Abstract
Organoids are three dimensional structures consisting of multiple cell types that recapitulate the cellular architecture and functionality of native organs. Over the last decade, the advent of organoid research has opened up many avenues for basic and translational studies. Following suit of other disciplines, research groups working in the field of male reproductive biology have started establishing and characterizing testicular organoids. The three-dimensional architectural and functional similarities of organoids to their tissue of origin facilitate study of complex cell interactions, tissue development and establishment of representative, scalable models for drug and toxicity screening. In this review, we discuss the current state of testicular organoid research, their advantages over conventional monolayer culture and their potential applications in the field of reproductive biology and toxicology.
Keywords: testis, organoid, morphogenesis, cell–cell interaction, toxicology
Introduction
Originally, the term organoid was used to refer to three-dimensional in vitro culture systems for tissue fragments [1, 2]. However, with recent successes in deriving organoids from primary dissociated cells and stem cells [3–13], the term organoid has evolved to include many different systems [14]. Fatehullah et al. proposed a definition of the term that currently appears to be the most accurate: ‘Here, we define an organoid as an in vitro 3D cellular cluster derived exclusively from primary tissue, embryonic stem cells, or induced pluripotent stem cells, capable of self-renewal and self-organization and exhibiting similar organ functionality as the tissue of origin’ [4]. Both primary cell and stem cell derived organoids fill different niches of biomedical research. Stem cell derived organoids can be used as an efficient model of organogenesis and development, whereas primary cell derived organoids are useful for drug-toxicity screening and studying the molecular mechanisms of organ specific functions.
Over the last decade, a large number of organoid systems from various organs have been reported, namely intestine [9], liver [15], vasculature [16], pancreas [17] and brain [18]. Because of their architectural and functional resemblance to their respective primary tissues in vivo, these organoids were shown to have widespread applications for the study of tissue development, disease modeling, and drug and toxicity screening.
Although organoids have been successfully derived from different organ systems, testicular organoids only gained attention relatively recently. Here, we discuss the different testicular organoids that have been reported and how such 3D testicular organoid model systems may play an important role in biomedical research, particularly in the field of reproductive toxicity.
Conventional Models of Reproductive Toxicology
Infertility affects 7% of all men [19], 23% of which is due to pathophysiological conditions. Environmental exposure to toxicants such as organic polychlorinated dibenzodioxins, dicarboximide fungicides and heavy metals also contributes to infertility [20–23]. Additionally, gonadotoxic effects from different chemotherapeutic modalities can lead to fertility impairment [24, 25]. Therefore, a robust model that can recapitulate the complex cell–cell communication of the testis in vivo is needed for early screening of different drug molecules and to study effects of different toxicants.
Animal Models
Testicular toxicity studies have traditionally been performed in rodents [26–29]. For example, Liu et al. characterized the testicular toxicity of 3-methyl-2-(1-hydroxyethyl) quinoxaline-N4-monoxide (M4), a metabolite of the synthetic antimicrobial agent Mequindox (MEQ). The authors reported that MEQ triggers oxidative stress, mitochondrial dysfunction and altered junctional protein expressions which lead to disrupted spermatogenesis in mice [26]. Animal models have also been widely used for assessing epigenetic effects of toxins in the testis [30–33]. Environmental exposure to chemicals such as Bisphenol A and phathlates can alter the methylation pattern of the promoter region of a number of different genes such as hippocalcin-like 1 (Hpcal1) genes [34]. It can also cause hypermethylation of estrogen receptor promoter regions in rodents [33]. Maternal exposure to Di-2-(ethylhexyl) phthalate (DEHP) leads to increased DNA methylation and upregulation of DNA methyltransferases in mouse testis [31, 32]. Although experiments such as these have provided important information about the effects of different drugs and toxicants, studies performed in rodents often translate poorly to humans because rodent physiology differs appreciably from humans or large animals [35, 36]. Rodents exhibit genomic responses to inflammatory diseases that are quite different from humans [37]. The activity of certain liver enzymes can also vary between rodents and humans [38] and whole animal models are expensive to maintain [39].
Two-Dimensional Monolayer Culture
Testicular cells (primary or immortalized) cultured on plastic tissue culture plates, due to their ease and low cost of maintenance, have been the standard platform for understanding male reproductive biology and for drug and toxicity screens in vitro. Co-cultures of primary and immortalized somatic (Sertoli, peritubular myoid and Leydig cells) cells and germ cells facilitated study of cell–cell and cell–ECM interactions [40–43]. In 1985, Hadley et al. using Sertoli cell 2D culture and Sertoli-myoid cell co-culture described the important role of the basement membrane in the testicular microenvironment [43]. Sertoli cells grown on reconstituted basement membrane could form polarized monolayers similar to in vivo. They also maintained tight junctions and undifferentiated germ cells [43]. Co-cultures of testicular cells have also been used to investigate the effects of hormones such as FSH; growth factors such as HGF, FGF2 and FGF9; signalling molecules, drugs and environmental toxicants such as Bisphenol A, and reactive oxygen species on testicular somatic and germ cells [42, 44–50]. Although, these 2D culture modalities have provided us with much information on testicular biology and toxicology, they often fail to mimic organ specific toxicity [51, 52].These 2D cultures, often grown on rigid and planar surfaces modify cellular architecture and can lead to inappropriate and biologically irrelevant cell–cell interactions [53–55].
