Abstract
Primary afferents are known to use glutamate as their principal fast neurotransmitter. However, it has become increasingly clear that peptides have an influential role in both mediating and modulating sensory transmission. Here we describe the transmission accounting for different acute pain states and itch transmitted via the transient receptor potential cation channel subfamily V member 1 (TRPV1) population by either ablating Trpv1–Cre-expressing neurons or inducing vesicular glutamate transporter 2 (VGLUT2) deficiency in Trpv1–Cre-expressing neurons. Furthermore, by pharmacological inhibition of substance P or calcitonin gene-related peptide (CGRP) signaling in Vglut2-deficient mice, we evaluated the contribution of substance P or CGRP to these sensory modulations, with or without the presence of VGLUT2-mediated glutamatergic transmission in Trpv1–Cre neurons. This examination, together with c-Fos analyses, showed that glutamate via VGLUT2 in the Trpv1–Cre population together with substance P mediate acute cold pain, whereas glutamate together with CGRP mediate noxious heat. Moreover, we demonstrate that glutamate together with both substance P and CGRP mediate tissue-injury associated pain. We further show that itch, regulated by the VGLUT2-mediated transmission via the Trpv1–Cre population, depends on CGRP and gastrin-releasing peptide receptor (GRPR) transmission because pharmacological blockade of the CGRP or GRPR pathway, or genetic ablation of Grpr, led to a drastically attenuated itch. Our study reveals how different neurotransmitters combined can cooperate with each other to transmit or regulate various acute sensations, including itch.
Keywords: CGRP, glutamate, itch, pain, substance P, Trpv1
Introduction
Nociceptive and pruritic responses are triggered by activation of sensory receptors expressed on primary afferents by external noxious stimuli or the release of itch- or pain-inducing agents. The neurons expressing the transient receptor potential cation channel subfamily V member 1 (TRPV1) constitute a population of somatosensory neurons involved in the transduction of various sensations, including acute heat, cold, and tissue injury-induced pain and itch (Lagerström et al., 2010; Mishra et al., 2011). However, the neurotransmitters required for the sensation of these diverse sensations have not yet been established fully.
Conditional genetic deletion of vesicular glutamate transporters (VGLUTs) provides an approach to silence glutamatergic signaling in defined neuronal populations (Lagerström et al., 2010, 2011; Liu et al., 2010; Scherrer et al., 2010; Rogoz et al., 2012). This has allowed for studies of the role of glutamate-mediated neurotransmission in acute and chronic pain states, which has begun to unravel interactions between the peptidergic and the glutamatergic neurotransmitter systems (Koga et al., 2011; Lagerström et al., 2011; Rogoz et al., 2014).
The molecular characterization of nociceptors has demonstrated a substantial heterogeneity, especially within C-fibers, dividing them into nonpeptidergic and peptidergic, the latter releasing calcitonin gene-related peptide (CGRP) and substance P (Snider and McMahon, 1998). Previously, substance P transmission has been connected mainly to the mediation of hyperalgesia associated with neuropathic or inflammatory conditions (Mansikka et al., 2000; Cahill and Coderre, 2002; Rogoz et al., 2014) but also contributes to the sensation of tissue-injury induced pain, as shown in studies of substance P-deficient mice (Zimmer et al., 1998). Whether substance P- or CGRP-positive primary afferents are required to sense acute thermal or mechanical stimuli has been unclear (McCoy et al., 2012), but it was reported recently that the CGRP-expressing population of neurons plays a role in encoding heat and can tonically cross-inhibit cold (McCoy et al., 2013).
Although substance P and CGRP have been associated with nociception, little is known regarding their role in pruriception. An early study on the subject demonstrated that intradermal injections of substance P in humans can produce flare and itching, suggesting a peptidergic involvement in peripheral transmission of itch (Hägermark et al., 1978). Later studies, using an animal model of atopic dermatitis, reported an increased release of CGRP and substance P during scratching (Katsuno et al., 2003) and a decreased sensitivity to itch after specific deletion of CGRP-positive neurons (McCoy et al., 2013).
Here, we have evaluated the contribution of the TRPV1 population and VGLUT2-mediated glutamate signaling via TRPV1 neurons to pain and itch transmission by a genetic approach. We have also combined the genetic approach with pharmacological interventions toward the substance P and CGRP systems to determine the role of these neuropeptides in the presence and absence of VGLUT2-mediated glutamatergic signaling from Trpv1–Cre neurons. This permitted us to compare transmission originating from the Trpv1–Cre population and examine the role of VGLUT2-mediated glutamate release from within this population, as well as the role of selected peptidergic primary afferent pathways.
Materials and Methods
Generation of transgenic animals
Mice heterozygous for the diphtheria toxin A (DTA) allele [Gt(ROSA)26Sortm1(DTA)Jpmb], hereafter called R26DTA/wt (Ivanova et al., 2005), were crossed with mice heterozygous for the Trpv1–Cre allele (Lagerström et al., 2010) to generate mice lacking TRPV1-positive neurons (R26DTA/wt;Trpv1–Cretg/wt) and controls (R26DTA/wt;Trpv1–Crew/w or R26wt/wt;Trpv1–Crew/w). The Trpv1–Cre mice were also crossed with Vglut2f/f mice (Wallén-Mackenzie et al., 2006) to generate mice in which Vglut2 was selectively removed from Trpv1–Cre-expressing neurons (Vglut2f/f;Trpv1–Cre). R26DTA/wt;Trpv1–Cretg/wt mice were further crossed with the reporter line tdTomato [Gt(ROSA)26Sortm14(CAG-tdTomato)Hze; Allen Brain Institute] to confirm deletion of all Trpv1–Cre-positive cells.
Genotyping by PCR
First, 1–2 mm tail was incubated in 75 μl of buffer consisting of 25 mm NaOH and 200 μm EDTA at 96°C for 45 min and placed on ice before the sample was neutralized with 75 μl of Tris-HCl (40 mm), pH 8.0. Mice were genotyped for the presence of the Trpv1–Cre, R26DTA, Vglut2f/f, and tdTomato alleles. The following primers were used: Trpv1–Cre, 5′-TGGGAAGGGTGACTCAGAAG (forward) and 5′- TCCCTCACATCCTCAGGTTC (reverse); R26DTA, 5′- GTTATCAGTAAGGGAGCTGCAGTGG, 5′-AAGACCGCGAAGAGTTTGTCCTC, and 5′- GGCGGATCACAAGCAATAATAACC; Vglut2f/f, 5′-CTGTCCACCTTTGTATCCCA (forward), 5′-GCAATCACATTTCACTGTTC (reverse, floxed allele), and 5′-CACACCCACTCCACTTGAGG (reverse, excised allele); and tdTomato, 5′-CTGTTCCTGTACGGCATGG (forward), 5′-GGCATTAAAGCAGCGTATCC (reverse), 5′-AAGGGAGCTGCAGTGGAGTA (forward), and 5′-CCGAAAATCTGTGGGAAGTC (reverse). The mutated gastrin-releasing peptide receptor (GRPR) allele was detected using the following primers: 5′- GATCTCTCGTGGGATCATTG (forward), 5′-CATCAACAAACTGAGCTAGAGT (reverse), and 5′-AGCCAGGTACTTGCTGGCAT (reverse).
Tissue preparation
Mice (>7 weeks old) were perfused (described previously by Gezelius et al., 2006). Dorsal root ganglia (DRGs) and lumbar and sacral spinal cord were isolated for immunohistochemistry. The isolated tissue was fixed in fresh 4% PFA for 2 h shaking on ice, followed by a graded series of sucrose solutions ending at 30% sucrose, before the tissue was mounted and frozen in O.C.T compound (Sakura Finetek) at −80°C. Sections of 16 μm were cut and mounted on Superfrost glass (Menzel-Gläser) and stored at −80°C.
Immunohistochemistry and in situ hybridization
The following criteria were used for immunohistochemical analysis: sections were briefly rinsed in 1× TBS, followed by an incubation in 10% methanol and 3–4% H2O2 in 1× TBS, before the sections were rinsed in 1× TBS and incubated overnight, or 48 h in the case of c-Fos staining, in 4°C in blocking buffer with 0.5% gelatin and 0.01% Triton X-100 in TBS supplied with primary antibodies. Primary antibodies used were chicken anti-VGLUT1 at 1:200 (Frontier Science), guinea pig anti-VGLUT3 at 1:200 (Millipore Bioscience Research Reagents), rabbit anti-CGRP at 1:1000 (Peninsula Laboratories), guinea pig anti-substance P at 1:200 (Abcam), goat anti c-Fos at 1:200 (Santa Cruz Biotechnology), rabbit anti-Cre at 1:1000 (Convance), guinea pig anti-VGLUT3 at 1:200 (gift from Hiroyuki Hioki, Department of Morphological Brain Science, Kyoto University, Japan), and Alexa Fluor 647-conjugated isolectin B4 (IB4) at 1:50 (Invitrogen). The sections were then rinsed repeatedly in 1× TBS, followed by incubation for 1 h at room temperature in blocking buffer with 0.5% gelatin and 0.01% Triton X-100 in TBS supplied with the secondary antibodies goat anti-chicken Alexa Fluor 488 at 1:400 (Invitrogen), goat anti-rabbit Alexa Fluor 594 at 1:800 (Invitrogen), goat anti-guinea pig Alexa Fluor 647 at 1:200 (Invitrogen), and donkey anti-goat Alexa Fluor 555 at 1:400 (Invitrogen). The VGLUT2 analysis was performed differently: the sections were rinsed three times in PBS, followed by 3 h incubation in blocking solution (5% goat serum, 1% Triton X-100, and 0.01% NaAz). The sections were then incubated overnight with rabbit or guinea pig anti-VGLUT2 at 1:200 (Frontier Science) and then rinsed in PBS. The secondary antibody used for visualizing fluorescent signal was goat anti-guinea pig Alexa Fluor 647 at 1:200 (Invitrogen) or Alexa Fluor 488 goat anti-rabbit at 1:200 (Invitrogen).
Cryo in situ was performed as described previously (Schaeren-Wiemers and Gerfin-Moser, 1993) with a few exceptions. Briefly, sections were fixed in 4% PFA, followed by repeated washes in 1× PBS, incubation with Proteinase K in 10 mm Tris-HCl, pH 8.0, for 8 min, followed by washes with 1× PBS and acetylation for 10 min. Sections were then rinsed briefly before incubation in hybridization buffer (50% formamide, 5× SSC, 5× Denhardt's solution, 500 μg/ml salmon sperm DNA, and 50 μg/ml tRNA) in 55°C for 1 h. Sections were then incubated with hybridization buffer supplied with the denaturated Vglut1 or Vglut2 probe in 55°C for 6 h. Sections were then briefly washed with 5× SSC and later 0.2× SSC for 1 h at 55°C before equilibration in 1× TBS at room temperature. The preblock was performed at room temperature for 1 h in 1× TBS supplied with 1× blocking reagent (Roche) before the sections were incubated with blocking buffer containing anti-digoxigenin alkaline phosphatase Fab fragments at 1:5000 (Roche) and left overnight in 4°C. Sections were later washed with 1× TBS supplied with 0.1% Tween 20 and 2 mm Levamisole (Sigma). Development was performed at 37°C using filtrated fast red (Roche) 0.1 nm Tris HCl, pH 8.2, solution. When the development was finished, the sections were again incubated with the secondary antibodies used before the in situ hybridization to strengthen the signal. Sections were then washed and closed with coverslips.
Imaging
Fluorescent images were viewed in an Olympus BX61WI microscope and analyzed using the Volocity software (Improvision).
