Key Points
Question
What is the value of left ventricular global longitudinal strain (GLS) for the prediction of cancer therapy–related cardiac dysfunction (CTRCD)?
Findings
In this systematic review and meta-analysis of 21 prognostic studies, worse absolute GLS during chemotherapy and a greater relative deterioration compared with baseline were associated with a higher risk of CTRCD. Additionally, there was significant publication bias and interstudy heterogeneity.
Meaning
Global longitudinal strain measured after treatment initiation has strong prognostic value for subsequent CTRCD, but available evidence is limited to specific regimens and cancer types and has publication bias and clinical heterogeneity, highlighting the need for larger prospective multicenter studies.
This systematic review and meta-analysis explores the prognostic and discriminatory value of echocardiographic left ventricular global longitudinal strain for the prediction of cancer therapy–related cardiac dysfunction.
Abstract
Importance
Echocardiographic left ventricular global longitudinal strain (GLS) detects early subclinical ventricular dysfunction and can be used in patients receiving potentially cardiotoxic chemotherapy. A meta-analysis of the prognostic value of GLS for cancer therapy–related cardiac dysfunction (CTRCD) has not been performed, to our knowledge.
Objective
To explore the prognostic value of GLS for the prediction of CTRCD.
Data Sources
Systematic search of the MEDLINE, Embase, Scopus, and the Cochrane Library databases from database inception to June 1, 2018.
Study Selection
Cohort studies assessing the prognostic or discriminatory performance of GLS before or during chemotherapy for subsequent CTRCD.
Data Extraction and Synthesis
Random-effects meta-analysis and hierarchical summary receiver operating characteristic curves (HSROCs) were used to summarize the prognostic and discriminatory performance of different GLS indices. Publication bias was assessed using the Egger test, and meta-regression was performed to assess sources of heterogeneity.
Main Outcomes and Measures
The primary outcome was CTRCD, defined as a clinically significant change in left ventricular ejection fraction with or without new-onset heart failure symptoms.
Results
Analysis included 21 studies comprising 1782 patients with cancer, including breast cancer, hematologic malignancies, or sarcomas, treated with anthracyclines with or without trastuzumab. The incidence of CTRCD ranged from 9.3% to 43.8% over a mean follow-up of 4.2 to 23.0 months (pooled incidence, 21.0%). For active treatment absolute GLS (9 studies), the high-risk cutoff values ranged from −21.0% to −13.8%, with worse GLS associated with a higher CTRCD risk (odds ratio, 12.27; 95% CI, 7.73-19.47; area under the HSROC, 0.86; 95% CI, 0.83-0.89). For relative changes vs a baseline value (9 studies), cutoff values ranged from 2.3% to 15.9%, with a greater decrease linked to a 16-fold higher risk of CTRCD (odds ratio, 15.82; 95% CI, 5.84-42.85; area under the HSROC, 0.86; 95% CI, 0.83-0.89). Both indices showed significant publication bias. Meta-regression identified differences in sample size and CTRCD definition but not GLS cutoff value as significant sources of interstudy heterogeneity.
Conclusions and Relevance
In this meta-analysis, measurement of GLS after initiation of potentially cardiotoxic chemotherapy with anthracyclines with or without trastuzumab had good prognostic performance for subsequent CTRCD. However, risk of bias in the original studies, publication bias, and limited data on the incremental value of GLS and its optimal cutoff values highlight the need for larger prospective multicenter studies.
Introduction
Advances in cancer treatment over the last decades have remarkably improved the survival rates of patients diagnosed as having solid and hematologic malignancies.1 Unfortunately, several chemotherapeutic agents (eg, anthracyclines, trastuzumab) commonly used in the treatment of these malignancies are known to have cardiotoxic effects.2 One of the most worrisome adverse effects is ventricular dysfunction and heart failure. While the incidence of overt heart failure is less than 5% typically, subclinical left ventricular (LV) dysfunction, defined by a threshold change in LV ejection fraction (LVEF), may be seen in up to 42% of patients with cancer in selected treatment groups.3,4,5 The development of LV dysfunction (cancer therapy–related cardiac dysfunction [CTRCD]) is associated with poor prognosis and contributes to long-term cardiovascular morbidity and mortality.6,7,8 In the absence of robust risk prediction models, there is an interest in the use of sensitive markers to detect early myocardial dysfunction,9 with the hope of instituting interventions to prevent CTRCD.
Among measures of myocardial function, echocardiography-measured peak systolic global longitudinal strain (GLS) is the most extensively studied marker and provides an easy, inexpensive, and quantitative assessment of global long-axis systolic function.10 Several studies have linked threshold changes in GLS or an absolute GLS value during cancer treatment with the subsequent development of CTRCD. However, these studies differ in the GLS cutoff values used and the populations studied.10,11,12 While the cardio-oncology expert consensus document of the American Society of Echocardiography (ASE) and European Association for Cardiovascular Imaging (EACVI) recommends the routine use of GLS in monitoring patients during cancer therapy when possible,5 the American Society for Clinical Oncology practice guideline does not.13 To understand the strength, robustness, and quality of the available evidence supporting this recommendation by the ASE/EACVI and to compare the different clinical approaches to using pretreatment and posttreatment GLS (absolute values vs absolute or relative changes), we performed a systematic review and meta-analysis of studies reporting on the prognostic and discriminatory performance of GLS measurement as a predictor of CTRCD.
Methods
Study Design and Guidelines
This study was performed according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (eMethods in the Supplement).14 The original study protocol was registered prospectively in PROSPERO (CRD42018102709). The primary aim was to assess the evidence regarding the use of different GLS indices and proposed cutoff values as predictors of CTRCD.
Study Eligibility
Studies were deemed eligible if they were prospective or retrospective cohort studies reporting on the prognostic or discriminatory performance of early GLS analysis for the development of CTRCD in patients with cancer of all ages undergoing chemotherapy with potentially cardiotoxic medications. The studies needed to have defined CTRCD based on clinical criteria, such as an absolute or relative decrease in the LVEF less than a specified threshold and/or development of heart failure symptoms. Echocardiographic assessment of GLS should have occurred shortly before or during the active course of chemotherapy treatment, with further subsequent follow-up visits to detect CTRCD based on LVEF changes. No further restrictions were applied, given the expected heterogeneity in the use of GLS indices and CTRCD definition (eg, Cardiac Review and Evaluation Committee [CREC] criteria vs ASE/EACVI criteria).4,5
Database Search
The MEDLINE, Embase, Scopus, and the Cochrane Library databases were searched for eligible studies from database inception to June 1, 2018. A combination of free-text words and MeSH subheadings were used, including the terms cardiotoxicity, heart failure, chemotherapy, anthracycline, trastuzumab, doxorubicin, daunorubicin, adriamycin, idarubicin, epirubicin, mitoxantrone, echocardiography, deformation, and strain, appropriately linked with the Boolean operators AND or OR. Case reports, comments, and editorials were excluded. No language restrictions were applied. Reference lists of selected articles were also searched manually to identify additional eligible studies. The full search algorithm for each database can be found in the eMethods in the Supplement.