Organ Culture
Organ culture methods were applied to address the lack of 3D cell–cell interactions of 2D culture. In organ culture, small testicular tissue fragments rather than single cells are placed in cultures [56]. In 2011, Sato et al. reported the birth of healthy mice after intra-cytoplasmic sperm injection of sperm produced by in vitro organ culture [57]. Since then a number of groups have reported using organ cultures to study spermatogenesis in rodents [58–61] and bovids [62]. These testis organ culture systems can also be used for assessing reproductive toxicity. A proof of principle was recently reported by Nakamura et al., where testis fragments were treated with increasing dosages of ethinylestradiol (EE), a well‐known testicular toxicant. EE treatment led to a reduction in viable germ cells and a reduction of estrogen receptor 1, cytochrome P450, family 11, subfamily a, and polypeptide 1 in a dose-dependent manner [63]. It was also shown that organ culture of rat fetal testes can recapitulate the epigenetic reprogramming in gonocytes [64] indicating that these models could be used for assessing testicular epigenetics.
Testicular Tissue and Cell Grafting
Although organ culture models have been used successfully to study testicular biology and toxicity in rodents, the system falls short when it comes to studying large animals or humans. Testicular organ culture also does not allow study of testicular morphogenesis. Autologous and xenogeneic transplantation of testicular tissue and cells were developed to address these shortcomings [65–69]. Autologous transplantation of cryopreserved prepubertal primate tissue supports production of fertilization competent sperm [69]. Xenotransplantation of testicular tissue from different animal species into immunodeficient mice also results in spermatogenesis [65–68]. Since the metabolism of toxicants such as phthalates is qualitatively similar between human and mouse [70], testis tissue xenografting is a unique model for toxicological assays. It also allows for reproductive toxicological studies on testicular tissue from non-human primates or humans where in vivo experiments cannot be performed due to ethical or regulatory issues [71, 72]. For example, chronic exposure of mice carrying testicular tissue fragments as grafts from pre-pubertal rhesus macaques to phthalate esters revealed that long-term, low-dose [0, 10, 500 mg/kg Di-n-Butyl and Di-(2-EthylHexyl)] exposures led to impaired steroidogenesis and spermatogenesis in a dose-dependent manner [72]. Reconstitution of functional testis tissue from xenografted testicular cells is a complementary bioassay where cells from a pre-pubertal donor are grafted ectopically to immunocompromised mice [73]. Xenografted cells are capable of re-establishing the germ cell niche environment and can support full spermatogenesis. The system can be utilized to study the effects of different environmental and experimental factors on reproductive function [74, 75].
In V itro Tubule Reconstitution
Although grafting of testicular tissue or cells provides a powerful platform to study testis function in different species, it is not without its shortcomings. The grafted tissue can experience hypoxic damage due to delayed vascularization from the host. It is also less accessible for manipulation and/or observation than an in vitro system. This led to the establishment of in vitro models of testicular reconstitution or morphogenesis where tubule like structures are generated from dissociated testicular single cells. Testicular cells from rodents and pigs cultured on supportive biomaterials such as ECM proteins or agar formed seminiferous tubule-like structures [76–79]. Dores et al. showed that in vitro tubule formation can be used as an assay to study the effect of an experimental agent namely Ciliobrevin D (an inhibitor of primary cilia) on testicular morphogenesis [78]. Since in vitro tubule formation depends entirely on cellular morphogenetic capacity with no external forces promoting a desired geometry, the tissue architecture of the de novo formed tubule may vary widely. It also requires a large number of cells, which can limit the utility of the system when dealing with limited samples such as those obtained from biopsies. Thus, model systems that allow reproducible recapitulation of architecture and function are needed. To address this need, testicular organoid models were investigated.
Testicular Organoid Models
3D testicular organoids can serve as an intermediate platform between 2D culture systems and animal models. Organoids can be used as a physiologically more relevant model system to study cell–cell interactions, development and tissue morphogenesis [80]. They also pave the way for high-throughput drug and toxicity screening with more reliable and biologically relevant readouts [3, 5, 7, 81].
So far, only a few groups have reported generating testicular organoids from testicular single cells [11–13, 82].
Baert et al. reported generation of human testicular organoids. Both adult and pre-pubertal (15-year-old) testicular cells were placed on decellularized adult testicular extra cellular matrix (ECM). Adult and prepubertal cells colonized, remodeled and compacted the ECM scaffold to generate spheroidal organoids. ECM scaffold-free cells also formed similar organoids. These organoids had no morphological similarity with human testis and did not produce a well-defined germ cell niche. However, they produced testosterone, inhibin B and several different cytokines such as interleukin 6. The Sertoli cells also expressed tight junction proteins similar to in vivo. The organoid model maintained undifferentiated germ cells for up to 4 weeks. This indicated the potential application of the system for studying effects of different drugs and toxicants on testicular paracrine signaling [82].
Alves-Lopes et al. described generation of testicular organoid from 20d old rat testicular cells using a novel three-layer Matrigel gradient system. The Sertoli and germ cells in this organoid system formed spherical tubular structures. Although, peritubular myoid cells were present, they did not appear to actively participate in the self organization process. Sertoli cells in the organoid gave rise to a functional blood testes barrier. The spherical tubules could maintain undifferentiated germ cells for up to 21 days. The authors also reported that their organoids were responsive to retinoic acid treatment similar to previous reports [83–85] and were sensitive to the pro-inflammatory cytokines tumor necrosis factor alpha (TNFα) and interleukin 1 alpha (IL1α) leading to impaired organoid formation, reduction of germ cell maintenance and loss of blood-testes barrier integrity as previously reported for testis in vivo [86–88]. This proof of principle points to the utility of the model for assaying the effect of experimental factors and drugs on testicular function [11].
