Abstract
Identification and validation of molecular targets are considered as key elements in new drug discovery and development. We have recently demonstrated that a novel synthetic iminoquinone analog, termed [7-(benzylamino)-1,3,4,8-tetrahydropyrrolo [4,3, 2-de]quinolin-8(1H)-one] (BA-TPQ), has significant anti-breast cancer activity both in vitro and in vivo, but the underlying molecular mechanisms are not fully understood. Herein, we report the molecular studies for BA-TPQ’s effects on JNK and its upstream and downstream signaling pathways. The compound up-regulates the JNK protein levels by increasing its phosphorylation and decreasing its polyubiquitination-mediated degradation. It activates ZAK at the MAPKKK level and MKK4 at the MAPKK level. It also up-regulates the TGFβ2 mRNA level, which can be abolished by the JNK-specific inhibitor SP600125, but not TGFβ pathway-specific inhibitor SD-208, indicating that both JNK and TGFβ signaling pathways are activated by BA-TPQ and that the JNK pathway activation precedes TGFβ activation. The pro-apoptotic and anti-growth effects of BA-TPQ are significantly blocked by both the JNK and TGFβ pathway inhibitors. In addition, BA-TPQ activates the ZAK-MKK4-JNK pathway in MCF7 cells, but not normal MCF10A cells, demonstrating its cancer-specific activities. In conclusion, our results demonstrate that BA-TPQ activates the ZAK-MKK4-JNK-TGFβ signaling cascade as a molecular target for its anticancer activity.
Keywords: Apoptosis, ATF3, breast cancer, cancer molecular target, DDIT3, Jun, MAP kinase, synthetic marine drugs, TGFβ, ZAK-MKK4-JNK
INTRODUCTION
Breast cancer poses a major health problem worldwide. It is the most common cancer in women [1]. Although several effective chemotherapeutic drugs are widely used for breast cancer treatment, including tamoxifen, doxorubicin, cyclophosphamide, methotrexate, fluorouracil, and paclitaxel, they often produce severe side effects. Additionally, advanced and recurrent breast cancer patients are resistant to these chemotherapeutic agents. Thus, there is an urgent need for the development of new drugs with multifaceted activity against advanced and drug-resistant breast cancer, while minimizing their side effects.
We have been interested in developing novel agents for breast cancer therapy and discovered a novel class of synthetic analogs of marine drugs that exert potent anticancer efficacy in vitro and in vivo [2-5]. One of the novel agents under preclinical development is [7-(benzylamino)-1,3,4, 8-tetrahydropyrrolo [4,3,2-de]quinolin-8(1H)-one] (BA-TPQ), a synthetic iminoquinone analog that is effective against both estrogen receptor positive and negative breast cancer cells, but has no apparent cytotoxicity to non-malignant breast epithelial cells [4]. Our recent microarray data demonstrate that the novel compound inhibits breast cancer growth by targeting multiple molecules and signaling pathways associated with cell cycle progression, proliferation, apoptosis, and DNA damage response [4]. Considering that identification and validation of molecular targets are a key element in new drug discovery and development, increasing efforts have been devoted to better understanding the molecular mechanisms of action for this compound.
Based on our preliminary microarray data [4], this investigation was focused on the JNK (Jun N-terminal Kinase) and TGFβ (Transforming growth factor beta) signaling pathways. The JNK signaling pathway is one of three major branches of the MAP kinase (Mitogen Activated Protein Kinase or MAPK) cascades in mammalian cells and plays a pivotal role in inducing cell apoptosis in response to a variety of internal and external stimuli, such as UV radiation, heat shock, inflammatory cytokines, endoplasmic reticulum stress, chemotherapy, and oxidative stress [6-9]. The JNK protein is activated by either MKK4 or MKK7 through phosphorylation, and the activated JNK further activates Jun by phosphorylating it at Ser63 and Ser73 [10, 11]. The activated Jun then forms a heterodimeric complex with AP-1 family members and acts as a transcription factor to regulate the expression of multiple genes related to cell growth, division, differentiation, and apoptosis [12, 13]. MKK4 and MKK7 phosphorylate and activate the JNK protein [14-16], and their activities are regulated by upstream MAPKKKs (MAP Kinase Kinase Kinase or MAP3K) [17]. At present, there are 14 MAP3Ks reported to activate the JNK cascade through phosphorylation of MKK4/7, including MEKK1, 2, and 4, MLK1-3, and DLK, among others [6, 18]. In response to an extracellular stimulus, the corresponding MAP3K is activated and then serves as an initiator of the MAPKKK-MAPKK-MAPK cascade to amplify, modulate, and integrate the extracellular signal into an intracellular response in a cell type- and stimulus-specific manner [6]. The TGFβ signaling pathway is involved in the regulation of cell proliferation, differentiation, invasion, and apoptosis. It is a potent regulator of both normal mammary gland development and mammary carcinogenesis [19]. TGFβ can function as either a tumor suppressor or promoter, depending on the stimuli, cell type, and cellular context [20].
The present study was designed to explore the molecular mechanisms of BA-TPQ-induced growth inhibition and apoptosis in breast cancer cells. Using both gene overexpressing and silencing technologies and employing specific pharmacological inhibitors, we were able to dissect the effects of BA-TPQ on the various levels of the ZAK-MKK4-JNK-TGFβ signaling cascade. Interestingly, BA-TPQ activated the ZAK-MKK4-JNK cascade in MCF7 cells, but not in non-tumorigenic MCF10A cells, suggesting that BA-TPQ specifically targets tumor cells as observed previously [4]. These results will facilitate the future development of BA-TPQ in both preclinical and clinical settings.
MATERIAL AND METHODS
Test Compound, Cell lines, Plasmids, Antibodies, Chemicals, and Reagents
The synthesis and purification of BA-TPQ were described previously [4]. The chemical structure is provided in supplemental data (Fig. S1). MCF7 and MCF10A cells were purchased from the ATCC and cultured under the conditions suggested by ATCC and reported previously [4]. ASK1 wild type (ASK1 wt) and knockout (ASK1 KO) mouse embryonic fibroblasts (MEFs) were a generous gift from Dr. Hidenori Ichijo at The University of Tokyo, Japan. The dominant negative HA-Jun vector was a kind gift from Dr. Dirk Bohmann, University of Rochester. The DDIT3 (9C8), ATF3 (C-19), and Jun (H-79) antibodies and Salubrinal (sc-202332) were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA). The antibodies against JNK (56G8), phospho-JNK (T183/Y185) (81E11), MKK4, phospho-MKK4 (S251/T261), smad2 (D43B4), and phospho-smad2 (S465/467) (138D4) were obtained from Cell signaling Technology Inc. (Beverly, MA). The ZAK (D1-18) antibody was from Abcam (Cambridge, MA). The Ub antibody was obtained from Millipore (Billerica, MA). The β-actin antibody, SD-208, thapsigargin, tunicamycin, protease inhibitor cocktail (P8340), and phosphatase inhibitor cocktails I (P2850) and II (P5726) were purchased from Sigma (St. Louis, MO). The siRNAs against Jun, ATF3, JNK, MKK4, ZAK and control siRNA pools were obtained from Dharmacon (Lafayette, CO). SP600125 was purchased from EMD Calbiochem (Gibbstown, NJ), and Protein G beads were purchased from GE Healthcare (Piscataway, NJ).
