Abstract
Training during a sensitive period in development may have greater effects on brain structure and behavior than training later in life. Musicians are an excellent model for investigating sensitive periods because training starts early and can be quantified. Previous studies suggested that early training might be related to greater amounts of white matter in the corpus callosum, but did not control for length of training or identify behavioral correlates of structural change. The current study compared white-matter organization using diffusion tensor imaging in early- and late-trained musicians matched for years of training and experience. We found that early-trained musicians had greater connectivity in the posterior midbody/isthmus of the corpus callosum and that fractional anisotropy in this region was related to age of onset of training and sensorimotor synchronization performance. We propose that training before the age of 7 years results in changes in white-matter connectivity that may serve as a scaffold upon which ongoing experience can build.
Introduction
Highly skilled musicians such as Yo-Yo Ma, Oscar Peterson, and Pablo Casals began training in early childhood, all before the age of 7 years. Such observations suggest that there may be a sensitive period when early musical training has greater effects on the brain and behavior than training later in life. Such periods of heightened sensitivity would likely interact with preexisting individual differences in ability, along with environmental factors, to result in the expertise observed in such outstanding musicians.
A sensitive period is defined as a developmental window where experience has long-lasting effects on the brain and behavior (Knudsen, 2004). Neurophysiological studies in animals show that exposure or training during specific periods in development can produce enhanced structural and functional plasticity in visual, auditory, and somatosensory regions of the brain (Hensch, 2005). Evidence for sensitive periods in humans comes from studies of second language learning showing that early exposure results in greater proficiency (Johnson and Newport, 1989; Kuhl, 2010), studies of deaf children showing that receiving cochlear implants earlier results in better language development (Sharma et al., 2007), and studies of blind persons showing greater neuronal reorganization following early blindness (Sadato et al., 2002; Frasnelli et al., 2011).
Musicians are an excellent model for investigating possible sensitive period effects on brain and behavior, as training often begins early and is quantifiable (Bengtsson et al., 2005; Wan and Schlaug, 2010; Penhune, 2011). Evidence for a possible sensitive period for musical training came from a study showing that the anterior corpus callosum (CC) was larger in musicians than non-musicians, and that the difference was greater for those who began training before the age of 7 years (Schlaug et al., 1995). Further, the extent of the representation of the left hand (Elbert et al., 1995) and motor cortex size (Amunts et al., 1997) have also been shown to be related to early onset of training.
However, none of these studies controlled for the fact that musicians who begin earlier typically have more training than those who begin later. Music and other forms of training induce gray and white matter changes (Hyde et al., 2009; Imfeld et al., 2009; Scholz et al., 2009), and brain structural measures have been shown to be related to the amount of training (Gaser and Schlaug, 2003; Bengtsson et al., 2005; Imfeld et al., 2009; Foster and Zatorre, 2010). Therefore, previously observed differences thought to be related to age of onset may be influenced by, or even artifacts of, differences in the duration of training. Further, previous studies did not demonstrate any relationship between differences in brain structure and performance, which is critical in establishing their relevance. Work from our laboratory has shown that early-trained musicians (ET; training begun before the age of 7 years) outperform late-trained musicians (LT; training begun after the age of 7 years) on auditory and visual sensorimotor synchronization tasks—even when matched for years of training and experience (Watanabe et al., 2007; Bailey and Penhune, 2012). Based on these studies, we hypothesized that early musical training might have a differential impact on plasticity in white-matter fibers connecting sensory and motor regions, resulting in better sensorimotor integration. To test this hypothesis, the current study used diffusion tensor imaging (DTI) to compare white-matter structure in ET and LT musicians matched for years of training and experience. We also specifically examined the relationship between brain structure and sensorimotor synchronization performance to test the hypothesis that structural changes induced by early learning would be directly related to behavioral enhancements.
Materials and Methods
Participants
We tested 36 highly trained musicians who were divided into two groups: ET, who began their training before age 7 (n = 18, 8 females); and LT, who began their training after age 7 (n = 18, 4 females). Groups were matched for years of musical experience (total years of training and practicing music), years of formal training (total years enrolled in music lessons), and hours of current practice as assessed by the Musical Experience Questionnaire developed in our laboratory (Bailey and Penhune, 2012) (Table 1). The age cutoff for ET and LT was based on previous studies (Schlaug et al., 1995; Watanabe et al., 2007). All musicians had at least 7 years of musical experience, were currently practicing, and were enrolled in a university music program or performing professionally. We also tested a group of nonmusician controls (NM; n = 17, 7 females) who had less than 3 years of musical experience and were not currently practicing an instrument or undergoing musical training. All participants were right-handed, neurologically normal, and were not taking any medication that could affect task performance. All participants completed an MR safety screening form and provided written informed consent. The experimental protocol was approved by the McGill University Montreal Neurological Hospital and Institute Research Ethics Board and the Concordia University Human Research Ethics Committee.
Table 1.
Group demographic variables
| ET | LT | NM | |
|---|---|---|---|
| n | 18 | 18 | 17 |
| Male/female | 10/8 | 14/4 | 10/7 |
| Age of onset of musical training (years) | |||
| Mean (SD) | 5.72 (±1.13) | 10.78 (±2.46) | — |
| Range | 3–7 | 8–18 | — |
| Age (years)* | |||
| Mean (SD) | 22.72 (±4.14) | 27.61 (±5.34) | 26.41 (±4.71) |
| Range | 18–32 | 19–35 | 21–36 |
| Years of formal training | |||
| Mean (SD) | 11.5 (±3.22) | 9.42 (±5.13) | 0.35 (±0.53) |
| Range | 3–16 | 1–20 | 0–1.58 |
| Years of experience | |||
| Mean (SD) | 16.72 (±3.89) | 16.58 (±4.88) | 0.68 (±0.61) |
| Range | 12–25 | 9.5–24 | 0–2 |
| Hours of current practice (hours per week) | |||
| Mean (SD) | 15 (±10.20) | 13.25 (±7.52) | — |
| Range | 3–35.5 | 4–34 | — |
*Significant difference in age between ET and LT; t(34) = 3.07, p < 0.05.
