Abstract
Affective cognitive control capacity (e.g., the ability to regulate emotions or manipulate emotional material in the service of task goals) is associated with professional and interpersonal success. Impoverished affective control, by contrast, characterizes many neuropsychiatric disorders. Insights from neuroscience indicate that affective cognitive control relies on the same frontoparietal neural circuitry as working memory (WM) tasks, which suggests that systematic WM training, performed in an emotional context, has the potential to augment affective control. Here we show, using behavioral and fMRI measures, that 20 d of training on a novel emotional WM protocol successfully enhanced the efficiency of this frontoparietal demand network. Critically, compared with placebo training, emotional WM training also accrued transfer benefits to a “gold standard” measure of affective cognitive control–emotion regulation. These emotion regulation gains were associated with greater activity in the targeted frontoparietal demand network along with other brain regions implicated in affective control, notably the subgenual anterior cingulate cortex. The results have important implications for the utility of WM training in clinical, prevention, and occupational settings.
Introduction
Humans vary enormously in their ability to keep a cool head in emotionally charged situations. The capacity to remain goal-focused when selecting and executing behavioral plans in the face of affectively salient distraction is a marker of success across the spectrum of human endeavor, from vocational and academic achievement to well-being (Barrett et al., 2004; Gray, 2004). Furthermore, deficits in this capacity for affective cognitive control characterize a wide range of neuropsychiatric conditions (Beck, 2008). Hitherto, efforts to improve affective cognitive control have been the preserve of extensive and costly psychotherapeutic, psychopharmacological, and invasive neurosurgical interventions (e.g., deep brain stimulation), usually restricted to neuropsychiatric samples (Harmer et al., 2003; Mayberg et al., 2005; DeRubeis et al., 2008). However, recent advances in cognitive neuroscience suggest that enhancement of affective cognitive control may be achievable via targeted computerized training that uses far fewer human and financial resources and that can be made widely available to the broader population via the Internet.
The key to this potential stems from research indicating that affective cognitive control relies to a great extent on the same frontoparietal (compare with multiple-demand network; Duncan, 2010) neural circuitry, including the dorsolateral prefrontal cortex (PFC), the inferior parietal and the anterior cingulate cortices (Banich et al., 2009), which is fundamentally involved in the performance of well-established working memory (WM) tasks (Miller, 2000; Brass et al., 2005; Owen et al., 2005). A primary role of this affective control network, which includes classical emotion regulation (ER) regions (e.g., BA25; Wager et al., 2008) in addition to the traditional frontoparietal multiple demand network, is to exert downregulatory effects on experienced emotional distress through projections to the amygdala and midbrain nuclei from the lateral and medial PFC components, including the dorsal and subgenual anterior cingulate (sgACC) (Ochsner and Gross, 2005; Wager et al., 2008; Etkin et al., 2011). Notably, these control-related regions are hypoactivated in neuropsychiatric disorders characterized by impoverished affective cognitive control (Price and Drevets, 2012). These insights from neuroscience lead to a clear hypothesis that repetitive WM training designed to augment the performance of this frontoparietal demand network has the potential to generate transferable gains in affective cognitive control, which are mediated by this shared underlying neural circuitry, especially when the WM training uses emotionally salient stimulus material.
In a preliminary behavioral test of this hypothesis (Schweizer et al., 2011), we showed that only emotional WM training (eWM; and not standard WM training) accrued transferable benefits to a laboratory measure of affective attentional processing, the emotional Stroop task. The specificity of these training gains to the affective domain can be accounted for by evidence showing that WM capacity and the capacity to use WM successfully in emotional contexts are partly separable cognitive abilities (Joormann et al., 2011; Schweizer and Dalgleish, 2011). We therefore propose that, while WM itself is a largely stable construct (Toga and Thompson, 2005; Craik and Bialystok, 2006; Shipstead et al., 2012), the ability to successfully deploy WM in emotional contexts (eWM) may be more plastic and amenable to training (however, for reviews on the promising effects of standard WM training on cognitive performance augmentation, see Buschkuehl et al., 2011; Morrison and Chein, 2011).
Here, we predicted that eWM would lead to transferable improvement on an ecologically valid measure of affective cognitive control-ER (Gross, 2002). As successful ER is dependent on the frontoparietal demand network outlined above, we hypothesized that the predicted transferrable benefits in ER accruing from eWM training would be mediated by training-related changes in frontoparietal activity. It should be noted, however, that the current study does not explicitly test whether eWM is more (or less) effective in improving ER capacity compared with other nonaffective types of WM training.
Materials and Methods
Participants
Thirty-four participants (20 women, age 23 ± 2.4 years, mean ± SD) were recruited through a University of Cambridge student bulletin and the Medical Research Council Cognition and Brain Sciences Unit community volunteer panel and randomly assigned to either placebo training or eWM training. Two participants who were randomized to the placebo training did not complete any training sessions after the pre-training assessment and were therefore excluded from the study. The placebo training (n = 15) and eWM training (n = 17) groups did not differ in age, gender, or education (F < 1).
Procedure
At the start of the study, all participants completed an individual pre-training assessment that comprised behavioral and neuroimaging assessments. Participants first provided the experimenter with written informed consent. They then completed the offline version of the eWM and placebo training tasks in a quiet testing room. After these tasks, participants completed the scan version of the eWM training and the ER task (described below) in the fMRI scanner. Participants then left the laboratory to complete their 20 d of training (for a detailed schedule, see task descriptions below). All participants in both groups completed more than the required 80% (16 d) of the training days. Indeed, all participants in both training groups completed 90% (18 d) of the training, and 65% and 47% of the eWM training group completed 19 and 20 d, respectively. In the placebo training group, compliance was even higher, with 67% completing 19 d and 53% completing 20 d of training. On the first or second day after training had been completed, participants returned to the laboratory for post-training behavioral and fMRI assessments, which were identical to the pre-training assessments. Participants were compensated with £10 per hour for the pre-training and post-training scanning sessions and £4.50 per training day. The two training groups received the same amount of compensation.
Tasks
eWM training.
The eWM training was based on the protocol described by Schweizer et al. (2011) and comprised an affective dual n-back task consisting of a series of trials each of which simultaneously presented a face (for 500 ms) on a 4 × 4 grid on a monitor and a word (for 500–950 ms) over headphones (Fig. 1A). Each picture-word pair was followed by a 2500 ms interval during which participants responded via button press if either/both stimuli from the pair matched the corresponding stimuli presented n positions back; 60% of the words (e.g., evil, rape) and faces (fearful, angry, sad, or disgusted expressions) were emotionally negative with the others affectively neutral in tone. Trial presentation order was randomized across training sessions.
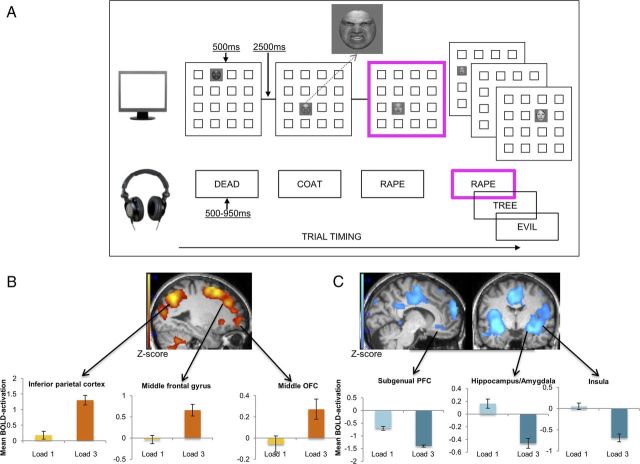
Figure 1.
A, Task design of the eWM training (dual n-back) task for a sample training block where n-back = 1. Stimuli with a bold pink border represent target stimuli for the current block. Participants respond with a button press if the target stimulus in either or both modalities matches the stimulus n positions back. In this n-back = 1 example, there is a match because, for the visuospatial modality, the current face appears in the same location as the face 1-position back; and for the auditory target, the word (RAPE) is the same as the word one-back. B, C, Task-demand-related BOLD activation that was observed comparing conditions of lower task-demand (n-back = 1) and higher task-demand (n-back = 3) at pre-training. All reported BOLD activation was significantly different across these conditions at the whole-brain level, with significance levels corrected for false discovery rates at PFDR < 0.05. Activation increases (B) and activation decreases (C) in condition n-back = 3 compared with n-back = 1. For a full overview of differential activation, see Table 1. Error bars indicate SEM.
