Abstract
In the hippocampus, at excitatory synapses between principal cell and oriens/alveus (O/A) interneurons, a particular form of NMDA-independent long-term synaptic plasticity (LTP) has been described (Lamsa et al., 2007). This type of LTP occurs when presynaptic activation coincides with postsynaptic hyperpolarization. For this reason it has been named “anti-Hebbian” to distinguish from the classical Hebbian type of associative learning where presynaptic glutamate release coincides with postsynaptic depolarization. The different voltage dependency of LTP induction is thought to be mediated by calcium-permeable (CP) AMPA receptors that, due to polyamine-mediated rectification, favor calcium entry at hyperpolarized potentials. Here, we report that the induction of this form of LTP needs CP-α7 nicotinic acetylcholine receptors (nAChRs) that, like CP-AMPARs, exhibit a strong inward rectification because of polyamine block at depolarizing potentials. We found that high-frequency stimulation of afferent fibers elicits synaptic currents mediated by α7 nAChRs. Hence, LTP was prevented by α7 nAChR antagonists dihydro-β-erythroidine and methyllycaconitine (MLA) and was absent in α7−/− mice. In addition, in agreement with previous observations (Le Duigou and Kullmann, 2011), in a minority of O/A interneurons in MLA-treated hippocampal slices from WT animals and α7−/− mice, a form of LTP probably dependent on the activation of group I metabotropic glutamate receptors was observed. These data indicate that, in O/A interneurons, anti-Hebbian LTP critically depends on cholinergic signaling via α7 nAChR. This may influence network oscillations and information processing.
Introduction
Two different forms of NMDA-independent long-term synaptic plasticity (LTP) have been described at synapses between principal cells and oriens/alveus (O/A) interneurons (Kullmann and Lamsa, 2008). The first one requires the activation of group I metabotropic glutamate receptors (mGluRs) and follows the Hebbian rule of associative learning since it occurs when afferent stimulation coincides with postsynaptic depolarization (Perez et al., 2001; Pelletier and Lacaille, 2008). The second one requires calcium entry via inwardly rectifying calcium-permeable (CP) α-amino-3-hydroxy-5-methylisoxazole propionic acid (AMPA) receptors and can be induced by hyperpolarizing the postsynaptic neuron during high-frequency stimulation of presynaptic fibers (Lamsa et al., 2007). This form of LTP has been named “anti-Hebbian” since the presynaptic activation coincides with postsynaptic quiescence. However, recent evidence indicates that group I mGluRs are also necessary for the induction of this type of LTP, suggesting that the induction cascades following activation of both CP-AMPARs and group I mGluRs converge on a common expression mechanism (Le Duigou and Kullmann, 2011).
Interestingly, O/A interneurons receive an important cholinergic innervation (Frotscher and Léránth, 1985) and are endowed with nicotinic acetylcholine receptors (nAChRs) which regulate their activity (Frazier et al., 1998; Alkondon et al., 1999; McQuiston and Madison, 1999; Griguoli et al., 2009). Among different nAChR subtypes, α7 nAChRs are highly permeable to calcium (Bertrand et al., 1993; Séguéla et al., 1993; Fucile, 2004) and like CP-AMPA receptors exhibit a strong inward rectification which favors calcium entry at relatively negative membrane potentials (Haghighi and Cooper, 1998).
Here, we tested the hypothesis that calcium entry via inwardly rectifying CP-nAChRs may account for anti-Hebbian LTP. We found that high-frequency stimulation (HFS) of afferent fibers elicited in O/A interneurons α7 nAChR-dependent synaptic currents. Pairing HFS of glutamatergic fibers with hyperpolarization of the postsynaptic neuron led to LTP that was blocked by the selective α7 nAChR antagonist methyllycaconitine (MLA). In addition, in α7−/− mice, the pairing protocol failed to produce LTP. In a minority of cells from MLA-treated WT or α7−/− mice, a form of LTP probably dependent on the activation of group I mGluRs occurred. These data indicate that, in O/A interneurons, anti-Hebbian LTP critically depends on cholinergic signaling via α7 nAChRs. This may influence network oscillations and information processing.
Materials and Methods
Animals.
We used C57BL/6 transgenic mice of either sex expressing EGFP in a subpopulation of somatostatin-containing GABAergic interneurons (GIN mice: Jackson Laboratories; Oliva et al., 2000; Minneci et al., 2007) and α7−/− mice of either sex (kindly provided by Dr. U. Maskos, Institut Pasteur, Paris). All experiments were performed in accordance with the European Community Council Directive of 24 November 1986 (86/609EEC) and were approved by local veterinary authorities. All efforts were made to minimize animal suffering and to reduce the number of animal used.
