Abstract
OBJECTIVES:
Physical function declines with aging and is accelerated for persons with cardiovascular disease (CVD). While CVD increases the risk of functional decline in late life, little is known about differences in trajectories of functional decline. To determine whether there is more than 1 trajectory of functional decline in Americans with cardiovascular disease (CVD) who are functionally independent.
DESIGN:
Secondary analysis of National Health and Aging Trends Study (NHATS). Latent class growth modeling was used to estimate trajectories of function over 4 years of follow-up.
SETTING:
Annual structured in-home interviews.
PARTICIPANTS:
Americans aged 65 and older with CVD who were functionally independent at baseline (N = 392).
MEASUREMENTS:
We compared trajectories of function in individuals with CVD with trajectories of those without and examined the association between risk factors (sex, age at baseline, education level, comorbidity) and trajectory group membership. Function was measured using the Short Physical Performance Battery.
RESULTS:
Three functional trajectories emerged: rapid functional decline (23.8%), gradual functional decline (44.2%), and stable function (32.0%). Similar trajectories were seen for those without CVD, with a smaller proportion in the rapid functional decline group (16.2%). Women, older participants, and those with less education and greater comorbidity were less likely to be in the stable function group than the rapid functional decline group.
CONCLUSION:
Although function declines in late life for independently functioning persons with CVD, some individuals remain stable, and others decline gradually or rapidly. Persons with CVD were more likely to experience rapid functional decline than those without, suggesting that CVD increases the risk of rapid functional decline. Risk factors predicted functional trajectory group membership, not just overall decline.
Keywords: aging, function, cardiovascular disease
Medical advances in the 20th century, including treatments for cardiovascular disease (CVD), have increased life expectancy.1,2 Because age is strongly associated with CVD,2 the number of newly diagnosed cases of coronary heart disease in individuals aged 65 and older is projected to increase 26% by 2040.3 Approximately 62% of adults with CVD are younger than 65, which will further exacerbate the burden of CVD on aging in the United States in coming years.4 Higher CVD prevalence will ultimately increase healthcare costs, which are estimated to triple to $818 billion by 2030.5
Increases in the aging population with CVD will challenge society, because CVD is associated with higher levels of functional limitations and disability.6,7 Although function declines at various rates with aging, it does so more quickly in those with more chronic conditions and poorer health.8–10 Recent research has found increased rates of functional decline in late life when individuals have CVD, such as coronary artery disease, hypertension, and myocardial infarction.11–14 In fact, the American Heart Association recently identified function as a priority in the assessment of outcomes for older adults with CVD.15
Functional decline for persons with CVD can be conceptualized through the disablement process.16 Pathology, such as CVD, leads to system level impairments in body systems (e.g., coronary and systemic vasculature), which progress to functional limitations (decrease in strength and aerobic endurance). As functional limitations accumulate and worsen, disability may develop. Risk factors, which accelerate development of functional limitations and resultant disability, can influence this process. Although CVD leads to a decrease in function in aging, little is known about the intra-individual risk factors that influence functional decline for older adults with CVD.
Previous studies examining late-life function have used variable-centered approaches, with analyses focusing on relationships between risk factors and a specific outcome. For instance, variable-centered approaches have identified CVD as an intra-individual risk factor that leads to a decrease in function and disability in late life. However, these approaches assume that all individual-level change in a sample can be described as a single pattern of change (trajectory) for an outcome of interest.17 Identification of 1 pattern of change for an entire sample may mask important differences between trajectory patterns within a population. Although variable-centered approaches demonstrated that CVD is a factor related to late-life functional decline, it is important to identify variation in trajectories for individuals with CVD because function may decline, be maintained, or even improve. In addition, factors that affect trajectory can be investigated, allowing for identification of risk factors associated with better or worse outcomes. Person-centered trajectory modeling strategies are used to identify different patterns of trajectory of an outcome. Person-centered analytical approaches examine an outcome individually and then use individual response patterns to group similar trajectories together, which in turn, identify multiple patterns of change over time.17
The purpose of the present study was to evaluate differences in functional trajectories for older Americans with CVD. The specific aim of this article was to examine functional trajectory patterns for persons with CVD who were functionally independent at baseline. We also compared trajectories of individuals with CVD with trajectories of those without to determine whether there were differences in trajectory patterns. The secondary aim was to determine the association between intra-individual risk factors and functional trajectory group membership. We hypothesized that being female, having lower levels of educational attainment, higher medical comorbidity, and being older at baseline would be associated with membership to a trajectory group with lower function because these factors have been found to be associated with functional decline in late life.11,18,19
METHODS
Data Source
Data for this study were from the National Health and Aging Trends Study (NHATS), a nationally representative, longitudinal cohort study that has followed individuals aged 65 and older annually since 2011.20 The dataset includes validated self-reported and performance-based measures to study trends in late-life disability and function. This investigation used the 2011 to 2015 rounds of the NHATS. Details on the NHATS study sample can be found at www.nhatsdata.org.
