Key Points
Question
Are 100% single-patient hospital rooms associated with reductions in the acquisition of common multidrug-resistant organisms and nosocomial infections compared with ward-type rooms?
Finding
In this time-series analysis of a move from a 417-bed hospital with ward-type rooms to a 350-bed facility with solely private rooms, the move was associated with reductions in the incidence of nosocomial vancomycin-resistant Enterococcus and methicillin-resistant Staphylococcus aureus colonization and vancomycin-resistant Enterococcus infection. However, no change in nosocomial Clostridioides difficile or methicillin-resistant Staphylococcus aureus infections was noted.
Meaning
Single-patient rooms may help prevent nosocomial multidrug-resistant organism colonization, but the extent to which single-patient rooms may be associated with infection rates likely owes to community colonization rates and factors associated with the transition from colonization to infection.
Abstract
Importance
Health care–associated infections are often caused by multidrug-resistant organisms and substantially factor into hospital costs and avoidable iatrogenic harm. Although it is recommended that new facilities be built with single-room, low-acuity beds, this process is costly and evidence of reductions in health care–associated infections is weak.
Objective
To examine whether single-patient rooms are associated with decreased rates of common multidrug-resistant organism transmissions and health care–associated infections.
Design, Setting, and Participants
A time-series analysis comparing institution-level rates of new multidrug-resistant organism colonization and health care–associated infections before (January 1, 2013-March 31, 2015) and after (April 1, 2015-March 31, 2018) the move to the hospital with 100% single-patient rooms. In the largest hospital move in Canadian history, inpatients in an older, tertiary care, 417-bed hospital in Montréal, Canada, that consisted of mainly mixed 3- and 4-person ward-type rooms were moved to a new 350-bed facility with all private rooms.
Exposures
A synchronized move of all patients on April 26, 2015, to a new hospital with 100% single-patient rooms equipped with individual toilets and showers and easy access to sinks for hand washing.
Main Outcomes and Measures
Rates of nosocomial vancomycin-resistant Enterococcus (VRE) and methicillin-resistant Staphylococcus aureus (MRSA) colonization, VRE and MRSA infection, and Clostridioides difficile (formerly known as Clostridium difficile) infection (CDI) per 10 000 patient-days.
Results
Compared with the 27 months before, during the 36 months after the hospital move, an immediate and sustained reduction in nosocomial VRE colonization (from 766 to 209 colonizations; incidence rate ratio [IRR], 0.25; 95% CI, 0.19-0.34) and MRSA colonization (from 129 to 112 colonizations; IRR, 0.57; 95% CI, 0.33-0.96) was noted, as well as VRE infection (from 55 to 14 infections; IRR, 0.30, 95% CI, 0.12-0.75). Rates of CDI (from 236 to 223 infections; IRR, 0.95; 95% CI, 0.51-1.76) and MRSA infection (from 27 to 37 infections; IRR, 0.89, 95% CI, 0.34-2.29) did not decrease.
Conclusion and Relevance
The move to a new hospital with exclusively single-patient rooms appeared to be associated with a sustained decrease in the rates of new MRSA and VRE colonization and VRE infection; however, the move was not associated with a reduction in CDI or MRSA infection. These findings may have important implications for the role of hospital construction in facilitating infection control.
This time-series analysis examines the changes in the rates of multidrug-resistant organisms and nosocomial infections after patients were moved from a hospital with ward-type rooms to one with 100% single-patient rooms.
