Abstract
Background:
Brain metastasis is a dreaded complication that significantly reduces the quality of life in breast cancer patients. The treatment options are limited by the inability of many chemotherapeutic agents to cross the blood–brain barrier. Surgery also has a limited role, except in few selected patients with oligometastasis. Therefore, whole-brain radiotherapy (WBRT) remains the available option that gives a gratifying result. However, the benefit of this treatment modality in our resource-poor environment needs to be investigated.
Materials and Methods:
The data of breast cancer patients with brain metastasis who were treated with WBRT using cobalt-60 equipment between 2005 and 2009 were retrospectively collected from the departmental medical record unit. The information extracted included biodata, presenting symptoms, imaging modality for confirmation of brain metastasis, treatment records, performance status pre-WBRT and 4 weeks post-WBRT, and other supportive treatments.
Results:
A total of 52 female patients were reviewed between 2005 and 2009. The mean age of patients was 44.7 years. The common clinical features on presentation were headache, nausea, and visual impairment in 30.8% of patients with the WHO performance status score ranging between 2 and 4. Patients with more than three brain deposits accounted for 71.2% of all the brain metastases. The mean radiation dose used for WBRT was 30 Gy in 10 fractions, and total responses recorded were 86.5% with 53.8% complete improvement in patients' performance status 4 weeks after WBRT treatment.
Conclusion:
WBRT is an effective treatment modality for patients with brain metastasis in our resource-poor environment. However, improvement of patients' performance status declined with advancing age.
Keywords: Brain metastasis, breast cancer, whole-brain radiotherapy, Métastases cérébrales, cancer de seins, Radiothérapie du cerveau entier
Résumé
Historique:
le cancer ou métastase du cerveau est une complication enchainée qui réduit d’une manière signifiante la qualité de vie des patients du cancer. Les modes de traitement sont limités par l’impossibilité de plusieurs agentschimiothérapiques de pénétrer la barrière sanguine du cerveau. L’opération surgi cale a aussi un rôle limité sauf dans quelques cas exceptionnels de patients sélectionnés avec des cas d’oligo-métastase alors, la radio thérapie complété du cerveau (WBRT) demeure l’unique option qui donne un résultat satisfaisant cependant l’avantage de ce mode de traitement dans notre environnement qui manque de ressources financière doit être vérifié.
Méthodes Et Matériels:
Les informations sur les patients du cancer de seins recueillies au département d’enregistrement et d’information de l’hôpital sur les patients du cancer de siens atteints de métastase du cerveau et qui ont été traitéspar le système (WBRT) en utilisant l’instrument cobalt-60 sont rassemblés en aspect rétrospectif au département du record médical. Les informations extraites comprennent des bio-data qui présentent des signes et de modalités confirmant de traces de métastase, les records du traitement, l’état de rétablissement et de récupération des patients avant l’application du système en question WBRT et d’autres traitements supplémentaires.
Résultats:
Un cas de 52 patients de genre féminin a été examiné entre 2005 - 2009 l’âge moyen des patients s’élève à 44.7 années. Les effets médicaux qui se sont manifestés en ces patients sont: Maux de tête, Pulsions cardiaques qui mènent aux vomissements, perte de vue régulière. Dans presque 30.8% des patients qui ont un taux de performance entre 2 et 4 auprès de l’Organisation Mondiale de la santé (OMS), des patients avec plus de trois traces de dépôts de métastase du cerveau sont à 71.2% de la totalité des patients. La dose moyenne de radiation utilisée lors d’administration de la méthode WBRT est 30GY en 10 fractions et le cas total des réponses enregistrées sont 86.5% avec 53,8% de rétablissements satisfaisants 4 semaines après le traitement WBRT.
Conclusion:
Le WBRT est un système très efficace en vue du traitement de la métastase du cerveau mais il est à remarquer deux choses fondamentales: Les patients les plus jeunes répondent plus aisément au traitement alors que les patients très âgés attirent d’autres complications qui rendent leur cas plus sévère et peut -être plus fatal. Le manque de ressources financières adéquats dans nos sociétés si pauvre est aussi un grand handicap à souligner en grande envergure car le système WBRT est couteux.
