Abstract
Previous studies in animal models and humans have shown that exposure to nutritional deficiencies in the perinatal period increases the risk of psychiatric disease. Less well understood is how such effects are modulated by the combination of genetic background and parent-of-origin (PO). To explore this, we exposed female mice from 20 Collaborative Cross (CC) strains to protein deficient, vitamin D deficient, methyl donor enriched or standard diet during the perinatal period. These CC females were then crossed to a male from a different CC strain to produce reciprocal F1 hybrid females comprising 10 distinct genetic backgrounds. The adult F1 females were then tested in the open field, light/dark, stress-induced hyperthermia, forced swim and restraint stress assays. Our experimental design allowed us to estimate effects of genetic background, perinatal diet, PO and their interactions on behavior. Genetic background significantly affected all assessed phenotypes. Perinatal diet exposure interacted with genetic background to affect body weight, basal body temperature, anxiety-like behavior and stress response. In 8 of 9 genetic backgrounds, PO effects were observed on multiple phenotypes. Additionally, we identified a small number of diet-by-PO effects on body weight, stress response, anxiety- and depressive-like behavior. Our data show that rodent behaviors that model psychiatric disorders are affected by genetic background, PO and perinatal diet, as well as interactions among these factors.
Keywords: anxiety, depression, development, gene-by-environment, methyl donors, multi-parental populations, protein, psychiatric disorders, stress, vitamin D
1 |. INTRODUCTION
It is now well-established that the risk for developing a psychiatric disorder is influenced by a combination of genetics, the environment and gene-by-environment interactions.1 The complex etiology of psychiatric disease, whereby risk is likely due to the actions of hundreds or thousands of genes as well as numerous and largely undefined environmental factors, has made it difficult to pinpoint mechanisms and improve treatment and prevention strategies.
One potentially modifiable environmental factor that has been linked to increased risk of psychiatric disorders is perinatal exposure to nutritional deficiencies. Longitudinal studies of the Dutch Hunger Winter and Chinese Famine cohorts have shown that perinatal nutritional deficiency increases risk for developing schizophrenia,2–7 affective disorders8–11 and addiction12 later in life. However, not all individuals exposed to perinatal nutritional deficiencies develop psychiatric disorders, suggesting potential interactions between the perinatal environment and genetic background. Published studies further suggest this interaction could be modulated by genomic imprinting, a phenomenon in which an allele is preferentially expressed depending on its parent-of-origin (PO);13 for example, the expression of imprinted genes IGF2, GNASAS and MEG3 were persistently altered in exposed vs unexposed siblings from the Dutch Hunger Winter cohort.14,15 Approximately 150 imprinted genes have been identified, many of which are highly expressed in the brain.16,17 Imprinted genes are known to be key regulators in prenatal development and postnatal growth,18 and dysregulation of imprinting has been shown to result in developmental disorders such as Prader-Willi, Angelman, Beckwith-Wiedmann and Silver-Russell syndromes, all of which result in growth and behavioral alterations.13
Although the Dutch Hunger Winter and Chinese Famine allowed for observational studies of a perinatal nutrition challenge in human populations, obvious ethical issues preclude controlled studies during this key developmental period in humans. Fortunately, rodent models of psychiatric disorders have been developed and offer clear advantages for studying genetic and environmental risk factors. The advantages include: a short gestation and time to adulthood; the ability to control and manipulate the environment; access to relevant tissues (ie, brain) for mechanistic studies; and access to well-characterized and replicable experimental populations along with advanced genetic tools.
A growing number of rodent studies show the persistent effect of perinatal nutritional deficiency on behavior in adulthood. Adult rodents exposed prenatally to vitamin D deficiency (VDD) show alterations in behaviors that model schizophrenia, including enhanced sensitivity to amphetamine,19 spontaneous hyperactivity20–24 and decreased sustained attentional processing.25,26 Rodents exposed to prenatal protein deficiency (PD) exhibit alterations in behavioral models of schizophrenia including enhanced sensitivity to amphetamine27 and decreased prepulse inhibition and startle response;28 behaviors that model affective disorders including increased depressive-like29–32 and anxiety-like behaviors;30,33 and addiction-like behaviors including enhanced sensitivity to cocaine.32,34 Rodents exposed to methyl donor deficiency (ie, choline, folate) during some part of the perinatal period display increased anxiety-like behaviors35,36 and alterations in learning ability and memory.36,37 A few studies in rodents have also reported strain differences in behavior after exposure to perinatal nutritional deficiencies,23,38,39 although genetic-background dependent effects are largely unexplored in animal models. Rodent models also support the hypothesis that perinatal nutritional deficiencies lead to the dysregulation of imprinted genes and mediate behavior. Two studies using mice have reported alterations in the imprinted genes Cdkn1c and Igf2 following perinatal exposure to PD32 or methyl donor deficiency.40
In this study, we used a recently established mouse resource, the Collaborative Cross (CC).41,42 The CC is a panel of recombinant inbred strains with each strain derived from an independent cross of 8 inbred founders. The 8 founders, composed of 5 classical laboratory strains and 3 wild-derived strains, capture 90% of the common genetic variation present in the domesticated house mouse.43 The CC was specifically designed for the study of complex phenotypes like behavior.44
In our study, we exposed females from 20 CC strains to 1 of 4 experimental diets prior to and during gestation and throughout the postnatal period until weaning. Strains were grouped into 10 pairs, and each strain within a pair was crossed with its CC strain partner, generating 10 genetically distinct types of reciprocal F1 hybrid female offspring (also known as recombinant inbred intercrosses, RIX). Based on this breeding strategy, all females within a reciprocal F1 pair were genetically identical except for the PO of the nuclear genome and, potentially, the mitochondria. These F1 females were subjected to a battery of commonly used behavioral models of psychiatric disorders (Figure 1).
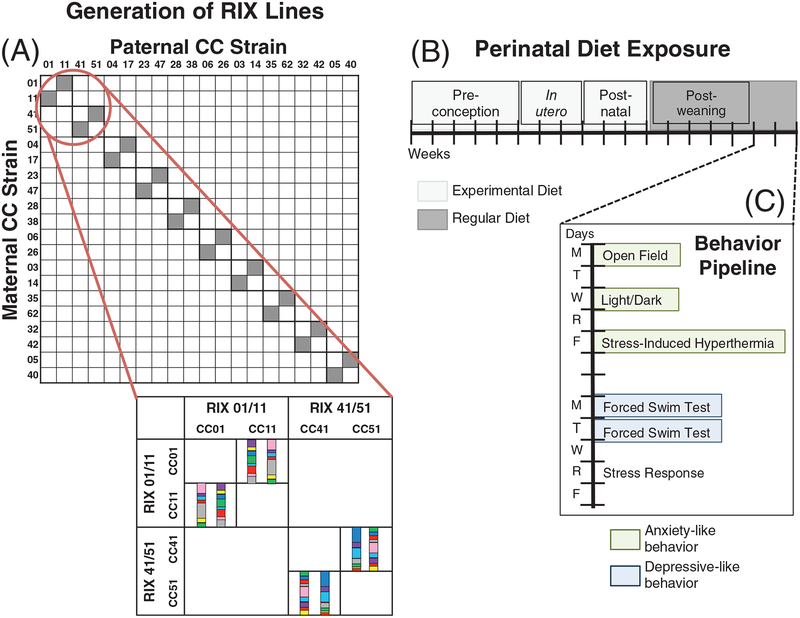
FIGURE 1.
Experimental design. A, Twenty different CC strains were used to generate 10 RIXs lines. Each RIX is a set of F1 reciprocal females that differ only in the parental origin of alleles. The CC alleles come from the 8 inbred strains (represented by 8 colors) that were used to generate the CC. Comparison of F1 reciprocal females within a RIX allows for detection of parent-of-origin effects. Comparison across RIX lines allows for detection of genetic background effects. B, Multiple dams from each CC strain were put on 1 of 4 diets (PD, ME, VDD and Std) for 5 weeks prior to mating, throughout gestation and the postnatal period. F1 females were weaned onto a regular diet and remained on that diet until the completion of behavioral testing. C, F1 females underwent a 2-week behavioral pipeline to assess anxiety- (OF, LD, SIH) and depressive-like (FST) behavior and stress response
Our experimental design takes advantage of this unique and powerful mouse population to detect the effects of exposure to nutritional deficiencies, genetic background, PO effects and importantly, their interactions to alter behavior in adulthood.
2 |. METHODS AND MATERIALS
2.1 |. CC strain selection
Generation of CC strains has previously been described in detail.42,45 Briefly, CC are recombinant inbred strains created from 8 inbred lines from the 3 major Mus musculus subspecies, domesticus (A/J, C57BL/6J, 129S1/SvImJ, NOD/ShiLtJ, NZO/HlLtJ, WSB/EiJ), castaneus (CAST/EiJ) and musculus (PWK/PhJ). CC mice were purchased from the Systems Genetics Core Facility (SGCF) at the University of North Carolina (UNC).46
The 20 CC strains used to generate the 10 RIX lines (Table S1, Supporting Information; Figure 1A) were selected using Rexplorer, a program developed at UNC for reciprocal cross strain selection (Oreper, Tarantino and Valdar, personal communication). Strain-pair selection aimed to maximize several criteria. (1) The number of known brain-imprinted loci, as defined from,17,47 that are heterozygous between 2 categories of haplotypes: those that are identical by descent with NOD/ShiLtJ, and those that are identical by descent with C57BL/6J. This criterion was based on pilot data suggesting that offspring from a reciprocal cross between these 2 strains had different behavioral responses to perinatal nutritional deficiencies (Oreper, Valdar and Tarantino, personal communication). Other criteria included: (2) maximum heterozygosity at all other haplotypes; (3) linkage disequilibrium between imprinted loci of interest in the 10-RIX population; and (4) predicted reproductive success (unpublished observations; http://csbio.unc.edu).
2.2 |. Perinatal diet exposure and breeding RIX lines
CC mice were purchased at approximately 4 to 5 weeks of age and acclimated for at least 1 week prior to diet exposure. After acclimation, dams were placed on 1 of 4 diets (Table S2 and see below) for 5 weeks. The average age at which dams were placed on the experimental diets was 41.4 ± 7.1 days. After 5 weeks on the experimental diet, dams were mated with a sire from a different CC strain to generate F1 offspring (Table S1, Figure 1A). The average age of dams at mating was 76.5 ± 7.3 days. CC dams remained on experimental diets throughout gestation and until litters were weaned, ensuring that the offspring were exposed throughout the entire perinatal period (Figure 1B). Sires were removed from the breeding cage once pregnancy was confirmed by visual inspection. We maximized the number of dams used in the study to ensure that behavioral observations in the F1 offspring were not attributable to a single dam. At least 3 dams per CC strain were used (Table S3). We also used only the first litter per dam to avoid differences in reproductive parity and to minimize variation in timing of exposure to experimental diets. Due to the large scale of this study, we did not cross-foster the F1 offspring; consequently, any observed PO effects could be due to genomic imprinting and/or maternal effects.
F1 offspring were weaned at postnatal day (PND) 23.1 ± 2.8 days onto standard laboratory chow (Harlan Teklad 2920; Envigo, Frederick MD, USA) Female offspring were co-housed with reciprocals to control for cage effects. Male offspring were provided at weaning to a collaborator and used for unrelated studies.48
All mice were housed in a specific pathogen free vivarium and maintained on a 12-hour light/dark (LD) cycle with lights on at 7 am. All procedures and animal care were approved by the UNC Institutional Animal Care and Use Committee and followed the guidelines set forth by the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
2.3 |. Nomenclature
The official name of each CC strain used in this study is provided in Table S1. For simplicity, we use only the last 2 digits of the name when referring to F1 hybrids in the text and figures. For each reciprocal F1 line the dam is listed first and the sire is listed second; for example CC(01 × 11)F1 refers to (CC001/Unc × CC011/Unc)F1. When referring to the collective group of F1 mice from a pair of reciprocal crosses, we simply list the 2 CC strains in numerical order separated by a forward slash; for example, RIX 01/11 includes mice from both reciprocal crosses, (CC001/Unc × CC011/Unc)F1 and (CC011/Unc × CC001/Unc)F1.
2.4 |. Experimental diets
Experimental diets were formulated by Dyets Inc. (Bethlehem, Pennsylvania): vitamin D deficient (#119266), protein deficient (7.5% casein; #102787), methyl donor enriched (ME; #518893) or standard control (Std; #AIN-93G). Table S2 shows the nutrient composition for all experimental diets. The PD and VDD diets were nutritionally matched to the Std diet. ME was matched to a methyl-donor-deficient diet that was used in pilot studies and eliminated from these experiments due to the inability of dams to produce viable offspring when exposed to the diet. All diets were administered in pelleted form and were available ad libitum to the dams during the perinatal period. The amount of diet consumed by each dam was not recorded.