Behavioral task
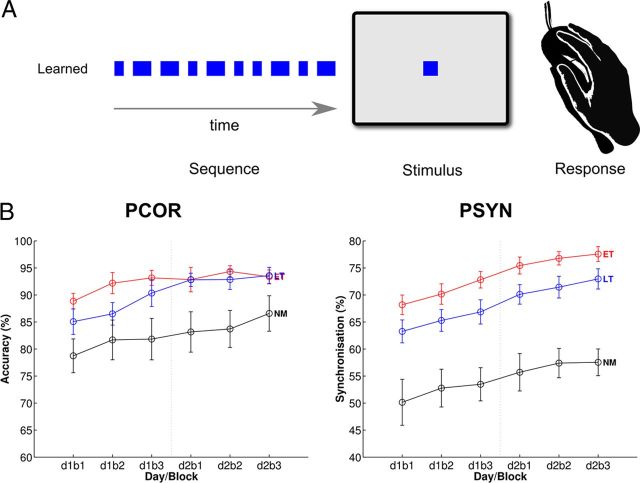
The temporal motor sequencing task (TMST) was used to assess motor timing and synchronization (Steele and Penhune, 2010; Penhune and Steele, 2012). The TMST (Fig. 1A) requires participants to tap in synchrony with a 10-element sequence of short and long visual cues that form a temporal sequence or rhythm. Previous work in our lab has shown that ET show better synchronization performance than LT on this task, even after 5 d of practice (Watanabe et al., 2006). In the present study, TMST performance was assessed on 2 consecutive days consisting of three blocks of 16 trials. Before testing, participants completed a block of training sequences to establish the mean and standard deviation of their short and long responses for scoring (described below) and practiced the sequence until they were able to reach 80% accuracy across three consecutive trials.
Figure 1.
Behavioral task and group performance data. A, Temporal motor sequence task. The learned sequence, visually presented stimuli, and response method are shown. B, Performance data from the TMST. PCOR and PSYN are shown across blocks. Group means for each measure are plotted for each day (d) and block (b): ET are shown in red, LT in blue, and NM in black. Error bars depict ±SEM. The vertical dotted line between d1b3 and d2b1 denotes the boundary between days of training.
Scoring.
Learning was assessed with two measures of performance: percentage correct (PCOR) and percentage synchronization (PSYN). PCOR is the percentage of long and short key-presses that fell within a 300 ms window around the visual stimulus and had a duration within 2 SD of each participant's mean for the short or long elements in the sequence (for additional scoring details, see Steele and Penhune, 2010). A score of 100% on PCOR represents perfect knowledge of the ordering of long/short elements within the sequence. PSYN is a measure of the synchronization of key-press response with visual stimuli, and represents a measure of sensorimotor integration. PSYN was calculated based only on correct responses and is the absolute lag between the onset and offset of the stimulus and the onset and offset of the response, divided by the stimulus duration. PSYN scores were subtracted from 100 to obtain a score that increased with performance. A score of 100% on PSYN indicates that the key press and release response exactly matched the onset and offset of the visual stimuli.
Analyses.
Omnibus F tests were used to assess learning on PCOR and PSYN and planned comparisons were conducted for all blocks (one-tailed t tests, α = 0.05, ET > LT and LT > NM compared separately for all blocks). Measures of final performance for PSYN, operationalized as performance on the last block of the second day of training, were calculated for use in behavioral and brain-behavior correlations (PSYN Final).
MRI data acquisition and analysis
We collected both standard high-resolution T1 (MPRAGE T1: TR = 2300 ms, TE = 2.98 ms, 1 × 1 × 1 mm) and diffusion-weighted images (99 directions, TR = 9340 ms, TE = 88 ms, b = 1000 s/mm2, 2 × 2 × 2 mm) on a Siemens Trio 3T MRI using a 32-channel head coil.
Diffusion imaging.
All imaging data were analyzed using the FMRIB Software Library (FSL 4.1.7) (Smith et al., 2004). Diffusion images were corrected for eddy current distortions before creating voxelwise maps of diffusion parameters. Images were then prepared using FSL's tract-based spatial statistics, which first nonlinearly aligns images to the FMIRB58_FA standard space template, calculates a mean fractional anisotropy (FA) image, and then thins it to produce the study-specific FA skeleton representing the centers of the tracts common to all participants (Smith et al., 2006). The aligned FA data were then projected onto individual FA skeletons that were subsequently used in permutation-based nonparametric statistical analyses. Skeletonized FA values were thresholded at FA > 0.20 before analyses. Volumetric (non-skeletonized) FA images were minimally smoothed (σ = 1 mm) before analyses. The same nonlinear warp and skeletonization parameters were used with the Tract-Based Spatial Statistics non-FA pipeline to create skeletonised and volumetric images of axial diffusivity (AD) and radial diffusivity (RD). Nonparametric permutation-based analyses were conducted with 5000 permutations for all analyses, with age and sex entered as covariates of no interest. Results were assessed for significance after multiple comparisons (α = 0.05) using threshold-free cluster enhancement (Smith and Nichols, 2009). Additional post hoc analyses were conducted at p < 0.10 to investigate the degree of overlap with previous findings. Presented p values are fully corrected for multiple comparisons.
Group differences and correlations.
We addressed the question of whether age of onset of training is related to white-matter organization in two complementary ways. First, we performed a whole-brain skeletonized between-group subtraction analysis to identify white-matter regions that may differ between musician groups matched on years of formal training and experience. This categorical contrast picks up group differences. We also performed a correlational analysis to examine white-matter differences that may be a function of age of onset of training. To this end, the age at which musicians began training was correlated with whole-brain skeletonized FA. Finally, to determine the global relationship between white-matter structure and performance on the TMST regardless of training-related variables, PSYN Final across all participants (ET, LT, NM) was correlated with skeletonized FA. Regions identified in these analyses were subsequently used as masks to extract FA, AD, and RD values for plotting, partial correlations, or one-tailed t tests to specify findings as required.