INTRODUCTION
In Nigeria, metastatic breast carcinoma is more common than locally advanced disease [1] and is associated with low survival in most patients across the world.[2,3] Blood-borne metastasis occurs even though the primary is small or impalpable, and most breast cancers have distant metastasis at the time of the first detection. Favored sites of dissemination are the lymph nodes, lungs, bones, liver, adrenals, brain, and meninges. The incidence of brain metastases in advanced breast cancer is relatively small (6%) compared to the bone.[4] However, some authors speculate that the incidence of brain metastases is rising as a result of improvements in the management of systemic disease and better diagnostic techniques.[5] More than 80% of patients with brain metastasis have multiple metastases with 50% having more than three deposits.[6,7]
The symptoms of brain metastases varied depending on the location of the lesion. Brain metastases are more common in the frontal and parietal lobes than the temporal and occipital lobes. The symptoms are generally progressive over days and weeks due to increasing tumor size and raised intracranial pressure. The most common symptoms are mental/cognitive changes and headaches.[8] Patients may also experience general weakness, seizures, nausea or vomiting, and vision disruptions such as double vision or partial blindness.
The treatment of brain metastases is multidisciplinary involving radiotherapy, corticosteroids, surgery, and systemic therapy such as chemotherapy and hormones. Corticosteroids are often used at the first sign of brain metastases. They are used to reduce intracranial pressure caused by edema. Anticonvulsants are used to minimize the frequency of seizures in patients who already had a previous seizure. However, prophylactic treatment of patients who have not had previous episodes of seizures can reduce the effectiveness of other adjuvant treatments and could result in other life-threatening conditions such as Stevens–Johnson syndrome.[9] Surgery still remains the mainstay of treatment in a patient with single brain metastasis. However, in multiple metastases, the role of surgery is for biopsy before commencement of radiotherapy. Chemotherapy normally has a limited role in the management of brain metastases. This is thought to be due to the inability of chemotherapeutic agents to cross the blood–brain barrier because of their size and hydrophilicity. This may explain the increasing occurrence of brain metastases in many cancer patients.[5] Whole-brain radiotherapy (WBRT) has been the standard treatment for patients with brain metastases for many years. Multiple studies have confirmed the efficacy of WBRT with respect to relief of neurologic complications, and 50%–75% of patients experience improvement in neurologic symptoms and quality of life after WBRT treatment.[10,11] Late side effects are rare, but studies have reported the following acute side effects which include nausea, vomiting, skin and ear toxicity, fatigue, and headaches.[12,13,14]
MATERIALS AND METHODS
Patients
We retrospectively collected data on breast cancer patients with brain metastases who were treated with WBRT between 2005 and 2009. The extracted information including biodata, presenting symptoms, imaging modality for confirmation of brain metastases, histology of primary sites, radiation doses and fractionations used for the treatment, and assessment of patients' performance status pre- and post-WBRT were conducted using the simple WHO performance status score to assess the improvement in quality of life. The score started from 0 to 5, where 0 means asymptomatic (fully active, able to carry on all predisease activities without restriction) and 5 stands for death. Side effects of radiotherapy during and 4 weeks after treatment were recorded.
Treatment
Cobalt-60 equipment with an average energy of 1.25 megavoltage was used for the treatment. Plain X-ray of the skull was used as a guide for the planning (two-dimensional planning). Two opposing lateral cranial fields were used to cover the entire brain as the target organ. Radiation doses ranging between 20 and 30 Gy in 5–10 fractions were used. Patients were covered with steroid (dexamethasone) an hour before, during the entire period of radiotherapy and some weeks after radiotherapy.
Statistical methods
A statistical analysis was performed using the Statistical Package for the Social Sciences version 20.0 (SPSS version 20) (SPSS Inc., Chicago, IL, USA), using an alpha value of 0.05; a Pearson correlation was conducted between radiation doses and responses in terms of symptom relief, we also cross-tabulate between responses and performance status after radiotherapy and finally age and performance status after WBRT. Results obtained were presented in tables, bar charts, and pie charts.
