infection | ||||
| Yes | 1 | 5 | 6 | 0.042 |
| No | 49 | 45 | 94 |
PSA=Prostate-specific antigen, LUTS=Lower urinary tract symptoms, BPH=Benign prostatic hyperplasia
Prebiopsy rectal flora and antibiotic sensitivity for all patients
Figure 2 shows the organism identified in the rectal flora, with Providencia stuartii, Escherichia coli, and Citrobacter freundii being the three most predominant. Figures 3 and 4 show the antibiotic sensitivity and resistant patterns of the organisms identified in the rectal flora, levofloxacin being the antibiotic that showed the highest sensitivity. There were 17 fluoroquinolone-resistant E. coli from the rectal swab culture, 15.8% were levofloxacin resistant, whereas 73.7% were resistant to ciprofloxacin.
Figure 2.
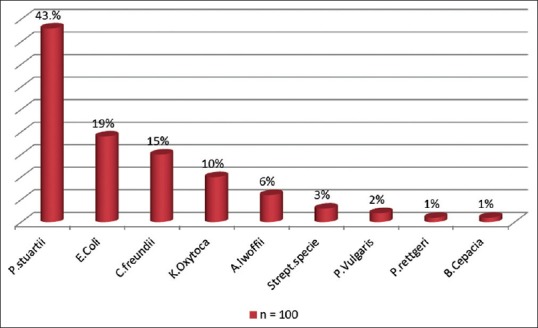
The prebiopsy bacterial flora of the rectum of all the patients
Figure 3.
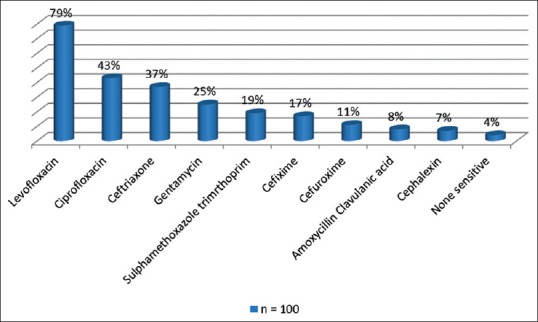
The prebiopsy antibiotic sensitivity pattern of the bacteria in the rectum of all the patients
Figure 4.
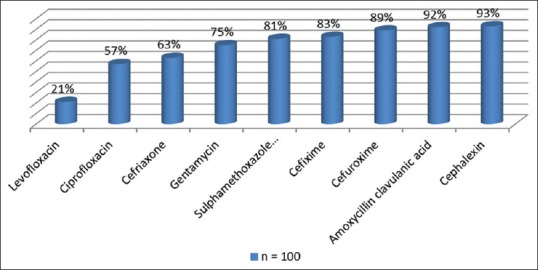
The prebiopsy antibiotic resistant pattern of the bacteria in the rectum of the patients
Incidence of postbiopsy sepsis and antibiotic sensitivity pattern
Of the fifty patients who had TA prophylaxis, one (2%) had positive postbiopsy urine culture, but there was no patient with febrile (symptomatic) urinary tract infection in this group. Conversely, among the fifty patients with EA prophylaxis, five (10%) had positive urine culture postbiopsy; three (6%) of whom had fever, chills, rigors, and required hospitalization, whereas the remaining two (4%) had only positive urine culture postbiopsy. Prophylactic antibiotic status was found to be a significant factor to the development of postbiopsy sepsis (P = 0.042).
The six patients who had postbiopsy infection had the prebiopsy rectal swab culture yielded E. coli resistant to the ciprofloxacin. The postbiopsy urine culture of these six patients yielded E. coli sensitive to levofloxacin, ceftriaxone, ceftazidime, and cefexime.
Risk factor for postbiopsy sepsis
The risk factor for postbiopsy sepsis was evaluated using multiple logistic regression [Table 2]. The prophylactic antibiotic status and the comorbidity of diabetes were found to be significant risk factors for sepsis. The risk for sepsis was 5.6 folds greater when EA prophylaxis was used compared to TA prophylaxis. In addition, men with diabetes have 22-fold increase in the incidence of sepsis following TRPB. The age, serum PSA, histology, lower urinary tract symptoms, and prostate size were not found to be significant risk factors for sepsis.