Probabilistic tractography.
Probabilistic tractography was used to better characterize the location and connectivity of findings. Significant voxels were first converted to binary masks in each individual's 1-mm-isotropic-transformed diffusion space and then used to seed a two-fiber model of probabilistic tractography (Behrens et al., 2007). Both fiber directions were randomly sampled 10,000 times for each voxel in the seed mask, averaged across groups, and thresholded for display. Thresholded tracts were converted into binary masks that were used to extract diffusion measures from each individual's nonlinearly registered voxelwise maps.
Results
Behavioral
Musician groups were well matched for musical training variables, with no significant differences in years of formal training, years of experience, or current hours of practice (Table 1). All musicians were currently playing one or more instruments that required the coordinated use of both hands and were highly trained, with a mean of 16.65 and range of 9.5–25 total years of experience. Nonmusicians had very little experience (mean, 0.68 years; range, 0–2 years). As expected, ET and LT differed on current age (ET: mean, 22.72 years; LT: mean, 27.61 years). There was no difference in age between musicians and nonmusicians (musicians: mean, 25.17 years; NM: mean, 26.41 years). The significant age difference and unequal number of males and females between groups led us to include both age and sex as covariates of no interest in the subsequent structural analyses. In addition, the relationship between our grouping variable, age of onset, and the other demographic measures was also assessed. Age of onset was significantly correlated with years of formal training (r = −0.41, p < 0.05) but not years of experience (p = 0.99) or hours of current practice (p = 0.83). Thus, to more precisely isolate the effects of age of onset across musician groups, we also used years of formal training as a covariate of no interest in correlational analyses described below.
Performance on the TMST across groups and blocks of training were assessed with 3 × 6 (group × block) repeated-measures ANOVA F tests and planned t tests. Accuracy, as measured by the percentage of correct responses on the learned sequence (PCOR), differed by group and block (group: F(2,50) = 6.18, p < 0.05, η2 = 0.20; block: F(5,250) = 8.89, p < 0.001, η2 = 0.15), with no interaction (group × block: F(10,250) = 0.85, p = 0.59). All groups improved across blocks, with musicians exhibiting better performance than non-musicians (Fig. 1B, left). Planned directional t tests revealed that ET had better performance than LT on block 2 (ET > LT: p < 0.05) and LT showed better performance than NM on blocks 3–6 (LT > NM: blocks 3–6, p < 0.05). Performance on the measure of sensorimotor synchronization (PSYN) also showed significant differences between groups (group: F(2,50) = 21.26, p < 0.001, η2 = 0.46; block: F(5,250) = 25.87, p < 0.001, η2 = 0.34), with no interaction (group × block: F(10,250) = 0.28, p = 0.99). Overall, synchronization performance differed between groups, was sustained across 2 d of training, and improved across blocks (Fig. 1B, right). Planned directional t tests revealed that ET had better synchronization performance than LT across all blocks (ET > LT: blocks 1–6, p < 0.05) and LT had better performance than NM (LT > NM: blocks 1–2, p < 0.05; blocks 3–6, p < 0.001). These results show that musicians have an initial advantage in sensorimotor synchronization that is sustained even after 2 consecutive days of training, and is in agreement with findings of a previous experiment using the same task (Watanabe et al., 2007). Because PSYN was more sensitive to between-group differences, PSYN Final was used as a regressor for investigating subsequent brain–behavior correlations.
Diffusion imaging
Group differences
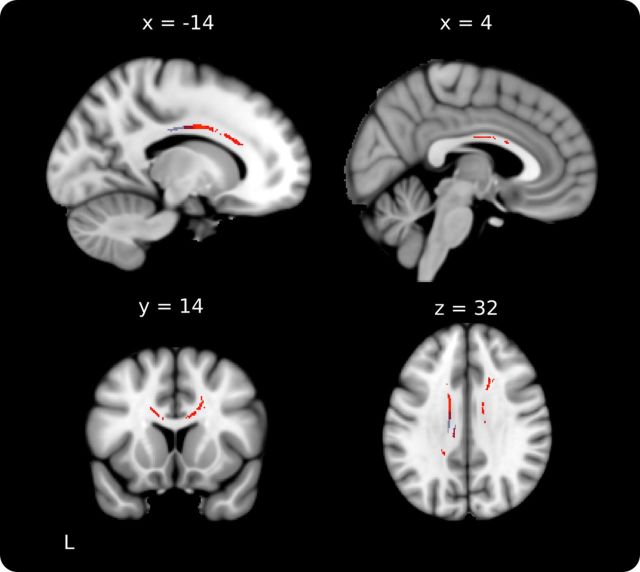
To determine the white matter structural differences related to early training, skeletonized FA values were compared between musician groups. ET had significantly greater FA than LT in a region of the corpus callosum including the posterior midbody and anterior portion of the isthmus (peak voxel: −14, −11, 32, t = 4.55; Fig. 2A). To confirm that voxels making up the skeleton were retrieved from the location identified in the group analysis, the significant region was deprojected onto each musician's normalized scan and reviewed. This review confirmed that the region of interest was in the same location in all individuals. To investigate whether we might also find group differences in a more anterior region of the CC as reported by others (Schlaug et al., 1995), the threshold for the skeletonized FA contrast was reduced to p < 0.10 (fully corrected). Consistent with previous studies, this analysis showed that ET had greater FA in a large portion of bilateral rostral body and midbody of the CC.
Figure 2.
ET versus LT group FA differences and extractions. A, ET > LT group difference in skeletonized FA (blue) in posterior midbody of the corpus callosum. The tract based on this seed connects the right and left sensorimotor cortices and is represented as the red-yellow underlay (where red represents a threshold of 1–10% of maximum particle count and bright yellow depicts 10% and greater). B, FA (top) and RD (bottom) values from the peak CC voxel plotted against group, age of onset, and PSYN Final. Values for ET are depicted in red, LT in blue, and NM in black. Group means are depicted with filled circles. Raw values were used for all plots while statistics were based on the corrected values as stated in the text. **p < 0.001.