Introduction
Health care–associated infections are common, costly, and harmful to patients. In a point prevalence survey from 2018, 3.2% of patients surveyed across 199 American hospitals had health care–associated infections.1 Similarly, the European Centers for Disease Prevention and Control reported a 5.7% prevalence of health care–associated infections in 947 acute care hospitals across 30 countries in 2012.2 In some instances (eg, health care–associated bloodstream infections), concerted infection control efforts have begun to positively affect the rates of health care–associated infections over time.3 Although slow and steady progress is being made, significant efforts are still required to eliminate this form of preventable iatrogenic harm.4
One proposed strategy to reduce the transmission of multidrug-resistant organisms and prevent certain health care–associated infections is to place patients in single-bed rooms to reduce the chance of transmission of organisms from neighbors and improve compliance with infection control measures.5,6 The Facility Guidelines Institute is a nonprofit organization that has developed guidelines for designing and building new health care facilities; since 2016, these guidelines have required the construction of all single-bed, low-acuity hospital rooms, which has resulted in billions of dollars of expenditures.7 Evidence linking hospital architectural design and the prevention of multidrug-resistant organism colonization and health care–associated infections is conflicting.2 Elements associated with the development of colonization and subsequent infection are complex and depend on a multitude of factors including, but not limited to, pre-existing rates of colonization, the concept of colonization pressure, institutional use of broad-spectrum antibiotics, facility cleaning, hand hygiene practices, and individual patient risk factors.
Following a major hospital relocation that took place on April 26, 2015, we sought to examine the outcome of changing from a hospital design of multiple occupancy rooms to 100% single-bed rooms on the incidence of nosocomial MRSA and VRE colonization and hospital-acquired methicillin-resistant Staphylococcus aureus (MRSA) infection, vancomycin-resistant Enterococcus (VRE) infection, and Clostridioides difficile (formerly known as Clostridium difficile) infection (CDI) rates. A time-series analysis compared institution-level rates of new multidrug-resistant organism colonization and health care–associated infections before (January 1, 2013-March 31, 2015) and after (April 1, 2015-March 31, 2018).
Methods
The Royal Victoria Hospital, part of the McGill University Health Centre, is a tertiary/quaternary care adult hospital in Montréal, Québec, that serves a catchment area of approximately 800 000 people. In addition to serving the needs of the immediate surrounding community, the Royal Victoria Hospital provides specialized care in many areas including, but not limited to, tertiary care for HIV/AIDS; tropical medicine; oncology, including referrals from other oncology centers; hematology (ie, apheresis center, lymphoma, and leukemia with autologous and allogeneic stem cell transplant); respirology, including referrals for chronic obstructive pulmonary disease and asthma, chronic ventilation and weaning, and cystic fibrosis; solid organ transplant (ie, liver, kidney, pancreas, heart); and cardiac, vascular, and other specialized surgical services. After the move, the inpatient respirology, oncology, hematology-oncology, and urology beds previously at another center were consolidated at the new hospital along with these services from the old hospital, and the Victoria Royal Hospital intensive care unit increased from 22 to 30 beds. The McGill University Health Centre Research Ethics Board approved this project, with a waiver of informed consent. Only aggregate deidentified data were used.
The original Royal Victoria Hospital was constructed in 1893 with innumerable renovations and additions performed in the following 122 years. As of 2013, the hospital featured a mix of 3- and 4-bed ward rooms and private rooms with an estimated capacity of 417 beds. On medical units, 80% of the rooms were multibed and 20% were private; on the surgical and oncological units, 60% of the rooms were multibed and 40% were private; and on the critical care units, 35% of the rooms were multiple occupancy and 65% were private. Multibed rooms shared 1 bathroom and sink with alcohol rinse dispensers located at the room’s main entrance. There were 1 to 2 hand hygiene sinks available in the corridor per 20- to 24-bed unit.
On April 26, 2015, in the largest hospital move ever to occur in Canada at that time, the original Royal Victoria Hospital closed, and all patients were moved to a newly constructed hospital by the end of the day. The new hospital consists entirely of single-patient rooms (350 adult beds, approximately 25 m2 per room) with in-room access to a dedicated sink for hand washing. With the exception of the emergency department and intensive care unit, all rooms have individual private bathrooms and showers. Each new room was also outfitted with new beds and furniture.