Study Selection and Data Extraction
Following removal of duplicate publications, the selected studies were screened by 2 independent reviewers (E.K.O. and D.G.K.), first based on the title and abstract and subsequently based on the full manuscript. The reference lists of selected publications were also manually searched to identify additional eligible studies. All disagreements were resolved by a third reviewer (P.N.K.). Predefined forms were used to manually extract information on the study design, populations, and outcomes for each eligible study.
Risk of Bias Assessment
Risk of bias was assessed using the Quality in Prognosis Studies (QUIPS) tool.15 Briefly, the following 6 fields were evaluated for each study: study participation, study attrition, prognostic factor measurement, outcome assessment, study confounding, and statistical analysis and reporting.
Strain Reporting
All references to changes in GLS in this article are based on absolute values per the ASE/EACVI recommendations.16 Therefore, an increase in GLS means that the value is more negative, while a decrease in GLS means that the value is less negative.
Statistical Analysis
Four different GLS-based indices were identified: (1) pretreatment absolute GLS, (2) active treatment absolute GLS, (3) absolute change in GLS, and (4) relative (percentage) change in GLS after treatment initiation compared with a pretreatment baseline value. Studies differed in their use of these indices as continuous or dichotomous predictors. We did not attempt to convert the dichotomous variables to continuous since the association of GLS with cardiac function and risk may not be linear, and current clinical practice relies on such thresholds to guide patient management.5 The prognostic performance of continuous predictors (if available in 2 or more studies) was summarized using an inverse-variance weighted random-effects model. We did not pool odds ratios (ORs) with hazard ratios and opted not to pool ORs that were adjusted for different covariates. For dichotomous GLS predictors of CTRCD, we estimated the number of events and nonevents in the high vs low GLS groups. The association of the cutoff values with sensitivity and specificity was assessed using the nonparametric Spearman rank coefficient. To account for the use of various cutoff values,17,18 the prognostic and discriminatory performance of GLS-based dichotomization was summarized using inverse-variance weighted random-effects models and hierarchical summary receiver operating characteristic curves (HSROCs) with Bayesian study estimates (if available in 4 or more studies), respectively.18 The 95% prediction intervals (PIs) are also presented to estimate the interval where a future observation may fall.17,19 The potential clinical utility of a GLS index was also evaluated by using the positive and negative likelihood ratios of the HSROC-derived (hypothetical) summary point to calculate the posttest risk of CTRCD based on a pretest probability. These were depicted using Fagan nomograms and conditional probability plots, assuming a pretest probability in the range reported in the included studies. Statistical heterogeneity was assessed by Higgins I2 (substantial heterogeneity if I2 was 50% or greater). Univariate meta-regression of the log(OR) with relevant study characteristics (eg, GLS cutoff values) was performed to assess potential sources of heterogeneity.17 Publication bias was assessed using funnel plots and the Egger test (with P < .10 demonstrating significant bias). All analyses were performed using the metan, metandi, metareg, and metabias packages of Stata version 14 (StataCorp). All P values were 2-tailed, and α was set at .05, unless specified otherwise.
Results
Selected Studies
Of the 2171 records retrieved, a total of 21 studies fulfilled the inclusion criteria for the study (eFigure and eTable 1 in the Supplement). The study and clinical characteristics are summarized in the Table and eTable 2 in the Supplement. All studies were published between 2011 and 2018 and included patients with breast cancer (13 studies), hematologic malignancies (4 studies), or a combination of malignancies (4 studies). A total of 17 studies were based on prospective cohorts. Most enrolled patients received anthracycline-based chemotherapy, while at least 1 subgroup in 13 studies received trastuzumab therapy. The definition of CTRCD was variable, with the CREC criteria based on 2-dimensional LVEF being used in 12 studies.4 Over a follow-up duration ranging from 4.2 to 23.0 months (Table), the incidence of CTRCD ranged from 9.3% to 43.8%, with a weighted pooled estimate of 21.0%. In 15 studies, GLS measurements were performed using GE EchoPAC software (General Electric).
Table. Summary of Included Studies.
| Source | Cancer Type, % | Treatment, % | Study Type | CTRCD Definitiona | Follow-up, mo | Age, y | Female, % | Baseline | Echo Vendor | Analysis Software Vendor | GLS Follow-up, mo | Study Size, No. | CTRCD, % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LVEF, % | GLS, −% | |||||||||||||
| Studies Examining the Prognostic Value of Absolute or Relative GLS Cutoff Values | ||||||||||||||
| Baratta et al,11 2013 | Breast, 44.4; lymphoma-leukemia, 30.6; other, 25.0 | Doxorubicin, 58.3; TZM, 22.2; mitoxantrone, 2.8; other, 16.7 | Prospective | CREC | Mean: 6 | Mean: 47 | 41.7 | Mean (SD): 65 (7) | Mean (SD): 20.3 (2.7)b | Vivid 7 (GE) | EchoPAC (GE) | 3 | 36 | 19.4 |
| Charbonnel et al,20 2017 | HL, 45; NHL, 49; acute leukemia, 6 | ANT, 100 | Prospective | ASE/EACVI | Mean: 12 | Median (IQR): 48 (30-64) | 36 | Median (IQR): 66 (62-69) | Median (IQR): 21.1 (19.3-23.1) | Vivid E9 (GE) | EchoPAC (GE) | 3 | 86 | 9.3 |