The 20 daily 20–30 min (depending on level of n-back achieved) training sessions consisted of 20 blocks of 20+ n trials. Ten trials in each block were “target trials” (six target trials per modality: visual; auditory, with two trials presenting both a visual and auditory target) presenting stimuli that matched the stimuli n positions back. If a target was detected correctly, a single, long, high-pitch tone was heard; incorrect (false hit or missed target) trials were followed by two short, low-pitch tones. Training started at n = 1; if 3+ consecutive trials were completed accurately, the level of n-back increased by one on the next block. Conversely, if five or more successive trials were completed inaccurately, the level of n-back decreased by 1 on the next block. Participants therefore continuously operated at their maximum performance level.
The task was modified for presentation in the MRI scanner at pre-training and post-training. In this scanner version there were 40 blocks consisting of 10+ n trials. Blocks were divided across emotional and neutral stimuli (alternated presentation from block to block) and varied quasi-randomly (never two consecutive blocks at same n-back) across levels of n-back, with n = 1, 2, 3, or 5. Each block was followed by a 20 s rest period resulting from the highly demanding nature of the task. Finally, unlike the training version, there was no feedback on performance during the scanner version of the task.
The performance-sensitive offline version of the task, which was administered at the pre-training and post-training assessments, started at n-back = 1 and presented two blocks of 20+ n trials at each consecutive level of n-back until participants failed both blocks at a given level.
Placebo training task.
The placebo training was identical to that described by Schweizer et al. (2011). It comprised a feature match task where each trial displayed two panels with 8–12 shapes in each panel. Participants indicated whether the panels were identical. This task makes minimal demands on WM resources. The training schedule was identical to that of the eWM training (i.e., 20 d of 20 min training sessions).
ER task.
The ER task was similar in format to tasks widely reported in the literature on affective cognitive control (Goldin et al., 2005). The ER task was presented in the MRI scanner at pre-training and post-training. Participants watched sets of 30 s film footage in three experimental conditions (10 films per condition): (1) Neutral: participants viewed affectively neutral film footage (e.g., a weather forecast) to which they were instructed to simply pay attention; (2) Attend: in this condition, emotionally aversive films (e.g., documentary footage of war scenes, accidents, terrorist atrocities, famine) were presented during which participants had to attend without attempting to regulate their emotions; and (3) Regulate: in this condition, participants were again presented with aversive film clips, but this time instructed to cognitively downregulate (Gross, 2002) their emotional distress as much as possible. The aversive films were all matched on emotionality based on independent ratings by assessors blind to experimental condition. The aversive clips were then separately randomized across the attend and regulate conditions and across pre-training and post-training sessions for each participant. The neutral films were all rated as emotionally neutral in tone and randomized across pre-and post-training for each participant. Each experimental condition was presented twice in the scanner in a blocked design with five trials in each block. A 45 s washout clip depicting emotionally calming footage followed negative blocks to normalize affect in preparation for the next trial. After each film clip, participants rated their experienced distress while viewing the film on a Likert scale ranging from 1 (extremely positive) to 10 (extremely negative), with 5 (neutral).
Neuroimaging
Image acquisition.
A 3T Siemens Tim Trio MRI scanner was used to collect 1200 (scanner version of the dual n-back task) and 745 (ER task) echoplanar imaging (EPI) volumes. All EPI data had 32 slices, matrix size of 64 × 64, echo time (TE) of 30 ms, repetition time (TR) of 2 s, field of view of 19.2×19.2 cm, flip angle of 78°, slice thickness of 3 mm, interslice distance of 0.75 mm, and in-plane resolution of 3 × 3 mm. High-resolution magnetization-prepared rapid-acquisition gradient echo anatomical images (TR of 2250 ms, TE of 2.99 ms, flip angle of 9°, inversion time of 900 ms, 256×240×192 isotropic 1 mm voxels) were collected for anatomic localization and coregistration. The total time each participant spent in the scanner was 79 min.
Imaging analyses.
SPM5 was used for data analysis (SPM5; Wellcome Department of Imaging Neuroscience, London, United Kingdom). Images were sinc-interpolated in time to correct for acquisition time differences and realigned spatially with respect to the first image using trilinear interpolation. The coregistered magnetization-prepared rapid-acquisition gradient echo image was segmented and normalized using affine and smoothly nonlinear transformations to the T1 template in Montreal Neurological Institute space. The normalization parameters were then applied to the EPIs, and all normalized EPI images were spatially smoothed with a Gaussian kernel of full-width half-maximum 8 mm.
For each participant, each session (pre-training, post-training) and each event type were modeled separately. Events modeled for the dual n-back task were as follows: emotional block at load 1, emotional block at load 2, emotional block at load 3, emotional block at load 5, neutral block at load 1, neutral block at load 2, neutral block at load 3, and neutral block at load 5. For the ER task, they were as follows: Neutral; Attend; Regulate; washout trials and button press response. Each event was modeled by using a regressor made from an on–off boxcar convolved with a canonical hemodynamic response function. Six estimated parameters of movement between scans (translation and rotation along x-, y-, and z-axes) were entered as covariates of no interest. Before running the model, the time course of the average brain signal was screened for spikes of high variance. Short periods of high variance are usually associated with brief subject movements as shown in the spatial realignment parameters. The high-variance scans were removed from the model by using a modified version of the SPM99 modeling routines (www.mrc-cbu.cam.ac.uk/Imaging/Common/missing_time.shtml). Low-frequency noise was removed with a standard high-pass filter of 120 s. The results estimated from single subject models were entered into second-level random effects analyses for standard SPM group inference (Penny et al., 2003).
All effects were investigated at the whole-brain level with a significance level of Puncorrected < 0.001, with a minimum cluster size of five voxels (where cluster definition required a more stringent level of correction we applied a significance level corrected for false detection rates PFDR < 0.05, this is reported where applicable). In addition to these whole-brain analyses, we specified frontoparietal (for the eWM and ER task) and emotion processing (for the ER task only) regions of interest (ROI) to enable neural hypothesis-driven analyses. However, if the whole-brain analyses revealed activation in regions congruent with our ROI, for simplicity we report only the whole-brain results.
Our a priori ROI for the eWM task were selected based on the literature identifying regions implicated in WM, affective and cognitive control (Miller, 2000; Owen et al., 2005; Banich et al., 2009; Duncan, 2010). The following ROI were included for the eWM task: (1–3) bilateral lateral PFC (inferior [L: −60:−30/10:48/−2:30; R: 30:66/12:50/−2:30], middle [L: −54:−18/−14:66/−2:64; R: 20:58/−12:64/−2:64], and superior [L: −36:−4/−12:72/−2:80; R: 10:38/−18:72/−2:76] frontal gyrus); (4) medial PFC (medial frontal gyrus [−16:20/16:72/−2:64]); (5) inferior parietal cortex [L: −60:−22/−84:−20/36:60; R: 26:62/−70:−28/38:58]; and (6) ACC [−16:18/−4:54/−10:34]. For the ER task, we examined blood oxygenated level-dependent (BOLD) activation in these same frontoparietal ROI, which are also known to be implicated in the performance of ER tasks within the literature, hence our predicted transfer effects (Wager et al., 2008; Banich et al., 2009). In addition to the executive control regions, we were interested in regions associated with affective control specifically (Banich et al., 2009): (7) orbitofrontal cortex (OFC) [−14:16/22:70/−16:−2]; (8) sgACC, and emotion processing (Dalgleish, 2004; Drevets et al., 2008): (9) amygdala [L: −30:−12/−8:4/−20:−12; R: 18:36/−8:6/−30:−12]; and (10) insula [L: −48:−24/−32:32/−20:22; R: 26:50/−30:32/−20:22]. We extracted average BOLD activation for ROI using MarsBAR AAL anatomical regions (Brett et al., 2002; Tzourio-Mazoyer et al., 2002), with the exception of the sgACC for which there is no anatomical MarsBAR ROI. The ROI for the sgACC was derived from the parameters presented in the reviews by Drevets et al. (2008) and Wager et al. (2008) [−3:3/21:32/−9:−2]. We examined activation in these selected ROI using a conventional level of significance rather than correcting for multiple comparisons. Our reasons for this were twofold: (1) the ROI under consideration are clearly derived from the literature a priori; and (2) averaging across all voxels within an anatomically defined ROI is itself very conservative because included in the average will likely be sizeable clusters of voxels not activated by the relevant contrast. Such averaging already therefore biases toward the null hypothesis, and additional correction for multiple tests would make the significance threshold very stringent indeed (Poldrack, 2007).