Nosocomial Infection Screening
All medical, surgical, and critical care inpatients are screened for MRSA and VRE on admission to the hospital and weekly thereafter using standard laboratory techniques described in the eMethods in the Supplement. Surveillance is mandated by provincial authorities, but there are no penalties or incentives tied to reporting. Surveillance is prospective and conforms to standard definitions that are applied by trained infection control personnel and validated by a hospital epidemiologist. Briefly, new nosocomial VRE8 and MRSA9 colonization was defined as a new positive screening test (or clinical isolate) occurring after 3 days of admission in the absence of a prior positive test. We also included patients with a positive admission swab who were previously negative and for whom the Royal Victoria Hospital was the only identifiable health care contact within the past 12 months. The VRE and MRSA infections were defined as the isolation of VRE or MRSA in a clinical specimen (eg, urine, surgical site wound swab, sterile sites, or other) in the context of signs or symptoms of infection. These infections included those with hospital onset as well as those occurring after discharge as appropriate, using National Healthcare Safety Network criteria.10 Clostridioides difficile testing is recommended only for patients with at least 3 liquid stools over 24 hours or for those with toxic megacolon.11 Nosocomial CDI was defined as per the provincial definition12 and included patients whose symptoms developed from 72 hours after admission up to 4 weeks post discharge. Direct stool polymerase chain reaction was the diagnostic test used over the entire study period.
Infection Control Procedures
Patients identified as having MRSA or VRE colonization are placed in contact isolation for the duration of hospitalization. In the old hospital, patients with the same organism could be placed in multibed rooms. Regular hospital disinfectant was used for daily and discharge MRSA and VRE room cleanings, with VRE requiring a double cleaning on discharge. On diagnosis, patients with CDI were placed in contact isolation and moved to single rooms with soap and water handwashing. These rooms received daily and discharge cleaning with bleach-based products. Throughout the entire study period, we had ongoing initiatives to monitor and improve compliance with hand hygiene, respect of contact precautions, and other infection control measures. More details are available in the eMethods in the Supplement. No new infection control measures were implemented to synchronize with the hospital move; however, after January 18, 2016, we began to use hydrogen peroxide vapor for discharge cleaning during local outbreaks of CDI or VRE infection. Chlorhexidine bathing was used throughout the study in preoperative preparation of patients undergoing cardiac surgery and transplant; there was also daily chlorhexidine bathing for patients in the intensive care unit (30 beds) for a brief period between April 1, 2017, and April 1, 2018.
Estimation of Cases Prevented
Using the time-series regression model restricted to before the move, we estimated the expected number of MRSA and VRE cases assuming no change. From these estimated values and their associated 95% CIs, we then subtracted the actual number of observed cases seen after the move. This method yielded an estimate of the number of MRSA and VRE colonization episodes prevented by the move with 95% CIs.
Statistical Analysis
We conducted an analysis using segmented interrupted time series with the timing of the move to the new hospital as the intervention, both immediately (change in level) and over time (change in trend).13 Data were available from fiscal period 1 2013-2014 until fiscal period 1 2018-2019 with each fiscal period representing 4 weeks and 13 periods per year. The outcomes were the per-period incidence (standardized per 10 000 patient-days) of nosocomial VRE colonization, MRSA colonization, VRE infection, MRSA infection, and CDI. These outcomes were first inspected graphically and then segmented Poisson regression models were fit. For VRE and CDI, aggregate data of monthly cases from the remainder of the province of Québec (excluding the Royal Victoria Hospital) were used to control for the underlying regional temporal trends. For MRSA colonization, provincial data do not exist; thus, we used the rate of MRSA that was identified as being acquired elsewhere to act as the control for regional temporal trends. Results are reported as incidence rate ratios (IRRs) comparing consecutive times with 95% CIs. Incidence rate ratios with 2-sided 95% CIs that did not cross 1 were considered statistically significant. Statistical analyses were carried out using Stata, version 15 (StataCorp LP).
