Abstract
α oscillations (8–14 Hz) greatly influence brain activity, yet we generally do not experience them consciously: the world does not appear to oscillate. Dedicated strategies must exist in the brain to prevent these oscillations from disrupting normal processing. Could suitable stimuli fool these strategies and lead to the conscious experience of our own brain oscillations? We describe and explore a novel illusion in which the center of a static wheel stimulus (with 30–40 spokes) is experienced as flickering when viewed in the visual periphery. The key feature of this illusion is that the stimulus fluctuations are experienced as a regular and consistent flicker, which our human observers estimated at ∼9 Hz during a psychophysical matching task. Correspondingly, the occipital α rhythm of the EEG was the only oscillation that showed a time course compatible with the reported illusion: when α amplitude was strong, the probability of reporting illusory flicker increased. The peak oscillatory frequency for these flicker-induced modulations was significantly correlated, on a subject-by-subject basis, with the individual α frequency measured during rest, in the absence of visual stimulation. Finally, although the effect is strongest during eye movements, we showed that stimulus motion relative to the retina is not necessary to perceive the illusion: the flicker can also be perceived on the afterimage of the wheel, yet by definition this afterimage is stationary on the retina. We conclude that this new flickering illusion is a unique way to experience the α rhythms that constantly occur in the brain but normally remain unnoticed.
Introduction
Neuronal oscillations, such as the α rhythm (Berger, 1929) (8–14 Hz), pervade the brain and influence sensory processing (Makeig et al., 2002; Thut et al., 2006; Hanslmayr et al., 2007; van Dijk et al., 2008). In particular, recent findings showed that visual processing is more efficient at certain phases of the ongoing α rhythm than others (Busch et al., 2009; Mathewson et al., 2009; Busch and VanRullen, 2010; Dugué et al., 2011; Scheeringa et al., 2011). It is surprising to realize, therefore, that the sensory outcome itself is unaffected: we do not perceive the world as oscillating. The brain must have developed strategies to conceal the consequences of oscillations from our perception. For instance, the brain could rely on one or more of the following mechanisms: (1) conscious experience may arise from brain regions far removed from those where α is produced and found to influence sensory processing; (2) attention, a major factor in the selection of information for awareness, is known to decrease the amplitude of α oscillations (Worden et al., 2000; Thut et al., 2006; Klimesch et al., 2007), thereby dampening their potential perceptual consequences; and (3) finally, one could envision that any recurring “temporal gaps” in perception produced by the periodic pulses of α inhibition (Jensen and Mazaheri, 2010) could be actively “filled in” using immediately preceding and/or following visual information to even out our conscious perceptual experience (similarly to the filling-in of the blind spot in early representations of visual space). Regardless of which of these (or other) strategies is actually used by the brain to conceal perceptual effects of α rhythms, it is tempting to ask whether suitable stimuli could fool these strategies and lead to the conscious experience of our own brain oscillations.
Here we report on a novel illusion in which the center of a stationary wheel made up of ∼30–40 spokes appears to flicker vividly and regularly (Fig. 1). The flicker occurs most strongly during small eye movements performed with the stimulus in the periphery. However, we show that stimulus motion relative to the retina is not crucial to perceive the illusory flicker: flicker can also be experienced on the afterimage of the stimulus; yet by definition, this afterimage is stationary on the retina. Using psychophysics, we demonstrate that the illusory flicker frequency peaks within the α range (∼9 Hz). To address the neuronal basis of the illusion, we recorded EEG while observers (N = 20) performed smooth pursuit eye movements, following with their gaze a slowly rotating dot around the stationary wheel. The flicker illusion was maximal for specific eye positions around the wheel. The only EEG frequency band that displayed a compatible time course was the α rhythm (8–14 Hz) over occipital electrodes: an increased α magnitude specifically coincided with the occurrence of illusory flicker. Furthermore, the frequency that was maximally modulated during illusory perception was significantly correlated with the individual α frequency on a subject-by-subject basis. We conclude that this new flickering illusion represents a unique way to consciously experience the brain's ongoing α rhythm.
Figure 1.