Pendergraft et al. described generation of a human testicular organoid system using adult germ cells and immortalized Leydig and Sertoli cells using the hanging drop culture system. The culture media was supplemented with solubilized human testis ECM. These organoids could be maintained in culture for up to 21 days and produced testosterone. Although the organoid lacked testicular tissue architecture, it appeared to support haploid germ cell transition. The authors assessed four different cytotoxic compounds: busulfan, cisplatin, doxorubicin and etoposide to evaluate the model’s utility for toxicity screening [12]. Organoids were exposed to increasing concentrations of the compounds for 48 h, which lead to a dose-dependent decrease in viability and increase in apoptotic cells. Organoids also displayed IC50 values significantly higher than corresponding 2D cultures. Another report by the same group described using organoids to model Zika virus infection. Testicular organoids were generated and then infected with Zika virus, effectively showing a reduction in testicular cell viability and decline in testosterone production. This suggests that testicular organoids can serve as a tool for infectious disease modeling [13].
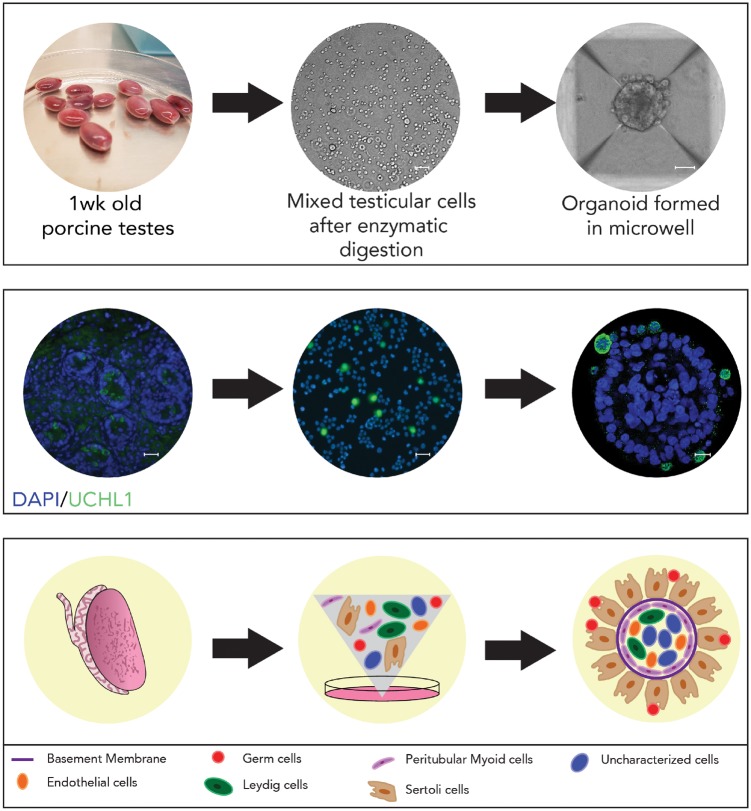
Recently, our group generated and characterized a testicular organoid model from pre-pubertal porcine testicular cells by using a microwell centrifugal aggregation system (see Fig. 1). The resulting organoids have a tissue architecture that is similar to testis in vivo [89]. These organoids have a clearly delineated exterior (seminiferous epithelium) and interior compartment (interstitial) separated by the basement membrane. Germ cells and Sertoli cells are in the exterior compartment. The peritubular myoid cells are localized along the interior of the basement membrane and the Leydig and endothelial cells are at the core of the interior compartment (see Fig. 1). We demonstrated that our organoid generation methodology is widely applicable across species, including mice, primates and humans. The Sertoli cells in these organoids express tight junction proteins. Germ cells in the organoids displayed an attenuated response to retinoic acid stimulation compared with conventional 2D culture indicating that the tissue architecture in the organoid modulates response to retinoic acid similar to testis in vivo [90]. Germ cells in organoids had fewer autophagosomes than those in 2D culture. Autophagy is a self-degradation and recycling mechanism that occurs at a basal level in every tissue [91, 92] and is an important process for normal protein turnover and maintenance of homeostasis [92, 93]. As a stress response mechanism autophagy serves to clear accumulating proteins and organelles crucial for the continuous renovation of the cell [94, 95]. Lower numbers of autophagosome in germ cells in organoids indicate reduced cellular stress when physiological cell interactions are maintained compared with cells in monolayer culture. Environmental toxicants can also trigger autophagy [93, 96] and autophagy as a biomarker for toxicity within the male reproductive tract has been described [97–99]. Exposure to increasing doses of di-(2-ethylhexyl) phthalate, a commonly used plasticizer, induced an increase in the number of autophagosomes in germ cells in a dose-dependent manner in 2D culture [99] and this observation could be replicated in organoids [89]. Similarly, exposure of cells to a small molecule inhibitor of primary cilia led to a loss of morphogenic capacity. These initial observations point towards the utility of testicular organoid systems for screening the effects of drugs and toxins on morphogenesis and cell function [89].
Figure 1:
testicular organoid formation in microwell culture. Top panel (left to right): 1-Week old porcine testes are enzymatically digested into single cells (Scale bar = 20 µm), which undergo self organization into an organoid (Scale bar = 50 µm) after 5 days of culture in microwell. Middle panel (left to right): Immunofluorescence characterization of testis cells and organoid. DAPI-nuclear stain, UCHL1-germ cell marker. Scale bars = 20 µm. Bottom panel (left to right): A schematic representation of testicular organoid formation from testis tissue-derived single cells undergoing self-organization in microwells. This figure has been modified from Sakib et al. [89].