To compare FA in the anterior midbody/isthmus between groups, we extracted FA, RD, and AD from the peak voxel identified in the skeletonized contrast. To visualize the group difference results, Figure 2B includes a plot of the extracted FA values by group (top left). There was a significant group difference in RD such that ET had lower values than LT and NM (ET < LT: t(34) = 3.59, p < 0.001; ET < NM: p = 0.06; LT < NM: p = 0.92; Fig. 2B, left). There were no significant group differences in AD (ET > LT: p = 0.07; ET > NM: p = 0.13; LT > NM: p = 0.60).
As an additional confirmation that the skeletonized group contrast accurately represented the location of group difference, we performed a smoothed whole-brain FA comparison between ET and LT. The results showed that the only location where ET had greater FA than LT was in a very similar region of the posterior midbody/isthmus of the CC (peak voxel: −12, −22, 32, t = 5.42, p < 0.05 fully corrected). This region overlaps with the skeletonized group difference.
Correlations with region of interest extractions
To further assess the relationship between age of onset of musical training and white matter in the CC, we correlated age of onset with extracted diffusion measures with age and sex, and years of formal training as covariates of no interest. Age of onset of musical training was significantly correlated with both FA and RD (FA: r = −0.40, p < 0.05; RD: r = 0.36, p < 0.05; Fig. 2B, middle). Together, these results demonstrate that white matter plasticity in the posterior midbody of the CC is differentially affected by the age at which musical training begins.
We also explored the possibility that the synchronization performance advantage of ET may be linked to enhanced FA in the midbody/isthmus of the CC. FA extracted from the peak voxel identified in the skeletonized group contrast was correlated with PSYN Final (Fig. 2B, right). There was a significant positive correlation across all participants (All: r = 0.30, p < 0.05); however, this effect was predominantly driven by the correlation within NM (ET: p = 0.67; LT: p = 0.80; NM: r = 0.57, p < 0.05). Consistent with a link between RD, greater myelination, and greater performance, the significant correlation between PSYN Final and FA in NM was driven by a significant correlation with RD (NM: r = −0.59, p < 0.05) while there was no relationship with AD (NM: p = 0.46). These findings indicate that while there is an overall relationship between variability in white matter integrity in the CC and synchronization performance, this effect is not significant for musicians who may be at ceiling for both diffusion measures and performance.
Correlations with age of onset
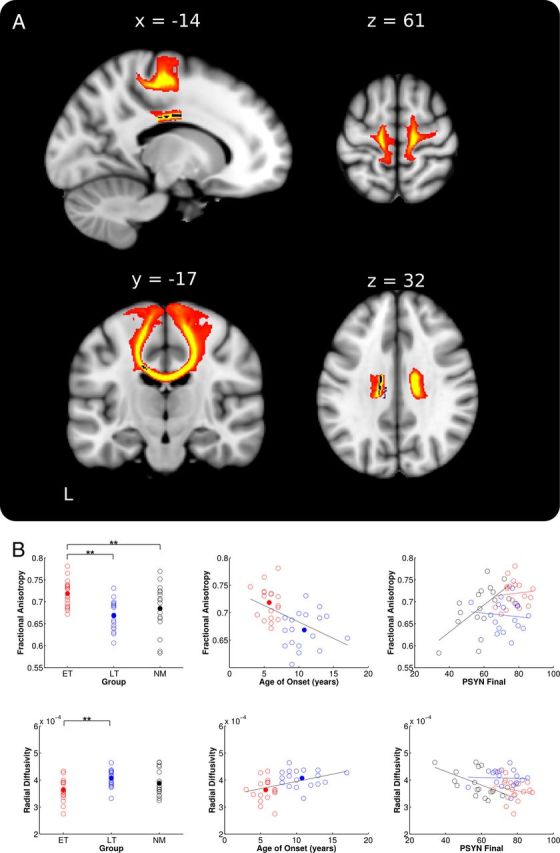
As an independent analysis to further establish the relationship between age of onset of musical training and FA, age of onset was regressed against whole-brain skeletonized FA. Age of onset was significantly correlated with FA in bilateral rostral body and midbody of the corpus callosum (Fig. 3), overlapping with the regions identified in the group-difference contrast. When years of formal training was included as an additional covariate of no interest, nearly identical results were obtained slightly below threshold (p < 0.08, fully corrected).
Figure 3.
Correlation between FA and age of onset of musical training. FA was significantly correlated with age of onset of musical training across musicians in bilateral rostral body and midbody of the corpus callosum (red). This region overlaps with the more posterior midbody location identified in the group contrast between ET and LT (overlayed in semitransparent blue visible in the top left and bottom right slices).
Probabilistic tractography
In a next step, fiber tractography was used to assess the structural connectivity of the posterior midbody/isthmus region. A seed mask was created from the significant CC cluster from the skeletonized ET–LT contrast, and the results were thresholded for display. The mean tract passed through the posterior midbody/isthmus of the CC to connect the right and left sensorimotor cortices (Fig. 2A). The tract identified here is consistent with CC connectivity reported in recent DTI-based human tractography studies (Hofer and Frahm, 2006; Chao et al., 2009). Mean diffusion parameters extracted from the tract-defined volume showed strikingly similar results to those found in the prior skeleton-based extractions (Fig. 2A). FA was greater in ET than LT (ET > LT: t(34) = 2.11, p < 0.05; ET > NM: p = 0.07; LT > NM: p = 0.72); there were no differences between groups on RD (ET < LT: p = 0.09; ET < NM: p = 0.16; LT < NM: p = 0.36) or AD (ET > LT: p = 0.21; ET > NM: p = 0.38; LT > NM: p = 0.71). There was no evidence for correlation between diffusion measures and age of onset (FA: p = 0.37; RD: p = 0.31). These results indicate that the group difference identified within the CC is also true for the tract that connects right and left sensorimotor cortex through this region.