Results
Pre-training eWM and ER task performance
At pre-training, mean eWM performance was n-back = 2.80 (SD, 0.09), as measured on the performance-sensitive version of the eWM training task completed outside of the scanner. The task starts at n-back = 1 and presents two blocks of 20+ n trials at each consecutive level of n-back until participants fail both blocks at a given level. The groups differed significantly in pre-training eWM performance with the placebo training (mean, 3.66; SD, 1.14) group performing significantly better compared with the eWM training (mean, 2.59; SD, 0.83) group (F(1,30) = 9.51, p = 0.004).
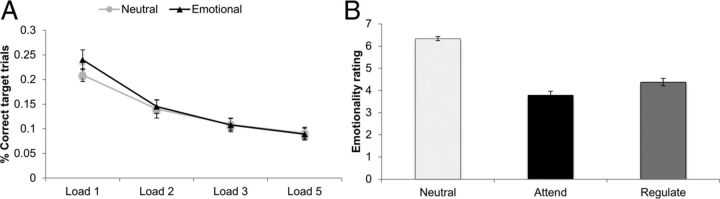
During fMRI, participants completed the adapted version of the eWM training task with fixed levels of n-back (1, 2, 3, and 5), which appeared in random order. Behaviorally, participants showed a linear effect of n-back level on eWM performance (computed as percentage correct trials) in the scanner, with performance deteriorating as a function of increasing levels of n (F(3,29) = 27.17, p < 0.001, η2 = 0.78, Fig. 2A).
Figure 2.
A, The graph represents mean performance accuracy on target trials at the levels of n-back = 1, 2, 3, and 5 in the emotional and neutral blocks during the eWM task in the scanner at pre-training. Error bars indicate SE. B, The graph reports emotionality ratings across ER task conditions at pre-training. Emotionality (experienced distress) while viewing the film clips was rated on a Likert scale ranging from 1 (extremely positive) to 10 (extremely negative), with 5 (neutral). The conditions were as follows: Neutral, neutral film clips were presented with the instruction to attend to the films without effortful ER; Attend, aversive film clips were presented with the instruction to attend to the films without effortful ER; Regulate, aversive film clips were presented with the instruction to downregulate negative emotions elicited by the films. Error bars indicate SEM.
To examine neural activation associated with eWM task-demands at pre-training (Table 1), we contrasted BOLD activation on n-back = 3 trials (in line with the mean peak performance at pre-training of n-back = 2.80) with activation on n-back = 1 trials. Consistent with our first hypothesis, the eWM task reliably activated the targeted frontoparietal circuitry and deactivated a range of brain regions involved in emotion processing, including the bilateral amygdalae, insular cortex and hippocampi, when performing the task under greater eWM load (Fig. 1B,C).
Table 1.
eWM training task-demand-related activation at pre-traininga
| Brain region | L/R | Cluster size (voxels) | Maxima of cluster x/y/z (mm)b | Z |
|---|---|---|---|---|
| Activation increasec | ||||
| Superior frontal gyrus | R | 55 | 24/57/03 | 3.56 |
| Middle frontal gyrus | L | 125 | −30/51/12 | 4.68 |
| R | 1216 | 27/15/51 | 4.80 | |
| R | 1216 | 27/09/60 | 4.74 | |
| Inferior frontal gyrus | L | 43 | −39/21/27 | 3.67 |
| Supplementary motor area/middle cingulate | L | 1216 | −06/18/45 | 4.73 |
| Middle orbitofrontal gyrus | R | 55 | 27/51/−12 | 3.66 |
| Inferior parietal lobe | R | 1671 | 42/−36/42 | 5.45 |
| Precuneus | R | 1671 | −09/−57/51 | 5.10 |
| Inferior temporal gyrus | R | 64 | 51/−57/−09 | 4.33 |
| Middle posterior insula | R | 18 | 30/24/−03 | 3.16 |
| Globus pallidus | L | 13 | −15/00/−03 | 3.10 |
| Caudate | L | 40 | −12/06/12 | 2.96 |
| Cerebellum | L | 266 | −12/−54/−45 | 4.59 |
| L | 266 | −36/−66/−48 | 3.90 | |
| R | 224 | 36/−54/−36 | 4.53 | |
| R | 224 | 33/−63/−51 | 3.89 | |
| R | 14 | 12/−54/−48 | 3.58 | |
| Activation decreased | ||||
| Medial prefrontal cortex (medial superior frontal gyrus) | R | 759 | 09/57/21 | 4.58 |
| R | 13 | 09/33/54 | 2.92 | |
| R | 11 | 12/42/42 | 2.77 | |
| R | 11 | 06/45/48 | 2.53 | |
| Subgenual prefrontal cortex | R | 759 | 00/42/−18 | 4.32 |
| Rolandic operculum | L | 3856 | −48/−24/18 | 6.11 |
| R | 1988 | 51/−21/18 | 5.47 | |
| Precentral gyrus | R | 45 | 21/−27/63 | 3.63 |
| Superior temporal gyrus | R | 97 | 60/−57/24 | 3.35 |
| Temporoparietal junction | L | 142 | −45/−63/24 | 3.63 |
| Middle temporal gyrus | R | 97 | 60/−60/09 | 3.87 |
| R | 97 | 54/−51/06 | 2.87 | |
| Fusiform gyrus | R | 19 | 39/−33/−18 | 3.64 |
| Amygdala/hippocampus | L | 3856 | −27/−06/−15 | 5.86 |
| R | 1988 | 27/−06/−15 | 4.99 | |
| Parahippocampal region | R | 88 | 12/−39/−06 | 3.04 |
| Insula | L | 3856 | −39/−06/−06 | 5.85 |
| R | 1988 | 39/−03/−09 | 4.84 | |
| Thalamus | L | 13 | −12/−24/03 | 2.74 |
| Cuneus | L | 17 | −03/−84/30 | 2.66 |
| R | 8 | 09/−87/36 | 2.67 | |
| Lingual gyrus | L | 8 | −12/−51/−03 | 2.62 |
| R | 88 | 15/−48/−09 | 3.01 | |
| Cerebellum | L | 43 | −30/−81/−36 | 3.85 |
| R | 29 | 33/−78/−36 | 3.20 | |
| R | 88 | 18/−54/−18 | 3.18 |
aRegions that showed differential activation at n-back = 3 compared with n-back = 1 back on the scanner version of the eWM training task at pre-training. Threshold for whole-brain correction at pFDR < 0.05.
bStereotaxic coordinates of peak voxels in MNI space.
ct test contrast: Load 3 > Load 1.
dt test contrast: Load 3 < Load 1.
Pre-training behavioral assessment showed reduced levels of reported distress to negative films on the ER Task in the Regulation condition compared with the Attend condition (F(2,29) = 91.45, p < 0.001, η2 = 0.86; Fig. 2B). Pre-training fMRI revealed that, as intended, downregulating negative emotions on the ER task (a contrast of the Regulate − Attend conditions) recruited regions from the same frontoparietal demand network as the eWM task, including the dorsolateral PFC and inferior parietal cortex. For a detailed overview of all areas activated by the ER task, see Tables 2 and 3.
Table 2.