Inclusion Criteria
Individuals were considered to have CVD if, at baseline (2011), they self-reported having heart disease or having had a myocardial infarction. Participants were included if they were community dwelling (resided at home or an independent living facility) and functionally independent, defined as a gait speed of 0.8 m/s or greater.21 (Supplementary Figure S1). Participants who died or were lost to follow-up were included in analyses if they met base-line inclusion criteria.
Measures
Functional status was measured annually using the Short Physical Performance Battery (SPPB), which is scored from 0 (lowest) to 12 (highest) and includes a balance assessment, average gait speed, and average time to complete 5 sit-to-stand transfers from a standard chair height.22 Scores are based on distribution-based cut-points designed for use in the NHATS.23 The SPPB does not have an established score that indicates functional independence. However, gait speed of 0.8 m/s or greater is associated with mobility independence in community-dwelling older adults.24 Therefore, gait speed was used to indicate independent functioning at baseline for inclusion in this study. Missing SPPB scores were imputed as averages of the functional scores around the missing time point (e.g., function at Time 2 was imputed as the average between Times 1 and 3). All other missing SPPB scores were treated as missing at random.
We examined the association between risk factors previously associated with a decrease in function in late life: sex,8,18 age at baseline,11 education,19 and comorbidity (number of chronic conditions).9,11 Sex was categorized as male or female. Education was categorized as less than high school, high school, and some college or more. Chronic conditions were categorized as 1 to 3 versus 4 or more. Baseline age was categorized as 65 to 69, 70 to 74, 75 to 79, 80 to 84, and 85 and older.
The Partners Healthcare institutional review board granted this study exempt status.
Analysis
Latent class growth modeling (LCGM) was used for trajectory modeling to classify individuals into distinct functional trajectory groups based on similarity in patterns of functional change over time.25 Individual trajectories were estimated using maximum likelihood and then grouped together based on trajectory similarity. LCGM models produce an estimated probability of an individual’s membership in a trajectory group (posterior probability),26 which indicates the reliability of the model to predict group membership for the sample.25 After establishing the number of trajectories, time-stable risk factors were added to the model to determine their association with trajectory group membership.27
We used SAS PROC TRAJ for LCGM (http://www.andrew.cmu.edu/user/bjones/). To determine the appropriate number of trajectory groups, function was regressed onto time in an iterative process, starting with 1 trajectory and increasing to 5 trajectories. Because the sample had 5 rounds of data for function, models were tested for cubic, quadratic, and linear trends for each trajectory group.25 A sensitivity analysis was conducted to identify functional trajectory groups in the NHATS who were independent at baseline without self-reported CVD. Trajectories of those with and without CVD were then compared to determine whether trajectory patterns were different.
Separate LCGM models were run with sex, age, education, and chronic conditions as time-stable covariates, which were included in the fully adjusted trajectory model if they were significant (p < .05) in predicting group membership trajectory. Rao Scott chi-square tests were used to examine the statistical significance of demographic differences between trajectory groups.
