Abstract
Competition between adult males for limited resources such as food and receptive females is shaped by the male pattern of pituitary growth hormone (GH) secretion that determines body size and the production of urinary pheromones involved in male-to-male aggression. In the brain, dopamine (DA) provides incentive salience to stimuli that predict the availability of food and sexual partners. Although the importance of the GH axis and central DA neurotransmission in social dominance and fitness is clearly appreciated, the two systems have always been studied unconnectedly. Here we conducted a cell-specific genetic dissection study in conditional mutant mice that selectively lack DA D2 receptors (D2R) from pituitary lactotropes (lacDrd2KO) or neurons (neuroDrd2KO). Whereas lacDrd2KO mice developed a normal GH axis, neuroDrd2KO mice displayed fewer somatotropes; reduced hypothalamic Ghrh expression, pituitary GH content, and serum IGF-I levels; and exhibited reduced body size and weight. As a consequence of a GH axis deficit, neuroDrd2KO adult males excreted low levels of major urinary proteins and their urine failed to promote aggression and territorial behavior in control male challengers, in contrast to the urine taken from control adult males. These findings reveal that central D2Rs mediate a neuroendocrine-exocrine cascade that controls the maturation of the GH axis and downstream signals that are critical for fitness, social dominance, and competition between adult males.
Introduction
Central D2 receptors (D2Rs) participate in important brain functions, including the control of locomotor activity and reward-seeking behavior. D2Rs are also found in pituitary lactotropes, where they mediate the tonic inhibitory control that dopamine (DA) exerts on prolactin (PRL) synthesis and release. The physiological significance of this inhibitory control has been appreciated in mice lacking D2Rs (Drd2−/−) that display hyperprolactinemia and develop pituitary hyperplasia (Kelly et al., 1997; Asa et al., 1999). Analysis of Drd2−/− mice also revealed the unexpected importance of D2Rs in the regulation of the growth hormone (GH) axis and control of body size, a differential phenotype that is considerable in males (Díaz-Torga et al., 2002). Drd2−/− mice display a shortfall of pituitary somatotropes, reduced GH and IGF-I serum levels, and are dwarfs (Díaz-Torga et al., 2002). The abnormally large lactotrope/somatotrope ratio observed in Drd2−/− mice is highly reminiscent of a similar phenomenon observed in lactating females, in which suckling-induced inhibition of tuberoinfundibular DA neurons reduces lactotrope D2R stimulation and promotes differentiation of somatolactotrope precursors into functional lactotropes (Porter et al., 1990; Porter et al., 1991). Therefore, it is conceivable that pituitary D2Rs play a critical role in establishing the terminal differentiation ratio of these two cell types from their common somatolactotrope precursor (Scully and Rosenfeld, 2002) and, therefore, in shaping the GH axis.
An alternative hypothesis that may account for the somatotrope shortfall and dwarfism of Drd2−/− mice is that lack of central D2Rs impairs growth hormone-releasing hormone (GHRH) or somatostatin (SST) function. In fact, the phenotype observed in Drd2−/− mice is similar to that present in lit/lit mice that lack functional GHRH receptors (Godfrey et al., 1993; Lin et al., 1993) and in mutant mice lacking Ghrh (Alba and Salvatori, 2004), because these two mouse models have fewer somatotropes and display dwarfism.
In the present study, we sought to determine whether GH insufficiency in Drd2−/− mice was due to the lack of D2Rs in pituitary lactotropes or in the brain by conducting a functional dissection strategy based on cell-specific Drd2 inactivation in conditional Drd2 mutant mice (Bello et al., 2011). We developed a strain of transgenic mice expressing cre from a mouse prolactin gene promoter, Tg(Prl-cre)1Mrub, that in parallel with transgenic mice expressing cre from a rat nestin promoter, Tg(Nes-cre)1Kln/J (Zimmerman et al., 1994; Tronche et al., 1999), were used to eliminate D2Rs selectively from pituitary lactotropes or from cells of neural origin, respectively. Our results showed that although male mice lacking D2Rs in pituitary lactotropes (lacDrd2KO) developed a normal GH axis and body size, those lacking D2Rs in the brain (neuroDrd2KO) showed an impaired GH axis and reduced body growth. Deficits in the male pattern of GH release are associated with feminization of sexually dimorphic liver proteins, including major urinary proteins (MUP; Udy et al., 1997), a group of pheromones excreted at high levels in the male urine and used in male-to-male social interactions (Chamero et al., 2007). Therefore, in this study, we also sought to investigate whether neuroDrd2KO male mice exhibited deficits in MUP levels and if their urine failed to induce territorial and aggressive behaviors in conspecific males.
Materials and Methods
Generation of mice lacking D2Rs in pituitary lactotropes.
A transgene construct carrying 2.9 kb of 5′ mouse Prl promoter sequence driving cre recombinase expression with a coding sequence for a nuclear localization was used to generate Prl-cre transgenic mice. The 4.2 kb Prl-cre transgene was microinjected into the pronuclei of fertilized B6D2F2 eggs. The selected line Tg(Prl-cre)1Mrub was backcrossed for five generations to C57BL/6J. Tg(Prl-cre)1Mrub (n = 5) mice were consecutively crossed to Drd2loxP/loxP mice (Bello et al., 2011) to generate Drd2loxP/loxP.Tg(Prl-cre) mice, named lacDrd2KO. Transgenic mice were produced at the Instituto de Investigaciones en Ingeniería Genética y Biología Molecular (CONICET, Argentina) and animal procedures were conducted in accordance with local regulations and the Guide for the Care and Use of Laboratory Animals (National Institutes of Health [NIH]).
Generation of mice lacking D2Rs in neurons.
To ablate D2Rs from cells of neural origin, Drd2loxP/loxP mice were crossed to B6.Cg-Tg(Nes-cre)1Kln/J (The Jackson Laboratory) to obtain cohorts of Drd2loxP/loxP (control) and Drd2loxP/loxP.B6.Cg-Tg(Nes-cre)1Kln/J (neuroDrd2KO) littermates.
Immunofluorescence.
Double transgenic mice, obtained by crossing Tg(Prl-cre)1Mrub mice with the cre reporter mouse line Ai14 (Madisen et al., 2010), received cabergoline (Laboratorios Beta) 0.5 mg/kg intraperitoneally to increase prolactin signal and 2 h later were perfused with paraformaldehyde 4% in phosphate buffered saline (PBS). Pituitaries were incubated in perfusion solution at 4°C overnight, then in PBS for 24 h, and finally in sucrose 10% in PBS for additional 24 h. Fixed pituitaries were mounted in gelatin cubes (gelatin 10%, sucrose 10% in PBS) and stored at −80°C until use. Coronal 12 μm sections were cut using a Leica CM1850 cryostat and mounted on Superfrost slides (Fisher Scientific). For prolactin and luteinizing hormone (LH) detection, an antigen retrieval procedure was performed incubating the sections in sodium citrate buffer 10 mm, pH 8.5, at 80°C for 10 min. Incubation with primary antibodies were performed overnight at 4°C. The primary antibodies used were as follows: goat anti-prolactin (sc-7805; Santa Cruz Biotechnology) diluted 1:200 in KPBS/0.3% Triton X-100 containing 2% normal donkey antiserum; rabbit anti-hGH (National Hormone and Pituitary Program [NHPP], NIH) diluted 1:1000 in KPBS/0.3% Triton X-100 containing 2% normal goat serum (NGS); rabbit anti-rat adrenocorticotropic hormone (rACTH; NHPP, NIH) diluted 1:1000 in KPBS/0.3% Triton X-100 containing 2% NGS; and rabbit anti-LH (NHPP, NIH) diluted 1:12,000 in KPBS/0.3% Triton X-100 containing 2% NGS. Sections were then washed twice in PBS for 20 min at room temperature and incubated for 2 h with a secondary antibody at room temperature. To detect GH, ACTH, and LH, a goat anti-rabbit Alexa Fluor 488 (Invitrogen) secondary antibody diluted 1:1000 in KPBS/0.3% Triton X-100 was used; to detect prolactin, a donkey anti-goat Alexa Fluor 488 secondary antibody diluted 1:500 in KPBS/0.3% Triton X-100 was used. Sections were washed twice in KPBS for 20 min and mounted in Vectashield (Vector Laboratories). Pituitary sections of neuroDrd2KO mice and control littermates were incubated with rabbit anti-GH antibody (NHPP, NIH) diluted 1:500 in KPBS/0.3% Triton X-100 containing 2% NGS. After washing twice for 20 min with KPBS, sections were incubated with donkey anti-rabbit FITC-coupled IgG (Santa Cruz Biotechnology) diluted 1:500 in KPBS/0.3% Triton X-100 for 2 h at room temperature. After washing twice in KPBS, sections were incubated with propidium iodide to stain cell nuclei. Finally, sections were rinsed in KPBS twice for 10 min, mounted in water, and coverslipped using Vectashield (Vector Laboratories). Confocal microscopy was performed to assess the number of cells expressing GH per pituitary.