2.5 |. Behavior assays
Only female F1 offspring were tested to ensure an identical genetic background, including the sex chromosomes, between reciprocal hybrids. All behavioral testing was performed during the light part of the LD cycle between 8:00 am and 12:00 pm. Animals were assessed in adulthood (average age at initiation of testing was 68.2 ± 5.3 days). Mice were screened in a 2-week behavioral pipeline that consisted of (in order of testing) open field (OF), LD, stress-induced hyperthermia (SIH), forced swim test (FST) and restraint stress test (Figure 1C). Mice were free from testing for at least 1 day between behaviors. Behavioral tests were administered in order of lowest to highest invasiveness/stress, in order to mitigate prior testing effects. We aimed to screen at least 20 females per RIX (10 of each reciprocal) for each of the 4 diet exposures. Actual sample sizes are provided in Table S1. Due to the large number of animals screened (N = 685), behavioral testing took place in 28 batches over a 2-year period. In each batch, we tested a minimum of 2 diet exposure groups and 2 RIX. Each RIX and diet exposure was distributed across at least 3 test batches to mitigate seasonal and batch effects. All testing equipment was cleaned with a 0.25% bleach solution between test subjects unless otherwise stated.
2.5.1 |. Body weight
Female F1 offspring were weighed at weaning (referred to as PND 21) and again at the initiation of behavioral testing (referred to as PND 60). We did not weigh dams so as not to induce stress associated with manipulation during the perinatal period.
2.5.2 |. Open field
The OF apparatus (ENV-515–16, Med Associates, St. Albans, Vermont) was a 43.2 × 43.2 × 33 cm arena consisting of a white Plexiglass floor and clear Plexiglass walls with infrared detection beams at 2.54 cm intervals on the x, y and z axes that tracked the animals’ position and activity automatically during the test session. The OF apparatus was enclosed in a sound-attenuating chamber (73.5 × 59 × 59 cm) fitted with 2 overhead light fixtures containing 28-V lamps. Mice were placed in the OF arena for 10 minutes and scored for total distance traveled (cm), number of vertical movements (rearing) and percent time spent in the center of the arena (defined as the 22.86 cm2 central part of the arena). Percent time spent in the center of the OF arena is commonly used as a measure of anxiety-like behavior. Data were analyzed post-session in 2-minute bins using commercially available software (Activity Monitor 5.1, Med Associates St. Albans, Vermont).
2.5.3 |. Light/dark
The LD apparatus consisted of a 42 × 42 × 30 cm OF arena (Versamax420 Animal Activity Monitoring System, AccuScan Instruments Inc., Columbus, Ohio) with a white Plexiglass floor and clear Plexiglass walls. The arena was surrounded by 16 photobeams along each side that allowed for tracking of both horizontal and vertical activity. The black Plexiglass LD box (40 × 21 × 13 cm) occupied one-half of the arenas and had a 10 × 3 cm opening to the light side and holes on all 4 sides that allowed detection of movement by the photo-beams. Mice were placed in the lighted area immediately adjacent to and facing the entry to the dark enclosure and left to freely investigate the apparatus for 10 minutes. The amount of time (seconds), number of transitions, distance moved (cm) and percent time in the dark and light zone was scored in 2-minute bins in post-session analyses using commercially available software (VersaMap version 1.7, AccuScan Instruments, Inc). Transitions into and percent time spent in the light side of the arena are used as measures of anxiety-like behavior.
2.5.4 |. Stress-induced hyperthermia
Mice were individually removed from the home cage and the initial temperature (SIH-T1) was measured by insertion of a lubricated digital thermometer probe (TH-5 Thermalert Monitoring Thermometer with RET-3 rectal probe, Physitemp Instruments, Clifton, New Jersey) 1 to 1.5 cm into the rectum for approximately 10 seconds. The animal was immediately returned to the home cage and 10 minutes later, the temperature measurement was repeated (SIH-T2). The difference in body temperature between T2 and T1 was calculated as the change in temperature (SIH-ΔT). This physiological response to a stressor is commonly used as a measure of anxiety-like behavior and is responsive to anxiolytic drugs.49
2.5.5 |. Forced swim test
The FST was conducted in a glass-polycarbonate cylinder (46 cm tall × 21 cm in diameter) filled with water to a depth of 15 cm and maintained at a temperature of 25°C to 28°C. Mice were tested for 6 minutes. Percent immobility during the last 4 minutes of the test period was recorded by video and scored using Ethovision 7.0 automated tracking software (Noldus, Leesburg, Virginia). Immobility was defined as the mouse making no movements other than those needed to stay afloat; this phenotype is thought to capture behavioral despair or depressive-like behavior. Mice were monitored continuously and removed from the apparatus if they were unable to keep their nose or heads above water for more than 30 seconds.
2.5.6 |. Restraint stress
Restraint was used to elicit a stress response that was quantified by measurement of corticosterone (CORT) levels in the serum. A retro-orbital blood sample was taken from unanesthetized mice to assess basal CORT levels, and then mice were immediately placed into a Broome-Style restraint tube (Plas Labs, Inc., Lansing, Michigan) for 10 minutes. Immediately upon removal from the restrainer, a second unanesthetized retro-orbital eye bleed was performed to assess stress-induced CORT levels (Stress CORT). Whole blood was centrifuged to isolate plasma and CORT levels (in ng/mL) were measured with a competitive radioimmunoassay (RIA) using the manufacturer’s protocol (MP Biomedicals, Santa Ana, California). Stress CORT minus basal CORT levels (change in CORT or ΔCORT) was calculated to assess stress reactivity. CORT RIAs were only performed on RIX lines that had an N > 4 for both sets of reciprocal females within a diet exposure group. Therefore, RIX 04/17 ME, RIX 23/47 Std, RIX 03/14 ME and PD and RIX 35/62 ME and PD groups were excluded from these analyses.
2.5.7 |. Missing data from the behavior pipeline
Equipment failure led to the loss of data from 14 females in the OF (3 RIX 01/11 PD, 4 RIX 01/11 ME, 3 RIX 41/51 Std and 4 RIX 23/47 PD) and 5 animals in the LD (1 RIX 01/11 ME and 4 RIX 04/17 VDD). Ten females died during restraint stress and 15 females did not have enough serum for CORT RIA analysis.
The entire dataset from this study will be deposited and made available in the Mouse Phenome Database (RRID:SCR_003212) for use in follow-up studies.
2.6 |. Statistical analysis
All statistical analyses were performed using R Studio 1.0.136 or SPSS v24 for Mac OS X 10.6+. Graphs were generated using Graph-pad Prism 7.0c for Mac OS X.
2.6.1 |. Analysis of breeding performance
Percent productive matings = ([# litters born/# dams mated] × 100), average litter size at birth, % survival to weaning = ([# pups at weaning/# pups born] × 100), and sex ratio = (# F pups weaning/total pups at weaning) were calculated for each CC reciprocal cross/diet combination. For each of these 4 measures, an analysis of variance (ANOVA) was performed to assess effects of PO, diet and diet-by-PO effects within each RIX. Overall effects of genetic background (RIX), diet and diet-by-RIX across all 20 CC reciprocal crosses was also analyzed by ANOVA.
2.6.2 |. Correlation of behavioral phenotypes
The Hmisc 4.0–2 package in R Studio was used to generate coefficient and P values using Spearman correlation.50 The P value threshold for significance was adjusted to P < .00026 to correct for 190 correlations. Correlation plots were generated using Corrplot 0.77 package.51
2.6.3 |. Analysis of effects of RIX, diet and diet-by-RIX
To assess the overall effect of genetic background (also referred to as RIX effect or RIX in the text), perinatal diet and perinatal diet-by-RIX interactions, we fit linear-mixed models to the behavior of all mice. Model fitting was performed using the lmer function from the lme4 1.1–12 R package.52 Our ‘base’ model had fixed effects of RIX, diet, diet-by-RIX and random effects of dam, sire, and behavior batch (Equation (1); lmer notation, where “(1|term)” indicates a random effect).
| (1) |
| (2) |
| (3) |
| (4) |
For behavioral tests, additional random effects were added to the base model (Equation (1)) as needed. For the OF, LD and FST phenotypes, test chamber was also included (Equation (2)). For the SIH phenotypes, test order was added (Equation (3)) whereas for restraint test phenotypes both test order and experimenter were added (Equation (4)), as 2 people were required to conduct that test. The same experimenter conducted all other behavioral assays across all batches.
P-values for fixed effects were calculated by type I ANOVA (sequential) tests, using the lmerTest 2.0–33 package53 (see Table 1 for a list of all phenotypes). To account for multiple testing, P values within each type of test (eg, tests of diet) were subject to false discovery rate (FDR) correction, with q values generated using the Shiny implementation of the q-value R package.54,55 When a significant diet effect was observed (P < .05), between-diet differences were examined by a Tukey’s honest significance difference (HSD) post hoc tests, with P values reported.
TABLE 1.
Effect of genetic background (RIX), perinatal diet and diet-by-RIX on behavioral phenotypes
| RIX | Diet | Diet-by-RIX | |||||
|---|---|---|---|---|---|---|---|
| Behavior test | Phenotype | P-value | q-value | P-value | q-value | P-value | q-value |
| Body weight | Weaning (PND 21) | 1.4E-05 | 1.5E-05 | 2.2E-16 | 2.2E-15 | 1.5E-07 | 7.4E-07 |
| Adulthood (PND 60) | 2.3E-15 | 2.9E-15 | 2.2E-16 | 2.2E-15 | .002 | .004 | |
| Open field | Total distance moved | 2.0E-16 | 3.4E-16 | .772 | .881 | .209 | .209 |
| % center time | 2.0E-16 | 3.4E-16 | .807 | .881 | .041 | .058 | |
| Vertical counts | 2.0E-16 | 3.4E-16 | .998 | .998 | .072 | .089 | |
| OF boli | 6.3E-14 | 7.4E-14 | .192 | .350 | .010 | .021 | |
| Light/dark | Total distance moved | 2.0E-16 | 3.4E-16 | .228 | .381 | .061 | .082 |
| Distance dark | 2.0E-16 | 3.4E-16 | .023 | .058 | .021 | .034 | |
| Distance light | 2.0E-16 | 3.4E-16 | .805 | .881 | .097 | .114 | |
| Total transitions | 2.0E-16 | 3.4E-16 | .837 | .881 | .130 | .136 | |
| % time light | 1.3E-04 | 1.4E-04 | .048 | .096 | .025 | .039 | |
| LD boli | 2.2E-16 | 3.4E-16 | .352 | .502 | .001 | .002 | |
| Stress-induced hyperthermia | SIH-T1 | 4.4E-16 | 6.3E-16 | 2.0E-04 | .001 | 3.6E-07 | 1.4E-06 |
| SIH-T2 | 2.2E-16 | 3.4E-16 | 1.9E-04 | .001 | .008 | .018 | |
| deltaSIH | .006 | .006 | .342 | .502 | 2.6E-04 | .001 | |
| Forced swim test | % immobile | 2.0E-16 | 3.4E-16 | .377 | .502 | .122 | .135 |
| FST Boli | 6.7E-16 | 8.9E-16 | .023 | .058 | .013 | .023 | |
| Restraint stress | Basal CORT | 2.2E-16 | 3.4E-16 | .038 | .085 | 1.5E-10 | 1.5E-09 |
| Stress CORT | 2.2E-16 | 3.4E-16 | .003 | .010 | 2.7E-13 | 5.4E-12 | |
| deltaCORT | 2.2E-16 | 3.4E-16 | 2.1E-05 | 1.4E-04 | 1.1E-08 | 7.6E-08 | |
Abbreviations: deltaCORT, Stress CORT − Basal CORT; deltaSIH, T2 − T1; SIH-T1, basal temperature; SIH-T2, post-stress temperature. Significant values are bolded.
2.6.4 |. Analysis of PO, diet and diet-by-PO effects within strain
PO, diet and diet-by-PO effects were assessed within each RIX, using linear-mixed models based on Equation (5):
| (5) |
| (6) |
| (7) |
| (8) |
where, a “Reciprocal” fixed effect term models PO effects and a “Diet:Reciprocal” fixed interaction effect models diet-by-PO. Equation (6) was used for OF, LD and FST phenotypes, Equation (7) was used for SIH phenotypes and Equation (8) was used for restraint stress phenotypes. P and q values as well as Tukey’s HSD post hocs for significant diet effects were generated in the same manner described above.