Model Fit and Selection
To assess model fit and determine the appropriate number of classes for the data, we used a number of criteria. First, we examined the Bayesian Information Criterion (BIC), and the BIC log Bayes factor approximation to compare models, which is equal to 2*(ΔBIC).28 A combination of larger BIC and adequate log Bayes factor is preferred.27,28 It has been recommended that the following values be used to interpret the log Bayes factor: 0 to 2 for weak evidence, 2 to 6 for moderate evidence, 6 to 10 for strong evidence, greater than 10 for very strong evidence.29
Each model produced individual posterior probabilities, which were used to indicate reliability of trajectory group assignment. To further assess model fit, average posterior probability was calculated for each trajectory group.25 An average posterior probability greater than 0.7 indicates that individuals in each trajectory have a similar pattern of change in the outcome variable over time.30 Finally, group membership percentages were used to support model selection. Group membership percentages indicate the percentage of the sample that belongs to a trajectory group. Group membership percentages of 5% or greater indicate adequate model selection.25
Predictors of Trajectory Membership
To assess the association between intra-individual risk factors and functional trajectory group membership, parameter estimates were produced for each risk factor included in the final trajectory model. To describe the odds of trajectory group membership, odds ratios were produced using parameter estimate exponentiation for sex, age, education, and comorbidity.26 All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Demographic Characteristics of Study Sample
Three hundred ninety-two individuals in the 2011 round of the NHATS were functionally independent and community dwelling. They were predominantly male and white and had a high level of education (Table 1). Approximately 62.2% of the sample was younger than 75 at baseline, and more than half had fewer than 4 chronic conditions. Average SPPB score at baseline was 9.05 (95% confidence interval (CI)=8.84–9.26).
Table 1.
Demographic Characteristics of National Health and Aging Trends Study Participants Who Were Independent in in 2011
| With CVD,n = 392 |
Without CVD, n = 1,666 |
|
|---|---|---|
| Sex, n (%)a | ||
| Male | 252 (64.6) | 805 (49.1) |
| Female | 140 (35.4) | 861 (50.9) |
| Race, n (%)a | ||
| White | 341 (93.0) | 1,332 (87.3) |
| Black | 36 (3.3) | 207 (4.2) |
| Other | 15(3.7) | 127 (8.4) |
| Education, n (%) | ||
| < High school | 61 (12.6) | 229 (11.9) |
| High school | 92 (22.2) | 407 (22.9) |
| ≥Some college | 239 (65.2) | 1,030 (65.2) |
| Age, n (%)a | ||
| 65–69 | 106 (35.2) | 542 (42.2) |
| 70–74 | 96 (27.0) | 480 (30.1) |
| 75–79 | 81 (19.2) | 332 (16.5) |
| 80–84 | 80 (14.3) | 213 (8.1) |
| ≥85 | 29 (4.3) | 99 (3.2) |
| Comorbidities, n (%) | ||
| 0 | 0 (0.0) | 269 (17.5) |
| 1–3 | 205 (53.0) | 1,279 (75.8) |
| ≥4 | 187 (47.0) | 118 (6.7) |
| Baseline Short Physical | 9.05 | 9.66 |
| Performance Battery score, mean (95% CI) | (8.84–9.26) | (9.55–9.77) |
p <.001.
Trajectory Modeling: Functional Trajectories for Older Adults with CVD
For all models tested (unadjusted, individually adjusted for separate risk factors, fully adjusted for risk factors), a 3-group trajectory solution demonstrated the best fit with the data (Supplementary Table S1). Model fit was assessed using a combination of clinical reasoning and model fit indices (described in the analysis section). When comparing the 3- and 4-group trajectory solutions, the 3-group solution had optimal model fit for 3 of the 4 model fit criteria. The 4-group model did not add clinical value, as 3 trajectory groups were similar in structure, with little difference in SPPB score. Additionally, 1 group represented less than 5% of the sample.25 When comparing the trajectory structure of the 2-group solution with that of the 3-group solution, the 2-group solution provided an overly simple explanation of function.
Three distinct functional trajectories emerged (Figure 1) that describe change in physical functioning over time, fully adjusted for intra-individual risk factors. We labeled these trajectories rapid functional decline (Group 1: 23.8%, quadratic trajectory pattern), gradual functional decline (Group 2: 44.2%, linear trajectory pattern), and stable function (Group 3: 32.0%, quadratic trajectory pattern). Slopes for the stable function and rapid functional decline groups were significantly different (χ2 = 29.68, p < .001). The magnitude of change in SPPB score for each trajectory group throughout follow-up can be found in Supplementary Table S2.
Figure 1.