In situ hybridization.
Brains and pituitaries were freshly removed and frozen at −40°C in isopentane, equilibrated at −20°C in Tissue-Tek, cryosectioned at 16 μm, and mounted on SuperFrost slides (Fisher Scientific), and stored at −70°C until use. Sections were thawed and maintained at room temperature for 30 min and then fixed in 4% paraformaldehyde in PBS for 30 min. After two washes in PBS, slides were incubated in 0.1 m triethanolamine, pH 8, for 3 min, followed by incubation with triethanolamine-acetic anhydride 0.0025% for 10 min. Slides were then rinsed twice in 2 × SSC (0.3 m NaCl, 0.03 m sodium citrate, pH 7.2), and, finally, dehydrated quickly in ascending ethanol concentrations. Sections were incubated with hybridization buffer (66% formamide, 260 mm NaCl, 1.3 × Denhardt's) for 1 h at 57°C and hybridization started after adding 5 × 106 to 107 cpm/ml of an antisense mouse Drd2 exon 2 [35S]-riboprobe and proceeded overnight at 57°C in a wet chamber. The next day, slides were washed 4 times with 4 × SSC and then incubated with RNase A (20 μg/ml in 0.5 m NaCl; 10 mm Tris-HCl pH 8; 1 mm EDTA pH 8) at 37°C for 30 min. Slides underwent consecutive washes with 2×, 1×, and 0.5 × SSC/1 mm DTT and a final wash with 0.1 × SSC/1 mm DTT at 65°C for 30 min. Finally, sections were dehydrated in ascending ethanol concentrations, covered in Kodak N-TB2 emulsion, and exposed for 15–20 d in a dark chamber until development for posterior analysis. For the digoxigenin-labeled probe, prehybridization was performed in a solution containing formamide 66%, NaCl 260 mm, 1.3 × Denhardt's, EDTA 1.3 mm, Tris-HCl 13 mm, pH 8.0, and dextran sulfate 13% at 57°C for 1 h. The probe was incubated at 65°C for 2 min in 0.5 mg/ml tRNA and 10 mm dithiothreitol and added to the prehybridization buffer at a final concentration of 85 ng/ml. Hybridization was performed at 70°C overnight. After hybridization, the sections were washed with 0.2% SSC at 72°C for 1 h and then twice with a solution containing 100 mm NaCl, 0.1% Triton X-100, and 100 mm Tris-HCl, pH 7.5. Sections were then incubated with the same solution plus 10% NGS for 4 h and then incubated with alkaline phosphatase-conjugated anti-digoxigenin Fab fragments antibody (1:3500; Roche) at 4°C overnight. The following day, samples were washed 5 times for 15 min each in PBS and then washed 4 times in a solution containing 100 mm NaCl, 50 mm MgCl2, 0.1% Tween 20, and 100 mm Tris-HCl, pH 9.5. Then samples were incubated with NBT/BCIP (Roche) in the above-mentioned buffer overnight at room temperature. Signal development was stopped with PBS, coverslipped with Mowiol (polyvinyl alcohol, glycerol in Tris 0.2 m, pH 8.5) and photographed.
Binding autoradiography.
Brain and pituitary sections were obtained as described for in situ hybridization, except they were stored at −20°C until use. Slides were thawed and maintained at room temperature for 30 min, and then preincubated in binding buffer (50 mm Tris-HCl, 120 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, pH 7.4) for 1 h. Next, slides were incubated for 1 h in binding buffer containing 1 nm [3H]-nemonapride. Nonspecific binding was determined in the presence of 10 μm S-(-)-sulpiride, and then sections were rinsed twice for 5 min in cold water, completely dried with cold air, and exposed for 15–30 d to [3H]-sensitive film (BIOMAX MR Scientific Imaging Film; Kodak) at room temperature in a dark cassette.
Radioimmunoassays.
Anterior pituitaries were dissected and homogenized in 0.2 ml of PBS. Protein contents were measured with the QUBIT Fluorometer and the QUANT-IT protein Assay Kit (Invitrogen). Aliquots of equal quantities of protein were used to measure GH content by RIA. Serum PRL and GH pituitary content were measured by RIA using kits provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; Dr. A.F. Parlow, NHPP, Torrance, CA). Results are expressed in terms of mouse PRL standard RP3 or mouse GH standard AFP-10783B, respectively. Intra-assay and interassay coefficients of variation were 7.2% and 12.8%, and 8.4% and 13.2%, for prolactin and GH, respectively. For IGF-1 RIA, serum samples (15 μl) and IGF-1 standards were subjected to the acid-ethanol cryoprecipitation method. IGF-1 was determined using antibody (UB2–495) provided by Drs. L. Underwood and J.J. Van Wyk, and distributed by the Hormone Distribution Program of the NIDDK. Recombinant human IGF-1 (rh IGF-1; Chiron) was used for radioligand and unlabeled standards. The assay sensitivity was 6 pg per tube. Intra-assay and interassay coefficients of variation were 8.2% and 14.1%, respectively. For testosterone RIA, an active testosterone RIA kit (DSL-4000; Diagnostic System Laboratories) was used.
Urine dosage of major urinary proteins.
Urine was collected from 6-month-old mice between 1500 and 1700 h and centrifuged briefly for 3 min at 8800 × g. Two microliters of the supernatant was boiled in SDS buffer. Samples were fractionated in 12% SDS-PAGE and subsequently stained with Coomassie blue. The 20 KDa MUP represents the major protein component of mouse urine.
Semiquantitative real-time PCR.
Hypothalami from Drd2loxP/loxP and neuroDrd2KO mice were excised to determine Ghrh and Sst mRNA levels. Tissue samples were immediately homogenized in TRIzol reagent (Invitrogen), RNA extracted, and first strand cDNA synthesized using oligo(dT)15 and MMLV reverse transcriptase (Epicenter Biotechnologies). Sense and antisense oligonucleotide primers were designed using PrimerExpress software (Applied Biosystems). The sequences of the primers were as follows: Ghrh sense: 5′-GCCATCTTCACCACCAA-3′; Ghrh antisense: 5′-CCTCCTGCTTGTTCATGATGT-3′; Sst sense: 5′-TCTGCATCGTCCTGGCTTT-3′; Sst antisense: 5′-CTTGGCCAGTTCCTGTTTCC-3′; cyclophilin sense: 5′-GTGGCAAGATCGAAGTGGAGAAAC-3′; cyclophilin antisense 5′-TAAAAATCAGGCCTGTGGAATGTG-3′. Semiquantitative determination of Ghrh, Sst, and cyclophilin cDNA were performed by kinetic PCR using 9.4 μl of TAQurate GREEN Real-Time PCR MasterMix (Epicenter Biotechnologies) containing 100 ng of cDNA, 0.4 μm primers, 10 mm Tris-HCl, 50 mm KCl, 3 mm MgCl2, 0.2 mm deoxy-NTP, and 1.25 U Taq polymerase in a final volume of 10 μl. After denaturation at 95°C for 10 min, the cDNA products were amplified with 40 cycles, each cycle consisting of denaturation at 95°C for 30 s, annealing at 61°C for 1 min, and extension at 72°C for 30 s. The accumulating DNA products were monitored by the ABI 7500 sequence detection system (Applied Biosystems) and data were stored continuously during the reaction. The calculations of the initial mRNA copy numbers in each sample were made according to the cycle threshold (CT) method as described previously (García-Tornadu et al., 2009). The relative Ghrh or Sst gene expression was normalized to that of the cyclophilin housekeeping gene using the standard curve method. Results are expressed as arbitrary units (AU) for comparison among samples. AU was defined as the expression level relative to a sample of wild-type mice (calibrator sample).
