Abstract
Recurrent apneas are important causes of hospitalization and morbidity in newborns. Gestational stress (GS) compromises fetal brain development. Maternal stress and anxiety during gestation are linked to respiratory disorders in newborns; however, the mechanisms remain unknown. Here, we tested the hypothesis that repeated activation of the neuroendocrine response to stress during gestation is sufficient to disrupt the development of respiratory control and augment the occurrence of apneas in newborn rats. Pregnant dams were displaced and exposed to predator odor from days 9 to 19 of gestation. Control dams were undisturbed. Experiments were performed on male and female rats aged between 0 and 4 d old. Apnea frequency decreased with age but was consistently higher in stressed pups than controls. At day 4, GS augmented the proportion of apneas with O2 desaturations by 12%. During acute hypoxia (12% O2), the reflexive increase in breathing augmented with age; however, this response was lower in stressed pups. Instability of respiratory rhythm recorded from medullary preparations decreased with age but was higher in stressed pups than controls. GS reduced medullary serotonin (5-HT) levels in newborn pups by 32%. Bath application of 5-HT and injection of 8-OH-DPAT [(±)-8-hydroxy-2-di-(n-propylamino) tetralin hydrobromide; 5-HT1A agonist; in vivo] reduced respiratory instability and apneas; these effects were greater in stressed pups than controls. Sex-specific effects were observed. We conclude that activation of the stress response during gestation is sufficient to disrupt respiratory control development and promote pathological apneas in newborn rats. A deficit in medullary 5-HT contributes to these effects.
Introduction
Recurrent apneas and respiratory instabilities are important causes of hospitalization and morbidity in premature newborns. Because the respiratory control system undergoes important development during early life, neonatal apneas typically resolve themselves by 36–40 weeks after conception. However, ∼10% of healthy premature infants and ∼5% of infants born at term show apneic events with O2 desaturations and bradycardias beyond this time (Poets, 2010; Hunt et al., 2011), and newborn males are more at risk of developing respiratory disorders than females (Mage and Donner, 2006). Despite its impact on infant morbidity, our understanding of such heterogeneity in respiratory control development remains limited.
Exposures to hypoxia, nicotine, or cocaine during gestation are established disruptors of respiratory development in newborns, and the mechanisms underlying their effects are diverse. However, considering that these stressors can stimulate corticosterone release in the mother (Damianopoulos and Carey, 1995; Stanulis et al., 1997) and that gestational stress (GS) or antenatal maternal anxiety is a risk factor for several neurological diseases (Talge et al., 2007; Field and Diego, 2008; Kinney et al., 2008), elevated stress hormones during gestation may be a common pathway that leads to disrupted ventilatory control in newborns. Little is known about the impact of stress hormones per se on respiratory control development. However, high maternal cortisol levels during pregnancy have been associated with an increased need for resuscitation measures at birth (Ponirakis et al., 1998). Furthermore, maternal anxiety and substance abuse exacerbate apneic events in the newborn and are established risk factors for sudden infant death syndrome (SIDS) (Kinney and Thach, 2009). Although these clinical observations argue that maternal stress during gestation disrupts respiratory control development, confounding factors related to maternal age/lifestyle and overall health of the infant limit the conclusions that can be drawn from these studies. Here, we used an animal model to better control environmental variables and tested the hypothesis that recurrent activation of stress response in gestating dams is sufficient to disrupt respiratory control development and promote respiratory disorders in newborn rats. We first compared respiratory activity of pups born to undisturbed dams with those born to females exposed to stress during pregnancy. We measured apnea frequency and respiratory variability in both intact animals and reduced medullary preparations; we then quantified the hyperventilatory response to respiratory stimuli (CO2 and hypoxia). Experiments were performed on males and females to assess sex-specific effects. At postnatal day 4 (P4), the physiological impact of GS was assessed by using pulse oxymetry to determine whether apneic events were associated with O2 desaturations and bradycardias.
Besides promoting neural development during early life (Hodges and Richerson, 2008), serotonin (5-HT) exerts a profound influence on respiratory rhythm and its stability (Richter et al., 2003). Consequently, abnormal 5-HT modulation of respiratory networks has been implicated in respiratory disorders, including SIDS (Hilaire et al., 2010). Because stress interferes with development and function of 5-HT neurons (Vázquez et al., 2000; Papaioannou et al., 2002), we determined whether disruption of 5-HT modulation contributes to the higher apnea frequency observed in pups born from stressed dams.
Materials and Methods
Animals
Experiments were performed on male and female Sprague Dawley rats aged between 0 and 4 d (P0, P2, and P4). These pups were born from 97 virgin females mated in our animal care facility. Dams were supplied with food and water ad libitum and maintained in standard animal care conditions (21°C, 12 h light/dark cycle: lights on at 7:00 A.M. and off at 7:00 P.M.). All experiments complied with the guidelines of the Canadian Council on Animal Care. The institutional animal care committee approved the specific protocols used in this study.
GS procedures
After mating, pregnancy was confirmed by vaginal smear [gestation day 0 (G0)]. Novelty, bright light, and predator odor activate the neuroendocrine response to stress in rodents (Chandramohan et al., 2007; Fendt and Endres, 2008). On G9, dams assigned to GS protocol were transported outside the animal care facility and placed in a brightly lit room in a clean cage (one dam per cage), each containing a piece of filter paper wetted with 35 μl of fox anal gland extract [2,5,-dihydro-2,4,5,-trimethylethiazoline (TMT)] (Pherotech). This predator odor induces fear and activates the neuroendocrine response to stress in rodents (Fendt and Endres, 2008). The protocol was performed at 9:00 AM daily from G9 to G19 and lasted 20 min. The stress procedure was performed under a fume hood located outside the main animal housing facility. At the end of the procedure, the TMT impregnated paper was removed from the cage, and a blood sample was taken (see below). Because the predator odor was still perceptible at that time, the dam remained in the experimental cage under the fume hood for 1 h before being returned to the animal care facility. Performing TMT exposure outside the animal care facility was necessary to prevent exposing other animals that were not part of the stress protocol. Accordingly, unstressed (control) dams remained in the animal care facility where they were not disturbed (except for regular care) throughout gestation.
Corticosterone measurement
At 9:20 AM on G12, a tail blood sample was taken either during normal care (control; n = 32) or immediately after TMT exposure (GS; n = 54). Blood collection and plasma corticosterone measurements were performed according to standard laboratory procedures (Fournier et al., 2007). Briefly, blood was transferred from the syringe into a tube containing EDTA (microvette 500; Sarstedt). Plasma was separated by centrifugation, quickly frozen at −80°C until assayed. Corticosterone levels were determined by an enzyme immunoassay (Assay Design). Corticosterone detection was done with a microplate spectrophotometer (μ-Quant; Bio-Tek Instruments). The corticosterone concentration was calculated from the four-parameter logistic standard curve using SigmaPlot 12.3 (Systat Software).
Respiratory measurements in vivo
Measurements of apneas, ventilatory activity, and basal O2 consumption (V̇O2) in newborn pups were performed using whole-body, flow-through plethysmography. Development of the hypoxic chemoreflex was compared between groups and sexes. The efficiency of the selective 5-HT1A agonist 8-OH-DPAT [(±)-8-hydroxy-2-di-(n-propylamino) tetralin hydrobromide; 0.05 mg/kg, i.p.] for the treatment of apneas was also compared. On P4, the physiological relevance of stress-related increase in apneic events was evaluated by (1) combining plethysmography with measurements of O2 saturation (SpO2) and heart rate with pulse oxymetry, (2) measuring the ventilatory response to CO2, and (3) comparing basal corticosterone levels between groups.
Definition of apneas, desaturations, and bradycardias in rodents.
There is no clear consensus regarding the clinical definition of pathological apneas in humans (Finer et al., 2006; Eichenwald et al., 2011). Thus, using a criteria commonly used in rodents (Han et al., 2002; Montandon et al., 2006; Zanella et al., 2008), an apnea was arbitrarily defined as an interruption of airflow for at least two breathing cycles (at P2 for instance, an apneic pause >0.75 s). The apnea frequency is the sum of both spontaneous and post-sigh apneas.
In the clinic, there is no consensus on the decrease of SpO2 or severity of bradycardia that should be considered pathologic (Finer et al., 2006). In rats, O2 desaturations and bradycardias were arbitrarily defined as a 5% fall below baseline (Bairam et al., 2012); however, the average SpO2 and heart rate decreases observed (see Fig. 8B,C) meet the 10% criteria often used in clinical studies (Ramanathan et al., 2001).
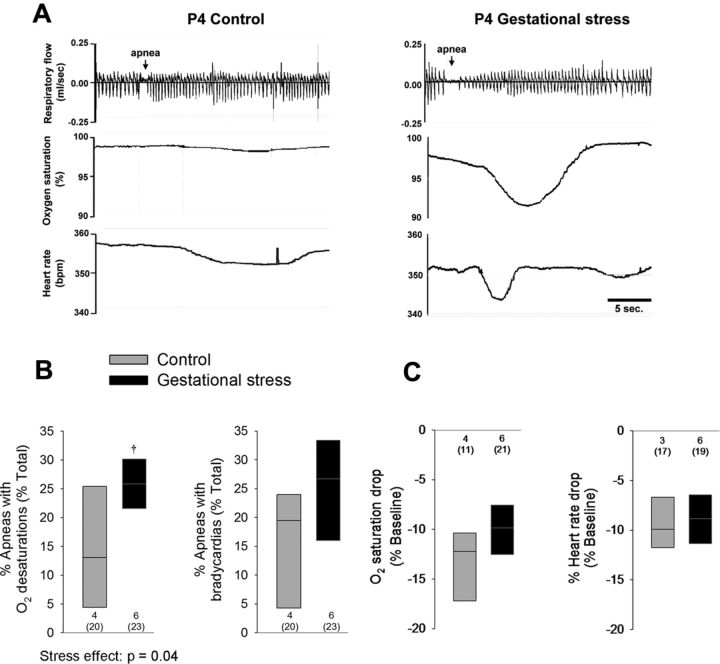
Figure 8.
