Abstract
Burst firing has been reported as a pathological activity of subthalamic nucleus (STN) neurons in Parkinson's disease. However, the origin of bursts and their causal link with motor deficits remain unknown. Here we tested the hypothesis that dopamine D5 receptors (D5Rs), characterized by a high constitutive activity, may contribute to the emergence of burst firing in STN. We tested whether inhibiting D5R constitutive activity depresses burst firing and alleviates motor impairments in the 6-OHDA rat model of Parkinson's disease. Intrasubthalamic microinjections of either an inverse agonist of D5Rs, flupenthixol, or a D2R antagonist, raclopride, were applied. Behavioral experiments, in vivo and in vitro electrophysiological recordings, and ex vivo functional neuroanatomy studies were performed. Using [5S]GTPγ binding autoradiography, we show that application of flupenthixol inhibits D5R constitutive activity within the STN. Furthermore, flupenthixol reduced evoked burst in brain slices and converted pathological burst firing into physiological tonic, single-spike firing in 6-OHDA rats in vivo. This later action was mimicked by calciseptine, a Cav1 channel blocker. Moreover, the same treatment dramatically attenuated motor impairment in this model and normalized metabolic hyperactivity in both STN and substantia nigra pars reticulata, the main output structure of basal ganglia in rats. In contrast, raclopride as well as saline did not reverse burst firing and motor deficits, confirming the selective action of flupenthixol on D5Rs. These results are the first to demonstrate that subthalamic D5Rs are involved in the pathophysiology of Parkinson's disease and that administering an inverse agonist of these receptors may lessen motor symptoms.
Introduction
Parkinson's disease (PD) is a neurological disorder characterized by a gradual degeneration of dopaminergic neurons in substantia nigra pars compacta (SNc), leading to a marked dopamine (DA) depletion in striatum, the primary projection region, as well as extrastriatal nuclei of the basal ganglia (Smith and Villalba, 2008). Component nuclei exhibit changes in discharge frequencies and/or firing patterns, as well as in dynamics of neuronal discharges, such as intrinsic oscillations or interneuronal synchronization (Galvan and Wichmann, 2008). However, it is not known whether these phenomena play a causal role in parkinsonian motor symptoms. Recent evidence in behaving rodents complements earlier data obtained in DA-depleted anesthetized animals and points to a pathological persistence of a post-decision stabilized state of cortical–basal ganglia networks (Mallet et al., 2008; Cruz et al., 2011; Leventhal et al., 2012). However, the parkinsonian symptoms are alleviated by subthalamic nucleus (STN) ablation (Bergman et al., 1990), high-frequency electrical stimulation (Benazzouz et al., 1993; Limousin et al., 1998), and pharmacological inhibition (Levy et al., 2001; Luo et al., 2002), suggesting that STN has a critical role in the symptoms.
STN holds a pivotal position in basal ganglia circuitry, exerting an excitatory drive on the output structures (Albin et al., 1989; Surmeier and Bevan, 2003). It is now thought to mediate a top-down executive cortical control over all behavioral programs treated by basal ganglia (Cavanagh et al., 2011; Haynes and Haber, 2013). It is well established that dopaminergic afferents from the SNc innervate the STN and that DA modulates the electrical activity of STN neurons through a variety of mechanisms via presynaptic and postsynaptic sites (Ni et al., 2001a,b; Cragg et al., 2004; Baufreton and Bevan, 2008; Rommelfanger and Wichmann, 2010). The changes in STN electrical activity have been examined in experimental models of the disease in vitro and in vivo, but there is no unifying view of STN activity in PD (Wilson and Bevan, 2011).
Among DA receptors, the D5 receptors (D5Rs) display a unique feature in vitro: a high agonist-independent constitutive activity (Tiberi and Caron, 1994; Demchyshyn et al., 2000). It has been also demonstrated in vitro that D5Rs potentiate burst firing in STN neurons (Baufreton et al., 2003, 2005). Although the action of receptors in the D2 family is not expected to be maintained in the DA-depleted state, the distinctive property of D5R of constitutive activity argues in favor of persistence of a significant part of their burst firing-potentiating action. Abnormal burst firing may interfere with normal information processing within the basal ganglia, thus contributing to PD motor symptoms.
Here we tested the hypothesis that inhibiting the constitutive activity of D5Rs may depress the burst firing of STN neurons, resulting in an alleviation of motor impairment in the 6-hydroxydopamine (6-OHDA) rat model of PD. We investigated the effects of intrasubthalamic microinjection of an inverse agonist of D5Rs, flupenthixol, on (1) in vitro and in vivo STN neuronal activity, (2) motor behavior, and (3) ex vivo functional neuroanatomy in the rat.
Materials and Methods
Animals
For in vitro experiments, P19–P28 Wistar rats of both sexes bred in the university animal facility were used. Adult male Wistar rats weighing 280–380 g were used for behavioral, in vivo electrophysiological recordings and ex vivo experiments. Animals (Charles River) were housed four per cage under artificial conditions of light (light/dark cycle, light on at 7:00 A.M.), temperature (24°C), and humidity (45%) with food and water available ad libitum. All animal experiments were performed in accordance with European Communities Council Directive 2010/63/UE. The study received approval from the local Ethics Committee (Bordeaux, France).