2.6.5 |. Analysis of variance explained by PO, diet and diet-by-PO effects within strain
The models from Equations (5) to (8) include random effects, complicating a typical percent variance explained computation. To overcome this difficulty, the random effects in Equations (5) to (8) were regressed out and a new simple linear model with only the effects of interest were fit, namely PO, diet and diet-by-PO effects. The percent variance explained was then calculated for each effect using the ratios of fitted sums-of-squares to total sums-of-squares from a sequential (ie, type 1 sums-of-squares) ANOVA table.
3 |. RESULTS
3.1 |. Effects of perinatal diet exposure on reproductive fitness
Number of dams, percent productive matings and survival of pups to weaning, number of pups born, average litter size and sex ratio for each of the 20 reciprocal crosses are provided in Table S3. CC014/Unc and CC062/Unc became unavailable during the study (CC014/Unc is now extinct; SGCF personal communication)56 and we were unable to produce a sufficient number of RIX 03/14 and RIX 35/62 F1 females exposed to PD and ME diets. Therefore, RIX 05/40 was added to the study and perinatal exposure was limited to PD and ME diets.
Genetic background affected success of matings, average litter size and survival to weaning but not sex ratio (Table S4). Exposure to ME lead to decreased mating productivity in comparison with the Std diet (P = .05). We also assessed the effects of diet exposure and PO on breeding productivity between reciprocal crosses of each RIX line (P values are provided in Table S4). There was a PO effect on percent productive matings and average litter size for 6 of the RIX lines. PO differences for percent survival to weaning were observed for 4 of the RIX lines. Only one RIX differed by PO for sex ratio (RIX 01/11).
In 5 of the reciprocal crosses, PO affected multiple measures of breeding success (Tables S3 and S4) but many of these effects seem to be diet-dependent. In RIX 01/11, CC001/Unc dams had more productive matings but fewer pups survived until weaning than CC011/Unc dams. Additionally, CC001/Unc dams had litters with a skewed sex ratio in favor of females compared with litters from CC011/Unc dams, although this finding appears to be specific to dams on the ME diet. In RIX 04/17, CC004/TauUnc dams had larger litter size and more pups that survived to weaning compared with CC017/Unc dams. However, reduced survival of pups from CC017/Unc dams was primarily observed in dams given a ME diet. In RIX 32/42, CC032/GeniUnc dams had more productive matings, larger litters and more pups that survived to weaning compared with CC042/GeniUnc dams. However, decreased survival of pups from CC042/GeniUnc dams was primarily observed in dams exposed to PD diet. There was also a significant difference of sex ratio, dependent on diet exposure in RIX 32/42. CC042/GeniUnc dams exposed to ME diet had litters with more males and dams on VDD diet had litters with increased numbers of females compared with CC032/GeniUnc dams.
3.2 |. Correlation of behavioral phenotypes
Spearman correlations between the 20 phenotypes measured in this study are shown in Figure S1. We observed the highest correlations among phenotypes measured in a single behavioral assay. However, we also observed significant correlations across behavioral assays. Locomotor phenotypes were positively correlated in the OF and LD assays (r[188] = .47 − .75; P < 1 × 10−14). Percent center time in the OF was significantly and positively correlated with percent time in the light (r[188] = .19; P = 1.2 × 10−6) and transitions (r[188] = .60; P < 1 × 10−14) in the LD test. Phenotypes from the 2 stress-based behavioral assays, SIH and restraint stress, were also significantly correlated. SIH basal temperature was negatively correlated with stress-induced CORT (r[188] = −.17; P = 2.8 × 10−5) and change in CORT (r[188] = −.21; P = 1.5 × 10−7) while change in body temperature was positively correlated with change in CORT (r[188] = .16; P = 5.8 × 10−5).
3.3 |. Overall effects of genetic background, perinatal diet and diet-by-RIX
A linear-mixed effects model was used to assess the overall effects of genetic background (RIX), perinatal diet and diet-by-RIX interactions. Table 1 presents the P and q values for each of the 20 phenotypes assessed in the 5 behavioral tests.
3.3.1 |. Genetic background affects all phenotypes
We assessed effects of genetic background across the 9 RIX lines, collapsed across diets, and found significant effects of RIX on all behavioral phenotypes (Table 1).
3.3.2 |. Perinatal exposure to PD and ME alters body weight, body, temperature and behavior in adulthood
3.3.2.1 |. Body weight
Overall effects of perinatal diet exposure were assessed across the 4 diet groups, collapsed across the 9 RIX lines. Females exposed to PD and ME weighed significantly less than either VDD- or Std-exposed females at weaning (P = 2.2 × 10−16, q = 2.2 × 10−15; Figure S2B; Table S5) and at the onset of behavioral testing (P = 2.2 × 10−16, q = 2.2 × 10−15; Figure S2D; Table S5). These data indicate that the effects of perinatal diet on body weight persisted into adulthood, well after exposure to PD and ME had ceased.
3.3.2.2 |. Body temperature
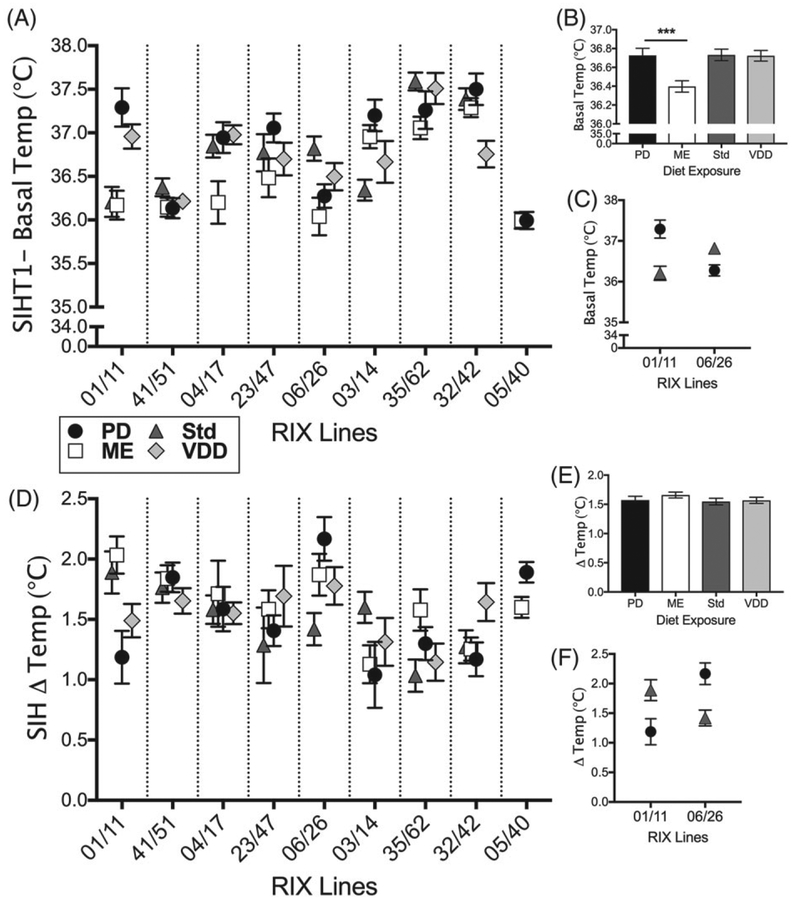
ME-exposed females had a lower basal temperature relative to all other groups (P = .0002, q = .001; Figure 2B; Table S5). Basal temperature in PD-exposed females did not differ from the other 3 groups, suggesting that basal temperature is not necessarily associated with the body weight changes described above (ie, lower body weight = normal basal temperature for PD and decreased basal temperature for ME).
FIGURE 2.
Basal- and stress-induced temperature are affected by the interaction of perinatal diet exposure and genetic background. Data points are means of diet exposure groups. Error bars are SEM. There was a significant diet-by-RIX effect on (A) basal body temperature (P = 3.6 × 10−7, q = 1.4 × 10−6) and (D) change in temperature following a stressor (P = 2.6 × 10−4, q = .001). There was an overall effect of perinatal diet on (B) basal temperature (P = .0002, q = .001) with ME-exposed females showing decreased basal temperature compared with PD-exposed females (post hoc***P < .001; N = 157, 177, 170, 180 for PD, ME, Std and VDD, respectively). (E) No significant overall effect of diet was observed for change in temperature (P > .05). An example of a diet-by-RIX interaction is shown for (C) basal and (F) change in temperature. C, RIX 01/11 PD-exposed females (N = 31) show increased basal temperature (post hoc P ≤ .02) and (F) decreased change in temperature in response to stress (post hoc P ≤ .03) compared with Std-exposed females (N = 28). C, An opposing effect is seen in RIX 06/26 wherein PD-exposed females (N = 12) have decreased basal temperature (post hoc P < .01) and (F) increased change in temperature in response to stress (post hoc P ≤ .009) compared with Std-exposed females (N = 21)
3.3.2.3 |. Anxiety-like behavior
Perinatal diet affected 2 measures of anxiety-like behavior: percent time in the light compartment in the LD test (P = .048, q = .096) and stress-induced temperature in the SIH test (SIH-T2; P = .0002, q = .001). ME-exposed females spent less time in the lighted compartment compared with all other groups although this difference was only significant in comparison with VDD-exposed females (Table S5). ME-exposed females also had a lower SIH-T2 temperature compared with all other groups, although this difference was only significant in comparison with females exposed to PD (Table S5). However, ME-exposed females were not significantly different from other diet groups for SIH change in temperature (Figure 2E). Therefore, the significant difference in SIH-T2 in the ME-exposed group may simply reflect differences in body temperature rather than response to the stressor.
3.3.2.4 |. Basal stress and stress reactivity
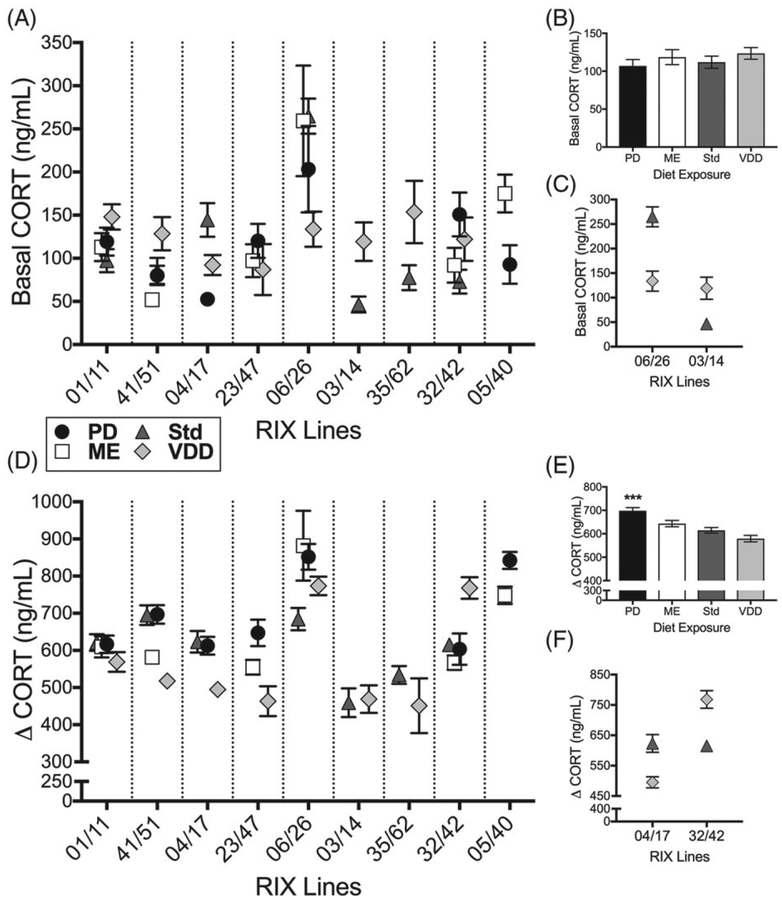
Perinatal diet altered basal CORT (P = .038, q = .085; Figure 3B), stress-induced CORT (P = .003, q = .010) and change in CORT (P = 2.1 × 10−5, q = 1.4 × 10−4; Figure 3E). PD-exposed females show significantly greater stress-induced CORT and change in CORT (Figure 3E) in comparison with both ME- and VDD-exposed females (Table S5). Post hoc comparisons did not identify any significant differences in basal CORT among the experimental diet groups.
FIGURE 3.