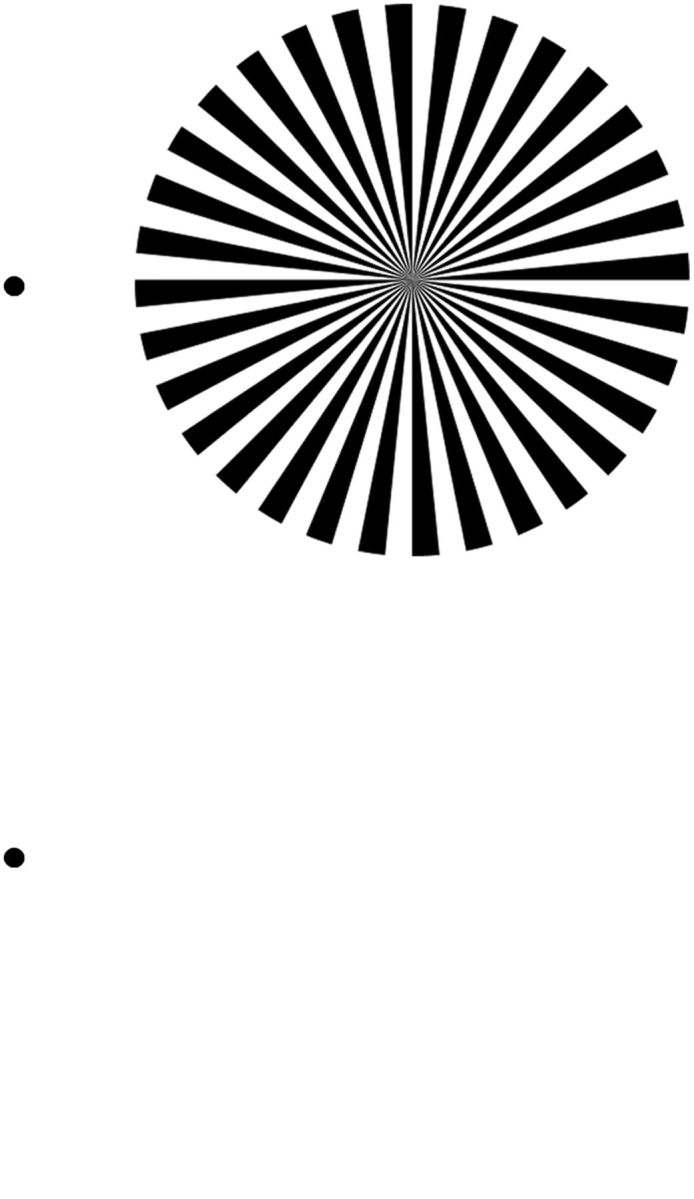
The flickering wheel illusion. The static wheel stimulus produces an impression of flicker when it is observed in the visual periphery. Small eye movements, as when reading these lines, can enhance the illusory effect. The flicker can also be perceived in the afterimage of the stimulus. To experience this, after fixating the top-left black dot for ∼10 s, switch your gaze to the lower dot: an impression of flicker should be fleetingly present within the wheel's afterimage on the right.
Materials and Methods
Subjects.
Ten volunteers (3 female, mean age 29.2 ± 4.8 years) participated in the psychophysical experiment and 21 (6 female; mean age 26.2 ± 4.0 years; one left handed) in the EEG experiment. All subjects showed normal or corrected to normal eye function. One participant was excluded from the psychophysical experiment because his mean reported flicker intensity was >2 SDs away from the group average and one subject was excluded from the EEG experiment because >50% of the EEG data contained artifacts.
Stimuli.
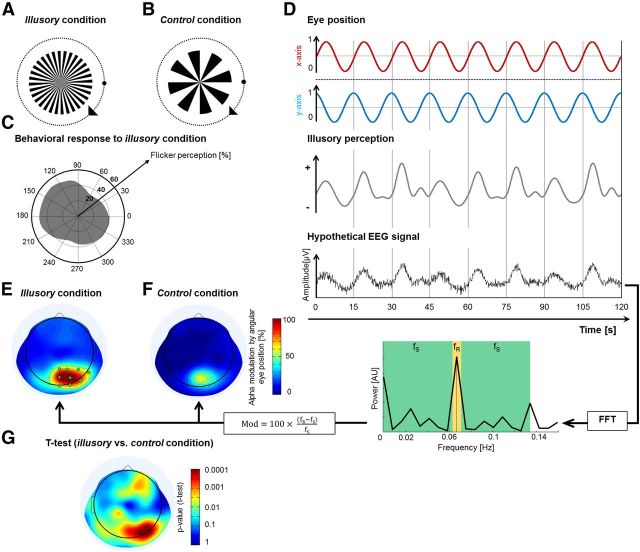
Stimuli were presented at 57 cm distance using a desktop computer (2.09 GHz Intel processor, Windows XP) with a cathode ray monitor (resolution: 800 × 600 pixels; refresh rate: 100 Hz) on a gray background (luminance: 11.5 cd/m2). Stimuli were designed and presented via the Psychophysics Toolbox (Brainard, 1997) running in MATLAB (MathWorks). For the matching task, the wheel stimuli (radius: 5 degrees of visual angle) were presented with different contrast levels (20%, 40%, 60%, 80%, and 100% Michelson contrast using uncorrected CRT γ values, corresponding to Michelson luminance contrast of 39%, 65%, 81%, 92%, and 96%, respectively) and spatial frequencies with 8, 16, 32, or 64 dark and bright alternating spokes. In the EEG smooth pursuit task, the fixation dot revolved (15 s/revolution) at 13 degrees from the wheel center. For the EEG smooth pursuit and the afterimage tasks, stimuli (radius: 7 degrees of visual angle) of the illusory condition were adjusted for optimal flicker perception (100% contrast and 32 spoke sectors), whereas stimuli of the control condition showed a different spatial frequency (8 spoke sectors) and did not lead to illusory perception. The 32 spoke wheels had a lower spatial frequency cutoff of 0.73 cycles per degree at their outer edge, this value increasing to infinity toward the wheel center.
Behavioral data analysis.
Behavioral data were analyzed using a one-way ANOVA for the matching task and a circular r test: Raleigh test for circular uniformity; circular statistics toolbox from MATLAB (Berens, 2009) for the smooth pursuit task.
EEG data acquisition and analysis.