RESULTS
Fifty-two female patients were reviewed between 2005 and 2009. Their mean age was 44.7 years, with standard deviation (SD) ±12.5 and age range of 21–71 years [Table 1]. Table 2 shows that headache/nausea/visual impairment (30.8%) was the common presenting symptom, followed by headache/cognitive changes (26.9%), headache/vomiting/seizure (26.9%), and headache/motor deficit (15.4%). Computed tomography scan (78.8%) and magnetic resonance imaging (21.2%) were the only two imaging modalities used for the confirmation of brain metastasis [Table 3]. Multiple brain metastases >3 lesions (71.2%) were the common findings in our breast cancer patients with brain metastasis, followed by 3 lesions of 17.3% and 2 lesions of 11.5% [Figure 1]. Table 4 shows the histology types of the primary site, where invasive ductal carcinoma accounted for 61.54%, followed by invasive lobular carcinoma of 11.54%, metaplastic carcinoma of 5.77%, mucinous carcinoma of 9.62%, and others of 11.5%. The mean radiation dose was 30 Gy, with SD ± 5 and range of 20–45 Gy [Figure 2]. Their corresponding mean fractionation number was 10#, with SD ± 2 and range of 5–12# [Figure 3]. Patients who presented with headache, vomiting, and seizures had the highest response rate of 78.6% complete and 21.4% partial, then followed by headache and cognitive changes of 50% complete and 42.9% partial, then headache and motor deficits of 75% complete and 12.5% partial, and finally, symptoms of headache, nausea, and visual impairment of 25% complete response and 43.8% partial response [Table 5]. The common acute side effect seen was alopecia of 67.3%, followed by body malaise and fatigue of 51.9%, fever of 15.4%, and ear tinnitus of 7.7% [Table 6]. Majority of patients presented with the WHO performance status score of 2 (46.2%), followed by score of 3 (44.2%) and that of 4 (9.6%); however, improvement in performance status was seen 4 weeks after WBRT with 53.8% of patients having score of 0 and 32.7% with score 2 [Table 7]. Table 8 shows a Pearson correlation between radiation doses and responses to WBRT, and patients treated with radiation doses ranging between 30 and 39 Gy have the highest response rate of 90.7%, followed by those treated with 20–29 Gy (83.3%) and those treated with ≥40 Gy (33.3%). A significant association between responses and improvement in performance status was observed with 53.8% having complete response after WBRT and a performance status of Score of 0, 32.7% with partial response have a score of 2, however, 13.5% of the patients were lost to follow up and their responses could not be acertained [Table 9]. Table 10 shows that the highest improvement in performance status (25%) was between 30 and 39 years' age group, followed by 40–49 years of 23.1%, 20–29 of 9.6%, and ≥70 years of 1.9%.
Table 1.
Age distribution of 52 patients presented with brain metastasis
| Age groups (years) | Number of patients (%) |
|---|---|
| 20-29 | 5 (9.6) |
| 30-39 | 14 (26.9) |
| 40-49 | 14 (26.9) |
| 50-59 | 10 (19.2) |
| 60-69 | 8 (15.4) |
| ≥70 | 1 (1.9) |
Table 2.
Clinical presentations of patients with brain metastasis
| Symptoms | Number of patients (%) |
|---|---|
| Headache, nausea, and visual impairment | 16 (30.8) |
| Headache and cognitive changes | 14 (26.9) |
| Headache, vomiting, and seizure | 14 (26.9) |
| Headache and motor deficit | 8 (15.4) |
Table 3.
Imaging modalities used for confirmation of brain metastasis
| Imaging modalities | Number of patients (%) |
|---|---|
| Computed tomography scan | 41 (78.8) |
| Magnetic resonance imaging | 11 (21.2) |
Figure 1.