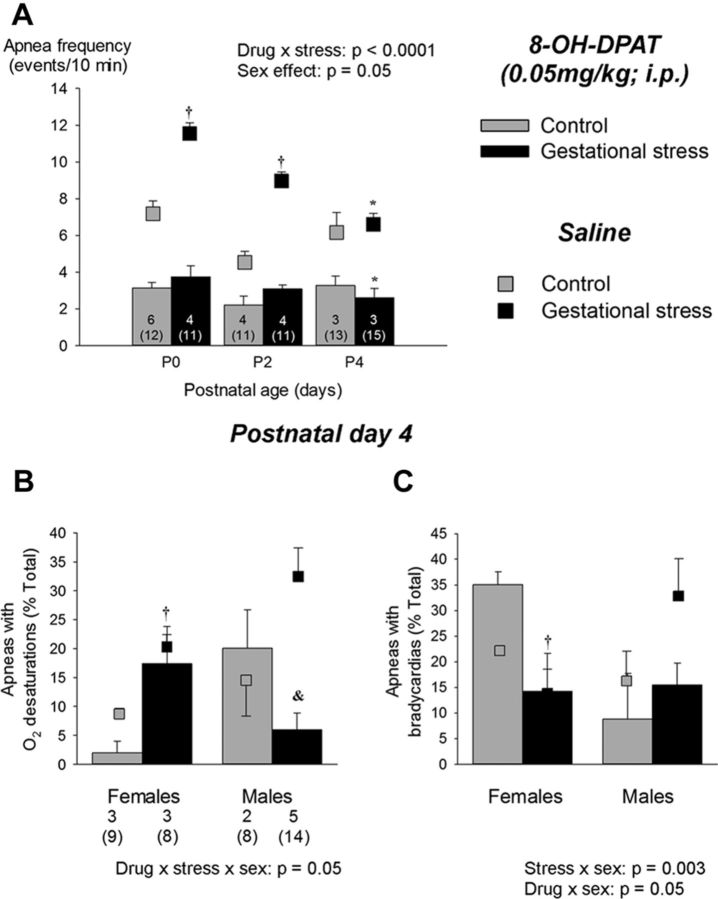
GS augments the physiological consequences of an apneic event in 4-d-old pups. A, Simultaneous recordings of ventilatory activity (plethysmography; top traces), SpO2, and heart rate (pulse oxymetry; bottom traces) in pups born to control dams (left traces) and pups born to dams subjected to GS (right traces). B, Box plots comparing the proportion of apneas with O2 desaturations and bradycardias in pups born to control (gray box) versus stressed dams (black boxes). C, Box plots comparing the nadir of O2 desaturations and decrease in heart rate during an apnea in each group of pups. These data were obtained from a total of 43 pups (20 controls and 23 stress) originating from 10 litters (4 controls and 6 stress). The numbers below and above the bars (B and C, respectively) indicate the number of litters sampled in each group; the numbers below (in parentheses) indicate the total number of pups used in this group. For details concerning box plots, see Figure 1 legend. †p < 0.05, statistically different from corresponding control value.
Whole-body plethysmography.
These methods have been described previously (Gulemetova and Kinkead, 2011). Briefly, air flow through the chamber was set to 0.1 L/min, and temperature inside the chamber was maintained at 34°C (P0/P2) or 32°C (P4) using a temperature control system (Physitemp). The system was calibrated by injecting a known volume (0.5 ml) into the chamber with a glass syringe at a rate corresponding to the air flow range typically generated by rat pups. Barometric pressure, chamber temperature and humidity, and the body temperature of the pup (Tb) were also measured in normoxia and at the end of hypoxia, hypercapnia, or the 8-OH DPAT protocol to correct the tidal volume (Vt) and thus minute ventilation (V̇e) and express values in milliliters BTPS (Drorbaugh and Fenn, 1955). Composition of the gas mixtures flowing in and out of the chamber was analyzed with an oxygen analyzer (model S-3A; Ametek) for subsequent calculation of V̇O2 (Mortola and Dotta, 1992).
Measurement of SpO2 and bradycardias.
An infrared emitter/sensor was placed around the neck of 4-d-old pups to measure SpO2 and heart rate (Mouse Ox; Starr Life Sciences). The pup was then placed in a plethysmography chamber to monitor apneas.
Apnea treatment with 8-OH-DPAT.
The apnea frequency, O2 desaturations, and bradycardias were measured after intraperitoneal injection of saline or the selective 5-HT1A agonist 8-OH-DPAT (0.05 mg/kg).
Basal corticosterone and testosterone levels.
Terminal blood samples were taken from 4-d-old pups that were not exposed to any stimulus or treatment. Sample handling and corticosterone measurement were performed according to the previously described methods (Fournier et al., 2011). Testosterone was measured in serum using an enzyme immunoassay (Cayman Chemical) and detected with a microplate spectrophotometer. The testosterone concentration was calculated with the spreadsheet provided by Cayman Chemical.
Protocol and data analyses
Normoxia.
Each pup was first placed in the plethysmograph for 10 min to acclimatize to the chamber and ambient temperature. The chamber was briefly opened, and buccal temperature was measured with a small thermocouple. Tb was not monitored continuously to minimize physical stress during the measurements. The chamber was closed, and the baseline (normoxic) ventilatory and V̇O2 measurements were started once the animal was calm and the breathing activity was stable. This baseline period typically lasted 20 min. Breathing frequency (fR), Vt, and V̇e were recorded using data acquisition software (IOX; EMKA Technologies). Baseline values of these variables were obtained by averaging results over the last 10 min that preceded hypoxia or hypercapnia.
Hypoxic ventilatory response.
Moderate hypoxia was initiated after baseline measurement by adding N2 to the inflowing gas mixture. The target level of hypoxia (FiO2 = 0.12) was reached within 2 min using preset gas mixing parameters to ensure that the hypoxia dynamic within the chamber was constant between experiments. Exposure lasted 20 min. The hypoxic ventilatory response (HVR) was first assessed by averaging fR over the first 8 min of hypoxia; this value was then expressed as a percentage change from the normoxic value. The HVR of newborn rats being biphasic (Saetta and Mortola, 1987; Bissonnette, 2000) (see Fig. 3A); this period was chosen because of our interest in obtaining a value reflecting the rapid increase in breathing associated with chemoreceptor activation at the onset of hypoxia. The late phase of the response was assessed by averaging values from the last 4 min of hypoxia (between minutes 16 and 20 of exposure).
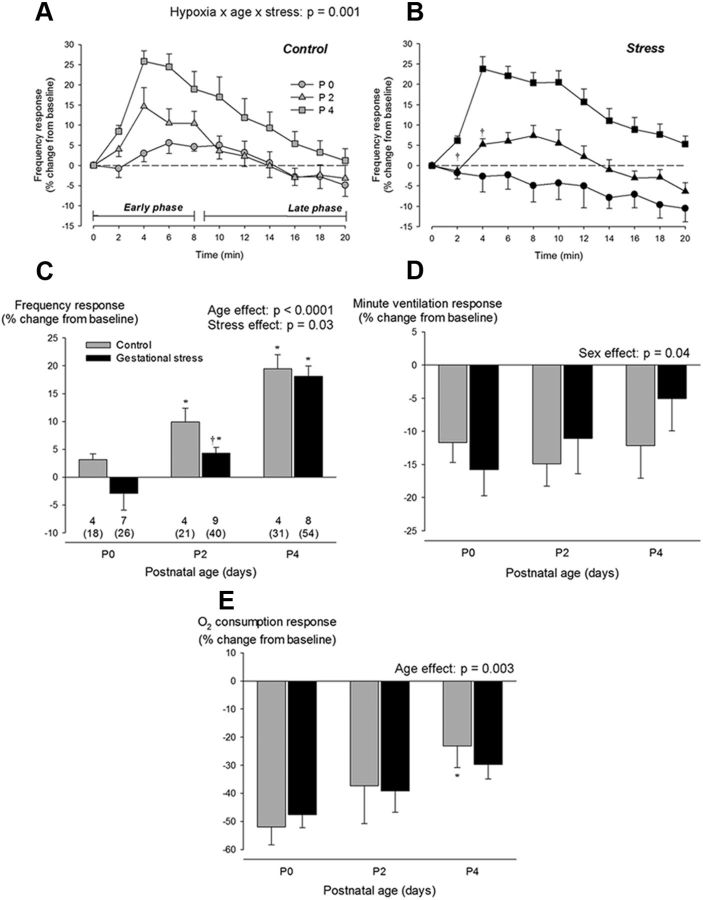
Figure 3.
Neonatal development of the ventilatory response to hypoxia in pups born to control dams (gray) versus pups born to dams subjected to GS (black). Time course of the fR response to hypoxia in three distinct age groups: P0 (circles), P2 (triangles), and P4 (squares). This representation illustrates the early (first 8 min) and late phases (minutes 9 to 20) of the response. Results are compared between control pups (A; gray) and pups born to dams subjected to GS (B; black). C, Comparison of the mean frequency response observed during the early phase of hypoxia. Effects of GS on V̇e (D) and V̇O2 (E) responses measured at the end of hypoxic exposure (late phase). Ventilatory measurements were performed using whole-body, flow-through plethysmography during exposure to moderate hypoxia (FiO2 = 0.12; 20 min). Values are expressed as percentage change from baseline values and are reported as means ± SEM. Note that mean values are based on litter means. These data were obtained from a total of 190 pups (70 controls and 120 stress) originating from 22 litters (7 controls and 15 stress). Numbers below the bars (C) indicate the number of litters used in each group, the number underneath (in parentheses) indicates the total number of pups sampled. *p < 0.05, statistically different from P0. †p < 0.05, statistically different from control value.
Hypercapnic ventilatory response.
Moderate hypercapnia (FiCO2 = 0.05) was initiated after baseline measurement by adding CO2 to the inflowing gas mixture. Exposure lasted 20 min. The response was assessed by averaging values from the last 5 min of hypercapnia (between minutes 15 and 20 of exposure).
Effect of a 5-HT1A agonist (8-OH-DPAT) on apnea frequency.
These experiments aimed to determine whether systemic administration of a 5-HT1A agonist could alleviate apneas in newborn pups. Before being placed in the recording chamber, pups were injected (intraperitoneally) with vehicle (saline) or 8-OH-DPAT (Sigma-Aldrich) dissolved in saline. The selected dose was 0.05 mg/kg, and the injected volume was 2 μl/g. Preliminary experiments showed that this dose effectively reduces apneas with minimal effects on fR. Higher doses (e.g., 0.1 mg/kg) augmented movement-related artifacts during recording. At 10 min after the injection, baseline measurement of respiratory activity was obtained (see previous protocol). The chamber was briefly opened to measure the Tb. Breathing was monitored under normoxic conditions for an additional 50 min. Tb was measured at the end of the experiment. In this series, apnea frequency was calculated at 60 min after injection; apneic events were counted between minutes 40 and 60 after injection.