Results
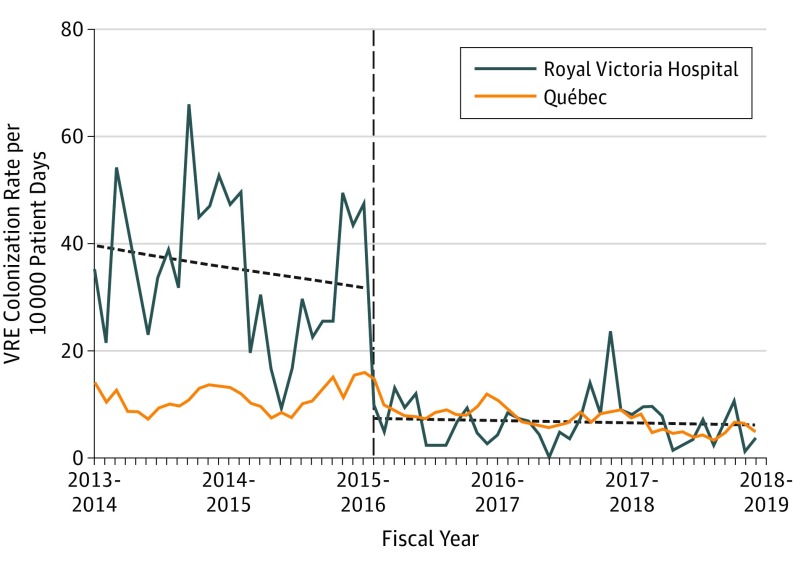
After the move, we observed an immediate and sustained reduction in the incidence of nosocomial VRE (Figure 1) and MRSA (Figure 2) colonization and of the incidence of VRE infection, but not of nosocomial CDI (Figure 3) or MRSA infection. The crude number of cases, admissions, patient days, and associated mean rates are presented in the Table. For VRE, there was no temporal trend before the move (IRR, 0.99; 95% CI, 0.98-1.0), there was an immediate level change (IRR, 0.25; 95% CI, 0.19-0.34), and there was no temporal trend after the move (IRR, 1.01; 95% CI, 1.00-1.03). We estimated that the move to the new hospital led to 818 colonization events prevented (95% CI, 676-1580). With this reduction in VRE colonization, we saw a concomitant reduction in VRE infections with an immediate-level change (IRR, 0.30; 95% CI, 0.12-0.75) without statistically significant changes in either of the temporal trends (preintervention: IRR, 1.01; 95% CI, 0.98-1.04; postintervention: IRR, 0.95; 95% CI, 0.88-1.00).
Figure 1. Nosocomial Vancomycin-Resistant Enterococcus (VRE) Colonization Rate Before and After Move to a New Hospital With All Private Rooms.
The rate of nosocomial VRE colonization (per 10 000 patient-days) by fiscal period from January 1, 2013, to March 31, 2018, in the Royal Victoria Hospital and province of Québec. The horizontal dashed line indicates the time of the move to the new hospital.
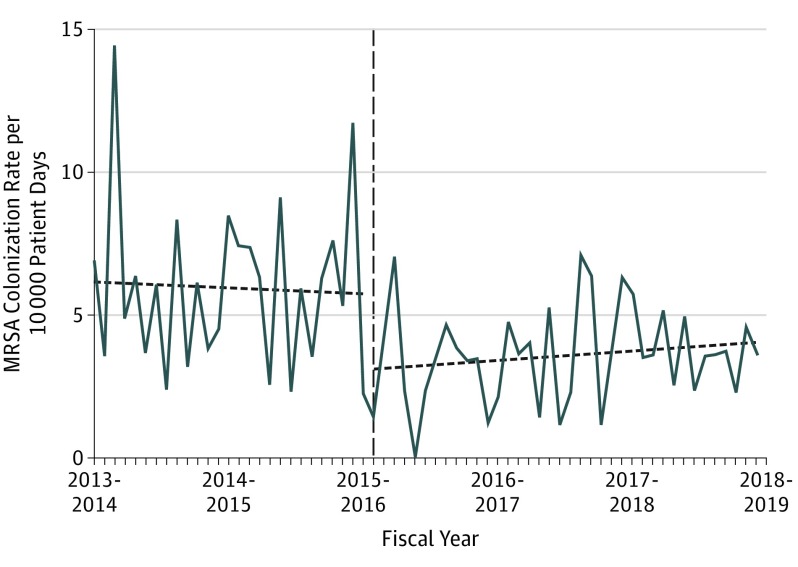
Figure 2. Nosocomial Methicillin-Resistant Staphylococcus aureus (MRSA) Colonization Rate After Move to a New Hospital With All Private Rooms.
The rate of nosocomial MRSA colonization (per 10 000 patient-days) by fiscal period from January 1, 2013, to March 31, 2018, in the Royal Victoria Hospital; the province of Québec does not include data on MRSA. The horizontal dashed line indicates the time of the move to the new hospital.