In vitro experiments
Experiments were performed on subthalamic neurons in 350- to 400-μm-thick slices. The animals were anesthetized using isoflurane and decapitated. The brain was removed, and three to four slices containing the STN were prepared in a saturated (95% O2/5% CO2) ice-cold solution containing the following (in mm): 250 sucrose, 26 NaHCO3, 7 MgCl2, 2 KCl, 1.15 NaH2PO4, 0.5 CaCl2, and 11 glucose, pH 7.35. They were incubated for at least 1 h at room temperature in a saturated (95% O2/5% CO2) solution containing the following (in mm): 124 NaCl, 3.6 KCl, 1.3 MgCl2, 1.25 HEPES, 2.4 CaCl2, 26 NaHCO3, and 10 glucose. The same solution was also used to continuously perfuse the bath at ∼4 ml/min. Pipettes were pulled from Harvard glass capillaries (GC150F10; Phymep). They contained 100 mm K-gluconate, 10 mm NaCl, 10 mm KCl, 11 mm EGTA, 10 mm HEPES, 1 mm CaCl2, 2 mm ATP-Mg, and 0.4 mm GTP-2Na at a pH of 7.25 and osmolarity of 290 mOsm and had a resistance of 10–14 MΩ. Signals were recorded using an Axopatch 1-D amplifier (Molecular Devices). They were digitized and stored using a Digidata 1322A and the pClamp suite version 9.2 (Molecular Devices), respectively. Both the blind and the visualized patch method were used on coronal and sagittal slices. The experiments on the 6-OHDA-treated animals and their control counterparts used sagittal slices and were entirely performed with the visualized patch-clamp method on a Carl Zeiss AxioExaminer Z1 upright microscope. All parameters (temperature, perfusion rate, composition of intrapipette and perfusion solutions, pipette resistance) were kept unchanged in the blind and visualized experiments. We observed that using pipettes with a resistance <10 MΩ, a usual condition in the visualized patch method, often led to rundown of burst competency, i.e., reduction or even disappearance of plateau potentials and rebound bursts in ∼30 min. The voltage-clamp mode was first used, and a −5 mV step was applied five times at −65 mV. Input and access resistance as well as cell capacity were measured from the average current offline. The current-clamp mode was used for the remaining part of the experiment. After recording a 20 s period of spontaneous electrical activity at Io, neurons were maintained at −65 mV by current injection, and perfusion was supplemented by antagonists of fast synaptic transmission (5–10 μm SR-95531 [2-(3-carboxypropyl)-3-amino-6-(4-methoxyphenyl)pyridazinium bromide] [GABAzine], 40 μm d-(−)-2-amino-5-phosphonopentanoic acid, 5 μm 6-cyano-7-nitroquinoxaline-2,3-dione, and 5 μm S(−)-raclopride (+) tartrate). Five hundred millisecond increasingly negative and 80 ms increasingly positive steps were successively injected to test for the presence of postinhibitory rebound bursts and plateau potentials, respectively. Plateau potentials are bursts evoked at negative potentials, with regenerative responses that outlast the stimulus. Neurons displaying a significant plateau potential (i.e., a depolarization sustained after the stimulation step termination allowing the discharge of at least 10 action potentials accounted for approximately half neurons and were found in all areas within STN). They were further challenged with 1–2 μm flupenthixol, fluphenazine, or butaclamol using depolarizing steps of 80–140 pA applied at 20 s intervals. Studies on recombinant human D5Rs have shown that doses of 1–5 μm efficiently decreased basal adenylate cyclase activity or AMPc accumulation in heterologous cell lines (Martin et al. 2001; D'Aoust and Tiberi, 2010). All the drugs, including cis-(z)flupenthixol di-hydrochloride, fluphenazine dihydrochloride salt, and (+)-butaclamol hydrochloride, were purchased from Sigma, prepared as stock solution, and kept at −20°C. Stock solutions of flupenthixol were not kept longer than 1 month. Bursts were evoked every 20–30 s, and their typical features (duration, number of action potentials, and discharge frequency) were measured offline throughout the recordings (usually lasting 15–20 min), using pClamp software. The control values were then calculated from the average of the five evoked bursts preceding drug application, whereas the test values were the mean of the five consecutive bursts showing the maximum change.
Ex vivo experiments
[35S]GTPγ binding autoradiography.
Agonist or antagonist receptor-stimulated [35S]GTPγ binding in the STN was measured by autoradiography, as described previously (Laffray et al., 2012). Ex vivo STN-containing brain coronal slices were incubated in [35S]GTPγ (0.04 nm) at 25°C for 30 min, in the absence (basal conditions) or presence of the drugs [D5 agonist SKF 38393 (2,3,4,5-tetrahydro-7,8-dihydroxy-1-phenyl-1H-3-benazepine HCl), D5 antagonist SCH 23390 (R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride), or D5 inverse agonist flupenthixol] at six different concentrations. Nonspecific binding was determined in the presence of 10 μm [35S]GTPγ. After exposure to beta-radiation-sensitive film, relative [35S]GTPγ autoradiographic binding was materialized by optical density (OD) values in the STN, measured using the Mercator image analysis system (ExploraNova). 14C standards were used to ascertain that all values were in a linear domain range. Experiments were designed as follow: three brain slices containing STN per animal, five animals per group (15 measurements for each contralateral and ipsilateral STN). Experiments were performed in triplicate (45 measurements).
Cytochrome oxidase histochemistry.
The cytochrome oxidase (COx) histochemical reaction was quantified using the metal-enhanced technique, as described previously (Blandini et al., 2007). COx activity was analyzed by densitometry, using the Mercator system, as described previously (Blandini et al., 2007). Digital images of each stained section were obtained, and OD was measured on four consecutive sections throughout the STN and the pars reticulata of substantia nigra (SNr).
In vivo experiments
DA depletion.
DA depletion was obtained by injection of 6-OHDA in the medial forebrain bundle of juvenile (17- to 19-d-old) and adult (280–300 g) rats. Seventeen- to 19-d-old rats were treated according to the protocol of Miguelez at al. (2012). Briefly, they were anesthetized with ketamine (75 mg/kg) and xylazine (10 mg/kg), and desipramine (20 mg/kg, i.p) was administered to avoid damage of the noradrenergic system. Thirty minutes later, 1 μl of 6-OHDA (4 μg/μl plus 0.1% ascorbic acid) was infused using stereotaxy at a rate of 0.5 μl/min into the right hemisphere in two points. The coordinates (relative to bregma) were as follows: anteroposterior (AP), −2.4 mm; mediolateral (ML), −1.2 mm; and dorsoventral (DV), −7.4 and −7.9 mm. Asymmetry of forelimb use was evaluated using the cylinder test 2 weeks later, and animals showing limb asymmetry were used for in vitro recordings during the following week.
Adult rats also received a stereotaxic unilateral injection of 6-OHDA (Sigma) or its saline vehicle into the medial forebrain bundle 3–4 weeks before behavioral testing and electrophysiological recordings, as described previously (Belujon et al., 2007). Briefly, each animal received a unilateral injection of 2.5 μl of 6-OHDA (Sigma; 5 mg/ml in sterile NaCl, 0.9% with 0.1% ascorbic acid) into the right medial forebrain bundle according to the brain atlas of Paxinos and Watson (1996). The coordinates (relative to bregma) were as follows: AP, −2.8 mm; ML, −2 mm; and DV, −8.4 mm.
The effectiveness of the 6-OHDA lesion in juvenile and adult rats was systematically assessed after all experiments using tyrosine hydroxylase immunoreactivity, as reported previously (Bouali-Benazzouz et al., 2009). To assess the behavioral effects of drug injection into the STN, adult rats were chronically implanted with guide cannula (Phymep). The tip of the guide cannula was lowered to 1 mm above the STN, at coordinates 3.8 mm posterior to bregma, 2.5 mm lateral to the midline, and 7 mm below the skull, according to the Paxinos and Watson brain atlas (Paxinos and Watson, 1996).
In vivo extracellular electrophysiology.
Single-unit recordings were made in rats anesthetized with urethane (1.2 g/kg, i.p.), as reported previously (Belujon et al., 2007; Chetrit et al., 2009). In a preliminary set of experiments, we recorded EEG simultaneously with single-unit activity urethane anesthesia. In these experiments, a stable slow cortical oscillation (∼1 Hz) was observed together with the bursty (6-OHDA rats) and tonic (control rats) firing (our unpublished data). A double-barreled pipette assembly, similar to that described previously (Akaoka and Aston-Jones, 1991), was used for STN neuronal activity recordings and simultaneous local microinjection of the drugs. Basal firing of the neurons was recorded for 20 min before drug injection to ascertain the stability of discharge activity, and then a drug or vehicle was injected directly into the STN in a volume of 20 nl of 0.9% NaCl, using brief pulses of pneumatic pressure (Picospritzer III; Parker).