| Gripp et al,21 2018 | Breast, 100 | ANT only, 83.7; ANT with TZM, 16.3 | Prospective | CREC | Mean (SD): 12.7 (1.0) | Mean: 50 | 100 | Mean: 68 | Mean: 20.4 | Vivid S6 (GE) | EchoPAC (GE) | 3 | 49 | 10.2 |
| Fallah-Rad et al,22 2011 | Breast, 100 | ANT with TZM, 12; FEC with TZM, 88 | Prospective | CREC | Mean: 12 | Mean (SD): 47 (9) | 100 | Mean: 63 | Mean: 20.1 | Vivid 7 (GE) | EchoPAC (GE) | 3 | 42 | 23.8 |
| Florescu et al,23 2014 | Breast, 100 | ANT, 100 | Prospective | CREC | Mean (SD): 4.2 (0.4) | Mean (SD): 51 (8) | 100 | Mean (SD): 60 (4) | Mean (SD): 23.1 (1.7) | Vivid 7 Dimension (GE) | EchoPAC (GE) | Mean: 2.1 | 40 | 35.0 |
| Guerra et al,24 2016 | Breast, 100 | CP, 62; ANT, 59; taxanes, 23; TZM, 17; 5-FU, 16c | Prospective | CREC/ESMO | Mean: 12 | Mean (SD): 56 (13) | 96 | Mean: 64 | Mean: 19.8 | Vivid 7 Pro (GE) | EchoPAC (GE) | 3 | 69 | 27.5 |
| Kang et al,25 2013 | NHL, 100 | ANT with R-CHOP, 100 | Prospective | CREC | Range: 4-6 | Mean (SD): 54 (14) | 45.3 | Mean (SD): 65 (4) | Mean (SD): 18.5 (1.7) | iE33 (Philips) | QLAB (Philips) | 2 to 3 | 75 | 18.7 |
| Milks et al,26 2018 | Breast, 100 | ANT only, 58.8; ANT with TZM, 41.2 | Retrospective | ASE/EACVI | NA | Mean (SD): 51 (11) | 100 | Mean: 59 | Mean: 22.4 | Several | Image-Arena (TomTec) | Clinical time points | 183 | 18.0 |
| Mornoş and Petrescu,27 2013 | Breast, 44.6; NHL, 20.3; HL, 17.6; ALL, 12.1; AML, 2.7; osteosarcoma, 2.7 | ANT, 100 | Prospective | CREC | Mean: 12 | Mean (SD): 51 (11) | 58.1 | Mean (SD): 61 (6) | Mean (SD): 21.2 (2.5) | Vivid 7 (GE) | EchoPAC Dimension (GE) | 1.5 | 74 | 13.5 |
| Mornoş et al,28 2014 | Breast, 44.1; NHL, 20.3; HL, 16.9; ALL, 13.6; AML, 3.4; osteosarcoma, 1.7 | ANT, 100 | Prospective | CREC | Mean: 8.4 | Mean (SD): 51 (10) | 59.3 | Mean (SD): 60 (6) | Mean (SD): 20.1 (3.7) | Vivid 9 (GE) | EchoPAC Dimension (GE) | 3 | 59 | 13.6 |
| Negishi et al,12 2013 | Breast (EGFR2+), 100 | TZM with or without ANT, 45.7; TZM with or without taxanes, 91.4c | Prospective | ≥10% Reduction in LVEF | Mean: 12 | Mean (SD): 50 (11) | 10 | Mean (SD): 62 (4) | Mean (SD): 20 (2) | Vivid 7 or E9 (GE) | EchoPAC (GE) | 6 | 81 | 29.6 |
| Paraskevaidis et al,29 2017 | BMT for NHL, 50; AML, 40; CML, 10 | BMT, 100d | Prospective | ASE/EACVI | Mean: 12 | Mean (SD): 45 (11) | 45 | Mean (SD): 59 (4)e | Mean (SD): 20 (2.2) | Vivid 7 (GE) | EchoPAC (GE) | 1 | 80 | 17.5 |
| Portugal et al,30 2017 | Breast, 100 | ANT only, 67; ANT with TZM, 33 | Prospective | ASE/EACVI | Mean: 5.4 | Mean (SD): 55 (13) | 100 | Mean (SD): 62 (8) | Mean (SD): 20.1 (3.5) | Vivid 7 or E9 (GE) | EchoPAC BT12 (GE) | Mean: 3.6 | 158 | 19.0 |
| Sawaya et al,31 2012 | Breast (EGFR2+), 100 | ANT with taxanes and TZM, 100 | Prospective | CREC | Mean: 15 | Mean (SD): 50 (18) | 100 | Mean (SD): 64 (5) | Mean (SD): 21 (2)b | Vivid 7 or E9 (GE) | EchoPAC (GE) | 3 | 81 | 32.1 |
| Sawaya et al,32 2011 | Breast (EGFR2+), 100 | ANT with taxanes and TZM, 100 | Prospective | CREC | Mean: 6 | Mean: 49 | 100 | Mean (SD): 65 (6) | Mean (SD): 20.5 (2.2)b | Vivid 7 or E9 (GE) |
EchoPAC (GE) | 3 | 43 | 20.9 |
| Tang et al,33 2017 | Breast, 100 | ANT, 100 | Retrospective | ASE/EACVI | Mean: 5.1 | Mean (SD): 49 (8) | 100 | Mean (SD): 65 (6) | Mean (SD): 17.9 (2.8) | Vivid E9 (GE) | EchoPAC (GE) | Median: 2.5 | 86 | 16.3 |
| Studies With No GLS Cutoff Values Identified or Examined | ||||||||||||||
| Fei et al,34 2016 | Breast, 100 | ANT with TZM, 100 | Retrospective | ASE/EACVI | Median (IQR): 17 (13-38) | Mean (SD): 47 (11) | 100 | Mean (SD): 68 (5) | Mean (SD): 22.2 (2) | Vivid 7 or E9 (GE) or iE33 (Philips) | 2D Cardiac Performance Analysis (TomTec) | Mean: 1.5 | 95 | 20.0 |
| Lorenzini et al,35 2013 | Breast, 100 | ANT with TZM, 100 | Prospective | CREC | Mean: 7.4 | Mean (SD): 53 (11) | NA | Mean (SD): 58 (4.8) | Mean: 19.9 | Vivid E9 (GE) | EchoPAC (GE) | 4 | 65 | 36.9 |
| Narayan et al,36 2016 | Breast, 100 | ANT only, 67; TZM only, 15; ANT with TZM, 18 | Prospective | ≥10% reduction in LVEF from baseline to <50% after treatment initiation | Median (IQR): 23 (11-29) | Median (IQR): 48 (41-57) | 100 | Median (IQR): 54 (51-56) | Median (IQR): 16.1 (17.7-14)f | Vivid 7 or E9 (GE) or iE33 (Philips) | 2D Cardiac Performance Analysis (TomTec) | Variable depending on treatment regimen | 135 | 15.6 |
| Narayan et al,37 2017 | Breast, 61; lymphoma, 29; leukemia, 6; sarcoma, 4 | ANT only, 92; ANT with TZM, 8 | Prospective | ≥10% reduction in LVEF from baseline to <50% after treatment initiation | Median (IQR): 7 (6-12) | Mean (SD): 53 (13) | 77 | Mean (SD): 50 (6) | Mean (SD): 14.9 (2.8)f | GE, Philips, and Sequoia platforms | 2D Cardiac Performance Analysis (TomTec) | 6 | 165 | 18.8 |
| Shaikh et al,38 2016 | AML, 100 | Mitoxantrone, 100 | Retrospective | CREC | Mean: 6 | Mean: 62 | 45 | Mean: 65 | Mean: 15.8 | Vivid 7 (GE) | TomTec | Mean: 1.8 | 80 | 43.8 |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myelocytic leukemia; ANT, anthracyclines; ASE, American Society of Echocardiography; BMT, bone marrow transplant; CML, chronic myeloid leukemia; CP, cyclophosphamide; CREC, Cardiac Review and Evaluation Committee; CTRCD, cancer therapy–related cardiac dysfunction; EACVI, European Association for Cardiovascular Imaging; EGFR, epidermal growth factor receptor; ESMO, European Society for Medical Oncology; FEC, combination of 5-FU, epirubicin, and CP; 5-FU, 5-fluorouracil; GLS, global longitudinal strain; HL, Hodgkin lymphoma; IQR, interquartile range; LVEF, left ventricular ejection fraction; NA, not applicable; NHL, non-Hodgkin lymphoma; R-CHOP, rituximab, doxorubicin, vincristine, cyclophosphamide, and prednisone; TZM, trastuzumab.