RNA Extraction and RT-PCR Assay
Total RNA was extracted from MCF7 cells using the Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Briefly, 2 μg of total RNA was used to synthesize the first strand cDNA with the superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) under the conditions recommended by the manufacturer. A 0.5 μl aliquot of cDNA was used as template for the PCR in a 20 μl reaction system. The primers used for amplification of the different genes were as follows: DDIT3 forward: CCT GAG GAG AGA GTG TTC AAG A, reverse: TCT TGC AGG TCC TCA TAC CA; ATF3 forward: AGG AGA AGA CGG AGT GCC T, reverse: GGT TTC TCT CAT CTT CTG GAG TC; Jun forward: CAG CCC AAA CTA ACC TCA CG, reverse: CAT GCT CTG TTT CAG GAT CTT G; TGFβ2 forward: CTC CTT CGA TGT AAC TGA TGC TG, reverse: TGG AGG TGC CAT CAA TAC C; XBP1 forward: GGG AAT GAA GTG AGG CCA, reverse: AAT GCC CAA CAG GAT ATC AGA; GAPDH forward: GGA GTC CAC TGG CGT CTT CAC, reverse: GAG GCA TTG CTG ATG ATC TTG AGG. PCR was performed using cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s followed by a final extension at 72°C for 5 min. The cycle numbers vary depending on mRNA abundance of genes detected. PCR products were resolved on 1.5% agarose gels and visualized by ethidium bromide staining.
Immunoblotting and Immunoprecipitation
MCF7 cells were seeded at a density of ~3 × 105/60 mm dish or 5 × 105/l00 mm dish. The following day, the cells were treated with BA-TPQ with or without the inhibitors (such as SP600125, or SD-208). For Western blot analysis, the cells were lysed in RIPA buffer consisting of 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.1% SDS, 1.0% sodium deoxycholate, 1% Triton X-100, and a protease inhibitor mixture from Sigma. Western blotting analysis was performed as described previously (4). For immunoprecipitation assays, cells were lysed in NP-40 lysis buffer consisting of 50 mM Tris-HCl (pH 8.0), 1.0% Nonidet P-40, 1.0 mM EDTA, 137 mM NaCl, and the protease inhibitor mixture from Sigma, and then the ubiquitin antibody was applied to co-precipitate ubiquitinated proteins.
Gene Silencing by siRNAs
MCF7 cells were transfected with the control or genespecific siRNA pools for 72 h. BA-TPQ was added 24 h or 48 h before cell harvesting for further analyses. The cells were collected for apoptosis assay, or lysed in RIPA buffer and detected for target protein expression by Western blot analysis.
Cell Survival Assay
Cells were plated in 96-well cell culture plates at a density of 3 × 103/well and treated the next day with the indicated agents for 72 h and the viable cell number was determined using the MTT assay as described previously [4].
Cell Cycle Distribution and Apoptosis Assays
Cells were seeded in 60-mm dishes at a density of 4 × 105/dish. The next day, the cells were treated with ZAK specific siRNA pool for 24 h, and then BA-TPQ was added to treat the cells for another 48 h; or pretreated with indicated inhibitor for 30 min, and then BA-TPQ was added for another 48 h. Apoptosis was determined by Annexin V-FITC staining using the BioVision Annexin V-FITC apoptosis kit according to manufacturer’s instructions (Biovision, Mountain View, CA). To determine the cell cycle distribution, cells were collected and fixed in 75% alcohol overnight, and the cell pellets were digested the next day with RNase A at 37°C for 20 min and stained with Propidium Iodide. Then, the cell cycle distribution was analyzed using a flow cytometry.
Statistical Analysis
All the data (mean ± SD) for cell apoptosis, cell cycle, or cell survival were derived from assays in triplicate and all the experiments were repeated twice or thrice. The significance of the differences among various treatment groups were analyzed by ANOVA.
RESULTS
BA-TPQ Induces the Expression of Jun, ATF3 and DDIT3 in MCF7 cells, Independent of Endoplasmic Reticulum Stress
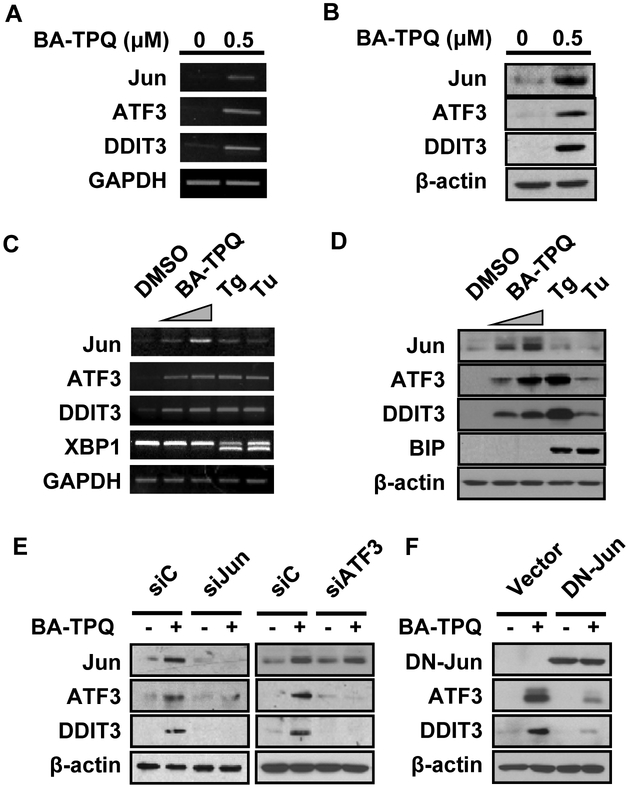
BA-TPQ inhibits cell growth and induces apoptosis in MCF7 cells [4]. To further elucidate the molecular mechanism of action of BA-TPQ, microarray analyses were employed to screen for signaling pathways or target molecules involved in this effect. We observed that the Jun (jun proto-oncogene), ATF3 (activating transcription factor 3) and DDIT3 (DNA-damage-inducible transcript 3) mRNA levels were all significantly induced by exposure to 0.5 μM BA-TPQ for 16 h (Jun: Fold=8.91, p=0.0037; ATF3: Fold=8.54, p=0.0101; DDIT3: Fold=21.31, p=0.0015). These alterations were further confirmed by RT-PCR (Fig. 1A) and Western blot analysis (Fig. 1B).
Fig. (1). BA-TPQ induces the expression of Jun, ATF3 and DDIT3, independent of endoplasmic reticulum stress.