In a set of experiments, we investigated the dose response of flupenthixol on STN neuron burst firing. Three doses (2, 4, and 6 μg) were tested in 6-OHDA-lesioned rats (n = 15). Flupenthixol significantly affected the burst parameters in a dose-dependent manner (Kruskal–Wallis test, burst frequency, F = 16.39, p = 0.0009; percentage of spikes in burst, F = 16.39, p = 0.001). In contrast to the dose of 2 μg, which did not affect the burst firing (Dunn's test, p > 0.05 for the two parameters), 4 and 6 μg turned the pathological burst firing into tonic, single-spike firing by significantly and dramatically decreasing the burst frequency (Dunn's test, p < 0.01) and the percentage of spikes in burst (Dunn's test, p < 0.01). However, any of the three doses did not induce a change in the firing rate of STN neurons (Kruskal–Wallis test, F = 0.53, p = 0.91). According to these results, only the dose of 4 μg has been used for all in vivo electrophysiological, behavioral, and metabolic studies.
Because D5Rs signaling has been shown to increase burst firing through potentiation of Cav1 channels in the STN in vitro (Baufreton et al., 2003), we tested the effect of the Cav1 channel inhibitor calciseptine using the same experimental protocol. It was purchased from Alomone Labs, and a dose of 6 ng in 20 nl of saline was injected. Calciseptine has been chosen for a number of reasons: (1) we showed previously that calciseptine occluded the action of SKF 81297 (6-chloro-2,3,4,5-tetrahydro-1-phenyl-1H-3-benzazepine hydrobromide) in vitro and reduced burst competency by virtually inhibiting plateau potentials (Baufreton et al. 2003); and (2) calciseptine is stable at room temperature and is not destroyed by exposition to light, a property that is necessary for using it in in vivo experiments.
Firing patterns were analyzed using the method developed by Kaneoke and Vitek (1996), as described previously (Tai et al., 2003). An algorithm using MATLAB computer software programming was used. Tonic versus bursty firing was distinguished by analyzing the distribution of the interspike intervals. Tonic firing had a Gaussian distribution, whereas burst firing had a Poisson distribution.
Behavioral assessment.
Spontaneous locomotor activity was measured using a photoelectric actimeter (Actitrack; Bioseb), as described previously (Belujon et al., 2007; Chetrit et al., 2009). All testing in the actimeter were done in an isolated room between 8:00 A.M. and 1:00 P.M. and consisted of three phases. (1) Spontaneous locomotor activity was recorded during 4 consecutive days (days 1–4; two times 10 min each) during which rats were habituated to manipulation by the experimenter. The first session of 10 min was considered as the daily habituation. Only the locomotor activity recorded during the second session of 10 min was used for data analysis. (2) Local injection of flupenthixol or raclopride (Sigma) directly into the STN was applied on the fifth day, just before the measurement of locomotor activity. (3) A post-challenge test of spontaneous locomotor activity was performed: 1 day after flupenthixol injection, rats were again reexposed to the actimeter.
The stepping test was used to monitor lesion-induced changes in forelimb akinesia in the rat that may be analogous to akinesia seen in PD (Olsson et al., 1995). The number of adjusting steps was measured on a smooth-surfaced table. The rat was held by the experimenter with one hand softly blocking the hindlimbs and with the other hand fixing the forelimb not to be monitored. In this way, the other forepaw had to bear the body weight. The rat was moved slowly sideways in both right and left directions by the experimenter with a speed of 0.9 m/5 s (Abedi et al., 2013). This was done for both the contralateral and ipsilateral forepaws. When the rat was moved along the table, the free forelimb had to step with the movement of the experimenter to keep balance. The number of adjusting steps for both directions and both paws was video recorded and counted. Values represent the number of adjusting steps.
Statistical analyses
Data are mean ± SEM. Statistical analyses were performed using Prism software (GraphPad Software). The Mann–Whitney and Wilcoxon's matched-pairs signed-ranks tests were used to compare behavioral and patch-clamp data from different groups and the same animals before and after treatment, respectively. The unpaired t test was used for COx histochemistry and [35S]GTPγ data. For in vivo electrophysiological analysis, firing rates and burst parameters before and after drug injection were compared using the Mann–Whitney test, and the distribution of the firing pattern was assessed using a χ2 test.
Results
D5R inverse agonists weaken STN burst firing in vitro
Application of flupenthixol and butaclamol, but not fluphenazine, to STN neurons in rat brain slices, at concentrations used on recombinant D5Rs (1–2 μm), significantly modified the specific features of evoked bursts (Fig. 1Aa), with partial reversibility. Flupenthixol was the only drug to significantly reduce mean burst duration as well as the average number of spikes in each burst, without any significant change in the intraburst firing frequency (Fig. 1Ab). Burst duration was reduced by 28% (1.11 ± 0.27 to 0.79 ± 0.22 s, n = 11, Wilcoxon's matched-pairs signed-rank test, p = 0.0186) and the average number of spikes in evoked burst by 42% (42 ± 9 to 24 ± 5, n = 11, p = 0.001), without any significant change in intraburst firing frequency (40 ± 5 and 38 ± 7 Hz, n = 11, p > 0.8311). Such results are the opposite of those we found during application of D1/D5R agonists (Baufreton et al., 2003). Thus, only flupenthixol acted as an inverse agonist of D5R on STN neurons. Furthermore, as shown in Figure 1Ac, depicting a neuron that fired spontaneously in bursts in the control condition, flupenthixol application resulted in an irregular discharge of action potentials and burst firing resumed on wash. Flupenthixol was also active on evoked bursts in brain slices obtained from DA-depleted, 6-OHDA lesioned animals (Fig. 1B). Burst duration and number of action potentials per burst were again reduced (1.40 ± 0.24 vs 1.17 ± 0.22 s, significantly different at p = 0.006; 58 ± 21 vs 41 ± 13, p = 0.002, respectively), whereas intraburst discharge frequency was unaltered (54 ± 10 vs 50 ± 8 Hz, p = 0.16). In contrast, we found no difference in the proportion of burst-competent neurons nor of neurons in the burst firing mode in normal and depleted states. We evoked sustained depolarizations over lasting the depolarizing stimuli in 18 of 23 neurons obtained from DA-intact rats and in 16 of 22 neurons from DA-depleted rats. This made flupenthixol the ideal drug for the following experiments.
Figure 1.

Flupenthixol weakens evoked bursts in burst-competent neurons and minimizes spontaneous burst firing in vitro. Aa, Representative examples of the action of flupentixol, butaclamol, and fluphenazine on bursts (top traces) evoked by depolarizing stimuli (bottom traces) in DA-intact animals. The time course of the action of flupenthixol on the duration of evoked burst (black squares), the number (gray diamonds), and the frequency (white squares) of action potentials (AP) is shown below the traces. Inverted triangles point to the three examples. Ab, Box-plot summary changes in typical features of evoked bursts after application of the three drugs. Ac, Flupenthixol turned burst firing into irregular firing in a spontaneously burst-firing neuron. B, Flupenthixol reduced evoked bursts in neurons from DA-depleted animals. Note that the bath contained fast synaptic transmission inhibitors and raclopride, a D2R inhibitor. Wilcoxon's matched-pairs signed-rank test, *p < 0.05, **p < 0.01, ***p < 0.001.