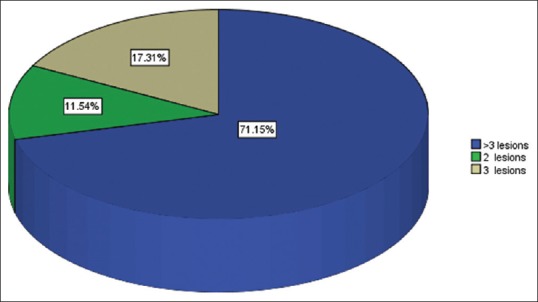
Number of brain metastasis among 52 breast cancer patients
Table 4.
Histological types of primary breast cancer
| Types of histology | Number of patients (%) |
|---|---|
| Invasive ductal | 32 (61.54) |
| Invasive lobular | 6 (11.54) |
| Metaplastic | 3 (5.77) |
| Mucinous | 5 (9.62) |
| Others | 6 (11.54) |
Figure 2.
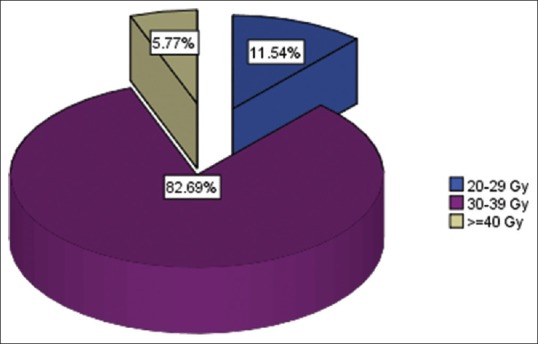
Range of radiation doses used for whole-brain radiotherapy treatments
Figure 3.
Range of fractionations used for whole-brain radiotherapy treatments
Table 5.
Responses inform of symptoms relief 4 weeks after whole-brain radiotherapy
| Symptoms | Number of patients | Responses to WBRT after 4 weeks |
||
|---|---|---|---|---|
| Complete (%) | Partial (%) | Unknown (%) | ||
| Headache/cognitive changes | 14 | 7 (50) | 6 (42.9) | 1 (7.1) |
| Headache/motor deficits | 8 | 6 (75) | 1 (12.5) | 1 (12.5) |
| Headache/nausea/visual impairment | 16 | 4 (25) | 7 (43.8) | 5 (31.2) |
| Headache/vomiting/seizure | 14 | 11 (78.6) | 3 (21.4) | 0 |
WBRT=Whole-brain radiotherapy
Table 6.
Side effects of whole-brain radiotherapy during and 4 weeks after treatment
| Side effects of WBRT | Number of patients (%) |
|---|---|
| Malaise/fatigue | 27 (51.9) |
| Fever | 8 (15.4) |
| Ear tinnitus | 4 (7.7) |
| Alopecia | 35 (67.3) |
WBRT=Whole-brain radiotherapy
Table 7.
The WHO performance status of the patients before and after whole-brain radiotherapy
| Performance status before WBRT | Number of patients (%) | Performance status after WBRT | Number of patients (%) |
|---|---|---|---|
| 2 | 24 (46.2) | 0 | 28 (53.8) |
| 3 | 23 (44.2) | 2 | 17 (32.7) |
| 4 | 5 (9.6) | Lost to follow-up | 7 (13.5) |
WBRT=Whole-brain radiotherapy
Table 8.
An association using Pearson correlation between radiation doses and responses to whole-brain radiotherapy treatment
| Radiation doses | Number of patients | Radiotherapy responses 4 weeks after WBRT |
P | ||
|---|---|---|---|---|---|
| Complete (%) | Partial (%) | Lost to follow-ups (%) | |||
| 20-29 | 6 | 3 (50) | 2 (33.3) | 1 (16.7) | 0.112 |
| 30-39 | 43 | 25 (58.1) | 14 (32.6) | 4 (9.3) | |
| ≥40 | 3 | 0 | 1 (33.3) | 2 (66.7) | |
WBRT=Whole-brain radiotherapy
Table 9.