Food intake.
Determination of food intake was performed in isolated adult Drd2loxP/loxP or neuroDrd2KO mice. Animals and food (regular chow) were weighed daily at the same hour during the light cycle (15:00 h) for 7 d.
Behavioral testing.
Haloperidol-induced catatonia was determined using the horizontal bar test in which the time of immobility after 30 min of a single injection of saline or 1.5 mg/kg haloperidol (Tocris Bioscience) was assessed. Five trials were performed for each animal with a 180 s cutoff for each trial. For the analysis, we considered the maximum time of immobility for each mouse over the five trials. The tube test, performed to assess social dominance, consisted of introducing two adult mice in opposite entrances of a cylindrical acrylic tube of 3.4 cm diameter and 30 cm length and allowing them to interact in the center of the tube by removing a gate placed at 15 cm from each entrance. It is expected that one of the animals will force the other to retreat from the tube by pushing it out. Successful trials were considered those in which a mouse retreated from the tube being pushed by the victor. We also observed atypical trials in which a mouse walked backwards by itself, not being forced by its opponent. Trials in which neither of the two mice pushed the other out during the 10 min test were considered null and discarded from the analysis. We assayed give pairs per genotype: C57BL/6J versus C57BL/6J females, Drd2loxP/loxP males, or neuroDrd2KO males. First, we trained all mice to enter the tube by both entrances. After training, each pair of confronting mice was assayed three times in the tube and the 15 trials considered in the analysis. A variant of the intruder-resident aggression test, using castrated wild-type (WT) mice as intruders, was used to determine the aggressive display promoted by the fresh urine of Drd2loxP/loxP or neuroDrd2KO male mice on isolated sex- and age-matched C57BL/6J mice (Hurst et al., 2001). C57BL/6J adult male mice were isolated for at least 1 month and then wild-type castrated adult male mice were swabbed on their genitals and back with 150 μl of saline or fresh collected urine from Drd2loxP/loxP or neuroDrd2KOmice. After 1 min of drying, intruders were introduced to the home cage of an isolated male for 3 min. All tests were videotaped and analyzed for determination of latency to first aggressive contact, consisting of kicking, biting, wrestling, or tumbling and total time spent attacking. The odor-stimulated scent-marking test was performed using as experimental subjects C57BL/6J adult male mice and, as stimulating odor sources, the cages in which Drd2loxP/loxP or neuroDrd2KO mice had been housed for the previous 48 h (test cages). The bedding of the test cages was removed but the cage itself was not cleaned. A rectangle (13 cm × 22 cm) of absorbent paper (Whatman, 3 mm Chr) was placed over the floor of each cage. Immediately, an adult male C57BL/6J previously isolated for 1 month was placed in each test cage. The subjects were allowed to explore the test cages for 15 min and then removed. The whole procedure took place during the first half hour of the dark period in the behavioral room to profit from the enhanced activity of the animals in that period. The papers were then removed from the test cages and photographed under UV light (UVP White/UV Transilluminator). Finally, the number of urine spots deposited on each paper was counted manually, assisted by Photoshop 7.0. Spots smaller than 1 mm or dragging marks were not considered in the analysis.
Statistics.
One- or two-way ANOVA, repeated-measures ANOVA, ANOVA on ranks (Kruskal-Wallis on ranks), Student's test, one-tailed χ2 analysis, and χ2 2 × 2 contingency tables were applied as appropriate. Fisher LSD or Dunnett's contrasts were analyzed post hoc. In all experiments, data are represented as means ± SEM.
Results
Mice lacking D2Rs in pituitary lactotropes display normal body growth curves
To inactivate Drd2 expression in pituitary lactotropes, we first generated transgenic mice carrying 2.9 kb of 5′ flanking sequences of the mouse prolactin gene ligated upstream of nuclear-targeted cre recombinase coding sequences. A selected pedigree, Tg(Prl-cre)1Mrub, expressed functional cre in most PRL-producing cells of the anterior pituitary in a highly selective manner as determined by immunofluorescence performed on coronal pituitary sections of double transgenic mice obtained by crossing Tg(Prl-cre)1Mrub mice with the cre reporter mouse line Ai14 (Madisen et al., 2010) that expresses the fluorescent protein td-tomato upon cre recombination (Fig. 1A). LacDrd2KO mice were obtained by successively breeding Tg(Prl-cre)1Mrub mice with Drd2loxP/loxP mice (Bello et al., 2011). In situ hybridization analysis using a Drd2 exon 2 antisense riboprobe showed that lacDrd2KO mice lacked D2Rs in pituitary lactotropes while retaining normal Drd2 expression in pituitary melanotropes of the intermediate lobe (Fig. 1B) and in all brain areas examined (Fig. 1C). To investigate whether ablation of D2R in lacDrd2KO mice was complete, we measured basal serum PRL levels and, more importantly, the acute effect of the D2R blocker haloperidol. LacDrd2KO mice displayed hyperprolactinemia that was particularly significant in females (Fig. 1D). The D2R antagonist haloperidol (3 mg/kg, i.p.) failed to increase serum PRL levels in lacDrd2KO mice of both genders, in contrast to the effect observed in Drd2loxP/loxP control mice, demonstrating the total absence of functional D2Rs in lactotropes of conditional lacDrd2KO mice (Fig. 1D). We also found that haloperidol was equally effective at inducing catatonia in lacDrd2KO and Drd2loxP/loxP control mice, in contrast to Drd2−/− mice, which were insensitive to the D2R blocker's effect (Fig. 1E), indicating that central D2R function is intact in lacDrd2KO mice. Therefore, lacDrd2KO mice are authentic lactotrope D2R-deficient mice. Body size and body weight curves of lacDrd2KO male mice were normal and identical to those observed in Drd2loxP/loxP male siblings (Fig. 1F,G), as well as GH axis parameters such as pituitary GH and serum IGF-I levels, which were also normal in lacDrd2KO males (Fig. 1H). These results indicate that the absence of D2Rs in pituitary lactotropes is not likely to account for the dwarfism found in Drd2−/− male mice.
Figure 1.