D5R in the STN display constitutive activity
In vitro experiments have shown that recombinant D5R exhibit constitutive activity (Tiberi and Caron, 1994; Demchyshyn et al., 2000). However, no data are available concerning the activated state of STN D5R. We used a [35S]GTPγ binding assay (Sim et al., 1995; O'Connor et al., 2005) to examine D5R-activated G-proteins in brain slices. SKF 38393, a D1/D5 agonist, stimulated dose-dependent [35S]GTPγ binding within the STN (maximum increase over basal level: 61%, one-way ANOVA, F = 12.26, p < 0.0001), indicating an increase in the activated state of local D5R (Fig. 2A). A D1/D5 antagonist, SCH 23390, had no effect (Fig. 2B; one-way ANOVA, F = 1.85, p = 0.1016). Flupenthixol had an inverse agonist effect on local D5R, decreasing [35S]GTPγ binding by 52% (Fig. 2C; one-way ANOVA, F = 21.90, p < 0.0001). Furthermore, as a specificity control, previous incubation with the D1/D5 antagonist blocked the effect of both the D1/D5 agonist and D5R inverse agonist (Fig. 2D). Together, these data provide the first evidence of D5R constitutive activity within the STN and confirm the inverse agonist property of flupenthixol.
Figure 2.
Constitutive activity of D5Rs in the rat STN blocked by flupenthixol. A–C, Histograms showing the dose–response effects of the D1/D5 receptor agonist SKF 38393 (A) and antagonist SCH 23390 (B), as well as the D5R inverse agonist flupenthixol (C) on [35S]GTPγ binding in the STN, assessed by OD. D, SCH 23390 blocked the effects of both SKF 38393 and flupenthixol. Data represent mean ± SEM of OD values. One-way ANOVA (A–C) and unpaired t test (D). *p < 0.05, **p < 0.01, compared with basal OD.
A D5R inverse agonist reverses STN burst firing
Extracellular recordings in urethane-anesthetized rats (n = 12) were used to test the hypothesis that flupenthixol reduced the burst discharge capacity of STN neurons in vivo. Because the large majority of STN neurons in sham-depleted animals display a tonic, not burst, discharge pattern (Fig. 3A,D), the effect of flupenthixol was studied only on DA-depleted rats (Fig. 3B). Single-unit recordings in the STN of these animals exhibited a higher percentage of burst-firing neurons than sham-depleted rats (50 vs 13.5%; Fig. 3C,D). Interestingly, local application of flupenthixol (4 μg) in the STN turned the pathological burst-firing activity into tonic, single-spike firing (n = 6 of 6, χ2 = 8.00, p = 0.0047; Fig. 3C–E), without any effect on tonically discharging neurons (n = 6 of 6, χ2 = 0.00, p = 1; Fig. 3E). Analysis of burst parameters showed that flupenthixol induced a dramatic decrease of the burst frequency (Mann–Whitney test, p = 0.042; Fig. 3F) and percentage of spikes in burst (p = 0.043; Fig. 3F). No significant modification in the firing rate was observed (p = 0.082; Fig. 3F).
Figure 3.

Flupenthixol reverses pathological burst-firing activity of STN neurons. A, A representative example of spike trains recorded in the STN in a sham-depleted rat. B, A representative photomicrograph of the striatum after immunohistochemistry of tyrosine hydroxylase in a brain slice from a DA-depleted rat. Note the extensive lesion of fibers in the striatum ipsilateral to the side that received stereotactic 6-OHDA injection into the medial forebrain bundle. C, Representative examples of spike trains recorded in the STN in DA-depleted rats before (left) and after local injection of flupenthixol (Flu, right). D, E, Histograms (D) and diagrams (E) of the effects of flupenthixol on STN neurons discharging in tonic single spike or burst mode. F, Histograms of burst frequency, percentage of spikes in burst, and firing rate of STN neurons before and after flupenthixol injection into the STN. G, Histograms of burst frequency, percentage of spikes in burst, and firing rate of STN neurons before and after local injection of calciseptine. Note that calciseptine in the same way as flupenthixol turned the pathological burst-firing activity into tonic, single-spike firing. Data represent mean ± SEM. D, χ2 test, **p < 0.01 compared with sham-depleted rats, $$p < 0.05 compared with DA-depleted rats. F, G, Mann–Whitney test, *p < 0.05, **p < 0.01; ns, not significant.
Because D5Rs signaling has been shown to increase burst firing through potentiation of Cav1 channels in the STN in vitro (Baufreton et al., 2003), we tested the action of the Cav1 channel inhibitor calciseptine in local application in vivo. As with flupenthixol, local application of calciseptine (6 ng) in the STN turned the pathological burst-firing activity into tonic firing (n = 5). Analysis of burst parameters showed that calciseptine dramatically decreased the burst frequency (Mann–Whitney test, p = 0.026; Fig. 3G) and percentage of spikes in burst (p = 0.002; Fig. 3G). No significant modification in the firing rate was observed (p = 0.81; Fig. 3G).
Because flupenthixol can exert a D2R antagonist activity, we examined the effect of local injection of a selective D2R antagonist, raclopride, on neuronal activity in the STN (n = 12 neurons) of 12 DA-depleted rats. Raclopride (4 μg) did not modify the firing pattern (χ2 test, χ2 = 0.00, p = 1) or the firing rate of any of the STN neurons tested (Mann–Whitney test, p = 0.986; Fig. 4). The burst frequency and the percentage of spikes in burst did not change (p = 0.625 and 0.437, respectively; Fig. 4). Saline injection under the same conditions did not induce any change in the firing rate (p = 0.54) or patterns of STN neurons (χ2 = 0.00, p = 1). Together, these results demonstrate that the impact of flupenthixol on firing patterns was mediated by D5Rs in the STN.
Figure 4.

Raclopride does not induce any effect on the pathological burst-firing activity of STN neurons. A, Representative examples of spike trains recorded in the STN in DA-depleted rats before (left) and after local injection of raclopride (Raclo, right) into the STN. B, C, Histograms (B) and diagrams (C) of the effects of raclopride on STN neurons discharging in tonic single-spike or burst mode. Note that raclopride did not reverse the pathological burst-firing activity into tonic, single-spike firing. D, Histograms of burst frequency, percentage of spikes in burst, and firing rate of STN neurons before and after raclopride injection into the STN. Data represent mean ± SEM. B, χ2 test, **p < 0.01 compared with sham-depleted rats. D, Mann–Whitney test, *p < 0.05; ns, not significant.
