Fig. 3. Mechanism of allosteric activation of the β2AR by Cmpd-6FA.
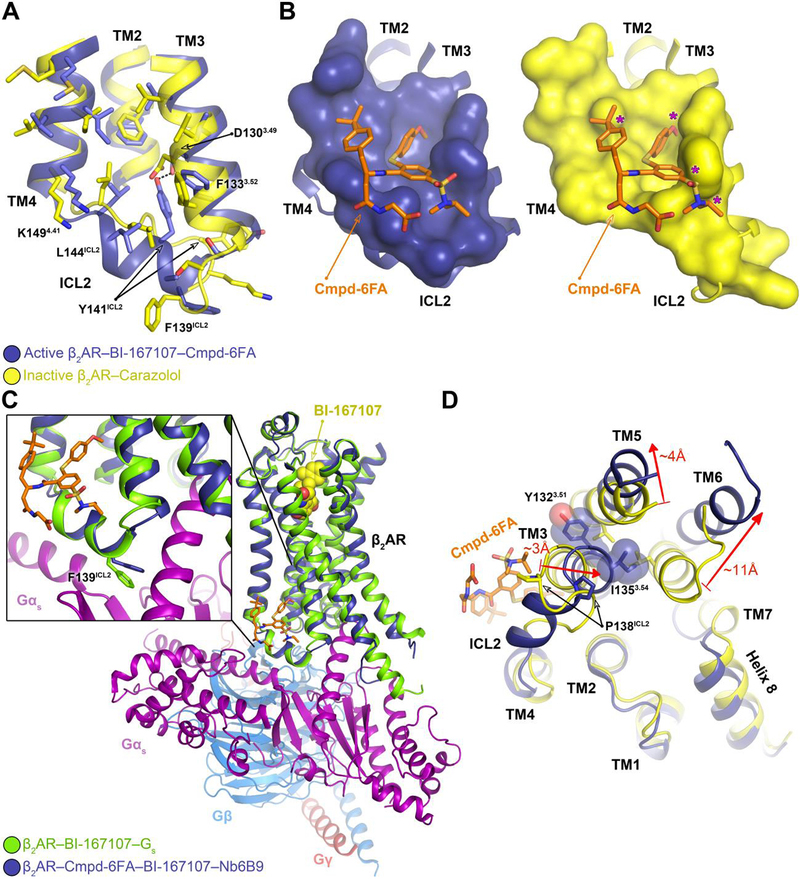
A, Superposition of the inactive β2AR bound to the antagonist carazolol (PDB code: 2RH1) and the active β2AR bound to the agonist BI-167107, Cmpd-6FA, and Nb6B9. Close-up view of the Cmpd-6FA binding site is shown. The residues of the inactive (yellow) and active (blue) β2AR are depicted and the hydrogen bond formed between Asp1303.49 and Tyr141ICL2 in the active-state is indicated by a black dashed line. B, Topography of Cmpd-6FA binding surface on the active β2AR (left, blue) and the corresponding surface of the inactive β2AR (right, yellow) with Cmpd-6FA (orange sticks) docked on top. Molecular surfaces are of only those residues involved in interaction with Cmpd-6FA. Steric clash between Cmpd-6FA and the surface of inactive β2AR is represented by a purple asterisk. C, Overlay of the β2AR bound to BI-167107, Nb6B9, and Cmpd-6FA with the β2AR–Gs complex (PDB code: 3SN6). The inset shows the position of Phe139ICL2 relative to the α-subunit of Gs. D, Superposition of the active β2AR bound to the agonist BI-167107, Nb6B9, and Cmpd-6FA (blue) with the inactive β2AR bound to carazolol (yellow) (PDB code: 2RH1) as viewed from the cytoplasm. For clarity, Nb6B9 and the orthosteric ligands are omitted. The arrows indicate shifts in the intracellular ends of the TMs 3, 5, and 6 upon activation and their relative distances.