A. MCF7 cells were treated with DMSO or 0.5 μM BA-TPQ for 16 h. Then, total cellular RNA was extracted, and RT-PCR was performed to detect the expression levels of the indicated genes. B. MCF7 cells were treated with DMSO or 0.5 μM BA-TPQ for 24 h. The cellular lysates were collected, and target protein expression was determined by Western blotting. C and D. MCF7 cells were treated with DMSO, or 0.5 μM or 0.75 μM BA-TPQ, or 2 μM thapsigargin (Tg) or 2 ng/μL tunicamycin (Tu) for 16 h for RT-PCR assay (C), or for 24 h for Western blotting (D). E. MCF7 cells were transfected with a control, Jun or ATF3 siRNA pool. After 48 h, the cells were exposed to DMSO or 0.5 μM BA-TPQ for an additional 24 h, and then cell lysates were harvested and subjected to Western blotting to detect the expression of the indicated proteins. F, MCF7 cells were transfected with a control vector or a dominant negative HA-Jun vector for 24 h prior to treatment with DMSO or 0.5 μM BA-TPQ for an additional 24 h. After that, Western blotting was performed to detect target protein expression as indicated. Of note, Jun was detected by HA antibody.
Because Jun, ATF3 and DDIT3 were reported to be induced during ER stress through the IRE1-ASK1-JNKJun/ATF3/DDIT3 and PERK-eIF2-ATF4-ATF3/DDIT3 signaling cascades in response to multiple insults [21, 22], and DDIT3 is commonly used as a biological marker for ER stress, we wonder if BA-TPQ induces MCF7 cell apoptosis via activating ER stress pathway. To verify this assumption, we blocked PERK pathway by its specific inhibitor, salubrinal, and then MCF7 cell survival and target protein expressions were detected. Contrary to our expectation, even 100 μM salubrinal pretreatments could neither effectively rescue MCF7 cell survival inhibited by BA-TPQ, nor prevent the increment of Jun, DDIT3 and ATF3 induced by BA-TPQ (data not shown). Since ASK1 is a unique upstream activator of JNK-Jun cascade during ER stress, we employed ASK1 wt and KO MEFs to check if ASK1 is an upstream regulator of Jun, ATF3 and DDIT3, and our data demonstrated that BA-TPQ induced the elevated expression of the above three proteins and MEF cell growth inhibition regardless of ASK1 status. Additionally, when MCF7 cells were treated with DMSO, 0.5 or 0.75 μM BA-TPQ, 2 μM thapsigargin (Tg) or 2 ng/μl tunicamycin (Tu) (Tg and Tu are known ER stress inducers). We found that both Tg and Tu induced the splicing of XBP1 mRNA and increased the expression of the BIP protein, two common events during ER stress, but BA-TPQ had no such effects, although there was an increase in the expression of DDIT3 and ATF3 following treatment of MCF7 cells with either BA-TPQ, or Tg or Tu (Fig. 1C and 1D). Of note, little induction of Jun at both mRNA and protein level was observed after MCF7 cells were treated with Tg and Tu, compared to BA-TPQ (Fig. 1C and 1D). Taken together, these results suggest that BA-TPQ probably induces an ER stress-independent MCF7 cell growth inhibition and apoptosis, or via a molecular mechanism that is a little different from the canonical endoplasmic reticulum stress cascade.
To gain insights into the detailed regulatory network among Jun, ATF3 and DDIT3, we knocked down Jun or ATF3 and determined their effects on the levels of the other two proteins. Our results showed that knockdown Jun by siRNA, or expression of a dominant negative mutant of Jun, decreased the expression of both ATF3 and DDIT3 (Fig. 1E, left panel; Fig. 1F), while ATF3 knockdown alone led to a reduction in the DDIT3 protein level, but not Jun (Fig. 1E, right panel), indicating that Jun probably regulates ATF3, which in turn regulates DDIT3 expression. It is also possible that Jun forms a complex with ATF3 that up-regulates DDIT3 expression, or that ATF3 interacts with DDIT3 to modulate their downstream targets [22, 23].
BA-TPQ Stabilizes and Activates JNK Pathway
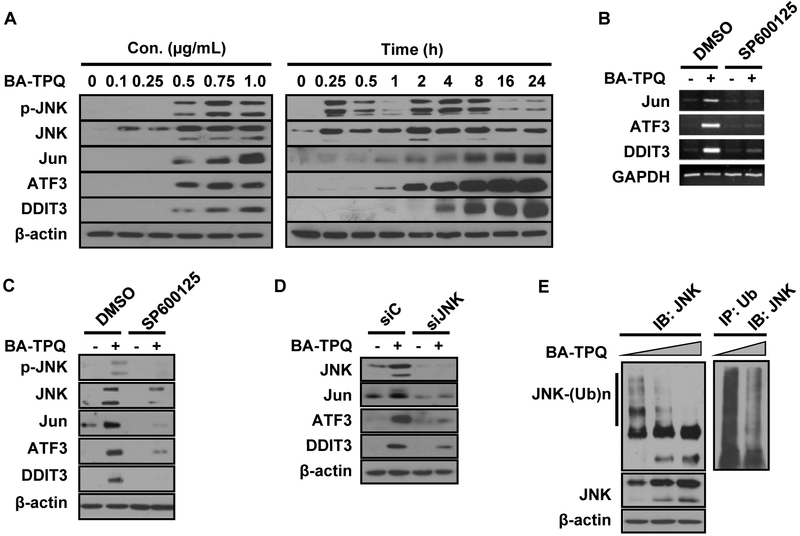
Since JNK is a key upstream activator of Jun [11, 17], we hypothesized that JNK was responsible for the induction of Jun, and the subsequent activation of ATF3 and DDIT3 by BA-TPQ. To test the hypothesis, we treated MCF7 cells with various concentrations of BA-TPQ for 24 h, or with 0.5 μM of BA-TPQ for various times, and determined the levels of phosphorylated and total JNK, as well as Jun, ATF3 and DDIT3 by Western blotting. As shown in the left panel of Fig. (2A), BA-TPQ treatment led to a dose-dependent increase in both phosphorylated JNK and the total JNK protein, together with the increased protein expression of Jun, ATF3 and DDIT3. We further examine time-dependence of the expression of these proteins. The activation of JNK in MCF7 cells was observed in a short period, only 15 min after exposure to BA-TPQ. However, as the duration of the treatment was increased, the level of phosphorylated JNK gradually decreased, and reached the lowest at the 1-h time point. The level then started to increase again, as shown in the 2-h time point. Correspondingly, the remarkably induced expressions were also observed at 2-h time point for Jun and ATF3, and at 4-h time point for DDIT3, their induced expressions showed a tendency of continuous increment even at 24-h time point (Fig. 2A, right panel). Although the exact molecular mechanism(s) and physiological significance of reduced expression and phosphorylation of JNK at 1-h time point were not known, we deduced that highest activities of serine/threonine protein phosphatase (such as PP1 or PP2A), or dual-specificity phosphatases (such as MAPK phosphatase 1 or MKP1) at 1-h time point were probably involved, as reported previously by other research groups [24, 25]. Meanwhile, our data denoted that the activated JNK (both phosphorylated and native JNK) prior and after 1-h time point probably have different functions, with the first increment of JNK dealing with promotion of cell survival, and when failed to do so, the second increment enhancing the expression of apoptosis related genes, including Jun ATF3 and DDIT3 to induce cell apoptosis.