Perinatal diet interacts with genetic background to alter measures of stress response. Data are means of diet exposure groups. Error bars are SEM. There was a significant diet-by-RIX effect on (A) basal CORT (P = 1.5 × 10−10, q = 1.5 × 10−9) and (D) change in CORT following a restraint stress (P = 1.1 × 10−8, q = 7.6 × 10−8). There was an overall effect of perinatal diet on (B) basal CORT (P = .038, q = .085) although post hoc tests showed no significant effects. E, There was a significant diet effect on change in CORT (P = 2.1 × 10−5, q = 1.4 × 10−4) with PD-exposed females showing increased change in CORT (post hoc ***P < .001; Ns = 141, 150, 157, 171 for PD, ME, Std and VDD). Example of a diet-by-RIX interaction is shown for (C) basal CORT and (F) change in CORT. C, In RIX 06/26, VDD exposure (N = 23) resulted in decreased basal CORT compared with Std exposure (N = 21) while in RIX 03/14, VDD-exposed females (N = 15) showed increased CORT compared with Std-exposed females (N = 21). F, RIX 32/42 VDD-exposed females (N = 25) showed increased change in CORT compared with Std-exposed females (N = 30; post hoc P ≤ .001) while RIX 04/17 VDD-exposed females (N = 27) showed decreased change in CORT compared with Std-exposed females (N = 27; post hoc P ≤ .001)
3.3.3 |. Genetic background interacts with perinatal diet to alter body weight, stress response and anxiety-like behavior
Fourteen of the 20 phenotypes exhibited significant diet-by-RIX effects based on P value, whereas 13 had a significant q value. Body weight, basal temperature, anxiety-like behavior and stress response all show a diet-by-RIX effect (see Table 1). In order to investigate these interactions, we analyzed each RIX independently to examine which genetic backgrounds were altered by perinatal diet and to determine if the diet-induced changes in a RIX matched the overall diet effects reported above. P and q values along with Tukey’s post hoc analyses of diet effects within a RIX are reported in Table S6.
3.3.3.1 |. Body weight
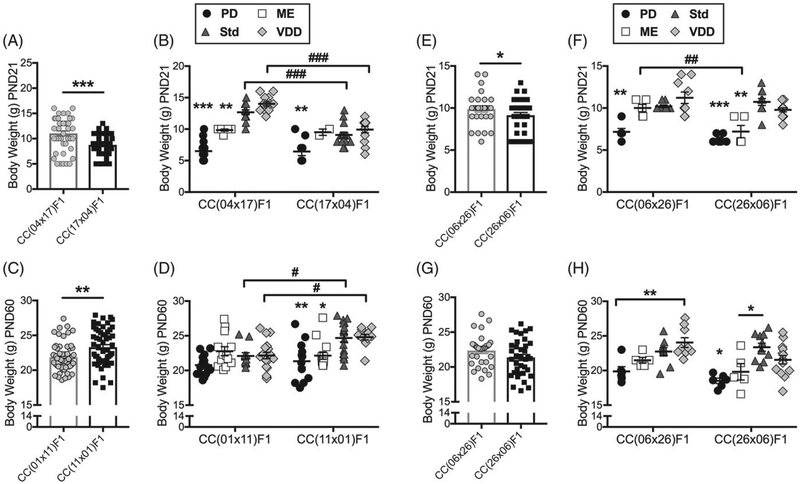
There was a significant diet-by-RIX effect on body weight at weaning (P = 1.5 × 10−7, q = 7.4 × 10−7; Figure S2A) and in adulthood (P = .002, q = .004; Figure S2C). For 6 of the RIX lines (RIXs 01/11, 41/51, 04/17, 23/47, 06/26 and 03/14), diet-specific differences in body weight at weaning persisted into adulthood. RIX lines 35/62 and 32/42 both exhibited diet-specific differences in body weight at only one of the time points—RIX 35/62 in adulthood and RIX 32/42 at weaning. RIX 05/40 was the only line for which perinatal diet did not differentially affect body weight, although only 2 diets were examined in this line.
3.3.3.2 |. Stress-induced hyperthermia
All 3 measures from the SIH assay showed a diet-by-RIX effect; basal temperature (P = 3.6 × 10−7, q = 1.4 × 10−6; Figure 2A), stress-induced temperature (P = .008, q = .018) and change in temperature (P = 2.6 × 10−4, q = .001; Figure 2D). Five of the RIX lines showed no effect of diet on basal temperature (RIXs 41/51, 04/17, 23/47, 35/62, 05/40) and 6 showed no effect of diet exposure on change in temperature following a stressor (RIXs 41/51, 04/17, 23/47, 03/14, 35/62, 32/42). To highlight a diet-by-RIX effect, the opposing effects of the PD compared with Std diet observed in RIX 01/11 and RIX 06/26 females is shown in Figure 2C,F. RIX 01/11 females exposed to PD showed increased basal temperature and a decreased change in temperature in response to stress while RIX 06/26 females exposed to a PD diet had a lower basal temperature and an increased change in temperature in response to stress. These data show that the effects of perinatal diet on basal temperature and stress-induced temperature change, a physiological measure of anxiety, are dependent on genetic background.
3.3.3.3 |. Basal stress and stress reactivity
Basal CORT (P = 1.5 × 10−10, q = 1.5 × 10−9; Figure 3A), stress-induced CORT (P = 2.7 × 10−13, q = 5.4 × 10−12) and change in CORT (P = 1.1 × 10−8, q = 7.6 × 10−8; Figure 3D) all showed significant diet-by-RIX effects. Diet had no effect on change in CORT in 4 lines (RIXs 01/11, 06/26, 03/14, 35/62). Interestingly, RIX 32/42 and RIX 04/17 females exposed to VDD showed diametrically opposed change in CORT levels. RIX 32/42 VDD-exposed females showed increased change in CORT while VDD-exposed RIX 04/17 females showed decreased change in CORT (Figure 3F). A similar opposing effect of exposure to VDD compared with Std is seen in basal CORT levels for RIX 06/26 and RIX 03/14, although this effect did not reach significance. VDD exposure in RIX 06/26 resulted in decreased basal CORT while the same dietary exposure in RIX 03/14 resulted in increased CORT (Figure 3C). Collectively, these data show that the effects of perinatal diet on basal stress and response to a stressor are also dependent on genetic background.
3.4 |. PO effects on behavior
A linear-mixed effects model was used to assess the effects of PO, by comparing reciprocal females within each RIX line. We report P and q values for PO in Table 2. RIX 32/42 was the only line that showed no effect of PO on behavioral phenotypes.
TABLE 2.
Parent-of-origin effects on behavior
| RIX 01/11 | RIX 41/51 | RIX 04/17 | RIX 23/47 | RIX 06/26 | RIX 03/14 | RIX 35/62 | RIX 32/42 | RIX 05/40 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotype | P | q | P | q | P | q | P | q | P | q | P | q | P | q | P | q | P | q |
| Weight PND 21 | .80 | .94 | .01 | .10 | 2E-05 | 3E-04 | 6E-08 | IE-06 | .03 | .12 | .55 | .78 | .47 | .56 | .20 | .83 | .05 | .14 |
| Weight PND 60 | IE-02 | .14 | .01 | .10 | IE-03 | 7E-03 | 2E-06 | 2E-05 | .11 | .27 | .86 | .89 | .47 | .56 | .32 | .83 | 2E-03 | .01 |
| OF total dist | .34 | .62 | .76 | .94 | .34 | .45 | .80 | .84 | IE-03 | .01 | .03 | .12 | .08 | .20 | .63 | .83 | .04 | .14 |
| OF % center | .71 | .91 | .43 | .78 | .21 | .35 | .26 | .64 | .070 | .20 | .05 | .15 | .12 | .28 | .40 | .83 | .14 | .27 |
| OF vert count | .02 | .15 | .02 | .10 | 5E-03 | .02 | .04 | .18 | 2E-04 | 4E-03 | .15 | .38 | .59 | .65 | .78 | .83 | .36 | .48 |
| OF boli | .61 | .91 | .27 | .68 | .03 | .07 | .65 | .81 | .38 | .55 | .49 | .75 | .47 | .56 | .85 | .85 | .15 | .28 |
| LD total dist | .10 | .29 | .27 | .68 | .62 | .68 | .71 | .83 | .20 | .37 | .08 | .24 | .04 | .16 | .54 | .83 | 2E-04 | 3E-03 |
| LD dist dark | .15 | .34 | .56 | .79 | .41 | .51 | .37 | .65 | .39 | .55 | .63 | .79 | .39 | .56 | .16 | .83 | 2E-03 | .01 |
| LD dist light | .12 | .31 | .14 | .57 | .88 | .88 | .65 | .81 | .13 | .29 | 5E-03 | .10 | .03 | .15 | .79 | .83 | .05 | .14 |
| LD transition | .32 | .62 | .59 | .79 | .26 | .40 | .39 | .65 | .44 | .59 | .03 | .12 | .03 | .15 | .35 | .83 | 6E-03 | .03 |
| LD % light | .98 | .98 | .41 | .78 | .32 | .45 | .30 | .65 | .18 | .36 | .01 | .12 | .03 | .15 | .73 | .83 | .79 | .84 |
| LD Boli | .66 | .91 | .95 | .95 | .55 | .65 | .61 | .81 | .52 | .65 | .31 | .69 | .87 | .87 | .66 | .83 | .12 | .26 |
| SIH-T1 | .73 | .91 | .25 | .68 | .02 | .06 | .90 | .90 | .84 | .84 | .89 | .89 | .26 | .44 | .24 | .83 | .65 | .82 |
| SIH-T2 | .72 | .91 | .07 | .34 | .04 | .09 | .20 | .64 | .79 | .83 | .64 | .79 | .30 | .47 | .68 | .83 | .80 | .84 |
| deltaSIH | .86 | .96 | .91 | .95 | 3E-03 | .01 | .44 | .68 | .66 | .73 | .73 | .86 | .22 | .41 | .52 | .83 | .23 | .33 |
| FST % Imb | .05 | .29 | .83 | .94 | .19 | .35 | 4E-03 | .03 | .36 | .55 | .02 | .12 | .18 | .36 | .56 | .83 | .10 | .26 |
| FST Boli | .10 | .29 | .36 | .78 | .69 | .73 | .33 | .65 | .04 | .12 | .35 | .69 | .08 | .20 | .12 | .83 | .22 | .33 |
| Basal CORT | .95 | .98 | .84 | .94 | .04 | .09 | .78 | .84 | 4E-03 | .02 | .86 | .89 | .82 | .86 | .68 | .83 | .17 | .29 |
| Stress CORT | .08 | .29 | .47 | .79 | 4E-05 | 4E-04 | .09 | .38 | 3E-03 | .02 | .45 | .75 | .02 | .15 | .17 | .83 | .90 | .90 |
| deltaCORT | .08 | .29 | .59 | .79 | IE-03 | 5E-03 | .25 | .64 | .66 | .73 | .49 | .75 | .07 | .20 | .36 | .83 | .77 | .84 |
Abbreviations: deltaCORT, Stress CORT − Basal CORT; deltaSIH, T2 − T1; Dist, distance; Imb, immobile; SIH-T1, basal temperature; SIH-T2, post-stress temperature; Vert, vertical. Significant values are bolded.
PO effects for body weight were observed in 6 of the 9 RIX (RIXs 01/11, 41/51, 04/17, 23/47, 06/26, 05/40). Five of these 6 lines (RIXs 01/11, 41/51, 04/17, 23/47, 06/26) also had a PO effect for vertical counts, or rearing, in the OF. In each case, the reciprocal group that weighed more was also the one that made more vertical movements in the OF. This might indicate that body weight influenced detection of vertical movements by the infrared sensors in the OF arena. However, we observed no significant correlation between weight at testing and OF vertical counts (r[188] = .06, P > .05; Figure S1).