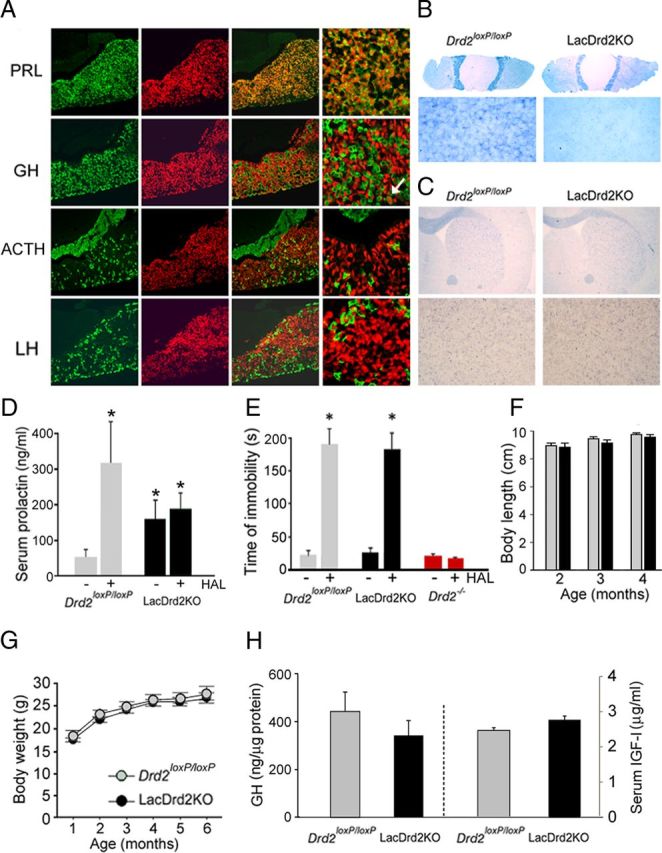
LacDrd2KO mice display hyperprolactinemia and normal body growth. A, Cre-mediated td-tomato expression in various pituitary cell types. Immunofluorescence using primary antisera for PRL, GH, ACTH, and LH (green) performed on coronal pituitary sections of Tg(Prl-cre)1Mrub mice crossed with the cre inducible td-tomato line Ai14 (first column). Identical sections show td-tomato fluorescence (red, second column). Superimposed images show coexpression of cre and PRL but not GH (with a few exceptions, see white arrow), ACTH or LH at lower (third column) and higher magnification (forth column). B, In situ hybridization using a Drd2 exon 2 antisense riboprobe in pituitary and brain sections of lacDrd2KO. Shown are whole pituitary sections and higher magnification of the anterior lobe and coronal brain sections (C) at the level of striatum and higher magnification. D, Serum PRL levels in Drd2loxP/loxP (n = 10) and lacDrd2KO (n = 11) females receiving saline (-) or haloperidol 3 mg/kg intraperitoneally (HAL, +). Two-way ANOVA *p < 0.01 versus saline-treated Drd2loxP/loxP mice. E, Haloperidol (1.5 mg/kg, i.p.)-induced catatonia. Latency to remove the front paws from an elevated horizontal bar was evaluated in Drd2loxP/loxP (n = 4), lacDrd2KO (n = 5), and Drd2−/− (n = 3) male mice. *p < 0.01 versus saline for each genotype. F, Nose to anus length of neuroDrd2KO and Drd2loxP/loxP male littermates. G, Body weight curves of Drd2loxP/loxP and lacDrd2KO male littermates. No significant differences were found between genotypes. H, Radioimmunoassay showed normal pituitary GH and serum IGF-I levels in lacDrd2KO mice relative to their Drd2loxP/loxP littermates. Bars and circles represent the mean ± SEM.
NeuroDrd2KO mice have reduced body weight and size
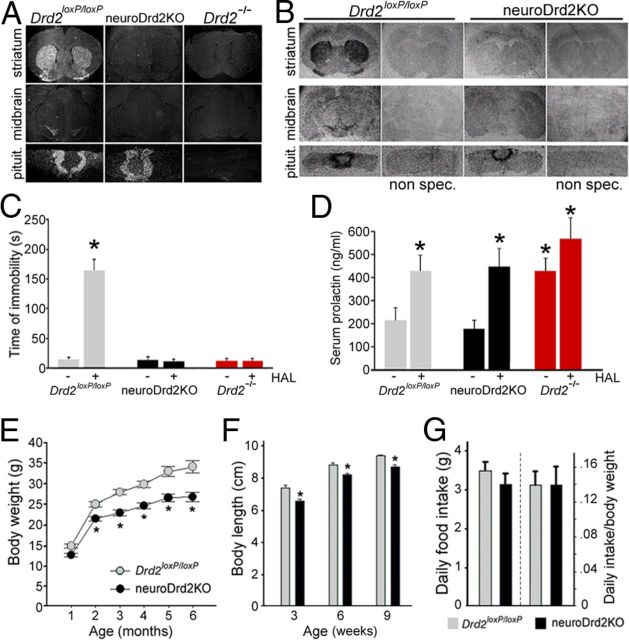
Transgenic mice expressing cre from a rat nestin promoter (Zimmerman et al., 1994; Tronche et al., 1999) were used in combination with Drd2loxP/loxP mice to inactivate Drd2 alleles in neural progenitors. As a result, neuroDrd2KO mice carry null Drd2 alleles in cells of neural origin and normally express Drd2 in other cell types, as demonstrated at the molecular and functional levels (Fig. 2). In situ hybridization assays using a Drd2 exon 2 antisense riboprobe (Fig. 2A) and [3H]-nemonapride binding autoradiography (Fig. 2B) revealed the absence of Drd2 mRNA and D2Rs, respectively, on coronal brain sections taken from neuroDrd2KO mice. NeuroDrd2KO mice failed to show haloperidol-induced catatonia, in contrast to Drd2loxP/loxP mice, but similar to Drd2−/− mice (Fig. 2C), indicating the absence of functional central D2Rs. In addition, D2R levels in the pituitary glands of neuroDrd2KO mice appeared to be similar to those observed in Drd2loxP/loxP mice (Fig. 2A,B) and functionally normal, as evidenced by the normal prolactin levels observed in neuroDrd2KO mice (Fig. 2D) and the increase observed after receiving a 3 mg/kg intraperitoneal haloperidol injection (Fig. 2D). Therefore, neuroDrd2KO mice lack D2Rs in the CNS but retain normally functional D2Rs in the anterior pituitary. NeuroDrd2KO male mice showed reduced body weight (Fig. 2E) and length (Fig. 2F) compared with their Drd2loxP/loxP male littermates. Body weight of neuroDrd2KO mice was 14.2% lower than Drd2loxP/loxP at 2 months of age and this difference increased over time, being 21.7% lower at 6 months (Fig. 2E). Body size of neuroDrd2KO mice at weaning was already significantly lower (10.2%) than control littermates and the difference persisted until reaching adulthood (Fig. 2F). The decreased growth exhibited by neuroDrd2KO male mice was not due to reduced feeding, because the daily food intakes of neuroDrd2KO and Drd2loxP/loxP siblings were similar (Fig. 2G). Therefore, these results demonstrate that the sole absence of D2Rs in the nervous system impairs normal growth rates and produces smaller mice.
Figure 2.
NeuroDrd2KO mice have reduced body weight and size. A, B, In situ hybridization assay using a Drd2 exon 2 [35S]-UTP antisense riboprobe (A) and [3H]-nemonapride binding autoradiography (B) in brain and pituitary coronal sections of Drd2loxP/loxP and neuroDrd2KO mice. Sections of Drd2−/− mice were included as negative controls. C, Haloperidol (HAL, 1.5 mg/kg, i.p.)-induced catatonia. Latency to remove the front paws from an elevated horizontal bar was evaluated in neuroDrd2KO mice and compared with Drd2loxP/loxP (n = 6) and Drd2−/− (n = 6) mice. *p < 0.01 haloperidol versus saline for each genotype. D, Serum PRL levels of female mice (n = 10–12) receiving haloperidol (HAL, 3 mg/kg, i.p.) or saline. *p < 0.05 haloperidol versus saline for each genotype. E, Body weight curves of neuroDrd2KO and Drd2loxP/loxP male mice (n = 7–12). Repeated-measures ANOVA genotype: F(1,14) = 14.06, p < 0.005. *p < 0.01 neuroDrd2KO versus Drd2loxP/loxP littermates, post hoc Fisher analysis. F, Nose to anus length of neuroDrd2KO and Drd2loxP/loxP male mice (n = 9–11) measured 3, 6, and 9 weeks after birth. Repeated-measures ANOVA genotype: F(1,16) = 21.35, p < 0.005. *p < 0.05 for neuroDrd2KO versus age-matched Drd2loxP/loxP littermates, post hoc Fisher analysis. G, Food consumption was measured for 7 consecutive days in adult neuroDrd2KO and Drd2loxP/loxP (n = 5–10) mice. Averaged food intake (left) and food intake relative to animal weight (right) is plotted. Bars and circles represent the mean ± SEM.
