Abstract
Metal-based multinuclear supramolecules with different functionalities designed by self-assembly represent a growing area of research due to their versatile applications, particularly as anticancer agents. Four novel boron dipyrromethene (BODIPY)-based octacationic heterometallic molecular squares, 3–6 were synthesized by self-assembly via reaction of dipyridyl BODIPY ligands with suitable 90° palladium and platinum acceptors. The formation of the as-synthesized molecular squares was confirmed by multinuclear NMR spectroscopy, elemental analysis, high resolution electrospray mass spectrometry, UV–vis spectroscopy, and fluorescence spectroscopy. The square molecular structures of 4 and 6 were further rationalized theoretically using the PM7 semi-empirical method. The activities of the supramolecules against cancer cells were tested using cell lines of various malignant and nonmalignant origins. Complexes 3–6 showed high cytotoxicity toward cancer cells but 7.0 to 15.2 times lower cytotoxic effects were observed against nonmalignant human kidney epithelial cells (HEK-293). Particularly, complexes 3–6 provided 2.1–6.0 times lower IC50 values as compared to cisplatin in HCT116 cells. Interestingly, BDP ligand-containing complexes (3 and 4) induced cytotoxicity through apoptosis, whereas BDPCC-based complexes (5 and 6) induced cell death by necrosis. This study presents a novel series of iron-based heteroatomic palladium and platinum complexes that exhibit substantial potential as drug candidates for anticancer therapy.
Introduction
Coordination-driven self-assembly for the design of multinuclear well-defined molecular structures is a convenient technique to develop large interesting supramolecules and metal organic compounds.1−6 Among the studied self-assembled supramolecules, macrocycles are commonly used owing to their different functionalities, which can be finely tuned based on the chemical and physical requirements.1−8 Since the initial development of palladium-based square-shaped macrocycles by Fujita et al. and Stang and Cao9,10 via self-assembly, several macrocycles with distinct triangular, square, and cage-type architectures have been reported.11−13 The size, shape, and functions of these architectures vary depending on the ligand and metal center used for the assembly.2−8,11,12 The overall properties of the architectures can be engineered to exhibit interesting physical and chemical properties that differ from those of the individual constituent materials.
Boron dipyrromethene (BODIPY) is a class of excellent fluorophores with important applications varying from biological labeling to light harvesting.14−16 Its physical and chemical properties together with its high stability are responsible for the attractiveness of this fluorescent material.17,18 The central BODIPY core can be extended further by adding different functionalizations without significant change to its optical properties. Recently, pyridine-functionalized BODIPY ligands were introduced by Hupp et al. to develop metal–organic frameworks with interesting light-harvesting properties.19 More recently, Lee et al. introduced dipyridyl BODIPY ligands for the synthesis of self-assembled ruthenium and iridium metalla-rectangles for anticancer applications.20−24 Furthermore, Nitschke et al. developed several BODIPY-based metal supramolecules with sensing, host–guest, and other interesting physical properties.25−27 Although several BODIPY ligands have been used to develop metal-based supramolecules, macrocycles based on palladium or platinum metals have been rarely reported. Pistolis et al. designed the first BODIPY-based platinum macrocycles with a square architecture that exhibited interesting physical properties.28 Since this first report, the same group has developed several other square and hexagonal architectures and studied their physical properties, including the host–guest chemistry.29,30 Additionally, only a few studies have been reported where the biological activities of palladium or platinum macrocycles based on BODIPY are investigated.31,32
Furthermore, a biological study of heterometallic macrocycles containing palladium or platinum with diphenyl ferrocenyl moieties was recently reported by Chi and co-workers.33 Herein, we present a novel series of diphenyl ferrocene-appended heteroatomic palladium and platinum complexes with square architectures synthesized from dipyridyl BODIPY ligands. The abilities of the synthesized supramolecules against proliferating cancer cells were determined and compared with those obtained from standard cisplatin treatment. Furthermore, their abilities to penetrate the cell membrane were investigated and examination of the probable mechanism of cell death was attempted.
Results and Discussion
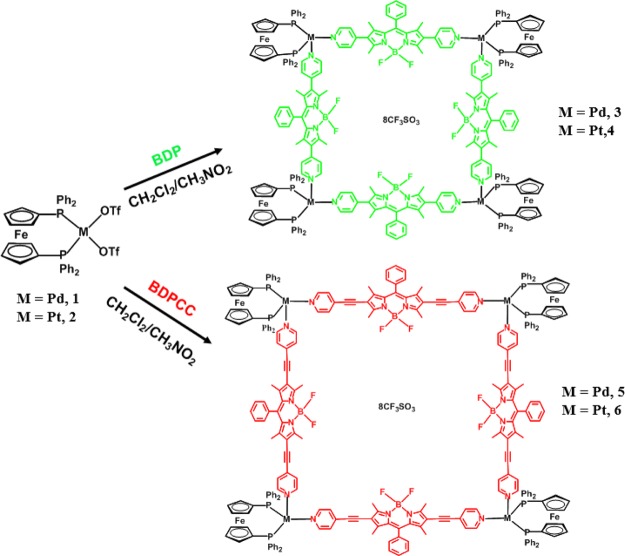
The reaction of heterometallic palladium iron triflate complex 1 and platinum iron triflate complex 2 with dipyridyl BODIPY (BDP and BDPCC) ligands in a mixed dichloromethane/nitromethane solution under nitrogen formed four novel self-assembled square complexes 3–6 (Scheme 1). The octanuclear complexes were isolated in good yields as their triflate salts and are soluble in common organic solvents. The complexes were fully characterized using different analytical techniques. Multinuclear NMR spectroscopy was used to examine the formation and purity of the new complexes. The 1H NMR spectra of complexes 3–6 in CDCl3 showed characteristic peaks corresponding to the starting precursor and BODIPY ligands (Experimental Section and Figure S1).
Scheme 1. Reaction Scheme for the Formation of Heteroatomic Molecular Squares 3–6 from 1, 2, BDP, and BDPCC.