Regions showing differential activation across ER task conditions at pre-training: main effect of typea
| Brain region | L/R | Cluster size (voxels) | Maxima of cluster x/y/z (mm)b | Z | Differential activation |
|---|---|---|---|---|---|
| Superior medial PFC | R | 34 | 6/60/30 | 3.98 | Attend and Regulate > Neutral |
| Middle temporal pole | R | 21 | 45/18/30 | 3.71 | Attend > Neutral |
| Middle temporal gyrus | L | 46 | −51/−39/−3 | 3.59 | Attend > Neutral |
| −54/−27/−6 | 3.35 | Attend > Neutral | |||
| −48/−21/−15 | 3.23 | Attend > Neutral | |||
| Fusiform gyrus | L | 53 | −30/−66/−6 | 4.23 | Neutral > Attend |
| −24/−81/−6 | 4.17 | Neutral > Attend | |||
| R | 13 | 30/−66/−3 | 3.23 | Neutral > Attend | |
| Cuneus | R | 121 | 9/−93/18 | 4.45 | Attend > Neutral |
| Vermis | L | 64 | −3/−60/−36 | 4.11 | Attend > Neutral |
| 0/−72/−30 | 3.59 | Attend > Neutral |
aRegions that showed differential activation across conditions (F contrast) at the whole-brain level with significance level thresholded at puncorrected < 0.001. Subsequent univariate analyses revealed the specific conditions that showed a significant difference (reported in the last column).
bStereotaxic coordinates of peak voxels in MNI space.
Table 3.
ROIs showing differential activation across ER task conditions at pre-traininga
| Brain region | L/R | Neutral, mean (SD) | Attend, mean (SD) | Regulate | Attend > Neutral, p (η2) | Regulate > Attend, p (η2) |
|---|---|---|---|---|---|---|
| Medial PFC | L | 0.16 (0.35) | 0.27 (0.39) | 0.29 (0.30) | <0.05 (0.17) | |
| R | 0.11 (0.30) | 0.20 (0.25) | 0.21 (0.26) | <0.05 (0.18) | ||
| Lateral PFC (middle frontal) | L | 0.11 (0.27) | 0.17 (0.29) | 0.27 (0.30) | <0.05 (0.13) | |
| R | 0.12 (0.30) | 0.22 (0.34) | 0.38 (0.26) | <0.01 (0.23) | ||
| OFC | L | 0.03 (0.30) | 0.11 (0.31) | 0.14 (0.24) | <0.05 (0.14) | |
| R | 0.10 (0.32) | 0.16 (0.36) | 0.25 (0.31) | <0.05 (0.13) | ||
| Inferior parietal cortex | L | 0.18 (0.36) | 0.25 (0.40) | 0.38 (0.35) | <0.05 (0.15) | |
| R | 0.23 (0.32) | 0.28 (0.35) | 0.35 (0.31) | <0.05 (0.14) |
aBOLD activation in the a priori specified ROIs across the different ER conditions. None of the other ROIs showed a significant effect of condition.
Finally, given our a priori assumption that the neural systems engaged in ER and eWM are overlapping, we conducted a conjunction analysis to confirm this hypothesis before further analyzing the training data. The analysis showed a significant overlap of the frontoparietal regions activated by the ER task and the eWM task at the whole-brain level (Table 4).
Table 4.
Regions conjointly activated by the eWM and ER tasks at pre-traininga
| Brain region | L/R | Cluster size (voxels) | Maxima of cluster x/y/z (mm)b | Z |
|---|---|---|---|---|
| Dorsolateral PFC | L | 82 | −33/45/6 | 5.74 |
| L | 30 | −42/12/39 | 5.36 | |
| −42/21/33 | 5.09 | |||
| L | 5 | −39/39/18 | 4.92 | |
| R | 112 | 36/45/18 | 5.71 | |
| 33/36/27 | 5.32 | |||
| 39/33/36 | 5.00 | |||
| R | 75 | 30/12/54 | 5.62 | |
| Superior frontal gyrus | 21/15/60 | 5.11 | ||
| Middle cingulate cortex | L | 90 | −9/24/33 | 4.98 |
| R | 6/21/36 | 5.37 | ||
| Superior frontal medial gyrus | 3/27/48 | 4.73 | ||
| Inferior parietal | L | 378 | −39/−57/45 | 7.53 |
| −42/−48/39 | 7.26 | |||
| R | 371 | 51/−39/45 | >10 | |
| 39/−54/42 | 7.06 | |||
| Precuneus | L | 91 | −9/−69/45 | 6.41 |
| −9/−66/48 | 6.26 | |||
| Insula | L | 8 | −33/15/6 | 4.82 |
| Rolandic opercelum | R | 45 | 45/15/6 | 5.72 |
| Insula | 39/18/−3 | 5.23 |
aRegions that emerged as being activated by both the eWM training task (Load 3 vs Load 1) and the ER task (Regulate vs Attend) in a conjunction analysis of the two tasks. Threshold for whole-brain correction was set at pFWE < 0.05.
bStereotaxic coordinates of peak voxels in MNI space.
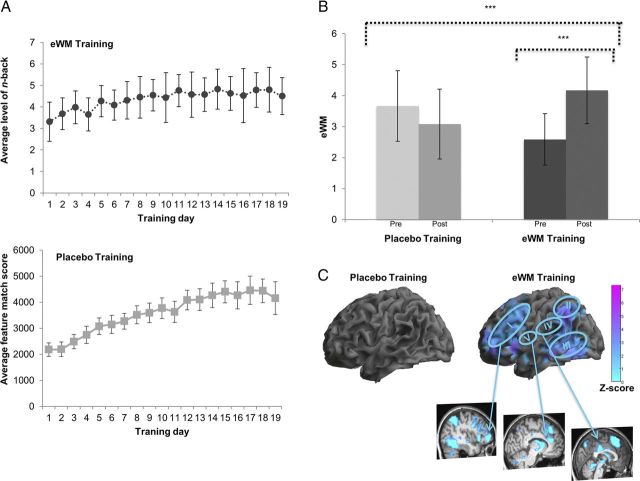
Training-related changes in eWM
Both training groups showed a linear improvement across training on their respective training tasks (Fig. 3A). However, only participants in the eWM (but not placebo) training group showed significant improvements in eWM performance (Fig. 3B). Post-training fMRI (contrasted with activation at pre-training) while performing the eWM task revealed that these behavioral improvements in the eWM training group relative to the placebo group were associated with reduced recruitment of the left inferior parietal cortex (Z = 4.18; −57/−48/39; k = 182; PFDR = 0.049) of the task-demand-related network identified at pre-training (Fig. 1B) and the middle temporal gyrus (Z = 4.37; 60/−45/0; k = 25; PFDR = 0.049). Examining pre-training to post-training activation changes in this network for each training group separately revealed no significant changes in the placebo group but BOLD activation reductions in the ventral and dorsal lateral PFC and the cingulate, inferior parietal and temporal cortices in the eWM training group (Fig. 3C).
Figure 3.
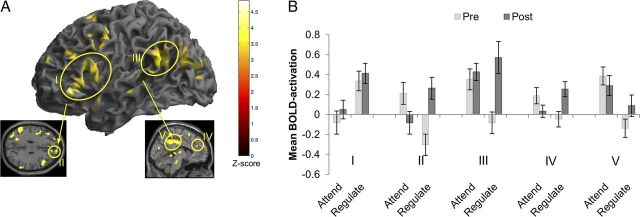
A, The graphs represent the eWM training (top graph) and placebo training (bottom graph) groups' performance on their respective tasks across training days. For the eWM training, the graph reports average level of n-back achieved across 20 blocks per day; and for the placebo training, a composite score (including raw score, number of attempts, and reaction time) on the feature match task is reported. Error bars indicate SD. B, The behavioral gains in eWM across training plotted as mean peak level of n-back achieved. A mixed-model ANOVA with time (pre-training and post-training) as the within-subjects factor and training (placebo, eWM) as the between-subjects factor yielded a significant interaction, which showed that the augmentation of eWM observed in the eWM training group was significantly greater than the change in the placebo training group (F(1,30) = 16.61, p < 0.001, longer dotted line). Repeated-measures analyses with time (pre-training, post-training) as the within-subjects factor were then conducted in the two groups separately. These revealed that the placebo training did not lead to any significant changes in eWM performance (Δ mean, −0.59; SD, 1.6; p = 0.18). In contrast, eWM training led to a significant pre-training to post-training increase in eWM performance (Δ mean, 1.59; SD, 1.2; p < 0.001, shorter dotted line). Moreover, eWM performance was significantly greater in the eWM training group compared with the placebo training group at post-training (t = −2.79 p = 0.009). ***p < 0.001, two-tailed significance level. Error bars indicate SDs. C, BOLD activation changes during the eWM task from pre-training to post-training conflated across all levels of n-back for the training groups. Significant interactions of training (placebo, eWM) × time (pre-training, post-training) are described in the main text. The absence of behavioral change after placebo training was mirrored by the absence of brain activation changes (left). In contrast, the behavioral gain in eWM after eWM training (right) was associated with decreased neural activation in the (I) left ventrolateral to dorsolateral PFC (Z = 4.10, −42/42/6), (II) bilateral inferior parietal cortex (Z = 4.87, −57/−48/39) and right precuneus (Z = 3.54, 12/−63/36), (III) inferior/middle temporal gyrus (Z = 5.76, 63/−33/−9), (IV) bilateral middle and posterior cingulum (Z = 3.74, −3/−24/33), and (V) left ACC (Z = 2.53, −1/10/25). All regions were significant at the whole brain with significance set at PFDR < 0.05.