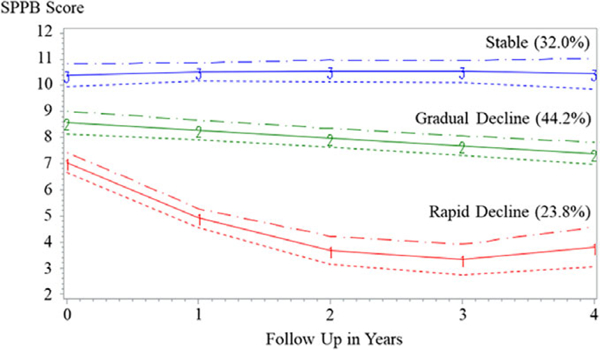
Functional trajectories in National Health and Aging Trends Study participants with cardiovascular disease (N=392).
Significant differences were found between trajectory groups in education, age, and comorbidity for those with and without CVD (Table 2). For persons with CVD, the stable function group had more education (79.3%), more individuals younger than 70 (53.7%), and lower comorbidity than the gradual and rapid functional decline groups. Conversely, the rapid functional decline group had less education, greater comorbidity, and more individuals aged 80 and older at baseline (41.8%).
Table 2.
Trajectory Group Demographic Characteristics for Persons with and Without Cardiovascular Disease (CVD)
| Characteristic | With CVD n = 392 |
Without CVD n = 1666 |
||||
|---|---|---|---|---|---|---|
| Group 1 (Rapid Functional Decline) n = 77 |
Group 2 (Gradual Functional Decline) n = 173 |
Group 3 (Stable Function) n = 142 |
Group 1 (Rapid Functional Decline) n = 226 |
Group 2 (Gradual Functional Decline) n = 848 |
Group 3 (Stable Function) n = 592 |
|
| Male | 55.7 | 62.8 | 69.6 | 46.0 | 47.4 | 52.0 |
| Racea | ||||||
| White | 91.8 | 91.5 | 95 | 82.3 | 86.0 | 90.1 |
| Black | 5.4 | 3.7 | 2.2 | 7.7 | 4.6 | 3.0 |
| Other | 2.9 | 4.8 | 2.8 | 9.9 | 9.4 | 6.9 |
| Educationb,c | ||||||
| < High school | 28.7 | 17.3 | 2.2 | 20.0 | 13.7 | 7.8 |
| High school | 23.3 | 25.5 | 18.5 | 26.0 | 28.1 | 16.0 |
| ≥Some college | 48.0 | 57.2 | 79.3 | 54.0 | 58.2 | 76.2 |
| Ageb,c | ||||||
| 65–69 | 16.1 | 23.4 | 53.7 | 21.2 | 36.3 | 54.5 |
| 70–74 | 25.0 | 29.7 | 25.1 | 24.3 | 30.0 | 31.7 |
| 75–79 | 17.1 | 25.0 | 14.4 | 26.4 | 20.1 | 9.6 |
| 80–84 | 28.9 | 17.6 | 5.6 | 15.9 | 9.9 | 3.9 |
| ≥85 | 12.9 | 4.3 | 1.2 | 12.2 | 3.6 | 0.3 |
| Comorbiditiesb,c | ||||||
| 0 | 0.0 | 0.0 | 0.0 | 13.2 | 13.1 | 23.8 |
| 1–3 | 41.0 | 46.3 | 63.9 | 69.5 | 80.5 | 71.8 |
| ≥4 | 59.0 | 53.7 | 36.1 | 17.3 | 6.4 | 4.3 |
Significant difference between trajectory groups in sample without CVD (p = .003).
Significant differences between trajectory groups in sample with CVD (p < .001).
Significant differences between trajectory groups in sample without CVD (p < .001).
Predictors of Trajectory Membership
Separate LCGM models revealed that sex, age at baseline, education, and comorbidity were significantly associated with trajectory group membership (Table 3). Predictors of group membership for the gradual functional decline and stable function groups were compared with those of the rapid functional decline group. Education and age were significant predictors of membership in the gradual functional decline group. Higher levels of education were associated with higher functioning and older age with lower functioning. Sex, age at baseline, education, and comorbidity predicted membership in the stable function group. Women were less likely than men to be in the stable function group (OR=0.41, 95% CI=0.24–0.99), and individuals with 4 chronic conditions or more were less likely to be in the stable function group than those with fewer than 4 conditions (OR=0.35, 95% CI=0.21–0.89). Individuals with more education had markedly greater odds of being in the stable function group, and persons aged 70 and older were less likely to be in the stable function group, with odds of membership declining with increasing age.