Fig. (2). BA-TPQ stabilizes and activates JNK, and as a result, the Jun-ATF3-DDIT3 cascade.
A. MCF7 cells were treated with increasing concentrations of BA-TPQ for 24 h (left panel), or treated with 0.5 μM BA-TPQ for various times indicated in the figure (right panel), then cell lysates were collected and subjected to Western blot analyses to detect the indicated proteins. B and C. MCF7 cells were pretreated with DMSO, or 10 μM of SP60015 for 30 min, and then DMSO or 0.5 μM BA-TPQ was added for an additional 16 h or 24 h. Then, RT-PCR or Western blotting was performed to detect target mRNA (B) or protein expression (C). D. MCF7 cells were transfected with a control or JNK siRNA pool. 48 h later, the cells were exposed to DMSO or 0.5 μM BA-TPQ for additional 24 h, and then the cell lysates were harvested and subjected to Western blotting to probe target protein expression as indicated in the figure. E. MCF7 cells were treated with increasing concentrations of BA-TPQ for 24 h. Then cells were lysed in RIPA buffer, and Western blotting was performed to evaluate the JNK protein level using a JNK antibody (left panel), or cell lysates were immunoprecipitated with an ubiquitin antibody, and probed with the JNK antibody (right panel).
To further support our findings that JNK induced increased expression of Jun, ATF3 and DDIT3, MCF7 cells were treated with either the vehicle, or with SP600125, a JNK pathway-specific inhibitor, for 30 min, prior to a 24-h treatment with the vehicle or BA-TPQ. As shown in Fig. (2B and C), SP600125 pretreatment blocked the up-regulation of Jun, ATF3 and DDIT3 at both the mRNA and protein levels. Similar results were obtained when specific siRNAs against JNK was used to knock down JNK expression (Fig. 2D). Collectively, these results demonstrate that the JNK signaling pathway plays a critical role in the BA-TPQ-induced up-regulation of Jun, ATF3 and DDIT3.
We next explored the underlying molecular mechanism(s) for increased expression of native JNK protein. We found that JNK1/2 mRNA level was not altered after BA-TPQ treatment (data not shown). However, BA-TPQ treatment resulted in a dose-dependent reduction in polyubiquitinated JNK and in contrast, a dose-dependent increment of native JNK in MCF7 cells (Fig. 2E, left panel), and the poly-ubiquitination was further confirmed by immunoprecipitation assays (Fig. 2E, right panel). These results implying that BA-TPQ probably increases JNK function by both inducing JNK phosphorylation and diminishing its polyubiquitination and degradation. However, the exact molecular mechanism(s) and function of polyubiquitinated JNK needs to be further determined.
The JNK Signaling Pathway is Responsible for the BA-TPQ-induced Apoptosis
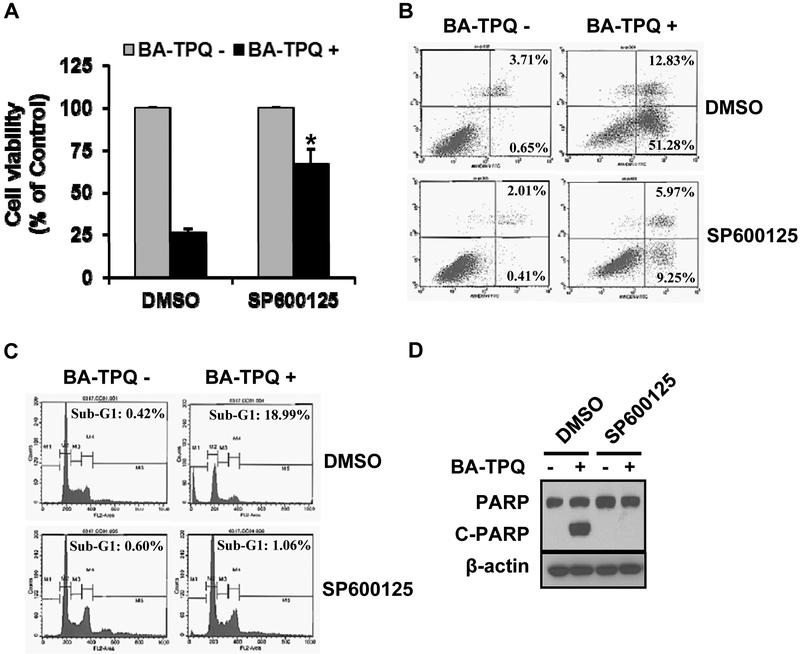
To test whether there was an association between cell growth inhibition and apoptosis and the activation of the JNK signaling pathway, we pre-treated MCF7 cells with vehicle or SP600125 for 30 min, and then added BA-TPQ to the cells for different times, followed by analysis of cell viability, apoptosis, cell cycle distribution, and PARP cleavage. BA-TPQ treatment significantly reduced the survival of cells (Fig. 3A), increased the number of apoptotic cells (Fig. 3B, arrested cells in the sub-G1 phase (Fig. 3C), and increased PARP cleavage (Fig. 3D); however, these effects were attenuated or completely abrogated by pretreatment with SP600125. These results further confirmed that JNK was the primary molecular target for BA-TPQ induced apoptosis in MCF7 cells.
Fig. (3). JNK pathway is critical for BA-TPQ-induced apoptosis.
A. MCF7 cells were treated with DMSO, or JNK inhibitor indicated in the figure with or without BA-TPQ for 72 h, and then the MTT assay was performed to assess cell viability. B-D. MCF7 cells were pretreated with DMSO or JNK inhibitors (indicated in the figure) for 30 min. Then, Cells were further treated with DMSO or 0.5 μM BA-TPQ for 48 h for apoptosis assay (B) or cell cycle analysis (C) by flow cytometry, or for 24 h to determine whether the compound induces PARP cleavage (D). * represents p<0.05.
BA-TPQ Activates JNK Signaling by Modulating the ZAK-MKK4 Cascade
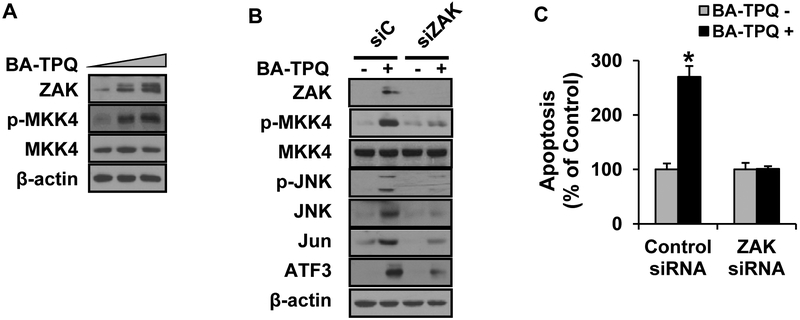
Next, we investigated the upstream molecule(s) responsible for phosphorylating JNK at the MAP3K and MAP2K levels after exposure to BA-TPQ. At present, there are 20 known MAP3Ks, and 14 of them were reported to be capable of activating the JNK protein [18, 26]. At MAP2K level, MKK4 and MKK7 are able to phosphorylate the JNK protein [18]. After a screening with the panel of kinases, we found that both ZAK and p-MKK4 were induced by BA-TPQ in a dose-dependent manner (Fig. 4A). When ZAK was knocked down by its specific siRNA pools, the activation or induced expression of p-MKK4, p-JNK, JNK, Jun, ATF3 and DDIT3 were decreased (Fig. 4B), further confirming that ZAK is the BA-TPQ sensor at the MAP3K level, and ZAK activates JNK via MKK4. As shown in Fig. (4C), the BA-TPQ-induced apoptosis was almost completely blocked after ZAK was knocked down by ZAK specific siRNAs, supporting that ZAK is an important upstream target of BA-TPQ in MCF7 cells.