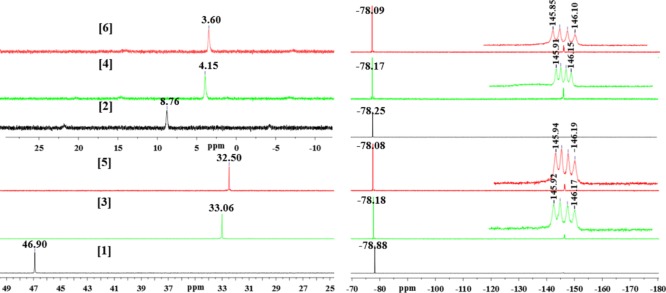
In addition, 31P and 19F NMR were used to confirm the formation of these macrocycles (Figure 1). The 31P NMR of the starting palladium precursor 1 showed a peak at 46.90 ppm. However, after self-assembly, this peak shifted upfield by approximately 13 ppm and peaks centered at 33.06 and 32.50 ppm appeared for 3 and 5, respectively. Similarly, the platinum precursor 2 showed a peak at 8.76 ppm, which after complexation with BDP and BDPCC ligands shifted upfield to peaks at 4.15 and 3.60 ppm for macrocycles 4 and 6, respectively. This upfield shift together with the single peak in the 31P NMR confirmed the presence of symmetric phosphorus nuclei in macrocycles 3–6. Similarly, the 19F NMR showed a sharp singlet at approximately −78 ppm, corresponding to the fluorine atom of the triflate molecule. In addition, a quartet between −145.85 and −146.19 ppm was observed in the 19F NMR spectra, originating from the fluorine atom of the BODIPY ligands in 3–6.
Figure 1.
31P NMR (left) and 19F NMR (right) of complexes 1–6 in acetone-d6.
Electrospray ionization mass spectrometry (ESI–MS) was used to confirm the formation of these macrocycles. ESI–MS spectra in acetonitrile showed tri-, tetra-, hexa-, and octa-cationic peaks corresponding to the loss of three to eight triflate anions. The peaks were centered at m/z 1287.57 and 569.16 for 3; m/z 1335.59 and 593.17 for 4; m/z 1376.68 and 613.68 for 5; and m/z 1424.60 and 637.75 for 6, originating from the tetra- and octacationic fragmentation, respectively. In addition, complex 4 showed a tricationic peak centered at m/z 1829.30, corresponding to the loss of three triflate anions, and the peaks centered at m/z 897.84 and 748.98 corresponded to the loss of six and seven triflate anions, respectively, for complex 6. The details of the peak assignments for these complexes are provided in the Experimental Section, and their spectra are shown in the Supporting Information (Figure S2).
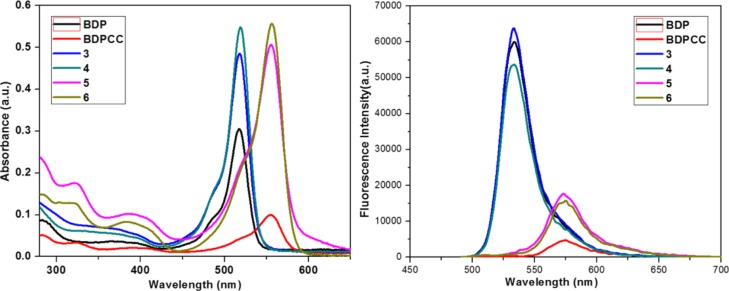
The photophysical properties of the free BODIPY ligands and prepared metal supramolecules were studied at room temperature in methanol solution. The absorption spectra of the supramolecules shown in Figure 2 exhibited major absorption peaks at around 518 nm for 3 and 4 and 556 nm for 5 and 6. These peaks originated from the BODIPY ligands present in these supramolecules and were assigned to the vibration band of the π–π* transition localized on the BODIPY chromophore. The emission properties of the free ligands and their corresponding metal complexes were also studied in methanol and are shown in Figure 2. The complexes were highly emissive due to the presence of the BODIPY chromophores. The emission peaks were centered at approximately 533 nm for 3 and 4 and 575 nm for 5 and 6.
Figure 2.
UV–vis (left) and fluorescence (right) spectra of the ligands BDP and BDPCC as well as supramolecules 3–6 in methanol.
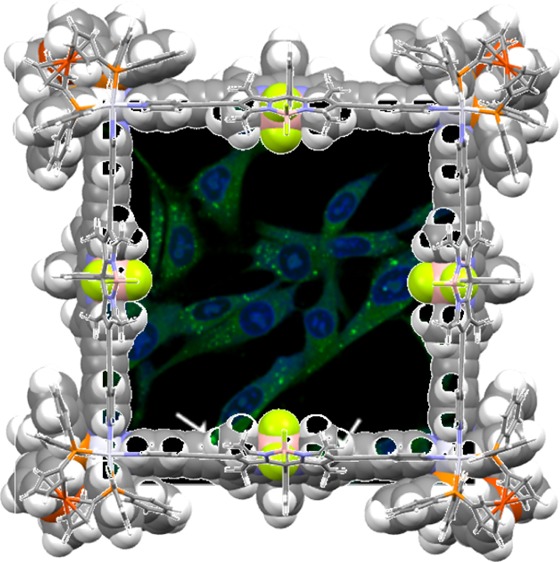
Our attempts to grow crystals suitable for single crystal X-ray study were not successful, so we investigated the structure of platinum-based complexes 4 and 6 using the PM7 semi-empirical method implemented in the Gaussian 16 software package.34,35 As expected from the molecular skeleton structures of 4 and 6 (see Scheme 1), we successfully obtained square structures from geometry optimizations, which were performed without symmetry constraints (Figure 3). The molecular size was estimated from the nearest Pt–Pt distance: 19.5 and 24.6 Å for 4 and 6, respectively. A variety of orientations of the BODIPY moiety were considered to obtain further insight into the structures of complexes 4 and 6 (Figure S3a,b). The geometric parameters related to the square structures are listed in Table S1. The torsional angles for the four Pt ions and interior angles for the local minimum structures of complex 4 (∼87°–93°) and 6 (∼90°) indicate that all molecular complexes adopted a square geometry. The interplanar angles between the square plane of four Pt ions and BODIPY moiety showed that steric hindrance is considerable in complex 4 compared to that of complex 6. The interplanar angles for the local minimum structures of complex 4 were determined to be ∼32°–52°, indicating a more tilted geometry of BODIPY from the perpendicular orientation with respect to the square plane of the complex compared to that of complex 6 (∼68°–73°). The strong effect of steric hindrance on the stability of complex 4 compared to that of complex 6 was also reflected in the larger variation of the relative energies in complex 4 depending on the orientation of BODIPY, ranging between ∼13 and ∼0.2 kcal/mol for complexes 4 and 6 (Figure S3a,b). Therefore, introducing the ethynyl group between the BODIPY and pyridyl groups may reduce the steric hindrance between the methyl groups of BODIPY and pyridyl group and leads to increased coplanarity of the BODIPY plane with the pyridyl group, which is favorable for improving the π-conjugated network.