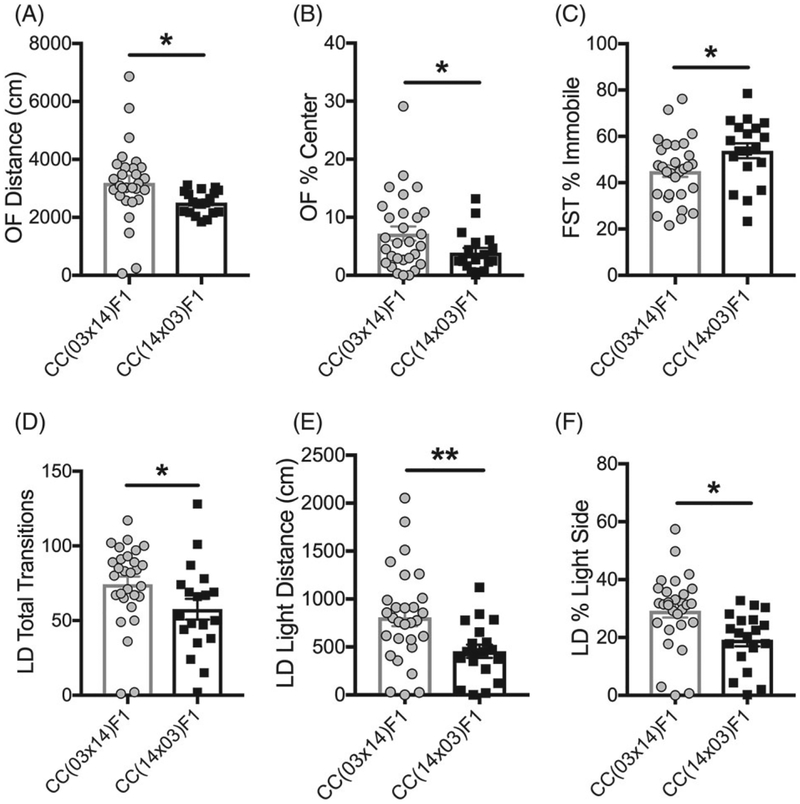
Several RIX displayed multiple PO effects across behavioral tests and are highlighted below. For example, CC(03 × 14)F1 females showed increased locomotion in the OF (Figure 4A), increased time spent in the center of the OF (Figure 4B), decreased immobility in the FST (Figure 4C) and increased transitions (Figure 4D), distance moved (Figure 4E) and time spent in the light side of the LD arena (Figure 4F) in comparison to CC(14 × 03)F1 females. These data indicate that female offspring from CC003/Unc dams have decreased anxiety- and depressive-like behavior compared with female offspring from CC014/Unc dams.
FIGURE 4.
Parent-of-origin affects anxiety- and depressive-like behaviors in RIX 03/14 females. Data points are individual animals with bars indicating means. Error bars are SEM. CC(03 × 14)F1 females (N = 29) showed (A) increased distance moved (*P = .031, q = .12), (B) increased time spent in the center of the OF (*P = .045, q = .15), (C) decreased percent immobility in the FST (*P = .02, q = .12), (D) increased transitions(*P = .026, q = .12), (E) increased distance moved on the light side (**P = .005, q = .10), and (F) increased percent time spent in the light side of the LD test(*P = .012, q = .12) in comparison to CC(14 × 03)F1 females (N = 19)
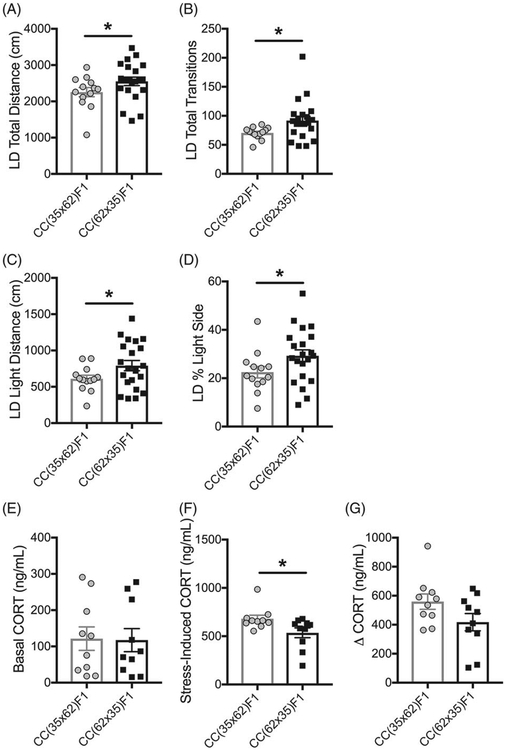
CC(62 × 35)F1 females showed increased locomotion (Figure 5A), transitions (Figure 5B), distance moved (Figure 5C) and time spent in the light side (Figure 5D) of the LD test compared with CC(35 × 62)F1 females. CC(62 × 35)F1 females also showed decreased CORT in response to a restraint stress (Figure 5F). These results indicate that female offspring from CC062/Unc dams have decreased anxiety-like behavior and are more resilient to stress compared with female offspring from CC035/Unc dams.
FIGURE 5.
Parent-of-origin affects locomotion, anxiety-like behavior and stress response in RIX 35/62 females. Data points are individual animals with bars indicating means. Error bars are SEM. CC(62 × 35)F1 females (N = 21) showed (A) increased total distance (*P = .041, q = .16), (B) increased transitions (*P = .03, q = .15), (C) increased distance on the light side (*P = .025, q = .15) and (D) increased percent time spent in the light side of the LD test (*P = .028, q = .15) compared with CC(35 × 62)F1 females (N = 13). (E) RIX 35/62 reciprocal females did not differ in basal CORT levels (P > .05). (F) CC(62 × 35)F1 females (N = 10) showed decreased CORT levels following a restraint stress (*P = .02, q = .15) which resulted in (G) decreased change in CORT compared with CC(35 × 62)F1 females (N = 10), although this effect did not reach statistical significance (P = .07). Stress response is only reported for females exposed to VDD and Std due to the low N for ME- and PD-exposed groups
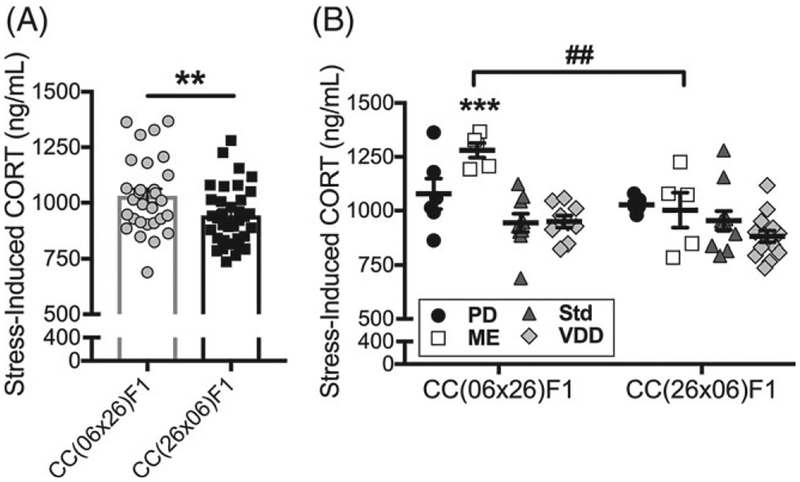
CC(06 × 26)F1 females showed increased locomotion (Figure S3A) and vertical counts in the OF (Figure S3B) and increased basal (Figure S3C) and stress-induced CORT levels (Figure S3D and Figure 6A) in comparison with CC(26 × 06)F1 females, but no reciprocal difference in change in CORT (Figure S3E). Collectively, these data indicate that female offspring from CC006/TauUnc dams have increased locomotion and exploratory behavior and a dysregulated hypothalamic-pituitary-adrenal (HPA) axis as reflected by increased basal CORT levels.
FIGURE 6.
Parent-of-origin and diet-by-PO effects on stress-induced CORT in RIX 06/26 females. (A) Data points are individual animals with bars indicating means. Error bars are SEM. There was a significant PO effect on stress-induced CORT levels in RIX 06/26 (**P = .003, q = .02) with CC(06 × 26)F1 females (N = 29) showing increased CORT compared with CC(26 × 06)F1 females (N = 35). (B) Data points shown are individual animals with mean (black bar). Error bars are SEM. There was a significant diet-by-PO effect on stress-induced CORT for RIX 06/26 (P = .018, q = .14). Post hoc analysis showed that within CC(06 × 26)F1, ME-exposed females (N = 5; ***P ≤ .001) had increased CORT compared with PD (N = 6), Std (N = 9) and VDD (N = 9) exposed females. No diet effects were observed within CC(26 × 06)F1 females (N = 5, 5, 11 and 14). This resulted in a significant difference between ME-exposed reciprocal females (##P ≤ .01)
Female offspring from CC005/TauUnc mothers exhibited increased locomotor activity as measured in both the OF and LD tests (Figure S4A–E) in comparison with female offspring from CC040/TauUnc mothers.
3.5 |. Perinatal diet interacts with PO in certain genetic backgrounds to alter body weight and behavior
We were particularly interested in assessing whether perinatal diet would induce behavioral differences between reciprocal females within a RIX. We found 18 significant diet-by-PO effects based on P value, although none were significant after multiple testing corrections (based on the q value; Table 3).
TABLE 3.
Diet-by-PO effects on behavior
| RIX 01/11 | RIX 41/51 | RIX 04/17 | RIX 23/47 | RIX 06/26 | RIX 03/14 | RIX 35/62 | RIX 32/42 | RIX 05/40 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotype | P | q | P | q | P | q | P | q | P | q | P | q | P | q | P | q | P | q |
| Weight PND 21 | .51 | .76 | .03 | .19 | .01 | .19 | .47 | .85 | .04 | .15 | .03 | .57 | .94 | .99 | .04 | .68 | .02 | .47 |
| Weight PND 60 | .03 | .32 | .24 | .46 | .65 | .76 | .18 | .85 | .04 | .15 | .34 | .77 | .37 | .70 | .56 | .71 | .92 | .97 |
| OF total dist | .56 | .76 | .09 | .26 | .07 | .26 | .44 | .85 | .77 | .82 | .99 | .99 | .81 | .99 | .17 | .68 | .79 | .97 |
| OF % center | .85 | .89 | .06 | .23 | .06 | .26 | .73 | .85 | .14 | .35 | .83 | .93 | .06 | .50 | .40 | .68 | .92 | .97 |
| OF vert count | .71 | .78 | .72 | .85 | .04 | .26 | .61 | .85 | .58 | .68 | .13 | .57 | .35 | .70 | .21 | .68 | .37 | .93 |
| OF Boli | .19 | .54 | .81 | .85 | .41 | .64 | .01 | .17 | .35 | .68 | .18 | .57 | .06 | .50 | .64 | .73 | .92 | .97 |
| LD total dist | .09 | .48 | .46 | .71 | .48 | .64 | .74 | .85 | .46 | .68 | .78 | .93 | .18 | .70 | .43 | .68 | .99 | .99 |
| LD dist dark | .10 | .48 | .78 | .85 | .91 | .96 | .73 | .85 | .61 | .68 | .82 | .93 | .08 | .50 | .54 | .71 | .65 | .97 |
| LD dist light | .12 | .49 | .28 | .46 | .10 | .28 | .49 | .85 | .18 | .40 | .87 | .93 | .22 | .70 | .51 | .71 | .67 | .97 |
| LD transition | .17 | .54 | .07 | .23 | .36 | .64 | .60 | .85 | .45 | .68 | .85 | .93 | .90 | .99 | .31 | .68 | .73 | .97 |
| LD % light | .61 | .76 | .62 | .85 | .26 | .64 | .23 | .85 | .51 | .68 | .18 | .57 | .14 | .70 | .69 | .73 | .42 | .93 |
| LD Boli | .03 | .32 | .94 | .94 | .08 | .26 | .04 | .36 | .58 | .68 | .11 | .57 | 1.00 | 1.00 | .12 | .68 | .47 | .93 |
| SIH-T1 | .63 | .76 | .19 | .46 | .90 | .96 | .56 | .85 | .02 | .14 | .75 | .93 | .48 | .74 | .44 | .68 | .21 | .85 |
| SIH-T2 | .47 | .76 | .02 | .19 | .59 | .73 | .82 | .87 | .54 | .68 | .88 | .93 | .38 | .70 | .67 | .73 | .72 | .97 |
| deltaSIH | .65 | .76 | .25 | .46 | .46 | .64 | .60 | .85 | .01 | .14 | .88 | .93 | .75 | .99 | .88 | .88 | .31 | .93 |
| FST % Imb | .35 | .71 | .74 | .85 | .05 | .26 | .91 | .91 | .53 | .68 | .25 | .63 | .88 | .99 | .15 | .68 | .07 | .70 |
| FST Boli | .51 | .76 | .24 | .46 | .96 | .96 | .20 | .85 | .07 | .23 | .82 | .93 | .31 | .70 | .44 | .68 | .12 | .78 |
| Basal CORT | .90 | .90 | .65 | .85 | .48 | .64 | .56 | .85 | .94 | .94 | .58 | .93 | .70 | .99 | .41 | .68 | .53 | .96 |
| Stress CORT | .29 | .71 | .02 | .19 | .36 | .64 | .68 | .85 | .02 | .14 | .20 | .57 | .27 | .70 | .28 | .68 | .19 | .85 |
| deltaCORT | .35 | .71 | .06 | .23 | .39 | .64 | .77 | .85 | .10 | .27 | .18 | .57 | .45 | .74 | .25 | .68 | .43 | .93 |
Abbreviations: deltaCORT, Stress CORT − Basal CORT; deltaSIH, T2 − T1; Dist, distance; Imb, immobile; SIH-T1, basal temperature; SIH-T2, post-stress temperature; Vert, vertical. Significant values are bolded.
