Abstract
Long-term potentiation (LTP) and long-term depression (LTD) are key mechanisms of synaptic plasticity that are thought to act in concert to shape neural connections. Here we investigated the influence of visual spatial attention on LTP-like and LTD-like plasticity in the human motor cortex. Plasticity was induced using paired associative stimulation (PAS), which involves repeated pairing of peripheral nerve stimulation and transcranial magnetic stimulation to alter functional responses in the thumb area of the primary motor cortex. PAS-induced changes in cortical excitability were assessed using motor-evoked potentials. During plasticity induction, participants directed their attention to one of two visual stimulus streams located adjacent to each hand. When participants attended to visual stimuli located near the left thumb, which was targeted by PAS, LTP-like increases in excitability were significantly enhanced, and LTD-like decreases in excitability reduced, relative to when they attended instead to stimuli located near the right thumb. These differential effects on (bidirectional) LTP-like and LTD-like plasticity suggest that voluntary visual attention can exert an important influence on the functional organization of the motor cortex. Specifically, attention acts to both enhance the strengthening and suppress the weakening of neural connections representing events that fall within the focus of attention.
Keywords: long-term depression, long-term potentiation, paired associative stimulation, spatial attention, spike-timing-dependent plasticity, transcranial magnetic stimulation
Introduction
Alterations in the strength of communication between neurons play a fundamental role in the capacity of the adult brain to adapt. “Hebbian” long-term potentiation (LTP) and long-term depression (LTD), which refer to increases and decreases in synaptic excitability, respectively, are key mechanisms thought to act in concert to shape neural connections (Malenka and Bear, 2004). Numerous factors affect the induction of LTP and LTD, but the influence of cognitive factors remains poorly understood. Here we focused on spatial attention as a potentially important cognitive modulator of plasticity.
Although LTP and LTD cannot be measured directly in the human brain, plasticity can be externally induced in the motor cortex using paired associative stimulation (PAS). PAS involves repeated pairing of peripheral nerve stimulation targeting a thumb muscle with transcranial magnetic stimulation (TMS) over the area of motor cortex representing that same muscle (Stefan et al., 2000). Depending on stimulus timing, PAS induces bidirectional increases or decreases in cortical excitability (Wolters et al., 2003) that reflect, respectively, LTP-like and LTD-like plasticity (Müller-Dahlhaus et al., 2010). Previous research has shown that LTP-like plasticity is enhanced when the limb undergoing PAS is monitored (Stefan et al., 2004), but methodological limitations preclude unequivocal attribution of those effects to attention (Kamke et al., 2012). Critically, the influence of attention on LTD-like plasticity, which acts together with LTP to allow changes in the behavior of neural circuits, has not been described.
Based on evidence that attention exerts an influence within and between sensory modalities (Macaluso, 2010), we reasoned that shifts in the focus of visual spatial attention should alter PAS-induced effects in the motor cortex. Moreover, such shifts of attention were predicted to differentially influence LTP- and LTD-like plasticity. Specifically, both attention and LTP are neural mechanisms for encoding stimulus relevance or importance, the former in a transitory manner and the latter on a longer time scale to strengthen neural connections. As an adjunct to LTP, however, LTD weakens neural connections that represent irrelevant sensory events. Irrelevant events, by definition, do not fall within the current focus of voluntary attention, suggesting that attending to a particular location may shift the balance of plasticity mechanisms in spatially related brain circuits to favor the induction of potentiation rather than depression. Accordingly, we hypothesized that, when visual attention is directed to a location in external space near to the hand targeted by PAS, LTP-like plasticity should be augmented and LTD-like plasticity reduced, compared with when attention is directed instead near to the other hand. Across three experiments, we found clear-cut support for this hypothesis.
Materials and Methods
Participants.