Cardiotoxicity definitions: ASE/EACVI, ≥10% reduction in LVEF from baseline to <53% after treatment; CREC, ≥10% reduction in LVEF from baseline to <55% or ≥5% reduction to <55% with heart failure symptoms; ESMO, ≥20% reduction in LVEF from baseline despite normal function or to an LVEF <50%.
Strain measured from 2 rather than all 3 apical views.
Patients received different combinations of treatments, so percentages do not sum to 100.
In this study, 89% of patients had previously received ANT.
LVEF measured using 3-dimensional echocardiography.
The LV endocardial border was manually traced from the parasternal short-axis view at the midpapillary level and apical 4-chamber views.
GLS Indices and CTRCD
Absolute GLS Before Treatment Initiation
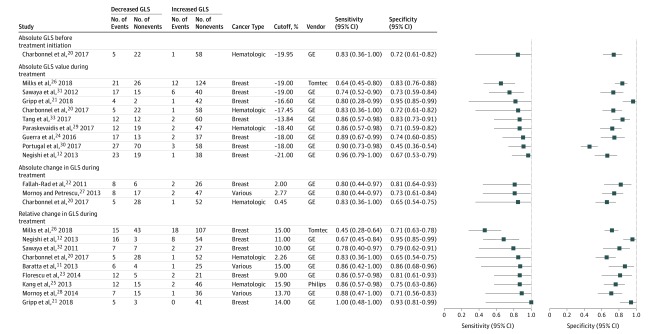
Four studies examined the association of GLS before treatment initiation with subsequent CTRCD.20,26,36,37 Following multivariable adjustment, 2 studies found no association,36,37 whereas 1 study26 reported a significant association (OR per 1% decrease, 1.48; 95% CI, 1.15-1.89) (eTable 3 in the Supplement). An independent study in patients with hematologic malignancy20 reported an area under the curve (AUC) of 0.76 (95% CI, 0.58-0.88), with an optimal cutoff value of −19.95% (sensitivity, 83%; specificity, 72%) for CTRCD (Figure 1).
Figure 1. Sensitivity and Specificity for Cancer Therapy–Related Cardiac Dysfunction Using Different Left Ventricular Global Longitudinal Strain (GLS) Indices and Reported Cutoff Values.
GE indicates GE EchoPAC software (General Electric); Philips, QLAB (Philips Medical System); and Tomtec, 2D Cardiac Performance Analysis (TomTec Imaging Systems).
Absolute GLS Value During Treatment
Eleven studies assessed the association of absolute GLS measured during treatment with the development of CTRCD,12,20,21,24,26,29,30,31,33,34,35 with 9 studies using the GE EchoPac system for analysis. All studies treating GLS as a continuous variable reported significant unadjusted and adjusted associations of lower GLS values with a higher risk of CTRCD (eTable 3 in the Supplement),21,26,34,35 with univariate AUCs for CTRCD discrimination ranging from 0.67 to 0.95.12,20,21,24,29,33
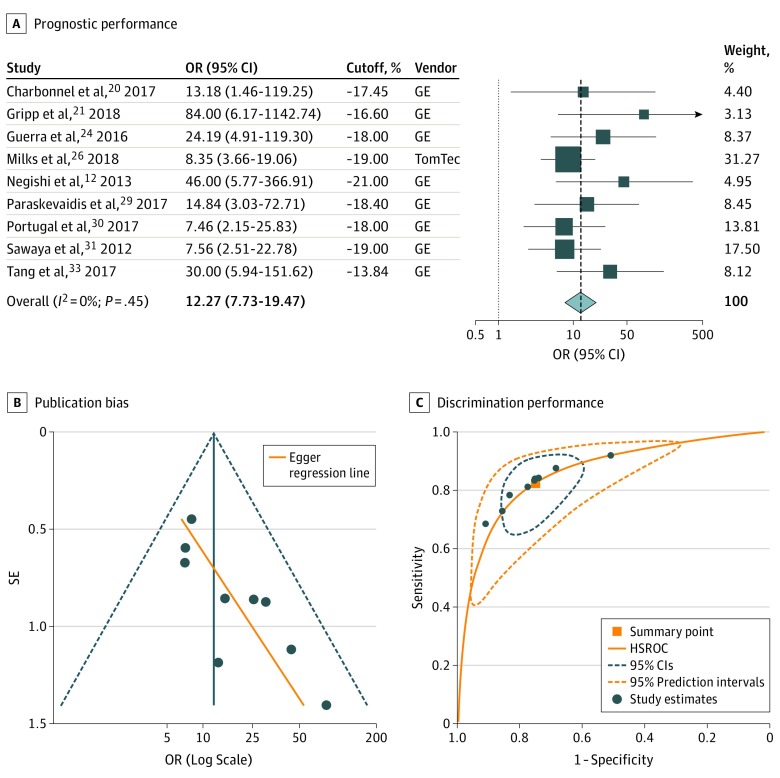
In 9 studies examining the value of specific GLS cutoff values,12,20,21,24,26,29,30,31,33 the threshold for detection of patients at risk of CTRCD ranged from −21.0% to −13.8% (median, −18.0%), with sensitivity ranging from 64% to 96% (median, 86%) and specificity ranging from 45% to 95% (median, 73%) (Figure 1). Study-specific cutoff values were independent of the reported specificity (ρ = −0.03; P = .94) and the reported sensitivity (ρ = 0.54; P = .13). The summary OR for a threshold-based low vs high treatment GLS was estimated at 12.27 (95% CI, 7.73-19.47; 95% PI, 7.03-21.42) (Figure 2A). Meta-regression analysis revealed no association of the GLS cutoff values with study size, average patient age, baseline LVEF, baseline GLS, cancer type or CTRCD incidence, or definition (eTable 4 in the Supplement), but there was evidence of significant funnel plot asymmetry, suggesting publication bias (Figure 2B). With regards to discrimination performance, hierarchical analysis resulted in an area under the HSROC of 0.86 (95% CI, 0.83-0.89) (Figure 2C). Considering a pretest probability of 21.0% (pooled estimate in all included studies), a GLS measurement lower or higher than the hypothetical summary point would be associated with a posttest CTRCD probability of 6.0% and 46.5%, respectively (Figure 3A).
Figure 2. Prognostic and Discriminatory Performance of Absolute Left Ventricular Global Longitudinal Strain (GLS) Measured After Initiation of Cardiotoxic Chemotherapy for Cancer Therapy–Related Cardiac Dysfunction .