Table 3.
Predictors of Functional Trajectory Group Membership for Persons with Cardiovascular Disease
| Characteristic | β Coefficient (Standard Error) | P-Value | Odds Ratio (95% Confidence Interval) |
|---|---|---|---|
| Gradual vs rapid functional decline | |||
| Female | −0.45 (0.37) | .22 | 0.64 (0.34–1.38) |
| Education (reference <high school) | |||
| High school | 1.62 (0.57) | .01 | 5.06 (1.29–9.27) |
| ≥Some college | 1.55 (0.45) | .01 | 4.70 (1.20–6.58) |
| ≥4 conditions | 0.04 (0.36) | .91 | 1.04 (0.54–2.08) |
| Age (reference <70) | |||
| 70–74 | −0.22 (0.62) | .73 | 0.81 (0.24–2.70) |
| 75–79 | 0.06 (0.65) | .93 | 1.06 (0.30–3.80) |
| 80–84 | −1.50 (0.60) | .01 | 0.22 (0.07–0.72) |
| ≥85 | −2.26 (0.73) | .01 | 0.10 (0.03–0.43) |
| Stable function vs rapid functional decline | |||
| Female | −0.90 (0.42) | .04 | 0.41 (0.24–0.99) |
| Education (reference <high school) | |||
| High school | 4.02 (1.54) | .01 | 55.59 (3.17–181.04) |
| ≥Some college | 4.41 (1.51) | .01 | 82.52 (5.66–274.35) |
| ≥4 conditions | −1.06 (0.43) | .01 | 0.35 (0.21–0.89) |
| Age (reference <70) | |||
| 70–74 | −1.31 (0.63) | .04 | 0.27 (0.08–0.92) |
| 75–79 | −1.75 (0.70) | .01 | 0.17 (0.04–0.69) |
| 80–84 | −3.79 (0.72) | <.01 | 0.02 (0.01–0.09) |
| ≥85 | −4.16 (0.87) | <.01 | 0.02 (0.01–0.09) |
Sensitivity Analysis Results
A similar fully adjusted 3-class trajectory model emerged for those without CVD (Supplementary Figure S2). A smaller proportion of the sample without CVD (16.2%) than of those with CVD (23.8%) were in the rapid functional decline group. Individuals with CVD started approximately 1 point lower on the SPPB than those without in each trajectory group.
DISCUSSION
For older adults with CVD who were functionally independent at baseline, patterns of function distinctly varied over 4 years of follow-up. Although 32.0% remained stable, a large proportion experienced functional decline, with 44.2% having gradual functional decline and 23.8% having rapid functional decline. Previous work has shown that CVD leads to accelerated functional decline with age. This is the first study to demonstrate that late-life functional decline is not universal for persons with CVD. Despite trajectory pattern similarities between those with and without CVD, a higher proportion of individuals with CVD (23.8%) than without (16.2%) belonged to the rapid functional decline group, demonstrating that CVD may increase the risk of functional decline, even in persons who are functionally independent.
Individuals without CVD were higher functioning at baseline, regardless of trajectory group membership. Previous work showed that a 1-point change in SPPB represents a substantial meaningful difference in functioning.24 Therefore, this study demonstrated that there are meaningful differences in baseline function in older adults with CVD. These differences in baseline functioning may reflect early functional limitations that result from body system level impairments associated with CVD.
When coupled with CVD, intra-individual risk factors increased the odds of experiencing accelerated functional decline, even in individuals who appeared to have good function. Consistent with our hypothesis, being female, older age, having less education, and greater comorbidity increased the risk of experiencing rapid functional decline in persons with CVD. Our findings are consistent with previous work that demonstrated deleterious effects on functioning in older women with CVD9,12 and individuals with less education31 and greater comorbidity.31 However, this study also revealed that risk factors predict the functional trajectory group to which an individual is likely to belong. This underscores the importance of using these risk factors to identify individuals who would benefit from clinical assessment of physical function to establish baseline function and to monitor for functional decline over time. Efficient identification of individuals at risk of accelerated functional decline can lead to earlier intervention, delaying further functional limitation and progression to disability.