To investigate brain activation changes across levels of n-back on the eWM task from pre-training to post-training, we first analyzed n-back = 3 trials. At post-training, this level of n-back was now comfortably within the eWM capacity for the eWM training group because of their performance gains over training, but on average continued to represent maximum capacity for the placebo training group where performance had not improved (Fig. 3B). eWM performance changes at this level (n-back = 3) were associated with significantly greater pre-training to post-training BOLD activation decreases in the eWM training group compared with placebo training in the frontoparietal demand network, including the dorsolateral PFC and middle temporal and occipital regions (Table 5).
Table 5.
Regions showing a greater activation decrease from pre-training to post-training after eWM training compared with placebo training for n-back = 3 trialsa
| Brain region | L/R | Cluster size (voxels) | Maxima of cluster x/y/z (mm)b | Z |
|---|---|---|---|---|
| Dorsolateral PFC | L | 10 | −48/39/18 | 3.77 |
| Superior frontal gyrus | R | 11 | 27/−6/63 | 3.40 |
| Supramarginal gyrus | L | 8 | −51/−30/33 | 3.46 |
| R | 31 | 63/−33/42 | 4.00 | |
| Middle temporal gyrus | L | 27 | −51/−63/15 | 4.16 |
| L | 17 | −60/−45/0 | 3.78 | |
| R | 58 | 60/−48/0 | 3.91 | |
| Middle occipital lobe | L | 29 | −45/−81/3 | 3.87 |
| R | 20 | 42/−81/9 | 3.77 |
aRegions that showed greater pre-training to post-training BOLD activation decreases at n-back = 3 in the eWM training group compared with the placebo training group. Threshold for whole-brain correction is puncorrected < 0.001.
bStereotaxic coordinates of peak voxels in MNI space.
Breaking this interaction down to examine pre-training to post-training activation changes in this network for each training group separately revealed no significant changes in the placebo group. In contrast, the eWM training showed a significant activation decrease at n-back = 3 in the temporal cortex and regions of the frontoparietal demand network, including the dorsolateral PFC and inferior parietal cortex, reflecting the now relatively easier challenge posed by the n-back = 3 trials (Table 6).
Table 6.
Regions showing activation changes from pre-training to post-training during the eWM task after eWM traininga
| Brain region | L/R | Cluster | Cluster size (voxels) | Maxima of cluster x/y/z (mm)b | Z |
|---|---|---|---|---|---|
| n-back = 3 | |||||
| Dorsolateral to middle PFC | L | 6 | 182 | −33/48/3 | 3.71 |
| 6 | −30/54/21 | 3.52 | |||
| R | 2 | 1686 | 45/12/18 | 4.95 | |
| 2 | 27/48/18 | 4.39 | |||
| Middle OFC | L | 6 | −45/51/−3 | 3.49 | |
| Inferior parietal cortex | L | 3 | 260 | −57/−48/39 | 4.56 |
| 3 | −39/−57/45 | 3.24 | |||
| Middle temporal gyrus | R | 1 | 1095 | 60/−30/−9 | 5.06 |
| Inferior temporal gyrus | L | 5 | 94 | −57/−36/−15 | 3.85 |
| Fusiform gyrus | L | 7 | −42/−54/−18 | 3.07 | |
| Insula | L | 4 | −36/15/−3 | 3.88 | |
| R | 2 | 42/21/3 | 4.06 | ||
| Thalamus | L | 4 | 556 | 9/−9/18 | 3.90 |
| Inferior occipital gyrus | L | 7 | 80 | −48/−69/−9 | 3.34 |
| Cerebellum | R | 8 | 21 | 36/−57/−45 | 3.25 |
| n-back = 5 | |||||
| Lateral PFC | R | 2 | 121 | 51/27/−3 | 4.28 |
| 2 | 48/24/9 | 3.99 | |||
| 4 | 36 | 48/15/24 | 3.54 | ||
| Supplementary motor area | R | 3 | 23 | 12/21/60 | 3.74 |
| Middle cingulate | L | 5 | 7 | −6/−24/33 | 3.20 |
| Angular gyrus | L | 1 | 211 | −54/−57/36 | 4.67 |
aRegions that showed pre-training to post-training BOLD activation increases at n-back = 5 in the eWM training group. Threshold for whole-brain correction was set at pFDR < 0.05.
bStereotaxic coordinates of peak voxels in MNI space.
We next examined training-related activation changes for n-back = 5 trials (using a post-training − pre-training contrast). As expected, this level of n-back was, on average, beyond the capacity of the placebo training group at post-training (mean, 3.08; SD, 1.13) but was closest to mean maximum capacity for the eWM training group (mean, 7.00; SD, 1.97). At this level of n-back, the eWM training group showed a significant BOLD activation increase compared with the placebo training within the frontoparietal demand network, including the lateral PFC and inferior parietal cortex, and the OFC (Table 7). Breaking this down for each group separately, there were no significant pre-training to post-training changes in the activation of this network in the placebo group, but there were significant activation increases in the eWM training group in the lateral PFC and middle cingulum (Table 6). These differential training effects reflect the fact that, with augmented executive effort, successful completion of the n-back = 5 trials was now attainable at post-training for the eWM training group.
Table 7.
ROIs showing a greater activation increase from pre-training to post-training during the eWM task after eWM training compared with placebo training for n-back = 5 trialsa
| Brain region | L/R | Placebo training |
eWM training |
F(1,31) | ||
|---|---|---|---|---|---|---|
| 5-back pre-training | 5-back post-training | 5-back pre-training | 5-back post-training | |||
| OFC | R | −0.08 (0.17) | −0.11 (0.26) | −0.06 (0.26) | 0.27 (0.25) | 8.39* |
| Lateral PFC (inferior frontal gyrus) | R | 0.08 (0.19) | 0.00 (0.35) | −0.13 (0.24) | 0.02 (0.24) | 5.07** |
| Inferior parietal cortex | R | 0.16 (0.35) | 0.33 (0.52) | 0.00 (0.39) | 0.30 (0.43) | 4.91** |
aBOLD activation for the ROIs, which showed a significantly different pre-training to post-training activation change related to task difficulty in the eWM training group compared with the placebo training group at the level of n-back = 5.
*p < 0.01.