Fig. (4). ZAK and MKK4 are upstream activators of JNK pathway induced by BA-TPQ.
A. MCF7 cells were treated with increasing concentrations of BA-TPQ for 24 h, and target proteins were detected by Western blotting. B. MCF7 cells were treated with a control, or ZAK-specific siRNA pool for 48 h prior to addition of DMSO or 0.5 μM BA-TPQ for an additional 24 h. Cell lysates were collected and subjected to Western blotting analysis to detect target protein expression as indicated in the figure. C. MCF7 cells were treated with a control siRNA pool or a siRNA pool specific for ZAK for 24 h, and then DMSO or 0.5 μM BA-TPQ was added to treat the cells for an additional 48 h. Then, MCF7 cell apoptosis assay was determined. * represents p<0.05.
BA-TPQ Activates TGFβ Signaling Located Downstream of JNK Pathway
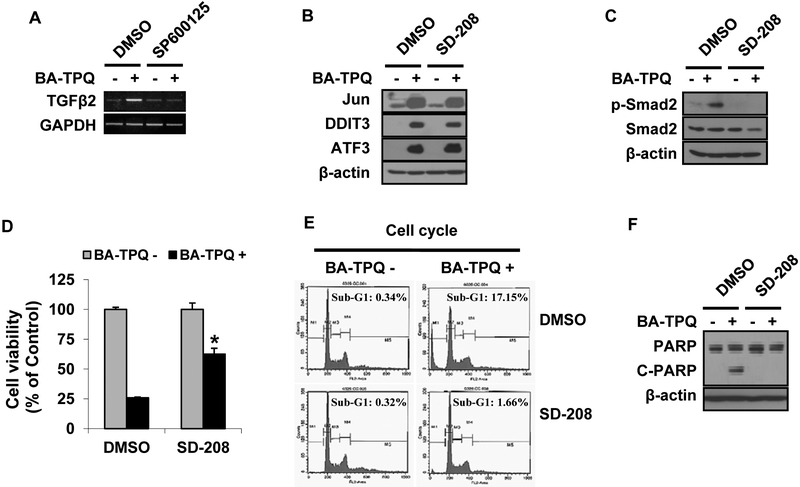
Our microarray data indicated that the mRNA level of TGFβ2 was increased in MCF7 cells after BA-TPQ treatment (Fold=2.39, p=0.046). Several reports have indicated that the TGFβ signaling pathway activates the JNK signaling pathway [27-29]. In the present study, we examined the linkage between the pathways and their roles in BA-TPQ induced apoptosis. As shown in Fig. (5A), BA-TPQ induced TGFβ2 expression. When the JNK signaling pathway was blocked by SP600125, BA-TPQ failed to induce TGFβ2 expression. However, blocking the TGFβ2 signaling pathway by SD-208, a TGFβ-specific inhibitor, did not prevent the induction of Jun, ATF3 or DDIT3, although it abrogated BA-TPQ induced phosphorylation of smad2, a downstream target of TGFβ pathway ((Fig. 5B and C). Therefore, these results indicate that JNK is located in the upstream of the TGFβ pathway. As shown in Fig. (5D-F), BA-TPQ reduced cell survival (Fig. 5D), arrested cells in sub-G1 phase (Fig. 5E), and increased PARP cleavage (Fig. 5F); and these effects were compromised by the pretreatment with SD-208, further indicating the importance of the TGFβ signaling pathway in BA-TPQ-induced apoptosis.
Fig. (5). Induction of TGFβ by JNK contributes to BA-TPQ-induced apoptosis.
A. MCF7 cells were pretreated with DMSO, or 10 μM of SP60015 for 30 min, and then DMSO or 0.5 μM BA-TPQ was added for an additional 16 h. Then, RT-PCR was performed to detect TGFβ mRNA expression. B and C. MCF7 cells were pretreated with DMSO or 1 μM SD-208 for 45 min prior to addition of DMSO or 0.5 μM BA-TPQ for another 24 h. Targeted protein expressions for Jun, ATF3 and DDIT3 (B) and smad2 and p-smad2 (C) were examined by Western blotting. D. MCF7 cells were pretreated with DMSO or 1 μM SD-208 for 45 min prior to addition of DMSO or 0.5 μm BA-TPQ for 72 h. Cells were then subjected to the MTT assay to determine cell viability. E and F, MCF7 cells were exposed to DMSO or 1 μM SD-208 for 45 min prior to addition of DMSO or 0.5 μM BA-TPQ. Cells were harvested at 48 h for cell cycle analysis (E) by flow cytometry, or were examined at 24 h by Western blotting for PARP cleavage (F). * represents p<0.05.