Fictive breathing measurements in vitro
After anesthesia and decerebration, the medulla and rostral spinal cord were dissected from pups (P0–P4). Using cranial nerves and blood vessels of the ventral brainstem as landmarks (Ruangkittisakul et al., 2007), the rostral brainstem was sectioned at the level of cranial nerve VI and the caudal cerebral artery and placed ventral side up in a recording chamber irrigated (5 ml/min) with artificial CSF (aCSF) containing the following (in mm): 129 NaCl, 3.35 KCl, 1.15 MgCl2, 30 d-glucose, 21 NaHCO3, 1.26 CaCl2, and 0.58 NaH2PO4. The superfusate was maintained at 26°C and equilibrated with a 95% O2/5% CO2 gas mixture, pH 7.4 ± 0.1. Bursts of inspiratory (phrenic)-related motor activity were recorded extracellularly from the C3 or C4 nerve rootlet using a suction electrode. The signal was processed using standard methods (Khemiri et al., 2012).
Protocol and data analyses.
The preparation was allowed to stabilize for 20–30 min, until stable rhythmic neural activity was recorded. Baseline recording was then performed for 5 min, and then the effect of 5-HT supplementation on fictive fR and respiratory variability was assessed by superfusing the preparation with aCSF containing 5-HT (5 μm; Sigma-Aldrich) for 20 min. The choice of this concentration was based on results from preliminary experiments and recent literature (Voituron et al., 2010). Variability of the “core” respiratory motor output was assessed by calculating the coefficient of variation (CV) of the interburst interval (CV = SD/mean × 100) during 5 min of baseline recording.
Measurement of medullary monoamines by HPLC
Tissue preparation.
After anesthesia, the medullary region corresponding to the one used for electrophysiological recording was excised from the animal and stored at −80°C. On the day of the analysis, each sample was thawed, weighted, and placed in 150 μl of methanol before being homogenized with a motorized pestle. The homogenate was then centrifuged at 12,000 rpm at 4°C for 15 min. The supernatant was filtered (pore size, 0.45 μm) and stored at −80°C until measurement by HPLC.
Monoamine analyses.
Before injection, 50 μl of internal standard (3,4-dihydroxybenzalamine hydrobromide) and 50 μl of distilled H20 were added to 50 μl of supernatant. One hundred microliters of this mixture were then injected with a Hamilton syringe into an HPLC apparatus consisting of a Varian ProStar 410 solvent delivery system (Varian Chromatography Systems) connected to a Decade II electrochemical detector containing a VT-03 electrochemical flow cell (both from Antec Leyden). Concentrations of noradrenaline (NA) and 5-HT were calculated relative to standards of known concentration and corrected for the known concentration of internal standard added to each sample. Each sample concentration was corrected for its initial dilution with methanol and medullary weight and expressed as nanomoles per gram.
Statistics
A mixed ANOVA model (mixed-effect model) was used to study the fixed effect of GS, age, and sex on selected variables of interest. The mixed procedure was used with litter nested within “gestational stress” as a random factor to take into account the correlation within animals from the same litter and thus ensure that between-group differences were not attributable to a litter effect. Several studies have discussed the advantages of this approach in developmental studies using multiparous species (Zorrilla, 1997; Wainwright et al., 2007). When ANOVA results indicated that a factor (or interaction between factors) was significant (p ≤ 0.05), the analysis was followed by Fisher's post hoc test, whenever appropriate. p values for ANOVA results are reported in the figures; results from post hoc tests are indicated by symbols in the figures. The number of litters in each group is indicated within the bars of the histograms; the total number of pups in each group is reported in parentheses. The relationship between maternal corticosterone and apnea frequency (see Fig. 2C) was analyzed using the least-square method. Analyses were performed using JMP (version 10; SAS Institute). Data are reported as mean ± 1 SEM. Note that mean values reported in the text, Table 1, and figures are based on litter means (i.e., a mean value was obtained for each litter, and these values were then averaged).
Figure 2.
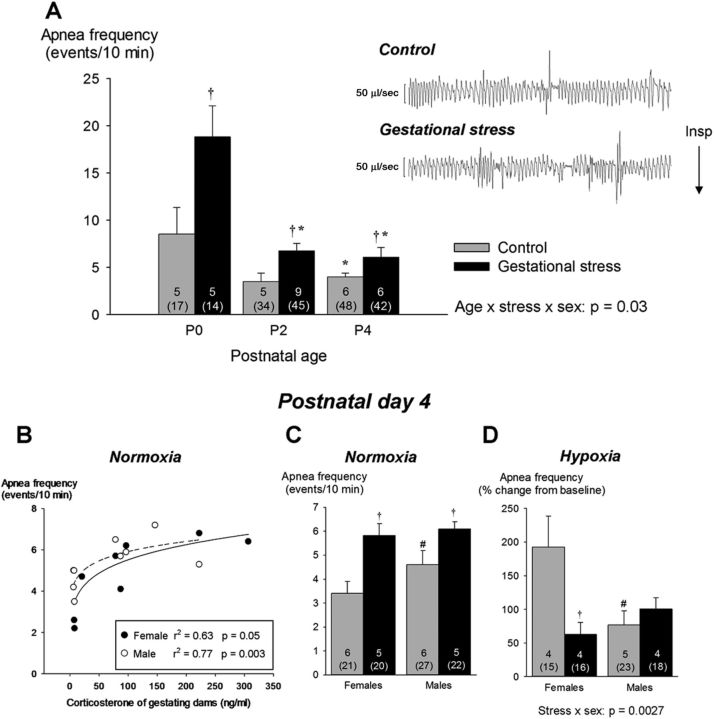
A, Effects of GS on apnea frequency during neonatal development (P0–P4); the right side of the panel presents 30 s segments of plethysmographic recordings illustrating respiratory instability and apneas in newborn pups (P0) born from control (top trace) and stressed (bottom trace) dams. Apnea frequency is the sum of spontaneous and post-sigh apneas measured during baseline recording (normoxia). Because sex-specific differences were apparent only at P4, the bottom panels present data from males and females separately. B, Relationships between maternal corticosterone measured on G12 and mean apnea frequency measured at P4 for each litter. Data for males and females are represented by open and filled circles, respectively. C, Apnea frequency measured in 4-d-old male and female pups under normoxic conditions. D, Apnea frequency observed at the end of hypoxic exposure. Values are expressed as means ± SEM. Note that the mean values are based on litter means. These data were obtained from a total of 200 pups (99 controls and 101 stress) originating from 27 litters (12 controls and 15 stress). In the figure, the numbers within bars indicate the number of litters used in each group, the number underneath (in brackets) indicate the total number of pups sampled. Gray bars, Controls; black bars, GS. *p < 0.05, statistically different from corresponding value measured in newborn (P0, <18 h old). †p < 0.05, statistically different from control value. #p < 0.05, statistically different from corresponding female value.
Table 1.
Comparison of body weights and selected physiological variables between pups born dams subjected to gestational stress versus pups born from undisturbed mothers (control)
| Control |
Stress |
Stress effect | Age effect | Sex effect | Factorial interaction | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| P0 | P2 | P4 | P0 | P2 | P4 | |||||
| Body weight (g) | ||||||||||
| Female | 6.3 ± 0.2& (15; 42) | 7.4 ± 0.3*& (12; 36) | 10.5 ± 0.3*& (24; 85) | 6.1 ± 0.1& (16; 33) | 7.0 ± 0.2*& (16; 47) | 10.3 ± 0.2*& (27; 92) | p = 0.30; F(1,86) = 1.1 | p < 0.0001; F(2,670) = 743.8 | p < 0.0001; F(1,609) = 39.9 | Age × sex × stress, p = 0.02; F(2,609) = 4.0 |
| Male | 6.5 ± 0.2 (13; 40) | 8.0 ± 0.2* (12; 34) | 10.4 ± 0.3* (25; 97) | 6.4 ± 0.1 (16; 34) | 7.3 ± 0.3* (17; 40) | 10.8 ± 0.3* (30; 110) | p = 0.30; F(1,86) = 1.1 | p < 0.0001; F(2,670) = 743.8 | p < 0.0001; F(1,609) = 39.9 | Age × sex × stress, p = 0.02; F(2,609) = 4.0 |
| fR (breaths/min) | 145 ± 2 (4; 18) | 165 ± 7* (4; 21) | 169 ± 5* (10; 74) | 143 ± 10 (7; 26) | 167 ± 7* (9; 40) | 167 ± 5* (15; 96) | p = 0.84; F(1,35) = 0.04 | p < 0.0001; F(2,251) = 16.6 | p = 0.58; F(1,245) = 0.3 | NS |
| Vt (ml BTPS/100 g) | 0.77 ± 0.08 | 0.93 ± 0.09 | 1.0 ± 0.1 | 1.3 ± 0.1† | 0.90 ± 0.14* | 1.1 ± 0.1* | p = 0.38; F(1,34) = 0.8 | p < 0.0001; F(2,256) = 12.2 | p = 0.10; F(1,237) = 2.8 | Age × stress, p < 0.0001; F(2,256) = 15.3 |
| V̇e (ml BTPS/min/100 g) | 111 ± 12 | 151 ± 11* | 174 ± 23 | 194 ± 37 | 156 ± 30* | 180 ± 19* | p = 0.44; F(1,34) = 0.6 | p = 0.01; F(2,257) = 4.4 | p = 0.16; F(1,237) = 2.8 | Age × stress, p < 0.0001; F(2,257) = 13.5 |
| V̇O2 (ml STPD/min/100 g) | 3.9 ± 0.4 | 4.9 ± 0.1* | 3.9 ± 0.3 | 4.3 ± 0.3 | 4.9 ± 0.4* | 4.1 ± 0.3 | p = 0.64; F(1,37) = 0.2 | p = 0.0005; F(2,227) = 7.8 | p = 0.63; F(1,250) = 0.2 | NS |
| V̇e/V̇O2 | 30 ± 3 | 33 ± 3 | 49 ± 8 | 49 ± 9 | 33 ± 5* | 47 ± 4* | p = 0.63; F(1,33) = 0.2 | p = 0.0008; F(2,257) = 7.3 | p = 0.18; F(1,240) = 1.8 | Age × stress, p < 0.0001; F(2,257) = 9.2 |
| Body temperature (°C) | 34.6 ± 0.3 | 33.9 ± 0.6 | 33.5 ± 0.2* | 33.8 ± 0.4 | 33.9 ± 0.4 | 33.1 ± 0.2 | p = 0.26; F(1,36) = 1.3 | p = 0.002; F(2,252) = 6.5 | p = 0.51; F(1,245) = 0.4 | NS |
Measurements were performed under normoxic conditions in three age groups. Number of litters (bold) and pups per group are reported in parentheses after body weights. Except for body weights, data from injected animals (8-OH-DPAT series) are not included. Because sex-related differences were observed only for body weights, results obtained from male and female pups were pooled for all other variables. Data are reported as means ± SEM.