**p < 0.05.
Changes in ER: behavioral effects and neural activation changes after training
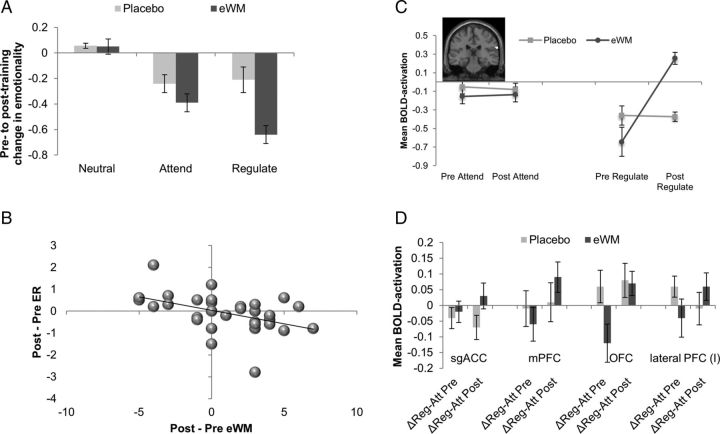
Analyses of the ER task confirmed our key hypothesis of behavioral transfer effects in the capacity for ER as a specific function of eWM training, which were associated with pre-training to post-training changes in BOLD activation in the frontoparietal demand network. Behaviorally, there was a significant time (pre-training, post-training) × training (placebo, eWM) × condition (Regulate, Attend) interaction (covarying baseline differences in mood for the Neutral condition, although this did not alter the pattern of findings), with significantly greater reduction in emotional distress to the negative films in the Regulate relative to the Attend condition compared with the placebo training group (F(1,27) = 4.91, p = 0.035, η2 = 0.15; Fig. 4A). We deconstructed this interaction by investigating the effects of training in each group separately. eWM training was associated with a significantly greater pre-training to post-training decrease in emotional distress for the Regulate relative to the Attend condition (F(1,15) = 6.06, p = 0.029, η2 = 0.32). In contrast, the placebo training group showed a nonsignificant pre-training to post-training increase in reported distress in the Regulate relative to the Attend condition (F(1,13) = 4.22, p = 0.061, η2 = 0.25). There was no effect of training on emotional reactivity (i.e., changes in reported distress for Attend compared with Neutral trials); that is, the interaction of time (pre-training, post-training) × training (placebo, eWM) × condition (Attend negative vs Attend neutral) was not significant (F(1,27) < 1). Pre-training to post-training improvements in ER capacity (i.e., a reduction in reported distress for Regulate compared with Attend trials) across all participants were significantly correlated with improvement in eWM (r = −0.43, p = 0.019; Fig. 4B). Pre-training to post-training changes in reported emotional reactivity, however, were not significantly associated with eWM changes (r = 0.14; p = 0.25). A mediation analysis provided further support for the transfer of eWM training to changes in ER capacity. Our mediation approach used the bootstrapping method devised by Preacher and Hayes (2004). A total of 1000 resamples of the data (with replacement) were executed using Hayes and Preacher's SPSS macro (http://www.afhayes.com/spss-sas-and-mplus-macros-and-code.html). The model revealed a significant indirect effect for eWM training gains to mediate between training group (placebo, eWM) and pre-training to post-training improvements in ER (Regulate, Attend), bootstrap index = 0.48, SE = 0.23, 95% bias-corrected CI = 0.11–1.05 (statistical significance is indicated if the CI does not cross 0) (Preacher and Hayes, 2004). This significant indirect effect overlay significant associations between training group and the mediator–eWM training gains (t = 3.98, p ≤ 0.001) and between eWM training gains and the outcome–improvement in ER (t = 2.03, p = 0.025).
Figure 4.
A, The graph reports mean changes (post-training − pre-training) in emotion ratings for each condition; lower change scores indicate reduced negative affect after training in that condition. B, The graph represents the association between pre-training and post-training changes in ER capacity and changes in eWM. The association is negative because the ER measure represents the pre-training to post-training reduction in reported emotional distress, whereas eWM reports increases in maximum level of n-back achieved at the offline post-training assessment. C, The figure shows the differential effects of eWM training compared with placebo training across time for the Regulate versus Attend conditions in the left superior temporal gyrus. The figure is represented at Puncorrected < 0.001. Error bars indicate SEM. D, The graphs depict the effect of eWM training compared with placebo training across time (pre-training, post-training) for the Regulate versus Attend conditions on BOLD activation in the ROI. Error bars indicate SEM.
Turning to the brain imaging analyses, we first examined pre-training to post-training BOLD activation changes across the ER task as a whole because each separate condition of the ER task required participants to exert some form of executive control over their emotions (e.g., the Attend condition required them to try not to downregulate their affective responses). Cognitive control was also exerted to keep current task-demands active and to switch between the different conditions (i.e., Regulate, Attend, and Neutral). These analyses revealed a pre-training to post-training increase in neural activity when performing the ER task in areas of the frontoparietal demand network implicated in both the ER task and eWM training task at pre-training. Whole-brain analyses showed that, compared with placebo training, eWM training led to significant BOLD activation increases from pre-training to post-training in sgACC (Z = 2.99, 3/18/−9), inferior OFC (Z = 2.98, −24/33/−6), inferior frontotemporal, (Z = 3.02, −39/6/−30), inferior parietal (Z = 2.99, 3/−42/57), and visuotemporal cortices (Z = 5.11, −9/−75/21). To support the assumption that the observed differential pre-training to post-training changes in BOLD activation during the ER task in the eWM training group changed within the network that was trained by the eWM training task, we ran a conjunction analysis on the interaction between task (eWM task, ER task), time (pre-training, post-training), and training (placebo, eWM). The analysis revealed a circumscribed (k = 11) cluster of shared activation in the left lateral PFC (Z = 3.04, −30/36/42).
We next investigated whether the training had differential effects across ER task conditions. Confirming our a priori hypothesis and in line with the behavioral findings, eWM training, relative to placebo training, had selective effects on BOLD activation during the Regulate relative to the Attend condition. Whole-brain analyses yielded a significant interactive effect of time (pre-training, post-training) × training (placebo, eWM) × condition (Attend, Regulate) on BOLD activation in the right superior temporal gyrus (bordering on the temporoparietal junction, Z = 3.89, 69/−27/21). The eWM group showed a significant activation increase compared with the Placebo group in the Regulate condition but not in the Attend condition (Fig. 4C). ROI analyses (Fig. 4D) on the same contrast (Regulate, Attend) further revealed greater pre-training to post-training increases after eWM training compared with placebo training in the sgACC (F(1,30) = 5.09, p = 0.032), medial PFC (F(1,30) = 4.40, p = 0.044), and at a trend level in the lateral PFC (F(1,30) = 3.14, p = 0.086) and OFC (F(1,30) = 3.56, p = 0.069).
We deconstructed these interactions to investigate pre-training to post-training activation changes in each training group separately. For the eWM training group, at the whole-brain level, these analyses showed a significant interaction of time (pre-training, post-training) by condition (Attend, Regulate) in the lateral (I) and medial (II) PFC, extending into the OFC, middle temporal gyrus (III), and the cingulate cortex (IV, V; Fig. 5A,B; for a list of all regions showing a significant interaction, see Table 8). ROI analyses in the eWM group confirmed that pre-training to post-training activation increases for the Regulate relative to Attend trials in the lateral and medial PFC, OFC, and ACC and showed additional effects in the sgACC, and the inferior parietal cortex (Table 9).
Figure 5.
A, The figure shows brain areas that showed greater BOLD activation increases (a contrast of post-training − pre-training) in the Regulate relative to the Attend condition for the eWM training group only at the whole-brain level of analysis. The figure was thresholded at Puncorrected < 0.001. For a full list of activation details, see Table 8. B, The histograms depict mean BOLD activation during the ER task at pre-training and post-training in the regions reported in Figure 5A, which showed an interactive effect of time (pre-training, post-training) and condition (Regulate, Attend) in the eWM training group. Error bars indicate SEM.
Table 8.
Regions showing a pre-training to post-training greater increase in BOLD activation during Regulate compared with Attend trials for the eWM training groupa
| Brain region | L/R | Cluster number | Cluster size (voxels) | Maxima of cluster x/y/z (mm)b | Z |
|---|---|---|---|---|---|
| Inferior OFC | L | 1 | 530 | −42/33/−3 | 4.62 |
| Medial PFC | R | 4 | 25 | 9/60/33 | 4.18 |
| Lateral PFC | L | 1 | 530 | −57/24/3 | 4.80 |
| L | 7 | 15 | −27/54/27 | 4.07 | |
| R | 2 | 207 | 60/24/15 | 4.30 | |
| Inferior frontal operculum | L | 1 | 530 | −57/15/9 | 4.50 |
| R | 2 | 207 | 45/12/21 | 4.31 | |
| R | 2 | 207 | 63/15/18 | 4.29 | |
| Lateral superior PFC | L | 6 | 42 | −18/9/60 | 3.21 |
| Supplementary motor area | L | 6 | 42 | −12/15/63 | 4.15 |
| R | 6 | 42 | 9/12/60 | 3.69 | |
| Anterior cingulate | R | 11 | 7 | 9/42/30 | 3.52 |
| Middle cingulate | L | 5 | 216 | −6/−21/39 | 3.97 |
| Precuneus | L | 5 | 216 | −6/−51/39 | 3.66 |
| Temporoparietal junction | L | 3 | 162 | −54/−54/24 | 4.30 |
| R | 9 | 10 | 63/−51/30 | 3.90 | |
| Superior temporal gyrus | R | 10 | 12 | 63/0/−6 | 3.87 |
| Middle temporal gyrus | L | 3 | 162 | −66/−42/12 | 3.63 |
| Middle occipital gyrus | L | 8 | 179 | −30/−78/21 | 4.04 |
| L | 8 | 179 | −42/−72/24 | 3.65 | |
| Cerebellum | R | 289 | 21/−78/−27 | 4.46 | |
| 12/−75/−30 | 4.45 |
aRegions that showed pre-training to post-training BOLD activation increases for the Regulate relative to the Attend condition in the eWM training group at the whole-brain level. Threshold for whole-brain correction is puncorrected < 0.001.
bStereotaxic coordinates of peak voxels in MNI space.
