Abstract
Doxorubicin (DOX) is widely used as a first-line chemotherapeutic drug for various malignancies. However, DOX causes severe cardiotoxicity, which limits its clinical uses. Oxidative stress is one of major contributors to DOX-induced cardiotoxicity. While autophagic flux serves as an important defense mechanism against oxidative stress in cardiomyocytes, recent studies have demonstrated that DOX induces the blockage of autophagic flux, which contributes to DOX cardiotoxicity. The present study investigated whether nicotinamide riboside (NR), a precursor of nicotinamide adenine dinucleotide (NAD)+, prevents DOX cardiotoxicity by improving autophagic flux. We report that administration of NR elevated NAD+ levels, and reduced cardiac injury and myocardial dysfunction in DOX-injected mice. These protective effects of NR were recapitulated in cultured cardiomyocytes upon DOX treatment. Mechanistically, NR prevented the blockage of autophagic flux, accumulation of autolysosomes, and oxidative stress in DOX-treated cardiomyocytes, the effects of which were associated with restoration of lysosomal acidification. Furthermore, inhibition of lysosomal acidification or SIRT1 abrogated these protective effects of NR during DOX-induced cardiotoxicity. Collectively, our study shows that NR enhances autolysosome clearance via the NAD+/SIRT1 signaling, thereby preventing DOX-triggered cardiotoxicity.
Introduction
Doxorubicin (DOX) was first isolated from Streptomyces peucetius and reported by Arcamone and colleagues in 1969 [1,2]. It is generally known that DOX is a broad-spectrum antitumor drug used in the treatment of a variety of malignant tumors, including hematologic malignancies and solid tumors. However, the use of DOX in clinical practice is limited by its dose-dependent cardiotoxic effects, which include consequences such as cardiomyocyte death via apoptosis [3,4], necroptosis [5] and ferroptosis [6], left ventricular (LV) dysfunction, dilated cardiomyopathy, and congestive heart failure in severe cases [7]. However, effective drugs to prevent DOX-induced cardiotoxicity have been limited. Thus, development of novel strategies to attenuate DOX-triggered cardiotoxicity, but not at the cost of its antineoplastic effects, is urgently required.
DOX-induced cardiotoxicity is mainly a result of oxidative stress [8,9]. This results from the generation of reactive oxygen species (ROS) during drug metabolism [10]. The role of oxidative stress has been strengthened by the fact that inhibition of ROS production protects the heart against DOX-induced injury [11–14]. Notably, the abundance and activity of the antioxidant enzymes superoxide dismutase and catalase are lower in the heart than in other organs, and they are additionally down-regulated in DOX cardiotoxicity [15]. While autophagic flux serves as an important defense mechanism against oxidative stress [16,17], recent studies have provided strong evidence showing that DOX induces autophagic flux blockage in the heart [18,19]. The blockage of autophagic flux cannot properly remove damaged organelles and unprocessed proteins resulting from oxidant stress in DOX-induced cardiotoxicity, which may augment oxidant stress. Indeed, autophagic flux blockage during lysosomal defect directly induces cellular toxicity [20]. Thus, improving autophagic flux may be a new therapeutic strategy to reduce DOX cardiotoxicity. However, effective approaches targetting autophagic flux have not been well investigated in preventing DOX cardiotoxicity.
Nicotinamide riboside (NR) is a pyridine-nucleoside form of vitamin B3 found in human diets [21]. NR is converted to nicotinamide adenine dinucleotide (NAD+) in a two-step reaction catalyzed by NR kinases 1/2 and nicotinic acid mononucleotide adenylyl-transferase [22,23]. NAD+ is a classical coenzyme mediating many redox reactions that also plays an important role in the regulation of NAD+-consuming enzymes, including sirtuins, poly-ADP-ribose polymerases (PARPs), and CD38/157 ectoenzymes [22,24]. In particular, redox reactions and sirtuins are implicated in regulating the autophagic process and oxidative stress. This raises an intriguing possibility that NR may represent a new agent to improve autophagic flux in preventing DOX cardiotoxicity. Thus, the present study was designed to examine whether NR improves autophagic flux and reduces oxidant stress, thereby protecting cardiomyocytes against DOX-induced injury both in vitro and in vivo.
Materials and methods
Animals
This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication, 8th edition, 2011). All experimental procedures were approved by the Animal Use Subcommittee at Western University, Canada (protocol number: 2016–059), and animal studies were conducted at Victoria Research Laboratories of Lawson Health Research Institute, London, Canada. Breeding pairs of C57BL/6 mice and CAG-RFP-EGFP-LC3 transgenic mice expressing RFP and EGFP (Tg-RFP-GFP-LC3, C57BL/6 background) were purchased from the Jackson Laboratory [25]. All animals were housed in a temperature and humidity controlled facility with 12-h light and dark cycles, water and food ad libitum.
Reagents
NR, EX-527, necrostatin-1, bafilomycin A1, chloroquine (CQ), and DOX were purchased from Sigma–Aldrich (Canada), and GSK872 from Cayman Chemical. Lysotracker Red (DND-99), LysoSensor yellow/blue DND-160, Amplex Red, MitoSOX™ Red, Annexin-V and Hoechst 33324 were purchased from Life Technologies.
Experimental protocol
Male mice (aged 2 months) were injected with a single dose of DOX (20 mg/kg, i.p., Sigma) [3,4]. Control mice received the same volume of sterile saline. NR (0, 100, 300, or 500 mg/kg, i.p.) was given 30 min prior to DOX injection. After 5 days of DOX injection, the animals were subjected to different experimental treatments.
To assess autophagic flux, bafilomycin A1 (1.5 mg/kg, i.p.) was administered to mice 2 h before killing.
To block autophagic flux or SIRT1 signaling, mice received a daily injection of CQ (50 mg/kg, i.p.) or EX-527 (10 mg/kg, i.p.), respectively, for 5 days immediately after DOX injection.
Echocardiography
Animals were lightly anesthetized with inhaled isoflurane (1%) and imaged on a warm handling platform using a 40 MHz linear array transducer attached to a preclinical ultrasound system (Vevo 2100, FUJIFILM Visual Sonics, Canada) with nominal in-plane spatial resolution of 40 μm (axial) × 80 μm (lateral). LV end-systolic inner diameter (LVIDs), LV end-diastolic inner diameter (LVIDd), and fractional shortening (FS) were analyzed. The pulsed wave Doppler measurements of maximal early (E) and late (A) transmitral velocities in diastole were performed in the apical view with the cursor at mitral valve inflow.
Cell membrane permeability to evans blue dye
Evans blue dye (EBD) was dissolved in saline and injected into mice (100 mg/kg body weight, i.p.). About 24 h later, the heart was harvested and embedded in optimal cutting temperature (OCT) compound (Sakura), snap frozen in liquid nitrogen, and cut into 5-μm cryosections. EBD uptakes (red) were visualized under fluorescence microscope.
Determination of autophagosomes and autolysosomes in Tg-RFP-GFP-LC3 mouse hearts
Since RFP (pKa 4.5) fluorescence is stable in an acidic environment, but GFP (pKa5.9) fluorescence is quenched in the acidic lysosomal compartment [26], autophagosomes (yellow) can be distinguished from autolysosomes (red), and flux can be determined directly. RFP/GFP analysis was performed as described [19]. Briefly, hearts were embedded in freezing matrix (OCT), and 8 μm frozen sections were prepared on a Leica CM3000 Cryostat (Leica Microsystems, Buffalo Grove, IL). Images were acquired on a fluorescent microscope.
Neonatal and adult mouse cardiomyocyte cultures
Neonatal mice (born in 24 h) were euthanized by decapitation. Neonatal cardiomyocytes were prepared and cultured according to methods we described previously [3,4]. Adult mice were killed by cervical dislocation. Hearts were isolated and perfused. Mouse ventricle cardiomyocytes were isolated according to methods previously described [4].
Measurement of lactate dehydrogenase and troponin I
The levels of troponin I in sera and lactate dehydrogenase (LDH) in culture media were measured using commercially available kits (Sigma–Aldrich, Canada) following the manufacturer’s instructions.