Table 2.
Risk factors for postbiopsy infection
| Variable | Univariate (P) | Multivariate (P) | OR | 95% CI of OR |
|---|---|---|---|---|
| Age | 0.084 | 0.162 | 0.051 | 0.970, 9.290 |
| Prostate size | 0.832 | 0.986 | 1.289 | 0.003, 9.923 |
| Serum PSA | 0.061 | 0.834 | 2.772 | 0.042, 6.984 |
| Histology | 0.247 | 0.335 | 2.634 | 0.368, 18.842 |
| LUTS | 0.623 | 0.995 | 2.754 | 0.345, 11.678 |
| Antibiotic group: Targeted versus empirical | 0.042 | 0.045 | 5.596 | 1.390, 38.108 |
| Comorbidity | 0.000 | 0.008 | 22.213 | 2.278, 216.612 |
CI=Confidence interval, OR=Odds ratio, PSA=Prostate-specific antigen, LUTS=Lower urinary tract symptoms, BPH=Benign prostatic hyperplasia
DISCUSSION
TRPB is currently the standard tissue-sampling technique for the histological diagnosis of cancer of the prostate. It is one of the most commonly performed urological procedures, with over a million patients reportedly undergoing prostate biopsy every year in the United States.[17] Although TRPB is generally considered a safe procedure, it has its own attendant complications ranging from minor problems such as pain, hematospermia, hematuria, and hematochezia to severe infectious complications such as urosepsis and acute prostatitis.[9]
There are two primary antibiotic prophylaxis strategies that have emerged over time to prevent postbiopsy-related infections, namely an EA or TA approach.[18] This study was designed to evaluate the infectious complication rate in a selected cohort of men who received a rectal swab culture and subsequent TA prophylaxis, compared to a group of same-risk men who had EA prophylaxis with ciprofloxacin before TRPB. The rationale for the use of rectal cultures to identify culpable organisms for appropriate antibiotics to be given in targeted prophylactic antibiotic use was formulated due to the increasing incidence of postbiopsy infective complications in EA prophylaxis for TRPB. In addition, inappropriate use of antibiotics is also a risk for the development of antibiotic resistance.
The findings from this study showed that the rate of infective complication was lower in the TA prophylactic group compared to the EA group (2% vs. 10%). This decrease in infective rate has been reported in similar studies in literature.[16,19,20,21,22] The incidence of urosepsis in the TA group was 0% compared to 6% in the EA group. In a similar study, with empiric single-drug antimicrobial prophylaxis for TRPB, the hospitalization rate for infections was 0%–6.3%,[23] which is similar to the result from our study. Dai et al.[21] and Summers et al.[22] have documented infective complication rates of 1.9% and 0.6%, respectively, among patients who had TA prophylaxis, whereas Duplesis's study [19] showed 2.9% infection rates among the EA group.
Furthermore, the presence of fluoroquinolone-resistant bacteria in the rectal flora increases the rate and potential severity of postbiopsy infections by 5 folds in patients receiving empiric fluoroquinolone prophylaxis.[24] Our study showed that fluoroquinolone-resistant organisms (two isolates of E. coli, two isolates of P. stuartii, and two isolates of C. freundi) were responsible for infection in all the six patients. In addition, this risk of postbiopsy infection was increased by 5.6 folds among the EA group compared to the TA group.
This finding supports the utilization of prebiopsy rectal cultures to identify patients at increased risk and to select targeted prophylaxis that is most likely to be effective. This corroborates other findings in the literature that fluoroquinolone-resistant bacteria are responsible for the increase in postbiopsy infective complications.[10,14,15] Furthermore, in all the six cases of postbiopsy infection, the bacterial isolates were all resistant to ciprofloxacin.