A, Forest plot representing the odds ratios (ORs) for each reported cutoff value in each study as well as the overall summary ORs. Weights are taken from random-effects analysis. The estimated 95% prediction intervals for the overall summary OR are 7.03 to 21.42. GE indicates GE EchoPAC software (General Electric); and TomTec, 2D Cardiac Performance Analysis (TomTec Imaging Systems). B, Funnel plot with Egger regression line for assessment of publication bias (P = .006). C, The orange square represents the summary operating point of the curve, a hypothetical point that summarizes the discriminatory value of all reported cutoff values (sensitivity, 0.82; 95% CI, 0.72-0.89; specificity, 0.75; 95% CI, 0.65-0.83; positive likelihood ratio, 3.27; 95% CI, 2.40-4.45; negative likelihood ratio, 0.24; 95% CI, 0.15-0.37). The area under the hierarchical summary receiver operating characteristic curve (HSROC) was 0.86 (95% CI, 0.83-0.89).
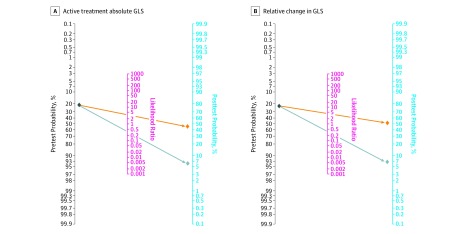
Figure 3. Posttest Probability of Cancer Therapy–Related Cardiac Dysfunction.
A, Fagan nomogram for calculation of posttest probabilities based on low (orange) or high (gray) absolute global longitudinal strain (GLS) measurements during chemotherapy treatment. The unconditional negative predictive value was 0.92 (95% CI, 0.84-0.99), and the unconditional positive predictive value was 0.52 (95% CI, 0.45-0.60). The positive likelihood ratio was 3.27, and the negative likelihood ratio was 0.24. B, Fagan nomogram for calculation of posttest probabilities based on a significant (orange) or nonsignificant (gray) relative change in GLS after initiation of chemotherapy. The calculation of positive and negative predictive values is based on an expected probability of cardiotoxicity between 9.3% and 43.8%. The Fagan nomogram is based on a pretest probability of 21.0%, which is the pooled cardiotoxicity estimate in our study. A positive or negative test is defined based on the summary points calculated from hierarchical summary receiver operating characteristic curves. The unconditional negative predictive value was 0.91 (95% CI, 0.83-0.98), and the unconditional positive predictive value was 0.57 (95% CI, 0.49-0.65). The positive likelihood ratio was 4.05, and the negative likelihood ratio was 0.27.
Absolute Change in GLS During Treatment
Six studies examined the prognostic and discriminatory value of the absolute change in GLS after treatment initiation (median [range] follow-up, 3 [1.5-6] months),20,22,27,36,37,38 with all 6 using the GE EchoPac system. In 2 studies with unadjusted risk estimates,27,38 the summary OR was 1.81 (95% CI, 0.44-7.40; I2 = 91%). Similarly, 2 of 3 studies reporting adjusted ORs also showed no significant association with CTRCD (eTable 3 in the Supplement).36,37,38 Univariate AUCs were 0.64,36 0.72,20 and 0.8427 (eTable 3 in the Supplement). In 3 studies reporting specific absolute change cutoff values, these ranged from 0.45% to 2.77% (median, 2.00%), with sensitivity ranging from 80% to 83% (median, 80%), specificity ranging from 65% to 81% (median, 73%), and a summary OR of 4.59 (95% CI, 2.64-7.98; I2 = 0%).20,22,27
Relative Change in GLS During Treatment
Nine studies assessed the value of the relative change in GLS measured shortly after chemotherapy initiation compared with baseline (median [range] follow-up, 3 [2-6] months).11,12,20,21,23,25,26,28,32 Most studies (7 of 9) used GE EchoPAC for GLS analysis. Among studies treating GLS as a continuous variable, the summary OR for the unadjusted association with subsequent CTRCD per 1% relative decrease in GLS was 1.19 (95% CI, 1.01-1.39),25,28,32 whereas one study reported a significant association after multivariable adjustment for age, traditional cardiovascular risk factors, and LVEF (eTable 3 in the Supplement).12 The univariate AUC for CTRCD in 6 studies ranged from 0.74 to 0.97 (median, 0.85).12,20,21,23,25,28
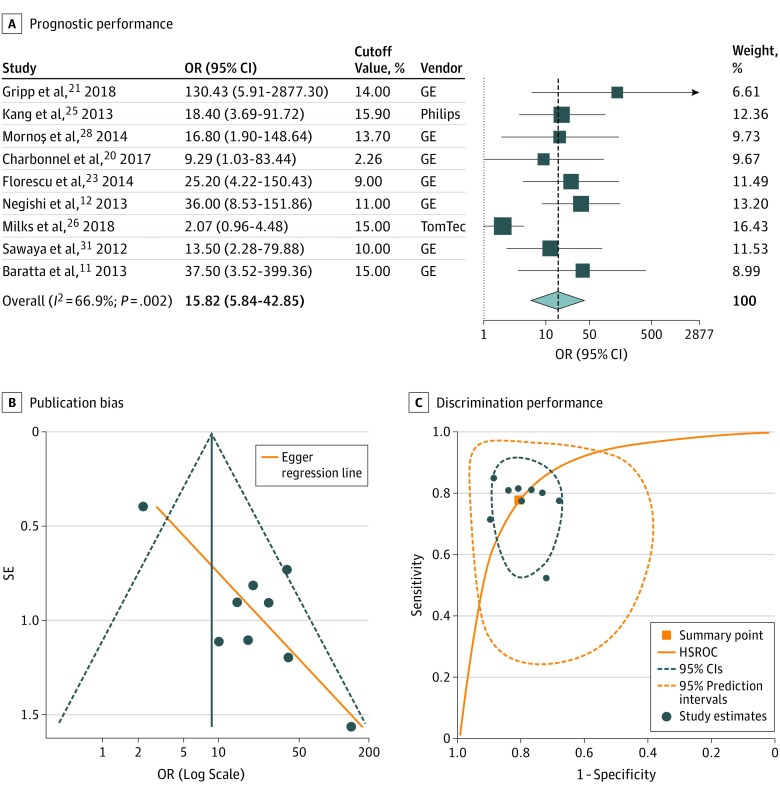
All 9 studies reported optimal cutoff values for relative change in GLS ranging from 2.3% to 15.9% (median, 13.7%), with a sensitivity and specificity between 45% and 100% (median, 86%) and 65% and 95% (median, 79%), respectively (Figure 1). Study-specific cutoff values were again independent of the reported sensitivity (ρ = 0.15; P = .71) and specificity (ρ = 0.11; P = .77). The summary OR for high vs low GLS was estimated at 15.82 (95% CI, 5.84-42.85), with significant heterogeneity and a wide 95% PI of 0.74 to 338.11 (Figure 4A). Meta-regression analysis revealed an inverse association between risk estimates and use of the ASE definition and study size but no association with the GLS cutoff values or other study characteristics (eTable 4 in the Supplement). Funnel plot analysis showed significant asymmetry, suggesting publication bias (Figure 4B). With regards to discrimination performance, hierarchical analysis resulted in an HSROC with an AUC of 0.86 (95% CI, 0.83-0.89) (Figure 4C). For a pretest probability of 21.0%, a greater relative decrease in GLS above the hypothetical summary point was associated with a posttest CTRCD probability of 51.8%, while a decrease below this summary point lowered the posttest probability to 6.7% (Figure 3B).