Hippocampal slices.
Hippocampal slices were obtained from postnatal day 14 (P14)–P21 animals, using a standard protocol (Griguoli et al., 2010). Briefly, after being anesthetized with CO2, the brain was quickly removed from the skull and placed in ice-cold artificial CSF (ACSF) containing (in mm): 130 NaCl, 10 glucose, 3.5 KCl, 1.2 NaH2PO4, 25 NaHCO3, 2 CaCl2, 1.3 MgCl2 (Sigma), saturated with 95% O2 and 5% CO2, pH = 7.3–7.4. Transverse hippocampal slices (300 μm thick) were cut with a vibratome and stored at room temperature (22−24°C) in a holding bath containing the same solution as above. After incubation for at least 1 h, an individual slice was transferred to a submerged recording chamber and continuously superfused at 33−34°C with oxygenated ACSF at a rate of 2–3 ml min−1.
Electrophysiology.
Recordings were made from visually identified O/A interneurons using the patch-clamp technique in different configurations. Patch electrodes were pulled from borosilicate glass capillaries. They had a resistance of 4–5 MΩ when filled with an intracellular solution containing (in mm): 100 MeCsO3S, 5 CsCl, 40 HEPES, 0.6 EGTA, 5 MgCl2, 8 NaCl, 1 QX314-Cl, 2 Na2-ATP, 0.3 NaGTP, pH 7.2–7.3; 280–300 mOsm. Whole-cell patch-clamp recordings in voltage-clamp mode were used to identify EPSCs mediated by CP-AMPA receptors. In these cases, spermine (100 μm) was added to the intrapipette solution. The stability of the patch was checked by repetitively monitoring the input and series resistance during the experiments. Cells exhibiting > 15% changes in either series resistance or holding current were rejected. The series resistance was < 20 MΩ and it was not compensated. Membrane potential values were corrected for a liquid junction potential of ∼10 mV. In O/A interneurons, monosynaptic CP-AMPA-mediated EPSCs were evoked by stimulation of axon collaterals of CA1 pyramidal neurons in the O/A border at 100–150 μm from the soma of the patched cell. We used single or double pulses (50 ms apart) repeated at 0.1 Hz (duration of each pulse 80–100 μs). nAChR-mediated EPSCs were elicited at −80 mV in the presence of DNQX (20 μm), dl-2-amino-5-phosphonopentaoic acid (dl-AP5; 100 μm), gabazine (10 μm), (+)-2-methyl-4-carboxyphenylglycine (LY367385, 100 μm), CGP 54656 hydrochloride (1 μm), atropine (1 μm) to block AMPA, NMDA, GABAA, mGluR1a, GABAB, muscarinic AChRs, respectively. In some experiments, physostigmine (2 μm) was also added to the extracellular solution to prevent the breakdown of acetylcholine. Afferent fibers were stimulated at 50–100 Hz for 0.5–1 s, respectively by bipolar stainless steel electrodes localized in the O/A border.
For LTP experiments, gramicidin-perforated patch recordings (in current-clamp mode) were used to preserve the intracellular milieu of the recorded cells (Kyrozis and Reichling, 1995) and to prevent disruption of anti-Hebbian LTP (Lamsa et al., 2007; Oren et al., 2009; Le Duigou and Kullmann, 2011). Gramicidin D (final concentration 100 μg ml−1, Sigma-Aldrich) was prepared freshly (<2 h before recording) by dissolving it in dimethyl sulfoxide (DMSO). This was diluted with a gramicidin-free intrapipette solution containing (in mm): 145 K-gluconate, 8 NaCl, 20–25 HEPES, 0.2 EGTA, 5 QX-314 Br (N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium bromide), pH 7.2; 295 mOsm. The presence of QX-314 in the filling solution allowed to detect patch rupture. Failure to generate action potentials upon injection of depolarizing current steps indicated rupture of the membrane in which case the experiment was abandoned. Series resistance was continuously monitored throughout the experiment, and recordings in bridge balance mode were started when this was <150 MΩ. Depolarizing currents were intermittently injected to evoke action potentials to verify the patch integrity and in the case of membrane rupture, the recording was discontinued. After establishing a baseline for 5–10 min, LTP was induced by stimulating afferent fibers twice (at 10 s interval) by HFS train (at 100 Hz for 1 s) while the postsynaptic cell (in current-clamp mode) was held at a potential between −90 mV and −100 mV to prevent firing. In the case of firing the cell was discarded. After HFS, recordings were continued for additional 20–30 min.
Data analysis.