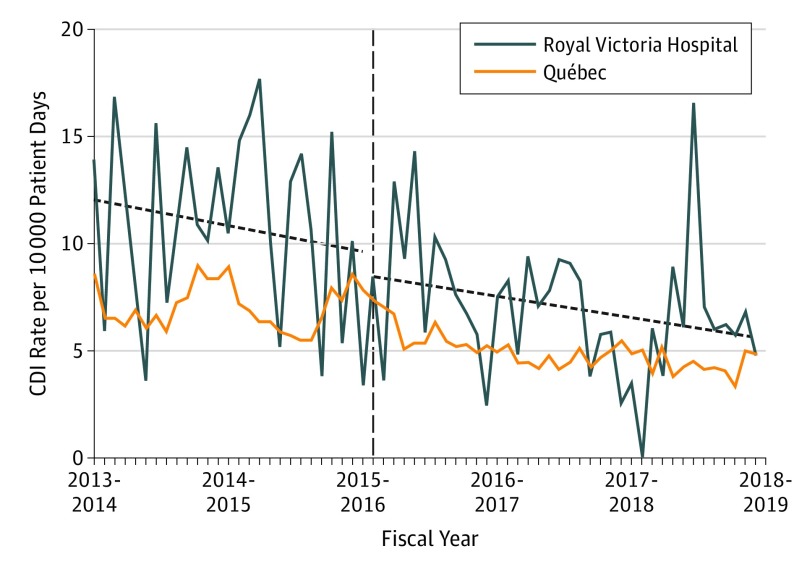
Figure 3. Nosocomial Clostridioides difficile Infection (CDI) Before and After Move to a New Hospital With All Private Rooms.
The rate of nosocomial CDI (per 10 000 patient-days) by fiscal period from January 1, 2013, to March 31, 2018, in the Royal Victoria Hospital and province of Québec. The horizontal dashed line indicates the time of the move to the new hospital.
Table. Number of Colonizations, Infections, Patient Days, and Mean Unadjusted Rates Before and After the Move.
| Variable | Before the Movea | After the Moveb | ||
|---|---|---|---|---|
| No. | Unadjusted Rate per 10 000 Patient-Days, Mean (95% CI) | No. | Unadjusted Rate per 10 000 Patient-Days, Mean (95% CI) | |
| VRE | ||||
| Colonizations | 766 | 35.0 (32.6-37.6) | 209 | 6.6 (5.7-7.5) |
| Infections | 55 | 2.5 (1.9-3.3) | 14 | 0.4 (0.2-0.7) |
| MRSA | ||||
| Colonizations | 129 | 5.9 (4.9-7.0) | 112 | 3.5 (2.9-4.2) |
| Infections | 27 | 1.2 (0.8-1.8) | 37 | 1.2 (0.8-1.6) |
| CDI infections | 236 | 10.8 (9.5-12.2) | 223 | 7.0 ( 6.1-8.0) |
Abbreviations: CDI, Clostridioides difficile infection; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus.
Before the move included 18 522 admissions contributing 218 868 patient-days.
After the move included 31 422 admissions contributing 318 257 patient-days.
For MRSA colonization, there was an immediate-level change (IRR, 0.57; 95% CI, 0.33-0.96) after the move without statistically significant preintervention or postintervention temporal trends (preintervention: IRR, 0.99; 95% CI, 0.97-1.02; postintervention: IRR, 1.01; 95% CI, 0.98-1.04). This change led to an estimated 93 fewer colonization events (95% CI, 78-134). There was no concomitant decrease in MRSA infections (IRR, 0.89; 95% CI, 0.34-2.29) or statistically significant changes in either of the temporal trends (preintervention: IRR, 0.98; 95% CI, 0.94-1.03; postintervention: IRR, 1.02; 95% CI, 0.97-1.07).