Correlations with sensorimotor synchronization performance
To directly test the global relationship between FA and performance on the TMST, PSYN Final was regressed against whole-brain skeletonized FA. Across all groups, PSYN Final was correlated with FA in an extensive region of the left temporal lobe (Fig. 4A), extending into the posterior limbs of the internal and external capsules. This same region was not present, even below threshold, in the right hemisphere. Mean diffusion values from the entire significant ROI were extracted to better represent the extensive area of interest. Mean FA did not differ between musician subgroups but differed between musicians and nonmusicians (ET > LT: p = 0.10; ET > NM: t(33) = 3.98, p < 0.001; LT > NM: t(33) = 2.56, p < 0.05; Fig. 4B, left). Again, differences in RD appear to be driving the FA differences (RD: ET < LT: p = 0.18; ET < NM: t(33) = 2.98, p < 0.05; LT < NM: t(33) = 2.07, p < 0.05). There were no AD differences between groups (AD: ET > LT: p = 0.24; ET > NM: p = 27; LT > NM: p = 52).
Figure 4.
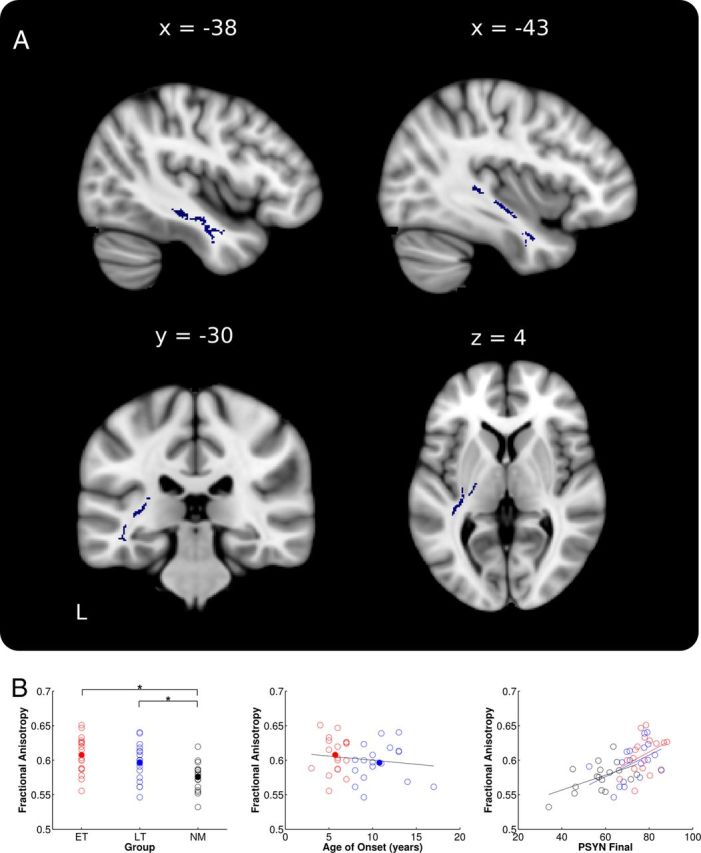
Whole-brain FA correlations with PSYN Final. A, Skeleton voxels significantly correlated with PSYN Final in left temporal lobe and posterior limb of the internal and external capsules (blue). B, Mean values extracted from the region of significant correlation plotted against group, age of onset, and PSYN Final. ET are shown in red, LT in blue, and NM in black. Group means are depicted with filled circles. Note that raw values were used for all plots while statistics were based on the corrected values as stated in the text. *p < 0.05.
We next correlated age of onset with extracted FA and RD values in this region to determine whether they showed a similar relationship to that found in the CC. Our results showed a significant negative correlation between age of onset and FA and a significant positive correlation between age of onset and RD when controlling for age, sex, and years of formal training (FA: r = −0.41, p < 0.05; RD: r = 0.38, p < 0.05; Fig. 4B, middle). In addition, the groupwise correlations with PSYN Final revealed that the overall significant correlation with FA was driven by correlations within LT and NM (ET: p = 0.74; LT: r = 0.59, p < 0.05; NM: r = 0.63, p < 0.05). Again, this finding appears to have been primarily driven by RD (ET: p = 0.77; LT: r = −0.62, p < 0.05; NM: r = −0.61, p < 0.05) and not AD (ET: p = 0.99; LT: p = 0.67; NM: p = 0.13).
Discussion
Our results show that early musical training has a differential impact on white matter structure and sensorimotor synchronization performance, providing evidence for a sensitive period where experience produces long-lasting changes in the brain and behavior. Consistent with previous findings, ET outperformed LT on a sensorimotor synchronization task across 2 d of practice (Watanabe et al., 2007). Group comparisons of diffusion imaging data showed that ET had greater FA and lower radial diffusivity in the posterior midbody/isthmus of the CC even when matched for years of formal training, years of experience, and hours of current practice. Fiber tractography showed that this region includes tracts that connect to the sensorimotor cortices in the two hemispheres. Extracted FA and radial diffusivity values in the CC correlated with age of onset of musical training. These correlations were confirmed by a whole-brain regression analysis showing that age of onset was negatively correlated with FA in the same region. Behavioral regression analysis showed that across all groups, synchronization performance was significantly correlated with FA in temporal lobe pathways. Crucially, FA in both the CC and temporal lobe was significantly correlated with the age of onset of musical training despite controlling for years of formal training.