Figure 4. Prognostic and Discriminatory Performance of Relative Change in GLS Compared With a Baseline Value for Cancer Therapy–Related Cardiac Dysfunction .
A, Forest plot representing the odds ratios (ORs) for each reported cutoff value in each study as well as the overall summary ORs. Weights are taken from random-effects analysis. The estimated 95% prediction intervals for the overall summary OR are 0.74 to 338.11. GE indicates GE EchoPAC software (General Electric); Philips, QLAB (Philips Medical System); and Tomtec, 2D Cardiac Performance Analysis (TomTec Imaging Systems). B, Funnel plot with Egger regression line for assessment of publication bias (P = .003). C, The orange square represents the summary operating point of the curve, a hypothetical point that summarizes the discriminatory value of all cutoff values (sensitivity, 0.78; 95% CI, 0.63-0.88; specificity, 0.81; 95% CI, 0.72-0.87; positive likelihood ratio, 4.05; 95% CI, 2.65-6.21; negative likelihood ratio, 0.27; 95% CI, 0.15-0.49). The area under the hierarchical summary receiver operating characteristic curve (HSROC) was 0.86 (95% CI, 0.83-0.89).
Incremental Prognostic Value of GLS Assessment
Two studies have assessed the incremental value of GLS beyond other risk factors for prognostication of CTRCD. Negishi et al12 reported significant improvement in the prognostic performance of a baseline model that included age, diabetes, hypertension, hyperlipidemia, smoking, and LVEF when adding the relative GLS change. However, Mornoş and Petrescu27 found no significant improvement when including the absolute GLS change on top of other echocardiographic and treatment parameters, such as LV twist, untwist rate, troponin levels, and anthracycline dose.
Sensitivity and Subgroup Analyses
Assessment of the methodological quality with the QUIPS tool revealed that most included studies were at high risk of bias (eTable 5 in the Supplement) predominantly owing to unclear study population selection and absence of adjustment for relevant risk factors (ie, troponin levels, anthracycline dose). After excluding retrospective studies, the threshold summary ORs as well as 95% PIs for active treatment absolute GLS (8 studies) and relative change in GLS (7 studies) were consistently greater than 1 (absolute: OR, 14.62; 95% CI, 8.38-25.53; 95% PI, 7.30-29.31; relative: OR, 24.18; 95% CI, 11.51-50.81; 95% PI, 9.13-64.00). However, the low number of studies precluded further heterogeneity analysis based on treatment type (ie, use of trastuzumab, anthracyclines, or both).
Discussion
In this study, we present a meta-analysis of the prognostic and discriminatory performance of GLS for CTRCD. We show that a threshold relative reduction in GLS compared with baseline or a lower absolute GLS value early during chemotherapy can be used to stratify individuals at high risk of developing CTRCD. Pretreatment GLS values and absolute changes are also associated with CTRCD; however, the number of published studies supporting the use of these metrics is limited. Notably, our analysis demonstrated possible publication bias in the literature and a high risk of bias in the published studies predominantly owing to unclear study population selection and absence of adjustment for relevant risk factors. Therefore, despite the encouraging data on the value of GLS in patients receiving cancer therapy, our meta-analysis suggests the need for larger prospective studies with carefully defined patient populations to determine the value of GLS for predicting CTRCD and to define the most optimal diagnostic thresholds.
Absolute Values vs Relative Change in GLS and CTRCD
The ASE/EACVI consensus document recommends GLS as the optimal deformation index for the early detection of subclinical LV dysfunction in patients with cancer, and ideally, measurements acquired during chemotherapy should be compared with baseline values.5 The document suggests that a relative change in GLS less than 8% is not meaningful, whereas a change greater than 15% is likely to indicate subclinical LV dysfunction.5 In our meta-analysis, in support of these recommendations, we observed a strong prognostic value for relative changes in GLS during treatment. However, absolute GLS values during treatment also had comparable prognostic and discriminatory performance for CTRCD. In this regard, in individuals without baseline echocardiography, low active treatment absolute GLS values can be used to identify patients at risk of CTRCD. This is important given that many patients are referred to cardiology or cardio-oncology clinics either without baseline imaging or baseline imaging performed without GLS. The threshold active treatment absolute GLS value to identify risk has been variable in the literature, with most studies suggesting a value between −18.0% and −19.0% (median, −18.0%), predominantly based on GE EchoPac analysis (8 of 9 studies). Of note, certain studies suggest that baseline GLS may also flag patients at risk of CTRCD, possibly through detection of preexisting subclinical cardiac dysfunction, which may be aggravated by chemotherapy.20 However, a higher level of evidence is needed to support these claims and any associated clinical recommendations.
Relative GLS Change Threshold for CTRCD
The relative GLS change of greater than 15% to detect subclinical myocardial dysfunction suggested in the ASE position statement was based on the best data available at that time, and it is unclear whether this is a sensitive or specific threshold. Our meta-regression suggests that the GLS thresholds used in the published studies did not explain the statistical heterogeneity in the prognostic or discriminatory performance of GLS indices, thus suggesting that lower thresholds for relative change in GLS may still be important in identifying CTRCD risk. This highlights the need for external validation of the recommended cutoff values in different centers and vendors, especially given the crucial role of GLS in guiding clinical decision-making.5
Another important consideration of the existing studies is that all but one29 of the studies included in our analysis used 2-dimensional echocardiography and Simpson biplane method39 to define CTRCD. Therefore, despite the fact that 3-dimensional echocardiography has been suggested as the preferred method in patients with cancer therapy owing to reduced measurement variability,5 we do not have adequate data on the association of GLS with CTRCD defined by 3-dimensional echocardiography. Also, since many of the studies were published prior to the ASE/EACVI consensus document, the confirmation of a change in LVEF or GLS with a repeated scan was not performed in most studies prior to the diagnosis of CTRCD.5 Regardless of whether an absolute GLS value or relative GLS change is used, these measures appear to have high negative predictive value for CTRCD in the reported range of cutoff values. Taken together, these findings could be used to update current guidelines on the use of GLS in patients receiving anthracyclines with or without trastuzumab therapy.