Figure 3.
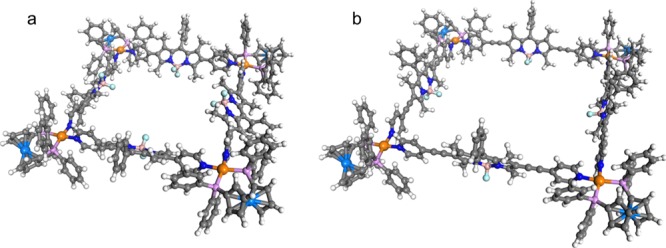
The PM7-optimized square geometries of complexes (a) 4 and (b) 6.
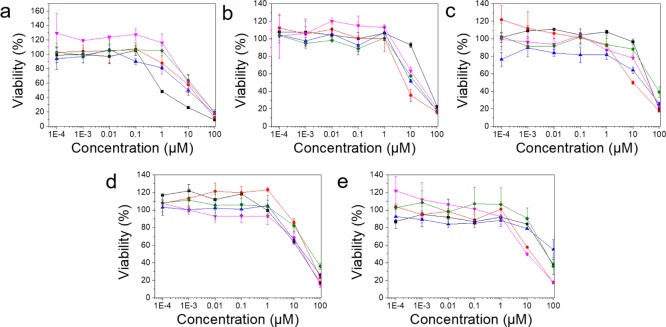
Owing to the growing interest and popularity of self-assembled metalla-architectures in biological systems, the anticancer properties of the synthesized complexes were studied in vitro. The cytotoxic activities of the prepared supramolecules were evaluated after incubation for 48 h with cancer cells of various origins: colorectal carcinoma (HCT116), prostrate adenocarcinoma (PC-3), breast adenocarcinoma (MCF-7), and lung adenocarcinoma (A549) (Figure 4). To qualify as a potent anticancer drug candidate, a complex must efficiently reduce the viability of cancer cells and exhibit minimal effects on the proliferation of normal cells. However, various metal-based anticancer agents cause significant toxicity to the normal cells. For example, cisplatin (cis-diamine-dichloro-platinum) exhibits cytotoxicity toward nonmalignant human embryonic kidney cells (HEK-293).36,37 Therefore, the respective cytotoxicities of 3–6 on cancer cells are compared with those obtained with nonmalignant HEK-293 cells. As indicated in Figure 4 and Table 1, all supramolecules show strong cytotoxic activities in all cell lines after 48 h incubation and high IC50 values of supramolecules were almost similar comparing the standard drug, cisplatin in most of the tested cells. For instance, the supramolecules inhibit the proliferation of HCT116, PC-3, and A549 with activity that is 2.1–6.0, 0.7–4.0, and 0.9–5.6 times, respectively, better than cisplatin. However, the IC50 values are higher by approximately 9.5–15.2 and 1.0–2.8 times, as compared to cisplatin for the HEK-293 and MCF-7 cells, respectively. More specifically, complex 4 shows 6.0, 4.0, and 3.7 times higher cytotoxicity toward HCT116, PC-3, and A549 cells, respectively, compared with cisplatin. However, it shows a slightly lower toxic effect against HEK-293 and MCF-7 cells. Overall, complexes show lower IC50 values against HCT116 and A549 comparing PC-3 and MCF-7 cells, exhibiting their selective cytotoxic affinities toward proliferating colorectal and lung cancer cells. Above all, our compounds were found to be 7.0 to 15.2 times less toxic than cisplatin in normal cells (HEK-293), and at the same time, have strong cytotoxicity in cancer cells.
Figure 4.
Cytotoxicity of supramolecules. The cytotoxic activities were analyzed using the conventional MTT assay in various cells: (a) HEK-293, (b) HCT116, (c) PC-3, (d) MCF-7, and (e) A549 cells. Black squares (■): cisplatin, pink reverse triangles (▼): 3, red circles (●): 4, green rhombus (⧫): 5, blue triangles (▲): 6.
Table 1. IC50 Values (μM) of the Prepared Supramolecules for HEK-293, HCT116, PC-3, MCF-7, and A549 Cells.