Potential Applications in Male Reproductive Biology and Toxicology
An effective model of in vitro spermatogenesis, particularly for non-rodent mammalian species remains elusive. As spermatogenesis is a multifactorial complex process which requires the coordination of germ cells and testicular somatic cells [100], an in vitro model that maintains testis specific cell associations is essential. Studies have shown that testis specific architecture is required for germ cell homeostasis [90] and a number of paracrine factors, such as glial cell line-derived neurotropic factor, colony-stimulating factor 1 [101–105] and signalling molecules such as wnt6 and wnt3a released by somatic cells are required for germ cell maintenance [106, 107]. Thus, a testicular organoid model with testis specific architecture and function can serve as a bridge between 2D culture and animal models. A 3D organoid composed of all different testicular cell types provides an accessible in vitro model to inform a more thorough understanding of how germ cells interact with their niche. Drug and toxicity screening in such models would provide more physiologically relevant readouts than 2D culture modalities [51, 108].
Primordial germ cells can undergo a series of epigenetic modifications such as the erasure of parental imprinting and demethylation during development. Environmental toxicants like bisphenol A and phthalates can cause epigenetic changes such as DNA methylation, histone modifications, and expression of different non-coding RNAs which can impact testicular functions [31–33]. Such epigenetic changes can happen to not only in germ cells but also in different testicular somatic cells in a transgenerational manner [109]. Studying such epigenetic mechanisms using a 3D organoid system may more accurately recapitulate the situation in vivo.
Testicular organoids generated from primary cells can also be an important tool for disease modeling. As the starting cell populations used are single cells [11–13, 82], different cell types can be isolated and genetically modified or exposed to environmental factors and then recombined to generate organoids with specific disease phenotypes. Testicular cancer tissue could be used to generate organoid models of testicular malignancy. Such models would be invaluable for early testing of pharmaceutical and chemotherapeutic interventions.
Organoids can also be a great boon for the field of development and regenerative medicine. Existing protocols for derivation of germ cells from induced pluripotent cells (iPSCs) remain inefficient [110, 111]. Combining these existing protocols with testicular organoids could enhance germ cell derivation efficiency. These protocols may also be combined with iPSC derived somatic cell derivation protocols [112–114] to generate entire testicular organoids from iPSCs. This could provide a powerful model for understanding testicular development as shown in other organoid systems [80, 115], allowing us to study and investigate therapeutic interventions to congenital male infertility syndromes.
Concluding Remarks
Testicular organoids that recapitulate testicular cytoarchitecture and function allow for a more thorough investigation of the germ cell niche. This in turn can lead to development of better interventions to regulate and modify germ cell proliferation and differentiation in vitro. It would also pave way for a more reliable model of testicular development and disease, and a platform to test experimental and environmental factors with readouts expected to mimic in vivo conditions more closely allowing for quicker translation to clinical applications.
Conflict of interest statement. None declared.
References
- 1. Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ.. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development 2001;128:3117–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ.. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 1989;105:223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Czerniecki SM, Cruz NM, Harder JL, Menon R, Annis J, Otto EA, Gulieva RE, Islas LV, Kim YK, Tran LM. et al High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell 2018;22:929–40.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fatehullah A, Tan SH, Barker N.. Organoids as an in vitro model of human development and disease. Nat Cell Biol 2016;18:246–54. [DOI] [PubMed] [Google Scholar]
- 5. Forsythe SD, Devarasetty M, Shupe T, Bishop C, Atala A, Soker S, Skardal A.. Environmental toxin screening using human-derived 3D bioengineered liver and cardiac organoids. Front Public Health 2018;6:103.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huch M, Koo B-K.. Modeling mouse and human development using organoid cultures. Development 2015;142:3113.. [DOI] [PubMed] [Google Scholar]
- 7. Jabs J, Zickgraf FM, Park J, Wagner S, Jiang X, Jechow K, Kleinheinz K, Toprak UH, Schneider MA, Meister M. et al Screening drug effects in patient‐derived cancer cells links organoid responses to genome alterations. Mol Syst Biol 2017;13:955.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matano M, Date S, Shimokawa M, Takano A, Fujii M, Ohta Y, Watanabe T, Kanai T, Sato T.. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med 2015;21:256–62. [DOI] [PubMed] [Google Scholar]
- 9. Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ. et al Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009;459:262–5. [DOI] [PubMed] [Google Scholar]