A D5 inverse agonist attenuates motor impairment
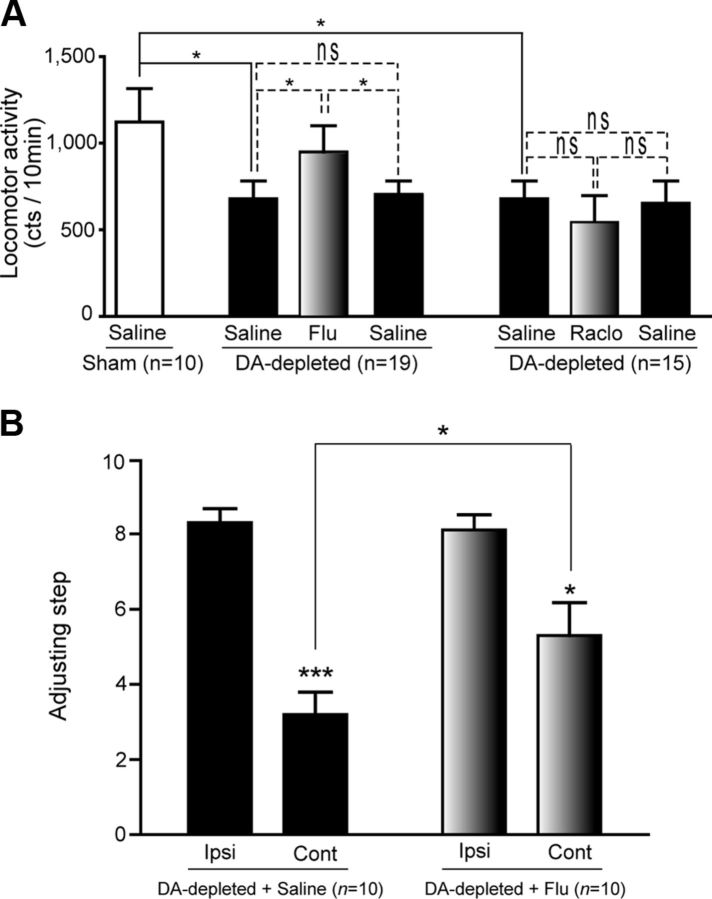
To test the hypothesis that flupenthixol reversed motor impairment induced by DA depletion, we assessed the behavior of adult male rats after intrasubthalamic injection of flupenthixol or saline. The outcome of DA depletion by 6-OHDA and flupenthixol treatment was assessed by analyzing spontaneous locomotor activity and asymmetry in forelimb motor activity. These motor behaviors were assessed 3–4 weeks after DA depletion. As shown previously (Belujon et al., 2007), DA depletion impaired locomotor activity, reducing mean scores by 44% in DA-depleted rats compared with sham-lesioned animals (Mann–Whitney test, p = 0.0313). Local flupenthixol injection (4 μg in 200 nl) significantly improved locomotor activity, which increased by 39%, compared with the measurements after a saline injection on the previous day (Fig. 5A; n = 19, Wilcoxon's test, p = 0.0144). To verify that this increase was attributable to flupenthixol injection, a post-challenge test was performed in which all rats received a saline injection on the next day. Rats reexposed to the actimeter had a similar locomotor activity compared with that observed after the first saline injection (Fig. 5A). Once again, in view of the antagonist activity of D2R, the effects of raclopride, a selective D2R antagonist, on locomotor activity were investigated. Injecting raclopride into the STN (4 μg in 200 nl) had no effect (Fig. 5A; n = 15, p = 0.7250), confirming that the beneficial impact of flupenthixol was attributable to its action on D5Rs in the STN. In agreement with previous studies (Tseng et al., 2005), unilateral DA depletion led to a 66% deficit in contralateral paw use (8.86 ± 0.49 ipsilateral vs 2.98 ± 0.53 contralateral, Wilcoxon's test, p < 0.0001; Fig. 5B). Local injection of flupenthixol into the STN on the DA-depleted side alleviated this asymmetry by 65.62 ± 0.53% (2.98 ± 0.53 vs 5.76 ± 1.03, Mann–Whitney test, p = 0.0137). Local flupenthixol application in the STN thus attenuated the motor impairments of DA-depleted rats.
Figure 5.
Flupenthixol attenuates motor impairment in DA-depleted rats. A, Locomotor activity in sham-lesioned rats (n = 10, white bars) and 6-OHDA unilaterally lesioned rats was measured before (black bars) and after (gray bars) local injection of flupenthixol (Flu, n = 19) or raclopride (Raclo, n = 15) into the STN (4 μg/200 nl). Only flupenthixol improved locomotor activity in 6-OHDA-lesioned rats. B, Asymmetry in paw use was partially but significantly reversed by local injection of flupenthixol into the STN (n = 10). Values are presented as the mean ± SEM. *p < 0.05, ***p < 0.0001; ns, not significant. Full and dashed lines, Mann–Whitney and Wilcoxon's matched-pairs signed-rank tests, respectively. Ipsi, Ipsilateral; Cont, contralateral.
A D5 inverse agonist normalizes metabolic activity
The metabolic correlates of motor improvement were investigated by measuring the expression of COx, an endogenous marker for neuronal activity (Hirsch et al., 2000), in the STN and its efferent structure, the SNr, the principal output structure of the basal ganglia in the rat. As reported previously (Vila et al., 2000; Benazzouz et al., 2004; Blandini et al., 2007), after DA depletion by 6-OHDA in rats (n = 4), the COx activity in the STN (74.5%, unpaired t test, p < 0.0001) and SNr (146.5%, p < 0.0001) was significantly higher than in sham-lesioned animals (n = 4; Fig. 6). This abnormal hyperactive metabolism was normalized by injecting flupenthixol into the STN (Fig. 6), using the same protocol applied as for behavioral experiments. Flupenthixol induced a significant decrease in COx levels in STN (78.6%, n = 4 rats, p = 0.0002) and SNr (151.2%, n = 4 rats, p = 0.0028), normalizing the values with those obtained in sham-lesioned animals (n = 4 rats; STN, p = 0.3828; SNr, p = 0.7193). These data show that the blockade of STN D5R constitutive activity reversed metabolic hyperactivity generated by DA depletion in both the STN and SNr.
Figure 6.
Flupenthixol normalizes the metabolic hyperactivity in the STN and SNr. A, C, Representative photomicrographs of the metabolic activity revealed by COx staining in the STN (A) and SNr (C). B, D, STN (B) and SNr (D) hyperactivity in DA-depleted rats (black bars) compared with sham-lesioned animals (white bars) was normalized by injecting flupenthixol (Flu) into the STN (gray bar). Data represent mean ± SEM of OD values. **p < 0.001, ***p < 0.0001; ns, not significant. Scale bar, 250 μm. Note that, in addition to the increased COx levels in the STN and SNr, this figure shows an increased COx level in the zona incerta (ZI), as described previously in hemiparkinsonian rats (Périer et al., 2000). ic, Internal capsule.
Discussion
Pathophysiology of PD is associated with abnormal spiking activities of basal ganglia neurons, including changes in firing rates, bursting activities, power of oscillatory frequencies, and synchrony. STN, a pivotal nucleus of basal ganglia, exhibits a marked proclivity to sustained burst firing in patients and nonhuman primate and rodent models of the disease (Bergman et al., 1994;Magill et al., 2001; Ni et al., 2001a,b; Galvan and Wichmann, 2008). The mechanism underlying pathological burst activity in STN is unknown. The causal link between the pathological firing and the manifestation of parkinsonian-like motor symptoms has not yet been determined. Our results show for the first time that D5Rs located in the STN display constitutive activity. Inhibition of the constitutive activity by the local injection of an inverse agonist of D5Rs, flupenthixol, depresses the prevalence of the burst firing and alleviates the motor impairment in the 6-OHDA rat model of PD.