Data from 32 right-handed volunteers were included. Twelve completed Experiment 1A (8 male; mean ± SD = 25.8 ± 5.4 years; range, 21–39 years), eight Experiment 1B (5 male; 23.5 ± 3.5 years; range, 21–31 years), and 12 Experiment 2 (8 male; 27.5 ± 5.8 years; range, 22–39 years). Data from two additional participants were excluded because of chance-level performance in the attention task (37% correct) or falling asleep after PAS. Experimental procedures were approved by the University of Queensland Medical Research Ethics Committee, and written informed consent was obtained from all participants. No participant was taking neuroactive medication, and there were no adverse reactions to TMS.
Physiological measures and PAS.
Electromyography and TMS procedures were the same as those reported previously (Kamke et al., 2012). In brief, cortical excitability was probed by measuring motor-evoked potential (MEP) amplitude and resting motor threshold (rMT) before and after PAS (Fig. 1A). MEPs were recorded using surface electrodes (Ag-AgCl) placed over the left abductor pollicis brevis (APB) and abductor digiti minimi (ADM). The TMS intensity used during PAS and to probe cortical excitability was that which produced an MEP of ∼0.5–1 mV before the plasticity intervention, whereas rMT was defined as the minimum TMS intensity required to evoke an MEP of ≥50 μV in at least five often consecutive pulses. Average MEP amplitude was determined from 21 TMS pulses (7 ± 1 s interpulse interval), with the first trial and trials containing voluntary muscle activity in the 200 ms pre-TMS period discarded from the analysis (<2.8% of trials).
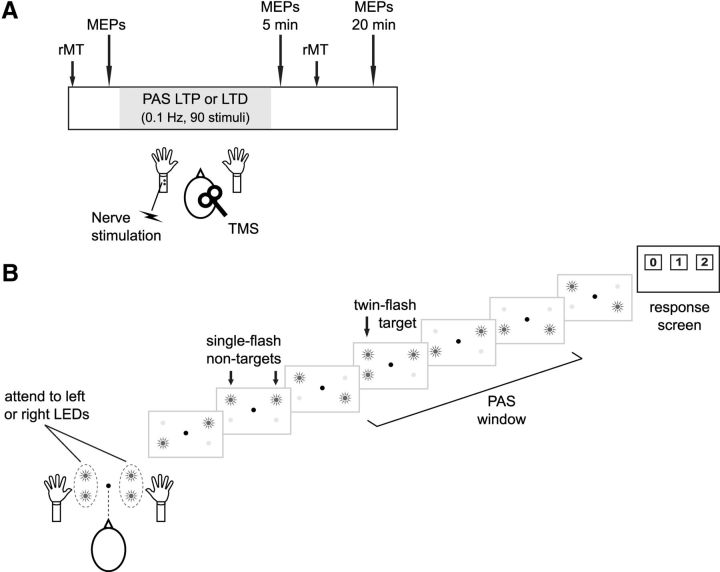
Figure 1.
Plasticity-inducing procedure and visual spatial attention task. A, PAS was used to induce either an LTP-like increase (Experiment 1) or an LTD-like decrease (Experiment 2) in excitability of the thumb representation in motor cortex. PAS produces coincident inputs to motor cortex by repetitively pairing peripheral nerve stimulation with TMS. Changes in excitability were probed by assessing the amplitude of MEPs and rMT. B, During PAS, participants fixated centrally and attended covertly to flashing LEDs located near their left hand or, in a separate session, near their right hand. A single trial is depicted, showing a series of flashes from one LED on either side of fixation (nontargets) as well as a target stimulus, which consisted of both LEDs flashing on one side. PAS occurred at a variable time-point within each trial.