Multiplex single-cell PCR
DRGs were dissected from Trpv1–Cre mice and collected in ice-cold Leibovitz's L-15 Medium (Invitrogen). The tissue was treated with dispase (Sigma) for 30 min at 37°C and then with trypsin (Invitrogen) for 15 min at 37°C. Trypsin was neutralized, and the tissue was triturated by several passages through glass pipettes of decreasing diameter to obtain a cell suspension that was centrifuged in a differential gradient to eliminate dead cells and debris. Cells were plated on poly-l-lysine-coated coverslips, left to adhere for 30 min at 37°C, and then washed with Krebs–Ringer buffer (KRB) (in mm: 140 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, 10 glucose, and 6 sucrose, pH 7.35) to eliminate non-attached cells. Coverslips were kept in KRB during single-cell collection. Cells were individually collected under RNase-free conditions using autoclaved borosilicate patch pipettes; each cell was collected by applying light negative pressure to the pipette; no intracellular pipette solution was used. The content of each pipette was transferred immediately into individual prechilled 200 μl tubes containing 6 μl of a freshly prepared solution of 20 U of RNaseOUT (Fermentas) and 8.3 mm DTT (Invitrogen), and the samples were immediately frozen on dry ice until use. Frozen samples were thawed on ice and subjected to cDNA synthesis for 1 h using 0.5 mm dNTPs mix, 1.25 μm random hexamers (Invitrogen), 40 U of RNase inhibitor (Fermentas), 100 U of Moloney murine leukemia virus reverse transcriptase (Invitrogen), 50 mm Tris-HCl, 75 mm KCl, and 3 mm MgCl2, pH 8.3. The reverse transcriptase enzyme was denatured, and the cDNAs were stored at −80°C until use. A first round of PCR was performed using 1.5 mm MgCl2, 10 pmol of each primer, 1.0 U of Maxima Hot Start Taq Polymerase (Fermentas), 20 mm Tris-HCl, and 10 mm KCl, pH 8.3, and 35 cycles with 55°C of annealing temperature. A second round of PCR was then performed using 10% of the first PCR reaction. Primers were designed based on sequences deposited in the GenBank database (www.ncbi.nlm.nih.gov/nucleotide). They were designed to bind different exons; therefore, they detect only mRNA and not genomic DNA (ladder, 100 bp; Fermentas). Right primers are followed by left primers: MAS-related GPR, member A3 (Mrgpra3), 5′-cctggttcctgttattctgg-3′and 5′-acattaataaaattcccata-3′; Mrgpra3 nested, 5′-gtactagattatagccctct-3′ and 5′-actggtccaccaacaccttc-3′; transient receptor potential cation channel subfamily A member 1 (Trpa1), 5′-agaaaggagagagcaagcaa-3′ and 5′-cttaactgcgtttaagacaa-3′; Trpa1 nested, 5′-tctcatttaaaccatcacaa-3′ and 5′-atagttacattaattcttat-3′; Natriuretic polypeptide b (Nppb), 5′-aaccccacccctactccgtg-3′ and 5′-attttctttctttttctacaa-3′; Nppb nested, 5′-agagacagctcttgaaggc-3′ and 5′-tttgaagtgatccatttat-3′; transient receptor potential cation channel subfamily M member 8 (Trpm8), 5′-ctgcctgaagaggaaattga-3′ and 5′-ttgctgtaacactcggtaa-3′; Trpm8 nested, 5′-agcaagacaaggacaactgg-3′ and 5′-taggagttcttggcgatctg-3′; Cre, 5′-ggaagatgctcctgtctgtg-3′ and 5′-gatttcagggatggacacac-3′; and Cre nested, 5′-tgaggatgtgagggactacc-3′ and 5′-ttctccatcagggatctgac-3′.
General behavior
All behavioral tests were performed on adult (>7 weeks old) female and male mice. Control mice were littermates and gender matched. All behavior analyses were performed in a controlled environment of 20–24°C, 45–65% humidity, and 12 h light/dark cycle. All animal procedures were approved by the local ethical committee in Uppsala following the Directive 2010/63/EU of the European Parliament and of the Council, the Swedish Animal Welfare Act (Swedish Animal Welfare Act: Swedish code of statues 1988:534), the Swedish Animal Welfare Ordinance (Swedish Animal Welfare Ordinance: Swedish code of statues 1988:539), and the provisions regarding the use of animals for scientific purposes (Animal Welfare Authority 2004:15 and The Swedish Board of Agriculture's regulations and general guidelines on laboratory animals 2012:26). All behavior experiments were performed by an observer blind to the genotype.
Pain behavior
Hargreaves test.
The mice were tested and acclimatized in transparent Plexiglas chambers with a glass floor for 30–60 min or until no exploratory behavior was observed. The Hargreaves heat source (IITC Life Science) was placed with the guide light pointing toward the plantar surface of a hindpaw and the thermal beam was started. A paw withdrawal would stop the test, and the time was monitored. The cutoff time was set to 20 s. The test was repeated at least three times for each animal, allowing at least 5 min between each test. To assess the contribution of substance P and CGRP in the acute heat responses, animals underwent the same procedure to obtain baseline values, followed by an intraperitoneal or an intrathecal injection of 5 mg/kg the substance P antagonist Win51078 (Sigma; dissolved in 10% DMSO) 10 min before the first Hargreaves measurement or an intraperitoneal or an intrathecal injection of 0.5 mg/kg the CGRP antagonist BIBN4096BS (1-piperidinecarboxamide, N-[2-[[5-amino-L-[[4-(4-pyridinyl)-l-piperazinyl]carbonyl]pentyl]amino]-1-[(3,5-dibromo-4-hydroxyphenyl)methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2H)-quinazolinyl; donated by Boehringer Ingelheim; dissolved in 10% DMSO) 30 min before the first Hargreaves measurement. The result was expressed as the mean withdrawal latency time for each animal and group ± SEM. To exclude possible effects from administration of 10% DMSO, we have compared a group of animals that repeatedly received saline injections at the time points and in the volumes corresponding to the drug injections, with a group of animals that received 10% DMSO injections.
Tail-immersion test.
The mouse was placed in a Plexiglas restrainer and left to settle for 10 min. Half of the tail was then immersed in −14°C to −15°C ethanol, and the time until the mouse would withdraw/rattle its tail was monitored (Mogil and Pasternak, 2001). The test was repeated two to three times. The animal was left to settle for 5 min between the tests. Cutoff was set to 20 s. For treatments with Win51078 and BIBN4096BS, the same procedure as above applies, with the exception that animals were injected (intraperitoneally or intrathecally) with Win51078 (Sigma; dissolved in 10% DMSO) 10 min before the measurement or 0.5 mg/kg BIBN4096BS (Boehringer Ingelheim; dissolved in 10% DMSO) 30 min before the measurement. The groups of Vglut2f/f;Trpv1–Cre animals used for the test without and with pharmacological treatment represent two separate sets of animals to avoid stress factors related to restraining. Results were expressed as mean withdrawal latency for each animal and group ± SEM.
Formalin test.
Each mouse was restrained using a tissue, gently keeping the right hindpaw isolated. Twenty microliters of 5% Formalin (37% formaldehyde; Sigma-Aldrich) in 0.9% saline (Sigma) were injected subcutaneously into the plantar surface of the right hindpaw using a Hamilton microsyringe (1710 TLL; 100 μl) with a 32 gauge needle. The mouse was observed in a transparent cage for the following 60 min to monitor pain behavior and licking and biting of the injected paw. The results were expressed in two different ways. First, the results were expressed as the mean time spent licking/biting 0–10 min (acute phase) and 10–60 min (inflammatory phase) for each group ± SEM. Second, the results from each 5 min interval was expressed as the mean time spent licking/biting for each group ± SEM and visualized in a graph showing the development in pain behavior over the 60 min. To assess the contribution of substance P, 5 mg/kg Win51078 (Sigma; dissolved in 10% DMSO) was injected intraperitoneally or intrathecally 10 min before the Formalin injection. To analyze CGRP contribution, 0.5 mg/kg BIBN4096BS was injected intraperitoneally (Boehringer Ingelheim; dissolved in 10% DMSO) 30 min before the measurement.
Assessment of c-Fos expression after heat, cold, and Formalin tests.
For assessment of c-Fos activity, mice underwent the same procedure as described in the heat, cold, and Formalin tests. In heat and cold measurements, the mice were stimulated twice, with 5 min between each measurement with Hargreaves or −15°C ethanol, respectively. The animals were then placed back in their home cage for 55 min after the last stimulation and before they were perfused (described previously by Gezelius et al., 2006). During the Formalin experiment, after 1 h of scoring the behavior, the mice were perfused. The spinal cords were dissected out and prepared for subsequent cryo-sectioning (as described above). Animals that underwent Win51078 or BIBN4096BS treatment were additionally injected intraperitoneally with Win51078 10 min before each measurement or BIBN4096BS 30 min before each measurement.
Itch behavior
Spontaneous itch behavior.
Before experiments, adult mice (7 weeks old) were given 5 min to acclimate in a plastic chamber (820 cm2) supplied with bedding from the home cage. The behavior was recorded with a digital video camera for 30 min. One bout of scratching by either hindpaw was defined as a scratching episode. Grooming was defined as episodes of biting/licking of the body and/or rubbing by either forepaw. The behavior was scored by an observer blind to the genotype using the software AniTracker version 1.0 (www.rsutils.com/downloads.html). The result was expressed as the mean number of scratch episodes for each group/30 min ± SEM. Vglut2f/f;Trpv1–Crew/w;Grpr+/0 male mice (controls), Vglut2f/f;Trpv1–Cretg/wt;Grpr+/0 (conditional knock-outs) male mice, and Vglut2f/f;Trpv1–Cretg/wt;Grpr−/0 (conditional knock-outs/full knock-out for Grpr) male mice were 8 weeks old when recorded.
Provocation of itch.
R26DTA/wt;Trpv1–Cretg/wt and control mice were recorded for 1 h before they were injected intradermally with 50 μl of NaCl using a 100 μl Hamilton syringe supplied with a 32 gauge needle. The animals were then recorded again for 1 h. The following day, the animals were again recorded for 1 h before they were injected intradermally with 100 μg of Compound 48/80 (Sigma) or 200 μg of chloroquine (CQ; Sigma) dissolved in 50 μl NaCl. The protocol ended with a 1 h recording of each mouse, directly after the injection. The behavior was scored by an observer that was blind to the treatment and genotype using the software Anitracker version 1.0 (www.rsutils.com/downloads.html).
Treatment of itch.
To assess the possible contribution of different peptides in the spontaneous scratch response, Vglut2f/f;Trpv1–Cre mice with obvious scratch behavior (with visible lesions on skin) were recorded on the first day for 1 h. On the second day, the mice were treated with the substance P antagonist Win51078, 5 mg/kg (Sigma; dissolved in 10% DMSO in NaCl), the CGRP antagonist BIBN4096BS, 0.3 mg/kg (Boehringer Ingelheim; dissolved in 10% DMSO in NaCl), or the GRPR antagonist RC3095, 10 mg/kg (Invitrogen, dissolved in NaCl). Win51078 and BIBN4096BS were injected intraperitoneally or intrathecally using a 100 μl Hamilton syringe, and RC3095 was injected intrathecally using a 10 μl Hamilton syringe to Vglut2f/f;Trpv1–Cre mice 10 min (or 30 min for BIBN4096BS) before the scratch recordings. To assess whether BIBN4096BS induces itch, control mice were injected intraperitoneally with 0.3 mg/kg (Boehringer Ingelheim; dissolved in 10% DMSO in NaCl) 30 min before a 30 min recording of the itch behavior. The behavior was scored by an observer that was blind to the treatment and genotype using the software Anitracker version 1.0 (www.rsutils.com/downloads.html).
Statistics
Nonparametric calculations of p values between two groups were conducted using Mann–Whitney test (Prism version 5.01; GraphPad Software). One-way ANOVA with Dunn's post hoc test was used for calculations of p values between three different groups of animals or pharmacological treatments on one group (before and after treatment; one variable) and two-way ANOVA with Bonferroni's post hoc test for calculation of p values for two groups in pharmacological treatments (two variables). For negative results, the power of the test was calculated, and, if necessary, the number of samples was increased.