Bias and Sources of Heterogeneity Between Studies
In most studies, the optimal GLS cutoff values were identified in post hoc analyses of ROC curves, thus introducing possible bias in their selection. We identified evidence of statistical heterogeneity between the included studies. This was attributed predominantly to differences in study size and CTRCD definition. The more contemporary studies were more likely to use the ASE/EACVI definition of CTRCD. Meta-regression analysis suggested that the use of the ASE/EACVI criteria for cardiotoxicity was associated with significantly lower prognostic performance compared with alternative definitions (eg, CREC criteria). This could reflect the fact that the ASE/EACVI definition uses a lower LVEF threshold for cardiotoxicity and does not include symptoms in the diagnostic criteria. We also identified significant publication bias, suggesting that studies that failed to show a significant prognostic or discriminatory value for GLS may have not been published at all or may have failed to examine the value of specific GLS cutoff values.
Strengths and Limitations
Strengths of our study include the systematic collection of evidence on the prognostic and discriminatory value of GLS for CTRCD and the first quantitative synthesis of previously reported data, to our knowledge. This enables a direct comparison of various GLS metrics (both absolute values as well as absolute and relative changes) for CTRCD discrimination using a range of cancer types and patient populations.
Our study also has some limitations. First, the ASE/EACVI recommendations acknowledge the importance of an integrated approach in the early diagnosis of CTRCD combining changes in strain parameters with circulating troponin levels.5 The incremental value of this approach is discussed in some studies12,31,40 but could not be explored quantitatively in this meta-analysis.
Second, the pooled incidence of CTRCD in our study (21.0%) may be particularly high, especially since most studies included patients with breast cancer with mean anthracycline doses of approximately 240 mg/m2. This does raise concerns about selection bias in the original studies and retrospective identification of eligible patients. Also, the CTRCD definition was based on changes in LVEF, which can be common, especially with the use of anthracyclines and trastuzumab, but may not translate to worse clinical outcomes. In our search, we identified 2 studies that examined the association of lower GLS measured before chemotherapy initiation with cardiac mortality or symptomatic heart failure,41,42 but to our knowledge, no studies have explored the prognostic value of postchemotherapy GLS for adverse events, such as cardiac mortality.
Additionally, despite the use of the broad term CTRCD based on ASE’s recommendations, most studies included in our analysis have focused on anthracyclines with or without trastuzumab therapy in patients with breast or hematological malignancies. However, knowing that heart failure is not only driven by these drugs and is often multifactorial (eg, traditional risk factors, radiation therapy), we have elected to use this broad term.43 Interestingly, our search did not reveal any prospective cohort reports on the role of GLS in pediatric patients or patients receiving nonanthracycline and nontrastuzumab regimens. Therefore, the results of this analysis, like the reported thresholds and conclusions of all individual studies, should be extrapolated with caution to different study populations, including different cancer types and/or treatments.
Conclusions
In patients receiving potentially cardiotoxic cancer therapy, deformation analysis by means of GLS can be used to detect early subclinical ventricular dysfunction before progression to CTRCD. An active treatment absolute GLS value or a relative change compared with baseline appears to have similar prognostic and discriminatory performance for subsequent CTRCD. Current evidence supports the use of GLS predominantly in patients with hematological and solid malignancies treated with anthracycline-based regimens with or without trastuzumab. However, significant statistical heterogeneity between published studies, variable GLS cutoff values, and publication bias highlight the need for higher-quality evidence in the form of large prospective cohorts. Finally, studies focusing on additional types of cancer, as well as novel cardiotoxic chemotherapeutic or immunotherapeutic agents, are needed to better understand the potential value of echocardiographic myocardial strain in these growing yet understudied populations.
eMethods. Database search algorithms.
eFigure. PRISMA flowchart.
eTable 1. Exclusion criteria for studies at the full manuscript review stage.
eTable 2. Summary clinical characteristics of the included study populations.
eTable 3. Prognostic and discriminatory value of continuous GLS for chemotherapy-induced cardiotoxicity.
eTable 4. Meta-regression analysis of prognostic odds ratios for threshold GLS changes.
eTable 5. Quality in Prognostic Studies (QUIPS) risk of bias tool.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Qadir H, Austin PC, Lee DS, et al. A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. 2017;2(1):88-93. doi: 10.1001/jamacardio.2016.3841 [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60(24):2504-2512. doi: 10.1016/j.jacc.2012.07.068 [DOI] [PubMed] [Google Scholar]
- 4.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20(5):1215-1221. doi: 10.1200/JCO.2002.20.5.1215 [DOI] [PubMed] [Google Scholar]
- 5.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911-939. doi: 10.1016/j.echo.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 6.Armenian SH, Armstrong GT, Aune G, et al. Cardiovascular disease in survivors of childhood cancer: insights into epidemiology, pathophysiology, and prevention. J Clin Oncol. 2018;36(21):2135-2144. doi: 10.1200/JCO.2017.76.3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981-1988. doi: 10.1161/CIRCULATIONAHA.114.013777 [DOI] [PubMed] [Google Scholar]
- 8.Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55(3):213-220. doi: 10.1016/j.jacc.2009.03.095 [DOI] [PubMed] [Google Scholar]
- 9.Hare JL, Brown JK, Leano R, Jenkins C, Woodward N, Marwick TH. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab. Am Heart J. 2009;158(2):294-301. doi: 10.1016/j.ahj.2009.05.031 [DOI] [PubMed] [Google Scholar]
- 10.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63(25, pt A):2751-2768. doi: 10.1016/j.jacc.2014.01.073 [DOI] [PubMed] [Google Scholar]
- 11.Baratta S, Damiano MA, Marchese ML, et al. Serum markers, conventional Doppler echocardiography and two-dimensional systolic strain in the diagnosis of chemotherapy-induced myocardial toxicity. Rev Argent Cardiol. 2013;81(2):151-158. doi: 10.7775/rac.v81.i2.2300 [DOI] [Google Scholar]