Magnetic stimulation was delivered using a Magstim 2002 stimulator and 70 mm figure-of-eight coil (#9925-00) over the APB hotspot, inducing a posterior-to-anterior current in cortex. Coil location and orientation were maintained using a frameless infrared stereotaxic system (Visor, ANT). For PAS, electrical stimulation (200 μs) was delivered at motor threshold intensity using a bar electrode (cathode proximal) to the left median nerve either 25 ms (Experiment 1) or 10 ms (Experiment 2) before the TMS pulse to induce LTP- and LTD-like plasticity, respectively (Stefan et al., 2000; Wolters et al., 2003). Ninety pairs of stimuli were delivered over 15 min (0.1 Hz), and cortical excitability was reprobed 5 and 20 min after PAS (Fig. 1A). To avoid carryover effects from PAS, which typically induces changes that last up to 90 min (Stefan et al., 2000; Wolters et al., 2003), experimental sessions were separated by at least 24 h. Furthermore, to reduce variability in PAS-induced effects, sessions were conducted in the afternoon (Sale et al., 2008).
Spatial attention task.
During PAS, participants monitored a bilateral stream of visual stimuli for targets appearing within the stream close to the hand receiving PAS (attend left) or for targets appearing within the stream adjacent to the hand on the other side (attend right; Fig. 1B). Participants sat at a table with their head in a chinrest, and arms were placed on foam wedges so that each thumb was within ∼5 cm of a vertical array of two light emitting diodes (LEDs; 5 mm diameter; 2.4° separation; 40° between arrays). Because overt gaze direction may alter activity in sensorimotor cortex (Baker et al., 1999; Taylor-Clarke et al., 2002), independent of any attentional effects, participants fixated centrally and covertly attended to the visual stimuli in all experiments.
As shown in Figure 1B, a trial consisted of one of the two LEDs in each array flashing randomly seven times (1.67 Hz, 300 ms ISI). The participants' task was to fixate centrally and covertly monitor either the left or right LED array for target stimuli. Targets consisted of both LEDs in the attended array flashing simultaneously, and there could be zero, one, or two targets in the attended array (presented in equal proportions). Targets did not appear in the first position within the flashing sequence and were always separated by at least one nontarget flash. The intensity of the LEDs was adjusted in pilot testing so that the targets did not capture attention because of a large change in luminance. At the end of each trial, participants responded by fixating one of three horizontally aligned response panels (2.5° × 2.5°; 10.5° separation) that appeared on a monitor behind the LED arrays (Fig. 1B). Eye gaze responses were used to avoid motor preparation and muscle activity in the hands, which might interfere with plasticity induction. The response was taken as the final panel fixated in the 2000 ms response period (EyeLink1000 system; SR Research). On each of the 90 trials, PAS occurred randomly between the third and sixth flash, but always before the second target when there was one. Feedback on accuracy was only provided during a practice block at the start of each session.
In Experiment 1A, participants completed three experimental sessions, in counterbalanced order, in which they fixated a continuously lit central LED and covertly detected targets in the left LED array (attend-left condition), the right LED array (attend-right condition), or did not detect targets (attend-neither condition). In the attend-neither condition, the left and right LED-arrays flashed at the same rate as in the other conditions; but to avoid spontaneous orienting to targets in the detection task, only nontarget stimuli were presented. In this condition, a saccade to the central (1 “target”) response panel was required on each trial. Because sight of one's own hands may influence activity in sensorimotor cortex, independent of attention and eye gaze effects (Forster and Eimer, 2005; Cardini et al., 2012), the PAS-LTP procedure was also administered with both hands hidden from view in control Experiment 1B. In this experiment, a black screen covered both arms below the elbow, such that no part of the body could be seen during PAS. In Experiment 2, PAS was used to induce LTD-like plasticity. Experiments 1B and 2 did not include the attend-neither condition, which was not critical to the hypothesis under investigation (see Discussion). Stimulus presentation was controlled by a PC running MATLAB (MathWorks) and the Cogent toolbox (LON, Wellcome Department of Imaging Neuroscience, United Kingdom).
Data analysis.