Results
A majority of Trpv1–Cre cells express VGLUT1, VGLUT2, substance P, and CGRP
We first set out to investigate the neurotransmitter phenotype of Trpv1–Cre neurons by analyzing the expression of the three VGLUTs and two neuropeptides in the Trpv1–Cre subpopulation of DRG neurons using immunohistochemistry and in situ hybridization. Our analyses showed that 33.1 ± 1.6% of VGLUT1-positive neurons coexpress Trpv1–Cre, which corresponds to 46.1 ± 2.2% of the Trpv1–Cre;tdTomato population (Fig. 1A). We could also show that VGLUT2, known to play an important role in sensory transmission (Lagerström et al., 2010; Liu et al., 2010; Rogoz et al., 2012), overlaps to a large extent (68.9 ± 1.8%) with Trpv1–Cre;tdTomato-positive DRG neurons, which corresponds to 53.5 ± 2.2% of the Trpv1–Cre;tdTomato-positive cells (Fig. 1B). Conversely, VGLUT3 showed almost no coexpression with Trpv1–Cre;tdTomato-positive neurons (7.4 ± 0.7%), corresponding to 4.3 ± 0.4% of Trpv1–Cre;tdTomato-positive neurons (Fig. 1C). The extensive overlap between the first two VGLUTs and Trpv1–Cre prompted us to study the overlap between the VGLUTs themselves, using in situ hybridization, to elucidate whether each VGLUT enables glutamatergic transmission in a specific Trpv1–Cre subpopulation or whether they are overlapping (Fig. 1G–I). Our analysis showed that 35.6 ± 2.4% of Vglut2-expressing cells overlap with Vglut1 mRNA expression, which corresponds to 57.1 ± 4.1% of the Vglut1 population. Vglut3 showed little overlap with both Vglut1 (2.4 ± 0.5%) and Vglut2 (11.1 ± 1.5%) and thus represents an almost entirely separate subpopulation. To investigate whether genetic ablation of Vglut2 from Trpv1–Cre cells affect the expression of the other VGLUTs, we examined the expression of Vglut1 and VGLUT3 in Vglut2f/f;Trpv1–Cre and control tissue using in situ and immunohistological analyses, respectively. The analysis showed that there were no differences in overlap between neither Vglut1-positive cells and Trpv1–Cre cells (p = 0.28, power of test = 0.99) nor VGLUT3-positive cells and Trpv1 cells (p = 0.37, power of test = 0.99) when comparing Vglut2f/f;Trpv1–Cre and control mice (n = 3 per genotype, 44 Vglut2f/f;Trpv1–Cre sections and 42 control sections analyzed, respectively). Hence, our data indicate that the glutamatergic signaling involved in Trpv1–Cre-mediated transmission is mediated by VGLUT1 and VGLUT2, coexpressed or expressed individually.
Figure 1.
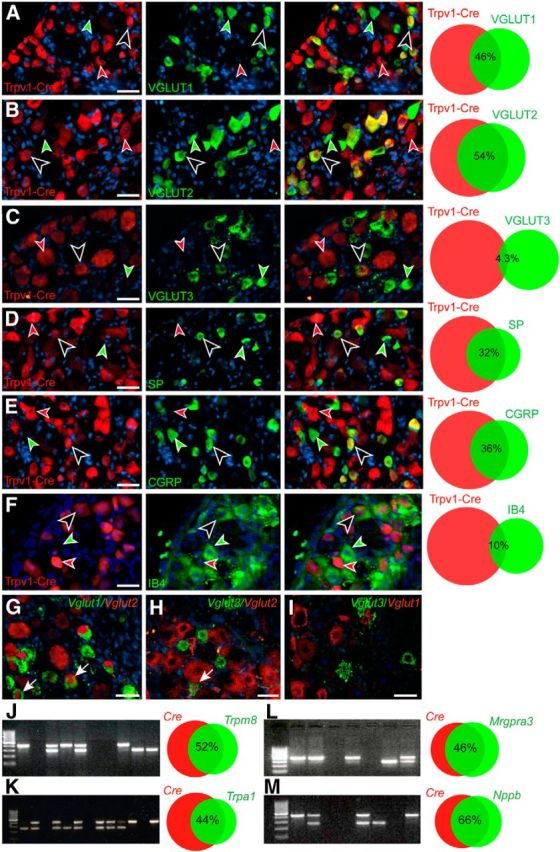
Peptidergic and glutamatergic markers are expressed robustly in Trpv1–Cre primary sensory neurons. The neurochemical phenotype of Trpv1–Cre;tdTomato-positive neurons was investigated using immunohistochemistry and in situ hybridization with different markers. A–C, Overlap analysis of Trpv1–Cre;tdTomato neurons with different VGLUTs. A, 62.1 ± 4.6 cells per section expressed VGLUT1 and 85.0 ± 5.0 cells per section expressed Trpv1–Cre;tdTomato; of these, 27.1 ± 1.8 coexpress Trpv1–Cre;tdTomato and VGLUT1 (n = 3 and 10 sections per animal). B, A total of 85.9 ± 6.6 cells per section expressed VGLUT2, and 110.7 ± 7.9 cells per section expressed Trpv1–Cre;tdTomato; of these, 60.1 ± 5.7 cells per section coexpress VGLUT2 and Trpv1–Cre;tdTomato (n = 4 and 10 sections per animal). C, VGLUT3 represents an almost distinct neuronal population from Trpv1–Cre;tdTomato in which only 4.8 ± 0.4 cells per section of the 119.0 ± 7.7 Trpv1–Cre;tdTomato cells per section expressed VGLUT3. A total of 72.4 ± 5.3 cells per section expressed VGLUT3 (n = 3 and 10 sections per animal). D, Approximately one-third (38.4 ± 4.7 cells per section) of the 110.8 ± 8.0 Trpv1–Cre;tdTomato cells per section were positive for substance P. A total of 53.6 ± 6.7 cells per section expressed substance P (n = 4 and 10 sections per animal). E, Similarly, approximately one-third (39.6 ± 3.3 cells per section) of the 113.3 ± 7.8 Trpv1–Cre;tdTomato cells per section express CGRP. A total of 63.5 ± 5.8 cells per section expressed CGRP (n = 4 and 10 sections per animal). F, Approximately 10% (6.56 ± 0.01 cells per section) of the 67.9 ± 3.8 Trpv1–Cre;tdTomato cells per section expressed IB4. A total of 31.9 ± 2.56 cells per section expressed IB4 (n = 3 animals per 39 sections). G, Vglut1 mRNA showed partial overlap with Vglut2 mRNA in the DRGs; of 64.3 ± 3.1 cells per section, 37.4 ± 1.8 cells per section overlap with Vglut2, which corresponds to 57.1 ± 4.1% of Vglut1-positive cells per section and 35.6 ± 2.4% of the 105.5 ± 5.3 Vglut2-positive cells per section (n = 3 and 10 sections per animal). H, Of 112.2 ± 7.7 Vglut2 mRNA-expressing cells per section, only 4.7 ± 0.4 cells per section overlap with the 42.7 ± 3.4 Vglut3 mRNA-positive cells per section (n = 3 and 10 sections per animal). I, Only 1.3 ± 0.2 cells per section coexpress Vglut1 and Vglut3 mRNA (n = 3 and 10 sections per animal). J, Single-cell PCR analysis show that, of 69 primary neurons analyzed, 17 neurons expressed both Cre and Trpm8, 16 neurons expressed only Cre, 9 neurons expressed only Trpm8, and 27 neurons expressed neither marker. K, Of 75 neurons analyzed, 18 expressed both Cre and Trpa1, 23 expressed only Cre, 6 expressed only Trpa1, and 28 expressed neither marker. L, Analyses of Mrgpra3 mRNA expression in primary afferent neurons revealed that, of 92 neurons analyzed, 28 expressed both Cre and Mrgpra3, 13 neurons expressed only Cre, 14 neurons expressed only Mrgpra3, and 37 expressed neither marker. M, Analyses of Nppb mRNA expression showed that, of 87 neurons analyzed, 27 expressed both Cre and Nppb, 16 expressed only Cre, 12 expressed only Nppb, and 32 expressed neither marker. A 100 bp ladder, size of PCR products: Cre, 405 bp; Trpm8, 290 bp; Trpa1, 261 bp; Mrgpra3, 284 bp; Nppb, 249 bp. Black/white arrows indicate a double-labeled cell. Green arrow indicates a cell singularly labeled with the marker in question. Red arrow highlight a Trpv1–Cre;tdTomato labeled cell. Scale bars, 60 μm. The Venn diagrams have been generated using the Venn diagram plotter (PNNL). Part of data presented in B, D, and E were also included in the study by Rogoz et al. (2014).
We next investigated the neuropeptide and receptor content of the TRPV1 population. We could observe that 73.3 ± 1.8% of the substance P-expressing neurons overlap with the Trpv1–Cre;tdTomato population, which corresponds to 32.3 ± 1.8% of the Trpv1–Cre;tdTomato population (Fig. 1D). Furthermore, 66.3 ± 2.4% of the CGRP-expressing cells overlap with the Trpv1–Cre;tdTomato population, which corresponds to 35.5 ± 1.6% of the Trpv1–Cre;tdTomato population (Fig. 1E). Our analysis also showed that 10% of the Trpv1–Cre;tdTomato population overlap with 21% of the IB4-positive population, which is considered to represent nonpeptidergic neurons (Fig. 1F). Moreover, our single-cell PCR analysis showed that 52% of the Trpv1–Cre population also expressed the cold receptor Trpm8, which corresponds to 65% of Trpm8-positive cells (Fig. 1J). Furthermore, 44% of Trpv1–Cre-positive cells coexpressed a receptor for noxious cold and Formalin; Trpa1, which corresponds to 75% of Trpa1-positive cells (Fig. 1K). These data indicate that Trpv1–Cre is affecting a substantial part of both Trpa1- and Trpm8-expressing neurons. We have also looked at two other markers related to itch transmission: Mrgpra3 and Nppb. Our analysis showed that 45% of Trpv1–Cre cells also express Mrgpra3, which corresponds to 50% of the Mrgpra3 population (Fig. 1L). Additionally, 66% of Trpv1–Cre cells coexpressed Nppb, which corresponds to 69% of the Nppb population (Fig. 1M).
In summary, we demonstrate that the Trpv1–Cre population contains components of the glutamatergic transmission machinery, as well as receptors for noxious cold, Formalin, and itch and the pain- and/or itch-associated neuropeptides substance P, Nppb, and CGRP, which presumably implicate a role for the Trpv1–Cre population in these sensory modalities.
Glutamate in peripheral TRPV1 neurons is, together with substance P, required for intact transmission of noxious cold sensation
To define the precise role of the Trpv1–Cre-expressing neurons in acute pain, the behavior phenotype of R26DTA/wt;Trpv1–Cre mice and controls was investigated and compared with the behavior phenotype of mice lacking Vglut2 in Trpv1–Cre neurons (Vglut2f/f;Trpv1–Cre mice; Lagerström et al., 2010). Our analysis showed that 99.1% of Trpv1–Cre-positive cells are lost in R26DTA/wt;Trpv1–Cre mice compared with control littermates (n = 2 per genotype, 23 sections/R26DTA/wt;Trpv1–Cre/tdTomato mice and 25 sections/R26wt;Trpv1–Cre/tdTomato mice), which indicates that expression of diphtheria toxin resulted in an almost complete deletion of the Trpv1–Cre population and therefore represents a suitable model for analyzing the role of the Trpv1–Cre population in sensory transmission.