Pharmacological validation
The lack of subtype-selective ligands limits the understanding of the respective role of D1Rs and D5Rs. Nevertheless, in our study, it is unlikely that the observed effects here are attributable to the action of drugs on D1R for the following arguments. (1) In situ hybridization studies found a high level of D5Rs mRNA in the STN (Svenningsson and Le Moine, 2002), whereas mRNA for D1Rs was not detected in rat (Boyson et al., 1986; Fremeau et al., 1991; Svenningsson and Le Moine, 2002) and human STN (Augood et al., 2000). (2) Using a highly selective antibody raised against a peptide sequence of cloned D5Rs, it has been shown that D5Rs were being expressed at postsynaptic sites within STN neurons, and single-cell RT-PCR profiling evidenced that burst-competent neurons only expressed D5Rs (Baufreton et al., 2003). Together, these evidences demonstrate that STN neurons express D5Rs but not D1Rs, suggesting that the effects observed in our study are attributable to the action of drugs on D5Rs but not on D1Rs. Then, to select a potent inverse agonist of D5Rs, three drugs, flupenthixol, butaclamol, and fluphenazine, were tested in the STN using whole-cell patch-clamp recordings in brain slices. They have been shown to behave as inverse agonists of D5Rs (Tiberi and Caron, 1994; Charpentier et al., 1996; Demchyshyn et al., 2000; Tumova et al., 2003; Tumova et al., 2004). We found that only flupenthixol, not butaclamol or fluphenazine, acted as an inverse agonist because it induced the opposite effects of an agonist, e.g., a reduction of burst duration and a decrease in the number of spikes in bursts (Baufreton et al., 2003). Accordingly, flupenthixol was selected and used in all the experiments of the study.
Our results of [35S]GTPγ binding provide the first evidence of D5R constitutive activity within the STN in vivo, in line with previous in vitro experiments using D5R expression in a heterologous system (Tiberi and Caron, 1994; Demchyshyn et al., 2000). We show that the agonist of receptors in the D1 family, SKF 38393, induced a dose-dependent activation of D5Rs in the STN. The D1 antagonist SCH 23390 alone had no effect, although it occluded the action of SKF 38393. Furthermore, [35S]GTPγ binding showed that D5R activation within the STN was inhibited by flupenthixol (thus behaving as an inverse agonist of D5Rs) and that SCH 23390 prevented this effect. Constitutive activity, the functional signature of D5Rs, has been suggested to contribute to burst firing in STN neurons in vitro (Baufreton et al., 2003, 2005). Our electrophysiological results provide evidence both in vitro and in vivo showing that flupenthixol dramatically reversed the pathological burst firing of STN neurons, which became a tonic single-spike firing. Flupenthixol-induced changes in the firing pattern were specific to STN bursty neurons because it did not modify the mean firing rate or the firing pattern of the tonically discharging STN neurons. These results strongly support the involvement of D5Rs in the burst firing of STN neurons in agreement with the previous study showing that D5R activation potentiated burst firing of STN neurons in brain slices (Baufreton et al., 2003). Calciseptine, a specific peptide antagonist of high threshold, slowly inactivating, Cav1-type calcium channels, reproduced the action of flupenthixol on firing in vivo. This new piece of evidence underlines the role of Cav channels in in vivo patterning, as suggested by a number of in vitro studies (Nakanishi et al., 1987; Atherton et al., 2010; Wilson and Bevan, 2011). Interestingly, flupenthixol-induced firing pattern normalization was paralleled by the improvement of motor impairment, previously induced by DA depletion. Indeed, we show that intrasubthalamic injection of flupenthixol increased locomotor activity and contralateral paw use. These results suggest the existence of a link between STN bursty activity and motor deficits, in line with a recent study demonstrating that STN electrical stimulation using negative constant currents decreased STN burst discharges and remedied locomotor deficits in 6-OHDA rats, whereas positive constant currents readily increased burst discharges and generate locomotor deficits in normal rats (Tai et al., 2012). It must be noted in this respect that clinical improvement in parkinsonian patients has been correlated with a shift from bursting activity to nonbursting discharge (Benedetti et al., 2004). To determine that the observed effects of flupenthixol were not attributable to an antagonistic action on D2Rs, we investigated the electrophysiological and behavioral effects of raclopride, which is a selective antagonist of D2Rs. Our results show that intrasubthalamic injection of raclopride had no electrophysiological or behavioral effects. This result rules out the involvement of D2Rs in the action of flupenthixol. Together, our data strongly support the implication of D5Rs in the pathophysiology of parkinsonian-like motor deficits and also the key role that STN firing abnormalities play in the manifestation of these deficits.
In several brain systems, burst firing patterns, which represent a prolonged period of densely populated spikes, have been shown to contribute to enhanced synaptic neurotransmitter release compared with tonic single-spike firing (Dutton and Dyball, 1979; Lundberg et al., 1986; Gonon, 1988). In the STN, the enhanced proportion of burst firing recorded after DA depletion may reflect functional neuronal hyperactivity exerting an exaggerated excitatory drive on the output structures of basal ganglia. This hyperactivity can be quantified by measuring the activity of COx using in situ hybridization or histochemistry approaches. Several studies, including ours (Vila et al., 2000; Tai et al., 2003; Benazzouz et al., 2004), have shown that, in 6-OHDA-lesioned rats, COx expression levels in the STN and its major efferent target, the SNr, increased dramatically compared with those of sham-lesioned animals. Accordingly, the present study shows similar increases in COx activity levels in 6-OHDA-lesioned rats, and flupenthixol normalized the pathological metabolic hyperactivity in both basal ganglia nuclei. These results are in line with previous studies showing that STN high-frequency stimulation, which is known to improve parkinsonian-like motor deficits, resulted in a normalization of COx expression in both STN and SNr (Salin et al., 2002; Tai et al., 2003; Benazzouz et al., 2004).
Our findings are the first to demonstrate the involvement of D5Rs, located in the STN, in the pathophysiology of PD because of their constitutive activity. Indeed, application of a D5R inverse agonist induced a switch from pathological burst firing to normal tonic firing, resulting in the attenuation of parkinsonian-like motor impairment. Additional experiments are needed to determine the relationship between burst firing and rhythmicity/synchrony. Given the pivotal position of the STN in basal ganglia, we suggest that selective action on the D5R subtype might lead to better-targeted drug therapy for PD. This not only represents a major paradigm shift in our understanding of the pathophysiology of PD but also opens up new avenues for therapeutic approaches based on specific pharmacological agents.
Footnotes
This work was supported by grants from the Fondation de France, the University Victor Segalen, the Centre National de la Recherche Scientifique (CNRS), and the Institut Fédératif de Recherche (Institut National de la Santé et de la Recherche Médicale 8; CNRS 13). J.C. and L.F. were supported by a fellowship from the Ministère de l'Education Nationale, de la Recherche et de la Technologie. We thank L. Cardoit for technical assistance.
The authors declare no competing financial interests.