EEG and EOG were recorded at 1024 Hz using a Biosemi system (64 active and 3 ocular electrodes) and downsampled offline to 128 Hz for data analysis via the MATLAB toolbox, eeglab (Delorme and Makeig, 2004). Individual electrode data were visually inspected, and channel data containing artifacts were interpolated by the mean of adjacent electrodes. Time-frequency transformations were generated over all channels using eeglab (Delorme and Makeig, 2004) with a function akin to a wavelet transform, starting with 3 cycles at 2 Hz and increasing linearly to 37.5 cycles at 50 Hz. To determine which oscillatory bands contributed to changes in brain activity during the perception of illusory flicker (smooth pursuit paradigm, illusory condition), a Fourier transform was performed on the amplitude envelope of the time-frequency transformed signals, separately for each frequency (which were then grouped into standard EEG frequency bands: δ, θ, α, β, γ). The resulting Fourier spectral amplitude at the frequency of revolution of the eyes around the wheel (0.067 Hz) was normalized by the amplitudes at surrounding frequencies (from 0 to 0.133 Hz, excluding the eye revolution frequency itself), revealing for each oscillatory band the amount of amplitude modulation by eye position (and, thus, by illusory flicker). The same procedure was also applied to EEG data of the control condition. To compare amplitude modulations during illusory and control conditions, we used a two-tailed paired t test (α level = 0.05). The time course of the amplitude modulation for illusory and control conditions was also analyzed for electrodes within a specific ROI (see Fig. 3E, green). Trials were epoched, each epoch corresponding to one revolution of the fixation dot around the wheel (i.e., 15 s); these epochs were aligned so as to be centered on the eye position of maximal flicker perception (averaged over all subjects).
Figure 3.
EEG correlates of illusory flicker in a smooth pursuit paradigm. A, In the “illusory” condition, the stimulus consisted of 32 black-and-white spoke sectors. Subjects (N = 20) followed the fixation dot around the wheel using smooth pursuit eye movements and pressed a button when they experienced illusory flicker. B, In the “control” condition, the stimulus contained only 8 black-and-white spoke sectors; although the task instructions were the same, no illusory flicker was reported. C, Distribution of flicker responses (in the illusory condition) as a function of the angular position of the eyes around the wheel (pooled over all 20 subjects). There was a significant tendency to perceive stronger illusory flicker at upper left positions: p < 10−6 (Rayleigh test for circular uniformity). D, Our analysis procedure is illustrated here with hypothetical data. As the eyes revolve around the wheel every 15 s, the perception of flicker rises and falls in each revolution (as demonstrated in C). We hypothesized that the relevant brain activity (e.g., the amplitude of a specific brain oscillation) would show a similar pattern with an increase once per revolution. In that case, a fast Fourier transform analysis would reveal a peak in the signal's power spectrum at the eye revolution frequency (1/15 s = 0.067 Hz). The amplitude value at that frequency (fR, marked in yellow) was compared with the surrounding frequencies (fS, marked in green) to reveal the percentage of signal modulation by angular eye position: Mod = 100*(fR − fS)/fS. E, Modulation of α amplitude by angular eye position. The analysis described in D was applied to the time course of fluctuations of oscillatory amplitude in the α band. The strong modulation over occipital electrodes suggests that occipital α amplitude changes systematically as a function of eye position, and thus as a function of flicker perception. F, The same analysis reveals weaker modulations for the control stimulus. G, Direct contrast between illusory and control conditions (two-tailed paired t test) reveals highly significant differences (p < 0.0001) of α modulation over occipital electrodes.
To evaluate whether the frequency band modulated during illusory perception was correlated to the individual α rhythm, we first determined, for each subject, the peak modulation frequency of these time-frequency representations (we applied a fast Fourier transform on the time dimension and used the first frequency component as a measure of illusory modulation, then extracted the peak frequency of the resulting amplitude spectrum). Fourteen of 20 subjects provided a clear individual peak frequency for these flicker-induced modulations (amplitude was 1.5 times as high as the amplitude at surrounding frequencies: 5–15 Hz). To determine the individual α frequency of each subject, we analyzed EEG data recorded during rest (eyes opened, data recorded from electrode Oz; epochs of variable duration from 3 to 60 s). Data were downsampled to 256 Hz, visually inspected for artifacts, and bandpass filtered between 0.3 Hz and 30 Hz. Power spectra were computed using Welch's modified periodogram method in MATLAB (Welch, 1967); we extracted the peak in the α frequency band (8–14 Hz), retaining only peak amplitudes that were at least 1.5 times as high as the average amplitude at surrounding frequencies (5–15 Hz). Ten of the previously retained 14 subjects yielded an exploitable individual α peak frequency.
Eye tracker data acquisition and analysis.
To precisely control eye movements during the smooth pursuit task, we performed a control experiment with a subgroup (N = 3) of our subjects where we simultaneously recorded EEG and eye movements using an eye tracker (IView Hi-Speed eye tracker; SensoMotoric Instruments). Participants were performing the same smooth pursuit task as explained above. Eye position data were recorded at 1250 Hz and downsampled offline to 128 Hz for data analysis. Data were then analyzed for artifacts and eye movements that exceeded a fixed limit: all data points that reflected eye positions deviating >3 degrees of visual angle from the fixation dot were flagged for rejection. Finally, we repeated the previously described EEG time-frequency analysis for these subjects after rejecting from our EEG data the correspondingly flagged time points.