Data were transferred to a computer hard disk after digitization with an A/D converter (Digidata 1322, Molecular Devices). Data acquisition (digitized at 20 kHz and filtered at 3.3 kHz) was performed with pClamp 9.2 software (Molecular Devices). Input resistance and capacitance of the cells were measured online with the membrane test feature of the pClamp software. The rectification index of CP-AMPA-mediated EPSCs was obtained by dividing the amplitude of the EPSC recorded at +30 mV by that predicted from linear extrapolation of the current–voltage (I–V) relationship measured at negative potentials (at −20 mV, −50 mV and −80 mV, respectively). For LTP experiments, we measured the initial slope of the EPSPs (3–5 ms from the onset) to restrict analysis to monosynaptic excitatory inputs (Maccaferri and McBain, 1996). The paired-pulse ratio (PPR) was measured as the mean peak amplitude of the synaptic response evoked by the second stimulus over that evoked by the first one. The unpaired t test was used to compare in individual cells the initial slope of synaptic responses obtained before and after pairing. EPSPs slopes obtained from single cells for each experimental condition (in control, during drug treatment, in α7−/− mice) during the first and last 5 min recordings were averaged and significance was calculated using the paired t test. Significance between independent experimental groups was calculated on the last 5 min after HFS using the one-way ANOVA. Bonferroni's post hoc test was used to evaluate the statistically significance between paired groups within the multiple comparison. A p value < 0.05 was considered as statistically significant. Values are given as mean ± SEM.
Drugs.
Drugs were applied in the bath via a three-way tap system, by changing the superfusion solution to one differing only in its content of drug(s). The ratio of flow rate to bath volume ensured complete exchange within 1–2 min. Drugs used were: dl-AP5 and SR 95531 hydrobromide (gabazine), purchased from Ascent Scientific; [S-(R*,R*)]-[3-[[1-(3,4-dichlorophenyl)ethyl]amino]-2-hydroxypropyl](cyclohexylmethyl) phosphinic acid (CGP 54656 hydrochloride), physostigmine, LY367385, 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP), philanthotoxin, and spermine purchased from Tocris Bioscience; MLA, dihydro-β-erythroidine (DHβE), and atropine from Sigma. Stock solutions were made in distilled water and then aliquoted and frozen at −20°C. CGP 54656 and DNQX were dissolved in DMSO. The final concentration of DMSO in the bathing solution was 0.1%. At this concentration, DMSO alone did not modify the membrane potential, input resistance or the firing properties of O/A interneurons.
Results
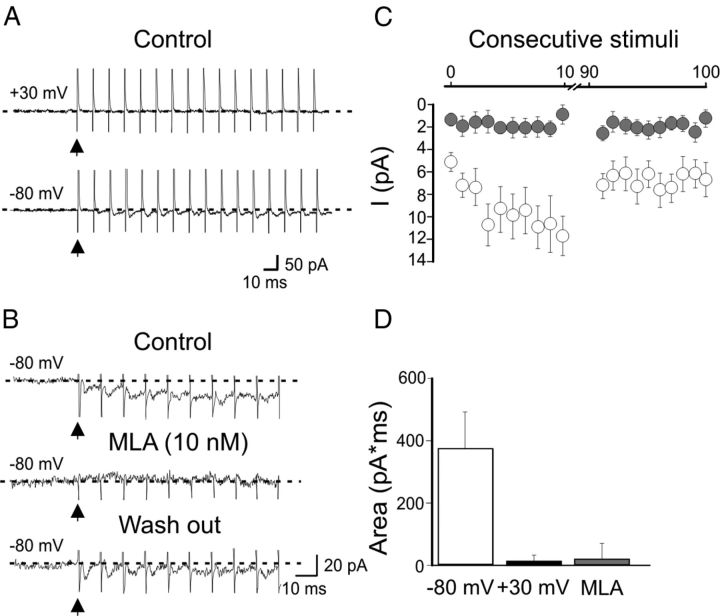
Whole-cell patch-clamp recordings in voltage-clamp mode were obtained from visually identified EGFP-positive cells in stratum oriens of the CA1 hippocampal region. We focused on those cells exhibiting round or fusiform cell bodies and horizontal dendrites running parallel to the alveus border that according to Goldin et al. (2007) belong in 50% of cases to oriens-lacunosum moleculare (O-LM) interneurons. Post hoc identification of some of these cells filled with biocytin revealed the classical morphology of O-LM interneurons (Griguoli et al., 2009, 2010). To verify the hypothesis that calcium