Cell viability
Cell Counting Kit-8 (CCK-8, Sigma–Aldrich, Canada) was used to determine viable cell number in neonatal cardiomyocytes following the manufacturer’s instructions.
Lysosomal pH measurement
Cardiomyocytes were incubated with LysoSensor yellow/blue DND-160 (1 mg/ml) for 5 min at 37°C. The LysoSensor dye is a ratiometric probe that produces yellow fluorescence in acidic environments but changes to blue fluorescence in neutral environments. The fluorescent signals were determined using a Spectra Max M5 Multi Mode Microplate Reader with excitation of 340 nm. The ratio of emission, 485/535 nm, was then calculated for each sample.
Caspase-3 activity
Caspase-3 activity in cell lysates was measured using a caspase-3 fluorescence assay kit (Biomol Research Laboratories, U.S.A.).
Determination of cellular DNA fragmentation
Cultured neonatal cardiomyocytes were prelabeled with BrdU (1 μg/ml). The DNA fragmentation was determined using a Cellular DNA Fragmentation ELISA kit (Roche Applied Science, Germany) according to the manufacturer’s instructions.
Annexin-V staining
Adult cardiomyocyte death was detected by Annexin-V and Hoechst 33324 staining as described previously [4]. At least 200 adult cardiomyocytes were examined for each sample.
Measurement of ROS production
Cell lysates or tissue lysates were incubated in a reaction buffer containing Amplex Red (0.05 mmol/l) and horseradish peroxidase (0.1 units/ml) at 37°C. The fluorescent signals were monitored by spectrofluorometer at 520/580 nm every 10 min. Mitochondrial superoxide generation was also assessed in living cardiomyocytes by using the MitoSOX™ Red mitochondrial superoxide indicator following manufacturer’s instructions.
Assessment of malondialdehyde production
The levels of malondialdehyde (MDA) in heart tissue lysates were measured using a TBARS assay kit (Cayman Chemical Company, U.S.A.) following the manufacturer’s instructions.
NAD+ assay
NAD+ was extracted from cells or tissues and measured by NAD/NADH Microplate Assay Kit (Cohesion Biosciences, U.K.) according to manufacturer’s instructions.
Western blot analysis
Western blot analysis was performed to determine the protein levels of LC3I/II (Catalog#: 2775), p62 (Catalog#: 5114), phosphorylated RIP3 (Thr231/Ser232, Catalog#: 57220) and total RIP3 (Catalog#: 15828), phosphorylated MLKL (Ser345, Catalog#: 37333) and total MLKL (Catalog#: 37705), acetylated p53 (Lys379, Catalog#: 2570) and total p53 (Catalog#: 2524), phosphorylated HA2X (Ser139, Catalog#: 9718) and total HA2X (Catalog#: 2595) using their specific antibodies from Cell Signaling Technology (1: 1000 dilution), USA. Antibody against GAPDH was from Santa Cruz Biotechnology, Inc (1:5000 dilution, Catalog#: sc-25778). Goat-Anti-Mouse IgG(H+L)-HRP conjugate (Catalog#: 170–6516), and Goat-Anti-Rabbit IgG(H+L)-HRP conjugate (Catalog#: 170–6515) were purchased from Bio-Rad (1:5000 dilution).
Statistical analysis
Data were provided as mean ± S.D. Student’s t test was employed for comparisons within two groups. ANOVA followed by Newman–Keuls test was performed for multigroup comparisons. A value of P<0.05 was regarded as statistically significant.
Results
Inhibitory effects of NR on DOX-induced injury in cardiomyocytes
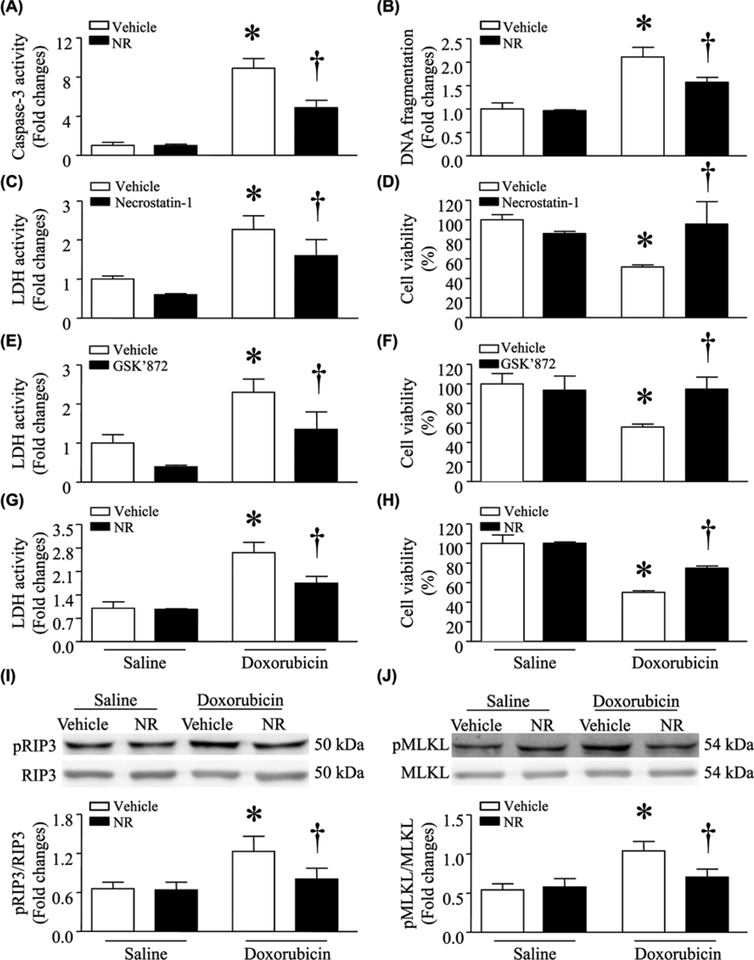
About 24 h after DOX treatment (0.1 μmol/l), apoptosis was significantly induced as determined by caspase-3 activity and DNA fragmentation in cultured neonatal cardiomyocytes (Figure 1A,B). DOX also increased the release of LDH in culture media and decreased cell viability, suggesting necrotic cell death (Figure 1C–H). These effects of DOX were attenuated by preincubation with NR (500 μmol/l) for 24 h.
Figure 1. Incubation with NR reduced DOX-induced injury in cultured cardiomyocytes.
Cultured neonatal mouse cardiomyocytes were incubated with NR (500 μmol/l) or vehicle for 24 h and then exposed to culture medium with DOX (1μmol/l) or saline for another 24 h. In separate experiments, cardiomyocytes were incubated with DOX or saline in combination with Necrostatin-1 or GSK’872 for 24 h. (A and B) Apoptosis was determined by caspase-3 activity and DNA fragmentation. (C, E, and G) LDH in culture media was measured. (D, F, and H) Cell viability was assessed. (I and J) The protein levels of total and phosphorylated RIP3 (pRIP3) and MLKL (pMLKL) were determined by western blot analysis. Upper panel: representative western blots for pRIP3 and pMLKL; lower panel: quantitation of pRIP3/GAPDH and pMLKL/GAPDH ratio. Data are mean ± S.D. from four different cell cultures with each in duplication. *P<0.05 versus saline + vehicle and †P<0.05 versus DOX + vehicle.
To determine necroptosis, we incubated cardiomyocytes with DOX or saline in the presence of inhibitors of necroptosis, necrostatin-1 (RIP1 inhibitor), or GSK872 (RIP3 inhibitor) for 24 h. Incubation with necrostatin-1 or GSK872 inhibited LDH release and reversed cell viability in DOX-induced cardiomyocytes, indicating the occurrence of necroptosis (Figure 1C–F). Furthermore, DOX increased the protein levels of phosphorylated RIP3 and phosphorylated MLKL, providing further evidence in support of necroptosis occurrence (Figure 1I,J). Interestingly, preincubation with NR also prevented necroptosis in DOX-stimulated cardiomyocytes as evidenced by inhibition of LDH release, normalization of cell viability, and prevention of RIP3 and MLKL phosphorylation (Figure 1G–J).