Results
Psychophysical measurements
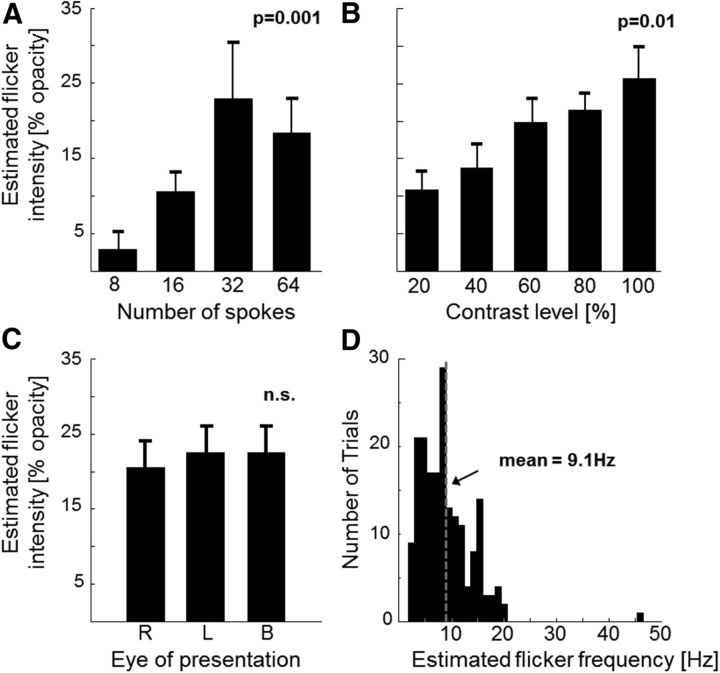
Our first experiment examined the influence of various psychophysical parameters on the perception of the illusion. We used different spatial frequencies (8, 16, 32, and 64 spoke sectors) and contrast levels (20%, 40%, 60%, 80%, and 100% contrast) of the stimulus pattern, and also tested whether binocular vision is needed to perceive the illusion. Participants (N = 9) followed a moving fixation dot by making small successive saccades from left to right along the horizontal midline of the screen.
Meanwhile, in one part of the screen (8 degrees above or below the horizontal midline), a static wheel stimulus (“test” stimulus, 5 degrees radius) was continuously presented. On the opposite side of the screen (below or above fixation) was a comparison stimulus (“reference”) comprising a circular pattern (same size as the “test”) textured with a black-and-white checkerboard, whose center flickered regularly; the flicker, created by superimposing a gray Gaussian window (SD 2.3 degrees) onto the stimulus center and sinusoidally modulating its opacity, was adjustable both in temporal frequency and intensity (i.e., opacity) via button presses. Participants were instructed to match the frequency and intensity of the artificial flicker in the reference stimulus with the illusory flicker (if any) perceived in the test stimulus. We found the greatest illusory effect for a stimulus pattern built up of 32 spoke sectors (Fig. 2A; one-way ANOVA, F(3,32) = 6.76, p = 0.001: 32 spoke and 64 spoke wheels induced significantly stronger illusion than 8 spokes) and having maximal contrast (Fig. 2B; one-way ANOVA, F(4,40) = 3.71, p = 0.01: 100% contrast wheels led to significantly stronger illusion than 20%).
Figure 2.
Psychophysical matching task. A–C, Estimated flicker intensity (% opacity of the sinusoidally modulated gray field superimposed at the center of the reference stimulus) for different spatial frequencies of the test pattern (i.e., different number of spokes within the wheel) (A), different contrast levels (B), and right-monocular (R), left-monocular (L), and binocular (B) viewing conditions (using a stimulus with 32 spoke sectors and 100% contrast) (C). p values are obtained from one-way ANOVAs. n.s., Not significant. D, Distribution of estimated frequencies of the perceived illusory flicker over trials. We analyzed for each subject (N = 9) and experimental condition with optimal flicker perception (32 spoke wheel and 100% contrast level) the three trials with strongest flicker intensity. A–C, Error bars indicate SEM.
Furthermore, the magnitude of the illusion was comparable whether the wheel was viewed with one or both eyes (Fig. 2C; one-way ANOVA, F(2,21) = 0.09, p > 0.05). Finally, the average flicker frequency reported by the participants was 9.1 ± 0.38 Hz (mean ± SEM; Fig. 2D). According to these psychophysical results, we used a 32 spoke wheel with maximal contrast in the following experiments.