For CDI, there was no immediate-level change (IRR, 0.95; 95% CI, 0.51-1.76) or statistically significant preintervention or postintervention temporal trends (preintervention: IRR, 0.99; 95% CI, 0.97-1.01; postintervention: IRR, 1.00; 95% CI, 0.98-1.02). Consequently, there could be no estimated level of the number of cases prevented due to the move; however, we observed that the entire province of Québec has been experiencing a downward trend in CDI over the study period with an annual IRR of 0.99 (95% CI, 0.98-1.00) described both in our data set and in other centers throughout Canada over the past decade.14
Discussion
The move to a new hospital with 100% single-patient rooms was associated with an immediate and durable reduction in the rates of nosocomial VRE and MRSA colonization and a reduction in VRE infection. There was no demonstrable association with MRSA infection rates or in CDI outside of a concurrent temporal trend in Québec. The changes in VRE and MRSA colonization appeared to be temporally associated with the move with an immediate apparent difference and no subsequent return to historical levels. This reduction occurred despite demographic changes that increased the high-risk tertiary care population, notably in hematology-oncology, complex surgery, and severe, end-stage respiratory diseases.
The move to a newly constructed facility with surfaces that were presumably free of VRE and MRSA are factors associated with the stepwise reduction that was immediately observed. Overall, low levels of colonization were seen in all hospital areas, with VRE infection rates reduced most prominently in high-risk areas (eg, intensive care unit, hematology-oncology, and transplant). Although there was an absolute decrease of nearly 35% in the rates of CDI over the studied years, this lower level seems more likely to represent the changes seen throughout the province of Québec and all of Canada.14 Although we have continued to use quality improvement measures in infection control throughout the period of study, including ongoing efforts at improving hand hygiene, none of these cointerventions corresponded temporally to the clear steplike changes in MRSA and VRE colonization rates occurring at the time of the hospital move. These cointerventions are, however, likely partly responsible for curtailing an increase in transmission back to historical levels.
Over the past 40 years, few studies have examined the outcome of the move of an entire facility. Vietri et al15 studied the association of relocation with MRSA colonization 3 after their 418-bed, 90% ward-type facility closed in 1996 and patients were relocated to a new 450-bed institution with 70% single-bed rooms. The prevalence of MRSA colonization did not decrease, but the study had limitations. First, there were only 2 times before the move (at 5 and 10 days) and 2 times after the move (at 30 days and 1 year), in contrast to our study, which spanned 65 consecutive months. Second, the protocol for surveillance was not well regimented or uniformly applied. Third, there were only 249 patients in total that were sampled. In addition, unlike our study, no data on regional trends were provided. Another study found no significant association between CDI and change of facility when a large acute care hospital in South West England with 10% single beds was relocated to a hospital with 75% single beds.16 However, the investigators found that bed-days lost from norovirus were considerably improved. Methicillin-resistant S aureus was not studied, as the rates were close to 0 at the time of the move, and data on VRE were not reported. One small report found a reduced incidence of CDI following a move to a hospital with all private rooms, but this study was methodologically limited with only 1 year of data available after the move and no consideration given to prevailing regional trends.17
A 2004 review that examined the association of architecture design with nosocomial infection rates found an overall trend toward a reduction in infections but cautioned that the findings were not generalizable because of a significant amount of confounding and small study populations.6 A more recent systematic review studied the association between single-bed rooms and nosocomial infection and colonization rates across 9 studies.2 These studies mainly examined the reorganization of specific units rather than entire facilities—a limitation that we addressed in our study. Results were mixed, but the authors concluded that single-patient rooms may be a useful part of a multifaceted strategy for reducing health care–associated infections.2
Moving to a facility with 100% private rooms did not appear to be associated with rates of CDI, and other studies have shown conflicting results.16,17,18,19 Because we have no data available to compare nosocomial C difficile colonization rates, we cannot evaluate the outcome of sole use of private rooms at preventing new colonization. Asymptomatic C difficile colonization in the community may be associated with nosocomial CDI,20 particularly when such patients are exposed to antibiotics. In addition, antimicrobial exposure may be associated with the development of nosocomial CDI more so than exposure to patients with C difficile colonization when infection control practices are established.21,22,23 Similarly, most MRSA infections in the Royal Victoria Hospital institution were diagnosed in patients who were admitted with MRSA colonization that had been acquired elsewhere. Infection by pathogens that are acquired outside of the institution, rather than during the hospital stay, will be largely unaffected by use of private rooms.24 Overall, our study suggests that rates of CDI and MRSA infection may be more heavily associated with factors other than the architectural design of the hospital when infection control practices are already in place.