- 10. Schepers A, Li C, Chhabra A, Seney BT, Bhatia S.. Engineering a perfusable 3D human liver platform from iPS cells. Lab Chip 2016;16:2644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alves-Lopes JP, Soder O, Stukenborg JB.. Testicular organoid generation by a novel in vitro three-layer gradient system. Biomaterials 2017;130:76–89. [DOI] [PubMed] [Google Scholar]
- 12. Pendergraft SS, Sadri-Ardekani H, Atala A, Bishop CE.. Three-dimensional testicular organoid: a novel tool for the study of human spermatogenesis and gonadotoxicity in vitrodagger. Biol Reprod 2017;96:720–32. [DOI] [PubMed] [Google Scholar]
- 13. Strange DP, Zarandi NP, Trivedi G, Atala A, Bishop CE, Sadri-Ardekani H, Verma S.. Human testicular organoid system as a novel tool to study Zika virus pathogenesis. Emerg Microb Infect 2018;7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simian M, Bissell MJ.. Organoids: a historical perspective of thinking in three dimensions. J Cell Biol 2017;216:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N. et al Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013;499:481–4. [DOI] [PubMed] [Google Scholar]
- 16. Morgan JP, Delnero PF, Zheng Y, Verbridge SS, Chen J, Craven M, Choi NW, Diaz-Santana A, Kermani P, Hempstead B. et al Formation of microvascular networks in vitro. Nat Protoc 2013;8:1820–36. [DOI] [PubMed] [Google Scholar]
- 17. Boj SF, Hwang C-I, Baker LA, Chio C II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS. et al Organoid models of human and mouse ductal pancreatic cancer. Cell 2015;160:324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, Maria N, Scholvin J, Goldman M, Kinney JP. et al Cell diversity and network dynamics in photosensitive human brain organoids. Nature 2017;545:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krausz C. Male infertility: pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab 2011;25:271–85. [DOI] [PubMed] [Google Scholar]
- 20. Kashir J, Heindryckx B, Jones C, De Sutter P, Parrington J, Coward K.. Oocyte activation, phospholipase C zeta and human infertility. Hum Reprod Update 2010;16:690–703. [DOI] [PubMed] [Google Scholar]
- 21. Cheng CY. Toxicants target cell junctions in the testis: insights from the indazole-carboxylic acid model. Spermatogenesis 2014;4:e981485.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharpe RM. Environmental/lifestyle effects on spermatogenesis. Philos Trans R Soc B 2010;365:1697–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siu ER, Mruk DD, Porto CS, Cheng CY.. Cadmium-induced testicular injury. Toxicol Appl Pharmacol 2009;238:240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee SH, Shin CH.. Reduced male fertility in childhood cancer survivors. Ann Pediatr Endocrinol Metab 2013;18:168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schrader M, Heicappell R, Muller M, Straub B, Miller K.. Impact of chemotherapy on male fertility. Onkologie 2001;24:326–30. [DOI] [PubMed] [Google Scholar]
- 26. Liu Q, Lei Z, Huang A, Lu Q, Wang X, Ahmed S, Awais I, Yuan Z.. Mechanisms of the testis toxicity induced by chronic exposure to mequindox. Front Pharmacol 2017;8:679.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaido KW, Hensley JB, Liu D, Wallace DG, Borghoff S, Johnson KJ, Hall SJ, Boekelheide K.. Fetal mouse phthalate exposure shows that Gonocyte multinucleation is not associated with decreased testicular testosterone. Toxicol Sci 2007;97:491–503. [DOI] [PubMed] [Google Scholar]
- 28. Akingbemi BT, Youker RT, Sottas CM, Ge R, Katz E, Klinefelter GR, Zirkin BR, Hardy MP.. Modulation of rat Leydig cell steroidogenic function by di(2-ethylhexyl)phthalate. Biol Reprod 2001;65:1252–9. [DOI] [PubMed] [Google Scholar]
- 29. Park JD, Habeebu SS, Klaassen CD.. Testicular toxicity of di-(2-ethylhexyl)phthalate in young Sprague-Dawley rats. Toxicology 2002;171:105–15. [DOI] [PubMed] [Google Scholar]
- 30. Akinjo OO, Gant TW, Marczylo EL.. Perturbation of epigenetic processes by doxorubicin in the mouse testis. Toxicol Res 2016;5:1229–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singh S, Li SS.. Epigenetic effects of environmental chemicals bisphenol A and phthalates. IJMS 2012;13:10143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu S, Zhu J, Li Y, Lin T, Gan L, Yuan X, Xu M, Wei G.. Dynamic effect of di-2-(ethylhexyl) phthalate on testicular toxicity: epigenetic changes and their impact on gene expression. Int J Toxicol 2010;29:193–200. [DOI] [PubMed] [Google Scholar]
- 33. Doshi T, Mehta SS, Dighe V, Balasinor N, Vanage G.. Hypermethylation of estrogen receptor promoter region in adult testis of rats exposed neonatally to bisphenol A. Toxicology 2011;289:74–82. [DOI] [PubMed] [Google Scholar]
- 34. Tang WY, Morey LM, Cheung YY, Birch L, Prins GS, Ho SM.. Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs of Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology 2012;153:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. González R, Dobrinski I.. Beyond the mouse monopoly: studying the male germ line in domestic animal models. ILAR J 2015;56:83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gutierrez K, Dicks N, Glanzner WG, Agellon LB, Bordignon V.. Efficacy of the porcine species in biomedical research. Front Genet 2015;6:293.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L. et al Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 2013;110:3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zanger UM, Schwab M.. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 2013;138:103–41. [DOI] [PubMed] [Google Scholar]