EEG α oscillations correlate with illusory flicker
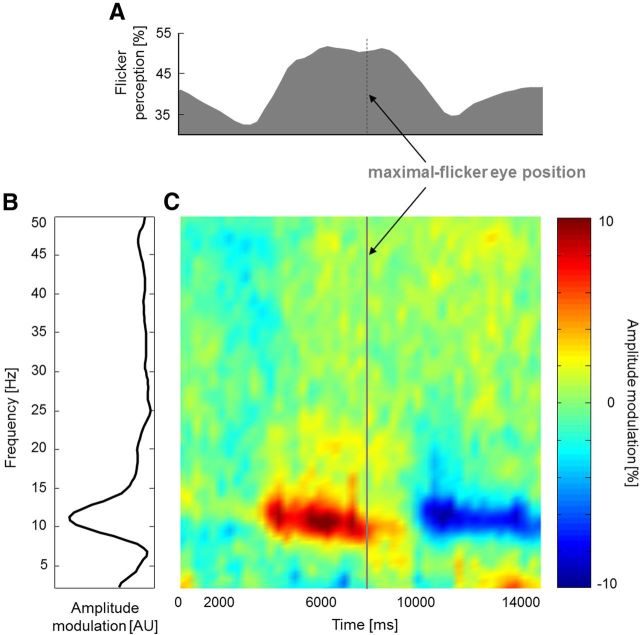
To investigate the neural correlates of illusory flicker, we recorded EEG continuously while observers performed smooth pursuit eye movements around the centered wheel stimulus (radius: 7 degrees of visual angle; Fig. 3A). The same procedure was also applied using a control stimulus that did not produce illusory flicker (Fig. 3B). We did not use saccadic eye movements in this case because they tend to generate large-scale EEG artifacts. Each trial lasted 2 min, during which the ocular pursuit target revolved around the wheel 8 times (revolution duration: 15 s). Subjects (N = 20) reported illusory flicker perception at any moment during the trial by pressing (at flicker onset) and releasing (at flicker offset) a button. The average probability of reporting flicker (in the “illusory” condition) was significantly higher for eye positions in the upper left quadrant of the screen (Fig. 3C; Rayleigh test for circular uniformity: p < 10−6). This nonuniform distribution of illusory percepts suggested the following analysis to determine any potential oscillatory correlate of the illusion (Fig. 3D): separately for each of the classical EEG frequency bands (δ= 2–4 Hz, θ = 4–8 Hz, α = 8–14 Hz, β = 14–30 Hz, and γ = 30–50 Hz), the amplitude envelope of oscillatory signals was extracted by means of a wavelet-based time-frequency transform; this envelope was then subjected to a Fourier transform to derive its amplitude spectrum. We reasoned that any oscillatory signal reflecting the perception of illusory flicker would display a peak in this amplitude spectrum at the revolution frequency of the eye (i.e., 1/15 cycles per second). The peak in the amplitude spectrum was measured as the percentage increase at the eye revolution frequency (fR = 0.067 Hz) with respect to an average of surrounding frequencies (from 0 to 0.133 Hz, excluding the eye revolution frequency itself). The α band displayed a clear response peak (∼100% increase) over occipital electrodes during the illusory condition (Fig. 3E); this peak was much decreased in the control condition (Fig. 3F), so that the difference between the two conditions was highly significant over occipital electrodes (Fig. 3G; two-tailed paired t test, p < 0.0001). The occipital topography, along with the absence of a response peak over frontal and ocular electrodes, suggests that the observed α modulation is truly related to the perception of flicker, rather than to the movements of the eyes.
In contrast, there was no measurable occipital response in other frequency bands (δ, θ, β, and γ). To summarize, the α rhythm was the only oscillation to be reliably modulated at the frequency of revolution of the eyes around the wheel and, thus, at the frequency at which the flickering illusion itself tended to recur during each trial. A similar conclusion was reached by observing the time-frequency representation of occipital EEG activity (Fig. 3E, electrodes of interest marked in green) during the course of an averaged 15 s smooth pursuit revolution epoch (Fig. 4).
Figure 4.
α fluctuations are positively related to illusory flicker. A, Percentage of reported flicker perception during one averaged smooth pursuit revolution epoch; the epoch was centered on the time of maximal illusory perception (N = 20). B, Frequency spectrum of amplitude modulation (9 selected occipital electrodes; Fig. 3E, green). C, Time course of amplitude modulations (same ROI) at each frequency (y-axis) during an averaged smooth pursuit revolution epoch (same x-axis as in A; N = 20). For each frequency, the modulation is computed as a percentage of deviation from the average amplitude over the entire epoch.
For increased readability, we aligned the epoch so that it was centered on the eye position that maximized the illusory flicker (averaged over all subjects). Not only was α the only reliably modulated frequency, but its modulation was also positively related to the flicker illusion: the likelihood of perceived flicker increased when α amplitude was high and decreased when it was low. We compared on a subject-by-subject basis the frequency of maximal modulation by the illusory flicker with the individual α frequency (measured during rest, in the absence of visual stimulation; see Materials and Methods). Over the group of 10 subjects for whom we could reliably determine both the individual α frequency and the individual frequency of maximal modulation by the illusory flicker, we found a strongly significant correlation between the two measures (p = 0.004, r = 0.813). In conclusion, the α rhythm appears to be the sole viable neural correlate of the flickering wheel illusion.
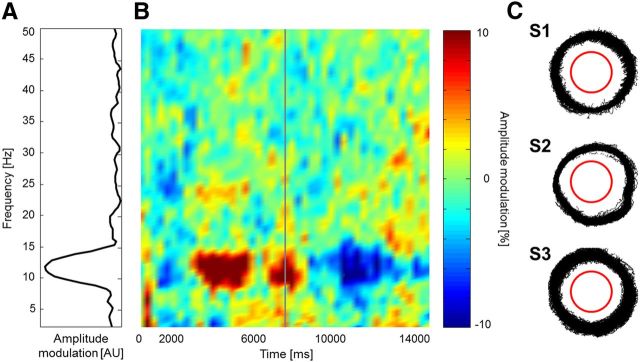
In a control experiment performed by a subgroup of subjects (N = 3), we repeated the smooth pursuit task while recording eye movements using an eye tracker. This was done to ensure that participants were effectively pursuing the fixation dot with their gaze. After rejecting epochs of EEG data showing high deviations of eye position from the fixation dot (>3 degrees visual angle), we repeated the EEG time-frequency analysis as above. The time-frequency decomposition in Figure 5 shows results highly compatible with those of the original experiment: α activity was positively related to the perceived illusory flicker.
Figure 5.
Smooth pursuit experiment with eye movement recordings. Three subjects repeated the previous experiment while their eye movements were continuously monitored. We performed the same analysis as in Figure 4, after discarding any time points with eye movements deviating by >3° from the fixation point. A, Frequency spectrum of amplitude modulation (9 selected occipital electrodes, as in Fig. 4B). B, Time course of amplitude modulations (same ROI) at each frequency (y-axis) during an averaged smooth pursuit revolution epoch (as in Fig. 4C). C, Superimposed eye traces recorded during the smooth pursuit task for each of the three participants (after discarding any time points with eye movements deviating by >3° from the fixation point). The red outline represents the outer edge of the wheel stimulus.