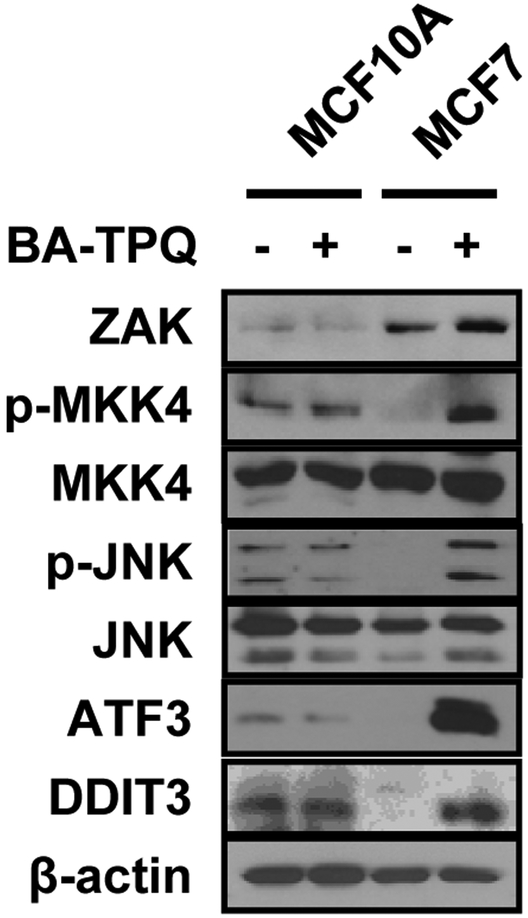
BA-TPQ Activates the ZAK-MKK4-JNK-ATF3/DDIT3 Signaling Pathway in a Breast Cancer Cell-specific Manner
After the observation that BA-TPQ induced MCF7 cell apoptosis through activation of the ZAK-MKK4-JNK-ATF3/DDIT3 signaling cascade, we determined whether this effect was specific to breast cancer cells. We treated MCF10A cells, a non-malignant immortalized breast epithelial cell line, and malignant MCF7 cells with vehicle or 0.5 μM BA-TPQ for 24 h, and then detected the signaling pathway molecules by Western blotting. As illustrated in Fig. (6), BA-TPQ activated the above pathway molecules in MCF7 cells. However, MCF10A cells did not respond to BA-TPQ exposure, showing the activation but no changes in MKK4-JNK-ATF3/DDIT3 signaling pathway. Of note, ZAK protein expression in MCF10A cells was much lower than that of MCF7 cells and was not induced by BA-TPQ, suggesting that ZAK is a sensor for BA-TPQ-induced activation of ZAK-MKK4-JNK-ATF3/DDIT3 pathway in MCF7 cancer cells, but not non-tumorigenic breast cells. This finding may help to explain the low expression of ZAK with high pathway activity in non-malignant MCF10A cells. As to ZAK, more studies need to be performed to clarify its expression regulation and function in cancerous MCF7 cells before and after exposure to BA-TPQ.
Fig. (6). BA-TPQ activates the ZAK-MKK4-JNK cascade and ATF3 and DDIT3 in a breast cancer cell-dependent fashion.
MCF10A and MCF7 cells were incubated with DMSO or 0.5 μM BA-TPQ for 24 h. After the treatment, cell lysates were collected and Western blotting was performed to probe target protein expression as shown in the figure.
DISCUSSION
Marine drugs have high potency and often exert their anticancer effects by targeting multiple molecules or signaling pathways [30]. Identification and validation of molecular targets will facilitate their development. BA-TPQ is a synthetic analog of a makaluvamine compound derived from marine sponges and has anticancer activity in both estrogen receptor positive MCF7 cells and estrogen receptor negative MDA-MB-468 cells, but has no obvious cytotoxicity against non-tumorigenic MCF10A breast epithelial cells [4]. Our microarray results suggest that this agent acts on multiple target molecules and signaling pathways, including the inhibition of the mRNA expression of E2F2 and E2F8, cyclin D1, cyclin E1 and E2, cyclin F, CDK6, CDC25A, and induction of several signaling pathways, such as p53, TNFα, and TGFβ pathways [4]. Following our observation of the up-regulation of Jun (8.9-fold increase) in our microarray studies of BA-TPQ treatment in MCF7 cells, we explored whether the JNK signaling pathway is responsible for its anticancer activity. Our results demonstrated that BA-TPQ treatment induced the phosphorylation of JNK and increased expression of native JNK, resulting in the activation of Jun. Subsequent pathway analyses revealed the activation of the ZAK-MKK4-JNK-TGFβ signaling cascade and the role of the activation in BA-TPQ-induced cell growth inhibition, cell cycle arrest, and apoptosis in MCF7 cells, but not MCF10A cells. These results support the notion that the test compound can be developed a novel anti-breast cancer agent.
The MAPK pathway is composed of three layers, with a number of proteins in each layer. For instance, at the MAPKK and MAPK levels, there are 7 and 11 kinases, respectively [6, 18]. To date, at least 20 kinases have been identified at the MAP3K level [18, 26]. ZAK, a member of the MAP3K family, has been reported to be activated in response to ionizing radiation, anisomycin stimulation, or UV radiation, among other stimuli, leading to the up-regulation of all three MAPK intermediates, and eventually to cell cycle arrest and/or apoptosis [31-33]. In our current study, we found that BA-TPQ activated the ZAK-MKK4-JNK cascade, while knockdown of ZAK down-regulated the phosphorylations of MKK4 and JNK and the expression of native JNK. These results suggest that ZAK could be a critical target for anticancer therapy. Future studies should examine how ZAK is activated by BA-TPQ.
In the present study, we showed that the activation of the ZAK-MKK4-JNK-Jun cascade was involved in BA-TPQ-induced apoptosis in MCF7 cells. JNK protein was induced at two different levels. First, BA-TPQ treatment inhibited JNK poly-ubiquitination and degradation, leading to an increase in native JNK protein. To our best knowledge, this should be the first report indicating that the expression of the native JNK protein can be regulated at the protein stability/degradation level. Second, BA-TPQ treatment activated the ZAK-MKK4 cascade, resulting in an increased activity of JNK. Of interest, the activity of Jun was also regulated at two levels. First, BA-TPQ induced a significant increase in Jun mRNA transcription, leading to elevated native Jun protein. Second, BA-TPQ treatment also led to an increase in the phosphorylation of Jun in MCF7 cells. Therefore, the dual regulation of the JNK-Jun cascade may greatly amplify the signal, enhancing the apoptosis-inducing capacity of the drug.
The TGFβ signaling pathway has a dichotomous function during breast cancer development, behaving as a tumor suppressor during early breast carcinogenesis, and as a tumor promoter in the later stages [19]. TGFβ2 was reported to be a potent growth inhibitor of human breast cancer cell lines, is significantly induced by tamoxifen, and was suggested to be a valid molecular biomarker for the antiproliferative effects of tamoxifen and its metabolites in breast cancer cells [34, 35]. In our current study, we found that BA-TPQ significantly increased the TGFβ2 mRNA level in MCF7 cells, which happened downstream of the activation of the JNK signaling pathway, suggesting that BA-TPQ induces apoptosis via the JNK-TGFβ signaling axis. Our finding is in contrast to two previous reports that suggest that the activation of the TGFβ pathway precedes the JNK pathway [27, 36], but is similar with another report that the activation of p38 MAPK lies upstream of the activation of the TGFβ signaling pathway in human breast cancer cells [37].