A significant PO effect for stress-induced CORT was observed in RIX 06/26 (Figure 6A). The PO effect is driven by a significant difference between CC(06 × 26)F1 females exposed to ME relative to other diets (post hoc P ≤ .001; Figure 6B); by contrast, stress-induced CORT in CC(26 × 06)F1 females was unaffected by diet.
Eight of the significant diet-by-PO effects involved body weight at weaning or in adulthood. For 5 of the RIX (RIXs 41/51, 04/17, 03/14, 32/42, 05/40), there was a significant diet-by-PO effect on body weight at weaning. For example, the PO effect on weaning weight in RIX 04/17 (Figure 7A) was due to increased weight in VDD- and Std-exposed CC(04 × 17)F1 females compared with ME and Std (post hoc P ≤ .01), a diet effect not observed in CC(17 × 04) F1 females (Figure 7B). Only one RIX showed a significant diet-by-PO effect on adult body weight (Figure 7C,D) only. VDD- and Std-exposed CC(11 × 01)F1 females weighed more than CC(01 × 11)F1 females (post hoc P ≤ .02; Figure 7D). Only one RIX showed a significant diet-by-PO effect on body weight at both weaning and in adulthood. CC(06 × 26)F1 females weighed more than CC(26 × 06)F1 females at weaning (Figure 7E). This effect was due to a difference in the ME-exposed groups across reciprocals (post hoc P ≤ .01; Figure 7F). In adulthood, CC(06 × 26)F1 females still weighed more than CC(26 × 06)F1 females although this effect was no longer significant (Figure 7G). However, there is still a significant diet-by-PO effect, and the distribution of diet effects on adult weight is similar to the distribution at weaning (Figure 7H). Collectively, these data show that body weight is strongly influenced by perinatal diet in a PO-specific manner across different genetic backgrounds.
FIGURE 7.
Perinatal diet interacts with parent-of-origin to affect body weight in multiple RIX lines. Data points are individual animals with bars indicating means. Error bars are SEM. There was a (A) PO effect (***P = 2.0 × 10−5, q = 3.0 × 10−4; N = 44, 39) and (B) diet-by-PO effect (P = .01, q = .19) on weight at weaning in RIX 04/17. Post hoc showed that CC(04 × 17)F1 PD (***P ≤ .001) and ME (**P ≤ .01) exposed females weighed less than Std and VDD groups (N = 12, 6, 12, 14) and within CC(17 × 04)F1, only PD-exposed females weighed less (**P ≤ .004; N = 9, 2, 15, 13). Reciprocal females in the Std and VDD exposure groups also differed (###P ≤ .001). For weight in adulthood in RIX 01/11, there was a (C) PO effect (**P = .01, q = .14; N = 54, 63) and (D) diet-by-PO effect (P = .03, q = .32). Post hoc showed that CC(11 × 01)F1 females exposed to PD (**P ≤ .002) and ME (*P ≤ .03) weighed less than Std and VDD groups (N = 12, 14, 17, 11) while within CC(01 × 11)F1 there was no effect of diet (N = 19, 13, 11, 20). Std (#P = .03) and VDD (#P = .02) exposure also differed across reciprocal females. In RIX 06/26, there was a PO effect on (E) weight at weaning (*P = .03, q = .12; N = 36, 30) and a similar pattern in (G) adulthood (P > .05) and a diet-by-PO effect on (F) weight at weaning (P = .04, q = .15) and (H) in adulthood (P = .04, q = .15). F, Post hoc showed that CC(26 × 06)F1 females exposed to PD (***P ≤ .001) and ME (**P ≤ .003) weighed less than Std and VDD groups (N = 6, 5, 11, 14) while in CC(06 × 26)F1, only PD-exposed females weighed less (**P ≤ .006; N = 6, 5, 10, 9). Across reciprocal females, ME-exposed groups differed (##P = .01). (H) Post hoc tests showed that CC(26 × 06)F1 females exposed to PD (*P ≤ .04) weighed less than Std and VDD groups but within CC(06 × 26)F1, PD-exposed females only weigh less than VDD (**P = .003). CC(26 × 06)F1 females exposed to ME and Std also differed (*P = .02)
3.6 |. Variance explained by PO, diet and diet-by-PO effects
Within each RIX line and for all 20 phenotypes measured, we assessed the percent variance explained by the effect of PO, diet and diet-by-PO (Table S7). Of particular interest in this study was the variance due to PO effects as PO effects on behavioral phenotypes are seldom reported. For phenotypes that exhibited a significant PO effect, variance explained ranged from 4% to 59%. Body weight at weaning in RIX 23/47 had the highest variance explained by PO while basal CORT in RIX 04/17 had the lowest percent variance explained by PO. For any given phenotype, however, variance explained depended on genetic background (Table S7).
4 |. DISCUSSION
4.1 |. Overall conclusions
We used reciprocal F1 females derived from 10 pairs of CC strains to investigate the effects of perinatal diet exposure, genetic background, PO and their interactions on 20 phenotypes. Genetic background significantly affected all phenotypes, whereas perinatal diet exposure affected fewer phenotypes—namely body weight, basal body temperature, anxiety-like behavior and stress response—but all in a manner dependent on genetic background. We also found significant PO effects in 8 of the 9 RIX lines and for a variety of behavioral phenotypes. To further investigate PO effects, we examined the interaction of PO with diet, identifying a small number of diet-by-PO effects; notably, body weight showed consistent diet-by-PO effects across 7 of the 9 RIX lines. Our data show that rodent behaviors that model psychiatric disorders are affected by genetic background, PO and perinatal diet, as well as by interactions among these factors. Below we compare our main findings with the current literature and highlight several interesting results that we believe warrant further investigation.
4.2 |. Genetic background effects
Significant effects of RIX were observed on all 20 phenotypes. This result was not surprising given that these phenotypes have a genetic component and the genetic backgrounds we tested covered a broad range of genetic diversity. The CC was designed to maximize genetic diversity and our choice of CC strain pairs in the 10 RIX lines was intended to maximize genetic heterozygosity so as to include novel combinations of alleles from the 3 major subspecies of M. musculus. The genetic diversity of the CC population resulted in an expanded phenotypic range relative to that observed in standard inbred strains (ie, Mouse Diversity Panel).57–61
The wide range of phenotypes in the CC has also led to the development of stable models of human disease not previously observed in traditional inbred strains.62 Our RIX lines also included clear phenotypic outliers. For example, RIX 41/51 and RIX 04/17 F1 mice were outliers for novelty-induced locomotion as measured in the OF and LD tests (Figure S5). Based on the literature linking novelty-induced locomotion with addiction-related behaviors,63 we are currently investigating these 2 lines in rodent models of addiction.
4.3 |. Diet effects
Although we chose perinatal diet as our environmental challenge based on existing literature in humans from the Dutch Hunger Winter and Chinese Famine,2,5,10 we acknowledge that we are not modeling human perinatal nutritional exposures per se. Rather, we are using these experimental diets as tools to induce behavioral changes based on evidence from published data in animal models,20,23,32,64,65 as well as data from our laboratory (Oreper, Valdar and Tarantino, personal communication). Our study was designed to compare phenotypes across diets and across genetic background rather than comparing each diet to a standard or treated animals to controls.
4.3.1 |. Effects of methyl enrichment
DNA methylation is an important mechanism for regulation of gene expression and is dependent on the availability of methyl donors mainly from diet.66 Disruption in DNA methylation has been implicated as a mechanism for increased risk for complex diseases, including psychiatric disorders.67 In our study, we exposed animals to methyl donor (choline) enrichment (Table S2). Perinatal exposure to ME resulted in lower body weight at weaning and adulthood, decreased basal body temperature and increased anxiety-like behavior in the LD test.
Choline supplementation during development is thought to be neuroprotective68 and is commonly used in animal models to ‘rescue’ the adverse effects of perinatal challenges such as iron deficiency,69 stress70 and alcohol exposure.71 Prenatal choline supplementation has also been shown to improve negative behavioral phenotypes in mouse models of neurodevelopmental disorders such as Rett Syndrome (Mecp272), autism (BTBR T+ltpr3tf/J; Reference 39) and Down Syndrome (Ts65Dn73).
We found that exposure to perinatal ME increased anxiety-like behavior in the LD test. Our findings are in contrast to studies in rats in which choline supplementation decreased anxiety in the LD65 and OF tests.64 However, the increased anxiety-like behavior we observed in ME-exposed females was specific to certain RIX lines, or genetic backgrounds (Table S5). Very few studies have examined the effect of methyl supplementation in different genetic backgrounds. Notably, one study comparing BTBR T+ltpr3tf/J (a mouse model of autism-related behaviors) to C57BL/6J mice did report differences in these 2 strains in response to perinatal choline supplementation on anxiety-like behavior in the elevated plus maze.39
4.3.2 |. Effects of PD
Proteins play a key role in brain development as they serve as key neurotransmitters and hormones. PD during the perinatal period has been used to induce intrauterine growth restriction and alter behaviors that model psychiatric disorders.74 In our study, perinatal PD led to decreased body weight at weaning and in adulthood. The findings of decreased weight at weaning are consistent with previous reports in rats27 and mice.29,30,32 However, the persistence of weight deficits into adulthood has not been consistent across studies.27,32
We also observed increased stress reactivity in response to perinatal PD—a finding that is consistent with previous studies in rats exposed to PD in utero.33,75 Previous studies have also reported increased depressive-like behaviors in response to perinatal PD.29–32 We did not observe any changes in depressive-like behavior in the FST in response to PD exposure. Nor did PD exposure alter anxiety-like behavior in the OF, LD and SIH assays. This finding is not particularly surprising given that the effects of exposure to PD on anxiety-like behavior have been equivocal across published studies.30,33,75–78 We did observe a significant diet-by-RIX effect on all 3 measures of anxiety-like behavior and stress response (Table 1). These data in combination with conflicting results in the literature support a hypothesis that the effects of exposure to PD on anxiety and stress response are dependent on genetic background.
4.3.3 |. Effects of VDD
Vitamin D is well known for its role in calcium homeostasis and bone formation, but it is also a neuroactive steroid involved in brain development and function.79 Exposure to VDD during development is hypothesized to increase risk for schizophrenia.80 As such, the effects of VDD during the perinatal period have been studied in rodents using behavioral models of schizophrenia.
In the present study, we observed very few overall effects of exposure to perinatal VDD. We considered the possibility that VDD diet exposure was not reducing endogenous vitamin D by the amount expected. It has been shown that exposure to unfiltered fluorescent lighting can induce production of endogenous Vitamin D3 in laboratory animals81 and we did not filter the lighting in our vivarium. However, we did observe significant decreases in serum vitamin D levels in VDD-exposed dams from several of the CC strains used in this study.48
Previous studies have assessed the role of VDD exposure on the stress axis in rodents and have reported either no effects22 or increased CORT levels.82 In our study, exposure to VDD induced RIX-specific alterations in basal and stress-induced CORT (Figure 3C, F) supporting a role for vitamin D in moderating the stress system.
We also considered the possibility that the behaviors examined in this study are not particularly sensitive to perinatal VDD exposure. However, the literature does report hyperlocomotion in rats exposed to VDD20–22,24 and we did not observe an overall or RIX-specific effect of VDD exposure on locomotion in either the OF or LD test.
4.3.4 |. Perinatal diet effects depend on genetic background
Our data highlight the importance of genetic background on the expression of body weight, basal temperature, anxiety-like behavior and stress response following exposure to perinatal dietary manipulations. We acknowledge that our assessment was limited to a small number of behaviors and did not include several that have been previously reported to be altered by perinatal diet (ie, learning and memory tests, response to psychostimulants, sensorimotor gating). Regardless of the limitations in behavioral assays employed, our data highlight the need to consider genetic background when investigating the role of perinatal diet exposure.
4.4 |. PO effects on behavior and physiology
We found PO effects in 8 of the 9 RIX lines for a number of behavioral phenotypes, body weight and basal body temperature. Although there are many studies that utilize F1 mice, few have used reciprocal F1 hybrids and even fewer have assessed the effects of PO on behavioral phenotypes. There is, however, a vast literature assessing the role of known imprinted genes on growth, physiology and behavior via reverse genetic approaches such as overexpression or gene knockouts (KO) (for a thorough review see Reference 18). Relevant findings from these studies will be discussed in relation to PO effects we observed.