*p < 0.05, different from P0.
†p < 0.05, different from corresponding control value.
&p < 0.05, different from corresponding male value. NS, Not significant. BTPS, Body temperature, ambient pressure, saturated; STPD, Standard temperature and pressure, dry.
Results
Stress protocol augments plasma corticosterone levels in gestating dams
On G12, plasma corticosterone levels measured in dams immediately after the fourth stress exposure were higher than controls (Fig. 1). The weight gain during gestation did not differ between groups (p = 0.47; data not shown).
Figure 1.
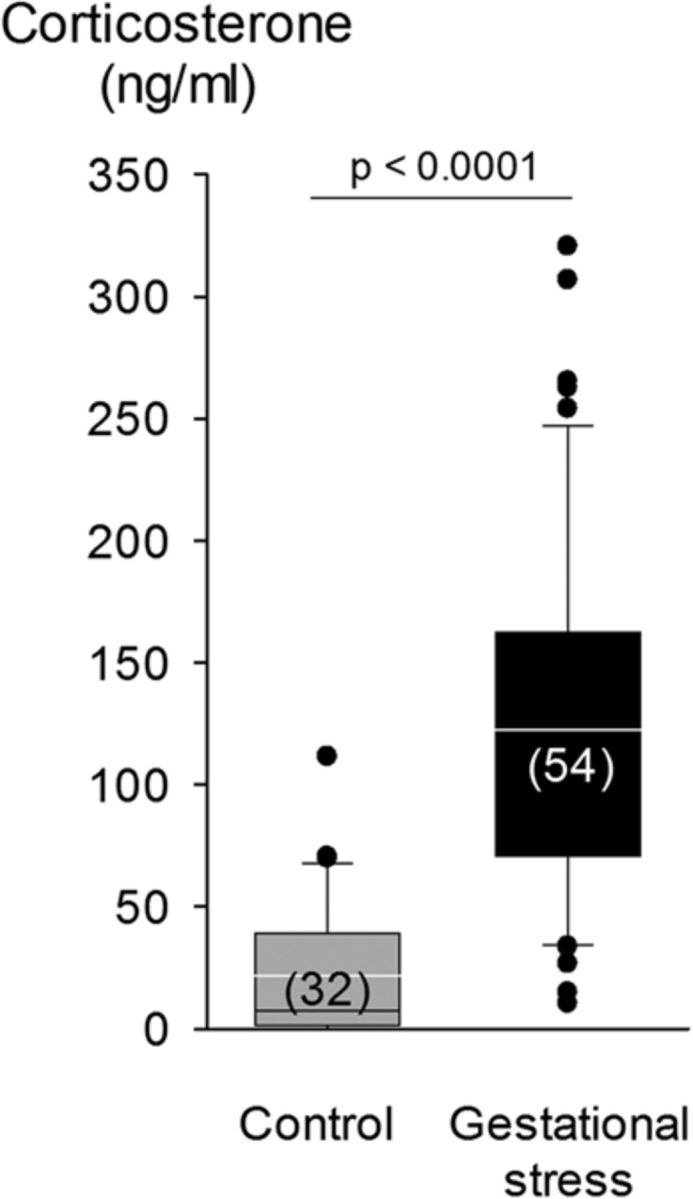
Box plot of the plasma corticosterone levels measured in gestating dams maintained under standard animal care conditions (control; gray bars; n = 32) and females subjected to GS (black bars; n = 54). Tail blood samples were taken at 9:20 A.M. on G12. Box boundaries correspond to the 25th and 75th percentiles (top and bottom, respectively); the line within the box indicates the median. Bars above and below show the 90th and 10th percentiles, respectively. Individual points are outside these limits.
GS increases apnea frequency in newborn pups
Baseline data from male and female pups were pooled because, except for body weight (Table 1) and apnea frequency (P4; Fig. 2B), ANOVAs revealed no sex-specific effect on any of the parameters studied. At birth, the Vt of GS pups was slightly higher than controls; this result contributes to the higher V̇e and relative hyperventilation (convective ratio) observed in this group (Table 1). The apnea frequency decreased progressively with age but was consistently higher in pups subjected to GS than controls (Fig. 2). At P4, the mean apnea frequency calculated for each litter correlated positively with the corticosterone levels measured in the mother during gestation (Fig. 2B); apneas were more frequent in males than females (Fig. 2C). The increase in apnea frequency was mainly attributable to an increase in post-sigh apneas; GS did not affect apnea duration (stress effect, p = 0.22; data not shown). Exposure to hypoxia augmented apnea frequency, especially in female pups born to control dams (Fig. 2D). Hypercapnia had no effect on this variable (p = 0.1; data not shown).
GS delays maturation of the HVR
In most pups, fR began to increase within the first 2 min of hypoxia (Fig. 3A,B). The hyperpnea observed during the early phase of hypoxia increased with age but was lower in GS pups than controls (Fig. 3C). Unlike controls, GS pups did not display a significant hyperventilation until P2. The HVR of newborns being biphasic (Fig. 3A,B), the V̇e measured during the last 4 min of hypoxia was not above baseline. The V̇e response did not change over the first 4 d of life and was not influenced by GS (Fig. 3D). Of note, the V̇e response of female pups was slightly less than males; this difference was most noticeable in 2-d-old stressed pups. Conversely, the decrease in V̇O2 that takes place during hypoxia in newborn diminished progressively with age but did not differ between groups or sexes (Fig. 3E).
GS reduces medullary 5-HT and NA levels in newborn pups
Medullary 5-HT levels changed with age; however, the maturation pattern differed between control and GS pups (Fig. 4A). At birth, medullary 5-HT levels of GS pups was 32% lower than controls. Medullary NA levels did not change with age but were lower in GS pups than controls (Fig. 4B).
Figure 4.
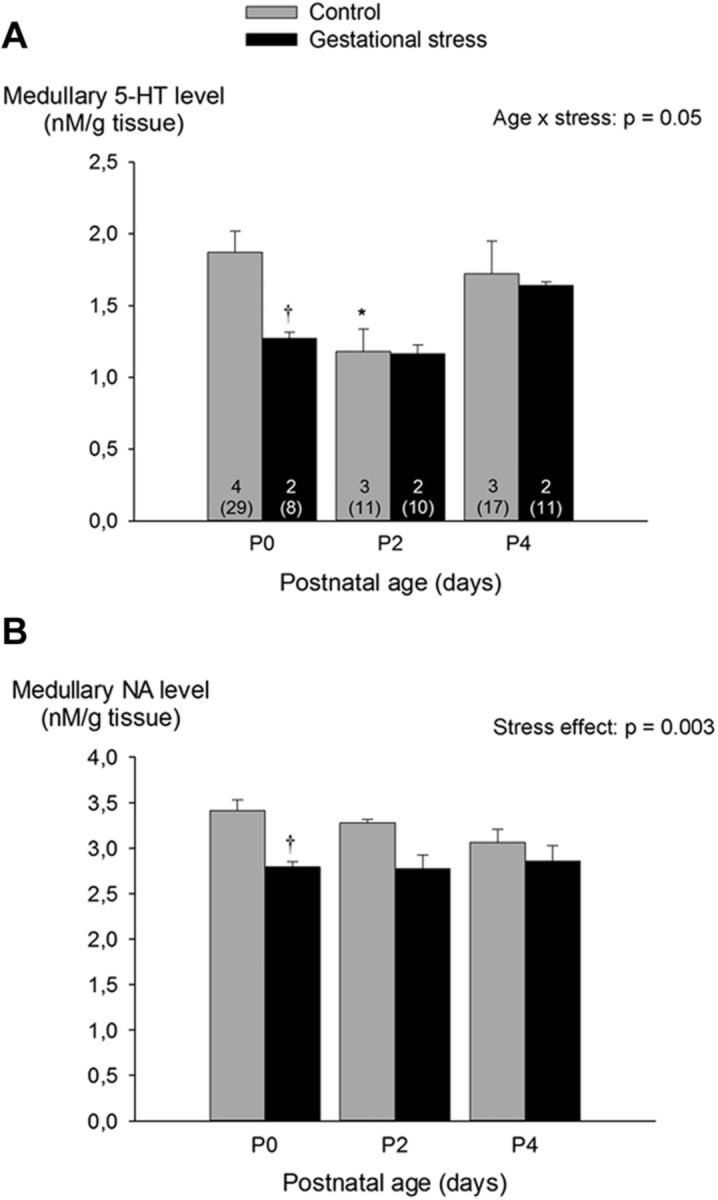
Effects of GS on medullary levels of 5-HT (A) and NA (B) in developing newborn rat pups. Measurements were performed by HPLC. Gray bars, Controls; black bars, GS. Values are expressed as means ± SEM. Note that mean values are based on litter means. These data were obtained from a total of 86 pups (57 controls and 29 stress) originating from nine litters (7 controls and 2 stress). Numbers within the bars (A) indicate the number of litters used in each group, and the number underneath (in parentheses) indicate the total number of medullas sampled. *p < 0.05, statistically different from P0. †p < 0.05, statistically different from corresponding control value.