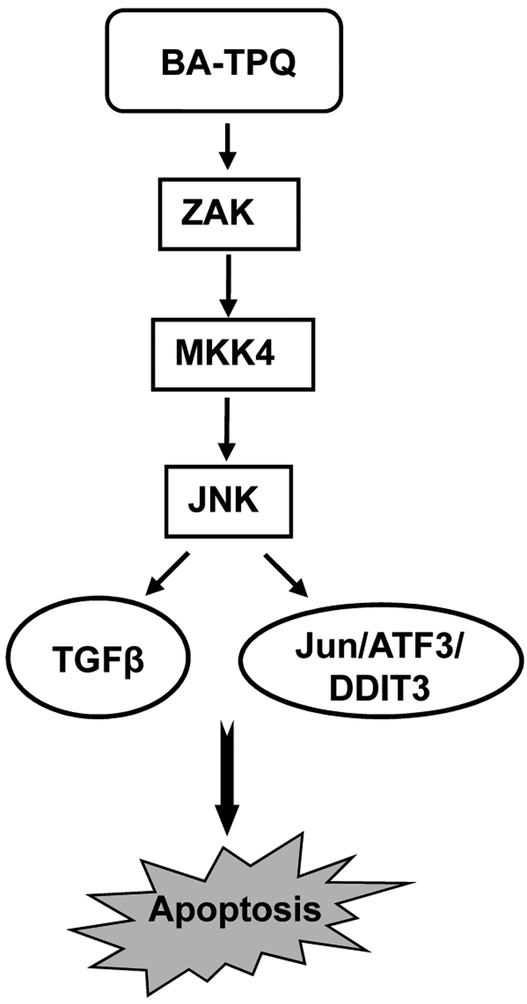
In summary, we have made several significant observations in the present study. The JNK pathway was in a minimally-active state in resting/unstressed MCF7 cells comparing to non-malignant immortalized MCF10A cells. BA-TPQ activated the ZAK-MKK4-JNK-TGFβ signaling cascade, leading to cell growth inhibition and apoptosis in MCF7 cells (Fig. 7). It regulated both JNK and its downstream target, Jun, by increasing phosphorylation and native protein levels. JNK was regulated at the posttranslational level by inhibition of its polyubiquitination, which may represent a novel mechanism of action for anticancer agents that exert pro-apoptotic effects via JNK pathway. It should be indicated that BA-TPQ and its analogs have been studied for various anticancer activities in preclinical models [2-5, 38-42], suggesting that they may have multiple molecular targets as anticancer agents, with one of the major targets being the oncogene MDM2 [43]. There is increasing evidence supporting that aiming at more than one molecular target may improve the potency and efficiency of anticancer agents. In conclusion, our results from the present study should facilitate not only the development of our currently tested compounds, but also the validation of the ZAK-MKK4-JNK-TGFβ pathway as a cancer drug target.
Fig. (7). Schematic model for BA-TPQ-induced MCF7 cell apoptosis.
BA-TPQ activates ZAK through an unknown mechanism. The activated ZAK phosphorylates MKK4, leading to the activation of JNK pathway. Then, the activated JNK up-regulates the expression of Jun, ATF3, DDIT3, and TGFβ signaling to induce apoptosis.
Supplementary Material
ACKNOWLEDGEMENTS
R.Z. was supported by NIH grants R01 CA112029 and R01 CA121211, and a grant (BCTR070731) from Susan G. Komen for the Cure. M.H.W. was supported by a NIH grant R01 CA91980. H.W. was supported by grants from One Hundred Talents Program of the Chinese Academy of Sciences and National Nature Science Foundation of China (81125020, 91029715 and 31070680). S. V. was supported by a UAB Breast Spore pilot grant and a collaborative Programmatic Development grant from UAB Comprehensive Cancer Center and a grant (1UL1RR025777) from the NIH National Center for Research Resources. We thank Dr. E. Rayburn, Ms. S. A. Nag, and Mr. S. Voruganti for assistance in preparation of the manuscript.
ABBREVIATIONS
- BA-TPQ
[7-(benzylamino)-1,3,4,8-tetrahydropyrrolo [4,3, 2-de]quinolin-8(1H)-one]
- JNK
Jun N-terminal Kinase
- MAPK
Mitogen Activated Protein Kinase
- MAPKKK
MAP Kinase Kinase Kinase or MAP3K
- MEF
mouse embryonic fibroblast
- siRNA
small interfering RNA
- TGFβ
Transforming growth factor beta
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
REFERENCES
- [1].American Cancer Society. Breast Cancer Facts & Figures 2011-2012. Atlanta: American Cancer Society, Inc. [Google Scholar]
- [2].Nag SN; Nadkarni DH; Qin JJ; Voruganti S; Murugesan S; Nguyen T; Xu S; Wang W; Wang H; Velu SE; Zhang R. Anticancer activity and molecular mechanisms of action of makaluvamines and analogues. Mol. Cell Pharm. 2012, 4, 69–81. [Google Scholar]
- [3].Wang W; Rayburn ER; Velu SE; Nadkarni DH; Murugesan S; Zhang R In vitro and in vivo anticancer activity of novel synthetic makaluvamine analogues. Clin. Cancer Res. 2009, 15,3511–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang W; Rayburn ER; Velu SE; Chen D; Nadkarni DH; Murugesan S; Chen D; Zhang R A novel synthetic iminoquinone, BA-TPQ, as an anti-breast cancer agent: in vitro and in vivo activity and mechanisms of action. Breast Cancer Res. Treat. 2010, 123,321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang XR; Xu H; Zhang X; Voruganti S; Murugesan S; Nadkarni DH; Velu SE; Wang MH; Wang W; Zhang R Preclinical evaluation of anticancer efficacy and pharmacological properties of FBA-TPQ, a novel synthetic makaluvamine analog. Marine Drugs 2012,10, 1138–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dhanasekaran DN; Reddy EP JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gabai VL; Yaglom JA; Volloch V; Meriin AB; Force T, Koutroumanis M; Massie B; Mosser DD; Sherman MY Hsp72-mediated suppression of c-Jun N-terminal kinase is implicated in development of tolerance to caspase-independent cell death. Mol. Cell. Biol. 2000, 20, 6826–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Davison K; Mann KK; Waxman S; Miller W Jr. JNK activation is a mediator of arsenic trioxide-induced apoptosis in acute promyelocytic leukemia cells. Blood 2004,103, 3496–3502. [DOI] [PubMed] [Google Scholar]
- [9].Urano F; Wang X; Bertolotti A; Zhang Y; Chung P; Harding HP; Ron D Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000, 237, 664–666. [DOI] [PubMed] [Google Scholar]
- [10].Smeal T; Binetruy B; Mercola D; Grover-Bardwick A; Heidecker G; Rapp UR; Karin M Oncoprotein mediated signalling cascade stimulates c-Jun activity by phosphorylation of serines 63 and 73. Mol. Cell. Biol. 1992, 12, 3507–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dérijard B; Hibi M; Wu IH; Barrett T; Su B; Deng T; Karin M; Davis RJ JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 1994, 76, 1025–1037. [DOI] [PubMed] [Google Scholar]
- [12].Karin M The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995, 270, 16483–16486. [DOI] [PubMed] [Google Scholar]
- [13].Karin M; Liu Z; Zandi E AP-1 function and regulation. Curr. Opin. Cell. Biol. 1997, 9, 240–246. [DOI] [PubMed] [Google Scholar]
- [14].Dérijard B; Raingeaud J; Barrett T; Wu IH; Han J; Ulevitch RJ; Davis RJ Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 1995, 267, 682–685. [DOI] [PubMed] [Google Scholar]
- [15].Sénchez I; Hughes RT; Mayer BJ; Yee K; Woodgett JR; Avruch J; Kyriakis JM; Zon LI Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature 1994, 372, 794–798. [DOI] [PubMed] [Google Scholar]
- [16].Tournier C; Whitmarsh AJ; Cavanagh J; Barrett T; Davis RJ Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc. Natl. Acad. Sci. USA 1997, 94, 7337–7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Davis RJ Signal transduction by the JNK group of MAP kinases.Cell 2000, 103, 239–252. [DOI] [PubMed] [Google Scholar]