entry via nAChRs may contribute to anti-Hebbian LTP, we first examined whether synaptically released ACh can activate nAChRs on O/A interneurons. HFS (at 50–100 Hz for 0.5–1 s) of afferent fibers in the O/A border, at 100–150 μm distance from the soma of the patched cell was delivered in the presence of 100 μm dl-AP5, 20 μm DNQX, 10 μm gabazine, 1 μm CGP 54656, 100 μm LY367385, 1 μm atropine to block NMDA, AMPA, GABAA, GABAB, mGluR1a, muscarinic ACh receptor, respectively. In 7 of 16 cells the acetylcholinesterase inhibitor physostigmine (2 μm) was added to the bathing solution to inhibit the breakdown of acetylcholine. This procedure elicited in 13 of 16 cells EPSCs that often summated giving rise to slow inward currents (36.4 ± 4.8 pA; n = 10; Fig. 1A,C). The mean peak amplitude of the first EPSC was 4.9 ± 0.8 pA, that of the tenth was to 11.1 ± 1.6 pA. After 50 ± 10 stimuli the EPSCs amplitude slowly declined to a steady-state level probably because of receptor desensitization. At +30 mV EPSCs were barely detectable (Fig. 1A,D). Individual EPSCs as well as the slow inward current were readily blocked by DHβE at the concentration of 50 μm which antagonizes all nAChRs (n = 4; data not shown) or by MLA (10–100 nm), a selective α7 nAChR antagonist, indicating that they were mediated by α7 nAChRs (n = 9; Fig. 1B). No differences between the two concentrations of MLA used were found and therefore data were pooled together. In three cells, a partial recovery was observed ∼ 10 min after washing out MLA (Fig. 1B). Physostigmine was not determinant for MLA-sensitive EPSCs as similar results were obtained in the absence of the acetylcholinesterase inhibitor (n = 6 of 13).
Figure 1.
Synaptic currents mediated by α7-nAChRs in O/A interneurons. A, nAChR-mediated EPSCs evoked in a O/A interneuron by repetitive stimulation of cholinergic fibers in the O/A border (100 Hz for 1 s) at −80 mV, at +30 mV. Note that synaptic currents could be detected at −80 mV but not at +30 mV due to their strong inward rectification. B, In another interneuron held at −80 mV, bath application of MLA (10 nm) blocked both the fast synaptic currents and the slow developing inward current. This effect was partially reversed 10 min after MLA was washed out. C, Peak EPSCs amplitude as a function of consecutive stimuli, before (white; n = 10) and during bath application of MLA (gray; n = 13). Vertical bars, SEM. D, Each column represents the charge transfer (pA*ms) obtained by summating respective areas underlying the first 5 consecutive EPSCs, recorded at −80 mV (white), at +30 mV (black) and at −80 mV in the presence of 10 nm MLA (gray).
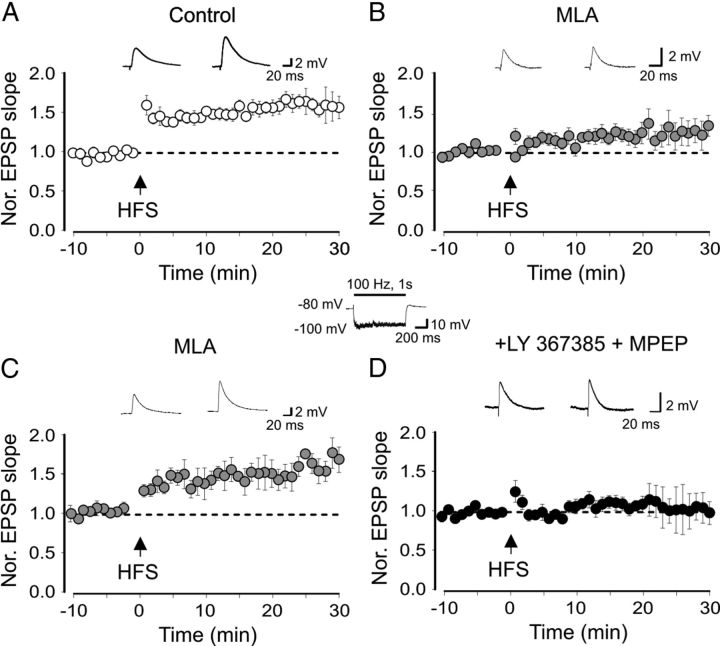
In 3 of 16 cases, no synaptic responses were detected probably because of failure of stimulating cholinergic afferents. At 0.1–1 Hz, only small amplitude, barely detectable EPSCs, difficult to isolate from the basal noise could be detected (data not shown). Next, we explored the possibility that α7 nAChRs contribute to anti-Hebbian LTP by pairing HFS of CA1 pyramidal cell collaterals with hyperpolarization of the postsynaptic cell at −90/−100 mV (Lamsa et al., 2007; Oren et al., 2009). At this potential, the postsynaptic cell failed to reach the firing threshold (see inset in Fig. 2). In the case of firing the experiment was aborted. To preserve