Is retinal motion of the wheel necessary?
Finally, we tested the role of any eye movements in the generation of the illusion: is the displacement of the stimulus pattern on the retina a necessary condition for flicker perception, or could the eye movements serve a more indirect role, for example, by enhancing or phase-resetting the α rhythm (Mulholland and Evans, 1965; Dewan, 1967; Fenwick and Walker, 1968; Fourment et al., 1976) that subsequently produces illusory flicker? Figure 1 illustrates that the flicker can also be experienced in the afterimage of the wheel stimulus; because the afterimage is, by definition, stabilized on the retina, this observation would tend to favor the second alternative. We tested this observation systematically on all 20 participants. They were asked to fixate the center of the screen. To the left or right of fixation (eccentricity: 10 degrees of visual angle), a wheel stimulus (radius: 7 degrees of visual angle) was presented for a fixed 6 s interval, so as to ensure adaptation; then the wheel disappeared, and another wheel simultaneously reappeared on the other side of fixation for another 6 s period, and so on. In each period, participants were instructed to pay attention to the afterimage (i.e., the side where the wheel had just disappeared) and report illusory flicker perception via button press (pressing when the illusion started, and releasing when it stopped). Flicker could be reported either on the side of the afterimage or on the side of the physically present stimulus (or both; two separate response buttons were provided). Two-thirds of the trials were performed with the illusory and one-third with the control stimulus (Fig. 3A,B). A total of 82.5 ± 5.0% (mean ± SEM) of all illusory trials showed responses to flicker perception in the afterimage (flicker perception period ≥ 100 ms). The control stimulus did not lead to illusory perception.
To summarize, the frequent perception of illusory flicker in the wheel's afterimage implies that displacements of the wheel on the retina are not strictly necessary to produce the illusion. This finding thus rules out eye movements (e.g., saccades and microsaccades) as a direct cause of the illusion, as well as any direct contribution from accommodation fluctuations (Alpern, 1958; Campbell et al., 1959; Kotulak and Schor, 1986; Winn and Gilmartin, 1992; Gray et al., 1993), small changes of the shape of the lens that distort the retinal image. This does not preclude, however, an indirect role for eye movements (or accommodation responses) in the flickering effect (e.g., by modulating the phase or amplitude of α rhythms in such a way that would eventually result in the perception of illusory flicker).
Discussion
Several other illusory effects have been described previously where a static pattern (Pirenne et al., 1958; Gregory, 1993; Troncoso et al., 2008), often under the influence of eye movements or accommodation fluctuations, appears to undergo dynamic changes. For example, our stimulus is a modified version of the well-known “Mackay rays” pattern, a wheel with ∼100–200 spokes that has been reported to produce slowly drifting and rotating illusory circles in its afterimage (MacKay, 1957). Other examples include Leviant's “Enigma” figure (Leviant, 1996; Kumar and Glaser, 2006; Troncoso et al., 2008) (based on Mackay rays), the scintillating luster illusion (Pinna et al., 2002) and the pursuit-pursuing illusion (Ito, 2012) (both based on Ehrenstein figures), the peripheral drift illusion (Fraser and Wilcox, 1979; Faubert and Herbert, 1999), the scintillating grid (Schrauf et al., 1997; VanRullen and Dong, 2003) (based on the Hermann grid), or the Ouchi illusion (Hine et al., 1997; Khang and Essock, 1997). Although it is likely that our flickering wheel illusion is related to one or more of these well-known effects and could thus share the same neuronal basis, what sets our illusion apart is the regular and cyclic (“flickering”) nature of the percept. This unique feature gave us the possibility to explore potential oscillatory correlates of the effect, which we identified in the α band.
In the perceptual matching task, participants estimated the flicker frequency in the α band (∼9 Hz; Fig. 2D). However, the distribution of reported frequencies was rather broad, with many reports occurring at lower frequencies (e.g., in the θ band, 4–8 Hz). We think that this may be explained by the intrinsic difficulty of the perceptual matching task. For example, previous studies have shown a general tendency for observers to underestimate perceived temporal frequency in the visual periphery (Yo and Wilson, 1993).
In the smooth pursuit task, EEG correlates of illusory flicker were again observed in the α band (Figs. 4 and 5). The peak of α activity, however, was not in perfect temporal alignment with the maximal strength of the illusion but occurred 1–2 s earlier. At least two factors may contribute to this temporal lag. First, the perception of the illusion could not be instantaneously reported by the participants because of the latency in the motor response; in addition, participants may have responded conservatively and waited before reporting a flicker percept (or its disappearance) when they were not fully confident in their perception. Second, one could also envision that illusory flicker may only become consciously visible after a minimal integration period of high α power has occurred; our results would then indicate that this integration period is on the order of 1–2 s.