| complexes | HEK-293 | HCT116 | PC-3 | MCF-7 | A549 |
|---|---|---|---|---|---|
| cisplatin | 1.38 ± 0.12 | 45.53 ± 0.12 | 44.64 ± 0.16 | 19.09 ± 0.22 | 57.11 ± 0.17 |
| 3 | 20.28 ± 0.37 | 21.83 ± 0.21 | 31.89 ± 0.09 | 19.80 ± 0.08 | 11.25 ± 0.17 |
| 4 | 13.11 ± 0.08 | 7.58 ± 0.16 | 11.25 ± 0.17 | 42.12 ± 0.29 | 15.51 ± 0.10 |
| 5 | 20.95 ± 0.09 | 15.51 ± 0.10 | 65.80 ± 0.09 | 53.86 ± 0.12 | 63.17 ± 0.10 |
| 6 | 9.67 ± 0.12 | 13.68 ± 0.13 | 20.13 ± 0.26 | 25.80 ± 0.09 | 10.16 ± 0.21 |
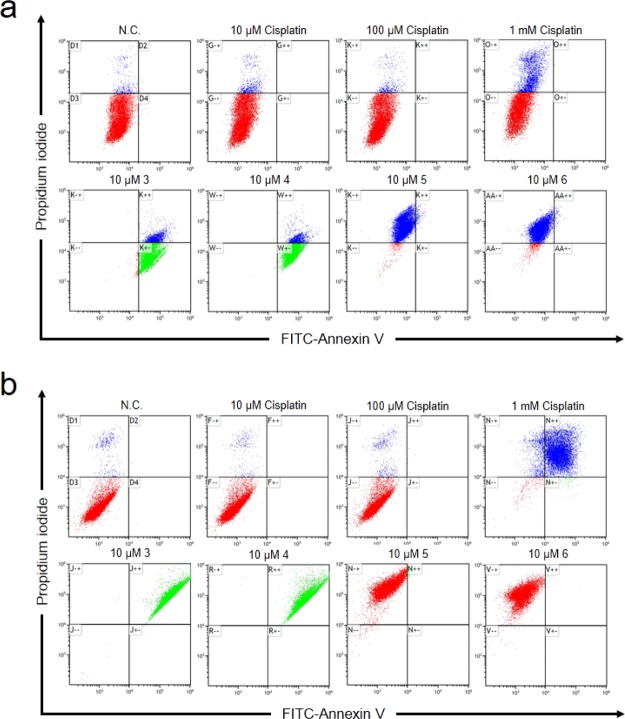
To identify the mechanism of cell death by the supramolecules, annexin-V FITC and propidium iodide (PI) dual-staining was conducted to distinguish between apoptosis- and necrosis-caused cell death by flow cytometry. Necrosis-based cell death was observed when cisplatin was used to treat HCT116 cells and the population of necrotic cells increased in a dose-dependent manner (Figure 5). Interestingly, cisplatin-induced necrosis was identified in the A549 cells exposed to 10 μM cisplatin, but significant apoptosis was observed when the dose was increased to 1 mM. Complexes 3 and 4 induced apoptosis in both HCT116 and A549 cells; both cell lines treated with complexes 5 and 6 displayed significant necrosis. Together, these experiments demonstrate that BDP ligand-containing complexes 3 and 4 exhibit cytotoxicity through apoptosis, but BDPCC-based complexes 5 and 6 induce cell death by necrosis.
Figure 5.
Flow cytometric analysis of the annexin-V/PI dual-staining of (a) HCT116 and (b) A549 cells after treatment with the prepared complexes and cisplatin.
When a fluorescent material shows cytotoxicity in cancer cells, the mechanism of action within the cells can be understood more easily via visualization using autofluorescence. In this regard, BODIPY-based complexes are easier to work with than the popular platinum-based anticancer drugs, such as cisplatin and carboplatin, as the processes of translocation and drug release can be easily tracked in BODIPY-based complexes.20,21,31,32 Based on their emission properties described in Figure 2, the intracellular penetration of 3–6 was validated. Confocal laser scanning microscopy investigations were performed on all types of cells previously tested (HEK-293, HCT116, PC-3, MCF-7, and A549) with complexes 3–6. Counterstaining with 4′,6-diamidino-2-phenylindole (DAPI) was performed to demarcate the nuclei. As seen in Figure 6, no fluorescence was detected in the untreated/control cells, except for that originating from the counterstain. In contrast, all cells treated with complexes 3–6 for 1 h displayed strong intracellular fluorescence, but fluorescence was not observed in the nuclei. These results indicate that complexes 3–6 were able to penetrate the cell membrane but failed to compromise the nuclear barrier. The results also indicate that the cell killing mechanism of all complexes was extranuclear. Previously, we obtained similar results using highly cytotoxic Pd-complexes localized in the cytoplasm of HeLa, MCF-7, and U87 cells.32 In this study, endocytosis may be the major mechanism of internalization. The cationic nature of the complexes is likely favorable for the cellular uptake because the potential of cell membrane is negative.38
Figure 6.
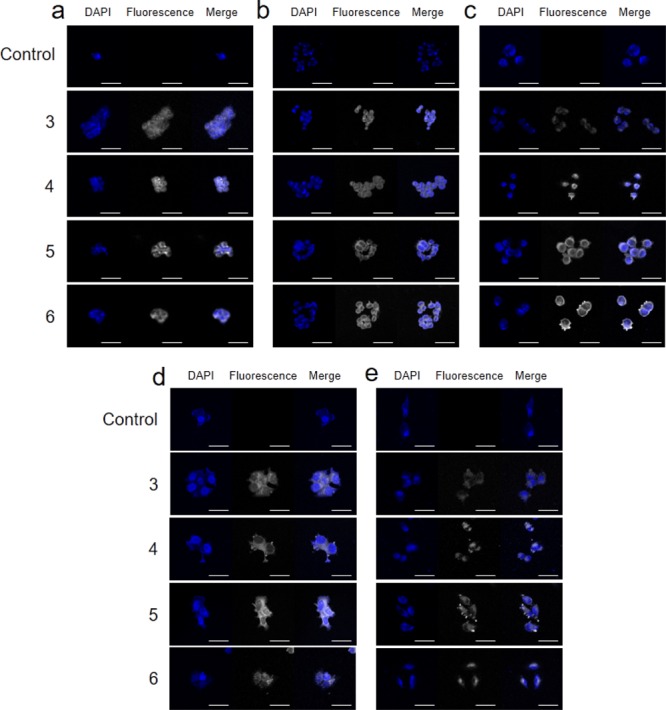
Intracellular compound localization. Cell nuclei are stained with DAPI (blue) in the absence (control) or presence of supramolecules (3–6) (white). The complexes are incubated in (a) HEK-293, (b) HCT116, (c) PC-3, (d) MCF-7, and (e) A549 cells. (scale bar = 50 μm).