NeuroDrd2KO mice have an impaired GH axis
We analyzed the relative number of somatotropes and GH content in pituitaries from neuroDrd2KO and control male mice. Immunofluorescence performed on pituitary slices taken from neuroDrd2KO male mice showed a 28.1% shortfall of GH-positive cells compared with control male mice (Fig. 3A,B). In agreement, a great reduction (63.2%) in GH content was determined by radioimmunoassay in pituitaries from neuroDrd2KO mice (Fig. 3B). Analysis of the hypothalamic content of Ghrh and Sst mRNAs using a semiquantitative RT-PCR assay showed that neuroDrd2KO males had 30% lower Ghrh mRNA levels compared with their Drd2loxP/loxP littermates (Fig. 3C), whereas hypothalamic Sst mRNA levels were not significantly altered (Fig. 3C). These results demonstrate that male mice lacking D2Rs in the nervous system develop an immature GH axis that limits the body growth program.
Figure 3.
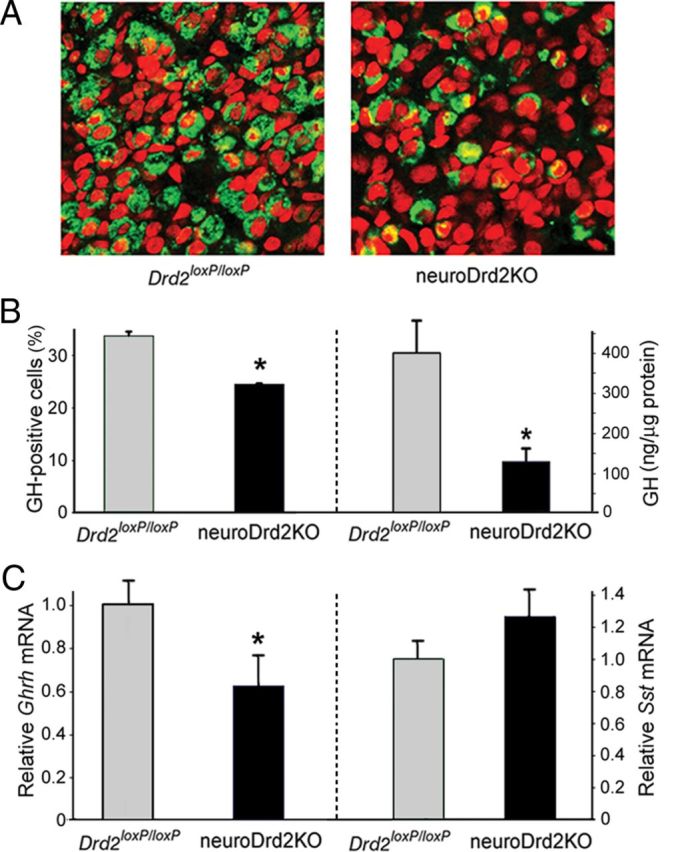
NeuroDrd2KO mice display an impaired GH axis. A, Immunofluorescence using an anti-hGH primary antiserum performed on 4 μm pituitary slices of Drd2loxP/loxP (n = 3) and neuroDrd2KO (n = 3) mice showed fewer pituitary somatotropes (green) in neuroDrd2KO mice relative to control mice. Cell nuclei were stained with propidium iodide (red). B, Right, Percentage of GH-positive cells, one-way ANOVA (OWA): F(1,4) = 18.01, *p < 0.02. Left, radioimmunoassay of GH concentration in anterior pituitary glands of Drd2loxP/loxP (n = 7) and neuroDrd2KO (n = 10) siblings (OWA: F(1,15) = 7.54, *p < 0.05). C, Right, relative hypothalamic mRNA levels of Ghrh in Drd2loxP/loxP (n = 9) and neuroDrd2KO (n = 9) mice determined by semiquantitative real-time PCR (OWA: F(1,16) = 6.32, *p < 0.05). Left, relative hypothalamic mRNA levels of Sst from Drd2loxP/loxP and neuroDrd2KO mice (n = 13). Samples were normalized to cyclophilin mRNA. Bars denote the mean ± SEM. All mice were 6–9 months old.
NeuroDrd2KO mice have low levels of the GH-dependent liver proteins IGF-I and MUP
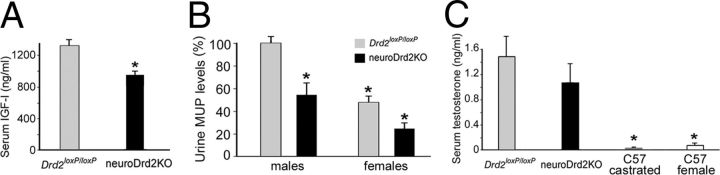
To determine whether the impaired GH axis found in neuroDrd2KO mice has downstream consequences in GH-dependent liver hormones, we measured IGF-I and MUP levels. Serum IGF-I levels were 18.7% lower in neuroDrd2KO male mice compared with those found in their control siblings (Fig. 4A), whereas MUP levels were markedly reduced (45.6%) in the urine of neuroDrd2KO male mice (Fig. 4B). Interestingly, MUP levels of the male mutants were similar to those found in the urine of their Drd2loxP/loxP female littermates (Fig. 4B). In contrast, serum testosterone levels in neuroDrd2KO male mice were similar to those found in control male littermates, in clear contrast to testosterone levels measured in adult wild-type females and castrated wild-type males (Fig. 4C). These results suggest that the reduced MUP levels were primarily the consequence of an impaired GH axis.
Figure 4.
NeuroDrd2KOmice have reduced IGF-I and MUPs and normal testostene levels. A, Radioimmunoassay of serum IGF-I levels in neuroDrd2KO (n = 14) and Drd2loxP/loxP (n = 20) mice (one-way ANOVA [OWA]: F(1,32) = 8.40, *p < 0.01). B, MUP levels in urine samples taken from male Drd2loxP/loxP (n = 17) and neuroDrd2KO (n = 12) adult mice and from their Drd2loxP/loxP (n = 16) and neuroDrd2KO (n = 9) female littermates. Two-way ANOVA showed significant main effects both for sex and genotype (sex effect: F(1,50) = 26.68, *p < 0.00001 and genotype effect: (1,50) = 17.84, *p < 0.0005). C, Serum testosterone levels determined by radioimmunoassay in neuroDrd2KO male mice (n = 9) compared with their Drd2loxP/loxP male littermates (n = 9). Castrated wild-type adult male mice (n = 4) and wild-type adult females (n = 5) were included as negative controls (OWA: F(3,23) = 5.0673, p < 0.008). *p < 0.05 for wild-type castrated males and wild-type females versus Drd2loxP/loxP and neuroDrd2KO male mice, post hoc Fisher's LSD. Bars represent the mean ± SEM.