Is the α rhythm the source or merely the consequence of the perceptual flicker, or is it both? The presence of similar, albeit weaker, α fluctuations when the smooth pursuit task is performed around a control stimulus that produces no illusion (Fig. 3F) indicates that these fluctuations can occur independently of the flicker (Mulholland and Evans, 1965; Dewan, 1967; Fenwick and Walker, 1968): they are not merely a consequence of the illusion. On the other hand, the increase of said α fluctuations when viewing the illusory stimulus (Figs. 3E, 4, and 5), together with the fact that our observers consistently placed the frequency of perceived flicker within the α band (Fig. 2D), suggests that the flicker illusion could “reverberate” and thereby enhance the existing α oscillations, much in the same way as a physically flickering stimulus ∼10 Hz evokes a steady-state response at the same frequency (Regan, 1966; Herrmann, 2001). Therefore, we speculate that α band oscillations are both a cause and a consequence of illusory flicker: they are necessary for flicker to arise (but certainly not sufficient; an appropriate stimulus pattern is also required), and they are also enhanced by this perceived flicker through reverberation mechanisms (VanRullen and Macdonald, 2012).
Not every geometric stimulus pattern can resonate with α oscillations and induce an oscillatory perception; this effect seems to be limited to sunburst or wheel patterns within a precise range of radial frequencies (between 30 and 40 spokes; Fig. 2A). What could be the relation specifically linking this type of geometric pattern to α oscillations? Interestingly, there are previous reports of visual hallucinations resembling geometric radial figures that can be induced by stroboscopic visual stimulation (i.e., an actual flicker) at a frequency ∼10 Hz (Shevelev et al., 2000; ter Meulen et al., 2009). The underlying mechanisms are little understood (Rule et al., 2011) but could rely on a correspondence between the spatial organization of visual cortex (retinotopy, cortical magnification, lateral connections) and the temporal dynamics of neuronal information propagation (neuronal time constants, conduction delays). A similar logic may be applied to explain the enhancement of α oscillatory activity upon presentation of our geometric stimulus pattern. Once this α activity reaches a critical threshold, the rapid alternation of favorable and less favorable phases for sensory processing (Busch et al., 2009; Mathewson et al., 2009) produces a “pulsed-inhibition” (Jensen and Mazaheri, 2010) that can become visible as a regular flicker in the center of the wheel.
Although the precise mechanisms linking the spatial wheel pattern to the temporal fluctuations at ∼10 Hz still remain to be elucidated, our report clearly implies that the illusion is a phenomenological counterpart of the α rhythm. α oscillations are constantly in your brain, affecting the way it processes visual inputs, but, unless you are a frequent LSD user (Dubois and VanRullen, 2011), you never consciously noticed them—until now.
Footnotes
This work was supported by Agence Nationale de la Recherche Grant 06JCJC-0154 and a European Young Investigator Award to R.V.
The authors declare no competing financial interests.
References
- Alpern M. Variability of accommodation during steady fixation at various levels of illuminance. J Opt Soc Am. 1958;48:193–197. doi: 10.1364/JOSA.48.000193. [DOI] [PubMed] [Google Scholar]
- Berens P. CircStat: a MATLAB Toolbox for Circular Statistics. J Stat Softw. 2009;31:1–21. [Google Scholar]
- Berger H. Über das Elektrenkephalogramm des Menschen. Arch Psychiatrie Nervenkrankheiten. 1929;87:527–570. doi: 10.1007/BF01797193. [DOI] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Busch NA, VanRullen R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proc Natl Acad Sci U S A. 2010;107:16048–16053. doi: 10.1073/pnas.1004801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. J Neurosci. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell FW, Robson JG, Westheimer G. Fluctuations of accommodation under steady viewing conditions. J Physiol. 1959;145:579–594. doi: 10.1113/jphysiol.1959.sp006164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dewan EM. Occipital α rhythm eye position and lens accommodation. Nature. 1967;214:975–977. doi: 10.1038/214975a0. [DOI] [PubMed] [Google Scholar]
- Dubois J, VanRullen R. Visual trails: do the doors of perception open periodically? PLoS Biol. 2011;9:e1001056. doi: 10.1371/journal.pbio.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugué L, Marque P, VanRullen R. Transcranial magnetic stimulation reveals attentional feedback to area V1 during serial visual search. PLoS One. 2011;6:e19712. doi: 10.1371/journal.pone.0019712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert J, Herbert AM. The peripheral drift illusion: a motion illusion in the visual periphery. Perception. 1999;28:617–621. doi: 10.1068/p2825. [DOI] [PubMed] [Google Scholar]
- Fenwick PB, Walker S. The effects of eye position on the α rhythm. Electroencephalogr Clin Neurophysiol. 1968;25:508. [PubMed] [Google Scholar]
- Fourment A, Calvet J, Bancaud J. Electrocorticography of waves associated with eye movements in man during wakefulness. Electroencephalogr Clin Neurophysiol. 1976;40:457–469. doi: 10.1016/0013-4694(76)90076-6. [DOI] [PubMed] [Google Scholar]
- Fraser A, Wilcox KJ. Perception of illusory movement. Nature. 1979;281:565–566. doi: 10.1038/281565a0. [DOI] [PubMed] [Google Scholar]
- Gray LS, Winn B, Gilmartin B. Effect of target luminance on microfluctuations of accommodation. Ophthalmic Physiol Opt. 1993;13:258–265. doi: 10.1111/j.1475-1313.1993.tb00468.x. [DOI] [PubMed] [Google Scholar]