Table 9.
ROIs showing a pre-training to post-training greater increase in BOLD activation during Regulate compared with Attend trials for the eWM training groupa
| Brain region | L/R | Attend |
Regulate |
F(1,16) | η2 | ||
|---|---|---|---|---|---|---|---|
| Pre-training | Post-training | Pre-training | Post-training | ||||
| Subgenual ACC | L | −0.03 (0.22) | 0.06 (0.20) | −0.01 (0.21) | 0.13 (0.26) | 4.65* | 0.24 |
| ACC | L | 0.27 (0.27) | 0.21 (0.40) | 0.09 (0.36) | 0.26 (0.38) | 5.68* | 0.28 |
| OFC | L | 0.14 (0.23) | 0.12 (0.26) | 0.04 (0.28) | 0.23 (0.31) | 8.21* | 0.35 |
| Medial PFC | L | 0.13 (0.16) | 0.11 (0.22) | 0.06 (0.19) | 0.11 (0.24) | 4.60* | 0.24 |
| Lateral PFC (middle frontal gyrus) | L | 0.14 (0.26) | 0.11 (0.28) | 0.01 (0.32) | 0.19 (0.30) | 7.62* | 0.34 |
| R | 0.21 (0.34) | 0.16 (0.40) | 0.06 (0.33) | 0.23 (0.41) | 4.85* | 0.24 | |
| Inferior parietal cortex | L | 0.34 (0.19) | 0.29 (0.26) | 0.21 (0.24) | 0.34 (0.28) | 5.23* | 0.26 |
aSignificant ROIs for the same contrast.
*p < 0.05.
As in the behavioral analyses, we tested whether pre-training to post-training BOLD activation changes during the eWM task mediated the effect of training type (placebo, eWM) on pre-training to post-training changes in (behavioral) ER (Regulate, Attend) capacity. As before, 1000 resamples of the data (with replacement) were executed using Hayes and Preacher's SPSS macro (http://www.afhayes.com/spss-sas-and-mplus-macros-and-code.html). The model revealed a significant indirect effect for change in lateral PFC activation to mediate the association between training group (placebo, eWM) and pre-training to post-training improvements in ER (Regulate, Attend), bootstrap index = −0.26, SE = 0.17, 95% bias-corrected CI = −0.71 to −0.03 (statistical significance is indicated if the CI does not cross 0) (Preacher and Hayes, 2004). No other ROI showed a mediating effect on ER capacity. This significant indirect effect overlay significant associations between training group and the mediator–change in lateral PFC activation (t = 2.18, p = 0.025) and between change in lateral PFC activation and the outcome–improvement in ER (t = 2.03, p = 0.026).
We also examined neural changes associated with emotional reactivity on the ER task as a function of training by comparing BOLD activation with Attend versus Neutral trials across groups from pre-training to post-training. Mirroring the behavioral data, whole-brain and ROI analyses yielded no significant interactive effects of time (pre-training, post-training) by condition (Attend, Neutral) in the placebo training group.
Discussion
The present findings show that 20 d of WM training on a dual n-back task populated with emotional stimuli (eWM task) (Schweizer et al., 2011) resulted in marked behavioral improvements on the trained task. Placebo training on a feature match paradigm low in WM demands generated no such improvements in eWM task performance. These behavioral gains, specific to the eWM training, were associated with increased efficiency of the frontoparietal cognitive control network (Miller, 2000; Duncan, 2010) from pre-training to post-training assessed with fMRI while participants performed the eWM task in the scanner. Furthermore, eWM training, and not placebo training, accrued clear behavioral and neural transfer effects across to a “gold standard” index of affective cognitive control, ER. Improved ER, specific to the eWM training group, was associated with increased recruitment of the same frontoparietal demand network implicated in eWM performance; this notably included the sgACC, a region crucially involved in mood regulation (Davidson et al., 2002; Drevets et al., 2008).
Our first experimental aim was to use fMRI at pre-training to confirm that the eWM task activated the extended frontoparietal affective control network (Wager et al., 2008; Banich et al., 2009) and deactivated limbic regions associated with emotion processing (Dalgleish, 2004). In line with this, pre-training task-demand-related BOLD activation increases were observed in the dorsolateral PFC and inferior parietal cortex while participants performed the eWM task. These regions overlap with those typically found during the performance of n-back tasks (for a meta-analysis, see Owen et al., 2005). We also found that task-demand-related decreased BOLD activation in emotion processing regions, in line with previous studies on WM that show decreased activation of emotion processing regions at higher levels of cognitive load (Kellermann et al., 2012).
Our prediction that augmented eWM capacity after training would be specific to the eWM training group was supported by the behavioral data. We anticipated that these specific behavioral gains in eWM would be associated with changes in the activation profile of the frontoparietal demand network from pre-training to post-training while participants performed the eWM task, reflecting the augmented efficiency of this network as a function of eWM training (Poldrack, 2000). Theorists argue that training leads to either (1) greater capacity to apply an initial strategy, which is associated with increased neural efficiency, or (2) the development of a new skill, which leads to functional reorganization (Kelly and Garavan, 2005). The BOLD-signal changes after practice, as observed in the current study, are an example of the former and proposed to indicate a “sharpened” (i.e., more efficient) response of the trained neural network (Poldrack, 2000). We anticipated that, after training, this increased efficiency of the neural regions subserving eWM performance in the eWM training group would manifest in different ways as a function of eWM load. This was supported by the data. For a lower level of load (n-back = 3) that represented eWM performance maximum capacity at pre-training but was comfortably within that capacity at post-training for the eWM training group, there was decreased frontoparietal activation from pre-training to post-training relative to the placebo training group. In contrast, for a higher load (n-back = 5) that was beyond performance maximum capacity (and was therefore unattainable) at pre-training for the eWM training group but became attainable at post-training, there was increased functional activation of the frontoparietal demand network from pre-training to post-training relative to the placebo-trained participants. These results (in combination with the previously discussed task-demands analyses at pre-training) suggest that increasingly effortful eWM performance is associated with increased activation in the task-demand-related frontoparietal demand network, including the inferior parietal and lateral and middle PFC, whereas improved eWM capacity is associated with decreased activation for a given task load in the same task-demand-related areas. This finding of differential patterns of eWM-related activation increase or decrease across the frontoparietal demand network from pre-training to post-training as a function of different levels of eWM load, and specific to eWM training relative to placebo training, potentially reconciles discrepant results in the extant literature, which have shown patterns of neural activation increase (Olesen et al., 2004) and decrease (Schneiders et al., 2011) across different experiments (for a recent review, see Buschkuehl et al., 2011). It should be noted, however, that there were behavioral pre-training performance differences on the eWM task with the placebo training group performing significantly better at pre-training. The eWM training group therefore had more room for performance improvement from pre-training to post-training compared with the placebo training group, which may account for part of the training effects.
Although improvement on the trained task itself is a sine qua non of cognitive training interventions, the fundamental aim of such protocols is to generate far transfer to untrained tasks (Subramaniam et al., 2012). Our central proposition was therefore to investigate, for the first time, putative cognitive and neural transfer effects from eWM training to an ecologically valid measure of affective cognitive control, ER capacity, which is dependent on the same underlying neural circuitry in the frontoparietal demand network as eWM, including the medial and lateral PFC and inferior parietal cortex (Banich et al., 2009). In support of the behavioral component of our core hypothesis, we showed that eWM training (compared with placebo training) yielded significantly improved ER capacity. Moreover, the greater the training gains in eWM performance, the greater the improvement in ER capacity from pre-training to post-training. Similarly, a formal mediation analysis showed that training-related improvements in eWM performance mediated the gains in ER capacity. This finding is highly encouraging as ER capacity is associated with a wide range of beneficial outcomes, such as better social relationships and better health (Gross, 2002), and impoverished ER is pervasive across psychopathology (Aldao et al., 2010). Augmenting ER capacity through a simple, easily administered eWM training with the possibility for easy dissemination therefore provides an exciting novel avenue to improve ER across a wide range of populations, including clinical and at risk groups.