GS reduces regularity of core medullary networks generating respiratory motor output
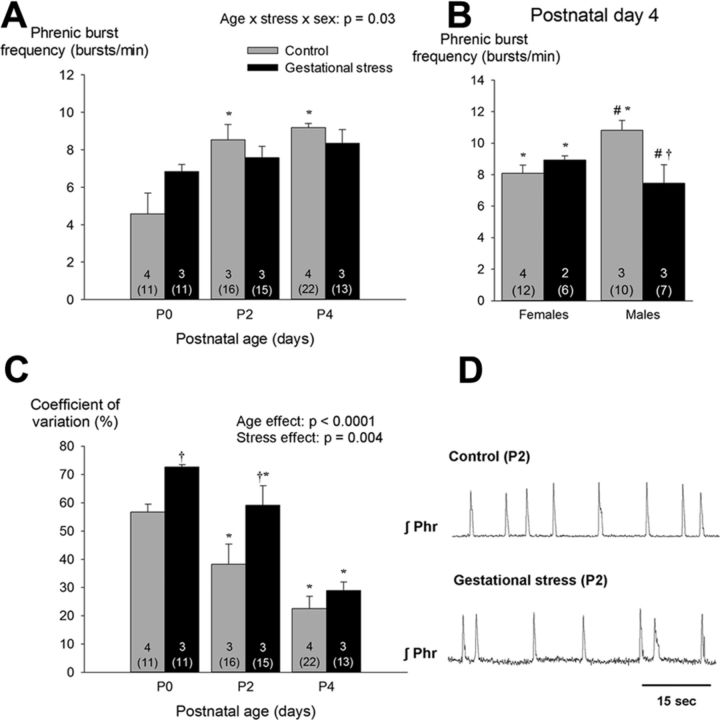
Basal phrenic burst frequency augmented with age (Fig. 5A). At P4, however, fictive fR of GS males was 31% lower than controls; this effect was not observed in females (Fig. 5B). The age-dependent increase in motor output concurred with a progressive reduction in the CV of the interburst interval (Fig. 5C). However, the variability inherent to medullary preparations from GS pups was greater than controls (Fig. 5C). This effect was not sex dependent.
Figure 5.
A, Effects of GS on age-dependent changes in basal phrenic burst frequency produced by in vitro medullary preparations. Because sex-specific differences were apparent only at P4, B presents data from males and females separately. C, Developmental change of the CV of the interburst interval between pups born from control dams (gray bars) versus dams subjected to GS (black bars). D, Phrenic neurograms (integrated signal) from 2-d-old pups illustrating differences in variability observed between preparations from stressed and control pups. Values are expressed as means ± SEM. Note that mean values are based on litter means. These data were obtained from a total of 88 pups (49 controls and 39 stress) originating from 14 litters (9 controls and 5 stress). In each panel, the numbers within the bars indicate the number of litters used in each group; the number underneath (in parentheses) indicate the total number of preparations used. *p < 0.05, statistically different from P0 (<18 h old). †p < 0.05, statistically different from corresponding control value. #p < 0.05, statistically different from corresponding female value.
The pathological consequences of GS are significant at P4
At P4, corticosterone levels of male pups born to stressed dams were higher than controls (Fig. 6A). Conversely, their testosterone levels were reduced substantially (Fig. 6B). The hypercapnic ventilatory response of female GS pups was 17% higher than controls (Fig. 7); this sex-specific effect was mainly mediated by an increased Vt response (Fig. 7C). Desaturations and bradycardias were mostly observed after spontaneous apneas. The proportion of apneas with O2 desaturation was higher in stressed pups than controls (Fig. 8A,B). Baseline SpO2 did not differ between groups (98.4 ± 0.4 vs 98.8 ± 0.1% for control and GS, respectively). During desaturating apneas, SpO2 decreased on average by 11 ± 1%; this value did not differ between groups (Fig. 8C). The proportion of apneas with bradycardias was not influenced by GS (Fig. 8B). During those events, the average decrease in heart rate was 9.2 ± 1.0%.
Figure 6.
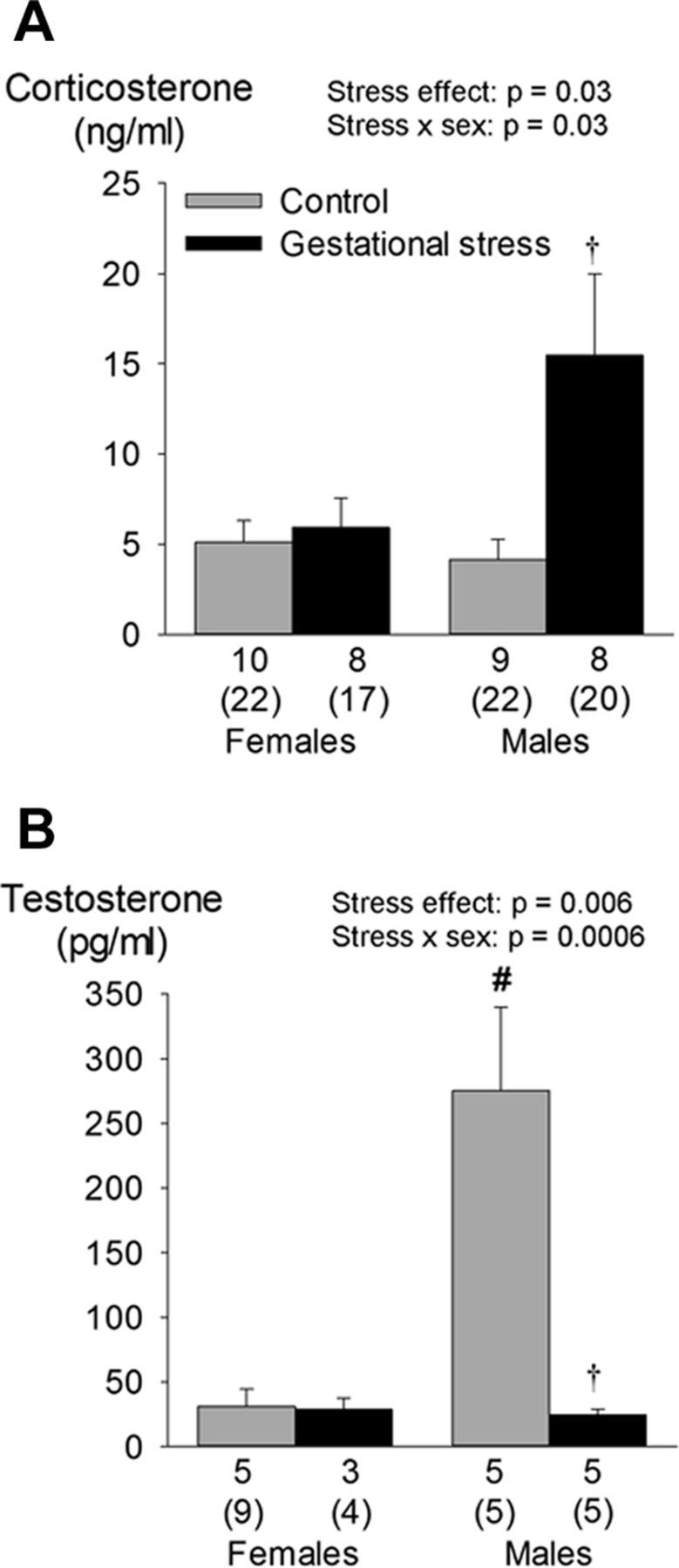
Effects of GS on circulating levels of corticosterone (A) and testosterone (B) in 4-d-old male and female pups. Values are expressed as means ± SEM, which are based on litter averages. These data were obtained from a total of 104 pups (58 controls and 46 stress) originating from 31 litters (16 controls and 15 stress). In each panel, the numbers underneath the bars indicate the number of litters sampled in each group; the numbers immediately below (in brackets) indicate the total number of pups used in this group. †p < 0.05 statistically different from corresponding control value. #p < 0.05, statistically different from corresponding female value.
Figure 7.
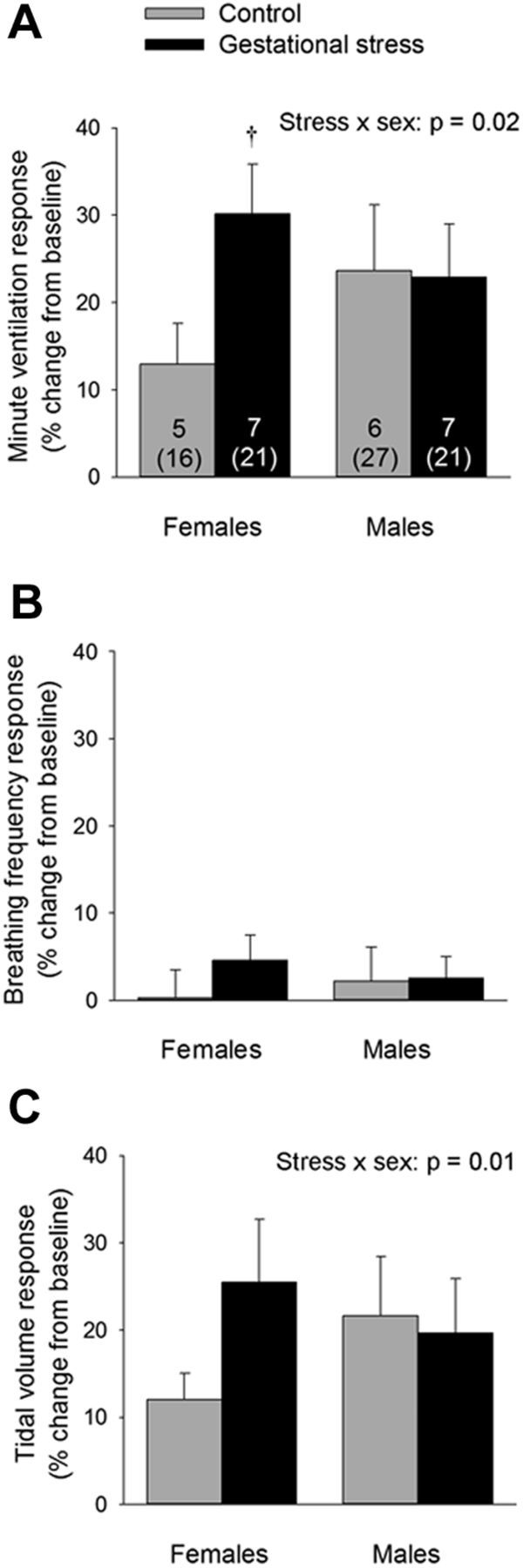
A, Comparison of the V̇e response to moderate hypercapnia (FiCO2 = 0.05; 20 min) between pups born to control dams (gray bars) versus pups born to dams subjected to GS (black bars). fR (B) and Vt (C) components of the response. Data were obtained over the last 5 min of hypercapnia and are expressed as a percentage change from baseline. Values are expressed as means ± SEM based on litter averages. These data were obtained from a total of 85 pups (43 controls and 42 stress) originating from 13 litters (6 controls and 7 stress). In A, the numbers within the bars indicate the number of litters sampled in each group; the numbers below (in parentheses) indicate the total number of pups used in this group. †p < 0.05, statistically different from corresponding control value.