- 39. Freires IA, Sardi JC, de Castro RD, Rosalen PL.. Alternative animal and non-animal models for drug discovery and development: bonus or burden? Pharm Res 2017;34:681–686. [DOI] [PubMed] [Google Scholar]
- 40. Richardson LL, Kleinman HK, Dym M.. Basement membrane gene expression by Sertoli and peritubular myoid cells in vitro in the rat. Biol Reprod 1995;52:320–30. [DOI] [PubMed] [Google Scholar]
- 41. Hofmann MC, Narisawa S, Hess RA, Millan JL.. Immortalization of germ cells and somatic testicular cells using the SV40 large T antigen. Exp Cell Res 1992;201:417–35. [DOI] [PubMed] [Google Scholar]
- 42. Kierszenbaum AL, Crowell JA, Shabanowitz RB, DePhilip RM, Tres LL.. Protein secretory patterns of rat Sertoli and peritubular cells are influenced by culture conditions. Biol Reprod 1986;35:239–51. [DOI] [PubMed] [Google Scholar]
- 43. Hadley MA, Byers SW, Suarez-Quian CA, Kleinman HK, Dym M.. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J Cell Biol 1985;101:1511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tung PS, Fritz IB.. Morphogenetic restructuring and formation of basement membranes by Sertoli cells and testis peritubular cells in co-culture: inhibition of the morphogenetic cascade by cyclic AMP derivatives and by blocking direct cell contact. Dev Biol 1987;120:139–53. [DOI] [PubMed] [Google Scholar]
- 45. van der Wee K, Hofmann MC.. An in vitro tubule assay identifies HGF as a morphogen for the formation of seminiferous tubules in the postnatal mouse testis. Exp Cell Res 1999;252:175–85. [DOI] [PubMed] [Google Scholar]
- 46. El Ramy R, Verot A, Mazaud S, Odet F, Magre S, Le Magueresse-Battistoni B.. Fibroblast growth factor (FGF) 2 and FGF9 mediate mesenchymal-epithelial interactions of peritubular and Sertoli cells in the rat testis. J Endocrinol 2005;187:135–47. [DOI] [PubMed] [Google Scholar]
- 47. Saez JM, Sanchez P, Berthelon MC, Avallet O.. Regulation of pig Leydig cell aromatase activity by gonadotropins and Sertoli cells. Biol Reprod 1989;41:813–20. [DOI] [PubMed] [Google Scholar]
- 48. Hung JH, Chen CY, Omar HA, Huang KY, Tsao CC, Chiu CC, Chen YL, Chen PH, Teng YN.. Reactive oxygen species mediate Terbufos-induced apoptosis in mouse testicular cell lines via the modulation of cell cycle and pro-apoptotic proteins. Environ Toxicol 2016;31:1888–1898. [DOI] [PubMed] [Google Scholar]
- 49. Tesarik J, Guido M, Mendoza C, Greco E.. Human spermatogenesis in vitro: respective effects of follicle-stimulating hormone and testosterone on meiosis, spermiogenesis, and Sertoli cell apoptosis. J Clin Endocrinol Metab 1998;83:4467–73. [DOI] [PubMed] [Google Scholar]
- 50. Gong X, Xie H, Li X, Wu J, Lin Y.. Bisphenol A induced apoptosis and transcriptome differences of spermatogonial stem cells in vitro. Acta Biochim Biophys Sin (Shanghai) 2017;49:780–791. [DOI] [PubMed] [Google Scholar]
- 51. Abbott A. Cell culture: biology's new dimension. Nature 2003;424:870–2. [DOI] [PubMed] [Google Scholar]
- 52. Mazzoleni G, Di Lorenzo D, Steimberg N.. Modelling tissues in 3D: the next future of pharmaco-toxicology and food research? Genes Nutr 2009;4:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yamada KM, Cukierman E.. Modeling tissue morphogenesis and cancer in 3D. Cell 2007;130:601–10. [DOI] [PubMed] [Google Scholar]
- 54. Horvath P, Aulner N, Bickle M, Davies AM, Nery ED, Ebner D, Montoya MC, Östling P, Pietiäinen V, Price LS. et al Screening out irrelevant cell-based models of disease. Nat Rev Drug Discov 2016;15:751.. [DOI] [PubMed] [Google Scholar]
- 55. Pampaloni F, Reynaud EG, Stelzer EH.. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 2007;8:839–45. [DOI] [PubMed] [Google Scholar]
- 56. Steinberger A, Steinberger E, Perloff WH.. Mammalian testes in organ culture. Exp Cell Res 1964;36:19–27. [DOI] [PubMed] [Google Scholar]
- 57. Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T.. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011;471:504–7. [DOI] [PubMed] [Google Scholar]
- 58. Nakamura N, Merry GE, Inselman AL, Sloper DT, Del Valle PL, Sato T, Ogawa T, Hansen DK.. Evaluation of culture time and media in an in vitro testis organ culture system. Birth Defects Res 2017;109:465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gholami K, Pourmand G, Koruji M, Ashouri S, Abbasi M.. Organ culture of seminiferous tubules using a modified soft agar culture system. Stem Cell Res Ther 2018;9:249.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sanjo H, Komeya M, Sato T, Abe T, Katagiri K, Yamanaka H, Ino Y, Arakawa N, Hirano H, Yao T. et al In vitro mouse spermatogenesis with an organ culture method in chemically defined medium. PLoS One 2018;13:e0192884–e0192884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reda A, Hou M, Winton TR, Chapin RE, Soder O, Stukenborg JB.. In vitro differentiation of rat spermatogonia into round spermatids in tissue culture. Mol Hum Reprod 2016;22:601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim K-J, Kim B-G, Kim Y-H, Lee Y-A, Kim B-J, Jung S-E, Cho Y-J, Lee S-H, Ryu B-Y.. In vitro spermatogenesis using bovine testis tissue culture techniques. Tissue Eng Regen Med 2015;12:314–323. [Google Scholar]
- 63. Nakamura N, Sloper DT, Del Valle PL.. Evaluation of an in vitro mouse testis organ culture system for assessing male reproductive toxicity. Birth Defects Res 2019;111:70–77. [DOI] [PubMed] [Google Scholar]