intracellular polyamines and to prevent LTP disruption (Lamsa et al., 2007), synaptic responses were recorded with gramicidin perforated patch, in the presence of dl-AP5 (100 μm), CGP 54656 (1 μm) and gabazine (2.5 μm) to block N-methyl-d-aspartate (NMDA), GABAB and GABAA receptors, respectively (Lamsa et al., 2007; Kullmann and Lamsa, 2008; Oren et al., 2009; Le Duigou and Kullmann, 2011). This protocol caused a persistent potentiation of AMPA-mediated EPSPs (30 min after induction, the mean EPSPs slope was 159 ± 14% of controls, n = 17; p = 0.0014; paired t test; Fig. 2A). In few cases (n = 6) two pulses (50 ms apart) were used to estimate LTP-induced changes in the PPR. Although a trend toward depression was observed (from 1.8 ± 0.2 to 1.4 ± 0.1; n = 6; data not shown), this did not reach a significant value (p = 0.06; paired t test) suggesting, in agreement with previous studies (Lamsa et al., 2007; Le Duigou and Kullmann, 2011), a presynaptic site of expression. Hyperpolarization of the postsynaptic cell in the absence of afferent stimulation failed to induce LTP (after 25 min the EPSP slope was 109 ± 8% of that obtained during the first 5 min; n = 3; data not shown). The selective α7 nAChR antagonist MLA (10 nm) strongly reduced synaptic potentiation (30 min after HFS the mean EPSPs slope was 127 ± 11% of controls; n = 17; Fig. 2B) that did not reach a statistical significant value (p = 0.06), suggesting the involvement of α7 nAChRs.
Figure 2.
Anti-Hebbian LTP requires the activation of α7 nAChRs. A, B, Mean EPSP slope (obtained before and after pairing, arrows), normalized to pre-pairing values in the absence (A) and presence (B) of MLA 10 nm. In A, mean values were obtained by pooling together six cells monitored 10 min before and 30 min after HFS and 11 cells monitored 5 min before and 20 min after HFS. In B, mean values were obtained by pooling together 10 cells monitored 10 min before and 30 min after HFS and seven cells monitored 5 min before and 20 min after HFS. C, Cells exhibiting LTP in the presence of MLA (6 of 15; included in the summary graph in B). D, In the presence of MLA plus mGluR1/5 antagonists LY367385 and MPEP, the pairing protocol (arrow) failed to produce LTP (n = 15). Insets above the graphs represent EPSPs evoked before (left) and 30 min after (right) pairing. The inset in the middle represents EPSPs recorded during the HFS (bar) delivered to a O/A interneuron maintained hyperpolarized at −100 mV.
Six cells of 17 exposed to MLA (included in the summary graph of Fig. 2B) exhibited a clear LTP (30 min after HFS the mean EPSPs slope was 167 ± 16% of controls; p = 0.003; Fig. 2C). This was probably dependent on the activation of group I mGluRs since it was prevented when LY367385 (100 μm) and MPEP (25 μm), selective antagonists for mGluR1/5, were added to MLA (see also Le Duigou and Kullmann, 2011). In this case, the mean slope of EPSPs was 98 ± 14% of the pre-pairing value; n = 15; p = 0.8 paired t test; Figure 2D). This suggests that, in some cases, concomitant activation of group I mGluRs by glutamate released from glutamatergic fibers during HFS contributes to generate local postsynaptic signals necessary for the LTP induction.
To further assess the involvement of α7 nAChRs in anti-Hebbian LTP, we used mice lacking α7 nAChRs (α7−/−; Orr-Urtreger et al., 1997).