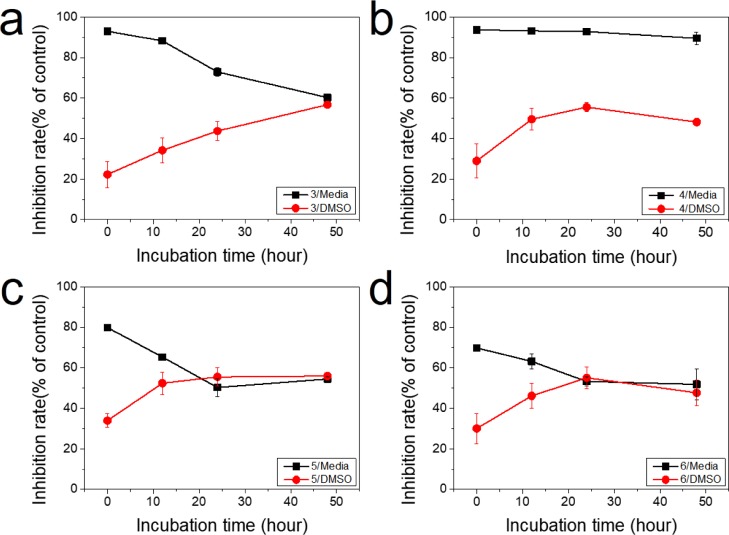
Determination of the biological activity of any organometallic complex largely relies on the understanding of its stability in biological media. To obtain more information regarding the stabilities of the synthesized supramolecules, 3–6 were pre-incubated in dimethyl sulfoxide (DMSO) or complete cell culture medium (RPMI 1640) at 100 μM for 0, 12, 24, or 48 h at 37 °C. Cytotoxic activities were then examined, as described in the Experimental Section against HCT116 cells. As shown in Figure 7, the cytotoxic activities of the complexes after preincubation in DMSO increased with the increasing preincubation time. On the other hand, the activities declined with preincubation time when the complexes were incubated in complete cell culture medium. Moreover, after incubation in DMSO, the cytotoxic activities were relatively stable for up to 48 h, likely because of the nonpolar nature of DMSO, allowing the complexes to be well dispersed. This is in contrast to the water-based culture media, where the relatively less soluble organometallic complexes tend to precipitate, leading to a reduced biological activity. Thus, we expect that the latter mechanism may have retarded the cell membrane penetration efficacy due to some precipitation.
Figure 7.
Inhibition rate of cell viability of the prepared complexes in HCT116 cells. (a) 3, (b) 4, (c) 5, and (d) 6. Red circles (●): supramolecules in DMSO, black squares (■): supramolecules in RPMI 1640.
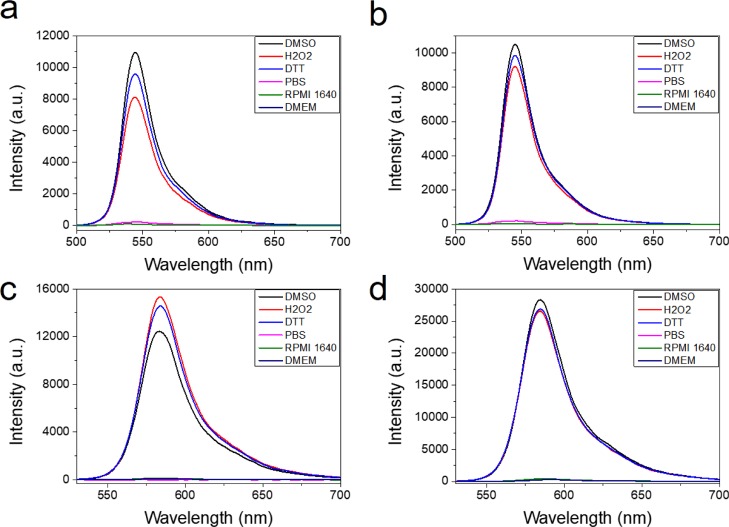
In support of the aforementioned observations, understanding the structural stability of the complexes under oxidative and reducing conditions is important. Thus, the fluorescence spectra of 3–6 in DMSO, 10 μM hydrogen peroxide (H2O2) in DMSO, 10 μM dithiothreitol (DTT) in DMSO, phosphate-buffered saline (PBS), and complete cell culture medium were analyzed (Figure 8).33 As shown in Figure 8, the maximum emission spectra of complexes 3, 4, and 6 are observed in DMSO and these slightly decrease under oxidative (H2O2) or reducing (DTT) conditions. Complex 5 shows the maximum fluorescence in H2O2, but no significant difference in the presence of DMSO is observed. However, the emission intensity disappears when dissolved in PBS as well as RPMI 1640 and DMEM cell culture media, likely due to the insolubility of the complexes. The emission spectra measured with the cell culture medium and PBS mimic the intracellular pH conditions. Together, these results indicate that 3–6 are stable under oxidative and reducing conditions.
Figure 8.
Fluorescence emission spectra of complexes 3–6 in DMSO, H2O2, DTT, PBS, RPMI 1640, and DMEM. (a) 3, (b) 4, (c) 5, and (d) 6
Conclusions
In conclusion, a series of novel BODIPY-based heterometallic palladium and platinum supramolecules with square architectures were designed and synthesized in good yields. The formation of the highly charged cationic complexes was confirmed by various analytical techniques and their square structures were further examined using the PM7 semi-empirical method. The anticancer properties of these complexes were studied using different cancer cell lines. Complexes showed similar toxicities toward the cancer cells compared to the standard drug, cisplatin in terms of their IC50 values. However, all complexes were found to be 7.0 to 15.2 times less toxic than cisplatin in normal cells (HEK-293), and at the same time, have strong cytotoxicity in cancer cells. Complex 3 was selective and better in killing lung cancer cells, whereas 4 and 5 displayed strong toxicity against HCT116 cells. Complexes 3 and 4 induced apoptosis in HCT116 and PC-3 cells, whereas 5 and 6 followed a necrotic mode of toxicity. Confocal microscopy studies indicated an extranuclear path of action for all prepared complexes. A further in-depth examination of the molecular mechanisms would certainly aid in understanding these drug candidates.
Experimental Section
General Information
The dipyridine-functionalized BODIPY ligands were synthesized using methods described previously.24 All other reagents were commercially obtained and used without further purification. 1H, 31P, and 19F NMR spectra were recorded using an Agilent 400-MR spectrometer with residual protonated solvent as an internal standard. UV–visible absorption spectra were recorded using a Thermo Scientific Genesys 10S UV–vis spectrometer, and fluorescence spectra were recorded on a Scinco FluoroMate FS-2 fluorescence spectrometer. ESI–MS spectra were recorded using a Waters Synapt G2Si spectrometer at KBSI, Korea. Elemental analysis was performed using an elemental analyzer (Elementar Analysensysteme GmbH, Germany) at KBSI, Busan. Infrared spectra were measured using an infrared Fourier vacuum spectrometer VERTEX 80v. Hyperion 2000 infrared microscope. The melting point was measured on a Stuart SMP30 melting point apparatus.