Central D2Rs, social dominance, and territorial behavior
The higher MUP content typically found in the urine of adult males is thought to promote a variety of male-specific behaviors that are critical for reproductive fitness, such as social recognition, territorial scent-marking, and aggression (Hurst et al., 2001; Chamero et al., 2007). To evaluate whether the feminized lower MUP levels found in the urine of neuroDrd2KO male mice modify these evolutionarily conserved behaviors, we designed a series of behavioral tests. First, we examined how adult male C57BL/6J WT mice reacted when confronted with either Drd2loxP/loxP or neuroDrd2KO male adults in a social dominance experimental paradigm called the double entrance tube test (Lijam et al., 1997). Normally in this test, when two adult male mice are placed at the opposite extremes of a cylindrical tube, they both gain access to the center and, after close mutual inspection, one moves forward, forcing the other to walk backwards until stepping down from the end of the tube (Fig. 5A, right). However, when confronting females, adult male mice do not show this behavior and back out spontaneously without showing signs of social dominance. When we challenged C57BL/6J adult males with sex- and age-matched Drd2loxP/loxP mice, we observed a similar number of retreats in mice of each genotype: C57BL/6J males backed out from the tube in seven of 15 trials, whereas Drd2loxP/loxP males backed out in the other eight (Fig. 5A). Conversely, when challenged with WT females, C57BL/6J adult males backed out of the tube in 12 of 15 encounters without showing social dominance behavior (Fig. 5A). Interestingly, C57BL/6J adult male mice challenged against sex- and age-matched neuroDrd2KO mice exhibited behavioral responses similar to those shown when challenged against C57BL/6J females. In this case, C57BL/6J males backed out of the tube spontaneously in 10 of 15 encounters and moved forward neuroDrd2KO males in only five trials (Fig. 5A). The different reaction of C57BL/6J adult males toward neuroDrd2KO adult males compared with that observed when confronting their Drd2loxP/loxP littermates could be due, at least in part, to the relatively smaller size of neuroDrd2KO mice and/or to their lower concentration of urinary MUP. To further investigate this latter hypothesis, we tested the ability of the urine taken from Drd2loxP/loxP and neuroDrd2KO male adults to promote territorial male-to-male aggression in an adapted version of the intruder-resident aggression test (Chamero et al., 2007). To this end, each C57BL/6J adult male resident mouse reared in isolation for 1 month was challenged with a castrated adult WT male intruder mouse placed into the resident's cage. Each castrated mouse was swabbed on its back and genital area with saline or freshly collected urine taken from Drd2loxP/loxP or neuroDrd2KO adult male mice. Upon close olfactory inspection of the intruder, resident mice exhibited aggressive behavior that varied in latency and intensity depending on the nature of the liquid swabbed on the intruder. Latency to the first attack was 65 ± 17 s when castrated mice received urine from Drd2loxP/loxP mice, and this value increased significantly when mice were swabbed with either saline or urine taken from neuroDrd2KO male adult mice (Fig. 5B, left). Moreover, 3 of 6 intruders swabbed with saline and 3 of 6 intruders swabbed with neuroDrd2KO male mice urine received no attacks from the resident males during the 3 min test. Therefore, when resident males were confronted with castrated males swabbed with urine taken from neuroDrd2KO mice, the latency to the first attack increased markedly and the time spent attacking decreased compared with castrated intruders swabbed with urine taken from Drd2loxP/loxP male adults (Fig. 5B, right). Finally, we performed a third experiment to investigate whether C57BL/6J adult male mice displayed different scent-marking behavior when placed in a cage where a sex- and age-matched Drd2loxP/loxP or a neuroDrd2KO mouse was previously housed. A stereotyped behavior commonly observed when a male mouse detects odorant cues from another adult conspecific male is the induction of widespread urine depositions that are interpreted as increased scent-marking activity to cover the area with its own scent (Desjardins et al., 1973). This behavior is greatly reduced when the adult male is placed in a location previously visited only by a castrated or a subordinate male (Hurst and Beynon, 2004). By measuring the number of urinary spots, we determined that C57BL/6J adult male mice reared in isolation displayed greater scent-marking activity when placed in a cage previously occupied by a Drd2loxP/loxP mouse rather than by a neuroDrd2KO male adult littermate (Fig. 5C). These results indicate that wild-type male adults do not exhibit typical male-to-male social and territorial behavior when exposed to neuroDrd2KO males or to their urine. Therefore, central D2Rs are necessary for the masculinization of the GH axis and, therefore, for the production of higher levels of pheromones typically found in the urine of normal males.
Figure 5.
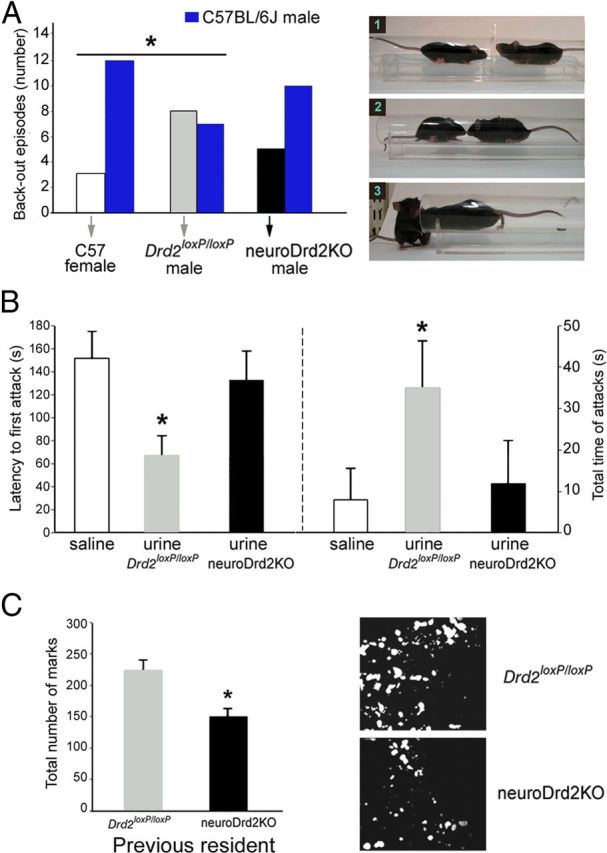
Urine from adult neuroDrd2KO males fails to promote social and territorial behaviors. A, Left, C57BL/6J adult females, Drd2loxP/loxP adult males and neuroDrd2KO adult males were challenged with C57BL/6J adult males in a social-dominance tube test. Fifteen trials were performed for each genotype. The behavior of C57BL/6J males was significantly different when confronting C57BL/6J females compared with Drd2loxP/loxP males (one-tailed χ2 *p < 0.05). C57BL/6J males confronting neuroDrd2KO males showed an intermediate phenotype because their behavior was not different from that observed when confronting either C57BL/6J females or Drd2loxP/loxP males (one-tailed χ2 p > 0.05). Right, Representative images of a typical test: (1) two mice approaching the center of the tube, (2) mutual inspection, and (3) the mouse on the right pushes the other out. B, Intruder-resident test. Isolated C57BL/6J adult male residents (n = 18) were challenged with C57BL/6J castrated males swabbed on their backs and genital areas with saline (n = 6), urine from Drd2loxP/loxP adult male mice (n = 6), or urine from neuroDrd2KO adult males (n = 6). Left, latency to the first resident's attack (one-way ANOVA [OWA]: F(2,15) = 4.55, p < 0.05). *p < 0.05 compared with saline, post hoc Fisher analysis. Right, total time spent attacking (Kruskal-Wallis OWA on ranks: H = 6.65 with 2 degrees of freedom, p < 0.05). *p < 0.05 compared with saline, post hoc Dunnett's comparison. C, Scent marking test. Left, C57BL/6J adult male mice were challenged in a cage previously occupied by a Drd2flox/flox (n = 5) or a neuroDrd2KO (n = 8) adult male mouse. Urine marks made by the visitor during 5 min were counted under UV light (OWA: F(1,11) = 14.66, *p < 0.005). Right, two representative examples of spotted urine are shown. Bars represent the mean ± SEM.