The protective effects of NR were further examined in isolated adult cardiomyocytes as they are more relevant to in vivo conditions in mice. As shown in Supplementary Figure S1, DOX induced a significantly higher proportion of cell death as determined by annexin V staining and LDH release, the effect of which was attenuated by preincubation with NR.
DOX significantly increased ROS formation and induced oxidative damage as evidenced by increased MDA production in cardiomyocytes. Preincubation with NR reduced ROS formation and prevented oxidative damage in DOX-induced cardiomyocytes (Supplementary Figure S2). These results suggest that NR protects cardiomyocytes against DOX-induced death, likely through the inhibition of oxidative stress as oxidative stress contributes to DOX-induced cardiomyocyte death [3].
Prevention of cardiac injury and dysfunction by NR in DOX-injected mice
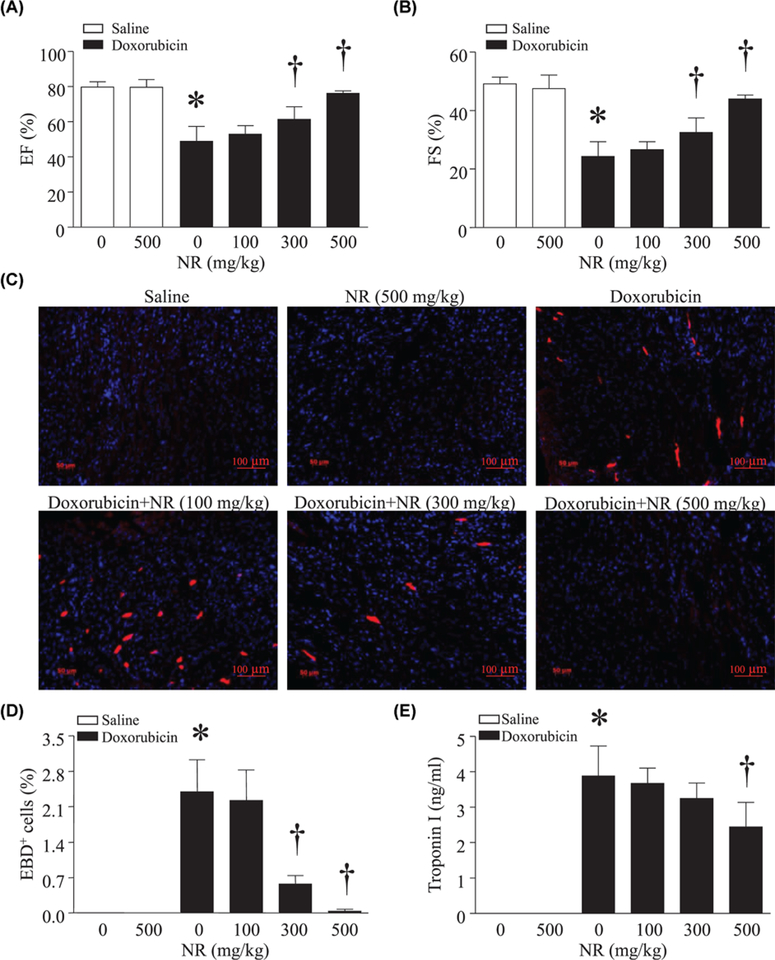
To investigate the in vivo effects of NR, we injected NR (100, 300, or 500 mg/kg, i.p.) or saline into mice. These doses of NR were chosen based on our recent study [27] and previous reports that showed that they were safe and efficiently increased the content of NAD+ in liver, lung, and muscle in mice [27–31]. About 30 min after NR injection, the animals received a single dose of DOX (20 mg/kg, i.p.). About 5 days later, DOX treatment decreased FS and ejection fraction, indicative of myocardial dysfunction. Preadministration of NR did not affect myocardial function in saline-injected mice, but dose-dependently attenuated myocardial dysfunction in DOX-injected mice (Figure 2A,B, Supplementary Table S1). DOX induced cardiac necrosis as evidenced by evans blue staining, which was inhibited by NR (Figure 2C,D). Consistently, as an indicator of cardiac injury, the plasma levels of troponin I were decreased by NR in a dose-dependent manner, further supporting the cardioprotective effects of NR (Figure 2E). Since the dose of 500 mg/kg for NR provided most effective protection, we chose this dose of NR for the following in vivo experiments.
Figure 2. Administration of NR reduced cardiac injury and improved myocardial function in DOX-injected mice.
Adult male mice received different doses of NR (0, 100, 300, or 500 mg/kg, i.p.) and 30 min later the animals were injected with DOX or saline. About 5 days after DOX injection, myocardial function was assessed by echocardiography (A and B). (C and D) Necrotic cell death was determined by evans blue dye positive (EBD+) cells. (C) Representative micro-pictures for evans blue staining (red) and nuclei staining (blue). (D) Quantitation of evans blue staining positive cells relative to total nuclei (%). (E) Troponin I was measured in blood. Data are mean ± S.D. from five different mice in each group. *P<0.05 versus saline + 0 and †P<0.05 versus DOX + 0.
In addition, NR reduced ROS formation and prevented oxidative damage in DOX-injected mouse hearts (Supplementary Figure S3). There was no death in all four groups of animals.
NR-mediated improvement in autophagic flux in cardiomyocytes and mouse hearts upon DOX treatment
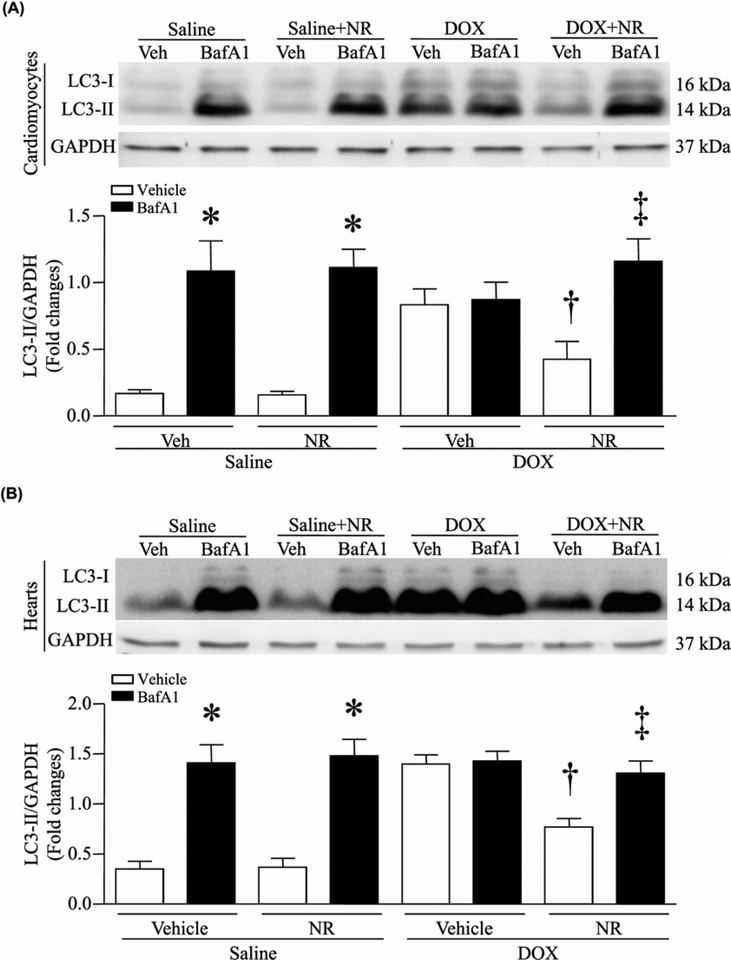
Since recent studies have implicated autophagic flux impairment in DOX cardiotoxicity [18,19], we evaluated changes in autophagic flux by analyzing LC3-II, an indicator of autophagy, in saline and DOX-treated cardiomyocytes in culture. DOX treatment increased the protein levels of LC3-II in cardiomyocytes (Figure 3A), suggesting the possibility of downstream blockage of autophagic vacuole processing. To address this possibility, we incubated cardiomyocytes with bafilomycin A1 (0.1 μmol/l), an inhibitor of late stage of autophagy [32]. Treatment with bafilomycin A1 induced a significant increase in the protein levels of LC3-II in saline-treated cardiomyocytes (Figure 3A). However, bafilomycin A1 did not further increase LC3-II protein levels in DOX-stimulated cardiomyocytes, suggesting that the accumulation of LC3-II in DOX-stimulated cardiomyocytes stems from inhibition of LC3-II turnover, implicating the blockage of autophagic flux. Interestingly, preincubation with NR normalized the protein levels of LC3-II in DOX-treated cardiomyocytes to that of saline-treated cardiomyocytes, but increased the protein levels of LC3-II in DOX-challenged cardiomyocytes when autophagic flux was blocked by bafilomycin A1 (Figure 3A). These results demonstrate that NR protects autophagic flux in DOX-stimulated cardiomyocytes. Notably, these actions of NR on autophagic flux were also recapitulated in DOX-injected mouse hearts in vivo (Figure 3B). Further evidence supporting the blockage of autophagic flux was that DOX increased the protein levels of p62 in mouse hearts and that this effect of DOX on p62 was prevented by NR (Supplementary Figure S4).