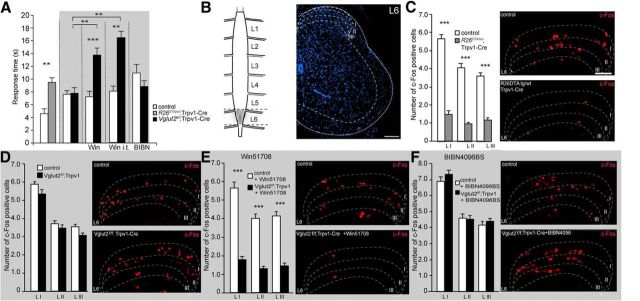
Although TRPV1 is associated traditionally with heat transmission, Trpv1–Cre-positive neurons also play a role in noxious cold transmission. TRPA1, known to detect noxious cold, is expressed extensively in TRPV1 neurons (Karashima et al., 2009; Mishra et al., 2011; Fig. 1K). Therefore, we decided to investigate noxious cold sensation associated with glutamate in Trpv1–Cre neurons. The sensation of cold pain was assessed by the tail-withdrawal test from a −15°C ethanol solution. The mean latency of tail withdrawal was 9.5 ± 0.71 and 4.6 ± 0.74 s for R26DTA/wt;Trpv1–Cre mice and controls, respectively (p = 0.008; Fig. 2A), confirming the role of TRPV1 neurons in cold pain transmission. Conversely, Vglut2-deficient mice did not display any difference in response time compared with control littermates (7.8 ± 0.9 and 7.6 ± 0.6 s, respectively; p = 0.71, power of test = 0.94; Fig. 2A). To test how substance P is affecting cold pain transmission in the presence of impaired or intact VGLUT2-mediated transmission, we injected Vglut2f/f;Trpv1–Cre mice and controls with the substance P receptor antagonist Win51708. This treatment resulted in a mean latency of withdrawal of 13.8 ± 1.1 and 7.2 ± 0.9 s for Vglut2f/f;Trpv1–Cre mice and controls, respectively (Fig. 2A). There was no difference between treated and untreated control mice (p = 0.71, power of test = 0.83), which suggests that substance P is redundant for cold transmission. However, when VGLUT2-mediated transmission was removed from Trpv1–Cre neurons, treated Vglut2f/f;Trpv1–Cre mice became less responsive than treated control mice (p = 0.0006) and untreated Vglut2f/f;Trpv1–Cre mice (p = 0.0012), which suggests a role for both substance P and VGLUT2-mediated glutamatergic transmission in cold pain. These results were confirmed by intrathecal administration of Win51708, which resulted in mean latencies of withdrawal of 16.4 ± 1.1 s compared with 8.0 ± 0.9 s for Vglut2f/f;Trpv1–Cre mice and controls, respectively (p = 0.0012).
Figure 2.
TRPV1 neurons are crucial for intact cold transmission, which is dependent on VGLUT2-mediated signaling from Trpv1–Cre neurons and substance P. A, R26DTA/wt;Trpv1–Cre mice are less sensitive to cold pain compared with control mice (n = 6 per genotype), whereas Vglut2f/f;Trpv1–Cre do not differ from control littermates (n = 7 per genotype). Win51708 injections resulted in decreased responses to cold in Vglut2f/f;Trpv1–Cre (n = 7 per genotype). Pretreatment with BIBN4096BS did not alter the behavior of either Vglut2f/f;Trpv1–Cre mice or control littermates (n = 6 per genotype). The animals were treated with vehicle for possible side effects; however, no changes in the behavior was observed after vehicle treatment. B, Schematic representation of c-Fos expression in the spinal cord. The major peak of expression was observed in a lumbar segment L6 and initial sections of sacral segment S1. C, Deletion of TRPV1 neurons resulted in a decreased number of c-Fos cells in the dorsal horn in laminae I–III in R26DTA/wt;Trpv1–Cre mice compared with control mice (n = 2 per genotype, 46 sections for controls, 39 sections for R26DTA/wt;Trpv1–Cre). C, Conversely, ablation of VGLUT2 from TRPV1 neurons did not affect the expression of c-Fos in dorsal horn in Vglut2f/f;Trpv1–Cre mice compared with controls (n = 2 per genotype, 36 sections for controls, 51 sections for Vglut2f/f;Trpv1–Cre. E, Pretreatment with the substance P antagonist Win51708 induced a decreased response of c-Fos in Vglut2f/f;Trpv1–Cre compared with controls (n = 2, 45 sections for controls, 42 sections for Vglut2f/f;Trpv1–Cre. F, Interestingly, injections of BIBN4096BS did not affect the expression of c-Fos in Vglut2f/f;Trpv1–Cre compared with controls (n = 2 per genotype, 51 sections for control, 45 sections for Vglut2f/f;Trpv1–Cre). Data represent means ± SEMs. Shown are Vglut2f/f;Trpv1–Cre and R26DTA/wt;Trpv1–Cre (gray) and the respective controls (white). ***p < 0.001. Mann–Whitney two-tailed test (A–D). Scale bars: B, 72 μm; C–F, 120 μm.
The role of CGRP in cold pain sensation was investigated by treating the Vglut2f/f;Trpv1–Cre mice and controls with the CGRP antagonist BIBN4096BS 30 min before the experiment. This treatment did not result in any significant changes between Vglut2f/f;Trpv1–Cre mice and controls (p = 0.24, power of test = 0.91) or between treated and untreated controls or between treated and untreated Vglut2f/f;Trpv1–Cre mice (p = 0.051, power of test = 0.91; p = 0.73, power of test = 0.85, respectively; Fig. 2A). In summary, these data demonstrate that VGLUT2-mediated glutamatergic transmission from Trpv1–Cre neurons together with substance P, but not CGRP, mediate acute cold transmission. We next analyzed the onset of c-Fos protein expression in the dorsal horn in response to tail immersion in −15°C ethanol. The experiment revealed a significant decrease in the number of c-Fos-positive cells in laminae I–III in segment L6 (Fig. 2B) in R26DTA/wt;Trpv1–Cre mice compared with control littermates (p < 0.0001; Fig. 2C), accompanying the observed difference in responses to acute cold stimulation (Fig. 2A). Ablation of Vglut2 from Trpv1–Cre neurons did not decrease the number of c-Fos-positive cells in Vglut2f/f;Trpv1–Cre mice compared with controls in laminae I–III (p = 0.06, power of test = 0.8; Fig. 2D). This is also supported by the behavioral data, which demonstrate that VGLUT2 alone is not crucial for acute cold sensation (Fig. 2A). Preinjection with Win51708 resulted in a decreased number of c-Fos-positive cells throughout laminae I–III in Vglut2f/f;Trpv1–Cre mice compared with injected controls (p < 0.0001; Fig. 2E), suggesting cooperative roles of glutamate and substance P in cold pain transmission. On the contrary, pharmacological blockage of CGRP transmission by BIBN4096BS did not alter the number of c-Fos-positive cells in the dorsal horn of the spinal cord in Vglut2f/f;Trpv1–Cre mice compared with controls (p = 0.41, power of test = 0.81; Fig. 2F).
In summary, our data suggest that VGLUT2-mediated glutamatergic transmission from Trpv1 neurons, together with substance P, transmit noxious cold sensation.
Heat nociception from primary afferents to the spinal cord is mediated mainly by Trpv1–Cre neurons and glutamate via VGLUT2 and CGRP
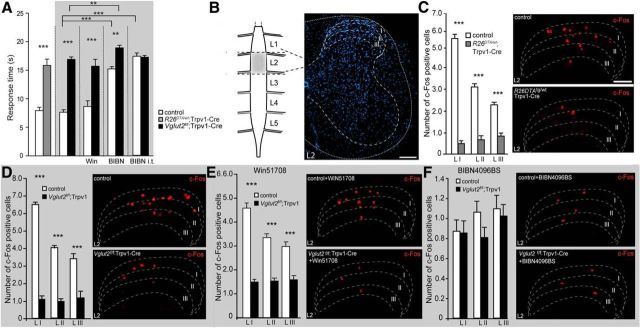
Acute thermal heat pain sensation was examined using the Hargreaves test, and the mean latency of withdrawal of a hindpaw was 15.8 ± 1.1 and 7.9 ± 0.6 s for R26DTA/wt;Trpv1–Cre mice and controls, respectively. This represented a significant difference between the groups (p < 0.0001; Fig. 3A) and is in agreement with previous findings (Mishra et al., 2011). When Vglut2 was selectively removed from the Trpv1–Cre population, Vglut2f/f;Trpv1–Cre mice also displayed a significantly decreased response time compared with control littermates (16.9 ± 0.4 compared with 7.6 ± 0.4 s, respectively; p < 0.0001), and the effect of the Vglut2 ablation was comparable with the response displayed by the R26DTA/wt;Trpv1–Cre mice (p = 0.92, power of test = 0.97), indicating that VGLUT2-mediated glutamatergic release is central for the role of the Trpv1–Cre population in heat transmission, which is consistent with our previous findings (Lagerström et al., 2010).
Figure 3.
Heat nociception is dependent on TRPV1 neurons and the transmitters glutamate and CGRP but not substance P. A, R26DTA/wt;Trpv1–Cre mice are less sensitive to thermal pain compared with control mice assessed by the Hargreaves test (n = 12–13 per genotype). Similarly Vglut2f/f;Trpv1–Cre mice displayed decreased sensitivity to Hargreaves stimulation (n = 10 per genotype). Pretreatment with Win51708 (intraperitoneally) did not alter the phenotype and still resulted in decreased sensitivity to heat in Vglut2f/f;Trpv1–Cre mice (n = 10 per genotype). Conversely, intraperitoneal pretreatment with BIBN4096BS led to decreased sensitivity to heat in both control littermates and Vglut2f/f;Trpv1–Cre mice compared with untreated mice of corresponding genotype (n = 6 per genotype). Intrathecal injections of BIBN4096BS also led to a decreased heat sensitivity in control mice compared with untreated control mice (n = 6 per genotype). B, Schematic illustration of the segment of spinal cord with the highest expression of c-Fos induced by Hargreaves stimulation, which was involving mostly segment L2 with some additional sections from the end of segment L1 and beginning of segment L3. C, Ablation of the Trpv1–Cre population resulted in a reduction of c-Fos-expressing spinal cord neurons after Hargreaves stimulation throughout laminae I–III at spinal segment L2 in R26DTA/wt;Trpv1–Cre mice compared with control mice (n = 2 per genotype, 65 sections for controls, 66 sections for R26DTA/wt;Trpv1–Cre). D, Likewise, genetic ablation of Vglut2 from Trpv1–Cre-positive neurons resulted in decreased reduction of c-Fos expression in Vglut2f/f;Trpv1–Cre mice compared with controls (n = 2 per genotype, 58 sections for controls, 65 sections for Vglut2f/f;Trpv1–Cre). E, Win51708 treatment resulted in a reduction of c-Fos-activated neurons in laminae I–III in Vglut2f/f;Trpv1–Cre compared with controls (n = 2 per genotype, 60 sections for controls and 77 sections for Vglut2f/f;Trpv1–Cre). F, Pretreatment with BIBN4096BS led to a drastic reduction of c-Fos-positive cells in laminae I–III in both Vglut2f/f;Trpv1–Cre mice and control littermates (n = 2 per genotype, 58 sections for controls and 65 sections for Vglut2f/f;Trpv1–Cre), representing no statistical difference between the genotypes. Data represent means ± SEMs. Shown are Vglut2f/f;Trpv1–Cre (black), R26DTA/wt;Trpv1–Cre (gray), and respective controls (white). **p < 0.01, ***p < 0.001. Mann–Whitney two-tailed test (A, C–F). Scale bars: B, 72 μm; C–F, 120 μm.