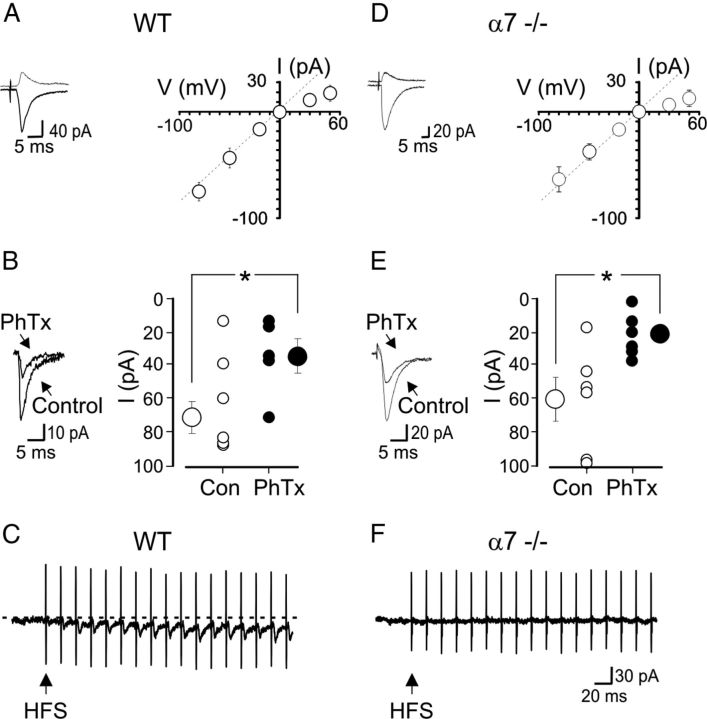
When recorded with a spermine-containing pipette solution, O/A interneurons exhibited synaptic currents mediated by CP-AMPA receptors, similar in all respects to those observed in WT animals (Fig. 3). In WT animals, stimulation of axon collaterals of CA1 pyramidal cells in the O/A border, at 100–150 μm distance from the soma of the patched cell (in the presence of 100 μm dl-AP5, 2.5 μm gabazine, and 1 μm CGP 54656 to block NMDA, GABAA and GABAB receptors, respectively), evoked monosynaptic EPSCs (EPSCs) that exhibited a characteristic inwardly rectifying I–V relationship (the rectification index was 0.5 ± 0.08; n = 16; Fig. 3A) and were sensitive to the polyamine toxin philanthotoxin (2 μm). At −80 mV in the absence or in the presence of the toxin, the peak amplitude of EPSCs was 73 ± 10 pA and 35 ± 10 pA, respectively; n = 5; p = 0.03, paired t test; Fig. 3B). As in WT animals, in α7−/− mice, CP-AMPA-mediated synaptic currents strongly rectified in the inward direction (the rectification index was 0.3 ± 0.05; n = 12; Fig. 3D) and were sensitive to philanthotoxin (at −80 mV, in the absence or in the presence of the toxin, 2 μm, the peak amplitude of EPSCs was 62 ± 12 pA and 24 ± 5 pA, respectively; n = 6; p = 0.007, paired t test; Fig. 3E). However, in contrast with WT mice (Fig. 3C) α7−/− mice did not exhibit α7 nAChR-mediated EPSCs following HFS of cholinergic fibers in the O/A border (Fig. 3F).
Figure 3.
α7−/− mice express CP-AMPAR- but not α7 nAChR-mediated EPSCs. A, On the left, AMPA-mediated synaptic currents evoked in one O/A interneuron at −50 and at +50 mV, respectively (in hippocampal slices from WT animals) by stimulation of afferent fibers, in the presence of dl-AP5 (100 μm), gabazine (2.5 μm) and CGP 54656 (1 μm), showing strong inward rectification. On the right, I–V relationship of inwardly rectifying AMPA-mediated synaptic currents (n = 5 at +50 mV, n = 16 at all other potentials). Vertical bars represent the SEM. Note that at +50, the EPSC value is below that expected in the absence of rectification (dashed line). B, On the left example of AMPA-mediated EPSC in the absence (Control) and in the presence of philanthotoxin (2 μm; PhTx). On the right, individual and mean (±SEM, vertical bars) peak current amplitude values obtained in the absence (Con; open circles) or in the presence (closed circles) of PhTx. C, In WT animals, HFS of afferent fibers in the O/A border (50 Hz for 0.5 s, arrow) elicited in a O/A interneuron fast EPSCs. Note EPSC summation with consequent stimuli and a slowly developing inward current. D–F, As in A–C but from α7−/− mice (the I–V relationship refers to 5 O/A interneurons at + 50 mV, and 16 at all other potentials). Note that in F, the HFS train (arrow) failed to elicit fast EPSCs or slow inward currents. In A, B and D, E, whole-cell recordings were performed with an intrapipette solution containing spermine (100 μm).