Figure 3. NR attenuated DOX-induced impairment of autophagic flux in cardiomyocytes.
(A) Cultured neonatal mouse cardiomyocytes were incubated with NR (500 μmol/l) or vehicle for 24 h, followed by DOX (DOX, 1 μmol/l) or saline in culture media for another 22 h. After that, bafilomycin A1 (BafA1) or vehicle (Veh) was added. About 2 h after addition of BafA1, western blot was performed to analyze the protein levels of LC3II and GAPDH. Upper panel: representative western blot for LC3II and GAPDH protein and lower panel: quantitation of LC3II/GAPDH ratio. (B) Mice received NR and 30 min later were injected with DOX or saline. About 5 days later after DOX injection, mice received BafA1 or Veh. About 2 h later, western blot was performed to analyze the protein levels of LC3II and GAPDH. Upper panel: representative western blot for LC3II and GAPDH protein and lower panel: quantitation of LC3II/GAPDH ratio. Data are mean + S.D. from four different cell cultures with each in duplication or five different hearts in each group. *P<0.05 versus saline + vehicle, †P<0.05 versus DOX + vehicle and ‡P<0.05 versus DOX + NR.
Inhibitory effects of NR on accumulation of autolysosomes and lysosomal alkalification in cardiomyocytes and mouse hearts upon DOX treatment
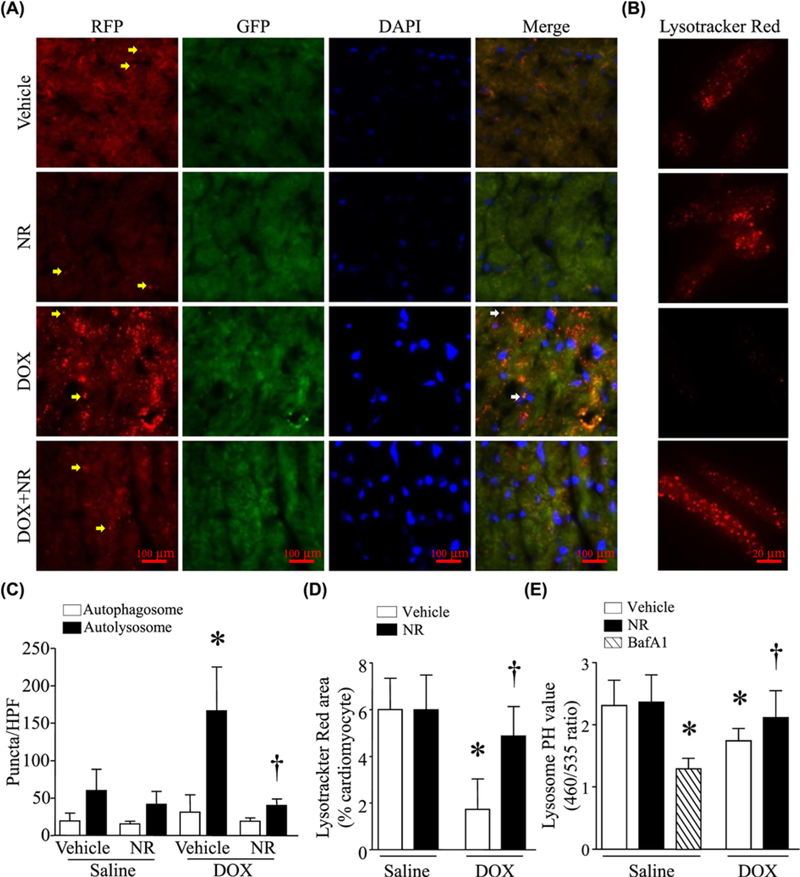
To localize the site of autophagic flux blockage, we employed a transgenic mouse model which harbors a tandem fluorescent RFP-GFP-LC3 reporter transgene driven by the universally expressed promoter CAG [25]. Tg-RFP-GFP-LC3 mice were injected with NR (500 mg/kg) or vehicle and 30 min later, received a single injection of DOX or saline. About 5 days after DOX injection, a significant increase in autolysosome numbers was observed in DOX-injected mouse hearts without changes in autophagosome numbers (Figure 4A,C), consistent with a recent report [19]. Injection of bafilomycin A1 in wild-type mice achieved changes similar to those in Tg-RFP-GFP-LC3 mice (Supplementary Figure S5). However, administration of NR reduced autolysosome numbers in hearts after DOX exposure (Figure 4A,C). These findings suggest that NR improves autophagic flux in DOX-induced cardiotoxicity at a point downstream of autolysosomal formation or at a point of autolysosomal degradation.
Figure 4. NR prevented accumulation of autolysosomes and lowered lysosomal pH in response to DOX.
(A) Adult mice received NR and then injected with DOX (DOX). About 5 days later, heart tissues were collected and prepared for frozen sectioning. The frozen heart sections were stained for nuclei using Hoechst33342. The signals were obtained under fluorescent microscopy. Representative micropictures for autolysosomes (yellow arrow) and autophagosomes (white arrow) in frozen heart sections are presented. (B) A representative Lysotracker Red staining (red) in adult cardiomyocytes. Adult cardiomyocytes were incubated with NR or vehicle for 30 min, followed by DOX for 24 h. Lysotracker Red was added to monitor lysosomal pH changes. (C) Quantititation of autophagosomes and autolysosomes per HPF in frozen heart sections. (D) Quantittation of Lysotracker Red signal area per adult cardiomyocytes. (E) Neonatal cardiomyocytes were incubated NR or vehicle for 30 min and then DOX or saline for 24 h. In a separate group, neonatal cardiomyocytes were incubated with bafilomycin A1 (BafA1) for 24 h. Lysosomal pH was determined. Data are mean ± S.D., n = five hearts in each group or four different cell cultures. *P<0.05 versus saline + vehicle and †P<0.05 versus DOX ± vehicle.
Abbreviation: HPF, high power field.
It has been reported that lysosome acidification was impaired in DOX cardiotoxicity, leading to accumulation of undegraded autolysosomes in the heart [19]. Therefore, we determined whether NR could protect lysosome acidification during DOX stimulation. We tracked lysosomes with LysoTracker Red, a fluorescent dye that labels acidic organelles in live cells. We observed that DOX treatment significantly decreased Lysotracker Red puncta in adult cardiomyocytes (Figure 4B,D). This effect of DOX was attenuated by NR. These results suggest that NR protects either lysosome acidification or biogenesis in DOX-treated cardiomyocytes. To test this further, we measured changes of lysosomal pH qualitatively using LysoSensor yellow/blue DND-160. DOX altered lysosome pH, a change similar to that caused by bafilomycin A1, an inhibitor of the vacuolar H+-ATPase (V-ATPase) which functions to acidify lysosome (Figure 4E), suggesting that DOX impairs lysosome acidification in cardiomyocytes. Pretreatment with NR improved the lysosome acidification in DOX-stimulated cardiomyocytes (Figure 4E).