References
- Abedi PM, Delaville C, De Deurwaerdère P, Benjelloun W, Benazzouz A. Intrapallidal administration of 6-hydroxydopamine mimics in large part the electrophysiological and behavioral consequences of major dopamine depletion in the rat. Neuroscience. 2013;236:289–297. doi: 10.1016/j.neuroscience.2013.01.043. [DOI] [PubMed] [Google Scholar]
- Akaoka H, Aston-Jones G. Opiate withdrawal-induced hyperactivity of locus coeruleus neurons is substantially mediated by augmented excitatory amino acid input. J Neurosci. 1991;11:3830–3839. doi: 10.1523/JNEUROSCI.11-12-03830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-X. [DOI] [PubMed] [Google Scholar]
- Atherton JF, Kitano K, Baufreton J, Fan K, Wokosin D, Tkatch T, Shigemoto R, Surmeier DJ, Bevan MD. Selective participation of somatodendritic HCN channels in inhibitory but not excitatory synaptic integration in neurons of the subthalamic nucleus. J Neurosci. 2010;30:16025–16040. doi: 10.1523/JNEUROSCI.3898-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augood SJ, Hollingsworth ZR, Standaert DG, Emson PC, Penney JB., Jr Localization of dopaminergic markers in the human subthalamic nucleus. J Comp Neurol. 2000;421:247–255. doi: 10.1002/(SICI)1096-9861(20000529)421:2<247::AID-CNE9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Baufreton J, Bevan MD. D2-like dopamine receptor-mediated modulation of activity-dependent plasticity at GABAergic synapses in the subthalamic nucleus. J Physiol. 2008;586:2121–2142. doi: 10.1113/jphysiol.2008.151118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufreton J, Garret M, Rivera A, de la Calle A, Gonon F, Dufy B, Bioulac B, Taupignon A. D5 (not D1) dopamine receptors potentiate burst-firing in neurons of the subthalamic nucleus by modulating an L-type calcium conductance. J Neurosci. 2003;23:816–825. doi: 10.1523/JNEUROSCI.23-03-00816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufreton J, Zhu ZT, Garret M, Bioulac B, Johnson SW, Taupignon AI. Dopamine receptors set the pattern of activity generated in subthalamic neurons. FASEB J. 2005;19:1771–1777. doi: 10.1096/fj.04-3401hyp. [DOI] [PubMed] [Google Scholar]
- Belujon P, Bezard E, Taupignon A, Bioulac B, Benazzouz A. Noradrenergic modulation of subthalamic nucleus activity: behavioral and electrophysiological evidence in intact and 6-hydroxydopamine-lesioned rats. J Neurosci. 2007;27:9595–9606. doi: 10.1523/JNEUROSCI.2583-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benazzouz A, Gross C, Féger J, Boraud T, Bioulac B. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci. 1993;5:382–389. doi: 10.1111/j.1460-9568.1993.tb00505.x. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Tai CH, Meissner W, Bioulac B, Bezard E, Gross C. High-frequency stimulation of both zona incerta and subthalamic nucleus induces a similar normalization of basal ganglia metabolic activity in experimental parkinsonism. FASEB J. 2004;18:528–530. doi: 10.1096/fj.03-0576fje. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M, Bergamasco B, Lopiano L. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat Neurosci. 2004;7:587–588. doi: 10.1038/nn1250. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Blandini F, Levandis G, Bazzini E, Nappi G, Armentero MT. Time-course of nigrostriatal damage, basal ganglia metabolic changes and behavioural alterations following intrastriatal injection of 6-hydroxydopamine in the rat: new clues from an old model. Eur J Neurosci. 2007;25:397–405. doi: 10.1111/j.1460-9568.2006.05285.x. [DOI] [PubMed] [Google Scholar]
- Bouali-Benazzouz R, Tai CH, Chetrit J, Benazzouz A. Intrapallidal injection of 6-hydroxydopamine induced changes in dopamine innervation and neuronal activity of globus pallidus. Neuroscience. 2009;164:588–596. doi: 10.1016/j.neuroscience.2009.07.034. [DOI] [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6:3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, Frank MJ. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci. 2011;14:1462–1467. doi: 10.1038/nn.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier S, Jarvie KR, Severynse DM, Caron MG, Tiberi M. Silencing of the constitutive activity of the dopamine D1B receptor. Reciprocal mutations between D1 receptor subtypes delineate residues underlying activation properties. J Biol Chem. 1996;271:28071–28076. doi: 10.1074/jbc.271.45.28071. [DOI] [PubMed] [Google Scholar]
- Chetrit J, Ballion B, Laquitaine S, Belujon P, Morin S, Taupignon A, Bioulac B, Gross CE, Benazzouz A. Involvement of basal ganglia network in motor disabilities induced by typical antipsychotics. PLoS One. 2009;4:e6208. doi: 10.1371/journal.pone.0006208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Baufreton J, Xue Y, Bolam JP, Bevan MD. Synaptic release of dopamine in the subthalamic nucleus. Eur J Neurosci. 2004;20:1788–1802. doi: 10.1111/j.1460-9568.2004.03629.x. [DOI] [PubMed] [Google Scholar]
- Cruz AV, Mallet N, Magill PJ, Brown P, Averbeck BB. Effects of dopamine depletion on information flow between the subthalamic nucleus and external globus pallidus. J Neurophysiol. 2011;106:2012–2023. doi: 10.1152/jn.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aoust JP, Tiberi M. Role of the extracellular amino terminus and first membrane-spanning helix of dopamine D1 and D5 receptors in shaping ligand selectivity and efficacy. Cell Signal. 2010;22:106–116. doi: 10.1016/j.cellsig.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Demchyshyn LL, McConkey F, Niznik HB. Dopamine D5 receptor agonist high affinity and constitutive activity profile conferred by carboxyl-terminal tail sequence. J Biol Chem. 2000;275:23446–23455. doi: 10.1074/jbc.M000157200. [DOI] [PubMed] [Google Scholar]
- Dutton A, Dyball RE. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979;290:433–440. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Duncan GE, Fornaretto MG, Dearry A, Gingrich JA, Breese GR, Caron MG. Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proc Natl Acad Sci U S A. 1991;88:3772–3776. doi: 10.1073/pnas.88.9.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24:19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- Haynes WI, Haber SN. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. J Neurosci. 2013;33:4804–4814. doi: 10.1523/JNEUROSCI.4674-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Périer C, Orieux G, François C, Féger J, Yelnik J, Vila M, Levy R, Tolosa ES, Marin C, Trinidad Herrero M, Obeso JA, Agid Y. Metabolic effects of nigrostriatal denervation in basal ganglia. Trends Neurosci. 2000;23:S78–S85. doi: 10.1016/S1471-1931(00)00021-5. [DOI] [PubMed] [Google Scholar]
- Kaneoke Y, Vitek JL. Burst and oscillation as disparate neuronal properties. J Neurosci Methods. 1996;68:211–223. doi: 10.1016/0165-0270(96)00081-7. [DOI] [PubMed] [Google Scholar]