For each experiment, behavioral data were compared using a two-tailed, repeated-measures t test. Baseline physiological measures (Table 1) were compared across attention conditions with one-way repeated-measures ANOVAs (Experiment 1A) or paired, two-tailed t tests (Experiments 1B and 2). Differences in rMT were investigated using a two-way, repeated-measures ANOVA. To assess the plasticity effects, post-PAS MEPs were expressed relative to the pre-PAS (baseline) level for each individual and repeated-measures ANOVA (Attention × post-PAS Time) was used to assess the changes. Significant effects were followed up with two-tailed t tests using the Bonferroni correction for multiple comparisons (the correction has been applied to the reported p value, so that p < 0.05 indicates significance). Statistical analysis was performed using SPSS (version 19, IBM).
Table 1.
Mean (SD) intensity of nerve stimulation, rMTs, and baseline (pre-PAS) MEPs in all experiments
| PES (mA) | rMT (% machine output) |
Baseline MEP (mV) |
|||
|---|---|---|---|---|---|
| Pre-PAS | Post-PAS | APB | ADM | ||
| Experiment 1A | |||||
| Attend-left | 6.28 (1.88) | 37.4 (4.9) | 37.6 (5.6) | 0.88 (0.20) | 0.59 (0.29) |
| Attend-neither | 6.57 (2.16) | 37.8 (5.1) | 37.8 (5.3) | 0.93 (0.23) | 0.61 (0.27) |
| Attend-right | 6.22 (2.58) | 36.9 (4.6) | 36.7 (4.0) | 0.94 (0.18) | 0.66 (0.42) |
| Experiment 1B | |||||
| Attend-left | 7.59 (2.77) | 37.9 (4.1) | 37.3 (4.1) | 0.76 (0.13) | 0.83 (0.61) |
| Attend-right | 7.44 (2.66) | 37.8 (4.4) | 37.9 (4.3) | 0.78 (0.12) | 0.71 (0.36) |
| Experiment 2 | |||||
| Attend-left | 8.11 (2.19) | 44.1 (6.6) | 45.0 (6.9) | 0.75 (0.18) | 0.69 (0.69) |
| Attend-right | 8.43 (2.73) | 43.6 (5.7) | 43.5 (5.9) | 0.80 (0.22) | 0.65 (0.72) |
PES, Peripheral electrical stimulation.
Results
Behavioral data
Analysis of eye-gaze responses for Experiment 1A revealed that detection accuracy was high, and did not differ, for conditions in which participants attended left (94.9 ± 8.8%; mean ± SD) and right (95.4 ± 4.9%); t(11) = −0.24, p > 0.80. It was also verified that participants maintained central fixation and made the required response in the attend-neither (no target) condition (99.3 ± 1.3% compliance). In Experiment 1B, detection accuracy was also high and did not differ between the left (93.3 ± 5.4%) and right (92.4 ± 7.0%) attention conditions (t(7) = 0.50, p > 0.63). Finally, accuracy in Experiment 2 was once again high and did not differ between the left (92.9 ± 6.8%) and right (89.8 ± 10.4%) attention conditions (t(11) = 1.65, p > 0.12).
Baseline physiological measures
Table 1 presents baseline physiological measures and rMT for all experiments. The intensity used for peripheral nerve stimulation and the amplitude of baseline MEPs did not vary across attention conditions within any of the experiments (Experiment 1A: all p > 0.66; Experiment 1B: all p > 0.14; Experiment 2: all p > 0.34). Similarly, rMT did not differ across the attention conditions or after PAS in Experiment 1A (Attention: F(2,22) = 1.35, p > 0.28, all other p > 0.75), Experiment 1B (Attention: F < 1, all other p > 0.10), or Experiment 2 (Attention: F(1,11) = 1.86, p > 0.20, all other p > 0.09).