5-HT-based treatment alleviates the consequences of stress-induced respiratory instability
Addition of 5-HT (5 μm) to the superfusion medium reduced the CV of the interburst interval; this “stabilizing” effect was greater in preparations from GS pups (Fig. 9A). These effects were not related to an increase in phrenic burst frequency (Fig. 9B,C). In GS pups, the frequency response to 5-HT application differed between males and females (Fig. 9B,C). In intact pups, intraperitoneal injection of the selective 5-HT1A receptor agonist 8-OH-DPAT reduced the apnea frequency; the effect was greatest in pups born to stressed dams (Fig. 10A). Compared with saline-injected pups, 8-OH-DPAT did not increase fR, V̇e, V̇O2, or Tb (data not shown). At P4, 8-OH-DPAT treatment reduced the fraction of apneas with O2 desaturation; this effect was greater in male pups (Fig. 10B). 8-OH-DPAT also reduced the proportion of apneas with bradycardias in males but not females (Fig. 10C).
Figure 9.
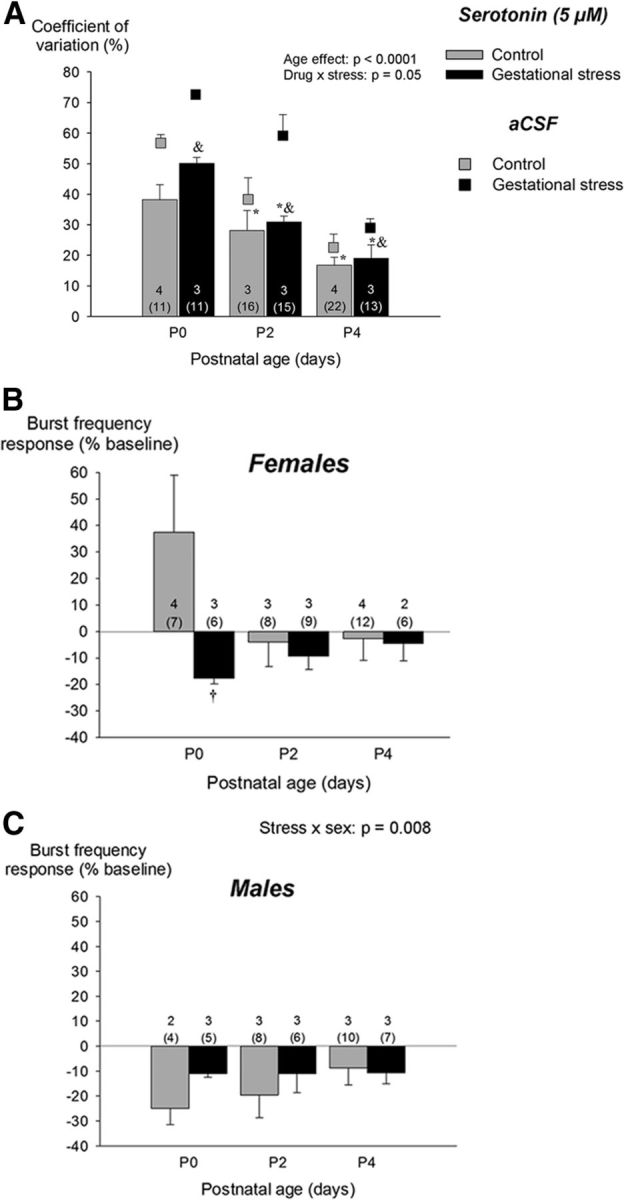
Effects of 5-HT (5 μm) bath application on inspiratory (phrenic) motor output produced by medullary preparation during the neonatal period (P0–P4). Results are compared between preparations from pups born from control dams (gray bars) and pups born from females subjected to stress during gestation (black bars). A, 5-HT reduces the CV of the interburst interval; data from untreated (aCSF) preparations is presented with squares to facilitate comparison. The change in burst frequency after 5-HT application is presented separately for females (B) and males (C). These data are reported as a percentage change from baseline (pre-5-HT). Values are expressed as means ± SEM based on litter averages. These results were obtained from a total of 88 pups (49 controls and 39 stress) originating from 14 litters (9 controls and 5 stress). In each panel, the numbers within the bars indicate the number of litters sampled in each group; the numbers below (in parentheses) indicate the total number of pups used in this group. *p < 0.05, statistically different from P0 (<18 h old). &p < 0.05, statistically different from corresponding aCSF (untreated) value.
Figure 10.
A, Effects of acute administration of the selective 5-HT1A agonist 8-OH-DPAT (0.05 mg/kg, i.p.) on apnea frequency in developing newborn pups (P0–P4). The effects are compared between pups born to control dams (gray bars) versus pups born to dams subjected to GS (black bars). Measurements were taken 60 min after the injection. The physiological consequences of this treatment were evaluated on P4 with pulse oxymetry to measuring the proportion of apneas with desaturations (B) or bradycardias (C). Because sex-specific effects were significant, males and females are presented separately. Values obtained after saline injection (sham) are reported with square symbols for comparison. Results are expressed as means ± SEM; means are based on litter averages. These data were obtained from a total of 184 pups (89 controls and 95 stress) originating from 27 litters (14 controls and 13 stress). The numbers within the bars (A, C) indicate the number of litters sampled in each group; the numbers below (in parentheses) indicate the total number of pups used in this group. *p < 0.05, statistically different from P0 (<18 h old). †p < 0.05, statistically different from corresponding control value. &p < 0.05, statistically different from corresponding saline value.
Discussion
Maternal socioeconomic status, malnutrition, and substance abuse interfere with brain development and are established risk factors for respiratory disorders and SIDS in infants (Darnall et al., 2006; Calhoun et al., 2010; Duncan et al., 2010). However, the nature, diversity, and complex interactions among these stressful situations for the gestating mother have made it difficult to identify a mechanism accounting for the deleterious consequences of poor fetal conditions on breathing at birth. Here, we demonstrate that GS alone is sufficient to disrupt respiratory regulation and promote apneic events with significant physiological consequences in rat pups; many of these effects are more important in males. We therefore propose that repeated activation of the neuroendocrine response to stress in the gestating mother is a key factor in the etiology of respiratory disorders in newborn.
Mechanisms by which GS promotes respiratory instability and apneas in newborn pups
Compared with humans, the CNS of pups born at term is immature (Clancy et al., 2001). This relative immaturity, combined with incomplete chemoreflex maturation at birth, make the newborn rat an excellent model to investigate respiratory control development and the factors influencing its trajectory. The progressive decline in both apnea frequency (in vivo) and rhythm variability (in vitro) observed after birth illustrate the maturation of the respiratory control system in this species. The fact that both variables were consistently greater in pups born to GS dams demonstrates that development of the neural circuits that regulate breathing is vulnerable to the hormonal changes induced by our stress protocol. The correlations between maternal corticosterone and apnea frequency at P4 support this interpretation. Furthermore, data showing that, at P4, the proportion of apneas with O2 desaturations is greater in GS pups than controls indicate that GS also affects cardio-respiratory coupling and that the physiological consequences of apneas are more important in stressed pups. The diverse experimental approaches used provide insight into the mechanisms contributing to this pathological phenotype.
The HVR increases during early life (Rigatto, 1992; Bissonnette, 2000). This maturation was observed in our experiments; however, the HVR of GS pups was lower than controls (especially at P0 and P2). This result is significant because O2 responsiveness is an important defense system against hypoxia and disruption of this reflex by GS may be threatening to the newborn (Leiter and Böhm, 2007; Kinney, 2009). Although it is interesting that this effect of GS is similar to the effects of prenatal nicotine (Huang et al., 2010) and cocaine (Moss et al., 1995; Lipton et al., 1996), it is unlikely that the reduced HVR alone explains the higher apnea frequency that characterized GS pups. In rat pups, the incidence of apneas correlates positively with the magnitude of the HVR (Julien et al., 2008). In preterm infants, an augmented (rather than reduced) O2 chemoreflex, as indicated by the rapid change in breathing that occurs during exposure to moderate hypoxia or hyperoxia, exacerbates periodic breathing and promotes apneas (Al-Matary et al., 2004; Gauda et al., 2004). Conversely, a reduced ventilatory response to CO2 contributes to respiratory disorders in the newborn, such as apnea of prematurity (Katz-Salamon, 2004; Gaultier and Gallego, 2005), and is a risk factor for SIDS (Gauda et al., 2004; Kinney et al., 2009). Here, GS augmented the ventilatory response to CO2 observed at P4 in females only. Although limited, this sex-specific effect is consistent with the persistent enhancement of the hypercapnic ventilatory response that emerges after neonatal stress (Genest et al., 2007). However, it does not explain why GS pups (especially males) produced more apneas than controls during normoxia.
In mammals, the rhythmic motor command driving lung ventilation emerges during fetal life. Because the frequency and the stability of the motor output produced by medullary preparations from newborn rodents and fetuses increases progressively with age, immaturity and/or deficiency in rhythmogenic mechanisms has been linked to respiratory instability and apneas in preterm infants (Hilaire and Duron, 1999; Gaultier and Gallego, 2005; Greer et al., 2006). Here, the instability measured in preparations from GS pups was larger than controls, and the frequency measured at P4 was lower in male GS pups than controls. Thus, disruption of development and function of the core networks generating and modulating respiratory rhythm likely contribute to the increased propensity for pathological apneas observed in GS pups. Results from 5-HT-based “treatment” support this interpretation.