CVD-related pathology exacerbates changes normally associated with aging, accelerating muscle loss and deconditioning.6 As a result, functional limitations develop, demonstrated in this study using SPPB score. Although 32.0% of those with CVD pathology did not demonstrate functional decline, the lowest functioning trajectory group experienced rapid functional decline and constituted nearly one-quarter of the sample. Clinicians may more efficiently identify persons at risk of functional decline by using the intra-individual risk factors shown to affect functional trajectory in this study. However, further study is needed to determine whether trajectories of function are modifiable, specifically for individuals in the rapid functional decline trajectory group. Identification of healthcare and community services and/or intervention strategies that can modify functional trajectories is important, as improving function may improve quality of life and decrease healthcare expenditures related to resultant disability in late life.
Limitations
Several limitations should be discussed. First, this study relied on self-report measures to indicate the presence of CVD. Participants may have underreported CVD, and they were not asked about other CVD (e.g. heart failure, valve disease). Severity of CVD could have affected baseline functional status, however, there were no indicators of CVD severity were available in the dataset. Further work is needed to identify functional trajectories of specific subpopulations of individuals with CVD, stratified according to severity of disease.
The presence of chronic conditions and overall comorbid status was limited to variables available in the NHATS data. Further study is needed to understand how indices of comorbidity and the presence of other chronic conditions (e.g., pulmonary disease, metabolic disorders) affect functional trajectories in persons with CVD in late life. In addition, there are many clinical and biological factors that may affect functional trajectories that should be explored in future research.
Our study sample was not adequately powered to determine the effect of race on trajectory group membership. Future studies should also examine functional trajectories of individuals with CVD aged 75 and older, because our sample was predominantly younger at baseline. There may be important differences in functional trajectories and risk factors that influence trajectory membership for individuals in the oldest age groups. Although this study identified important associations between risk factors and functional trajectories, we were unable to establish the specific causal influence of risk factors studied on functional trajectories in late life.
CONCLUSIONS
This study revealed that, for individuals with CVD who were functionally independent at baseline, 3 distinct trajectories of function emerged over a 4-year follow-up period: stable function (32.0%), gradual functional decline (44.2%), and rapid functional decline (23.8%). Older adults with CVD have a higher rate of rapid functional decline (23.8%) than those without (16.2%). Intra-individual risk factors such as being female, being aged 70 and older, having less education, and having more chronic conditions increased the odds of rapid functional decline in late life. These risk factors may be used to identify persons who would benefit from functional screening to potentially interrupt the rapid pattern of functional decline. Further work is needed to fully examine functional trajectories for specific populations and to clearly identify risk factors that attenuate impairments, leading to functional limitations and resultant disability.
Supplementary Material
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
Conflict of Interest: Dr. A. Jette is an investigator with NHATS. The other authors have no conflicts of interest to disclose.