By eliminating VGLUT2-mediated glutamatergic signaling from the Trpv1–Cre population, the role of other less dominant transmitters in the primary afferents could be investigated. By the administration of Win51708 to Vglut2f/f;Trpv1–Cre mice and control littermates, we induced an additional block of substance P signaling in mice with impaired or intact VGLUT2-mediated transmission, respectively. In acute heat sensation experiments, the pharmacological manipulation did not result in any changes in response time in Vglut2f/f;Trpv1–Cre (15.7 ± 1.2 s) or control (8.6 ± 1.0 s) mice when compared with untreated mice of corresponding genotype (p = 0.91, p = 0.35; power of test = 0.82, power of test = 0.91, respectively), indicating that substance P is not crucial for transmitting acute noxious heat (Fig. 3A). We next investigated the role of CGRP in acute heat in mice with impaired or intact VGLUT2-mediated transmission by administrating BIBN4096BS to Vglut2f/f;Trpv1–Cre mice and control littermates. Block of CGRP receptors resulted in decreased sensitivity to heat stimuli in treated control animals (15.2 ± 0.4 s; p = 0.0002) and in treated Vglut2f/f;Trpv1–Cre mice (18.9 ± 0.4 s; p = 0.008) when compared with untreated mice of corresponding genotype, indicating that CGRP plays an important role in acute heat transmission (Fig. 3A). Also, when the CGRP antagonist was delivered intrathecally, control mice became less responsive to noxious heat compared with untreated control mice (p = 0.0002), confirming the role of CGRP in the mediation of noxious heat.
In summary, the Trpv1–Cre population is central for mediating acute heat pain and the main neurotransmitter is glutamate, as revealed by the R26DTA/wt;Trpv1–Cre and the Vglut2f/f;Trpv1–Cre analyses, respectively. Substance P was shown to have no significant role in acute heat transmission neither in control mice nor when VGLUT2-mediated transmission was removed from the Trpv1–Cre-expressing cells. Conversely, treatment with the CGRP antagonist increased the response latency to acute heat in Vglut2f/f;Trpv1–Cre and control mice, showing that CGRP also plays a role in acute heat transmission.
We next compared how ablation of the Trpv1–Cre population, deletion of Vglut2 from the Trpv1–Cre population, and/or blocking of substance P or CGRP transmission affected downstream activation of second-order neurons in the spinal cord. We did this by examining the number of c-Fos protein-expressing neurons in the dorsal horn after heat stimulation using the Hargreaves setup (Fig. 3C–F). The analyses revealed a significant decrease in the number of c-Fos-positive nuclei in laminae I–III in segment L2 (Fig. 3B) in R26DTA/wt;Trpv1–Cre mice compared with control littermates (p < 0.0001), reflecting the observed difference in responses to acute heat stimulation (Fig. 3A,C). Furthermore, Vglut2f/f;Trpv1–Cre mice also displayed a significant decrease in the number of c-Fos-positive nuclei compared with littermate controls at the same spinal level (p < 0.0001; Fig. 3D), which is in concert with the observed decreased sensitivity to heat of Vglut2f/f;Trpv1–Cre mice compared with controls (Fig. 3A). Additional preinjection of Win51708 did not notably affect the c-Fos expression in the dorsal spinal cord of Vglut2f/f;Trpv1–Cre mice, reiterating the drastic reduction of c-Fos-expressing cells in Vglut2f/f;Trpv1–Cre animals compared with control mice (p < 0.0001; Fig. 3E). Both Vglut2f/f;Trpv1–Cre and control mice displayed a similar number of c-Fos-positive neurons compared with untreated Vglut2f/f;Trpv1–Cre mice and their littermate controls (p > 0.05 and p > 0.05; power of test = 0.83 and power of test = 0.87, respectively). Interestingly, pretreatment with BIBN4096BS resulted in a significant reduction of c-Fos-positive cells in laminae I–III in control mice compared with untreated (p < 0.0001) but not in Vglut2f/f;Trpv1–Cre mice compared with untreated (p = 0.2–0.4, power of test = 0.83), which reflects the behavioral data. Furthermore, there was no difference between c-Fos expression between Vglut2f/f;Trpv1–Cre and control mice (p > 0.05, power of test = 0.8). These data support a role for CGRP transmission independently and, together with VGLUT2-mediated glutamatergic transmission from Trpv1–Cre primary afferent neurons, in acute heat transmission.
Glutamatergic, substance P, and CGRP-mediated signaling in Trpv1–Cre neurons is crucial for Formalin-induced nociception
We next assessed inflammatory pain by the Formalin test, which is considered to model tissue-injury induced pain. The typical biphasic response induced by Formalin was observed in control littermates, whereas the R26DTA/wt;Trpv1–Cre mice displayed an attenuated response in the second phase (Fig. 4A). The mean observed pain behavior in the first phase was 62.9 ± 7.6 and 59.6 ± 4.1 s for R26DTA/wt;Trpv1–Cre mice and controls, respectively (p = 0.88, power of test = 0.98), whereas the mean observed pain behavior in the second phase was 150.6 ± 21.8 for R26DTA/wt;Trpv1–Cre mice and 331.2 ± 17.8 s for control animals (p = 0.0003). Thus, no significant change in the acute response was observed, but the secondary response was significantly attenuated in the R26DTA/wt;Trpv1–Cre mice, demonstrating a role for the Trpv1–Cre-expressing population in Formalin-induced nociception.
Figure 4.
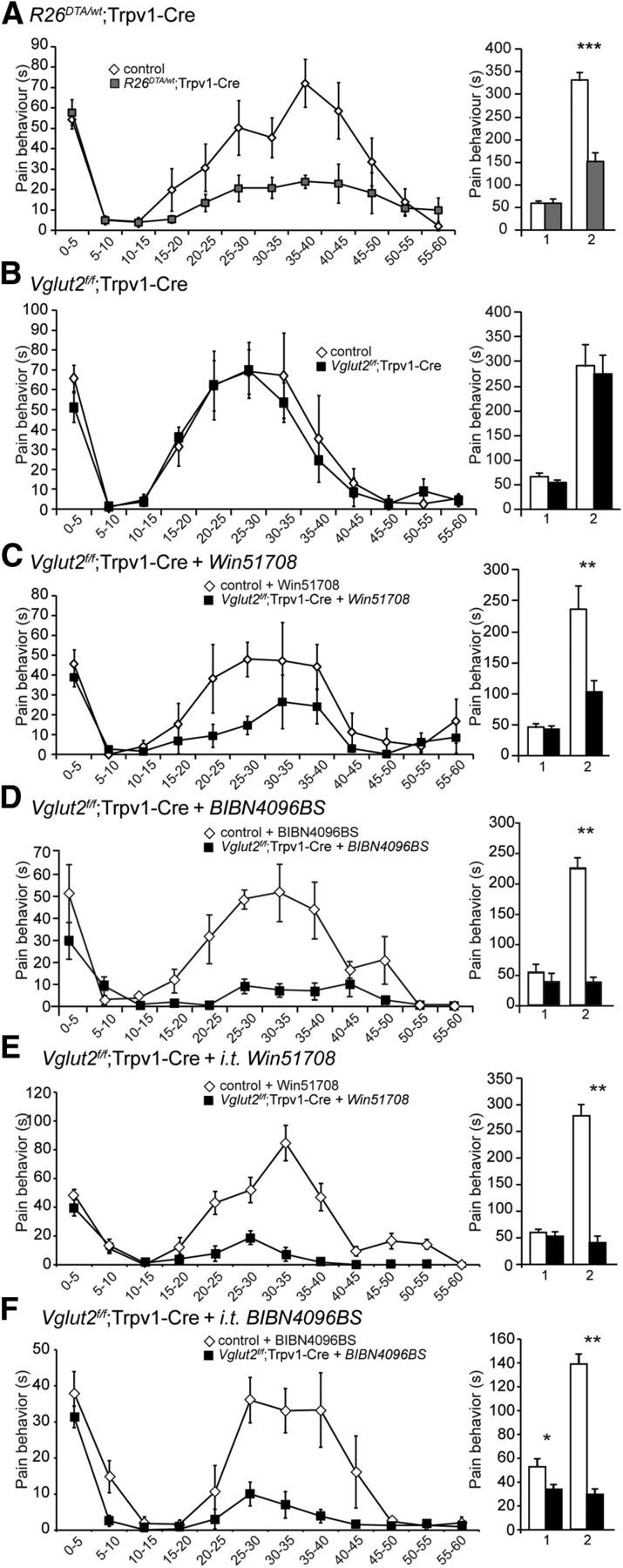
The second phase of the Formalin response depends on substance P-, CGRP-, and VGLUT2-mediated glutamatergic transmission. A, R26DTA/wt;Trpv1–Cre mice display an attenuated nociceptive response to Formalin in the second phase (10–60 min after the Formalin injection) but not to the first phase (0–10 min; n = 8 per genotype). The left displays the cumulative time spent licking/biting of the injected paw/time interval, and the right displays the cumulative time of nociceptive behavior/phase. B, No difference in either phase was observed between Vglut2f/f;Trpv1–Cre and control littermates (n = 8 per genotype). C, The second phase of the Formalin-induced nociceptive response was attenuated in Vglut2f/f;Trpv1–Cre mice pretreated with the substance P antagonist Win51708. Vglut2f/f;Trpv1–Cre mice and littermate controls were given Win51708 (5 mg/kg, i.p.) in DMSO (10 mg/ml) 10 min before the intraplanar Formalin injection (n = 8 per genotype). D, Similarly, BIBN4096BS injections resulted in decreased responses in the second phase of the Formalin-induced nociceptive behavior in Vglut2f/f;Trpv1–Cre mice compared with littermate controls (n = 6 per genotype). E, Intrathecal injection of Win51708 10 min prior to the formalin injection resulted in an attenuated second phase in the formalin response in Vglut2f/f;Trpv1-Cre mice compared to control mice (n = 6/genotype). F, Intrathecal BIBN4096BS injection 30 min prior to the formalin injection attenuated both the first and the second phase of the formalin response in Vglut2f/f;Trpv1-Cre mice compared to control mice (n = 6/genotype). Mann–Whitney test, two-tailed. *p < 0.05, **p < 0.01, ***p < 0.001. Data represent means ± SEMs. Shown are Vglut2f/f;Trpv1–Cre and R26DTA/wt;Trpv1–Cre (gray) and the respective controls (white).
Conversely, when Vglut2 was selectively removed from the Trpv1–Cre population, these mice responded similarly to control littermates in both phases (Fig. 4B). The mean observed nociceptive behavior in the first phase was 52.8 ± 7.6 for Vglut2f/f;Trpv1–Cre mice and 67.0 ± 6.4 s for control animals (p = 0.16, power of test = 0.92), whereas the mean observed pain behavior in the second phase was 276.1 ± 37.8 for Vglut2f/f;Trpv1–Cre mice and 291.6 ± 44.3 for control littermates (p = 0.72, power of test = 0.95). Thus, these results suggest that VGLUT2-mediated glutamatergic transmission does not play a role in Formalin-induced nociceptive behavior mediated by the Trpv1–Cre population, which is consistent with our previous findings (Lagerström et al., 2010). However, it was shown previously that both VGLUT2-mediated and substance P-mediated neurotransmission is required for the development of the second phase of Formalin-induced pain in the Nav1.8 population of primary afferents (Lagerström et al., 2011) and that substance P-deficient mice show attenuated Formalin-induced behavior (Zimmer et al., 1998). Therefore, we treated the Vglut2f/f;Trpv1–Cre mice and controls with Win51708 before the Formalin injection to evaluate whether substance P was also affecting mice with impaired VGLUT2-mediated glutamatergic transmission in the Trpv1–Cre population. The Win51708 treatment did not affect the first phase, but it significantly attenuated the second phase of the Formalin response in the Vglut2f/f;Trpv1–Cre mice compared with control mice (Fig. 4C). The response in the second phase was comparable with the response from the R26DTA/wt;Trpv1–Cre mice (p > 0.05, power of test = 0.93), indicating that both VGLUT2- and substance P-mediated transmission participate to evoke the nociceptive behavior induced by Formalin. The mean observed behavior in the first phase was 41.7 ± 5.9 s for treated Vglut2f/f;Trpv1–Cre mice and 45.8 ± 7.0 s for treated control animals (p = 0.65, power of test = 0.96), and the mean observed behavior for the second phase was 102.1 ± 20.2 s for treated Vglut2f/f;Trpv1–Cre mice and 236.6 ± 38.0 s for treated control animals (p = 0.0063). There was no difference between the first phase in the R26DTA/wt;Trpv1–Cre mice compared with treated Vglut2f/f;Trpv1–Cre mice (p > 0.05, power = 0.85). Also, the substance P antagonist treatment did not affect the first or second phase in control mice compared with untreated control mice (p > 0.05 for both phases, power phase I = 0.97, phase II = 0.92), showing that the Formalin response is not solely mediated by substance P.