Synthetic Procedures for Supramolecules 3 and 5
A mixture of Pd(dppf)2(OTf)2 (30 mg, 0.030 mmol) and BDP or BDPCC ligands (0.030 mmol)) in a dry dichloromethane/nitromethane mixture (3 + 3 mL) was stirred under nitrogen at room temperature for 45 min. The reaction was stopped and the contents were filtered to remove any undissolved particles. The solvent was reduced to half, and DEE was slowly added to precipitate the dark red/pink solid. The solid was removed by filtration, recrystallized in dichloromethane, and dried under vacuum.
Complex 3: yield = 39 mg (90%). mp >250 °C. ESI–MS (CH3CN) m/z: 1377.66 [M – 4OTf]4+; 808.98 [M – 6OTf]6+; 569.16 [M – 8OTf]8+. Calcd (%) for C260H212B4F32Fe4N16O24P8Pd4S8·8CH2Cl2: C, 50.07; H, 3.58; N, 3.49. Found: C, 49.89; H, 3.64; N, 3.62. IR ν (cm–1): 833, 1018, 1103, 1161, 1245, 1437, 1526, and 1611. 1H NMR (CDCl3): δ 8.75 (b, 16H, Hpy), 7.88–7.83 (m, 32H, Hph), 7.61–7.48 (m, 60H, Hph), 7.12 (d, 8H, Hph), 6.70 (d, 16H, Hpy), 4.85 (s, 16H, Hcp), 4.60 (s, 16H, Hcp), 2.12 (s, 24H, CH3), and 0.89 ppm (s, 24H, CH3). 31P{1H} NMR (CDCl3): δ 33.06 ppm (s). 19F NMR (CDCl3): δ −78.18 ppm (s, OTf), −145.92 to −146.17 (q, BF2).
Complex 5: yield = 38 mg (85%). mp >250 °C. ESI–MS (CH3CN) m/z: 1376.68 [M – 4OTf]4+; 613.68 [M – 8OTf]8+. Calcd (%) for C276H212B4F32Fe4N16O24P8Pd4S8·4CH2Cl2: C, 53.54; H, 3.53; N, 3.57. Found: C, 53.53; H, 3.69; N, 3.72. IR: ν (cm–1) 827, 1024, 1087, 1160, 1250, 1432, 1527, and 2200. 1H NMR (CDCl3): δ 8.64 (b, 16H, Hpy), 7.85–7.82 (m, 32H, Hph), 7.57–7.50 (m, 60H, Hph), 7.17 (d, 8H, Hph), 6.88 (d, 16H, Hpy), 4.79 (s, 16H, Hcp), 4.57 (s, 16H, Hcp), 2.56 (s, 24H, CH3), and 1.36 ppm (s, 24H, CH3). 31P{1H} NMR (CDCl3): δ 32.50 ppm (s). 19F NMR (CDCl3): δ −78.08 ppm (s, OTf), −145.94 to −146.19 (q, BF2).
Synthetic Procedures for Supramolecules 4 and 6
A mixture of Pt(dppf)2(OTf)2 (30 mg, 0.027 mmol) and BDP or BDPCC ligands (0.027 mmol)) in a dry dichloromethane/nitromethane mixture (3 + 3 mL) was stirred under nitrogen at room temperature for 45 min. The reaction was stopped and filtered to remove any undissolved particles. The solvent was reduced to half, and DEE was slowly added to precipitate the dark red/pink solid. The solid was removed by filtration, recrystallized in dichloromethane, and dried under vacuum.
Complex 4: yield = 36 mg (85%). mp >250 °C. ESI–MS (CH3CN) m/z: 1829.30 [M – 3OTf]3+; 1335.59 [M – 4OTf]4+; 593.17 [M – 8OTf]8+. Calcd (%) for C260H212B4F32Fe4N16O24P8Pt4S8·3CH2Cl2: C, 49.21; H, 3.44; N, 3.48. Found: C, 49.29; H, 3.49; N, 3.61. IR: ν (cm–1) 842, 1028, 1068, 1097, 1178, 1254, 1439, 1526, and 1619. 1H NMR (CDCl3): δ 8.78 (b, 16H, Hpy), 7.84 (b, 32H, Hph), 7.61 (b, 60H, Hph), 7.10 (d, 8H, Hph), 6.72 (d, 16H, Hpy), 4.79 (s, 16H, Hcp), 4.56 (s, 16H, Hcp), 2.11 (s, 24H, CH3), and 0.89 ppm (s, 24H, CH3). 31P{1H} NMR (CDCl3): δ 4.15 ppm (s). 19F NMR (CDCl3): δ −78.17 ppm (s, OTf), −145.91 to −146.15 (q, BF2).
Complex 6: yield = 34 mg (78%). mp >250 °C. ESI–MS (CH3CN) m/z: 1424.60 [M – 4OTf]4+; 897.84 [M – 6OTf]6+; 748.96 [M – 7OTf]7+; 637.75 [M – 8OTf]8+. Calcd (%) for C276H212B4F32Fe4N16O24P8Pt4S8·5CH2Cl2: C, 50.22; H, 3.33; N, 3.33. Found: C, 50.35; H, 3.39; N, 3.29. IR: ν (cm–1) 830, 1025, 1094, 1180, 1254, 1438, 1611, and 2202. 1H NMR (CDCl3): δ 8.66 (d, 16H, Hpy), 7.83 (b, 32H, Hph), 7.53–7.49 (m, 60H, Hph), 7.15 (d, 8H, Hph), 6.88 (d, 16H, Hpy), 4.73 (s, 16H, Hcp), 4.52 (s, 16H, Hcp), 2.54 (s, 24H, CH3), and 1.34 ppm (s, 24H, CH3). 31P{1H} NMR (CDCl3): δ 3.60 ppm (s). 19F NMR (CDCl3): δ −78.09 ppm (s, OTf), −145.85 to −146.10 (q, BF2).
Acknowledgments
This research was supported by the National Research Foundation of Korea and funded by the Ministry of Science, ICT & Future Planning (NRF2018R1D1A1B07045863), as well as the Post-Doctoral Research Program (2018) in the Incheon National University.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01328.