Discussion
In this study, we conducted a differential cell-specific ablation strategy to eliminate D2Rs selectively from pituitary lactotropes and, independently, from brain neurons. Whereas lacDrd2KO mice displayed normal body growth, neuroDrd2KO mice showed deficits in the GH axis that led to reduced body weight and length, recapitulating the dwarf phenotype observed in Drd2−/− mutants. Based on the analysis of lacDrd2KO mice, we concluded that the absence of lactotrope D2R is not responsible for the altered lactotrope/somatotrope ratio previously observed in Drd2−/− mice, ruling out its participation in the terminal differentiation of these two cell types. Furthermore, neuroDrd2KO mice showed fewer somatotropes, diminished pituitary GH content, and lower levels of hypothalamic Ghrh expression. Therefore, central D2Rs are essential for the normal maturation of the GH axis and full development of the body growth plan. Selective ablation of D2Rs in the brain while maintaining normal Drd2 expression in the pituitary was achieved by using a strain of transgenic mice expressing cre from a nestin promoter. A similar strategy has been used previously to eliminate the transcription factor CREB from the CNS (Mantamadiotis et al., 2002) because the nestin gene (Nes) is expressed in embryonic dividing cells derived from the neuroectoderm but not from the oral ectoderm (Zimmerman et al., 1994; Tronche et al., 1999) and is particularly absent from progenitors and differentiated cells of all anterior pituitary cell lineages (Krylyshkina et al., 2005; Gleiberman et al., 2008). In fact, neuroDrd2KO mice showed normal Drd2 expression in the intermediate and anterior lobe of the pituitary but complete absence of expression in the CNS. The differential response to haloperidol-induced hyperprolactinemia and catatonia observed in lacDrd2KO and neuroDrd2KO mice confirmed, at the functional level, our successful attempt to inactivate Drd2 expression selectively in the pituitary or in the brain.
Identifying the Drd2-expressing neurons in the CNS controlling the maturation of the GH axis is still pending, although our own studies ruled out the participation of DA neurons expressing D2 autoreceptors (Bello et al., 2011) and striatal medium spiny neurons of the indirect extrapyramidal pathway (Kaplan et al., 2012), because these two conditional Drd2 knock-out models exhibited normal growth curves. Because D2Rs couple to inhibitory signals that diminish neuron activity, a candidate neuronal type is a group of periventricular SST neurons that make synaptic contacts with GHRH arcuate neurons to exert a tonic inhibition of Ghrh expression and GHRH release (Liposits et al., 1988; McCarthy et al., 1992; Müller et al., 1999) and control the rhythmic oscillations of GHRH neuronal firing that establish the male pattern of GH release (Müller et al., 1999; Low et al., 2001). However, a semiquantitative RT-PCR determination only found a tendency for increased hypothalamic Sst mRNA levels in neuroDrd2KO mice that never reached statistical significance (Fig. 3C).
The present study demonstrates that central D2Rs regulate a neuroendocrine/exocrine cascade that controls body growth and participates in male-to-male territorial behavior. This multistep signaling cascade initiates in brain centers that regulate the hypothalamic level Ghrh expression and GHRH release, which then relays to the pituitary to control the pattern of GH release. GH surges from the pituitary of male rodents are characterized by high-amplitude and low-frequency pulses intercalated among long nadir periods, which permit the resensitization of GH receptor signaling by a Janus kinase/signal transducer and activation of transcription (JAK-STAT) complex (Waxman et al., 1991) that specifically involves STAT5a and STAT5b (Udy et al., 1997; Davey et al., 1999; Park et al., 1999). Only when long interpulse nadirs of GH levels are attained in puberal males are high transcriptional rates of Igf1 and other somatomedins that promote body growth observed in the liver and extrahepatic sites (Low et al., 2001). The lower serum levels of IGF-I observed in neuroDrd2KO mice are consistent with their smaller body size and weight, a phenotype that in the wild could be detrimental for reproductive fitness due to reduced competitiveness among males. The sexually dimorphic liver proteins that depend on the male pattern of GH release is manifold and include MUPs (McIntosh and Bishop, 1989). This group of pheromones secreted into the blood and then excreted in the urine are known to promote social recognition and territorial dominance between males (Hurst et al., 2001; Chamero et al., 2007). Therefore, an emerging downstream finding disclosed by this study is that the urine of mice lacking D2Rs in the nervous system showed a lower content of MUP and, therefore, lost the signaling cues that trigger territorial behavior in other males. Typically, MUP levels in males are much higher than in females (Stopková et al., 2007) and this difference not only depends on the sexually dimorphic pattern of GH pulsatility, but also on testosterone concentration, because castrated males have feminized MUP levels and females treated with testosterone display male MUP levels (Hastie et al., 1979). Because testosterone concentration was normal in neuroDrd2KO mice, the reduced MUP levels are likely to be the sole consequence of an impaired GH axis resulting from lack of Drd2 expression in the CNS. MUPs are encoded by >30 genes, mainly synthetic, in a locus of mouse chromosome 4 (Hastie et al., 1979; Cheetham et al., 2007). The large allelic variation at this locus and the relative amount of each MUP in the mouse urine contributes to generate a great combinatorial diversity of individual patterns that are believed to provide, in the wild, a unique biological identity card for each conspecific (Cheetham et al., 2007). In fact, male mice use MUPs to scent-mark and countermark territories (Hurst and Beynon, 2004). The ability of MUPs to create a chemical barrier for territorial defense and to promote territorial protection adds to the adaptive behavioral inventory that males use to gain access to receptive females. Therefore, this study shows that central D2R regulate a neuroendocrine-exocrine cascade that controls the maturation of the GH axis and downstream signals that play important roles in territorial behavior and competition between adult males.
Throughout vertebrate evolution, the D2R has played key roles in the complex repertoire of adaptive functions that improve fitness, reproductive success, and survival. For example, D2Rs in the nucleus accumbens and striatum participate in neural circuits that assign reinforcing and hedonic value to nutritious food and reproductive sex (Berridge and Robinson, 1998; Kelley and Berridge, 2002; Palmiter, 2008). Moreover, this system acquired the exquisite capacity to anticipate and predict the close availability of food and sexual mates by providing incentive salience to conditioned environmental stimuli (Berridge and Robinson, 1998; Schultz, 2007). D2Rs are fundamental also in motor coordination, locomotor activity, and executive planning, all functions that allow animals to expand the territories used to search for natural rewards. In addition, it has been shown that central D2Rs participate in establishing the social dominance ranking within males (Morgan et al., 2002). In mammalian females, D2Rs of pituitary lactotropes mediate a functional switch that is essential for reproduction: although tuberoinfundibular dopamine normally inhibits PRL release to ensure fertility, D2R stimulation is suppressed before labor to greatly increase circulating PRL levels that exert lactogenic and social bonding properties that are necessary to raise the newborns efficiently. In summary, our study reports two additional adaptive properties of central D2Rs: (1) high levels of somatotropic hormones allow adult male mice to fulfill their genetic body growth program and reach a fit body size and, in parallel, (2) adult males excreting large amounts of MUPs in their urine are able to protect their territories by scent-marking an area that potential intruders will recognize as being defended by a fully developed active male.
Footnotes
This work was supported in part by the Howard Hughes Medical Institute (International Research Scholar Grant to M.R.), Tourette Syndrome Association (to M.R.), Universidad de Buenos Aires (to M.R.), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina, to D.B.-V.), and the Agencia Nacional de Promoción Científica y Tecnológica (to M.R. and D.B.V.). D.N., M.I.P.-M., E.P.B., G.M.L., D.M.G., M.P., and I.G.T., were recipients of doctoral fellowships from CONICET. We thank the National Hormone and Pituitary Program (National Institute of Diabetes and Digestive and Kidney Diseases) and Dr. A.F. Parlow for mouse RIA kits and antibodies, Guillermo Lanuza for providing Ai14 mice, and Vanina Rodriguez and Irina García Suárez for excellent technical assistance.
The authors declare no competing financial interests.