Impact of autophagic flux blockage on the protective effects of NR in DOX-induced cardiotoxicity
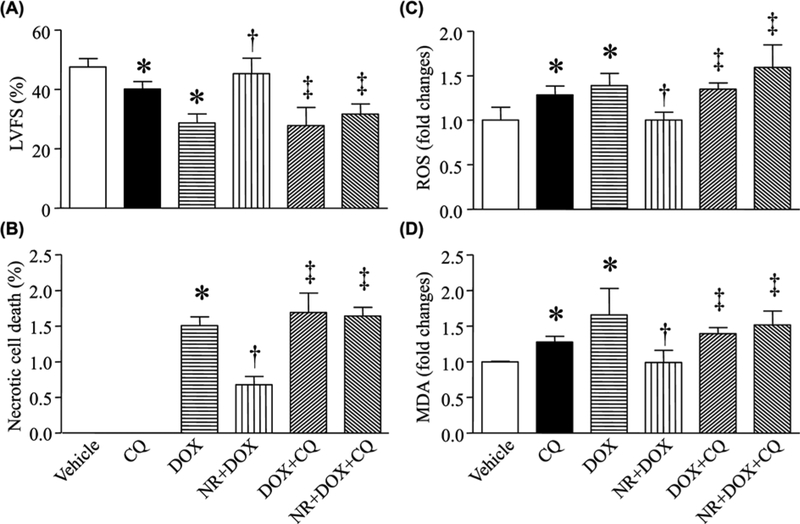
To investigate whether NR reduced DOX-induced cardiotoxicity by improving autophagic flux, we preincubated cardiomyocytes with CQ (blocking autophagic flux), bafilomycin A1, or vehicle in combination with NR, and then with DOX or saline. Consistently, NR prevented apoptosis, reduced LDH release and increased cell viability during DOX stimulation. All these protective effects of NR were offset by CQ and bafilomycin A1 in DOX-stimulated cardiomyocytes (Figure 5A–D, G–J). In a mouse model of DOX-induced cardiotoxicity, daily injection of CQ after DOX exposure reversed the protective effects of NR on cardiac cell death and myocardial dysfunction in hearts (Figure 6A,B, Supplementary Table S2).
Figure 5. Incubation with CQ and bafilomycin A1 offset the negative effects of NR on cell injury in DOX-simulated cardiomyocytes.
Neonatal cardiomyocytes were preincubated with NR in combination with CQ, bafilomycin A1 (BafA1), or vehicle for 30 min and then with DOX or saline for 24 h. Apoptosis was determined by caspase-3 activity (A and G) and DNA fragmentation (B and H). LDH in culture media was measured (C and I). Cell viability was measured (D and J). ROS production (E and K) and MDA (F and L) were assayed. Data are mean ± S.D., n = four different cell cultures. *P<0.05 versus vehicle, ‡P<0.05 versus DOX + vehicle and ‡P<0.05 versus DOX + NR.
Figure 6. Administration of CQ offset the negative effects of NR on cardiac injury in DOX-injected mice.
Mice received NR or vehicle and 30 min later were injected with DOX (DOX) or saline. After that, the animals received daily injection of CQ or vehicle for a total of 5 days. (A) Myocardial function was assessed by echocardiography. (B) Necrotic cell death was determined using EBD. (C) ROS production was measured and (D) MDA was determined in heart tissue lysates. Data are mean ± S.D., n = four different cell cultures. *P<0.05 versus vehicle, †P<0.05 versus DOX and ‡P<0.05 versus DOX + NR.
Since autophagic flux blockage has been shown to promote ROS production and subsequent cyto-toxicity [20], we showed that NR inhibited ROS formation and MDA production in DOX-induced cardiomyocytes and mouse hearts, the effects of which were abrogated by CQ (Figures 5E,F & 6C,D) and bafilomycin A1 (Figure 5K,L).
Prevention of cardiac injury by NR-mediated SIRT1 signaling in DOX-induced toxicity
The content of NAD+ was reduced in DOX-stimulated cardiomyocytes (Supplementary Figure S6). Since NR is converted into NAD+, which activates SIRT1. In support of this, we showed that administration of NR elevated the content of NAD+ in cardiomyocytes (Supplementary Figure S7A) and mouse hearts (Supplementary Figure S7B). To determine whether NR increased SIRT1 activity of de-acetylation, we preincubated cardiomyocytes with NR and EX-527 (a selective inhibitor of SIRT1) in combination or alone, followed by DOX. In line with our recent report [3], DOX increased p53 acetylation in cardiomyocytes (Supplementary Figure S7C). Preincubation with NR prevented DOX-induced p53 acetylation. However, this inhibitory effect of NR on p53 acetylation was abrogated by coincubation with EX-527 (Supplementary Figure S7C). These results indicate that NR induced SIRT1 activation in cardiomyocytes.
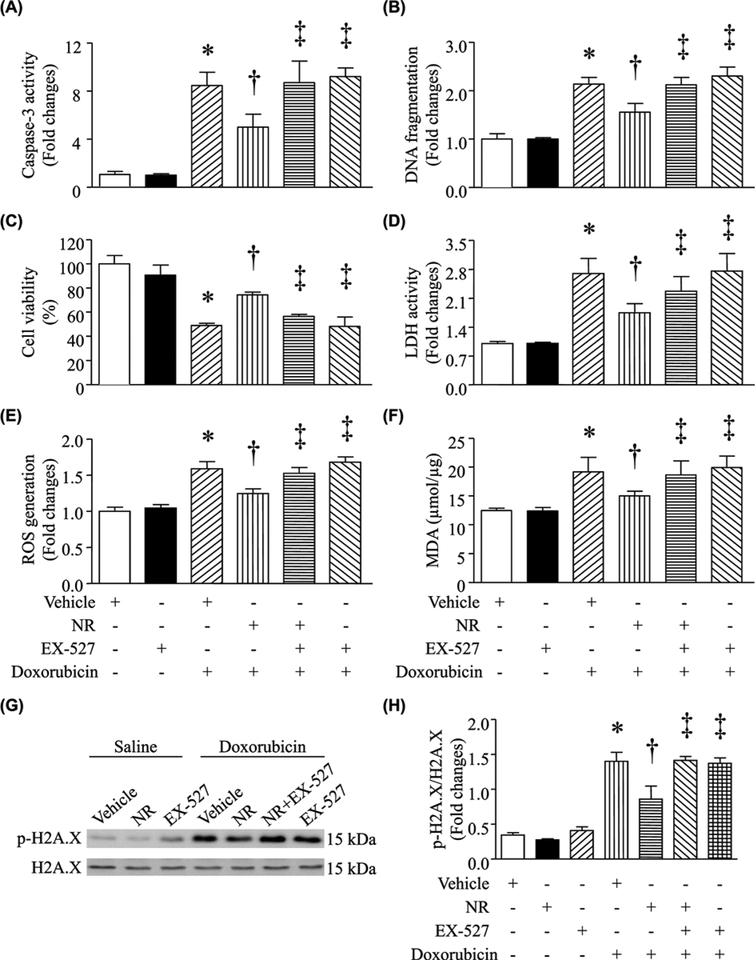
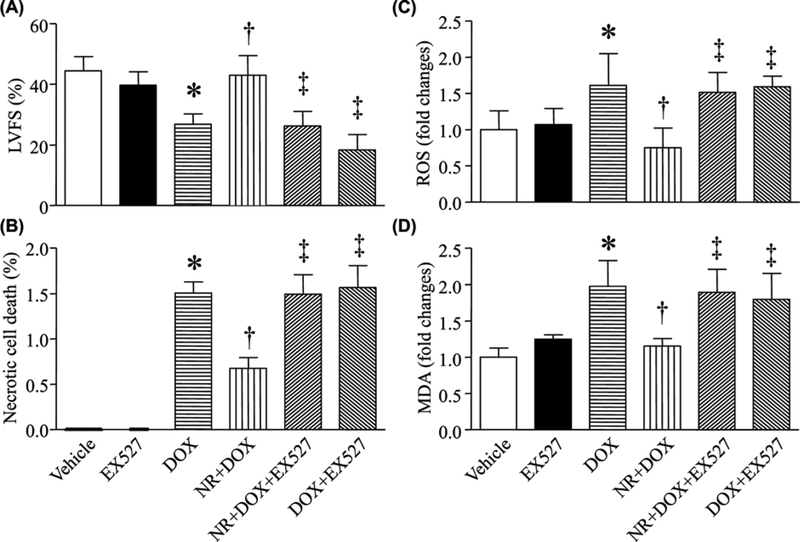
We then examined whether the protective effects of NR were mediated through NAD+/SIRT1 signaling. Coincubation with EX-527 reversed the effects of NR on apoptosis, LDH release, cell viability, ROS production, and MDA production induced by DOX in cardiomyocytes (Figure 7A–F). To further understand the involvement of SIRT1, we examined the protein levels of phosphorylated H2AX, an indicator of oxidative damage in DOX-stimulated cardiomyocytes. DOX treatment manifested as an increase in phosphorylated H2AX protein levels in cardiomyocytes (Figure 7G,H). However, preincubation with NR prevented DOX-induced H2AX phosphorylation. Similarly, this effect of NR was offset in DOX-stimulated cardiomyocytes by EX-527 (Figure 7G,H). At last, preadministration of EX-527prevented the protective effects of NR on cardiac cell death, ROS production, oxidative damage, and myocardial dysfunction in DOX-injected mice (Figure 8, Supplementary Table S3). These results further reinforce the role of SIRT1 signaling in the context of cardiomyocyte protection.