An association using Pearson correlation between responses to whole-brain radiotherapy and the WHO performance status after whole-brain radiotherapy
| WBRT responses | Number of patients | Performance status score after WBRT |
P | |
|---|---|---|---|---|
| 0 | 2 | |||
| Complete (%) | 28 | 28 (53.8) | 0 | 0.000 |
| Partial (%) | 17 | 0 | 17 (32.7) | |
| Lost to follow-up (%) | 7 (13.5) | 0 | 0 | |
WBRT=Whole-brain radiotherapy
Table 10.
An association using Pearson correlation between age groups and the WHO performance status
| Age groups (Years) | Performance status scores 4 weeks post-WBRT |
P | ||
|---|---|---|---|---|
| 0 | 2 | Lost to follow-up | ||
| 20-29 | 3 | 2 | 0 | 0.010 |
| 30-39 | 12 | 1 | 1 | |
| 40-49 | 8 | 4 | 2 | |
| 50-59 | 3 | 5 | 2 | |
| 60-69 | 2 | 4 | 2 | |
| ≥70 | 0 | 1 | 0 | |
WBRT=Whole-brain radiotherapy
DISCUSSION
Brain metastasis constituted about 8.9% of total patients (584) recruited in the main study. Multiple brain metastases were the common pattern of presentations, with 71.15% of them having more than three brain metastases. A similar study supported our finding with 6% incidence of brain metastases in breast cancer patients,[4] but the incidence was speculated to be beyond the quoted figures, and reasons could be as a result of improvements in the management of systemic disease and better diagnostic techniques.[5] Similarly, >80% of patients were reported to have multiple metastases, and 50% of them have more than three brain metastases.[6,7]
Although majority of our patients were treated with 30 Gy in 10 fractions, we have recorded no dose–response relationship in terms of symptom relief with P = 0.112. However, for those who have a complete response to WBRT, they showed a significant improvement in performance status (P = 0.000) with about 53.8% of them fully active, able to carry on all predisease activities without restriction as against to their poor performance status at presentation. Multiple studies have confirmed a similar efficacy of WBRT with respect to relief of neurologic complications associated with brain metastases and improvements in quality of life.[10,11] Borgelt et al. conducted a similar study where they analyzed 1830 patients and reported a neurological improvement of 60%–90% posttreatment with patients being able to carry out their normal activities.[15] Upward of 90% of patients with headaches, seizures, and increased intracranial pressure experience complete relief upon being treated with WBRT.[15] However, we recorded a complete response rate of 50% among patients treated with WBRT secondary to features of raised intracranial pressure. The differences in the response rates between the two studies might be attributed to differences in their sample sizes. Despite numerous success stories with regard to the efficacy of WBRT, patients' performance status after WBRT was found to decline with advancing age in this study, P = 0.010. Contrary to our finding, Tyldesley et al. showed that the decline in rate of palliative radiotherapy (PRT) among elderly patients was not justified by a decline in their functional status.[16] However, Paszat et al. reported their finding which indicates that elderly patients over the age of 80 years were 0.08 as less likely to receive PRT as compared to patients <40 years.[17] Reasons behind the controversies regarding the use of PRT among elderly people and decline in their performance status remain unknown. It may be that supportive care alone, without WBRT, is an appropriate treatment for elderly people, particularly those with very poor performance status. WBRT is well tolerated with few acute side effects comparable to what was obtained in the previous studies.[12,13,14] Alopecia (67.3%) was the common side effect seen among our patients treated with WBRT, followed by body fatigue and malaise of 51.9%.