- Gregory RL. A comment: MacKay rays shimmer due to accommodation changes. Proc Biol Sci. 1993;253:123. doi: 10.1098/rspb.1993.0090. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Aslan A, Staudigl T, Klimesch W, Herrmann CS, Bäuml KH. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37:1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Herrmann CS. Human EEG responses to 1–100 Hz flicker: resonance phenomena in visual cortex and their potential correlation to cognitive phenomena. Exp Brain Res. 2001;137:346–353. doi: 10.1007/s002210100682. [DOI] [PubMed] [Google Scholar]
- Hine T, Cook M, Rogers GT. The Ouchi illusion: an anomaly in the perception of rigid motion for limited spatial frequencies and angles. Percept Psychophys. 1997;59:448–455. doi: 10.3758/BF03211911. [DOI] [PubMed] [Google Scholar]
- Ito H. Illusory object motion in the centre of a radial pattern: the Pursuit-Pursuing illusion. Iperception. 2012;3:59–87. doi: 10.1068/i0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory α activity: gating by inhibition. Front Hum Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khang BG, Essock EA. A motion illusion from two-dimensional periodic patterns. Perception. 1997;26:585–597. doi: 10.1068/p260585. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG α oscillations: the inhibition-timing hypothesis. Brain Res Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kotulak JC, Schor CM. Temporal variations in accommodation during steadystate conditions. J Opt Soc Am A. 1986;3:223–227. doi: 10.1364/JOSAA.3.000223. [DOI] [PubMed] [Google Scholar]
- Kumar T, Glaser DA. Illusory motion in Enigma: a psychophysical investigation. Proc Natl Acad Sci U S A. 2006;103:1947–1952. doi: 10.1073/pnas.0510236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviant I. Does “brain power” make Enigma spin? Proc R Soc Lond B Biol Sci. 1996;263:997–1001. doi: 10.1098/rspb.1996.0147. [DOI] [Google Scholar]
- Mackay DM. Some further visual phenomena associated with regular patterned stimulation. Nature. 1957;180:1145–1146. doi: 10.1038/1801145a0. [DOI] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. To see or not to see: prestimulus α phase predicts visual awareness. J Neurosci. 2009;29:2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland T, Evans CR. An unexpected artefact in the human electroencephalogram concerning the α rhythm and the orientation of the eyes. Nature. 1965;207:36–37. doi: 10.1038/207036a0. [DOI] [PubMed] [Google Scholar]
- Pinna B, Spillmann L, Ehrenstein WH. Scintillating lustre and brightness induced by radial lines. Perception. 2002;31:5–16. doi: 10.1068/p3281. [DOI] [PubMed] [Google Scholar]
- Pirenne MH, Compbell FW, Robson JG, MacKay DM. Moving visual images produced by regular stationary patterns. Nature. 1958;181:362–363. doi: 10.1038/181362a0. [DOI] [PubMed] [Google Scholar]
- Regan D. Some characteristics of average steady-state and transient responses evoked by modulated light. Electroencephalogr Clin Neurophysiol. 1966;20:238–248. doi: 10.1016/0013-4694(66)90088-5. [DOI] [PubMed] [Google Scholar]
- Rule M, Stoffregen M, Ermentrout B. A model for the rrigin and properties of flicker-induced geometric phosphenes. PLOS Comp Biol. 2011;7:e1002158. doi: 10.1371/journal.pcbi.1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa R, Mazaheri A, Bojak I, Norris DG, Kleinschmidt A. Modulation of visually evoked cortical fMRI responses by phase of ongoing occipital α oscillations. J Neurosci. 2011;31:3813–3820. doi: 10.1523/JNEUROSCI.4697-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauf M, Lingelbach B, Wist ER. The scintillating grid illusion. Vision Res. 1997;37:1033–1038. doi: 10.1016/S0042-6989(96)00255-6. [DOI] [PubMed] [Google Scholar]
- Shevelev IA, Kamenkovich VM, Bark ED, Verkhlutov VM, Sharaev GA, Mikhailova ES. Visual illusions and travelling α waves produced by flicker at α frequency. Int J Psychophysiol. 2000;39:9–20. doi: 10.1016/S0167-8760(00)00105-7. [DOI] [PubMed] [Google Scholar]
- ter Meulen BC, Tavy D, Jacobs BC. From stroboscope to dream machine: a history of flicker-induced hallucinations. Eur Neurol. 2009;62:316–320. doi: 10.1159/000235945. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. α-Band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso XG, Macknik SL, Otero-Millan J, Martinez-Conde S. Microsaccades drive illusory motion in the Enigma illusion. Proc Natl Acad Sci U S A. 2008;105:16033–16038. doi: 10.1073/pnas.0709389105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen JM, Oostenveld R, Jensen O. Prestimulus oscillatory activity in the α band predicts visual discrimination ability. J Neurosci. 2008;28:1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRullen R, Dong T. Attention and scintillation. Vision Res. 2003;43:2191–2196. doi: 10.1016/S0042-6989(03)00412-7. [DOI] [PubMed] [Google Scholar]
- VanRullen R, Macdonald JS. Perceptual echoes at 10 Hz in the human brain. Curr Biol. 2012;22:995–999. doi: 10.1016/j.cub.2012.03.050. [DOI] [PubMed] [Google Scholar]
- Welch PD. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroacoustics. 1967;15:70–73. doi: 10.1109/TAU.1967.1161901. [DOI] [Google Scholar]
- Winn B, Gilmartin B. Current perspective on microfluctuations of accommodation. Ophthalmic Physiol Opt. 1992;12:252–256. doi: 10.1111/j.1475-1313.1992.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific α-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20:RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yo C, Wilson HR. Peripheral temporal frequency channels code frequency and speed inaccurately but allow accurate discrimination. Vision Res. 1993;33:33–45. doi: 10.1016/0042-6989(93)90056-3. [DOI] [PubMed] [Google Scholar]