- Laffray S, Bouali-Benazzouz R, Papon MA, Favereaux A, Jiang Y, Holm T, Spriet C, Desbarats P, Fossat P, Le Feuvre Y, Decossas M, Héliot L, Langel U, Nagy F, Landry M. Impairment of GABAB receptor dimer by endogenous 14-3-3ζ in chronic pain conditions. EMBO J. 2012;31:3239–3251. doi: 10.1038/emboj.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal DK, Gage GJ, Schmidt R, Pettibone JR, Case AC, Berke JD. Basal ganglia beta oscillations accompany cue utilization. Neuron. 2012;73:523–536. doi: 10.1016/j.neuron.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Lang AE, Dostrovsky JO, Pahapill P, Romas J, Saint-Cyr J, Hutchison WD, Lozano AM. Lidocaine and muscimol microinjections in subthalamic nucleus reverse Parkinsonian symptoms. Brain. 2001;124:2105–2118. doi: 10.1093/brain/124.10.2105. [DOI] [PubMed] [Google Scholar]
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Rudehill A, Sollevi A, Theodorsson-Norheim E, Hamberger B. Frequency- and reserpine-dependent chemical coding of sympathetic transmission: differential release of noradrenaline and neuropeptide Y from pig spleen. Neurosci Lett. 1986;63:96–100. doi: 10.1016/0304-3940(86)90020-0. [DOI] [PubMed] [Google Scholar]
- Luo J, Kaplitt MG, Fitzsimons HL, Zuzga DS, Liu Y, Oshinsky ML, During MJ. Subthalamic GAD gene therapy in a Parkinson's disease rat model. Science. 2002;298:425–429. doi: 10.1126/science.1074549. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience. 2001;106:313–330. doi: 10.1016/S0306-4522(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MW, Scott AW, Johnston DE, Jr, Griffin S, Luedtke RR. Typical antipsychotics exhibit inverse agonist activity at rat dopamine D1-like receptors expressed in Sf9 cells. Eur J Pharmacol. 2001;420:73–82. doi: 10.1016/S0014-2999(01)00982-7. [DOI] [PubMed] [Google Scholar]
- Miguelez C, Morin S, Martinez A, Goillandeau M, Bezard E, Bioulac B, Baufreton J. Altered pallido-pallidal synaptic transmission leads to aberrant firing of globus pallidus neurons in a rat model of Parkinson's disease. J Physiol. 2012;590:5861–5875. doi: 10.1113/jphysiol.2012.241331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H, Kita H, Kitai ST. Electrical membrane properties of rat subthalamic neurons in an in vitro slice preparation. Brain Res. 1987;437:35–44. doi: 10.1016/0006-8993(87)91524-1. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gao D, Bouali-Benazzouz R, Benabid AL, Benazzouz A. Effect of microiontophoretic application of dopamine on subthalamic nucleus neuronal activity in normal rats and in rats with unilateral lesion of the nigrostriatal pathway. Eur J Neurosci. 2001a;14:373–381. doi: 10.1046/j.0953-816x.2001.01644.x. [DOI] [PubMed] [Google Scholar]
- Ni Z, Bouali-Benazzouz R, Gao D, Benabid AL, Benazzouz A. Intrasubthalamic injection of 6-hydroxydopamine induces changes in the firing rate and pattern of subthalamic nucleus neurons in the rat. Synapse. 2001b;40:145–153. doi: 10.1002/syn.1036. [DOI] [PubMed] [Google Scholar]
- O'Connor KA, Porrino LJ, Davies HM, Childers SR. Time-dependent changes in receptor/G-protein coupling in rat brain following chronic monoamine transporter blockade. J Pharmacol Exp Ther. 2005;313:510–517. doi: 10.1124/jpet.104.078451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15:3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic; 1996. [DOI] [PubMed] [Google Scholar]
- Périer C, Vila M, Féger J, Agid Y, Hirsch EC. Functional activity of zona incerta neurons is altered after nigrostriatal denervation in hemiparkinsonian rats. Exp Neurol. 2000;162:215–224. doi: 10.1006/exnr.1999.7331. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Wichmann T. Extrastriatal dopaminergic circuits of the basal ganglia. Front Neuroanat. 2010;4:139. doi: 10.3389/fnana.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin P, Manrique C, Forni C, Kerkerian-Le Goff L. High-frequency stimulation of the subthalamic nucleus selectively reverses dopamine denervation-induced cellular defects in the output structures of the basal ganglia in the rat. J Neurosci. 2002;22:5137–5148. doi: 10.1523/JNEUROSCI.22-12-05137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Childers SR. In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5′-[gamma-[35S]thio]-triphosphate binding. Proc Natl Acad Sci U S A. 1995;92:7242–7246. doi: 10.1073/pnas.92.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Villalba R. Striatal and extrastriatal dopamine in the basal ganglia: an overview of its anatomical organization in normal and Parkinsonian brains. Mov Disord. 2008;23(Suppl 3):S534–S547. doi: 10.1002/mds.22027. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Bevan MD. “The little engine that could”: voltage-dependent Na(+) channels and the subthalamic nucleus. Neuron. 2003;39:5–6. doi: 10.1016/S0896-6273(03)00400-8. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C. Dopamine D1/5 receptor stimulation induces c-fos expression in the subthalamic nucleus: possible involvement of local D5 receptors. Eur J Neurosci. 2002;15:133–142. doi: 10.1046/j.0953-816x.2001.01840.x. [DOI] [PubMed] [Google Scholar]
- Tai CH, Boraud T, Bezard E, Bioulac B, Gross C, Benazzouz A. Electrophysiological and metabolic evidence that high-frequency stimulation of the subthalamic nucleus bridles neuronal activity in the subthalamic nucleus and the substantia nigra reticulata. FASEB J. 2003;17:1820–1830. doi: 10.1096/fj.03-0163com. [DOI] [PubMed] [Google Scholar]
- Tai CH, Pan MK, Lin JJ, Huang CS, Yang YC, Kuo CC. Subthalamic discharges as a causal determinant of parkinsonin motor deficits. Ann Neurol. 2012;72:464–476. doi: 10.1002/ana.23618. [DOI] [PubMed] [Google Scholar]
- Tiberi M, Caron MG. High agonist-independent activity is a distinguishing feature of the dopamine D1B receptor subtype. J Biol Chem. 1994;269:27925–27931. [PubMed] [Google Scholar]
- Tseng KY, Kargieman L, Gacio S, Riquelme LA, Murer MG. Consequences of partial and severe dopaminergic lesion on basal ganglia oscillatory activity and akinesia. Eur J Neurosci. 2005;22:2579–2586. doi: 10.1111/j.1460-9568.2005.04456.x. [DOI] [PubMed] [Google Scholar]
- Tumova K, Iwasiow RM, Tiberi M. Insight into the mechanism of dopamine D1-like receptor activation. Evidence for a molecular interplay between the third extracellular loop and the cytoplasmic tail. J Biol Chem. 2003;278:8146–8153. doi: 10.1074/jbc.M208059200. [DOI] [PubMed] [Google Scholar]
- Tumova K, Zhang D, Tiberi M. Role of the fourth intracellular loop of D1-like dopaminergic receptors in conferring subtype-specific signaling properties. FEBS Lett. 2004;576:461–467. doi: 10.1016/j.febslet.2004.09.059. [DOI] [PubMed] [Google Scholar]
- Vila M, Périer C, Féger J, Yelnik J, Faucheux B, Ruberg M, Raisman-Vozari R, Agid Y, Hirsch EC. Evolution of changes in neuronal activity in the subthalamic nucleus of rats with unilateral lesion of the substantia nigra assessed by metabolic and electrophysiological measurements. Eur J Neurosci. 2000;12:337–344. doi: 10.1046/j.1460-9568.2000.00901.x. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Bevan MD. Intrinsic dynamics and synaptic inputs control the activity patterns of subthalamic nucleus neurons in health and in Parkinson's disease. Neuroscience. 2011;198:54–68. doi: 10.1016/j.neuroscience.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]