- [18].Johnson GL; Nakamura K The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim. Biophys. Acta. 2007, 1773, 1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee JD; Blobe GC In: Breast Cancer in the Post-Genomic Era, Giordano A; Normanno N.Ed.; Humana Press: New York, 2009, Vol. 9, pp. 137–149. [Google Scholar]
- [20].Massagué J TGF-beta signal transduction. Annu. Rev. Biochem. 1998, 67, 753–791. [DOI] [PubMed] [Google Scholar]
- [21].Oyadomari S; Mori M Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [DOI] [PubMed] [Google Scholar]
- [22].Schröder M; Kaufman RJ ER stress and the unfolded protein response. Mutat. Res. 2005, 569, 29–63. [DOI] [PubMed] [Google Scholar]
- [23].Jiang HY; Wek SA; McGrath BC; Lu D, Hai T; Harding HP; Wang X; Ron D; Cavener DR; Wek RC Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol. Cell. Biol. 2004, 24, 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhao Q; Wang X; Nelin LD; Yao Y; Matta R; Manson ME; Baliga RS; Meng X; Smith CV; Bauer JA; Chang CH; Liu Y MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J. Exp. Med. 2006, 203, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aguilar JL; Kulkarni R; Randis TM; Soman S; Kikuchi A; Yin Y; Ratner AJ Phosphatase-dependent regulation of epithelial mitogen-activated protein kinase responses to toxininduced membrane pores. PLoS One 2009, 4, e8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cuevas BD; Abell AN; Johnson GL Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene 2007, 26, 3159–3171. [DOI] [PubMed] [Google Scholar]
- [27].Perlman R; Schiemann WP; Brooks MW; Lodish HF; Weinberg RA TGF-beta-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat. Cell. Biol. 2001, 3, 708–714. [DOI] [PubMed] [Google Scholar]
- [28].Yamashita M; Fatyol K; Jin C; Wang X; Liu Z; Zhang YE TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol. Cell 2008, 31, 918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hofmann TG; Stollberg N; Schmitz ML; Will H HIPK2 regulates transforming growth factor-beta-induced c-Jun NH (2)-terminal kinase activation and apoptosis in human hepatoma cells. Cancer Res. 2003, 63, 8271–8277. [PubMed] [Google Scholar]
- [30].Barthomeuf C; Bourguet-Kondracki ML; Kornprobst JM Marine metabolites overcoming or circumventing multidrug resistance mediated by ATP-dependent transporters: a new hope for patient with tumors resistant to conventional chemotherapy. Anticancer Agents Med. Chem. 2008, 8, 886–903. [DOI] [PubMed] [Google Scholar]
- [31].Liu TC; Huang CJ; Chu YC; Wei CC; Chou CC; Chou MY; Chou CK; Yang JJ Cloning and expression of ZAK, a mixed lineage kinase-like protein containing a leucinezipper and a sterile-alpha motif. Biochem. Biophys. Res. Commun. 2000, 274, 811–816. [DOI] [PubMed] [Google Scholar]
- [32].Gotoh I; Adachi M; Nishida E Identification and characterization of a novel MAP kinase kinase kinase, MLTK. J. Biol. Chem. 2001, 276,4276–4286. [DOI] [PubMed] [Google Scholar]
- [33].Gross EA; Callow MG; Waldbaum L; Thomas S; Ruggieri R MRK, a mixed lineage kinase-related molecule that plays a role in gamma-radiation-induced cell cycle arrest. J. Biol. Chem. 2002, 277,13873–13882. [DOI] [PubMed] [Google Scholar]
- [34].Zugmaier G; Ennis BW; Deschauer B; Katz D; Knabbe C; Wilding G; Daly P; Lippma ME; Dickson RB Transforming growth factors type beta 1 and beta 2 are equipotent growth inhibitors of human breast cancer cell lines. J. Cell. Physiol. 1989, 141, 353–361. [DOI] [PubMed] [Google Scholar]
- [35].Buck MB; Coller JK; Mürdter TE; Eichelbaum M; Knabbe C TGFbeta2 and TbetaRII are valid molecular biomarkers for the antiproliferative effects of tamoxifen and tamoxifen metabolites in breast cancer cells. Breast Cancer Res. Treat. 2008, 107, 15–24. [DOI] [PubMed] [Google Scholar]
- [36].Mazars A; Tournigand C; Mollat P; Prunier C; Ferrand N; Bourgeade MF; Gespach C; Atfi A Differential roles of JNK and Smad2 signaling pathways in the inhibition of c-Myc-induced cell death by TGF-beta. Oncogene 2000, 19, 1277–1287. [DOI] [PubMed] [Google Scholar]
- [37].Buck MB; Pfizenmaier K; Knabbe C Antiestrogens induce growth inhibition by sequential activation of p38 mitogen-activated protein kinase and transforming growth factor-beta pathways in human breast cancer cells. Mol. Endocrinol. 2004, 18, 1643–1657. [DOI] [PubMed] [Google Scholar]
- [38].Nadkarni DH; Wang F; Wang W; Rayburn ER; Ezell SJ; Murugesan S; Velu SE; Zhang R Synthesis and in vitro antilung cancer activity of novel 1, 3, 4, 8-tetrahydropyrrolo [4, 3, 2-de]quinolin-8(1H)-one alkaloid analogs. Med. Chem. 2009, 5, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang F; Ezell SJ; Zhang Y; Wang W; Rayburn ER; Nadkarni DH; Murugesan S; Velu SE; Zhang R FBA-TPQ, a novel marine-derived compound as experimental therapy for prostate cancer. Invest. New Drugs 2010, 28, 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ezell SJ; Li H; Xu H; Gurpinar E; Zhang X; Rayburn ER; Sommers CI; Yang X; Velu SE; Wang W; Zhang R Preclinical pharmacology of BA-TPQ, a novel synthetic iminoquinone anticancer agent. Marine Drugs 2010, 8, 2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li H; Ezell SJ; Zhang X; Wang W; Xu H; Rayburn ER; Zhang X; Gurpinar E; Yang X; Sommers CI; Velu SE; Zhang R Development and validation of an HPLC method for quantitation of BA-TPQ, a novel iminoquinone anticancer agent, and an initial pharmacokinetic study in mice. Biomed. Chromatogr. 2011, 25, 628–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chen T; Xu Y; Guo H; Liu Y; Hu P; Yang X; Li X; Ge S; Velu SE; Nadkarni DH; Rayburn ER; Zhang R; Wang H Experimental therapy of ovarian cancer with a novel synthetic makaluvamine analog; anticancer activity in vitro and in vivo and mechanisms of action. Plos One 2011, 6, e20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Qin JJ; Nag SN; Voruganti S; Wang W; Zhang R Natural product MDM2 inhibitors: anticancer activity and mechanisms of action. Curr. Med. Chem. 2012, 19, 5705–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.