In addition, patients with comorbidity of diabetes are particularly at risk. The risk of postbiopsy infection increased 22 folds among patients undergoing TRPB if they were diabetic.
Therefore, the use of rectal swab cultures for TA prophylaxis will reduce infective complications compared to EA prophylaxis which may be associated with more infective complications and antibiotic resistance. In addition, when EA prophylaxis must be considered at all, levofloxacin would be a much better choice than ciprofloxacin because of the less resistance pattern from the rectal bacterial flora.
Reducing the postbiopsy infection would lead to less hospitalization and reduced morbidity and mortality following TRPB. In addition, TA prophylaxis for patients undergoing TRPB would potentially reduce the overall cost of treatment, especially in a resource-limited environment like ours, which is very important.
CONCLUSION
Targeted antimicrobial prophylaxis achieved a lower rate of infectious complications in patients who had prostate biopsy. This finding suggests that this individualized method of prophylaxis should be considered for patients undergoing TRPB.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cancer Facts and Statistics | American Cancer Society. Cancer.org. 2019. [Last accessed on 2014 Apr 18]. Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-027766.pdf .
- 2.Brawley OW. Prostate cancer epidemiology in the United States. World J Urol. 2012;30:195–200. doi: 10.1007/s00345-012-0824-2. [DOI] [PubMed] [Google Scholar]
- 3.Osegbe DN. Prostate cancer in Nigerians: Facts and Nonfacts. J Urol. 1997;157:1340–3. [PubMed] [Google Scholar]
- 4.Eke N, Sapira MK. Prostate cancer in Port Harcourt, Nigeria: Features and outcome. Niger J Surg Res. 2002;4:34–44. [Google Scholar]
- 5.Mohammed AZ, Edino ST, Ochicha O, Gwarzo AK, Samaila AA. Cancer in Nigeria: A 10-year analysis of the Kano cancer registry. Niger J Med. 2008;17:280–4. doi: 10.4314/njm.v17i3.37396. [DOI] [PubMed] [Google Scholar]
- 6.Ikuerowo SO, Omisanjo OA, Bioku MJ, Ajala MO, Mordi VP, Esho JO, et al. Prevalence and characteristics of prostate cancer among participants of a community-based screening in Nigeria using serum prostate specific antigen and digital rectal examination. Pan Afr Med J. 2013;15:129. doi: 10.11604/pamj.2013.15.129.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aron M, Rajeev TP, Gupta NP. Antibiotic prophylaxis for transrectal needle biopsy of the prostate: A randomized controlled study. BJU Int. 2000;85:682–5. doi: 10.1046/j.1464-410x.2000.00576.x. [DOI] [PubMed] [Google Scholar]
- 8.Otrock ZK, Oghlakian GO, Salamoun MM, Haddad M, Bizri AR. Incidence of urinary tract infection following transrectal ultrasound guided prostate biopsy at a tertiary-care medical center in Lebanon. Infect Control Hosp Epidemiol. 2004;25:873–7. doi: 10.1086/502312. [DOI] [PubMed] [Google Scholar]
- 9.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: Data from SEER-medicare. J Urol. 2011;186:1830–4. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carignan A, Roussy JF, Lapointe V, Valiquette L, Sabbagh R, Pépin J, et al. Increasing risk of infectious complications after transrectal ultrasound-guided prostate biopsies: Time to reassess antimicrobial prophylaxis? Eur Urol. 2012;62:453–9. doi: 10.1016/j.eururo.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 11.Patel U, Dasgupta P, Amoroso P, Challacombe B, Pilcher J, Kirby R, et al. Infection after transrectal ultrasonography-guided prostate biopsy: Increased relative risks after recent international travel or antibiotic use. BJU Int. 2012;109:1781–5. doi: 10.1111/j.1464-410X.2011.10561.x. [DOI] [PubMed] [Google Scholar]