Corpus callosum and bimanual coordination
DTI analyses showed that ET had greater FA and reduced radial diffusivity in the posterior midbody/isthmus of the CC and that those who began earlier had higher FA. The posterior midbody contains the fibers that connect the sensorimotor cortices of the two hemispheres (Hofer and Frahm, 2006; Chao et al., 2009). This region undergoes significant developmental changes between the ages of 6 and 8 years (Westerhausen et al., 2011), when our ET would have begun their training. Individual differences in FA in this subregion of the CC have been shown to be less strongly influenced by genetics (Chiang et al., 2009), and are thus more likely to be influenced by environmental factors such as musical training. Consistent with this, 6-year-olds who received 15 months of musical training showed increased volume in a similar region of the CC (Hyde et al., 2009), and FA in this region in adult musicians has been linked to hours of practice before the age of 11 years (Bengtsson et al., 2005). Playing a musical instrument requires the coordinated action of the two hands and interhemispheric interactions mediated by the CC have been shown to play a prominent role in bimanual coordination (Swinnen, 2002). The size of the CC and FA have been shown to be related to bimanual task performance in children (Kurth et al., 2012) and adults (Johansen-Berg et al., 2007; Muetzel et al., 2008; Gooijers et al., 2013). Further, the size of the primary motor cortex connected through this region has been shown to be related to the age of onset of musical training (Amunts et al., 1997). Early musical training, by requiring practice of bimanual skills, may place greater demands on interhemispheric interactions between sensorimotor regions, thus promoting the development of enhanced connections that are indexed by increased FA. Contrary to expectations, we found no evidence that LT differed from non-musicians, even though ET and LT had the same amount of musical training while non-musicians had almost none. This lends further strength to the argument that the onset of training, rather than the amount of experience or practice, is the driving factor behind the observed FA differences. Finally, whereas musicians were specifically selected for extensive musical training, the control group was merely selected to have little or no experience; hence, the wide range of FA values in this group could reflect a diversity of adaptations that obscure possible differences with the LT group.
In addition to differences in the CC, we found that FA in the left temporal lobe was significantly correlated with synchronization performance and with age of onset across musician groups. This region includes fibers from auditory cortex that connect to the motor and parietal cortices through the arcuate fasciculus (Petrides and Pandya, 1988; Glasser and Rilling, 2008). Importantly, synchronization performance on our task has previously been shown to recruit both auditory and motor regions in non-musicians (Steele and Penhune, 2010) and structural differences in the arcuate fasciculus have been hypothesized to support the stronger auditory–motor associations found in musicians (Wan and Schlaug, 2010; Halwani et al., 2011). Finally, white matter in the temporal lobes and arcuate fasciculus continues to develop into adulthood (Lenroot and Giedd, 2006; Hasan et al., 2010), making it susceptible to the effects of childhood training.
Together, our findings indicate that early musical training enhances the development of white matter pathways in the CC and temporal lobe that support interhemispheric interaction and sensorimotor integration. Enhanced white matter plasticity in ET in these regions may be the result of an interaction between training during an early sensitive period and on-going practice. Thus, early training may induce initial changes in white-matter connectivity that serve as a scaffold on which later training continues to build.
Evidence for sensitive periods
Evidence for the effects of experience on brain structure and function during specific periods of early development has been found in the auditory (Chang and Merzenich, 2003; de Villers-Sidani et al., 2007), somatosensory (Fox, 1992), and visual (Wiesel and Hubel, 1963; Hubel and Wiesel, 1970) domains (Knudsen, 2004; for review, see Hensch, 2004). Rat pups exposed to specific frequencies between days 9–13 of life show expanded functional representation for these frequencies as adults (de Villers-Sidani et al., 2007). Studies with congenitally deaf cats have shown microstructural changes in the dendrites of auditory cortex (Wurth et al., 2001) and changes in cortical excitability that can be ameliorated by early cochlear implantation (Klinke et al., 1999; Kral et al., 2000). Human studies show that deaf children who receive implants before 3–4 years of age show better auditory/speech perception than those who receive implants later (Svirsky et al., 2004; Sharma et al., 2007). Kral and Eggermont (2007) have hypothesized that such plasticity is a result of the interaction between bottom-up sensory information and top-down feedback from higher-order areas involved in functions such as language, attention, and motivation or reward. It has also been proposed that there may be a sequence of overlapping sensitive periods that occur as progressively more complex functions come online (de Villers-Sidani and Merzenich, 2011). Thus, early experience may produce changes in lower-level processes on which later experience can build.
White matter plasticity as measured by FA is hypothesized to be based on experience-dependent neuronal firing (Fields, 2005; Zatorre et al., 2012); thus, interaction between different functional regions may be particularly important for neuronal change. Musical training is a rich source of bottom-up stimulation to the sensory and motor systems, and places demands on cognitive systems involved in auditory–motor integration, attention, and memory (Zatorre et al., 2007; Wan and Schlaug, 2010). Further, cortical plasticity has also been shown to be influenced by the reward value of stimuli (Beitel et al., 2003; Fritz et al., 2007) and music has been shown to engage the reward system (Blood and Zatorre, 2001; Salimpoor et al., 2011). Thus, musical training may be particularly effective in driving structural changes.
Mechanisms of experience-dependent plasticity
Differences in FA may reflect variation in white matter features, such as axon myelination, diameter, packing density, and geometry (Beaulieu, 2002; Alexander et al., 2007). When we decomposed FA into axial and radial diffusivity, our findings were shown to be primarily driven by lower radial diffusivity in ET. Increases in radial diffusivity have been linked to decreased myelin protein content (Song et al., 2002), dysmyelination (Sun et al., 2008; Klawiter et al., 2011), and axon degeneration (Pierpaoli et al., 2001). By inference, lower radial diffusivity values have thus been interpreted as indexing greater myelin integrity. In keeping with this interpretation, greater radial diffusivity in the CC of ET is a possible indicator of greater myelination. Increased FA in the CC of mice following training has also been related to increased expression of a myelin marker (Blumenfeld-Katzir et al., 2011). As described above, changes in white matter may arise from experience-dependent, temporally synchronized neuronal firing in connected regions (Fields, 2005; Zatorre et al., 2012). Neuroimaging studies have shown greater functional connectivity in musicians between auditory and motor regions (Zatorre et al., 2007; Chen et al., 2008), as well as between premotor cortex and thalamus (Krause et al., 2010). By stimulating interactions between sensory and motor regions—and between these regions and systems important for attention, learning, and memory—musical training may drive synchronized firing and thus neural change.
Effects of training or preexisting differences?