Figure 7. Incubation with EX-527 offset the negative effects of NR on cell injury in DOX-simulated cardiomyocytes.
Neonatal cardiomyocytes were preincubated with NR in combination with EX-527 or vehicle for 30 min and then with DOX or saline for 24 h. Apoptosis was determined by caspase-3 activity (A) and DNA fragmentation (B). Cell viability (C) and LDH in culture media were measured (D). ROS production (E) and MDA (F) were assayed. (G and H) Western blot analysis was performed to determine phosphorylated H2A.X (p-H2A.X) and total H2A.X. (G) A representative western blots for p-H2A.X and H2A.X. (H) Quantitation of p-H2A.X/H2A.X ratio. Data are mean ± S.D., n = four different cell cultures. *P<0.05 versus vehicle, †P<0.05 versus DOX + vehicle and ‡P<0.05 versus DOX + NR.
Figure 8. Administration of EX-527 abrogated the negative effects of NR on cardiac injury in DOX-injected mice.
Mice received NR or vehicle and 30 min later were injected with DOX (DOX) or saline. After that, the animals received daily injection of EX-527 or vehicle for a total of 5 days. (A) Myocardial function was assessed by echocardiography. (B) Necrotic cell death was determined using evans blue dye. (C) ROS production was measured and (D) MDA was determined in heart tissue lysates. Data are mean ± S.D., n = four to 11 different hearts. *P<0.05 versus vehicle, †P<0.05 versus DOX and ‡P<0.05 versus DOX + NR.
Discussion
DOX cardiotoxicity remains a leading cause of mortality in patients undergoing chemotherapy. Indeed, childhood cancer survivors treated with anthracyclines have significantly higher risks for heart failure, myocardial infarction, pericardial disease, and valvular abnormalities than their sibling controls [33]. Moreover, coadministration with trastuzumab – an antihuman epidermal growth factor receptor-2 monoclonal antibody used in the treatment of breast cancers – leads to exacerbated cardiotoxicity in adults [34]. Unfortunately, so far specific therapeutic approaches to prevent DOX cardiotoxicity are largely limited. Dexrazoxane is the only drug available specifically for DOX cardiotoxicity [35]. Although the American Society of Clinical Oncology moderately recommends the use of dexrazoxane in adult patients whose DOX doses exceed 250 mg/m2, the literature is conflicting when considering pediatric populations. One systematic review and meta-analysis found that children treated with DOX and dexrazoxane had a 2.37 relative risk to develop a secondary malignant neoplasm (p = 0.06) [36], yet several clinical trials found no difference in such malignancies or overall mortality [37]. These inconsistencies illustrate the need to develop treatments that prevent DOX cardiotoxicity, while maintaining its antineoplastic effects. In the present study, we discovered dose-dependent preventive effects of NR in DOX cardiotoxicity, with the most optimal effects seen at our highest studied dose of 500 mg/kg. This finding has a high translational potential because NR is available as Niagen™ (ChromaDex Inc.), which is an over-the-counter, FDA-approved, oral formulation [38]. The lowest-observed-adverse-effect level for this formulation was found to be 1000 mg/kg/day in rodents, which supports our NR dosing of 500 mg/kg. Because of its favorable safety profile, NR could easily be applied to clinical trials. Interestingly, during the preparation of this manuscript, two papers were published which reported that administration of NR protected cardiac function in mouse models of dilated cardiomyopathy triggered by cardiac-specific inducible inactivation of Serum Response Factor or lamin A/C gene mutations, and mouse model of pressure overload hypertrophy induced by transverse aorta constriction [39,40]. Thus, our study together with these two reports suggests that NR may represent a new therapeutic agent to protect against cardiac injury and prevent heart failure.
The oxidative stress caused by DOX contributes significantly to the development of cardiomyopathy [8,9]. Through inhibiting oxidative stress pathways, DOX-induced cardiotoxicity may be attenuated [11–14]. The accumulation of ROS from mitochondria and other sources including NADPH oxidase in combination with the naturally lower level of antioxidant enzymes in cardiomyocytes contributes to DNA fragmentation, mitochondrial dysfunction, and the ultimate apoptosis and necroptosis of cardiomyocytes as well as cardiac endothelial dysfunction [41,42]. These complex pathways ultimately help elucidate the mechanism of DOX-induced cardiotoxicity. By examining the levels of phosphorylated H2AX, ROS production, and lipid peroxidation, we demonstrated that NR could inhibit oxidant stress and further inhibit the apoptosis and necroptosis of cardiomyocytes.
Autophagy is a series of processes through which damaged or unnecessary proteins and cytoplasmic organelles are degraded by eukaryotic cells, allowing for the organized degradation and recycling of cellular building blocks [43]. The autophagic flux refers to the whole process of autophagy, including autophagosome formation, maturation, fusion with lysosomes, subsequent breakdown, and the release of macromolecules back into the cytosol [44]. The autophagic flux is involved in cell growth, survival, development, and death and performs a protective function in physiological as well as some pathological conditions, including cardiac diseases [45]. Studies have shown that autophagic flux may attenuate DOX-induced cardiotoxicity, but this process is simultaneously impaired by DOX treatment [18,19], leading to accumulation of undegraded autolysosomes in DOX-treated cardiomyocytes. In the present study, for the first time, we demonstrate that NR improved autophagic flux in DOX-treated cardiomyocytes and mouse hearts by maintaining lysosome acidification and facilitating clearance of autolysosomes. As a result, restoration of autophagic flux helped to reduce DOX-induced ROS and oxidized molecules as well organelles in DOX cardiotoxicity. In order to prove that NR exerted its protective effects specifically through autophagic flux, we blocked this pathway in a confirmatory experiment with CQ. Consequently, the protective effects of NR in DOX-induced cardiomyocyte injury including oxidative damage, apoptosis, and necroptosis were abolished when autophagic flux was impaired. These data indicate that autophagic flux blockage contributes to cardiomyocyte apoptosis and necroptosis in response to DOX. The inhibitory effect of NR on necroptosis may be associated with a reduction of ROS production as ROS was reported to mediate necroptosis in cardiomyocytes [46]. Nevertheless, it remains to be determined whether NR or autophagic flux blockage plays a role in ferroptosis in DOX-induced cardiotoxicity.