The results also supported the neuroscientific component of our transfer hypothesis, which posited that because eWM and ER share underlying neural substrates, training in one context would benefit performance in the other by augmenting the efficiency of this common neural network. The data showed that the eWM training-driven improvement in ER capacity was associated with augmented BOLD activation during ER in the eWM training group relative to the placebo training group in the frontoparietal demand network that was targeted by the eWM training. This network, especially the lateral and medial PFC, is thought to be essential to successful ER by downregulating emotional activation in subcortical emotion processing areas, including the amygdala (Wager et al., 2008). Moreover, compared with the placebo training, the eWM training group showed increased activation in the superior temporal cortex and lateral and medial (sgACC and OFC) PFC, during Regulate compared with Attend trials. A conjunction analysis revealed a cluster in the left lateral PFC, which showed a significant BOLD activation for both the eWM and ER tasks. Interestingly, however, we found no significant training-specific changes in activation in the subcortical brain regions associated with emotion processing during the ER task from pre-training to post-training. Perhaps the most important evidence of “neural transfer” in the data were the increased BOLD activation in the sgACC during ER at post-training that was recorded in the eWM training group only. The sgACC has been reliably shown to be involved in successful downregulation of emotional distress through the use of various effortful cognitive control strategies (Wager et al., 2008). The mechanism of action is likely to be sgACC's coupling with the amygdale, and the strength of this functional coupling is predictive of ER success (Banks et al., 2007). It is also worth noting that the inhibitory effect of the sgACC on amygdala activation is reduced in those at genetic risk for depression (Pezawas et al., 2005) and in currently depressed individuals, who are characterized by poor ER capacity (Anand et al., 2005). Moreover, depressed individuals show reduced gray matter volumes in the sgACC and resting-state hypoactivation of the region compared with healthy controls (Davidson et al., 2002). The role of the sgACC in mood regulation is further evidenced by studies of deep brain stimulation in the sgACC that report symptom alleviation in previously treatment-resistant patients (Mayberg et al., 2005). Our current finding that engagement of the sgACC during ER can be augmented after an eWM training that is dependent on affective cognitive control is encouraging for the treatment and prevention of mood and other highly prevalent neuropsychiatric disorders that are characterized by dysfunctional sgACC activation (Rauch et al., 2003). The reported imaging findings based on ROI analyses, however, should be interpreted with a degree of caution because they were based on a significance threshold uncorrected for multiple comparisons. Finally, it is important to note that, unlike our previous work (Schweizer et al., 2011), the present study did not compare the eWM training with standard WM training using affectively neutral material. It is therefore possible that transferable ER gains may also be accrued through nonaffective WM training paradigms.
In conclusion, the present study is the first to find support for behavioral and neural transfer effects after eWM training to an ecologically valid measure of affective cognitive control, ER capacity. The data significantly enhance our understanding of the neural substrates underlying cognitive training transfer effects. The findings also underscore the promise of cognitive training protocols populated with affective stimuli as a means to boost affective control in healthy populations where such control is critical (e.g., professionals, such as physicians, pilots, and stock-brokers required to make decisions in stressful situations). Finally, the results highlight the potential of eWM training for clinical groups with impoverished affective cognitive control.
Footnotes
This work was supported by the United Kingdom Medical Research Council (MC US A060 0019). Susanne Schweizer was supported by the Gates Cambridge Trust.
The authors declare no competing financial interests.
References
- Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clin Psychol Rev. 2010;30:217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, Heller W. Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neurosci Biobehav Rev. 2009;33:613–630. doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala: frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Tugade MM, Engle RW. Individual differences in working memory capacity and dual-process theories of the mind. Psychol Bull. 2004;130:553–573. doi: 10.1037/0033-2909.130.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Brass M, Ullsperger M, Knoesche TR, von Cramon DY, Phillips NA. Who comes first? The role of the prefrontal and parietal cortex in cognitive control. J Cogn Neurosci. 2005;17:1367–1375. doi: 10.1162/0898929054985400. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16:37. [Google Scholar]
- Buschkuehl M, Jaeggi SM, Jonides J. Neuronal effects following working memory training. Dev Cogn Neurosci. 2011;2:S167–S179. doi: 10.1016/j.dcn.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FI, Bialystok E. Cognition through the lifespan: mechanisms of change. Trends Cogn Sci. 2006;10:131–138. doi: 10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Dalgleish T. The emotional brain. Nat Rev Neurosci. 2004;5:583–589. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD, Drevets WC, Farah MJ, Kagan J, McClelland JL, Nolen-Hoeksema S, Peterson BS. Neural and behavioral substrates of mood and mood regulation. Biol Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Hutcherson CA, Ochsner KN, Glover GH, Gabrieli JD, Gross JJ. The neural bases of amusement and sadness: a comparison of block contrast and subject-specific emotion intensity regression approaches. Neuroimage. 2005;27:26–36. doi: 10.1016/j.neuroimage.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Gray JR. Integration of emotion and cognitive control. Curr Dir Psychol Sci. 2004;13:46–48. [Google Scholar]
- Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Hill SA, Taylor MJ, Cowen PJ, Goodwin GM. Toward a neuropsychological theory of antidepressant drug action: increase in positive emotional bias after potentiation of norepinephrine activity. Am J Psychiatry. 2003;160:990–992. doi: 10.1176/appi.ajp.160.5.990. [DOI] [PubMed] [Google Scholar]
- Joormann J, Levens SM, Gotlib IH. Sticky thoughts: depression and rumination are associated with difficulties manipulating emotional material in working memory. Psychol Sci. 2011;22:979–983. doi: 10.1177/0956797611415539. [DOI] [PubMed] [Google Scholar]
- Kellermann TS, Sternkopf MA, Schneider F, Habel U, Turetsky BI, Zilles K, Eickhoff SB. Modulating the processing of emotional stimuli by cognitive demand. Soc Cogn Affect Neurosci. 2012;7:263–273. doi: 10.1093/scan/nsq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex. 2005;15:1089–1102. doi: 10.1093/cercor/bhi005. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Morrison AB, Chein JM. Does working memory training work? The promise and challenges of enhancing cognition by training working memory. Psychon Bull Rev. 2011;18:46–60. doi: 10.3758/s13423-010-0034-0. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Holmes A, Friston KJ. Random effects analysis. In: Ashburner J, Friston KJ, Penny WD, editors. Human brain function, II. Ed 2. Oxford, United Kingdom: Elsevier; 2003. [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Imaging brain plasticity: conceptual and methodological issues—a theoretical review. Neuroimage. 2000;12:1–13. doi: 10.1006/nimg.2000.0596. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis in fMRI. Soc Cogn Affect Neurosci. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, Whalen PJ, Makris N. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14:913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- Schneiders JA, Opitz B, Krick CM, Mecklinger A. Separating intra-modal and across-modal training effects in visual working memory: an fMRI investigation. Cereb Cortex. 2011;21:2555–2564. doi: 10.1093/cercor/bhr037. [DOI] [PubMed] [Google Scholar]
- Schweizer S, Dalgleish T. Emotional working memory capacity in posttraumatic stress disorder (PTSD) Behav Res Ther. 2011;49:498–504. doi: 10.1016/j.brat.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer S, Hampshire A, Dalgleish T. Extending brain-training to the affective domain: increasing cognitive and affective executive control through emotional working memory training. PLoS ONE. 2011;6:e24372. doi: 10.1371/journal.pone.0024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipstead Z, Redick TS, Engle RW. Is working memory training effective? Psychol Bull. 2012;138:628–654. doi: 10.1037/a0027473. [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73:842–853. doi: 10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Genetics of brain structure and intelligence. Annu Rev Neurosci. 2005;28:1–23. doi: 10.1146/annurev.neuro.28.061604.135655. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]