- 64. Rwigemera A, Joao F, Delbes G.. Fetal testis organ culture reproduces the dynamics of epigenetic reprogramming in rat gonocytes. Epigenet Chromatin 2017;10:19.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Honaramooz A, Snedaker A, Boiani M, Scholer H, Dobrinski I, Schlatt S.. Sperm from neonatal mammalian testes grafted in mice. Nature 2002;418:778–81. [DOI] [PubMed] [Google Scholar]
- 66. Zeng W, Snedaker AK, Megee S, Rathi R, Chen F, Honaramooz A, Dobrinski I.. Preservation and transplantation of porcine testis tissue. Reprod Fertil Dev 2009;21:489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pukazhenthi BS, Nagashima J, Travis AJ, Costa GM, Escobar EN, Franca LR, Wildt DE.. Slow freezing, but not vitrification supports complete spermatogenesis in cryopreserved, neonatal sheep testicular xenografts. PLoS One 2015;10:e0123957.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu Z, Nie YH, Zhang CC, Cai YJ, Wang Y, Lu HP, Li YZ, Cheng C, Qiu ZL, Sun Q.. Generation of macaques with sperm derived from juvenile monkey testicular xenografts. Cell Res 2016;26:139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fayomi AP, Peters K, Sukhwani M, Valli-Pulaski H.. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 2019;363:1314–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kluwe WM. Overview of phthalate ester pharmacokinetics in mammalian species. Environ Health Perspect 1982;45:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jahnukainen K, Ehmcke J, Schlatt S.. Testicular xenografts: a novel approach to study cytotoxic damage in juvenile primate testis. Cancer Res 2006;66:3813–8. [DOI] [PubMed] [Google Scholar]
- 72. Rodriguez-Sosa JR, Bondareva A, Tang L, Avelar GF, Coyle KM, Modelski M, Alpaugh W, Conley A, Wynne-Edwards K, França LR. et al Phthalate esters affect maturation and function of primate testis tissue ectopically grafted in mice. Mol Cell Endocrinol 2014;398:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Honaramooz A, Megee SO, Rathi R, Dobrinski I.. Building a testis: formation of functional testis tissue after transplantation of isolated porcine (Sus scrofa) testis cells. Biol Reprod 2007;76:43–7. [DOI] [PubMed] [Google Scholar]
- 74. Dores C, Dobrinski I.. De novo morphogenesis of testis tissue: an improved bioassay to investigate the role of VEGF165 during testis formation. Reproduction 2014;148:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dores C, Rancourt D, Dobrinski I.. Stirred suspension bioreactors as a novel method to enrich germ cells from pre-pubertal pig testis. Andrology 2015;3:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Reda A, Hou M, Landreh L, Kjartansdottir KR, Svechnikov K, Soder O, Stukenborg JB.. In vitro spermatogenesis – optimal culture conditions for testicular cell survival, germ cell differentiation, and steroidogenesis in rats. Front Endocrinol (Lausanne) 2014;5:21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yokonishi T, Sato T, Katagiri K, Komeya M, Kubota Y, Ogawa T.. In vitro reconstruction of mouse seminiferous tubules supporting germ cell differentiation. Biol Reprod 2013;89:15.. [DOI] [PubMed] [Google Scholar]
- 78. Dores C, Alpaugh W, Su L, Biernaskie J, Dobrinski I.. Primary cilia on porcine testicular somatic cells and their role in hedgehog signaling and tubular morphogenesis in vitro. Cell Tissue Res 2017;368:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gassei K, Schlatt S, Ehmcke J.. De novo morphogenesis of seminiferous tubules from dissociated immature rat testicular cells in xenografts. J Androl 2006;27:611–8. [DOI] [PubMed] [Google Scholar]
- 80. Lancaster MA, Knoblich JA.. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 2014;345:1247125. [DOI] [PubMed] [Google Scholar]
- 81. McCauley HA, Wells JM.. Pluripotent stem cell-derived organoids: using principles of developmental biology to grow human tissues in a dish. Development (Cambridge, England) 2017;144:958–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Baert Y, De Kock J, Alves-Lopes JP, Soder O, Stukenborg JB, Goossens E.. Primary human testicular cells self-organize into organoids with testicular properties. Stem Cell Rep 2017;8:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Endo T, Freinkman E, de Rooij DG, Page DC.. Periodic production of retinoic acid by meiotic and somatic cells coordinates four transitions in mouse spermatogenesis. Proc Natl Acad Sci USA 2017;114:E10132.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li H, Palczewski K, Baehr W, Clagett-Dame M.. Vitamin A deficiency results in meiotic failure and accumulation of undifferentiated spermatogonia in prepubertal mouse testis. Biol Reprod 2011;84:336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. van Pelt AM, de Rooij DG.. Retinoic acid is able to reinitiate spermatogenesis in vitamin A-deficient rats and high replicate doses support the full development of spermatogenic cells. Endocrinology 1991;128:697–704. [DOI] [PubMed] [Google Scholar]