PAS-induced effects
PAS-LTP (Experiments 1A and 1B)
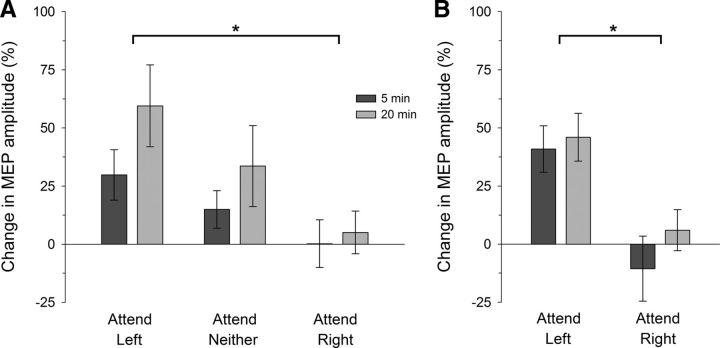
The mean change in MEP amplitude for the targeted (left APB) muscle after PAS with the hands visible (Experiment 1A) is shown in Figure 2A. After PAS, the expected increase in MEPs was largest in the attend-left condition and for the 20 min post-PAS intervals. ANOVA revealed a reliable difference in MEPs across attention conditions (Attention: F(2,22) = 3.74, p = 0.040, ηp2 = 0.254), but not between the post-PAS intervals (post-PAS Time: F(1,11) = 2.91, p > 0.11; Attention × Time, F(2,22) = 0.44, p > 0.64). A significant difference was found between the left- and right-attention conditions (t(11) = 2.84, p = 0.048), but not between the attend-neither and attend-left (t(11) = −1.19, p > 0.77) or the attend-neither and attend-right (t(11) = 1.55, p > 0.44) conditions (p values have been adjusted for three comparisons).
Figure 2.
PAS-induced LTP-like effects under different spatial attention conditions. Mean MEP amplitudes for the muscle targeted by PAS (left APB) are shown relative to the pre-PAS (baseline) level for the two post-PAS time intervals. After PAS, the increase in MEPs was significantly larger in the attend-left than the attend-right condition, both when the hands were visible (A, Experiment 1A) or hidden from view (B, Experiment 1B) during PAS: *p < 0.05. Error bars indicate within-subjects SEM (Cousineau, 2005). A, Legend applies to both panels.
To investigate any influence of hand visibility on this attention effect, the participants' hands were occluded from view during PAS in Experiment 1B. As shown in Figure 2B, the associated PAS-induced increase in MEPs in the targeted muscle was larger in the attend-left than the attend-right condition. ANOVA confirmed that the difference in MEPs across the attention conditions was reliable (Attention: F(1,7) = 6.97, p = 0.033, ηp2 = 0.499) and did not vary significantly across the post-PAS time intervals (post-PAS Time: F(1,7) = 1.09, p > 0.33; Attention × Time, F(1,7) = 0.47, p > 0.51).
To assess the somatotopic specificity of these PAS-induced effects, additional analysis on MEPs simultaneously recorded from a nearby muscle in the same hand (the left ADM) was undertaken. During PAS, neurons in motor cortex representing the ADM are activated by the TMS pulse; but because the ADM is not innervated by the median nerve, there is less associated input from the peripheral stimulation. In Experiment 1A, there was a slight increase in mean MEP amplitudes recorded from the ADM after PAS. Critically, there was no difference across the left- (15.7 ± 14.1%; mean ± SE), neither- (14.0 ± 14.1%), and right- (4.0 ± 9.3%) attention conditions, nor over the post-PAS time intervals (all F < 1). Similarly, responses recorded from the nontargeted ADM muscle in Experiment 1B revealed a small but highly variable increase in mean MEP amplitudes. Importantly, there was again no difference in MEPs across the left- (18.2 ± 20.5%; mean ± SE) and right- (9.6 ± 7.9%) attention conditions, or over the post-PAS time intervals (all F < 1.2, p > 0.32).