Stress-induced 5-HT deficit and respiratory instability in newborn
During gestation, activation of glucocorticoid receptors has age- and region-specific effects on brain monoaminergic systems of pups (Peters, 1982; Muneoka et al., 1997). Administration of dexamethasone to gestating dams augments expression of the presynaptic 5-HT transporter in 14-d-old pups, thus reducing 5-HT levels in the synaptic cleft (Slotkin et al., 1996). Tryptophan hydroxylase regulates 5-HT synthesis, and, in rats, its activity is greatly influenced by corticosterone such that adverse conditions exert region- and stress-specific effects on the central serotonergic system (Chaouloff, 2000). Although acute stress augments tryptophan hydroxylase activity (Azmitia and McEwen, 1974), chronic stress reduces the number of 5-HT neurons and terminals (Kitayama et al., 1989). Moreover, GS persistently reduces tyrosine hydroxylase mRNA expression in noradrenergic neurons in the dorsal pons (Green et al., 2011). Our results demonstrate that GS results in medullary 5-HT and NA deficiency in newborns. Although this effect did not persist beyond birth (P0), transient reduction in medullary monoamines by GS (especially 5-HT) may be sufficient to affect respiratory control development. In addition to its prominent role in autonomic regulation, 5-HT also exerts neurotrophic influence on neural circuit development during the prenatal and postnatal periods (Hodges and Richerson, 2008; Hilaire et al., 2010). Consistent with this crucial role, reduction/elimination of 5-HT and NA neurons in rodents causes important respiratory disorders, including respiratory instability and apneas (Viemari et al., 2005; Hodges et al., 2008; Hilaire et al., 2010). In humans, brainstem 5-HT and NA deficiency are associated with SIDS (Paterson et al., 2009; Duncan et al., 2010). Several risk factors have been evoked to explain these effects, but the nature of the mechanisms leading to monoamine deficiency (and related respiratory disorders) remain unknown (Kinney and Thach, 2009). The present results allow us to propose that elevation of corticosterone (cortisol in humans) during pregnancy contributes to medullary 5-HT and NA deficiency in newborns. Although both monoamines could be important, we chose to focus on the 5-HT system because of the following: (1) 5-HT agonists (especially 5-HT1A) are highly effective in stabilizing breathing in both animals and humans (Richter et al., 2003; Dutschmann et al., 2009; Manzke et al., 2009); (2) 5-HT1A receptors are the most extensively expressed subtype within the central respiratory network (Richter et al., 2003); (3) 5-HT1A receptors are greatly reduced in the brainstem of SIDS victims (Ozawa and Okado, 2002; Kinney et al., 2009; Duncan et al., 2010); and (4) the male/female difference in 5-HT1A receptors may explain the increased risk of SIDS in males versus females (Kinney et al., 2009).
The increase in respiratory rhythm stability following 5-HT bath application (in vitro) along with the reduction of apneas following 8-OH-DPAT injection (in vivo) support an important role for 5-HT. These results and the fact that the effects of 5-HT agents were greater in GS pups indicate that a deficit in medullary 5-HT contributes to respiratory disorders in GS newborn. Considering that the prevalence of many respiratory disorders (including SIDS) is greater in males (Mage and Donner, 2006) and the reduction in 5-HT1A receptor binding observed in SIDS victims is greater in males than females (Kinney et al., 2009), it is interesting that reduction of apneic events with O2 desaturations by 8-OH-DPAT administration was significant only in male pups that experienced GS. In adult rodents, the expression of 5-HT1A receptors is reduced by corticosteroids and augmented by testosterone (Chalmers et al., 1993; Flügge et al., 1998). Stress generally interferes with androgen secretion and GS reduces testosterone levels in male offspring (Weisz and Ward, 1980; Kapoor and Matthews, 2005). The endocrine profile observed in 4 d old pups is consistent with these reports; the sex- and stress specific cardio-respiratory responses to 8-OH-DPAT injection further support the notion that the balance between glucocorticoids and androgen is a key determinant of the behaviors modulated by 5-HT1A receptors.
Perspectives
Factors underlying respiratory disorders of newborns are numerous. Unlike research in human subjects, the rat allows better control over the nature, intensity, and duration of the stress paradigm while eliminating confounding factors such as maternal life style. Though the increase in corticosterone induced by our stress paradigm was relatively modest, repeated exposure to this protocol was sufficient to augment apneic events and respiratory instability in newborn rats. Non-invasive measurement of SpO2 and heart rate at P4 showed that these apneas are physiologically significant and with time, could compromise brain development (Baird, 2004; Talge et al., 2007; Zhao et al., 2011). Animal models have inherent limitations. Stress has broad effects on the mother and the pups and the deleterious consequences of chronic corticosterone elevation on development were not limited to breathing in pups. Nevertheless, the significant impact that GS exerts on the development of respiratory control still raises important questions and point to new factors in the etiology of respiratory disorders of newborns. Such novel findings are essential to the development of prevention strategies and interventions. In that regard, the efficiency of 5-HT agonists in alleviating instability and apneas suggest that these agents may have therapeutic potential in this vulnerable population.
Footnotes
This research was supported by Canadian Institutes of Health Research Grants MOP 119337 (R.K.) and MOP 119272 (A.B.), the Canada Research Chair in Respiratory Neurobiology (R.K.), and the Foundation of Stars. We thank Hélène Crépeau (consultant in statistics, Department of Mathematics, Université Laval, Québec City, Québec, Canada) and Mélanie Pelletier and Sylvie Viger (animal care specialists).
The authors declare no competing financial interests.
References
- Al-Matary A, Kutbi I, Qurashi M, Khalil M, Alvaro R, Kwiatkowski K, Cates D, Rigatto H. Increased peripheral chemoreceptor activity may be critical in destabilizing breathing in neonates. Semin Perinatol. 2004;28:264–272. doi: 10.1053/j.semperi.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Jr, McEwen BS. Adrenalcortical influence on rat brain tryptophan hydroxylase activity. Brain Res. 1974;78:291–302. doi: 10.1016/0006-8993(74)90553-8. [DOI] [PubMed] [Google Scholar]
- Bairam A, Niane LM, Joseph V. Role of ATP and adenosine on carotid body function during development. Respir Physiol Neurobiol. 2012 doi: 10.1016/j.resp.2012.06.016. Advance online publication. Retrieved November 28, 2012. [DOI] [PubMed] [Google Scholar]
- Baird TM. Clinical correlates, natural history and outcome of neonatal apnoea. Semin Neonatol. 2004;9:205–211. doi: 10.1016/j.siny.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1391–R1400. doi: 10.1152/ajpregu.2000.278.6.R1391. [DOI] [PubMed] [Google Scholar]
- Calhoun SL, Vgontzas AN, Mayes SD, Tsaoussoglou M, Sauder K, Mahr F, Karippot A, Wisner K, Bixler EO. Prenatal and perinatal complications: is it the link between race and SES and childhood sleep disordered breathing? J Clin Sleep Med. 2010;6:264–269. [PMC free article] [PubMed] [Google Scholar]
- Chalmers DT, Kwak SP, Mansour A, Akil H, Watson SJ. Corticosteroids regulate brain hippocampal 5-HT1A receptor mRNA expression. J Neurosci. 1993;13:914–923. doi: 10.1523/JNEUROSCI.13-03-00914.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramohan Y, Droste SK, Reul JM. Novelty stress induces phospho-acetylation of histone H3 in rat dentate gyrus granule neurons through coincident signalling via the N-methyl-d-aspartate receptor and the glucocorticoid receptor: relevance for c-fos induction. J Neurochem. 2007;101:815–828. doi: 10.1111/j.1471-4159.2006.04396.x. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Serotonin, stress and corticoids. J Psychopharmacol. 2000;14:139–151. doi: 10.1177/026988110001400203. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Damianopoulos EN, Carey RJ. Evidence for N-methyl–aspartate receptor mediation of cocaine induced corticosterone release and cocaine conditioned stimulant effects. Behav Brain Res. 1995;68:219–228. doi: 10.1016/0166-4328(94)00175-f. [DOI] [PubMed] [Google Scholar]
- Darnall RA, Ariagno RL, Kinney HC. The late preterm infant and the control of breathing, sleep, and brainstem development: a review. Clin Perinatol. 2006;33:883–914. doi: 10.1016/j.clp.2006.10.004. abstract x. [DOI] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Waki H, Manzke T, Simms AE, Pickering AE, Richter DW, Paton JFR. The potency of different serotonergic agonists in counteracting opioid evoked cardiorespiratory disturbances. Philos Trans R Soc B Biol Sci. 2009;364:2611–2623. doi: 10.1098/rstb.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenwald EC, Zupancic JA, Mao WY, Richardson DK, McCormick MC, Escobar GJ. Variation in diagnosis of apnea in moderately preterm infants predicts length of stay. Pediatrics. 2011;127:e53–e58. doi: 10.1542/peds.2010-0495. [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T. 2,3,5-Trimethyl-3-thiazoline (TMT), a component of fox odor—just repugnant or really fear-inducing? Neurosci Biobehav Rev. 2008;32:1259–1266. doi: 10.1016/j.neubiorev.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M. Cortisol: the culprit prenatal stress variable. Int J Neurosci. 2008;118:1181. doi: 10.1080/00207450701820944. [DOI] [PubMed] [Google Scholar]
- Finer NN, Higgins R, Kattwinkel J, Martin RJ. Summary proceedings from the apnea-of-prematurity group. Pediatrics. 2006;117:S47–S51. doi: 10.1542/peds.2005-0620H. [DOI] [PubMed] [Google Scholar]
- Flügge G, Kramer M, Rensing S, Fuchs E. 5HT1A-receptors and behaviour under chronic stress: selective counteraction by testosterone. Eur J Neurosci. 1998;10:2685–2693. [PubMed] [Google Scholar]
- Fournier S, Allard M, Gulemetova R, Joseph V, Kinkead R. Chronic corticosterone elevation and sex-specific augmentation of the hypoxic ventilatory response in awake rats. J Physiol. 2007;584:951–962. doi: 10.1113/jphysiol.2007.141655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier S, Joseph V, Kinkead R. Influence of juvenile housing conditions on the ventilatory, thermoregulatory, and endocrine responses to hypoxia of adult male rats. J Appl Physiol. 2011;111:516–523. doi: 10.1152/japplphysiol.00370.2011. [DOI] [PubMed] [Google Scholar]