CONCLUSION
WBRT is an effective treatment modality for patients with brain metastasis in our resource-poor environment. Patients' performance status declined with advancing age. Therefore, supportive care alone, without WBRT, is recommended for the treatment of elderly patients, particularly those ≥70 years. The study concerning the benefit of WBRT in advanced aged patients requires further investigations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Adisa AO, Arowolo OA, Akinkuolie AA, Titiloye NA, Alatise OI, Lawal OO, et al. Metastatic breast cancer in a Nigerian tertiary hospital. Afr Health Sci. 2011;11:279–84. [PMC free article] [PubMed] [Google Scholar]
- 2.Gennari A, Conte P, Rosso R, Orlandini C, Bruzzi P. Survival of metastatic breast carcinoma patients over a 20-year period: A retrospective analysis based on individual patient data from six consecutive studies. Cancer. 2005;104:1742–50. doi: 10.1002/cncr.21359. [DOI] [PubMed] [Google Scholar]
- 3.Falkson G, Holcroft C, Gelman RS, Tormey DC, Wolter JM, Cummings FJ. Ten-year follow-up study of premenopausal women with metastatic breast cancer: An Eastern Cooperative Oncology Group Study. J Clin Oncol. 1995;13:1453–8. doi: 10.1200/JCO.1995.13.6.1453. [DOI] [PubMed] [Google Scholar]
- 4.Patanaphan V, Salazar OM, Risco R. Breast cancer: Metastatic patterns and their prognosis. South Med J. 1988;81:1109–12. [PubMed] [Google Scholar]
- 5.Gavrilovic IT, Posner JB. Brain metastases: Epidemiology and pathophysiology. J Neurooncol. 2005;75:5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen TD, DeAngelis LM. Brain metastases. Neurol Clin. 2007;25:1173. doi: 10.1016/j.ncl.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 8.Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin. 2003;21:1–23, vii. doi: 10.1016/s0733-8619(02)00035-x. [DOI] [PubMed] [Google Scholar]
- 9.Delattre JY, Safai B, Posner JB. Erythema multiforme and Stevens-Johnson syndrome in patients receiving cranial irradiation and phenytoin. Neurology. 1988;38:194–8. doi: 10.1212/wnl.38.2.194. [DOI] [PubMed] [Google Scholar]
- 10.Gelber RD, Larson M, Borgelt BB, Kramer S. Equivalence of radiation schedules for the palliative treatment of brain metastases in patients with favorable prognosis. Cancer. 1981;48:1749–53. doi: 10.1002/1097-0142(19811015)48:8<1749::aid-cncr2820480810>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Oneschuk D, Bruera E. Palliative management of brain metastases. Support Care Cancer. 1998;6:365–72. doi: 10.1007/s005200050178. [DOI] [PubMed] [Google Scholar]
- 12.Antonadou D, Paraskevaidis M, Sarris G, Coliarakis N, Economou I, Karageorgis P, et al. Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. J Clin Oncol. 2002;20:3644–50. doi: 10.1200/JCO.2002.04.140. [DOI] [PubMed] [Google Scholar]
- 13.Harwood AR, Simson WJ. Radiation therapy of cerebral metastases: A randomized prospective clinical trial. Int J Radiat Oncol Biol Phys. 1977;2:1091–4. doi: 10.1016/0360-3016(77)90114-6. [DOI] [PubMed] [Google Scholar]
- 14.Chatani M, Matayoshi Y, Masaki N, Inoue T. Radiation therapy for brain metastases from lung carcinoma: The second prospective randomized trial. Nihon Igaku Hoshasen Gakkai Zasshi. 1994;54:1380–7. [PubMed] [Google Scholar]
- 15.Borgelt B, Gelber R, Kramer S, Brady LW, Chang CH, Davis LW, et al. The palliation of brain metastases: Final results of the first two studies by the radiation therapy oncology group. Int J Radiat Oncol Biol Phys. 1980;6:1–9. doi: 10.1016/0360-3016(80)90195-9. [DOI] [PubMed] [Google Scholar]
- 16.Tyldesley S, Zhang-Salomons J, Groome PA, Zhou S, Schulze K, Paszat LF, et al. Association between age and the utilization of radiotherapy in Ontario. Int J Radiat Oncol Biol Phys. 2000;47:469–80. doi: 10.1016/s0360-3016(00)00440-5. [DOI] [PubMed] [Google Scholar]
- 17.Paszat LF, Mackillop WJ, Groome PA, Zhang-Salomons J, Schulze K, Holowaty E. Radiotherapy for breast cancer in Ontario: Rate variation associated with region, age and income. Clin Invest Med. 1998;21:125–34. [PubMed] [Google Scholar]