- 12.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26(5):493-498. doi: 10.1016/j.echo.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 13.Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35(8):893-911. doi: 10.1200/JCO.2016.70.5400 [DOI] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, et al. ; Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group . Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 15.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280-286. doi: 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 16.Voigt JU, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28(2):183-193. doi: 10.1016/j.echo.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 17.Riley RD, Elia EG, Malin G, Hemming K, Price MP. Multivariate meta-analysis of prognostic factor studies with multiple cut-points and/or methods of measurement. Stat Med. 2015;34(17):2481-2496. doi: 10.1002/sim.6493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Kim KW, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers—part II: Statistical Methods of Meta-Analysis. Korean J Radiol. 2015;16(6):1188-1196. doi: 10.3348/kjr.2015.16.6.1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7):e010247. doi: 10.1136/bmjopen-2015-010247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charbonnel C, Convers-Domart R, Rigaudeau S, et al. Assessment of global longitudinal strain at low-dose anthracycline-based chemotherapy, for the prediction of subsequent cardiotoxicity. Eur Heart J Cardiovasc Imaging. 2017;18(4):392-401. [DOI] [PubMed] [Google Scholar]
- 21.Gripp EA, Oliveira GE, Feijó LA, Garcia MI, Xavier SS, Sousa AS. Global longitudinal strain accuracy for cardiotoxicity prediction in a cohort of breast cancer patients during anthracycline and/or trastuzumab treatment. Arq Bras Cardiol. 2018;110(2):140-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fallah-Rad N, Walker JR, Wassef A, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57(22):2263-2270. doi: 10.1016/j.jacc.2010.11.063 [DOI] [PubMed] [Google Scholar]
- 23.Florescu M, Magda LS, Enescu OA, Jinga D, Vinereanu D. Early detection of epirubicin-induced cardiotoxicity in patients with breast cancer. J Am Soc Echocardiogr. 2014;27(1):83-92. doi: 10.1016/j.echo.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 24.Guerra F, Marchesini M, Contadini D, et al. Speckle-tracking global longitudinal strain as an early predictor of cardiotoxicity in breast carcinoma. Support Care Cancer. 2016;24(7):3139-3145. [DOI] [PubMed] [Google Scholar]
- 25.Kang Y, Cheng L, Li L, et al. Early detection of anthracycline-induced cardiotoxicity using two-dimensional speckle tracking echocardiography. Cardiol J. 2013;20(6):592-599. doi: 10.5603/CJ.2013.0158 [DOI] [PubMed] [Google Scholar]
- 26.Milks MW, Velez MR, Mehta N, et al. Usefulness of integrating heart failure risk factors into impairment of global longitudinal strain to predict anthracycline-related cardiac dysfunction. Am J Cardiol. 2018;121(7):867-873. doi: 10.1016/j.amjcard.2017.12.022 [DOI] [PubMed] [Google Scholar]
- 27.Mornoş C, Petrescu L. Early detection of anthracycline-mediated cardiotoxicity: the value of considering both global longitudinal left ventricular strain and twist. Can J Physiol Pharmacol. 2013;91(8):601-607. doi: 10.1139/cjpp-2012-0398 [DOI] [PubMed] [Google Scholar]
- 28.Mornoş C, Manolis AJ, Cozma D, Kouremenos N, Zacharopoulou I, Ionac A. The value of left ventricular global longitudinal strain assessed by three-dimensional strain imaging in the early detection of anthracyclinemediated cardiotoxicity. Hellenic J Cardiol. 2014;55(3):235-244. [PubMed] [Google Scholar]
- 29.Paraskevaidis IA, Makavos G, Tsirigotis P, et al. Deformation analysis of myocardial layers detects early cardiac dysfunction after chemotherapy in bone marrow transplantation patients: a continuous and additive cardiotoxicity process. J Am Soc Echocardiogr. 2017;30(11):1091-1102. doi: 10.1016/j.echo.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 30.Portugal G, Moura Branco L, Galrinho A, et al. Global and regional patterns of longitudinal strain in screening for chemotherapy-induced cardiotoxicity. Rev Port Cardiol. 2017;36(1):9-15. doi: 10.1016/j.repc.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 31.Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5(5):596-603. doi: 10.1161/CIRCIMAGING.112.973321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107(9):1375-1380. doi: 10.1016/j.amjcard.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Q, Jiang Y, Xu Y, Xia H. Speckle tracking echocardiography predicts early subclinical anthracycline cardiotoxicity in patients with breast cancer [retracted]. J Clin Ultrasound. 2017;45(4):222-230. doi: 10.1002/jcu.22434 [DOI] [PubMed] [Google Scholar]
- 34.Fei HW, Ali MT, Tan TC, et al. Left ventricular global longitudinal strain in HER-2 + breast cancer patients treated with anthracyclines and trastuzumab who develop cardiotoxicity is associated with subsequent recovery of left ventricular ejection fraction. Echocardiography. 2016;33(4):519-526. doi: 10.1111/echo.13168 [DOI] [PubMed] [Google Scholar]
- 35.Lorenzini C, Corsi C, Aquilina M, et al. Early detection of cardiotoxicity in chemotherapy-treated patients from real-time 3D echocardiography. Comput Cardiol (2013). 2013;2013(40):249-252. [Google Scholar]
- 36.Narayan HK, French B, Khan AM, et al. Noninvasive measures of ventricular-arterial coupling and circumferential strain predict cancer therapeutics-related cardiac dysfunction. JACC Cardiovasc Imaging. 2016;9(10):1131-1141. doi: 10.1016/j.jcmg.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narayan HK, Wei W, Feng Z, et al. Cardiac mechanics and dysfunction with anthracyclines in the community: results from the PREDICT study. Open Heart. 2017;4(1):e000524. doi: 10.1136/openhrt-2016-000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaikh AY, Suryadevara S, Tripathi A, et al. Mitoxantrone-induced cardiotoxicity in acute myeloid leukemia-a velocity vector imaging analysis. Echocardiography. 2016;33(8):1166-1177. doi: 10.1111/echo.13245 [DOI] [PubMed] [Google Scholar]
- 39.Nijjer SS, Pabari PA, Stegemann B, et al. The limit of plausibility for predictors of response: application to biventricular pacing. JACC Cardiovasc Imaging. 2012;5(10):1046-1065. doi: 10.1016/j.jcmg.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 40.Mavinkurve-Groothuis AM, Marcus KA, Pourier M, et al. Myocardial 2D strain echocardiography and cardiac biomarkers in children during and shortly after anthracycline therapy for acute lymphoblastic leukaemia (ALL): a prospective study. Eur Heart J Cardiovasc Imaging. 2013;14(6):562-569. doi: 10.1093/ehjci/jes217 [DOI] [PubMed] [Google Scholar]
- 41.Ali MT, Yucel E, Bouras S, et al. Myocardial strain is associated with adverse clinical cardiac events in patients treated with anthracyclines. J Am Soc Echocardiogr. 2016;29(6):522-527.e3. doi: 10.1016/j.echo.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 42.Mousavi N, Tan TC, Ali M, Halpern EF, Wang L, Scherrer-Crosbie M. Echocardiographic parameters of left ventricular size and function as predictors of symptomatic heart failure in patients with a left ventricular ejection fraction of 50-59% treated with anthracyclines. Eur Heart J Cardiovasc Imaging. 2015;16(9):977-984. [DOI] [PubMed] [Google Scholar]
- 43.Abdel-Qadir H, Thavendiranathan P, Austin PC, et al. The risk of heart failure and other cardiovascular hospitalizations after early stage breast cancer: a matched cohort study [published online January 31, 2019]. J Natl Cancer Inst. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Database search algorithms.
eFigure. PRISMA flowchart.
eTable 1. Exclusion criteria for studies at the full manuscript review stage.
eTable 2. Summary clinical characteristics of the included study populations.
eTable 3. Prognostic and discriminatory value of continuous GLS for chemotherapy-induced cardiotoxicity.
eTable 4. Meta-regression analysis of prognostic odds ratios for threshold GLS changes.
eTable 5. Quality in Prognostic Studies (QUIPS) risk of bias tool.