- Gauda EB, McLemore GL, Tolosa J, Marston-Nelson J, Kwak D. Maturation of peripheral arterial chemoreceptors in relation to neonatal apnoea. Semin Neonatol. 2004;9:181–194. doi: 10.1016/j.siny.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Gaultier C, Gallego J. Development of respiratory control: evolving concepts and perspectives. Respir Physiol Neurobiol. 2005;149:3–15. doi: 10.1016/j.resp.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Genest SE, Gulemetova R, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation induces sex-specific augmentation of the hypercapnic ventilatory response in awake rat. J Appl Physiol. 2007;102:1416–1421. doi: 10.1152/japplphysiol.00454.2006. [DOI] [PubMed] [Google Scholar]
- Green MK, Rani CS, Joshi A, Soto-Piña AE, Martinez PA, Frazer A, Strong R, Morilak DA. Prenatal stress induces long term stress vulnerability, compromising stress response systems in the brain and impairing extinction of conditioned fear after adult stress. Neuroscience. 2011;192:438–451. doi: 10.1016/j.neuroscience.2011.06.041. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Funk GD, Ballanyi K. Preparing for the first breath: prenatal maturation of respiratory neural control. J Physiol. 2006;570:437–444. doi: 10.1113/jphysiol.2005.097238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulemetova R, Kinkead R. Neonatal stress increases respiratory instability in rat pups. Respir Physiol Neurobiol. 2011;176:103–109. doi: 10.1016/j.resp.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Han F, Subramanian S, Price ER, Nadeau J, Strohl KP. Periodic breathing in the mouse. J Appl Physiol. 2002;92:1133–1140. doi: 10.1152/japplphysiol.00785.2001. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Duron B. Maturation of the mammalian respiratory system. Physiol Rev. 1999;79:325–360. doi: 10.1152/physrev.1999.79.2.325. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Voituron N, Menuet C, Ichiyama RM, Subramanian HH, Dutschmann M. The role of serotonin in respiratory function and dysfunction. Respir Physiol Neurobiol. 2010;174:76–88. doi: 10.1016/j.resp.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008;164:222–232. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Brown AR, Cross SJ, Cruz J, Rice A, Jaiswal S, Fregosi RF. Influence of prenatal nicotine exposure on development of the ventilatory response to hypoxia and hypercapnia in neonatal rats. J Appl Physiol. 2010;109:149–158. doi: 10.1152/japplphysiol.01036.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CE, Corwin MJ, Weese-Mayer DE, Davidson Ward SL, Ramanathan R, Lister G, Tinsley LR, Heeren T, Rybin D Collaborative Home Infant Monitoring Evaluation (CHIME) Study Group. Longitudinal assessment of hemoglobin oxygen saturation in preterm and term infants in the first six months of life. J Pediatrics. 2011;159:377–383.e1. doi: 10.1016/j.jpeds.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien C, Bairam A, Joseph V. Chronic intermittent hypoxia reduces ventilatory long-term facilitation and enhances apnea frequency in newborn rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1356–R1366. doi: 10.1152/ajpregu.00884.2007. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG. Short periods of prenatal stress affect growth, behaviour and hypothalamo–pituitary–adrenal axis activity in male guinea pig offspring. J Physiol. 2005;566:967–977. doi: 10.1113/jphysiol.2005.090191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz-Salamon M. Delayed chemoreceptor responses in infants with apnoea. Arch Dis Child. 2004;89:261–266. doi: 10.1136/adc.2003.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khemiri H, Seaborn T, Gestreau C, Soliz J. Erythropoietin and its antagonist regulate hypoxic fictive breathing in newborn mice. Respir Physiol Neurobiol. 2012;183:115–121. doi: 10.1016/j.resp.2012.05.027. [DOI] [PubMed] [Google Scholar]
- Kinney DK, Munir KM, Crowley DJ, Miller AM. Prenatal stress and risk for autism. Neurosci Biobehav Rev. 2008;32:1519–1532. doi: 10.1016/j.neubiorev.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC. Brainstem mechanisms underlying the sudden infant death syndrome: evidence from human pathologic studies. Dev Psychobiol. 2009;51:223–233. doi: 10.1002/dev.20367. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol. 2009;4:517–550. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama I, Cintra A, Janson AM, Fuxe K, Agnati LF, Eneroth P, Aronsson M, Harfstrand A, Steinbush HW, Visser TJ, Goldstein M, Vale W, Gustafsson JA. Chronic immobilization stress: evidence for decreases of 5-hydroxy-tryptamine immunoreactivity and for increases of glucocorticoid receptor immunoreactivity in various brain regions of the male rat. Neural Transm. 1989;77:93–130. doi: 10.1007/BF01248925. [DOI] [PubMed] [Google Scholar]
- Leiter JC, Böhm I. Mechanisms of pathogenesis in the sudden infant death syndrome. Respir Physiol Neurobiol. 2007;159:127–138. doi: 10.1016/j.resp.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Lipton JW, Davidson TL, Carvey PM, Weese-Mayer DE. Prenatal cocaine: effect on hypoxic ventilatory responsiveness in neonatal rats. Respir Physiol. 1996;106:161–169. doi: 10.1016/s0034-5687(96)00075-8. [DOI] [PubMed] [Google Scholar]
- Mage DT, Donner M. Female resistance to hypoxia: does it explain the sex difference in mortality rates? J Womens Health (Larchmt) 2006;15:786–794. doi: 10.1089/jwh.2006.15.786. [DOI] [PubMed] [Google Scholar]
- Manzke T, Dutschmann M, Schlaf G, Mörschel M, Koch UR, Ponimaskin E, Bidon O, Lalley PM, Richter DW. Serotonin targets inhibitory synapses to induce modulation of network functions. Philos Trans R Soc B Biol Sci. 2009;364:2589–2602. doi: 10.1098/rstb.2009.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon G, Bairam A, Kinkead R. Long-term consequences of neonatal caffeine on ventilation, occurrence of apneas, and hypercapnic chemoreflex in male and female rats. Pediatr Res. 2006;59:519–524. doi: 10.1203/01.pdr.0000203105.63246.8a. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Dotta A. Effects of hypoxia and ambient temperature on gaseous metabolism of newborn rats. Am J Physiol. 1992;263:R267–R272. doi: 10.1152/ajpregu.1992.263.2.R267. [DOI] [PubMed] [Google Scholar]
- Moss IR, Laferrière A, Faltus RE. Prenatal cocaine alters diaphragmatic EMG responses to hypoxia in developing swine. Am J Respir Crit Care Med. 1995;152:1961–1966. doi: 10.1164/ajrccm.152.6.8520763. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Mikuni M, Ogawa T, Kitera K, Kamei K, Takigawa M, Takahashi K. Prenatal dexamethasone exposure alters brain monoamine metabolism and adrenocortical response in rat offspring. Am J Physiol. 1997;273:R1669–R1675. doi: 10.1152/ajpregu.1997.273.5.R1669. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Okado N. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics. 2002;33:142–149. doi: 10.1055/s-2002-33678. [DOI] [PubMed] [Google Scholar]
- Papaioannou A, Dafni U, Alikaridis F, Bolaris S, Stylianopoulou F. Effects of neonatal handling on basal and stress-induced monoamine levels in the male and female rat brain. Neuroscience. 2002;114:195–206. doi: 10.1016/s0306-4522(02)00129-x. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Hilaire G, Weese-Mayer DE. Medullary serotonin defects and respiratory dysfunction in sudden infant death syndrome. Respir Physiol Neurobiol. 2009;168:133–143. doi: 10.1016/j.resp.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters DA. Prenatal stress: effects on brain biogenic amine and plasma corticosterone levels. Pharmacol Biochem Behav. 1982;17:721–725. doi: 10.1016/0091-3057(82)90353-7. [DOI] [PubMed] [Google Scholar]
- Poets CF. Apnea of prematurity: what can observational studies tell us about pathophysiology? Sleep Medicine. 2010;11:701–707. doi: 10.1016/j.sleep.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Ponirakis A, Susman EJ, Stifter CA. Negative emotionality and cortisol during adolescent pregnancy and its effects on infant health and autonomic nervous system reactivity. Dev Psychobiol. 1998;33:163–174. [PubMed] [Google Scholar]
- Ramanathan R, Corwin MJ, Hunt CE, Lister G, Tinsley LR, Baird T, Silvestri JM, Crowell DH, Hufford D, Martin RJ, Neuman MR, Weese-Mayer DE, Cupples LA, Peucker M, Willinger M, Keens TG Group fTCHIMES. Cardiorespiratory events recorded on home monitors. JAMA. 2001;285:2199–2207. doi: 10.1001/jama.285.17.2199. [DOI] [PubMed] [Google Scholar]
- Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med. 2003;9:542–548. doi: 10.1016/j.molmed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Rigatto H. Maturation of breathing control in the fetus and newborn infant. In: Beckerman RC, Brouilette RT, Hunt CE, editors. Respiratory control disorders in infants and children. Baltimore: Williams and Wilkins; 1992. pp. 61–75. [Google Scholar]
- Ruangkittisakul A, Secchia L, Bornes TD, Palathinkal DM, Ballanyi K. Dependence on extracellular Ca2+/K+ antagonism of inspiratory centre rhythms in slices and en bloc preparations of newborn rat brainstem. J Physiol. 2007;584:489–508. doi: 10.1113/jphysiol.2007.142760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetta M, Mortola JP. Interaction of hypoxic and hypercapnic stimuli on breathing pattern in the newborn rat. J Appl Physiol. 1987;62:506–512. doi: 10.1152/jappl.1987.62.2.506. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Barnes GA, McCook EC, Seidler FJ. Programming of brainstem serotonin transporter development by prenatal glucocorticoids. Dev Brain Res. 1996;93:155–161. doi: 10.1016/0165-3806(96)00027-2. [DOI] [PubMed] [Google Scholar]
- Stanulis ED, Matulka RA, Jordan SD, Rosecrans JA, Holsapple MP. Role of corticosterone in the enhancement of the antibody response after acute cocaine administration. J Pharmacol Exp Ther. 1997;280:284–291. [PubMed] [Google Scholar]
- Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez DM, López JF, Van Hoers H, Watson SJ, Levine S. Maternal deprivation regulates serotonin 1A and 2A receptors in the infant rat. Brain Res. 2000;855:76–82. doi: 10.1016/s0006-8993(99)02307-0. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Peña F, Zanella S, Bévengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voituron N, Shvarev Y, Menuet C, Bevengut M, Fasano C, Vigneault E, El Mestikawy S, Hilaire G. Fluoxetine treatment abolishes the in vitro respiratory response to acidosis in neonatal mice. PLoS One. 2010;5:e13644. doi: 10.1371/journal.pone.0013644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright PE, Leatherdale ST, Dubin JA. Advantages of mixed effects models over traditional ANOVA models in developmental studies: a worked example in a mouse model of fetal alcohol syndrome. Dev Psychobiol. 2007;49:664–674. doi: 10.1002/dev.20245. [DOI] [PubMed] [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- Zanella S, Watrin F, Mebarek S, Marly F, Roussel M, Gire C, Diene G, Tauber M, Muscatelli F, Hilaire G. Necdin plays a role in the serotonergic modulation of the mouse respiratory network: implication for Prader-Willi syndrome. J Neurosci. 2008;28:1745–1755. doi: 10.1523/JNEUROSCI.4334-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Gonzalez F, Mu D. Apnea of prematurity: from cause to treatment. Eur J Pediatr. 2011;170:1097–1105. doi: 10.1007/s00431-011-1409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]