REFERENCES
- 1.Oeppen J, Vaupel J. Broken limits to life expectancy. Science 2002;296: 1029–1031. [DOI] [PubMed] [Google Scholar]
- 2.Paneni F, Canestro C, Libby P et al. The aging cardiovascular system understanding it at the cellular and clinical levels. J Am Coll Cardiol 2017;69: 1952–1967. [DOI] [PubMed] [Google Scholar]
- 3.Odden M, Coxson P, Moran A et al. The impact of the aging population on coronary heart disease in the U.S. Am J Med 2011;124:827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mensah G, Brown D. An overview of cardiovascular disease burden in the United States. Health Aff 2007;26:38–48. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich P, Trogdon J, Khavjou O et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011;123:933–944. [DOI] [PubMed] [Google Scholar]
- 6.Fried L, Tangen C, Walston J et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56A:M146–M156. [DOI] [PubMed] [Google Scholar]
- 7.Bandeen-Roche K, Seplaki C, Huang J et al. Frailty in older adults: A nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci 2015;70:1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beckett L, Brock D, Lemke J et al. Analysis of change in self-reported physical function among older persons in four population studies. Am J Epidemiol 1996;143:766–778. [DOI] [PubMed] [Google Scholar]
- 9.Stenholm S, Westerlund H, Head J et al. Comorbidity and functional trajectories from midlife to old age: The Health and Retirement Study. J Gerontol A Biol Sci Med Sci 2015;70:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman A, Sanders J, Kizer J et al. Trajectories of function and biomarkers with age: The CHS All Stars Study. Int J Epidemiol 2016;45:1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, van Belle G, Kukull W et al. Preditors of functional change: A longitudinal study of nondemented people aged 65 and older. J Am Geriatr Soc 2002;50:1525–1534. [DOI] [PubMed] [Google Scholar]
- 12.Vaughan L, Leng X, La Monte M et al. Functional independence in late-life: Maintaining physical functioning in older adulthood predicts daily life function after age 80. J Gerontol B Psychol Sci Soc Sci 2016;71:S79–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho C, Alessi C, Cho M et al. The association between chronic illness and functional change among participants in a comprehensive geriatric assessment program. J Am Geriatr Soc 1998;46:677–682. [DOI] [PubMed] [Google Scholar]
- 14.Levine D, Davydow D, Hough C et al. Functional disability and cognitive impairment after hospitalization for myocardial infarction and stroke. Circ Cardiovasc Qual Outcome 2014;7:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forman D, Arena R, Boxer R et al. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: A scientific statement for healthcare professionals from the American Heart Association. Circulation 2017;135:e894–e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verbrugge L, Jette A. The disablement process. Soc Sci Med 1994; 38:1–14. [DOI] [PubMed] [Google Scholar]
- 17.Jung T, Wickrama K. An introduction to latent class growth analysis and growth mixture modeling. Soc Personal Psychol Compass 2008;2:302–317. [Google Scholar]
- 18.Botoseneanu A, Allore H, Mendes de Leon C et al. Sex differences in concomitant trajectories of self-reported disability and measured physical capacity in older adults. J Gerontol A Biol Sci Med Sci 2016;71:1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X, Liang J, Bennett J et al. Socioeconomic stratification and multidimensional health trajectories: Evidence of convergence in later old age. J Gerontol B Psychol Sci Soc Sci 2015;70:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasper JD, Freedman VA. National Health and Aging Trends Study User Guide: Rounds 1–6 Final Release. Baltimore: Johns Hopkins University School of Public Health, 2017. Available at www.NHATS.org. [Google Scholar]
- 21.Middleton A, Fritz S, Lusardi M. Walking speed: The functional vital sign. J Aging Phys Activ 2015;23:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guralnik J, Simonsick E, Ferruci L et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol Med Sci 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 23.Kasper JD, Freedman VA, Niefeld MR. Construction of performance-based summary measure of physical capacity in the National Health and Aging Trends Study NHATS Technical Paper #4. Baltimore: Johns Hopkins University School of Public Health, 2012. Available at www.NHATS.org. [Google Scholar]
- 24.Perera S, Mody S, Woodman R et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. [DOI] [PubMed] [Google Scholar]
- 25.Andruff H, Carraro N, Thompson A et al. Latent class growth modelling: A tutorial. Tutor Quant Methods Psychol 2009;5:11–24. [Google Scholar]
- 26.Nagin D Group-Based Modeling of Development. Cambridge, MA: Harvard University Press, 2005. [Google Scholar]
- 27.Jones B, Nagin D. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Soc Method Res 2007;35:542–571. [Google Scholar]
- 28.Jones B, Nagin D, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Soc Method Res 2001;29:374–393. [Google Scholar]
- 29.Louvet B, Gaudreau P, Menaut A et al. Longitudinal patterns of stability and change in coping across three competitions: A latent class growth analysis. J Sport Exerc Psychol 2007;29:100–117. [DOI] [PubMed] [Google Scholar]
- 30.Nagin D Analyzing developmental trajectories: A semiparametric, group-based approach. Psychol Methods 1999;4:139–157. [DOI] [PubMed] [Google Scholar]
- 31.Stuck A, Walthert J, Nikolaus T et al. Risk factors for functional status decline in community-living elderly people: A systematic literature review. Soc Sci Med 1999;48:445–469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