CGRP-deficient mice show attenuated responses to Formalin (Zhang et al., 2001), and we next evaluated whether CGRP-mediated transmission may compensate for the loss of VGLUT2-mediated glutamatergic transmission in the Trpv1–Cre population. The CGRP component of Formalin-induced nociceptive behavior was investigated by injecting Vglut2f/f;Trpv1–Cre mice and controls with BIBN4096BS 30 min before the Formalin injection. The BIBN4096BS treatment did not affect the first phase, but it significantly attenuated the second phase of the Formalin response in the Vglut2f/f;Trpv1–Cre mice compared with control mice (Fig. 4D). The mean observed behavior in the first phase was 38.6 ± 11.9 s for Vglut2f/f;Trpv1–Cre mice compared with 53.8 ± 12.7 s for control littermates (p = 0.48, power of test = 0.8), and the mean observed behavior for the second phase was 37.7 ± 7.3 s for Vglut2f/f;Trpv1–Cre mice compared with 225.2 ± 14.1 s for control animals (p = 0.0022). These results suggest that VGLUT2-mediated glutamatergic signaling transmitted by the Trpv1–Cre population together with CGRP- and substance P-mediated signaling are responsible for the second phase of the Formalin response. These results were confirmed by intrathecal administration of BIBN4096BS or Win51708, which both resulted in attenuated responses in the second phase in Vglut2f/f;Trpv1–Cre mice compared with controls (p = 0.0022 and p = 0.0022, respectively; Fig. 4E,F).
We next analyzed the onset of c-Fos protein expression in the dorsal horn that followed Formalin injection and compared how ablation of the Trpv1–Cre population, deletion of Vglut2 from the Trpv1 population, and/or blocking of substance P or CGRP transmission affects downstream activation of second-order neurons (Fig. 5). Formalin injections of R26DTA/wt;Trpv1–Cre and control mice revealed a significant decrease in the number of c-Fos-positive nuclei in laminae I–III in segment L3/L4 compared with control littermates (Fig. 5A; p < 0.0001), reflecting the observed difference in behavior during Formalin-induced inflammation (Fig. 4A). Similarly, Vglut2f/f;Trpv1–Cre mice did not show any decrease in the number of c-Fos-positive nuclei compared with littermate controls at the same spinal level (Fig. 5B; p = 0.0988,), in this case reflecting the absence of a behavioral difference (Fig. 4B). Furthermore, the phenotypic alteration in Vglut2f/f;Trpv1–Cre mice, observed after injection of Win51078 (Fig. 4C), was confirmed by the c-Fos pattern (Fig. 5C). A drastic reduction of c-Fos-positive cells was observed in Win51708-treated Vglut2f/f;Trpv1–Cre mice, especially in laminae I–III, compared with Win51708-treated control animals (p = 0.006). Similarly, pretreatment of Vglut2f/f;Trpv1–Cre mice with BIBN4096BS resulted in significant reduction of c-Fos-positive cells in laminae I–III compared with BIBN4096BS-treated controls, highlighting the important role of substance P and CGRP transmission together with VGLUT2-mediated transmission in Formalin-induced inflammation (p < 0.0001). Hence, substance P and/or CGRP together with VGLUT2-mediated glutamatergic signaling from the Trpv1–Cre population are transmitting Formalin-induced nociception, and the decimation of these transmitters leads to less activation of second-order interneurons, tentatively explaining the observed phenotypes.
Figure 5.
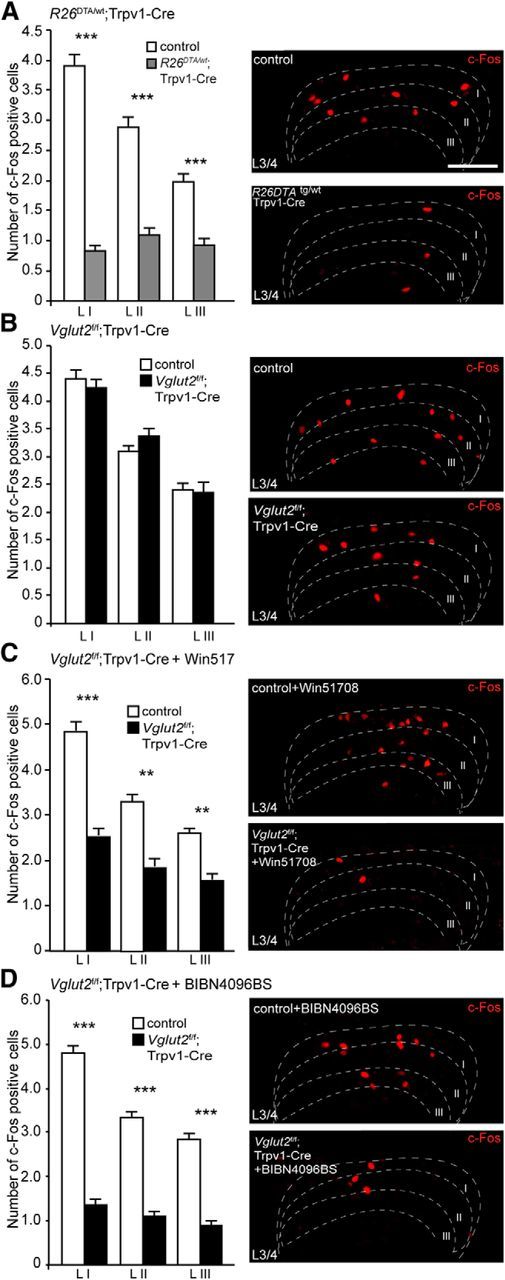
The contribution of VGLUT2, substance P, and CGRP to the development and transmission of Formalin-induced inflammation is apparent at second-order neurons. A, R26DTA/wt;Trpv1–Cre mice were shown to have decreased levels of c-Fos-positive cells in the dorsal horn of the spinal cord compared with control animals (n = 2 per genotype, 51 sections for controls, 41 sections for R26DTA/wt;Trpv1–Cre; n = 2 per genotype, 52 sections for controls, 42 sections for R26DTA/wt;Trpv1–Cre). B, Ablation of Vglut2 from Trpv1–Cre neurons did not result in any significant changes in the number of observed c-Fos cells between Vglut2f/f;Trpv1–Cre and control mice (n = 2 per genotype, 58 sections for controls, 52 sections for Vglut2f/f;Trpv1–Cre; n = 2 per genotype, 59 sections for controls, 53 sections for Vglut2f/f;Trpv1–Cre). C, Injection of Win51708 before the experiment resulted in a decreased number of c-Fos activated cells in the dorsal horn of the spinal cord (n = 2 per genotype, 59 sections for controls, 47 sections for Vglut2f/f;Trpv1–Cre; n = 2 per genotype, 60 sections for controls; 48 for Vglut2f/f;Trpv1–Cre). D, Similarly, pretreatment with BIBN4096BS resulted in a decreased number of c-Fos activated cells in Vglut2f/f;Trpv1–Cre compared with controls (n = 2 per genotype, 49 sections for controls, 57 sections for Vglut2f/f;Trpv1–Cre; n = 2 per genotype, 50 sections for controls, 73 sections for Vglut2f/f;Trpv1–Cre). Mann–Whitney test, two-tailed. **p < 0.001, ***p < 0.001. Data represent means ± SEMs. Shown are Vglut2f/f;Trpv1–Cre (black), R26DTA/wt;Trpv1–Cre (gray), and respective controls (white). Scale bar, 100 μm.
TRPV1 primary neurons are crucial for itch transmission, and the sensation is dependent on VGLUT2, CGRP, and GRPR
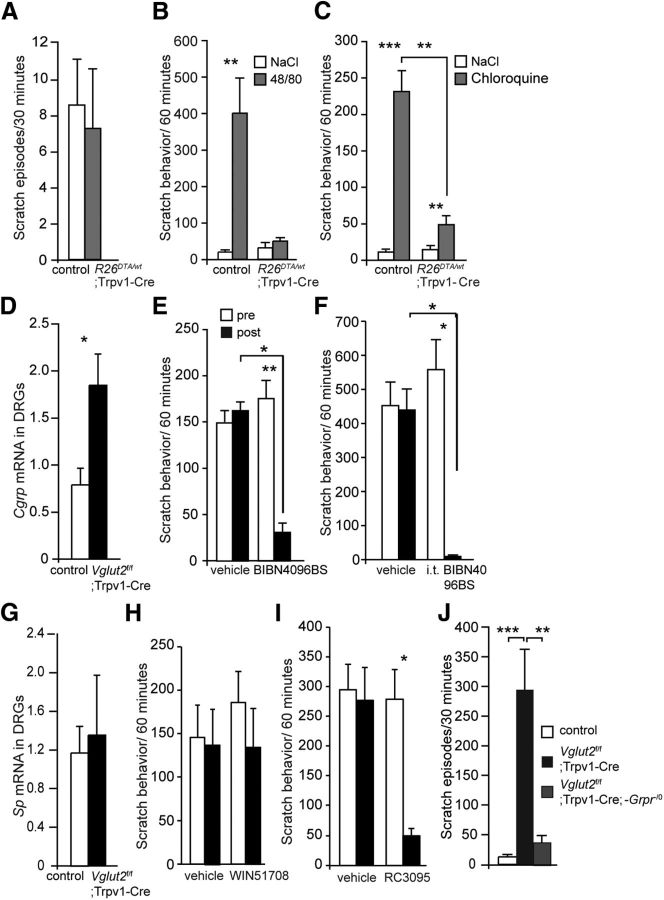
Primary afferents not only mediate nociceptive input to the spinal cord, they also transduce pruritogenic signals. To investigate the role of the Trpv1–Cre population in itch transmission, we first examined the spontaneous itch behavior in R26DTA/wt;Trpv1–Cre mice and controls and examined alterations in the number of spontaneous scratch episodes. R26DTA/wt;Trpv1–Cre mice displayed a mean of 7.2 ± 3.3 scratch episodes/30 min compared with 8.5 ± 2.5 scratch episodes/30 min in control littermates, representing no apparent difference in spontaneous itch between the groups (p = 0.53, power of test = 0.84; Fig. 6A). During intradermal injections of 48/80, which induces mast cell degranulation and subsequent histamine release, R26DTA/wt;Trpv1–Cre mice displayed no significant difference in scratch frequency between vehicle and 48/80 injection (31.3 ± 13.0 and 49.8 ± 6.8 scratch episodes/h, respectively; p = 0.99, power of test = 0.97; Fig. 6B). Conversely, control littermates displayed a significantly elevated scratch behavior after the intradermal 48/80 injection, reaching levels of 20.5 ± 3.7 scratch episodes/h for the vehicle and 400.0 ± 95.6 scratch episodes/h for the 48/80 treatment, respectively (p = 0.0001; Fig. 6B), indicating that the Trpv1–Cre population is mediating histaminergic itch induced by mast cell degranulation.
Figure 6.