- 86. Sarkar O, Mathur PP, Cheng CY, Mruk DD.. Interleukin 1 alpha (IL1A) is a novel regulator of the blood-testis barrier in the rat. Biol Reprod 2008;78:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yan HH, Mruk DD, Lee WM, Cheng CY.. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J 2008;22:1945–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cheng CY, Mruk DD.. The blood-testis barrier and its implications for male contraception. Pharmacol Rev 2012;64:16–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sakib S, Uchida A, Valenzuela-Leon P, Yu Y, Valli-Pulaski H, Orwig K, Ungrin M, Dobrinski I.. Formation of organotypic testicular organoids in microwell culture. Biol Reprod 2019;100:1648–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lord T, Oatley MJ, Oatley JM.. Testicular architecture is critical for mediation of retinoic acid responsiveness by undifferentiated spermatogonial subtypes in the mouse. Stem Cell Rep 2018;10:538–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mizushima N. Autophagy: process and function. Genes Dev 2007;21:2861–73. [DOI] [PubMed] [Google Scholar]
- 92. Ravanan P, Srikumar IF, Talwar P.. Autophagy: the spotlight for cellular stress responses. Life Sci 2017;188:53–67. [DOI] [PubMed] [Google Scholar]
- 93. Aki T, Funakoshi T, Unuma K, Uemura K.. Impairment of autophagy: from hereditary disorder to drug intoxication. Toxicology 2013;311:205–215. [DOI] [PubMed] [Google Scholar]
- 94. Mizushima N, Komatsu M.. Autophagy: renovation of cells and tissues. Cell 2011;147:728–41. [DOI] [PubMed] [Google Scholar]
- 95. Feng Y, Yao Z, Klionsky DJ.. How to control self-digestion: transcriptional, post-transcriptional, and post-translational regulation of autophagy. Trends Cell Biol 2015;25:354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Scherz-Shouval R, Elazar Z.. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci 2011;36:30–8. [DOI] [PubMed] [Google Scholar]
- 97. Liu ML, Wang JL, Wei J, Xu LL, Yu M, Liu XM, Ruan WL, Chen JX.. Tri-ortho-cresyl phosphate induces autophagy of rat spermatogonial stem cells. Reproduction 2015;149:163–70. [DOI] [PubMed] [Google Scholar]
- 98. Han SP, Zhou DX, Lin P, Qin Z, An L, Zheng LR, Lei L.. Formaldehyde exposure induces autophagy in testicular tissues of adult male rats. Environ Toxicol 2015;30:323–31. [DOI] [PubMed] [Google Scholar]
- 99. Valenzuela-Leon P, Dobrinski I.. Exposure to phthalate esters induces an autophagic response in male germ cells. Environ Epigenet 2017;3:dvx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Oatley JM, Brinster RL.. The germline stem cell niche unit in mammalian testes. Physiol Rev 2012;92:577–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M. et al Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2000;287:1489–93. [DOI] [PubMed] [Google Scholar]
- 102. Kubota H, Avarbock MR, Brinster RL.. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA 2004;101:16489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Oatley MJ, Racicot KE, Oatley JM.. Sertoli cells dictate spermatogonial stem cell niches in the mouse testis. Biol Reprod 2011;84:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL.. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development 2009;136:1191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yoshida S, Sukeno M, Nakagawa T, Ohbo K, Nagamatsu G, Suda T, Nabeshima Y-I.. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 2006;133:1495–1505. [DOI] [PubMed] [Google Scholar]
- 106. Takase HM, Nusse R.. Paracrine Wnt/beta-catenin signaling mediates proliferation of undifferentiated spermatogonia in the adult mouse testis. Proc Natl Acad Sci USA 2016;113:E1489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chassot AA, Le Rolle M, Jourden M, Taketo MM, Ghyselinck NB, Chaboissier MC.. Constitutive WNT/CTNNB1 activation triggers spermatogonial stem cell proliferation and germ cell depletion. Dev Biol 2017;426:17–27. [DOI] [PubMed] [Google Scholar]
- 108. Chapin RE, Boekelheide K, Cortvrindt R, van Duursen MB, Gant T, Jegou B, Marczylo E, van Pelt AM, Post JN, Roelofs MJ. et al Assuring safety without animal testing: the case for the human testis in vitro. Reprod Toxicol 2013;39:63–8. [DOI] [PubMed] [Google Scholar]
- 109. Guerrero-Bosagna C, Savenkova M, Haque MM, Nilsson E, Skinner MK.. Environmentally induced epigenetic transgenerational inheritance of altered Sertoli cell transcriptome and epigenome: molecular etiology of male infertility. PLoS One 2013;8:e59922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Easley CAT, Phillips BT, McGuire MM, Barringer JM, Valli H, Hermann BP, Simerly CR, Rajkovic A, Miki T, Orwig KE. et al Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep 2012;2:440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhao Y, Ye S, Liang D, Wang P, Fu J, Ma Q, Kong R, Shi L, Gong X, Chen W. et al In vitro modeling of human germ cell development using pluripotent stem cells. Stem Cell Rep 2018;10:509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rodríguez Gutiérrez D, Eid W, Biason-Lauber A.. A human gonadal cell model from induced pluripotent stem cells. Front Genet 2018;9:498–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bucay N, Yebra M, Cirulli V, Afrikanova I, Kaido T, Hayek A, Montgomery AM.. A novel approach for the derivation of putative primordial germ cells and sertoli cells from human embryonic stem cells. Stem Cells 2009;27:68–77. [DOI] [PubMed] [Google Scholar]
- 114. Yang Y, Su Z, Xu W, Luo J, Liang R, Xiang Q, Zhang Q, Ge RS, Huang Y.. Directed mouse embryonic stem cells into leydig-like cells rescue testosterone-deficient male rats in vivo. Stem Cells Dev 2015;24:459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM. et al Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015;526:564–8. [DOI] [PubMed] [Google Scholar]