Limitations and Strengths
Our study had several limitations. We present a time-series analysis before and after the move to a facility with only private rooms but cannot model for all of the other contemporaneous interventions in infection control and antimicrobial stewardship that were ongoing as part of continuous quality improvement efforts. Nonetheless, we believe that the immediate changes seen that uniquely correspond to the move suggest positive outcomes. Interventions that were singularly put in place after the move have been useful in the maintenance of the success reported herein from the private room system. We were also limited by our inability to present data on asymptomatic C difficile colonization or on nosocomial acquisition of colonization with other important pathogens, such as carbapenemase-resistant Enterobacteriaceae, because we did not uniformly and routinely screen for these organisms before the move. Evidence for a decrease in these pathogens would provide an even more compelling argument for private rooms. In addition, we were not able to present individual patient data and were limited to institution-level surveillance data. Nonetheless, the strengths of this study lie in the robust time-series analysis with findings measured at multiple time points both before and after the move and our ability to demonstrate a sustained improvement over 3 years. Furthermore, the use of the provincial rates of VRE colonization and CDI as a point of comparison is helpful in strengthening the demonstrated association by reducing the chance that the outcome was associated with a regional temporal change.
Conclusions
After the Royal Victoria Hospital moved to a new facility with exclusively single-patient rooms, we observed a notable and sustained decrease in the rates of new MRSA and VRE colonization and VRE infection, but not of CDI or MRSA infection. This study adds observational evidence that could support the recommendations to build single-bed facilities to decrease the transmission of specific multidrug-resistant organisms and health care–associated infections and may have implications for infection control strategies for planned renovation or construction of new inpatient units and hospitals.
eMethods. Detailed Methodology
References
- 1.Magill SS, O’Leary E, Janelle SJ, et al. ; Emerging Infections Program Hospital Prevalence Survey Team . Changes in prevalence of health care-associated infections in US hospitals. N Engl J Med. 2018;379(18):1732-1744. doi: 10.1056/NEJMoa1801550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stiller A, Salm F, Bischoff P, Gastmeier P. Relationship between hospital ward design and healthcare-associated infection rates: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2016;5:51. doi: 10.1186/s13756-016-0152-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson DJ, Miller BA, Chen LF, et al. The network approach for prevention of healthcare-associated infections: long-term effect of participation in the Duke Infection Control Outreach Network. Infect Control Hosp Epidemiol. 2011;32(4):315-322. doi: 10.1086/658940 [DOI] [PubMed] [Google Scholar]
- 4.Johnson NB, Hayes LD, Brown K, Hoo EC, Ethier KA; Centers for Disease Control and Prevention (CDC) . CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors—United States, 2005-2013. MMWR Suppl. 2014;63(4):3-27. [PubMed] [Google Scholar]
- 5.Bracco D, Dubois MJ, Bouali R, Eggimann P. Single rooms may help to prevent nosocomial bloodstream infection and cross-transmission of methicillin-resistant Staphylococcus aureus in intensive care units. Intensive Care Med. 2007;33(5):836-840. doi: 10.1007/s00134-007-0559-5 [DOI] [PubMed] [Google Scholar]
- 6.Dettenkofer M, Seegers S, Antes G, Motschall E, Schumacher M, Daschner FD. Does the architecture of hospital facilities influence nosocomial infection rates? a systematic review. Infect Control Hosp Epidemiol. 2004;25(1):21-25. doi: 10.1086/502286 [DOI] [PubMed] [Google Scholar]
- 7.Voigt J, Mosier M, Darouiche R. Private rooms in low acuity settings: a systematic review of the literature. HERD. 2018;11(1):57-74. doi: 10.1177/1937586717702597 [DOI] [PubMed] [Google Scholar]
- 8.Institut national de santé publique du Québec. Surveillance provinciale des infections à entérocoque résistant à la vancomycine au Québec. 2017. https://www.inspq.qc.ca/sites/default/files/documents/infectionsnosocomiales/protocole_erv_2017.pdf. Published 2017. Accessed May 9, 2019.