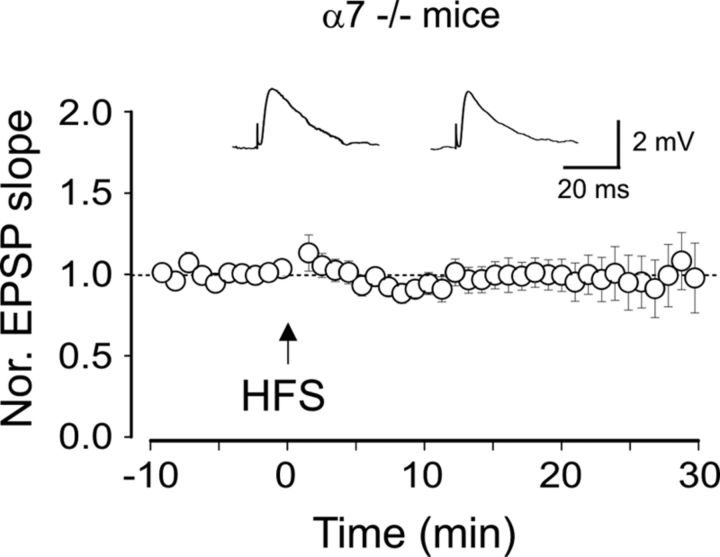
Furthermore, in α7−/− mice, the pairing procedure failed to induce LTP (30 min after HFS the mean EPSPs slope was 97 ± 21% of controls; n = 18; p = 0.8, paired t test), further confirming the crucial role of α7 nAChRs in this form of synaptic plasticity (Fig. 4). However, one of 18 cells, included in the summary graph of Figure 4, exhibited LTP (20 min after HFS the mean EPSPs slope was 147% of controls; data not shown) which was probably dependent on the activation of group I mGluRs.
Figure 4.
Lack of anti-Hebbian LTP in α7−/− mice. The pairing protocol (arrow) fails to induce LTP (n = 18). Insets represent EPSPs evoked before (left) and 30 min after (right) pairing.
For statistical analysis the mean EPSP slope values, obtained during the last 5 min after HFS, in controls, in MLA, in MLA plus group I mGluR antagonists, and in α7−/− mice were compared using ANOVA one-way analysis. The statistical significance between single groups in the multi comparison analysis was assessed using Bonferroni's post hoc test. Control group was statistically different from all the other groups (p < 0.001) as well as MLA-treated group expressing LTP (p < 0.001); no statistically significance was found between MLA plus group I mGluR antagonists and α7−/− mice (p > 0.05).
No statistically significant differences were found in baseline values obtained in different experimental groups before HFS train.
Discussion
The present data highlight the role of α7 nAChRs in anti-Hebbian LTP. Previous studies have shown that nAChRs are highly expressed in O/A interneurons at presynaptic and postsynaptic sites. While at presynaptic sites nAChRs regulate transmitter release mainly via α7 nAChRs subtypes (McGehee et al., 1995; Gray et al., 1996; Maggi et al., 2003), at postsynaptic sites they mediate fast αBGTx-sensitive and slow DHβE-sensitive responses to nicotine (McQuiston and Madison, 1999; Griguoli et al., 2009) mediated by α7 and non-α7 nAChR subtypes, respectively. In addition, our data indicate that in O/A interneurons α7 nAChRs can be synaptically activated by endogenous ACh released from cholinergic fibers. Like CP-AMPA receptors, α7 nAChRs are highly permeable to calcium (Bertrand et al., 1993; Séguéla et al., 1993; Fucile, 2004) and exhibit a strong inward rectification due to the voltage-dependent block of spermine (Haghighi and Cooper, 1998). Interestingly, nAChRs are several times more sensitive to spermine block than AMPA and kainate receptors (Haghighi and Cooper, 1998). The progressive reduction in channel conductance as the membrane potential depolarizes facilitates calcium entry at negative potentials, an ideal condition for the induction of anti-Hebbian LTP. Although the activation of presynaptic nAChRs by endogenously released ACh cannot be excluded, the involvement of postsynaptic receptors is supported by the observation that LTP can be induced only in perforated patch, which preserves the cytoplasmic content of polyamines, and when presynaptic glutamatergic fibers fire in concomitance with quiescence of the postsynaptic neuron, deliberately hyperpolarized via the recording pipette (Lamsa et al., 2007).
The LTP observed in a minority of neurons from MLA-treated WT mice was probably dependent on the activation of group I mGluRs since it was blocked by selective antagonists (see also Le Duigou and Kullmann, 2011). The additional voltage-independent rise of intracellular calcium via metabotropic receptors may activate a converging transduction pathway. Although it would be of interest to know what is the relative contribution of different calcium signals mediated by different receptor types, the perforated patch technique, necessary for anti-Hebbian LTP detection, hampers the use of conventional methods to measure intracellular calcium dynamics. Whatever the mechanisms, the present findings point to nAChRs as the key players in the induction of anti-Hebbian LTP in O/A interneurons. This may strongly influence feedback inhibitory circuits, network oscillations and information processing in the hippocampus. Whether nAChRs are instrumental in inducing LTP at glutamatergic synapses of other hippocampal interneuron types known to express calcium-permeable AMPA receptors (Szabo et al., 2012) remain to be established.