NR is converted into NAD+ in cells, the active coenzyme that activates SIRT1. SIRT1, a NAD+-dependent histone deacetylase, belongs to the human sirtuins family, which regulates transcription, genome stability, longevity, and metabolism in mammals. NAD+/SIRT1 signaling plays roles in metabolism, energy balance, ageing maladies, and longevity control [22,23]. As in our recent report [3] and the present study, incubation with NR prevents p53 acetylation, indirectly supporting occurrence of de-acetylation by SIRT1. Furthermore, the present study shows that administration of EX-527 (a selective inhibitor of SIRT1) attenuated the protective effects of NR on cardiac cell death, ROS production, oxidative damage, and myocardial dysfunction in DOX-injected mice. These results demonstrate that NR exerts its cardio-protective effects through NAD+/SIRT1 signaling in DOX stimulation.
SIRT1 has been engaged in different steps of autophagy. SIRT1 has been linked to protective autophagy [47]. SIRT1 de-acetylates FOXOs to exploit their regulatory role in controlling autophagy-related genes mainly working as part of the core machinery (Ulk2, Pik3c3, Beclin1, Atg4B, Lc3b, and Atg12, etc.) [48–50]. Activation of SIRT1 inhibits the mTOR pathway and thus, promotes autophagy [51]. More recent studies have implicated SIRT1 in regulation of TFEB. SIRT1 directly induced de-acetylation of TFEB, leading to its nuclear translocation [52]. It was also reported that SIRT1 inhibited mTOR, resulting in de-phosphorylation of TFEB and its nuclear translocation [51,53]. Nuclear translocation of TFEB induces genes for lysosomal biogenesis and function [53]. Notably, our study suggests that SIRT1 may directly improve autophagic function by restoring V-ATPase activity and lysosome acidification as a recent study localized the site of autophagic flux blockage as a defect in lysosomal acidification in DOX-induced cardiotoxicity [19]. DOX has been shown to uncouple the V0 (ATPase domain) and V1 (hydrolytic domain) subunits. It has been shown in yeast that the d subunit of the V0 domain is responsible for V0-V1 coupling [54]; however, no studies to date have established a link between SIRT1 and this ATPase, which merits future investigations.
It is important to mention that boosting NAD+ by NR dietary supplement showed antitumor effects in liver cancer [55]. Although it is currently known how NR exhibits opposite effects in cardiomyocytes and tumor cells (inhibiting versus promoting cell death), these findings support that it is safe in using NR to prevent DOX-induced cardiotoxicity for patients with cancer.
Conclusion
Our study for the first time shows that NR maintains lysosomal acidification and enhances autolysosome clearance via SIRT1 signaling thereby preventing DOX cardiotoxicity. Given its recognition as an effective dietary supplement to increase NAD+ levels and its favorable safety profile, NR may be a potential candidate for clinical trials.
Supplementary Material
Clinical perspectives.
Effective drugs to prevent DOX-induced cardiotoxicity, but not at the cost of its antineoplastic effects, have been limited and are urgently required.
NR prevents DOX-triggered cardiotoxicity by enhancing autolysosome clearance and reducing oxidative stress via the NAD+/SIRT1 signaling.
NR may be a potential candidate for clinical trials to prevent DOX-induced cardiotoxicity.
Funding
The present study was supported by the Natural Science Foundation of Jiangsu Province [grant number BK20171216] and the Heart and Stroke Foundation of Canada [grant numbers G-17–0018361 and G-19–0026380].
Abbreviations
- CQ
chloroquine
- EBD
Evans blue dye
- FS
fractional shortening
- LDH
lactate dehydrogenase
- LV
left ventricle
- MDA
malondialdehyde
- MLKL
mixed lineage kinase domain like pseudokinase
- NAD
nicotinamide adenine dinucleotide
- OCT
optimal cutting temperature
- NR
nicotinamide riboside
- RIP
receptor-interacting serine/threonine-protein kinase
- ROS
reactive oxygen species
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Arcamone F, Cassinelli G, Fantini G, Grein A, Orezzi P, Pol C et al. (1969) Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol. Bioeng 11, 1101–1110, 10.1002/bit.260110607 [DOI] [PubMed] [Google Scholar]
- 2.Bonadonna G, Monfardini S, De Lena M and Fossati-Bellani F (1969) Clinical evaluation of adriamycin, a new antitumour antibiotic. Br. Med. J 3, 503–506, 10.1136/bmj.3.5669.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma J, Wang Y, Zheng D, Wei M, Xu H and Peng T (2013) Rac1 signalling mediates DOX-induced cardiotoxicity through both reactive oxygen species-dependent and -independent pathways. Cardiovasc. Res 97, 77–87, 10.1093/cvr/cvs309 [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Zheng D, Wei M, Ma J, Yu Y, Chen R et al. (2013) Over-expression of calpastatin aggravates cardiotoxicity induced by DOX. Cardiovasc. Res 98, 381–390, 10.1093/cvr/cvt048 [DOI] [PubMed] [Google Scholar]
- 5.Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F et al. (2016) CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat. Med 22, 175–182, 10.1038/nm.4017 [DOI] [PubMed] [Google Scholar]
- 6.Fang X, Wang H, Han D, Xie E, Yang X, Wei J et al. (2019) Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl Acad. Sci. U.S.A 116, 2672–2680, 10.1073/pnas.1821022116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N et al. (2017) Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol 35, 893–911, 10.1200/JCO.2016.70.5400 [DOI] [PubMed] [Google Scholar]
- 8.Štěrba M, Popelová O, Vávrová A, Jirkovský E, Kovaříková P, Geršl V et al. (2013) Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxid. Redox Signal 18, 899–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Šimůnek T, Štěrba M, Popelová O, Adamcová M, Hrdina R and Geršl V (2009) Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol. Rep 61, 154–171, 10.1016/S1734-1140(09)70018-0 [DOI] [PubMed] [Google Scholar]
- 10.Kalyanaraman B, Perez-Reyes E and Mason RP (1980) Spin-trapping and direct electron spin resonance investigations of the redox metabolism of quinone anticancer drugs. Biochim. Biophys. Acta 630, 119–130, 10.1016/0304-4165(80)90142-7 [DOI] [PubMed] [Google Scholar]
- 11.Venditti P, Balestrieri M, De Leo T and Di Meo S (1998) Free radical involvement in DOX-induced electrophysiological alterations in rat papillary muscle fibres. Cardiovasc. Res 38, 695–702, 10.1016/S0008-6363(98)00034-0 [DOI] [PubMed] [Google Scholar]
- 12.Shioji K, Kishimoto C, Nakamura H, Masutani H, Yuan Z, Oka S et al. (2002) Overexpression of thioredoxin-1 in transgenic mice attenuates adriamycin-induced cardiotoxicity. Circulation 106, 1403–1409, 10.1161/01.CIR.0000027817.55925.B4 [DOI] [PubMed] [Google Scholar]
- 13.Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virag L et al. (2003) Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of DOX-induced cardiac dysfunction. Circulation 107, 896–904, 10.1161/01.CIR.0000048192.52098.DD [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, McLaughlin D, Robinson E, Harvey AP, Hookham MB, Shah AM et al. (2010) Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with DOX chemotherapy. Cancer Res. 70, 9287–9297, 10.1158/0008-5472.CAN-10-2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalivendi SV, Kotamraju S, Zhao H, Joseph J and Kalyanaraman B (2001) DOX-induced apoptosis is associated with increased transcription of endothelial nitric-oxide synthase. Effect of antiapoptotic antioxidants and calcium. J. Biol. Chem 276, 47266–47276, 10.1074/jbc.M106829200 [DOI] [PubMed] [Google Scholar]
- 16.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T et al. (2004) The role of autophagy during the early neonatal starvation period. Nature 432, 1032–1036, 10.1038/nature03029 [DOI] [PubMed] [Google Scholar]
- 17.Wen X, Wu J, Wang F, Liu B, Huang C and Wei Y (2013) Deconvoluting the role of reactive oxygen species and autophagy in human diseases. Free Radic. Biol. Med 65, 402–410, 10.1016/j.freeradbiomed.2013.07.013 [DOI] [PubMed] [Google Scholar]
- 18.Bartlett JJ, Trivedi PC, Yeung P, Kienesberger PC and Pulinilkunnil T (2016) DOX impairs cardiomyocyte viability by suppressing transcription factor EB expression and disrupting autophagy. Biochem. J 473, 3769–3789, 10.1042/BCJ20160385 [DOI] [PubMed] [Google Scholar]