Preexisting factors, whether genetic or environmental, may also influence both the propensity to begin training early and the observed differences in brain structure and behavior. Genetic factors have been linked to the ability to acquire absolute pitch (Zatorre, 2003) and to measures of musical aptitude (Ukkola et al., 2009). However, other evidence strongly suggests that preexisting differences are not the only cause of the observed enhanced callosal connectivity in ET. As described above, white matter in this region of the CC may be under less strong genetic control than other regions (Chiang et al., 2009), undergoes normative developmental plasticity between the ages of 6 and 8 years (Westerhausen et al., 2011), and changes as a result of training (Hyde et al., 2009). Nevertheless, the only possible direct tests for a sensitive period would come from studies using randomized designs (musical vs nonmusical training, with age as a parameter) or from longitudinal studies assessing changes in brain structure and performance across development. The present findings can serve to motivate such studies, providing specific hypotheses concerning neural and behavioral correlates of early training.
In conclusion, our findings provide compelling evidence that early musical training can produce long-lasting changes in behavior and the brain. We propose that early training interacts with preexisiting individual differences in brain organization and ongoing maturational processes to produce differential changes in white matter structure. Early musical experience may promote plasticity in motor and auditory connectivity that serves as a scaffold upon which ongoing training can build.
Footnotes
This work was supported by the Natural Sciences and Engineering Research Council of Canada Postgraduate Doctoral Scholarships to C.J.S. and J.A.B. and a grant from the Canadian Institutes of Health Research (MOP-220211 to V.B.P. and R.J.Z.). We thank the staff at the McConnel Brain Imaging Centre of McGill University for their assistance with scanning.
The authors declare no competing financial interests.
References
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Schlaug G, Jäncke L, Steinmetz H, Schleicher A, Dabringhaus A, Zilles K. Motor cortex and hand motor skills: structural compliance in the human brain. Hum Brain Mapp. 1997;5:206–215. doi: 10.1002/(SICI)1097-0193(1997)5:3<206::AID-HBM5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bailey J, Penhune VB. A sensitive period for musical training: contributions of age of onset and cognitive abilities. Ann N Y Acad Sci. 2012;1252:163–170. doi: 10.1111/j.1749-6632.2011.06434.x. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitel RE, Schreiner CE, Cheung SW, Wang X, Merzenich MM. Reward-dependent plasticity in the primary auditory cortex of adult monkeys trained to discriminate temporally modulated signals. Proc Natl Acad Sci U S A. 2003;100:11070–11075. doi: 10.1073/pnas.1334187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci U S A. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld-Katzir T, Pasternak O, Dagan M, Assaf Y. Diffusion MRI of structural brain plasticity induced by a learning and memory task. PLoS One. 2011;6:e20678. doi: 10.1371/journal.pone.0020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- Chao YP, Cho KH, Yeh CH, Chou KH, Chen JH, Lin CP. Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum Brain Mapp. 2009;30:3172–3187. doi: 10.1002/hbm.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Penhune VB, Zatorre RJ. Moving on time: brain network for auditory-motor synchronization is modulated by rhythm complexity and musical training. J Cogn Neurosci. 2008;20:226–239. doi: 10.1162/jocn.2008.20018. [DOI] [PubMed] [Google Scholar]
- Chiang MC, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, Klunder AD, Toga AW, McMahon KL, de Zubicaray GI, Wright MJ, Srivastava A, Balov N, Thompson PM. Genetics of brain fiber architecture and intellectual performance. J Neurosci. 2009;29:2212–2224. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Merzenich MM. Lifelong plasticity in the rat auditory cortex: basic mechanisms and role of sensory experience. Prog Brain Res. 2011;191:119–131. doi: 10.1016/B978-0-444-53752-2.00009-6. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007;27:180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- Fields RD. Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist. 2005;11:528–531. doi: 10.1177/1073858405282304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster NE, Zatorre RJ. Cortical structure predicts success in performing musical transformation judgments. Neuroimage. 2010;53:26–36. doi: 10.1016/j.neuroimage.2010.06.042. [DOI] [PubMed] [Google Scholar]
- Fox K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J Neurosci. 1992;12:1826–1838. doi: 10.1523/JNEUROSCI.12-05-01826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasnelli J, Collignon O, Voss P, Lepore F. Crossmodal plasticity in sensory loss. Prog Brain Res. 2011;191:233–249. doi: 10.1016/B978-0-444-53752-2.00002-3. [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, David SV, Shamma SA. Does attention play a role in dynamic receptive field adaptation to changing acoustic salience in A1? Hear Res. 2007;229:186–203. doi: 10.1016/j.heares.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Schlaug G. Gray matter differences between musicians and nonmusicians. Ann N Y Acad Sci. 2003;999:514–517. doi: 10.1196/annals.1284.062. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Rilling JK. DTI tractography of the human brain's language pathways. Cereb Cortex. 2008;18:2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Gooijers J, Caeyenberghs K, Sisti HM, Geurts M, Heitger MH, Leemans A, Swinnen SP. Diffusion tensor imaging metrics of the corpus callosum in relation to bimanual coordination: effect of task complexity and sensory feedback. Hum Brain Mapp. 2013;34:241–252. doi: 10.1002/hbm.21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halwani GF, Loui P, Rüber T, Schlaug G. Effects of practice and experience on the arcuate fasciculus: comparing singers, instrumentalists, and non-musicians. Front Psychol. 2011;2:156. doi: 10.3389/fpsyg.2011.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Kamali A, Abid H, Kramer LA, Fletcher JM, Ewing-Cobbs L. Quantification of the spatiotemporal microstructural organization of the human brain association, projection and commissural pathways across the lifespan using diffusion tensor tractography. Brain Structure and Function. 2010;214:361–373. doi: 10.1007/s00429-009-0238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Ann Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde KL, Lerch J, Norton A, Forgeard M, Winner E, Evans AC, Schlaug G. The effects of musical training on structural brain development. Ann N Y Acad Sci. 2009;1169:182–186. doi: 10.1111/j.1749-6632.2009.04852.x. [DOI] [PubMed] [Google Scholar]