Footnotes
This work was supported by a grant from Ministero Istruzione Università e Ricerca (Grant MIUR-PRIN 2008) to E.C. We thank Christophe Mulle, Christophe Blanchet, Nelson Rebola, and Mario Carta for useful comments on the manuscript.
References
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Galzi JL, Devillers-Thiéry A, Bertrand S, Changeux JP. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor. Proc Natl Acad Sci U S A. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV. Synaptic potentials mediated via alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J Neurosci. 1998;18:8228–8235. doi: 10.1523/JNEUROSCI.18-20-08228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M, Léránth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol. 1985;239:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Fucile S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35:1–8. doi: 10.1016/j.ceca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Goldin M, Epsztein J, Jorquera I, Represa A, Ben-Ari Y, Crépel V, Cossart R. Synaptic kainate receptors tune oriens-lacunosum moleculare interneurons to operate at theta frequency. J Neurosci. 2007;27:9560–9572. doi: 10.1523/JNEUROSCI.1237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Griguoli M, Scuri R, Ragozzino D, Cherubini E. Activation of nicotinic acetylcholine receptors enhances a slow calcium-dependent potassium conductance and reduces the firing of stratum oriens interneurons. Eur J Neurosci. 2009;30:1011–1022. doi: 10.1111/j.1460-9568.2009.06914.x. [DOI] [PubMed] [Google Scholar]
- Griguoli M, Maul A, Nguyen C, Giorgetti A, Carloni P, Cherubini E. Nicotine blocks the hyperpolarization-activated current Ih and severely impairs the oscillatory behavior of oriens-lacunosum moleculare interneurons. J Neurosci. 2010;30:10773–10783. doi: 10.1523/JNEUROSCI.2446-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi AP, Cooper E. Neuronal nicotinic acetylcholine receptors are blocked by intracellular spermine in a voltage-dependent manner. J Neurosci. 1998;18:4050–4062. doi: 10.1523/JNEUROSCI.18-11-04050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa K. Roles of distinct glutamate receptors in induction of anti-Hebbian long-term potentiation. J Physiol. 2008;586:1481–1486. doi: 10.1113/jphysiol.2007.148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- Lamsa KP, Heeroma JH, Somogyi P, Rusakov DA, Kullmann DM. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science. 2007;315:1262–1266. doi: 10.1126/science.1137450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Duigou C, Kullmann DM. Group I mGluR agonist-evoked long-term potentiation in hippocampal oriens interneurons. J Neurosci. 2011;31:5777–5781. doi: 10.1523/JNEUROSCI.6265-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Long-term potentiation in distinct subtypes of hippocampal nonpyramidal neurons. J Neurosci. 1996;16:5334–5343. doi: 10.1523/JNEUROSCI.16-17-05334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Le Magueresse C, Changeux JP, Cherubini E. Nicotine activates immature “silent” connections in the developing hippocampus. Proc Natl Acad Sci U S A. 2003;100:2059–2064. doi: 10.1073/pnas.0437947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J Neurosci. 1999;19:2887–2896. doi: 10.1523/JNEUROSCI.19-08-02887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minneci F, Janahmadi M, Migliore M, Dragicevic N, Avossa D, Cherubini E. Signaling properties of stratum oriens interneurons in the hippocampus of transgenic mice expressing EGFP in a subset of somatostatin-containing cells. Hippocampus. 2007;17:538–553. doi: 10.1002/hipo.20291. [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren I, Nissen W, Kullmann DM, Somogyi P, Lamsa KP. Role of ionotropic glutamate receptors in long-term potentiation in rat hippocampal CA1 oriens-lacunosum moleculare interneurons. J Neurosci. 2009;29:939–950. doi: 10.1523/JNEUROSCI.3251-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Urtreger A, Göldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, Dani JA, Patrick JW, Beaudet AL. Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci. 1997;17:9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier JG, Lacaille JC. Long-term synaptic plasticity in hippocampal feedback inhibitory networks. Prog Brain Res. 2008;169:241–250. doi: 10.1016/S0079-6123(07)00014-3. [DOI] [PubMed] [Google Scholar]
- Perez Y, Morin F, Lacaille JC. A hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc Natl Acad Sci U S A. 2001;98:9401–9406. doi: 10.1073/pnas.161493498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo A, Somogyi J, Cauli B, Lambolez B, Somogyi P, Lamsa KP. Calcium-permeable AMPA receptors provide a common mechanism for LTP in glutamatergic synapses of distinct hippocampal interneuron types. J Neurosci. 2012;32:6511–6516. doi: 10.1523/JNEUROSCI.0206-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]