- 19.Li DL, Wang ZV, Ding G, Tan W, Luo X, Criollo A et al. (2016) DOX blocks cardiomyocyte autophagic flux by inhibiting lysosome acidification. Circulation 133, 1668–1687, 10.1161/CIRCULATIONAHA.115.017443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Button RW, Roberts SL, Willis TL, Hanemann CO and Luo S (2017) Accumulation of autophagosomes confers cytotoxicity. J. Biol. Chem 292, 13599–13614, 10.1074/jbc.M117.782276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chi Y and Sauve AA (2013) Nicotinamide riboside, a trace nutrient in foods, is a vitamin B3 with effects on energy metabolism and neuroprotection. Curr. Opin. Clin. Nutr. Metab. Care 16, 657–661, 10.1097/MCO.0b013e32836510c0 [DOI] [PubMed] [Google Scholar]
- 22.Belenky P, Bogan KL and Brenner C (2007) NAD+ metabolism in health and disease. Trends in Biochem. Sci 32, 12–19, 10.1016/j.tibs.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 23.Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS and Brenner C (2007) Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell 129, 473–484, 10.1016/j.cell.2007.03.024 [DOI] [PubMed] [Google Scholar]
- 24.Mouchiroud L, Houtkooper RH and Auwerx J (2013) NAD(+) metabolism: a therapeutic target for age-related metabolic disease. Crit. Rev. Biochem. Mol. Biol 48, 397–408, 10.3109/10409238.2013.789479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Wang ZV, Hill JA and Lin F (2014) New autophagy reporter mice reveal dynamics of proximal tubular autophagy. J. Am. Soc. Nephrol 25, 305–315, 10.1681/ASN.2013040374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Llopis J, McCaffery JM, Miyawaki A, Farquhar MG and Tsien RY (1998) Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl Acad. Sci. U.S.A 95, 6803–6808, 10.1073/pnas.95.12.6803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong G, Zheng D, Zhang L, Ni R, Wang G, Fan GC et al. (2018) Administration of nicotinamide riboside prevents oxidative stress and organ injury in sepsis. Free Rad. Biol. Med 123, 125–137, 10.1016/j.freeradbiomed.2018.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y et al. (2012) The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 15, 838–847, 10.1016/j.cmet.2012.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsstrom S et al. (2014) Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol. Med 6, 721–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryu D, Zhang H, Ropelle ER, Sorrentino V, Mazala DA, Mouchiroud L et al. (2016) NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci. Transl. Med 8, 361ra139, 10.1126/scitranslmed.aaf5504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P et al. (2016) NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352, 1436–1443, 10.1126/science.aaf2693 [DOI] [PubMed] [Google Scholar]
- 32.Klionsky DJ, Elazar Z, Seglen PO and Rubinsztein DC (2008) Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy 4, 849–850, 10.4161/auto.6845 [DOI] [PubMed] [Google Scholar]
- 33.Franco VI and Lipshultz SE (2015) Cardiac complications in childhood cancer survivors treated with anthracyclines. Cardiol. Young 25, 107–116, 10.1017/S1047951115000906 [DOI] [PubMed] [Google Scholar]
- 34.Nemeth BT, Varga ZV, Wu WJ and Pacher P (2017) Trastuzumab cardiotoxicity: from clinical trials to experimental studies. Br. J. Pharmacol 174, 3727–3748, 10.1111/bph.13643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cvetkovic RS and Scott LJ (2005) Dexrazoxane : a review of its use for cardioprotection during anthracycline chemotherapy. Drugs 65, 1005–1024, 10.2165/00003495-200565070-00008 [DOI] [PubMed] [Google Scholar]
- 36.Shaikh F, Dupuis LL, Alexander S, Gupta A, Mertens L and Nathan PC (2016) Cardioprotection and second malignant neoplasms associated with dexrazoxane in children receiving anthracycline chemotherapy: a systematic review and meta-analysis. J. Natl Cancer Inst 108, djv357, 10.1093/jnci/djv357 [DOI] [PubMed] [Google Scholar]
- 37.Seif AE, Walker DM, Li Y, Huang YS, Kavcic M, Torp K et al. (2015) Dexrazoxane exposure and risk of secondary acute myeloid leukemia in pediatric oncology patients. Pediatr. Blood Cancer 62, 704–709, 10.1002/pbc.25043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conze DB, Crespo-Barreto J and Kruger CL (2016) Safety assessment of nicotinamide riboside, a form of vitamin B3. Hum. Exp. Toxicol 35, 1149–1160, 10.1177/0960327115626254 [DOI] [PubMed] [Google Scholar]
- 39.Diguet N, Trammell S, Tannous C, Deloux R, Piquereau J, Mougenot N et al. (2018) Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation 137, 2256–2273, 10.1161/CIRCULATIONAHA.116.026099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vignier N, Chatzifrangkeskou M, Rodriguez BM, Mericskay M, Mougenot N, Bonne G et al. (2018) Rescue of biosynthesis of nicotinamide adenine dinucleotide (NAD+) protects the heart in cardiomyopathy caused by lamin A/C gene mutation. Human Mol. Genet 27, 3870–3880, 10.1093/hmg/ddy278 [DOI] [PubMed] [Google Scholar]
- 41.Takemura G and Fujiwara H (2007) DOX-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog. Cardiovasc. Dis 49, 330–352, 10.1016/j.pcad.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 42.Incalza MA, D’Oria R, Natalicchio A, Perrini S, Laviola L and Giorgino F (2018) Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol 100, 1–19, 10.1016/j.vph.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 43.Klionsky DJ and Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290, 1717–1721, 10.1126/science.290.5497.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang XJ, Chen S, Huang KX and Le WD (2013) Why should autophagic flux be assessed? Acta. Pharmacol. Sin 34, 595–599, 10.1038/aps.2012.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dirks-Naylor AJ (2013) The role of autophagy in DOX-induced cardiotoxicity. Life Sci. 93, 913–916, 10.1016/j.lfs.2013.10.013 [DOI] [PubMed] [Google Scholar]
- 46.Zhu P, Hu S, Jin Q, Li D, Tian F, Toan S et al. (2018) Ripk3 promotes ER stress-induced necroptosis in cardiac IR injury: a mechanism involving calcium overload/XO/ROS/mPTP pathway. Redox Biol 16, 157–168, 10.1016/j.redox.2018.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE et al. (2008) A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl Acad. Sci. U.S.A 105, 3374–3379, 10.1073/pnas.0712145105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y et al. (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015, 10.1126/science.1094637 [DOI] [PubMed] [Google Scholar]
- 49.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W et al. (2004) Mammalian SIRT1 represses forkhead transcription factors. Cell 116, 551–563, 10.1016/S0092-8674(04)00126-6 [DOI] [PubMed] [Google Scholar]
- 50.Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA and Sadoshima J (2010) Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ. Res 107, 1470–1482, 10.1161/CIRCRESAHA.110.227371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ et al. (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 15, 675–690, 10.1016/j.cmet.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bao J, Zheng L, Zhang Q, Li X, Zhang X, Li Z et al. (2016) Deacetylation of TFEB promotes fibrillar Abeta degradation by upregulating lysosomal biogenesis in microglia. Protein Cell 7, 417–433, 10.1007/s13238-016-0269-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lapierre LR, Kumsta C, Sandri M, Ballabio A and Hansen M (2015) Transcriptional and epigenetic regulation of autophagy in aging. Autophagy 11, 867–880, 10.1080/15548627.2015.1034410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Owegi MA, Pappas DL, Finch Jr, M.W., Bilbo SA., Resendiz CA., Jacquemin LJ. et al. (2006) Identification of a domain in the V0 subunit d that is critical for coupling of the yeast vacuolar proton-translocating ATPase. J. Biol. Chem 281, 30001–30014, 10.1074/jbc.M605006200 [DOI] [PubMed] [Google Scholar]
- 55.Djouder N (2015) Boosting NAD(+) for the prevention and treatment of liver cancer. Mol. Cell Oncol 2, e1001199, 10.1080/23723556.2014.1001199 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.