References
- Alba M, Salvatori R. A mouse with targeted ablation of the growth hormone-releasing hormone gene: a new model of isolated growth hormone deficiency. Endocrinology. 2004;145:4134–4143. doi: 10.1210/en.2004-0119. [DOI] [PubMed] [Google Scholar]
- Asa SL, Kelly MA, Grandy DK, Low MJ. Pituitary lactotroph adenomas develop after prolonged lactotroph hyperplasia in dopamine D2 receptor-deficient mice. Endocrinology. 1999;140:5348–5355. doi: 10.1210/endo.140.11.7118. [DOI] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noaín D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, Rubinstein M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- Cheetham SA, Thom MD, Jury F, Ollier WE, Beynon RJ, Hurst JL. The genetic basis of individual-recognition signals in the mouse. Curr Biol: CB. 2007;17:1771–1777. doi: 10.1016/j.cub.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Davey HW, Park SH, Grattan DR, McLachlan MJ, Waxman DJ. STAT5b-deficient mice are growth hormone pulse-resistant. Role of STAT5b in sex-specific liver p450 expression. J Biol Chem. 1999;274:35331–35336. doi: 10.1074/jbc.274.50.35331. [DOI] [PubMed] [Google Scholar]
- Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- Díaz-Torga G, Feierstein C, Libertun C, Gelman D, Kelly MA, Low MJ, Rubinstein M, Becú-Villalobos D. Disruption of the D2 dopamine receptor alters GH and IGF-I secretion and causes dwarfism in male mice. Endocrinology. 2002;143:1270–1279. doi: 10.1210/endo.143.4.8750. [DOI] [PubMed] [Google Scholar]
- García-Tornadu I, Díaz-Torga G, Risso GS, Silveyra P, Cataldi N, Ramirez MC, Low MJ, Libertun C, Becú-Villalobos D. Hypothalamic orexin, OX1, alphaMSH, NPY and MCRs expression in dopaminergic D2R knockout mice. Neuropeptides. 2009;43:267–274. doi: 10.1016/j.npep.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Gleiberman AS, Michurina T, Encinas JM, Roig JL, Krasnov P, Balordi F, Fishell G, Rosenfeld MG, Enikolopov G. Genetic approaches identify adult pituitary stem cells. Proc Natl Acad Sci U S A. 2008;105:6332–6337. doi: 10.1073/pnas.0801644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey P, Rahal JO, Beamer WG, Copeland NG, Jenkins NA, Mayo KE. GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat Genet. 1993;4:227–232. doi: 10.1038/ng0793-227. [DOI] [PubMed] [Google Scholar]
- Hastie ND, Held WA, Toole JJ. Multiple genes coding for the androgen-regulated major urinary proteins of the mouse. Cell. 1979;17:449–457. doi: 10.1016/0092-8674(79)90171-5. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Beynon RJ. Scent wars: the chemobiology of competitive signalling in mice. BioEssays. 2004;26:1288–1298. doi: 10.1002/bies.20147. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DH, Cavaggioni A, Beynon RJ. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–634. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- Kaplan AR, Doyle T, Casey E, Free RB, Sibley DR, Rubenstein M, Alvarez VA. Distinct roles of dopamine D2 receptors in dorsal and ventral striatum on motor and drug-related behaviors. Paper presented at the Society for Neuroscience Annual Meeting; 2012; New Orleans, LA. 2012. [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Asa SL, Zhang G, Saez C, Bunzow JR, Allen RG, Hnasko R, Ben-Jonathan N, Grandy DK, Low MJ. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron. 1997;19:103–113. doi: 10.1016/s0896-6273(00)80351-7. [DOI] [PubMed] [Google Scholar]
- Krylyshkina O, Chen J, Mebis L, Denef C, Vankelecom H. Nestin-immunoreactive cells in rat pituitary are neither hormonal nor typical folliculo-stellate cells. Endocrinology. 2005;146:2376–2387. doi: 10.1210/en.2004-1209. [DOI] [PubMed] [Google Scholar]
- Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, Stevens KE, Maccaferri G, McBain CJ, Sussman DJ, Wynshaw-Boris A. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- Lin SC, Lin CR, Gukovsky I, Lusis AJ, Sawchenko PE, Rosenfeld MG. Molecular basis of the little mouse phenotype and implications for cell type-specific growth. Nature. 1993;364:208–213. doi: 10.1038/364208a0. [DOI] [PubMed] [Google Scholar]
- Liposits Z, Merchenthaler I, Paull WK, Flerkó B. Synaptic communication between somatostatinergic axons and growth hormone-releasing factor (GRF) synthesizing neurons in the arcuate nucleus of the rat. Histochemistry. 1988;89:247–252. doi: 10.1007/BF00493148. [DOI] [PubMed] [Google Scholar]
- Low MJ, Otero-Corchon V, Parlow AF, Ramirez JL, Kumar U, Patel YC, Rubinstein M. Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J Clin Invest. 2001;107:1571–1580. doi: 10.1172/JCI11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Martin Villalba A, Tronche F, Kellendonk C, Gau D, Kapfhammer J, Otto C, Schmid W, Schütz G. Disruption of CREB function in brain leads to neurodegeneration. Nat Genet. 2002;31:47–54. doi: 10.1038/ng882. [DOI] [PubMed] [Google Scholar]
- McCarthy GF, Beaudet A, Tannenbaum GS. Colocalization of somatostatin receptors and growth hormone-releasing factor immunoreactivity in neurons of the rat arcuate nucleus. Neuroendocrinology. 1992;56:18–24. doi: 10.1159/000126203. [DOI] [PubMed] [Google Scholar]
- McIntosh I, Bishop JO. Differential expression in male and female mouse liver of very similar mRNAs specified by two group 1 major urinary protein genes. Mol Cell Biol. 1989;9:2202–2207. doi: 10.1128/mcb.9.5.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Müller EE, Locatelli V, Cocchi D. Neuroendocrine control of growth hormone secretion. Physiol Rev. 1999;79:511–607. doi: 10.1152/physrev.1999.79.2.511. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann New York Acad Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Liu X, Hennighausen L, Davey HW, Waxman DJ. Distinctive roles of STAT5a and STAT5b in sexual dimorphism of hepatic P450 gene expression. Impact of STAT5a gene disruption. J Biol Chem. 1999;274:7421–7430. doi: 10.1074/jbc.274.11.7421. [DOI] [PubMed] [Google Scholar]
- Porter TE, Hill JB, Wiles CD, Frawley LS. Is the mammosomatotrope a transitional cell for the functional interconversion of growth hormone- and prolactin-secreting cells? Suggestive evidence from virgin, gestating, and lactating rats. Endocrinology. 1990;127:2789–2794. doi: 10.1210/endo-127-6-2789. [DOI] [PubMed] [Google Scholar]
- Porter TE, Wiles CD, Frawley LS. Evidence for bidirectional interconversion of mammotropes and somatotropes: rapid reversion of acidophilic cell types to pregestational proportions after weaning. Endocrinology. 1991;129:1215–1220. doi: 10.1210/endo-129-3-1215. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Scully KM, Rosenfeld MG. Pituitary development: regulatory codes in mammalian organogenesis. Science. 2002;295:2231–2235. doi: 10.1126/science.1062736. [DOI] [PubMed] [Google Scholar]
- Stopková R, Stopka P, Janotová K, Jedelský PL. Species-specific expression of major urinary proteins in the house mice (Mus musculus musculus and Mus musculus domesticus) J Chem Ecol. 2007;33:861–869. doi: 10.1007/s10886-007-9262-9. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schütz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci U S A. 1997;94:7239–7244. doi: 10.1073/pnas.94.14.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, Pampori NA, Ram PA, Agrawal AK, Shapiro BH. Interpulse interval in circulating growth hormone patterns regulates sexually dimorphic expression of hepatic cytochrome P450. Proc Natl Acad Sci U S A. 1991;88:6868–6872. doi: 10.1073/pnas.88.15.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, Mann J, Vassileva G, McMahon A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]