1H NMR, ESI–MS, and theoretical structural data (PDF)
Author Contributions
∥ G.G. and Y.Y. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Cook T. R.; Vajpayee V.; Lee M. H.; Stang P. J.; Chi K.-W. Biomedical and Biochemical Applications of Self-Assembled Metallacycles and Metallacages. Acc. Chem. Res. 2013, 46, 2464–2474. 10.1021/ar400010v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty R.; Mukherjee P. S.; Stang P. J. Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles. Chem. Rev. 2011, 111, 6810–6918. 10.1021/cr200077m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.-F.; Jin G.-X. Half-Sandwich Iridium- and Rhodium-based Organometallic Architectures: Rational Design, Synthesis, Characterization, and Applications. Acc. Chem. Res. 2014, 47, 3571–3579. 10.1021/ar500335a. [DOI] [PubMed] [Google Scholar]
- Mirtschin S.; Slabon-Turski A.; Scopelliti R.; Velders A. H.; Severin K. A Coordination Cage with an Adaptable Cavity Size. J. Am. Chem. Soc. 2010, 132, 14004–14005. 10.1021/ja1063789. [DOI] [PubMed] [Google Scholar]
- Kim D. H.; Singh N.; Oh J.; Kim E.-H.; Jung J.; Kim H.; Chi K.-W. Coordination-Driven Self-Assembly of a Molecular Knot Comprising Sixteen Crossings. Angew. Chem., Int. Ed. 2018, 57, 5669–5673. 10.1002/anie.201800638. [DOI] [PubMed] [Google Scholar]
- Kim T.; Singh N.; Oh J.; Kim E.-H.; Jung J.; Kim H.; Chi K.-W. Selective Synthesis of Molecular Borromean Rings: Engineering of Supramolecular Topology via Coordination-Driven Self-Assembly. J. Am. Chem. Soc. 2016, 138, 8368–8371. 10.1021/jacs.6b04545. [DOI] [PubMed] [Google Scholar]
- Therrien B. Transporting and Shielding Photosensitisers by Using Water-Soluble Organometallic Cages: A New Strategy in Drug Delivery and Photodynamic Therapy. Chem.—Eur. J. 2013, 19, 8378–8386. 10.1002/chem.201301348. [DOI] [PubMed] [Google Scholar]
- Therrien B. Biologically relevant arene ruthenium metalla-assemblies. CrystEngComm 2015, 17, 484–491. 10.1039/c4ce02146k. [DOI] [Google Scholar]
- Fujita M.; Yazaki J.; Ogura K. Preparation of a macrocyclic polynuclear complex, [(en)Pd(4,4’-bpy)]4(NO3)8 (en = ethylenediamine, bpy = bipyridine), which recognizes an organic molecule in aqueous media. J. Am. Chem. Soc. 1990, 112, 5645–5647. 10.1021/ja00170a042. [DOI] [Google Scholar]
- Stang P. J.; Cao D. H. Transition Metal Based Cationic Molecular Boxes. Self-Assembly of Macrocyclic Platinum(II) and Palladium(II) Tetranuclear Complexes. J. Am. Chem. Soc. 1994, 116, 4981–4982. 10.1021/ja00090a051. [DOI] [Google Scholar]
- Saha M. L.; Yan X.; Stang P. J. Photophysical Properties of Organoplatinum(II) Compounds and Derived Self-Assembled Metallacycles and Metallacages: Fluorescence and its Applications. Acc. Chem. Res. 2016, 49, 2527–2539. 10.1021/acs.accounts.6b00416. [DOI] [PubMed] [Google Scholar]
- Suzuki K.; Kawano M.; Fujita M. Solvato-Controlled Assembly of Pd3L6 and Pd4L8 Coordination ″Boxes′′. Angew. Chem., Int. Ed. 2007, 46, 2819–2822. 10.1002/anie.200605084. [DOI] [PubMed] [Google Scholar]
- Lu Y.; Zhang H.-N.; Jin G.-X. Molecular Borromean Rings Based on Half-Sandwich Organometallic Rectangles. Acc. Chem. Res. 2018, 51, 2148–2158. 10.1021/acs.accounts.8b00220. [DOI] [PubMed] [Google Scholar]
- Loudet A.; Burgess K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. 10.1021/cr078381n. [DOI] [PubMed] [Google Scholar]
- Kowada T.; Maeda H.; Kikuchi K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. 10.1039/c5cs00030k. [DOI] [PubMed] [Google Scholar]
- Boens N.; Leen V.; Dehaen W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. 10.1039/c1cs15132k. [DOI] [PubMed] [Google Scholar]
- Lakshmi V.; Rajeswara Rao M.; Ravikanth M. Halogenated boron-dipyrromethenes: synthesis, properties and applications. Org. Biomol. Chem. 2015, 13, 2501. 10.1039/c4ob02293a. [DOI] [PubMed] [Google Scholar]
- Solomonov A. V.; Marfin Y. S.; Rumyantsev E. V. Design and applications of dipyrrin-based fluorescent dyes and related organic luminophores: From individual compounds to supramolecular self-assembled systems. Dyes Pigm. 2019, 162, 517–542. 10.1016/j.dyepig.2018.10.042. [DOI] [Google Scholar]
- Lee C. Y.; Farha O. K.; Hong B. J.; Sarjeant A. A.; Nguyen S. T.; Hupp J. T. Light-Harvesting Metal-Organic Frameworks (MOFs): Efficient Strut-to-Strut Energy Transfer in Bodipy and Porphyrin-Based MOFs. J. Am. Chem. Soc. 2011, 133, 15858–15861. 10.1021/ja206029a. [DOI] [PubMed] [Google Scholar]
- Gupta G.; Das A.; Ghate N. B.; Kim T.; Ryu J. Y.; Lee J.; Mandal N.; Lee C. Y. Novel BODIPY-based Ru(II) and Ir(III) metalla-rectangles: cellular localization of compounds and their Antiproliferative activities. Chem. Commun. 2016, 52, 4274–4277. 10.1039/c6cc00046k. [DOI] [PubMed] [Google Scholar]
- Gupta G.; Das A.; Panja S.; Ryu J. Y.; Lee J.; Mandal N.; Lee C. Y. Self-Assembly of Novel Thiophene-Based BODIPY RuII Rectangles: Potential Antiproliferative Agents Selective Against Cancer Cells. Chem.—Eur. J. 2017, 23, 17199–17203. 10.1002/chem.201704368. [DOI] [PubMed] [Google Scholar]