4.4.1 |. Body weight
PO effects were observed for body weight in 6 RIX lines and in 4 of these lines, body weight differences persisted from weaning into adulthood. This result is not surprising, given the vast amount of literature implicating imprinted genes on growth.18 Additional studies are necessary to determine whether the specific genes responsible for body weight PO effects in our study are known imprinted genes or new genes. Of note, in this study we assessed body weight but no other measures of growth (ie, body length). Follow-up studies using both body mass and body size measurements are necessary to determine whether strain, diet or PO effects on body weight were due to the changes in the actual size of the mice (ie, length) or reflect only weight differences (ie, overweight or underweight).
4.4.2 |. Locomotor behavior
We found PO effects on locomotor behavior in 4 of the RIX lines. In 2 of these RIXs, we also observed PO effects on body weight, although the relationship between body weight and locomotion is opposite in the 2 RIX lines (RIX 06/26 vs RIX 05/40, Table 2). Hyperactivity, body weight and metabolism have been linked previously in studies manipulating the imprinted genes Asb483 and Kcnq1.84,85 These RIX lines can be examined in follow-up studies to determine whether locomotor activity and body weight PO effects share a common genetic basis.
4.4.3 |. Anxiety- and depressive-like behaviors and stress response
We found a consistent PO effect among RIX 03/14 reciprocal females on anxiety- and depressive-like behavior (Figure 4). Interestingly, these behavioral phenotypes are very similar to that observed in KO mice for the imprinted gene, Sgce. Sgce KO mice display increased anxiety-like and exploratory behavior in the OF and increased depressive-like behavior in the FST.86 There is also an abundance of evidence from the literature that establishes a role for imprinted genes such as Sgce, Nesp55, Htr2a, Peg3 and Snord116 in mediating anxiety-like behavior.87–90 Studies also show a role of Nesp55 in mediating addiction-related behaviors.89,91 It would be interesting to assess PO effects on addiction-related behaviors in RIX 03/14 females.
RIX 35/62 reciprocal females displayed PO effects on anxiety-like behavior and stress response. Two other RIX also displayed PO effects on measures of stress response. A previous study using F1 reciprocals of spontaneously hypertensive rat and Wistar-Kyoto rats reported a PO effect on the cardiovascular activity in response to an acute stressor.92 A recent study also reported PO effects on stress reactivity using reciprocal F1 of C57BL/6J and 129S1/SvlmJ mice.93 These studies, together with our findings, indicate that further work is needed to examine the HPA axis in regards to imprinted gene functions and other effects due to PO.
4.5 |. Perinatal diet interaction with PO
We identified perinatal diet-by-PO effects on body weight, exploratory behavior, basal temperature, anxiety- and depressive-like behavior and stress response. PO effects on body weight were the most severely affected by perinatal diet, as evidenced by our observation of diet-by-PO effects in 7 of the RIX lines (Table 2, Figure 7).
Although we cannot directly compare our diet-by-PO findings on behavior with previously published studies due to methodological differences, there are a few studies that have reported changes in imprinted gene expression due to perinatal diet alterations, which might indicate a mechanism for these effects.32,40 Vucetic et al32 found that perinatal PD caused a reduction in the methylation at the promotor of the imprinted gene Cdkn1c, along with a correlated increase in its expression in the prefrontal cortex, nucleus accumbens and hypothalamus. Cdkn1c is involved in differentiation and specification of dopamine neurons and the authors also reported an alteration in dopamine-mediated behaviors in these mice. The link between exposure to PD, imprinted gene expression differences and dopamine-mediated behaviors provides a potential avenue for future mechanistic studies in our RIX lines.
Collectively, our findings support the role of PO on body weight and behavioral phenotypes that model psychiatric disorders. It is interesting that RIX 32/42 was the only RIX that showed no PO effects on any behavioral phenotype measured. One possible explanation for this result is lower genetic diversity at imprinted loci in the CC strains used to generate RIX 32/42 mice. Importantly, the PO effects we observed are dependent on genetic background. Of note, our use of the term PO effect, by definition, assumes an interaction with genetic background. We assessed the variance explained due to PO to obtain a sense of the importance of these effects on the phenotypes measured. On average, variance explained by PO was less than that explained by strain. In fact variance explained by PO effects depended on both strain and the specific behavioral assay, ranging from 4% to 59% (with the highest value for body weight). Nonetheless, it is important to note that within RIX comparisons describe differences between 2 experimental groups that are genetically identical and differ only in the parental origin of the genome and the maternal environment; therefore, even small effects can be biologically relevant and useful for developing interventions and treatments.
Although we focused our discussion on imprinted genes, we acknowledge that they are likely not the sole source of the observed PO effects. Future studies examining gene expression in these RIX lines will likely help identify specific genes responsible. We expect that some of these genes will be at known imprinted loci, while others will be novel in the context of behavioral effects. Additionally, PO effects can be due to maternal effects independent of imprinting,94 and further studies will be necessary to disentangle their respective contributions to the PO effects observed in this study. Lastly, our study illustrates how the CC and RIX lines derived from the CC can provide an ideal experimental model for jointly examining genetic background and PO, being reproducible, genetically diverse and uniquely designed to support systems genetics studies.
Supplementary Material
ACKNOWLEDGMENTS
Funding for this study was provided by R01MH100241 from NIMH to W.V. and L.M.T. D.O. also received partial support from a PhRMA Foundation predoctoral informatics fellowship.
Funding information
National Institute of Mental Health, Grant/Award number: R01MH100241;
Pharmaceutical Research and Manufacturers of America Foundation
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Lee R, Avramopoulos D. Chapter 1 – Introduction to epigenetics in psychiatry Epigenetics in Psychiatry. Boston, MA: Academic Press; 2014:3–25. [Google Scholar]
- 2.Brown AS, Susser ES. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr Bull. 2008;34:1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoek HW, Brown AS, Susser E. The Dutch famine and schizophrenia spectrum disorders. Soc Psychiatry Psychiatr Epidemiol. 1998;33: 373–379. [DOI] [PubMed] [Google Scholar]
- 4.Hoek HW, Susser E, Buck KA, Lumey LH, Lin SP, Gorman JM. Schizoid personality disorder after prenatal exposure to famine. Am J Psychiatry. 1996;153:1637–1639. [DOI] [PubMed] [Google Scholar]
- 5.St Clair D, Xu MQ, Wang P, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA. 2005;294:557–562. [DOI] [PubMed] [Google Scholar]
- 6.Susser E, Neugebauer R, Hoek HW, et al. Schizophrenia after prenatal famine – further evidence. Arch Gen Psychiatry. 1996;53:25–31. [DOI] [PubMed] [Google Scholar]
- 7.Xu MQ, Sun WS, Liu BX, et al. Prenatal malnutrition and adult schizophrenia: further evidence from the 1959–1961 Chinese famine. Schizophr Bull. 2009;35:568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown AS, Susser ES, Hoek HW, Neugebauer R, Lin SP, Gorman JM. Schizophrenia and affective disorders after prenatal famine. Biol Psychiatry. 1996;39:173–173. [Google Scholar]
- 9.Brown AS, Susser ES, Lin SP, Neugebauer R, Gorman JM. Increased risk of affective-disorders in males after 2nd-trimester prenatal exposure to the Dutch-Hunger-Winter of 1944–45. Br J Psychiatry. 1995; 166:601–606. [DOI] [PubMed] [Google Scholar]
- 10.Brown AS, van Os J, Driessens C, Hoek HW, Susser ES. Further evidence of relation between prenatal famine and major affective disorder. Am J Psychiatry. 2000;157:190–195. [DOI] [PubMed] [Google Scholar]
- 11.Stein AD, Pierik FH, Verrips GHW, Susser ES, Lumey LH. Maternal exposure to the Dutch famine before conception and during pregnancy quality of life and depressive symptoms in adult offspring. Epidemiology. 2009;20:909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franzek EJ, Sprangers N, Janssens ACJW, Van Duijn CM, De Wetering BJMV. Prenatal exposure to the 1944–45 Dutch ‘hunger winter’ and addiction later in life. Addiction. 2008;103:433–438. [DOI] [PubMed] [Google Scholar]
- 13.Ishida M, Moore GE. The role of imprinted genes in humans. Mol Asp Med. 2013;34:826–840. [DOI] [PubMed] [Google Scholar]
- 14.Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–17049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobi EW, Lumey L, Talens RP, et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet. 2009;18:4046–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies W, Isles AR, Wilkinson LS. Imprinted gene expression in the brain. Neurosci Biobehav Rev. 2005;29:421–430. [DOI] [PubMed] [Google Scholar]
- 17.Williamson CM, Blake A, Thomas S, et al. Mouse Imprinting Data and References. Oxfordshire, UK: MRC Harwell; 2013. [Google Scholar]
- 18.Cleaton MA, Edwards CA, Ferguson-Smith AC. Phenotypic outcomes of imprinted gene models in mice: elucidation of pre- and postnatal functions of imprinted genes. Annu Rev Genomics Hum Genet. 2014; 15:93–126. [DOI] [PubMed] [Google Scholar]
- 19.Kesby JP, Cui X, O’Loan J, McGrath JJ, Burne TH, Eyles DW. Developmental vitamin D deficiency alters dopamine-mediated behaviors and dopamine transporter function in adult female rats. Psychopharmacology. 2010;208:159–168. [DOI] [PubMed] [Google Scholar]
- 20.Burne TH, Becker A, Brown J, Eyles DW, Mackay-Sim A, McGrath JJ. Transient prenatal vitamin D deficiency is associated with hyperlocomotion in adult rats. Behav Brain Res. 2004;154:549–555. [DOI] [PubMed] [Google Scholar]
- 21.Burne TH, O’Loan J, McGrath JJ, Eyles DW. Hyperlocomotion associated with transient prenatal vitamin D deficiency is ameliorated by acute restraint. Behav Brain Res. 2006;174:119–124. [DOI] [PubMed] [Google Scholar]
- 22.Eyles DW, Rogers F, Buller K, et al. Developmental vitamin D (DVD) deficiency in the rat alters adult behaviour independently of HPA function. Psychoneuroendocrinology. 2006;31:958–964. [DOI] [PubMed] [Google Scholar]
- 23.Harms LR, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH. Developmental vitamin D deficiency alters adult behaviour in 129/SvJ and C57BL/6J mice. Behav Brain Res. 2008;187:343–350. [DOI] [PubMed] [Google Scholar]
- 24.Kesby JP, Burne TH, McGrath JJ, Eyles DW. Developmental vitamin D deficiency alters MK 801-induced hyperlocomotion in the adult rat: an animal model of schizophrenia. Biol Psychiatry. 2006;60:591–596. [DOI] [PubMed] [Google Scholar]
- 25.Harms LR, Turner KM, Eyles DW, Young JW, McGrath JJ, Burne TH. Attentional processing in C57BL/6J mice exposed to developmental vitamin D deficiency. PLoS One. 2012;7:e35896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner KM, Young JW, McGrath JJ, Eyles DW, Burne TH. Cognitive performance and response inhibition in developmentally vitamin D (DVD)-deficient rats. Behav Brain Res. 2013;242:47–53. [DOI] [PubMed] [Google Scholar]
- 27.Palmer AA, Brown AS, Keegan D, et al. Prenatal protein deprivation alters dopamine-mediated behaviors and dopaminergic and glutamatergic receptor binding. Brain Res. 2008;1237:62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer AA, Printz DJ, Butler PD, Dulawa SC, Printz MP. Prenatal protein deprivation in rats induces changes in prepulse inhibition and NMDA receptor binding. Brain Res. 2004;996:193–201. [DOI] [PubMed] [Google Scholar]
- 29.Belluscio LM, Alberca CD, Pregi N, Canepa ET. Altered gene expression in hippocampus and depressive-like behavior in young adult female mice by early protein malnutrition. Genes Brain Behav. 2016; 15:741–749. [DOI] [PubMed] [Google Scholar]
- 30.Belluscio LM, Berardino BG, Ferroni NM, Ceruti JM, Canepa ET. Early protein malnutrition negatively impacts physical growth and neurological reflexes and evokes anxiety and depressive-like behaviors. Physiol Behav. 2014;129:237–254. [DOI] [PubMed] [Google Scholar]