Itch regulated by VGLUT2 is mediated by CGRP and GRPR. A, R26DTA/wt;Trpv1–Cre did not differ in the observed levels of spontaneous scratch from control littermates at the age of 7 weeks (n = 8 and 9 per genotype, respectively). B–D, Itch was induced in R26DTA/wt;Trpv1–Cre mice and controls using 48/80 and CQ. B, Intradermal injections of 48/80 did not increase the number of scratch episodes in R26DTA/wt;Trpv1–Cre, whereas control animals displayed elevated itch responses (n = 6 per genotype). C, Similarly, intradermal CQ injections resulted in an increased scratch behavior in control mice and R26DTA/wt;Trpv1–Cre mice (n = 6 per genotype). D, Vglut2f/f;Trpv1–Cre mice displayed increased levels of Cgrp mRNA in DRGs compared with control mice (n = 4 per genotype). E, Intraperitoneal injection of the CGRP antagonist BIBN4096BS resulted in decreased scratch responses in Vglut2f/f;Trpv1–Cre mice compared with the level of scratch episodes before the BIBN4096BS injection (n = 6). F, Also, intrathecal injections of BIBN4096BS resulted in decreased scratch responses in Vglut2f/f;Trpv1–Cre mice compared with the level of scratch episodes before the BIBN4096BS injection (n = 6). G, There was no significant difference in substance p mRNA levels observed between Vglut2f/f;Trpv1–Cre mice and controls (n = 4 per genotype). H, Intraperitoneal injections of the substance P antagonist Win51078 in Vglut2f/f;Trpv1–Cre mice did not result in a change in the number of scratch episodes (n = 8). I, Pharmacological blockade of the GRPR receptor by intrathecal injections of the GRPR antagonist RC3095 resulted in a significantly reduced number of scratch episodes in Vglut2f/f;Trpv1–Cre mice compared with vehicle treatment and before and after the injection (n = 6). J, Genetic removal of the Grpr gene reduced the elevated levels of itch in Vglut2f/f;Trpv1–Cre mice to levels comparable with control littermates (n = 10 per genotype). Mann–Whitney test, two-tailed (A, D, G); one-way ANOVA with Dunn's post hoc test (E, F, H–J); two-way ANOVA (B, C). *p < 0.05, **p < 0.001, ***p < 0.001. Data represent means ± SEMs. Shown are R26DTA/wt;Trpv1–Cre (gray), Vglut2f/f;Trpv1–Cre (black), and respective controls in A, E, and H (white).
CQ, a drug that has long been used in the treatment and prevention of malaria, induces itch as a major side effect (Adebayo et al., 1997). CQ inflicts itch by interacting with the receptor MrgprA3 (Liu et al., 2009), and because half of the MrgprA3-expressing population is affected by Trpv1–Cre (Fig. 1L), we decided to investigate whether ablation of the Trpv1–Cre population would affect CQ-induced itch. Intradermal CQ injection resulted in an increased scratch behavior in littermate controls (10.8 ± 2.1 and 231.5 ± 28.1 scratch episodes/h for vehicle and CQ, respectively; p = 0.0005), and interestingly, also in R26DTA/wt;Trpv1–Cre mice (14.7 ± 3.0 and 49.5 ± 7.4 scratch episodes/h for vehicle and CQ, respectively; p = 0.0005; Fig. 6C). Notably, the response was attenuated compared with the control response (p = 0.002). In summary, the loss of 48/80-induced itch in R26DTA/wt;Trpv1–Cre mice and the attenuated responses to the histamine-independent pruritogen CQ suggest that histamine-dependent itch is mediated by the TRPV1 population, whereas histamine-independent itch also is mediated by additional neuronal populations.
When VGLUT2-mediated transmission from Trpv1–Cre afferents is ablated, such mice develop profoundly elevated itch and the development of small wounds attributable to intense scratching. Induction of itch, by removal of excitatory transmission from Trpv1–Cre afferents, indicates that glutamate, via VGLUT2, regulates itch rather than mediating the sensation itself (Lagerström et al., 2010; Liu et al., 2010; Rogoz et al., 2012). Because both previous findings and ours indicate that the TRPV1 population mediates itch (Imamachi et al., 2009; Mishra et al., 2011), this apparent paradox may be better understood by examining the neurotransmitters responsible for the itch transmission regulated by VGLUT2 and glutamate. qPCR analysis showed that the levels of Cgrp mRNA in DRGs were upregulated in Vglut2f/f;Trpv1–Cre mice compared with controls (p = 0.0286; Fig. 6D), which prompted us to evaluate whether the CGRP antagonist BIBN4096BS could block the itch phenotype displayed by Vglut2f/f;Trpv1–Cre mice. Both intraperitoneal and intrathecal injections of BIBN4096BS drastically attenuated the itch phenotype compared with vehicle treatment (p < 0.05 and p < 0.05, respectively; Fig. 6E,F), suggesting that CGRP mediates itch. BIBN4096BS treatment did not affect the itch behavior in control mice [p = 1.0; 7.8 ± 2.7 and 8.7 ± 3.1 scratches/30 min after vehicle and BIBN4096BS treatment (n = 6), respectively]. However, substance p mRNA levels in DRGs were not found to be upregulated in Vglut2f/f;Trpv1–Cre mice compared with control mice (Fig. 6G). Also, pharmacological treatment with the substance P antagonist Win51708 on Vglut2f/f;Trpv1–Cre mice did not result in any significant difference in the scratching frequency when comparing vehicle and Win51708 treatment (p > 0.05, power of test = 0.93; Fig. 6H). These findings suggest that VGLUT2-mediated glutamatergic transmission from Trpv1–Cre neurons regulates itch mediated by CGRP but not substance P.
The GRPR-mediated signaling pathway in the spinal cord dorsal horn has been shown to mediate both histamine-dependent and -independent itch (Sun and Chen, 2007; Sun et al., 2009). To identify whether VGLUT2-regulated itch from Trpv1–Cre neurons depends on GRPR transmission, Vglut2f/f;Trpv1–Cre mice were treated intrathecally with the GRPR antagonist RC3095, which resulted in an attenuated scratch behavior in the Vglut2f/f;Trpv1–Cre mice when compared with controls (p < 0.05; Fig. 6I). To further examine whether the itch induced by Vglut2 ablation from Trpv1–Cre neurons is mediated by the GRP/GRPR pathway, Grpr null mice (Grpr−/−) were bred with Vglut2f/f;Trpv1–Cre mice. In control mice, spontaneous scratching behavior was consistently low (12.6 ± 1.9 scratches/30 min), whereas Vglut2f/f;Trpv1–Cre mice displayed a robust scratching behavior (294.2 ± 67.5 scratch episodes/30 min; p < 0.05). In contrast, the number of scratch episodes decreased considerably in Vglut2f/f;Trpv1–Cre; Grpr−/0 mice (38.5 ± 9.6 scratch episodes/h, p < 0.05; Fig. 6J), and was comparable with control levels (p > 0.05), indicating that a large part of the itch sensation was relayed via the GRP/GRPR pathway in Vglut2f/f;Trpv1–Cre mice.
In summary, the Trpv1–Cre population was found to mediate primarily histaminergic itch and regulate CGRP- and GRPR-dependent itch through VGLUT2-mediated glutamatergic transmission.
Discussion
Here we describe the transmission accounting for different acute pain states and itch. First, our study corroborates and builds on the knowledge that the Trpv1–Cre population is central for detecting itch, acute heat, cold, and tissue-injury associated pain. We next provide evidence that glutamate via VGLUT2 in the Trpv1–Cre population together with substance P mediate acute cold pain, whereas glutamate together with CGRP mediate noxious heat. Moreover, we show that glutamate together with both substance P and CGRP mediate tissue-injury associated pain (Table 1). The neurons and transmitters responsible for these modalities were further studied using targeted intrathecal injections and analysis of second-order neuron activation in the dorsal horn of the spinal cord. Some effects persisted when switching from systemic intraperitoneal to intrathecal injections, suggesting that the transmission under study is central rather than peripheral and is likely to take place at the first synapse in the dorsal horn. Finally, we demonstrate that CGRP and GRPR play important roles in the mediation of itch originating fromVGLUT2-mediated transmission from Trpv1–Cre neurons. At the spinal level, GRPR-positive neurons have been described as a labeled line for itch sensation in the spinal cord (Sun and Chen, 2007; Sun et al., 2009). These results are in agreement with our data, because we could reverse the profound scratch behavior in Vglut2f/f;Trpv1–Cre mice by antagonistic treatment or genetic deletion of the Grpr gene.
Table 1.
Summary of the role of different neurotransmitters and the Trpv1–Cre population in acute noxious and pruritic transmission in the first synapse
| Trpv1–Cre neurons | VGLUT2-mediated transmission in Trpv1–Cre neurons | Substance P in the first synapse | CGRP in the first synapse | |
|---|---|---|---|---|
| Noxious heat | Yesa | Yesb | No | Yes |
| Noxious cold | Yesa | Yes, together with substance P | Yes, together with VGLUT2-mediated transmission | No |
| Formalin, first phase | No | Yes, together with CGRP | No | Yes, together with VGLUT2-mediated transmission |
| Formalin, second phase | Yes | Yes, together with substance P and CGRP | Yes, together with VGLUT2-mediated transmission and CGRP | Yes, together with VGLUT2-mediated transmission and substance P |
| Mediating itch | Yesa | No | No effect on VGLUT2-regulated itch | Yes, mediates VGLUT2-regulated itch |
| Regulating itch | Yesb | Yesb | ND | ND |
The findings are based on genetic ablation of the Trpv1–Cre population (R26DTA/wt;Trpv1–Cre), genetic ablation of Vglut2 from the Trpv1–Cre population (Vglut2f/f;Trpv1–Cre), intrathecal injections of peptidergic antagonists and c-Fos analysis of second-order neurons in the spinal cord dorsal horn. ND, Not determined.
aConsistent with Mishra et al. (2011).
bConsistent with Lagerström et al. (2010).
The use of pharmacological treatments and the systemic administrations are potential caveats of this study. Both CGRP and substance P receptors are expressed in the PNS and CNS; thus, the effects of systemic treatments have an unclear origin. Therefore, we decided to confirm the data obtained from systemic injections by intrathecal injections. This treatment confirmed an effect originating from the CNS and raises the possibility that the antagonistic site of action is in the first synapse. The selectivity of the drugs is another potential confounding factor. However, BIBN4096BS does not have any relevant affinity for a set of 75 different receptors or enzyme systems (Doods et al., 2000). Similarly, Win51708 was tested both pharmacologically and biochemically for its selective action on the substance P system (Venepalli et al., 1992).
Glutamatergic and peptidergic components compensate for each other and are both crucial for acute thermal nociception
The diverse role of the Trpv1–Cre population as a whole emphasizes the importance of specifically expressed neurotransmitters and receptors in Trpv1–Cre subpopulations to accurately conduct the role of the population. Our study suggests that the two thermal sensations, heat or cold pain, involve partly different combinations of neurotransmitters. Acute noxious heat transmission involves TRPV1 activity (Caterina et al., 1997), VGLUT2-mediated glutamatergic transmission, and CGRP signaling, whereas cold transmission relies on TRPA1 activation (Karashima et al., 2009), VGLUT2-mediated glutamatergic transmission, and substance P. In support of the differential use of peptidergic neurotransmitters in acute heat and cold pain, and a possible compensatory role of glutamate, it was shown previously that loss of CGRPα does not affect the behavioral responses to acute noxious heat (Zhang et al., 2001). However, mice with specific ablation of CGRP-expressing cells were shown to be less sensitive to noxious heat and capsaicin (McCoy et al., 2013). This indicates that loss of CGRP, but not the neuronal population, can be compensated for by another transmitter, which according to our data can be glutamate acting via VGLUT2.
Synaptic transmission through substance P signaling has been shown to be at least in part involved in cold stress-induced hyperalgesia (Satoh et al., 1992). Additionally, intense cold stimulus was shown to induce c-Fos expression in neurokinin-1 receptor-expressing neurons in the superficial horn (Doyle and Hunt, 1999), which supports our findings. The role of substance P in heat is more contradictory; Zimmer et al. (1998) have shown that disruption of substance P signaling through genetic ablation of the tachykinin-1 gene results in longer response latencies in the hotplate test, whereas the behavioral response to the tail flick was unaffected. However, genetic disruption of the substance P receptor gene did not result in any differences in acute heat pain (De Felipe et al., 1998; Zimmer et al., 1998), which is consistent with our findings that substance P is not required for heat pain transmission.