- 9.Institut national de santé publique du Québec. Surveillance provinciale des bactériémies à Staphylococcus aureus au Québec. 2018; https://www.inspq.qc.ca/sites/default/files/documents/infectionsnosocomiales/protocole_sarm_2018.pdf. Published 2018. Accessed May 9, 2019.
- 10.Centers for Disease Control and Prevention. National Healthcare Safety Network Tracking infections in acute care hospitals/facilities. https://www.cdc.gov/nhsn/acute-care-hospital/index.html. Published November 28, 2018. Accessed May 9, 2019.
- 11.McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. doi: 10.1093/cid/cix1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institut national de santé publique du Québec. Surveillance provinciale des diarrhées à Clostridium difficile au Québec. https://www.inspq.qc.ca/sites/default/files/documents/infectionsnosocomiales/protocole_dacd_2017.pdf. Published April 2017. Accessed May 9, 2019.
- 13.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz KC, Golding GR, Choi KB, et al. ; Canadian Nosocomial Infection Surveillance Program . The evolving epidemiology of Clostridium difficile infection in Canadian hospitals during a postepidemic period (2009-2015). CMAJ. 2018;190(25):E758-E765. doi: 10.1503/cmaj.180013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vietri NJ, Dooley DP, Davis CE Jr, Longfield JN, Meier PA, Whelen AC. The effect of moving to a new hospital facility on the prevalence of methicillin-resistant Staphylococcus aureus. Am J Infect Control. 2004;32(5):262-267. doi: 10.1016/j.ajic.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 16.Darley ESR, Vasant J, Leeming J, et al. Impact of moving to a new hospital build, with a high proportion of single rooms, on healthcare-associated infections and outbreaks. J Hosp Infect. 2018;98(2):191-193. doi: 10.1016/j.jhin.2017.06.027 [DOI] [PubMed] [Google Scholar]
- 17.Heddema ER, van Benthem BH. Decline in incidence of Clostridium difficile infection after relocation to a new hospital building with single rooms. J Hosp Infect. 2011;79(1):93-94. doi: 10.1016/j.jhin.2011.03.028 [DOI] [PubMed] [Google Scholar]
- 18.Ellison J, Southern D, Holton D, et al. Hospital ward design and prevention of hospital-acquired infections: a prospective clinical trial. Can J Infect Dis Med Microbiol. 2014;25(5):265-270. doi: 10.1155/2014/685402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teltsch DY, Hanley J, Loo V, Goldberg P, Gursahaney A, Buckeridge DL. Infection acquisition following intensive care unit room privatization. Arch Intern Med. 2011;171(1):32-38. doi: 10.1001/archinternmed.2010.469 [DOI] [PubMed] [Google Scholar]
- 20.Kong LY, Eyre DW, Corbeil J, et al. Clostridium difficile: investigating transmission patterns between infected and colonized patients using whole genome sequencing. Clin Infect Dis. 2019;68(2):204-209. doi: 10.1093/cid/ciy457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owens RC Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;46(suppl 1):S19-S31. doi: 10.1086/521859 [DOI] [PubMed] [Google Scholar]
- 22.Brown K, Valenta K, Fisman D, Simor A, Daneman N. Hospital ward antibiotic prescribing and the risks of Clostridium difficile infection. JAMA Intern Med. 2015;175(4):626-633. doi: 10.1001/jamainternmed.2014.8273 [DOI] [PubMed] [Google Scholar]
- 23.Forster AJ, Daneman N, van Walraven C. Influence of antibiotics and case exposure on hospital-acquired Clostridium difficile infection independent of illness severity. J Hosp Infect. 2017;95(4):400-409. doi: 10.1016/j.jhin.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 24.McDonald LC, Diekema DJ. Point-counterpoint: active surveillance for carriers of toxigenic Clostridium difficile should be performed to guide prevention efforts. J Clin Microbiol. 2018;56(8):e00782-e18. doi: 10.1128/JCM.00782-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Detailed Methodology