- 31.de Godoy MA, de Souza AS, Lobo MA, et al. Effects of protein restriction during gestation and lactation on cell proliferation in the hippocampus and subventricular zone: functional implications. Protein restriction alters hippocampal/SVZ cell proliferation. Brain Res. 2013; 1496:10–27. [DOI] [PubMed] [Google Scholar]
- 32.Vucetic Z, Totoki K, Schoch H, et al. Early life protein restriction alters dopamine circuitry. Neuroscience. 2010;168:359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reyes-Castro LA, Rodriguez JS, Charco R, et al. Maternal protein restriction in the rat during pregnancy and/or lactation alters cognitive and anxiety behaviors of female offspring. Int J Dev Neurosci. 2012;30:39–45. [DOI] [PubMed] [Google Scholar]
- 34.Valdomero A, Bussolino DF, Orsingher OA, Cuadra GR. Perinatal protein malnutrition enhances rewarding cocaine properties in adult rats. Neuroscience. 2006;137:221–229. [DOI] [PubMed] [Google Scholar]
- 35.Ferguson SA, Berry KJ, Hansen DK, Wall KS, White G, Antony AC. Behavioral effects of prenatal folate deficiency in mice. Birth Defects Res A Clin Mol Teratol. 2005;73:249–252. [DOI] [PubMed] [Google Scholar]
- 36.Konycheva G, Dziadek MA, Ferguson LR, et al. Dietary methyl donor deficiency during pregnancy in rats shapes learning and anxiety in offspring. Nutr Res. 2011;31:790–804. [DOI] [PubMed] [Google Scholar]
- 37.Berrocal-Zaragoza MI, Sequeira JM, Murphy MM, et al. Folate deficiency in rat pups during weaning causes learning and memory deficits. Br J Nutr. 2014;112:1323–1332. [DOI] [PubMed] [Google Scholar]
- 38.Harms LR, Cowin G, Eyles DW, Kurniawan ND, McGrath JJ, Burne TH. Neuroanatomy and psychomimetic-induced locomotion in C57BL/6J and 129/X1SvJ mice exposed to developmental vitamin D deficiency. Behav Brain Res. 2012;230:125–131. [DOI] [PubMed] [Google Scholar]
- 39.Langley EA, Krykbaeva M, Blusztajn JK, Mellott TJ. High maternal choline consumption during pregnancy and nursing alleviates deficits in social interaction and improves anxiety-like behaviors in the BTBR T+Itpr3tf/J mouse model of autism. Behav Brain Res. 2015;278: 210–220. [DOI] [PubMed] [Google Scholar]
- 40.Waterland RA, Lin JR, Smith CA, Jirtle RL. Post-weaning diet affects genomic imprinting at the insulin-like growth factor 2 (Igf2) locus. Hum Mol Genet. 2006;15:705–716. [DOI] [PubMed] [Google Scholar]
- 41.Churchill GA, Airey DC, Allayee H, et al. The collaborative cross, a community resource for the genetic analysis of complex traits. Nat Genet. 2004;36:1133–1137. [DOI] [PubMed] [Google Scholar]
- 42.Srivastava A, Morgan AP, Najarian ML, et al. Genomes of the mouse collaborative cross. Genetics. 2017;206:537–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts A, Pardo-Manuel de Villena F, Wang W, McMillan L, Threadgill DW. The polymorphism architecture of mouse genetic resources elucidated using genome-wide resequencing data: implications for QTL discovery and systems genetics. Mamm Genome. 2007; 18:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Threadgill DW, Churchill GA. Ten years of the collaborative cross. Genetics. 2012;190:291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collaborative Cross C The genome architecture of the collaborative cross mouse genetic reference population. Genetics. 2012;190: 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welsh CE, Miller DR, Manly KF, et al. Status and access to the collaborative cross population. Mamm Genome. 2012;23:706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crowley JJ, Zhabotynsky V, Sun W, et al. Analyses of allele-specific gene expression in highly divergent mouse crosses identifies pervasive allelic imbalance. Nat Genet. 2015;47:353–U102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xue J, Schoenrock SA, Valdar W, Tarantino LM, Ideraabdullah FY. Maternal vitamin D depletion alters DNA methylation at imprinted loci in multiple generations. Clin Epigenetics. 2016;8:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adriaan Bouwknecht J, Olivier B, Paylor RE. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev. 2007;31:41–59. [DOI] [PubMed] [Google Scholar]
- 50.Harrell FE, Dupont WCFC & et al. , (2017) Hmisc: Harrell Miscellaneous. p. R package [Google Scholar]
- 51.Wei T & Simko V (2016) corrplot: Visualization of a Correlation Matrix. p. R package. [Google Scholar]
- 52.Bates D, Machler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 53.Kuznetsova A, Brockhoff PB & Christensen RHB (2016) lmerTest: in Linear Mixed Effects Models. p. R package. [Google Scholar]
- 54.Bass JD, Dabney A & Robinson D (2015) qvalue: Q-value estimation for false discovery rate control. R package. [Google Scholar]
- 55.Storey JD. A direct approach to false discovery rates. J R Stat Soc SerB. 2002;64:479–498. [Google Scholar]
- 56.Shorter JR, Odet F, Aylor DL, et al. Male infertility is responsible for nearly half of the extinction observed in the mouse collaborative cross. Genetics. 2017;206:557–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gralinski LE, Ferris MT, Aylor DL, et al. Genome wide identification of SARS-CoV susceptibility loci using the collaborative cross. PLoS Genet. 2015;11:e1005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levy R, Mott RF, Iraqi FA, Gabet Y. Collaborative cross mice in a genetic association study reveal new candidate genes for bone microarchitecture. BMC Genomics. 2015;16:1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mosedale M, Kim Y, Brock WJ, et al. Editor’s highlight: candidate risk factors and mechanisms for Tolvaptan-induced liver injury are identified using a collaborative cross approach. Toxicol Sci. 2017;156: 438–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venkatratnam A, Furuya S, Kosyk O, et al. S highlight: collaborative cross mouse population enables refinements to characterization of the variability in Toxicokinetics of trichloroethylene and provides genetic evidence for the role of PPAR pathway in its oxidative metabolism. Toxicol Sci. 2017;158:48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vered K, Durrant C, Mott R, Iraqi FA. Susceptibility to Klebsiella pneumoniae infection in collaborative cross mice is a complex trait controlled by at least three loci acting at different time points. BMC Genomics. 2014;15:865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rogala AR, Morgan AP, Christensen AM, et al. The collaborative cross as a resource for modeling human disease: CC011/Unc, a new mouse model for spontaneous colitis. Mamm Genome. 2014;25: 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. [DOI] [PubMed] [Google Scholar]
- 64.Glenn MJ, Adams RS, McClurg L. Supplemental dietary choline during development exerts antidepressant-like effects in adult female rats. Brain Res. 2012;1443:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Plyusnina IZ, Os’kina IN, Shchepina OA, Prasolova LA, Trut LN. A maternal methyl-containing diet alters learning ability in the Morris swimming test in adult rats. Neurosci Behav Physiol. 2007;37: 425–428. [DOI] [PubMed] [Google Scholar]
- 66.Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr. 2002;132:2333S–2335S. [DOI] [PubMed] [Google Scholar]
- 67.Grayson DR, Guidotti A. The dynamics of DNA methylation in schizophrenia and related psychiatric disorders. Neuropsychopharmacology. 2013;38:138–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bekdash RA. Choline and the brain: an epigenetic perspective. Adv Neurobiol. 2016;12:381–399. [DOI] [PubMed] [Google Scholar]
- 69.Kennedy BC, Dimova JG, Siddappa AJ, Tran PV, Gewirtz JC, Georgieff MK. Prenatal choline supplementation ameliorates the long-term neurobehavioral effects of fetal-neonatal iron deficiency in rats. J Nutr. 2014;144:1858–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schulz KM, Pearson JN, Gasparrini ME, et al. Dietary choline supplementation to dams during pregnancy and lactation mitigates the effects of in utero stress exposure on adult anxiety-related behaviors. Behav Brain Res. 2014;268:104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomas JD, Idrus NM, Monk BR, Dominguez HD. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res A Clin Mol Teratol. 2010;88:827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nag N, Berger-Sweeney JE. Postnatal dietary choline supplementation alters behavior in a mouse model of Rett syndrome. Neurobiol Dis. 2007;26:473–480. [DOI] [PubMed] [Google Scholar]
- 73.Moon J, Chen M, Gandhy SU, et al. Perinatal choline supplementation improves cognitive functioning and emotion regulation in the Ts65Dn mouse model of Down syndrome. Behav Neurosci. 2010;124: 346–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tarantino L, Reyes T, Palmer A. Animal models of prenatal protein malnutrition relevant for schizophrenia In: Brown A, Patterson P, eds. The Origins of Schizophrenia. New York, NY: Columbia University Press; 2012:300–334. [Google Scholar]
- 75.Reyes-Castro LA, Rodriguez JS, Rodriguez-Gonzalez GL, et al. Pre- and/or postnatal protein restriction developmentally programs affect and risk assessment behaviors in adult male rats. Behav Brain Res. 2012;227:324–329. [DOI] [PubMed] [Google Scholar]
- 76.Almeida SS, Tonkiss J, Galler JR. Prenatal protein malnutrition affects exploratory behavior of female rats in the elevated plus-maze test. Physiol Behav. 1996;60:675–680. [DOI] [PubMed] [Google Scholar]
- 77.Francolin-Silva AL, da Silva Hernandes A, Fukuda MT, Valadares CT, Almeida SS. Anxiolytic-like effects of short-term postnatal protein malnutrition in the elevated plus-maze test. Behav Brain Res. 2006; 173:310–314. [DOI] [PubMed] [Google Scholar]
- 78.Furuse T, Miyake K, Kohda T, et al. Protein-restricted diet during pregnancy after insemination alters behavioral phenotypes of the progeny. Genes Nutr. 2017;12(1)1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–105. [DOI] [PubMed] [Google Scholar]
- 80.Does McGrath J. ‘imprinting’ with low prenatal vitamin D contribute to the risk of various adult disorders? Med Hypotheses. 2001;56: 367–371. [DOI] [PubMed] [Google Scholar]
- 81.McDowell LR. Vitamins in Animal Nutrition Comparative Aspects to Human Nutrition. San Diego, CA: Academic Press; 1989. [Google Scholar]
- 82.Tesic D, Hawes JE, Zosky GR, Wyrwoll CS. Vitamin D deficiency in BALB/c mouse pregnancy increases placental transfer of glucocorticoids. Endocrinology. 2015;156:3673–3679. [DOI] [PubMed] [Google Scholar]
- 83.Li JY, Chai BX, Zhang W, Wang H, Mulholland MW. Expression of ankyrin repeat and suppressor of cytokine signaling box protein 4 (Asb-4) in proopiomelanocortin neurons of the arcuate nucleus of mice produces a hyperphagic, lean phenotype. Endocrinology. 2010; 151:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boini KM, Graf D, Hennige AM, et al. Enhanced insulin sensitivity of gene-targeted mice lacking functional KCNQ1. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1695–R1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Casimiro MC, Knollmann BC, Ebert SN, et al. Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange-Nielsen syndrome. Proc Natl Acad Sci U S A. 2001;98:2526–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yokoi F, Dang MT, Li J, Li Y. Myoclonus, motor deficits, alterations in emotional responses and monoamine metabolism in epsilon-sarcoglycan deficient mice. J Biochem. 2006;140:141–146. [DOI] [PubMed] [Google Scholar]
- 87.Champagne FA, Curley JP, Swaney WT, Hasen NS, Keverne EB. Paternal influence on female behavior: the role of Peg3 in exploration, olfaction, and neuroendocrine regulation of maternal behavior of female mice. Behav Neurosci. 2009;123:469–480. [DOI] [PubMed] [Google Scholar]
- 88.Ding F, Li HH, Zhang S, et al. SnoRNA Snord116 (Pwcr1/MBII-85) deletion causes growth deficiency and hyperphagia in mice. PLoS One. 2008;3:e1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Plagge A, Isles AR, Gordon E, et al. Imprinted Nesp55 influences behavioral reactivity to novel environments. Mol Cell Biol. 2005;25: 3019–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weisstaub NV, Zhou M, Lira A, et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313: 536–540. [DOI] [PubMed] [Google Scholar]
- 91.Dent CL, Humby T, Lewis K, et al. Impulsive choices in mice lacking imprinted Nesp55. Genes Brain Behav. 2016;15:693–701. [DOI] [PubMed] [Google Scholar]
- 92.Woodworth CH, Knardahl S, Sanders BJ, Kirby RF, Johnson AK. Dam strain affects cardiovascular reactivity to acute stress in BHR. Physiol Behav. 1990;47:139–144. [DOI] [PubMed] [Google Scholar]
- 93.Chan JC, Houghton AB, Bale TL. Strained in planning your mouse background? Using the HPA stress axis as a biological readout for back-crossing strategies. Neuropsychopharmacology. 2017;42:1749–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.