- 12.Loeb S, van den Heuvel S, Zhu X, Bangma CH, Schröder FH, Roobol MJ, et al. Infectious complications and hospital admissions after prostate biopsy in a European randomized trial. Eur Urol. 2012;61:1110–4. doi: 10.1016/j.eururo.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 13.Carlson WH, Bell DG, Lawen JG, Rendon RA. Multi-drug resistant E. coli urosepsis in physicians following transrectal ultrasound guided prostate biopsies – Three cases including one death. Can J Urol. 2010;17:5135–7. [PubMed] [Google Scholar]
- 14.Feliciano J, Teper E, Ferrandino M, Macchia RJ, Blank W, Grunberger I, et al. The incidence of fluoroquinolone resistant infections after prostate biopsy – Are fluoroquinolones still effective prophylaxis? J Urol. 2008;179:952–5. doi: 10.1016/j.juro.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 15.Steensels D, Slabbaert K, De Wever L, Vermeersch P, Van Poppel H, Verhaegen J, et al. Fluoroquinolone-resistant E. coli in intestinal flora of patients undergoing transrectal ultrasound-guided prostate biopsy – Should we reassess our practices for antibiotic prophylaxis? Clin Microbiol Infect. 2012;18:575–81. doi: 10.1111/j.1469-0691.2011.03638.x. [DOI] [PubMed] [Google Scholar]
- 16.Taylor AK, Zembower TR, Nadler RB, Scheetz MH, Cashy JP, Bowen D, et al. Targeted antimicrobial prophylaxis using rectal swab cultures in men undergoing transrectal ultrasound guided prostate biopsy is associated with reduced incidence of postoperative infectious complications and cost of care. J Urol. 2012;187:1275–9. doi: 10.1016/j.juro.2011.11.115. [DOI] [PubMed] [Google Scholar]
- 17.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–37. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 18.Womble PR, Linsell SM, Gao Y, Ye Z, Montie JE, Gandhi TN, et al. A statewide intervention to reduce hospitalizations after prostate biopsy. J Urol. 2015;194:403–9. doi: 10.1016/j.juro.2015.03.126. [DOI] [PubMed] [Google Scholar]
- 19.Duplessis CA, Bavaro M, Simons MP, Marguet C, Santomauro M, Auge B, et al. Rectal cultures before transrectal ultrasound-guided prostate biopsy reduce post-prostatic biopsy infection rates. Urology. 2012;79:556–61. doi: 10.1016/j.urology.2011.09.057. [DOI] [PubMed] [Google Scholar]
- 20.Liss MA, Kim W, Moskowitz D, Szabo RJ. Comparative effectiveness of targeted vs. empirical antibiotic prophylaxis to prevent sepsis from transrectal prostate biopsy: A Retrospective analysis. J Urol. 2015;194:397–402. doi: 10.1016/j.juro.2015.03.110. [DOI] [PubMed] [Google Scholar]
- 21.Dai J, Leone A, Mermel L, Hwang K, Pareek G, Schiff S, et al. Rectal swab culture-directed antimicrobial prophylaxis for prostate biopsy and risk of postprocedure infection: A cohort study. Urology. 2015;85:8–14. doi: 10.1016/j.urology.2014.09.035. [DOI] [PubMed] [Google Scholar]
- 22.Summers SJ, Patel DP, Hamilton BD, Presson AP, Fisher MA, Lowrance WT, et al. An antimicrobial prophylaxis protocol using rectal swab cultures for transrectal prostate biopsy. World J Urol. 2015;33:2001–7. doi: 10.1007/s00345-015-1571-y. [DOI] [PubMed] [Google Scholar]
- 23.Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, et al. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64:876–92. doi: 10.1016/j.eururo.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 24.Van Besien J, Uvin P, Van den Abeele AM, Merckx L. Prevalence, risk factors, and clinical relevance of fluoroquinolone-resistant organisms in rectal cultures: Should we target antibiotic prophylaxis prior to prostate biopsy? Adv Urol. 2016;2016:5392107. doi: 10.1155/2016/5392107. [DOI] [PMC free article] [PubMed] [Google Scholar]