- Gupta G.; Das A.; Lee J.; Mandal N.; Lee C. Y. Self-Assembled BODIPY-Based Iridium Metallarectangles: Cytotoxicity and Propensity to Bind Biomolecules. ChemPlusChem 2018, 83, 339–347. 10.1002/cplu.201800035. [DOI] [PubMed] [Google Scholar]
- Gupta G.; Das A.; Lee S. W.; Ryu J. Y.; Lee J.; Nagesh N.; Mandal N.; Lee C. Y. BODIPY-based Ir(III) rectangles containing bis-benzimidazole ligands with highly selective toxicity obtained through self-assembly. J. Organomet. Chem. 2018, 868, 86–94. 10.1016/j.jorganchem.2018.04.034. [DOI] [Google Scholar]
- Gupta G.; Cherukommu S.; Srinivas G.; Lee S. W.; Mun S. H.; Jung J.; Nagesh N.; Lee C. Y. BODIPY-based Ru(II) and Ir(III) organometallic complexes of avobenzone, a sunscreen material: Potent anticancer agents. J. Inorg. Biochem. 2018, 189, 17–29. 10.1016/j.jinorgbio.2018.08.009. [DOI] [PubMed] [Google Scholar]
- Neelakandan P. P.; Jiménez A.; Nitschke J. R. Fluorophore incorporation allows nanomolar guest sensing and white-light emission in M4L6 cage complexes. Chem. Sci. 2014, 5, 908–915. 10.1039/c3sc53172d. [DOI] [Google Scholar]
- Neelakandan P. P.; Jiménez A.; Thoburn J. D.; Nitschke J. R. An Autocatalytic System of Photooxidation-Driven Substitution Reactions on a FeII4L6 Cage Framework. Angew. Chem. 2015, 127, 14586–14590. 10.1002/ange.201507045. [DOI] [PubMed] [Google Scholar]
- Musser A. J.; Neelakandan P. P.; Richter J. M.; Mori H.; Friend R. H.; Nitschke J. R. Excitation Energy Delocalization and Transfer to Guests within MII4L6 Cage Frameworks. J. Am. Chem. Soc. 2017, 139, 12050–12059. 10.1021/jacs.7b06709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaloudi-Chantzea A.; Karakostas N.; Raptopoulou C. P.; Psycharis V.; Saridakis E.; Griebel J.; Hermann R.; Pistolis G. Coordination-Driven Self Assembly of a Brilliantly Fluorescent Rhomboid Cavitand Composed of Bodipy-Dye Subunits. J. Am. Chem. Soc. 2010, 132, 16327–16329. 10.1021/ja1064679. [DOI] [PubMed] [Google Scholar]
- Kaloudi-Chantzea A.; Karakostas N.; Pitterl F.; Raptopoulou C. P.; Glezos N.; Pistolis G. Efficient supramolecular synthesis of a robust circular light-harvesting Bodipy-dye based array. Chem. Commun. 2012, 48, 12213–12215. 10.1039/c2cc36825k. [DOI] [PubMed] [Google Scholar]
- Kaloudi-Chantzea A.; Martinou E.; Seintis K.; Karakostas N.; Giastas P.; Pitterl F.; Oberacher H.; Fakis M.; Pistolis G. Formation of a highly-ordered rigid multichromophoric 3D supramolecular network by combining ionic and coordination-driven self-assembly. Chem. Commun. 2016, 52, 3388–3391. 10.1039/c5cc10335e. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Zhang Y.; Yu G.; Crawley M. R.; Fulong C. R. P.; Friedman A. E.; Sengupta S.; Sun J.; Li Q.; Huang F.; Cook T. R. Highly Emissive Self-Assembled BODIPY-Platinum Supramolecular Triangles. J. Am. Chem. Soc. 2018, 140, 7730–7736. 10.1021/jacs.8b04929. [DOI] [PubMed] [Google Scholar]
- Gupta G.; Das A.; Park K. C.; Tron A.; Kim H.; Mun J.; Mandal N.; Chi K.-W.; Lee C. Y. Self-Assembled Novel BODIPY-Based Palladium Supramolecules and Their Cellular Localization. Inorg. Chem. 2017, 56, 4615–4621. 10.1021/acs.inorgchem.7b00260. [DOI] [PubMed] [Google Scholar]
- Mishra A.; Chang Lee S.; Kaushik N.; Cook T. R.; Choi E. H.; Kumar Kaushik N.; Stang P. J.; Chi K.-W. Self-Assembled Supramolecular Hetero-Bimetallacycles for Anticancer Potency by Intracellular Release. Chem.—Eur. J. 2014, 20, 14410–14420. 10.1002/chem.201403372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. J. P. Optimization of Parameters for Semiempirical methods VI: more modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. 10.1007/s00894-012-1667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; Li X.; Caricato M.; Marenich A. V.; Bloino J.; Janesko B. G.; Gomperts R.; Mennucci B.; Hratchian H. P.; Ortiz J. V.; Izmaylov A. F.; Sonnenberg J. L.; Williams-Young D.; Ding F.; Lipparini F.; Egidi F.; Goings J.; Peng B.; Petrone A.; Henderson T.; Ranasinghe D.; Zakrzewski V. G.; Gao J.; Rega N.; Zheng G.; Liang W.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Throssell K.; Montgomery J. A.; Peralta J. E. Jr.; Ogliaro F.; Bearpark M. J.; Heyd J. J.; Brothers E. N.; Kudin K. N.; Staroverov V. N.; Keith T. A.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A. P.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Millam J. M.; Klene M.; Adamo C.; Cammi R.; Ochterski J. W.; Martin R. L.; Morokuma K.; Farkas O.; Foresman J. B.; Fox D. J.. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford CT, 2016.
- Sadeghi F.; Etebari M.; Roudkenar M. H.; Jahanian-Najafabadi A. Lipocalin2 Protects Human Embryonic Kidney Cells against Cisplatin-Induced Genotoxicity. Iran. J. Pharm. Res. 2018, 17, 147–154. [PMC free article] [PubMed] [Google Scholar]
- Lease N.; Vasilevski V.; Carreira M.; de Almeida A.; Sanaú M.; Hirva P.; Casini A.; Contel M. Potential Anticancer Heterometallic Fe-Au and Fe-Pd Agents: Initial Mechanistic Insights. J. Med. Chem. 2013, 56, 5806–5818. 10.1021/jm4007615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. 10.2147/ijn.s36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.