Itch conducted via a subpopulation of TRPV1 neurons depends on VGLUT2, GRP/GRPR, and CGRP for regulation and transmission
Previous studies have shown that the Trpv1 population is important for both the mediation and regulation of itch (Imamachi et al., 2009; Lagerström et al., 2010; Mishra et al., 2011). Our results give additional support for these findings; the R26DTA/wt;Trpv1–Cre mice showed attenuated responses to both histaminergic and nonhistaminergic pruretic agents, and the Vglut2f/f;Trpv1–Cre mice developed a spontaneous itch phenotype. Itch is a complex sensation that may be transmitted and regulated via multiple signaling pathways from the periphery to the spinal cord (LaMotte et al., 2014). We here investigated the role of two neuropeptides in itch transmission, substance P and CGRP, which are both released by primary afferent neurons and partially expressed by the TRPV1 population. Previous reports have indicated that substance P could contribute to pruritus, because ablation of dorsal horn neurons expressing the neurokinin-1 receptor resulted in reduced responses to pruritogens in rats (Carstens et al., 2010). However, in our study, the chronic itch in Vglut2f/f;Trpv1–Cre mice was not reversible by substance P antagonist treatment, indicating that substance P is not required for itch regulated by VGLUT2-mediated transmission. Interestingly, it was shown previously that a neurokinin-1 antagonist failed to inhibit scratching induced by histamine, 5-HT, and bovine adrenal medulla 8–22 but could inhibit scratching when induced by CQ or a proteinase-activated receptor 2 (PAR2) agonist (Akiyama et al., 2013). These data imply that different transmitters regulate different types of itch, in which substance P is involved in the regulation of nonhistaminergic itch induced by CQ or PAR2 agonists (Akiyama et al., 2013).
Recently, mice deficient of CGRP-expressing neurons were shown to have a decreased response to both histamine- and CQ-induced itch (McCoy et al., 2013). We here observed that itch regulated by VGLUT2-mediated transmission from Trpv1–Cre neurons was blocked by intrathecal CGRP antagonist treatment, suggesting that CGRP is an itch mediator in the TRPV1 population and that CGRP-mediated transmission is regulated by VGLUT2. The TRPV1 receptor is predominantly associated with histaminergic itch (Imamachi et al., 2009), and VGLUT2-regulated itch is partly histaminergic (Lagerström et al., 2010). These results, together with our present findings, indicate that CGRP is involved in transmitting histaminergic itch via TRPV1 neurons.
Our findings show the importance of peptide signaling in sensory modulation in which CGRP antagonists could be beneficial in chronic pruritic conditions. We have shown previously that CGRP antagonists attenuate heat hyperalgesia associated with inflammation (Rogoz et al., 2014), and our recent findings show that also acute noxious heat and tissue injury-induced pain involves CGRP transmission, which further emphasizes the potential of CGRP targeted treatments. CGRP antagonists are currently evaluated for use in the clinic for migraine treatment (Bell, 2014). Substance P shows importance for cold noxious pain and shares the importance of CGRP in inflammation-induced hyperalgesia (Rogoz et al., 2014) and tissue injury-induced pain. Our study did not find a role for substance P in VGLUT2-regulated itch, but substance P antagonists are efficient in treating atopic itch (Ständer et al., 2010), in which histaminergic antagonists show little therapeutic effect.
Conclusions
Our study shows how different neurotransmitters cooperate with each other in acute sensations. We suggest that complementary signaling of glutamate and substance P is essential for intact cold sensation and that CGRP signaling together with VGLUT2-mediated transmission from TRPV1 neurons is a main pathway of acute heat transmission. Finally, we propose that CGRP and GRPR are central for the conduction of itch regulated by VGLUT2-mediated transmission from TRPV1 neurons.
Footnotes
This work was supported by grants from the Swedish Research Council (Medicine), Uppsala University, and the foundations of R. Söderberg, K. and A. Wallenberg, Å. Wiberg, M. Bergwall, G. and J. Anér, Jeanssons, and the Royal Swedish Academy of Sciences. K.K. is a Royal Swedish Academy of Sciences Research Fellow supported by a grant from the Knut and Alice Wallenberg Foundation. M.C.L. is a Ragnar Söderberg Fellow in Medicine. We thank Edda Blümel and Felicia Båvenholm for technical support.
The authors declare no competing financial interests.
References
- Adebayo RA, Sofowora GG, Onayemi O, Udoh SJ, Ajayi AA. Chloroquine-induced pruritus in malaria fever: contribution of malaria parasitaemia and the effects of prednisolone, niacin, and their combination, compared with antihistamine. Br J Clin Pharmacol. 1997;44:157–161. doi: 10.1046/j.1365-2125.1997.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Davoodi A, Nagamine M, Blansit K, Horwitz A, Carstens MI, Carstens E. Roles for substance P and gastrin-releasing peptide as neurotransmitters released by primary afferent pruriceptors. J Neurophysiol. 2013;109:742–748. doi: 10.1152/jn.00539.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell IM. Calcitonin gene-related peptide receptor antagonists: new therapeutic agents for migraine. J Med Chem. 2014 doi: 10.1021/jm500364u. doi: 10.1021/jm500364u. Advance online publication. Retrieved September 12, 2014. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Coderre TJ. Attenuation of hyperalgesia in a rat model of neuropathic pain after intrathecal pre- or post-treatment with a neurokinin-1 antagonist. Pain. 2002;95:277–285. doi: 10.1016/S0304-3959(01)00410-9. [DOI] [PubMed] [Google Scholar]
- Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport. 2010;21:303–308. doi: 10.1097/WNR.0b013e328337310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- De Felipe C, Herrero JF, O'Brien JA, Palmer JA, Doyle CA, Smith AJ, Laird JM, Belmonte C, Cervero F, Hunt SP. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, Eberlein W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle CA, Hunt SP. Substance P receptor (neurokinin-1)-expressing neurons in lamina I of the spinal cord encode for the intensity of noxious stimulation: a c-Fos study in rat. Neuroscience. 1999;89:17–28. doi: 10.1016/S0306-4522(98)00276-0. [DOI] [PubMed] [Google Scholar]
- Gezelius H, Wallén-Mackenzie A, Enjin A, Lagerström M, Kullander K. Role of glutamate in locomotor rhythm generating neuronal circuitry. J Physiol Paris. 2006;100:297–303. doi: 10.1016/j.jphysparis.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Hägermark O, Hökfelt T, Pernow B. Flare and itch induced by substance P in human skin. J Invest Dermatol. 1978;71:233–235. doi: 10.1111/1523-1747.ep12515092. [DOI] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova A, Signore M, Caro N, Greene ND, Copp AJ, Martinez-Barbera JP. In vivo genetic ablation by Cre-mediated expression of diphtheria toxin fragment A. Genesis. 2005;43:129–135. doi: 10.1002/gene.20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuno M, Aihara M, Kojima M, Osuna H, Hosoi J, Nakamura M, Toyoda M, Matsuda H, Ikezawa Z. Neuropeptides concentrations in the skin of a murine (NC/Nga mice) model of atopic dermatitis. J Dermatol Sci. 2003;33:55–65. doi: 10.1016/S0923-1811(03)00155-5. [DOI] [PubMed] [Google Scholar]
- Koga K, Chen T, Li XY, Descalzi G, Ling J, Gu J, Zhuo M. Glutamate acts as a neurotransmitter for gastrin releasing peptide-sensitive and insensitive itch-related synaptic transmission in mammalian spinal cord. Mol Pain. 2011;7:47. doi: 10.1186/1744-8069-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerström MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, Olund C, Smith C, Mendez JA, Chen ZF, Wood JN, Wallén-Mackenzie A, Kullander K. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerström MC, Rogoz K, Abrahamsen B, Lind AL, Olund C, Smith C, Mendez JA, Wallén-Mackenzie Å, Wood JN, Kullander K. A sensory subpopulation depends on vesicular glutamate transporter 2 for mechanical pain, and together with substance P, inflammatory pain. Proc Natl Acad Sci U S A. 2011;108:5789–5794. doi: 10.1073/pnas.1013602108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci. 2014;15:19–31. doi: 10.1038/nrn3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansikka H, Sheth RN, DeVries C, Lee H, Winchurch R, Raja SN. Nerve injury-induced mechanical but not thermal hyperalgesia is attenuated in neurokinin-1 receptor knockout mice. Exp Neurol. 2000;162:343–349. doi: 10.1006/exnr.1999.7336. [DOI] [PubMed] [Google Scholar]
- McCoy ES, Taylor-Blake B, Zylka MJ. CGRPalpha-expressing sensory neurons respond to stimuli that evoke sensations of pain and itch. PLoS One. 2012;7:e36355. doi: 10.1371/journal.pone.0036355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy ES, Taylor-Blake B, Street SE, Pribisko AL, Zheng J, Zylka MJ. Peptidergic CGRPalpha primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron. 2013;78:138–151. doi: 10.1016/j.neuron.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 2011;30:582–593. doi: 10.1038/emboj.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- Rogoz K, Lagerström MC, Dufour S, Kullander K. VGLUT2-dependent glutamatergic transmission in primary afferents is required for intact nociception in both acute and persistent pain modalities. Pain. 2012;153:1525–1536. doi: 10.1016/j.pain.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Rogoz K, Andersen HH, Kullander K, Lagerström MC. Glutamate, substance P, and calcitonin gene-related peptide cooperate in inflammation-induced heat hyperalgesia. Mol Pharmacol. 2014;85:322–334. doi: 10.1124/mol.113.089532. [DOI] [PubMed] [Google Scholar]
- Satoh M, Kuraishi Y, Kawamura M. Effects of intrathecal antibodies to substance P, calcitonin gene-related peptide and galanin on repeated cold stress-induced hyperalgesia: comparison with carrageenan-induced hyperalgesia. Pain. 1992;49:273–278. doi: 10.1016/0304-3959(92)90151-Z. [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Low SA, Wang X, Zhang J, Yamanaka H, Urban R, Solorzano C, Harper B, Hnasko TS, Edwards RH, Basbaum AI. VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc Natl Acad Sci U S A. 2010;107:22296–22301. doi: 10.1073/pnas.1013413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/S0896-6273(00)81003-X. [DOI] [PubMed] [Google Scholar]
- Ständer S, Siepmann D, Herrgott I, Sunderkötter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLoS One. 2010;5:e10968. doi: 10.1371/journal.pone.0010968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venepalli BR, Aimone LD, Appell KC, Bell MR, Dority JA, Goswami R, Hall PL, Kumar V, Lawrence KB, Logan ME, Scensny PM, Seelye JA, Tomczuk BE, Yanni JM. Synthesis and substance P receptor binding activity of androstano[3,2-b]pyrimido[1,2-a]benzimidazoles. J Med Chem. 1992;35:374–378. doi: 10.1021/jm00080a025. [DOI] [PubMed] [Google Scholar]
- Wallén-Mackenzie A, Gezelius H, Thoby-Brisson M, Nygård A, Enjin A, Fujiyama F, Fortin G, Kullander K. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J Neurosci. 2006;26:12294–12307. doi: 10.1523/JNEUROSCI.3855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hoff AO, Wimalawansa SJ, Cote GJ, Gagel RF, Westlund KN. Arthritic calcitonin/alpha calcitonin gene-related peptide knockout mice have reduced nociceptive hypersensitivity. Pain. 2001;89:265–273. doi: 10.1016/S0304-3959(00)00378-X. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Baffi J, Usdin T, Reynolds K, König M, Palkovits M, Mezey E. Hypoalgesia in mice with a targeted deletion of the tachykinin 1 gene. Proc Natl Acad Sci U S A. 1998;95:2630–2635. doi: 10.1073/pnas.95.5.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]