- Imfeld A, Oechslin MS, Meyer M, Loenneker T, Jancke L. White matter plasticity in the corticospinal tract of musicians: A diffusion tensor imaging study. Neuroimage. 2009;46:600–607. doi: 10.1016/j.neuroimage.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Della-Maggiore V, Behrens TE, Smith SM, Paus T. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuroimage. 2007;36:T16–T21. doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JS, Newport EL. Critical period effects in second language learning: the influence of maturational state on the acquisition of English as a second language. Cognitive Psychology. 1989;21:60–99. doi: 10.1016/0010-0285(89)90003-0. [DOI] [PubMed] [Google Scholar]
- Klawiter EC, Schmidt RE, Trinkaus K, Liang HF, Budde MD, Naismith RT, Song SK, Cross AH, Benzinger TL. Radial diffusivity predicts demyelination in ex-vivo multiple sclerosis spinal cords. Neuroimage. 2011;55:1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinke R, Kral A, Heid S, Tillein J, Hartmann R. Recruitment of the auditory cortex in congenitally deaf cats by long-term cochlear electrostimulation. Science. 1999;285:1729–1733. doi: 10.1126/science.285.5434.1729. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Kral A, Eggermont JJ. What's to lose and what's to learn: development under auditory deprivation, cochlear implants and limits of cortical plasticity. Brain Res Rev. 2007;56:259–269. doi: 10.1016/j.brainresrev.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Congenital auditory deprivation reduces synaptic activity within the auditory cortex in a layer-specific manner. Cereb Cortex. 2000;10:714–726. doi: 10.1093/cercor/10.7.714. [DOI] [PubMed] [Google Scholar]
- Krause V, Schnitzler A, Pollok B. Functional network interactions during sensorimotor synchronization in musicians and non-musicians. Neuroimage. 2010;52:245–251. doi: 10.1016/j.neuroimage.2010.03.081. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Brain mechanisms in early language acquisition. Neuron. 2010;67:713–727. doi: 10.1016/j.neuron.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Mayer EA, Toga AW, Thompson PM, Luders E. The right inhibition? Callosal correlates of hand performance in healthy children and adolescents callosal correlates of hand performance. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22060. Advanced online publication. Retrieved June 18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Muetzel RL, Collins PF, Mueller BA, M Schissel A, Lim KO, Luciana M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. Neuroimage. 2008;39:1918–1925. doi: 10.1016/j.neuroimage.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhune VB. Sensitive periods in human development: evidence from musical training. Cortex. 2011;47:1126–1137. doi: 10.1016/j.cortex.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Steele CJ. Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav Brain Res. 2012;226:579–591. doi: 10.1016/j.bbr.2011.09.044. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J Comp Neurol. 1988;273:52–66. doi: 10.1002/cne.902730106. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- Sadato N, Okada T, Honda M, Yonekura Y. Critical period for cross-modal plasticity in blind humans: a functional MRI study. Neuroimage. 2002;16:389–400. doi: 10.1006/nimg.2002.1111. [DOI] [PubMed] [Google Scholar]
- Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 2011;14:257–262. doi: 10.1038/nn.2726. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Jäncke L, Huang Y, Staiger JF, Steinmetz H. Increased corpus callosum size in musicians. Neuropsychologia. 1995;33:1047–1055. doi: 10.1016/0028-3932(95)00045-5. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Gilley PM, Dorman MF, Baldwin R. Deprivation-induced cortical reorganization in children with cochlear implants. Int J Audiol. 2007;46:494–499. doi: 10.1080/14992020701524836. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Steele CJ, Penhune VB. Specific increases within global decreases: a functional magnetic resonance imaging investigation of five days of motor sequence learning. J Neurosci. 2010;30:8332–8341. doi: 10.1523/JNEUROSCI.5569-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Cross AH, Song SK. Evolving Wallerian degeneration after transient retinal ischemia in mice characterized by diffusion tensor imaging. Neuroimage. 2008;40:1–10. doi: 10.1016/j.neuroimage.2007.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svirsky MA, Teoh SW, Neuburger H. Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiol Neurootol. 2004;9:224–233. doi: 10.1159/000078392. [DOI] [PubMed] [Google Scholar]
- Swinnen SP. Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci. 2002;3:348–359. doi: 10.1038/nrn807. [DOI] [PubMed] [Google Scholar]
- Ukkola LT, Onkamo P, Raijas P, Karma K, Järvelä I. Musical aptitude is associated with AVPR1A-haplotypes. PLoS One. 2009;4:e5534. doi: 10.1371/journal.pone.0005534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Schlaug G. Music making as a tool for promoting brain plasticity across the life span. Neuroscientist. 2010;16:566–577. doi: 10.1177/1073858410377805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Savion-Lemieux T, Penhune VB. The effect of early musical training on adult motor performance: evidence for a sensitive period in motor learning. Exp Brain Res. 2007;176:332–340. doi: 10.1007/s00221-006-0619-z. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Luders E, Specht K, Ofte SH, Toga AW, Thompson PM, Helland T, Hugdahl K. Structural and functional reorganization of the corpus callosum between the age of 6 and 8 years. Cereb Cortex. 2011;21:1012–1017. doi: 10.1093/cercor/bhq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wurth N, Heid S, Kral A, Klinke R. Morphology of neurons in the primary auditory cortex (AI) in normal and congenitally deaf white cats—study of Dil labelled cells. In: Elsner N, Eysel U, editors. From molecular neurobiology to clinical neuroscience: proceedings of the 27th Gottingen Neurobiology Conference; Germany: Georg Thieme Verlag; 2001. p. 318. [Google Scholar]
- Zatorre RJ. Absolute pitch: a model for understanding the influence of genes and development on neural and cognitive function. Nat Neurosci. 2003;6:692–695. doi: 10.1038/nn1085. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Chen JL, Penhune VB. When the brain plays music: auditory-motor interactions in music perception and production. Nat Rev Neurosci. 2007;8:547–558. doi: 10.1038/nrn2152. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]