PAS-LTD (Experiment 2)
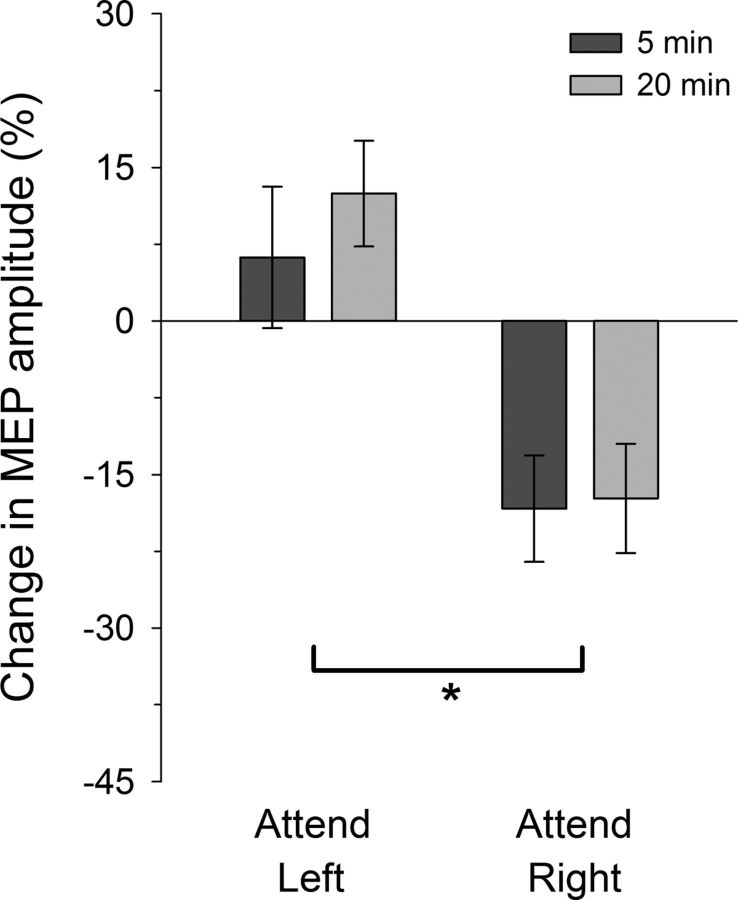
The mean change in MEP amplitude for the targeted (left APB) muscle after PAS-LTD is shown in Figure 3. As expected, MEP amplitudes were reduced after the PAS procedure, but this effect only appears when attention was directed to the LEDs located nearer the right hand. ANOVA confirmed a significant difference in MEPs across the attention conditions (Attention: F(1,11) = 9.42, p = 0.011, ηp2 = 0.461), but no difference between the post-PAS intervals (post-PAS Time: F(1,11) = 0.40, p > 0.54; Attention × Time, F(1,11) = 0.36, p > 0.55). Additional analyses revealed that the mean change in MEPs in the attend-left condition did not differ from zero (t(11) = 1.39, p > 0.19), but that MEPs in the attend-right condition were significantly reduced (t(11) = −3.01, p = 0.012). Similar to Experiment 1, responses recorded from the nontargeted ADM muscle revealed a variable increase in mean MEP amplitudes, but no difference across the left- (17.6 ± 10.3%; mean ± SE) and right- (7.3 ± 11.7%) attention conditions or over the post-PAS time intervals (all F < 1).
Figure 3.
PAS-induced LTD-like effects under different spatial attention conditions. After PAS, the decrease in MEPs in the targeted APB muscle was significant larger in the attend-right than the attend-left condition: *p < 0.05. Error bars indicate within-subjects SEM.
Discussion
This study investigated the influence of spatial attention on plasticity in the human motor cortex. Plasticity was induced using the PAS procedure, which has been shown to produce bidirectional, NMDA-dependent changes in cortical excitability that resemble spike-timing-dependent LTP and LTD (Müller-Dahlhaus et al., 2010). Larger LTP-like effects and reduced LTD-like effects were found when attention was focused on visual stimuli located near the thumb targeted by PAS than when attention was directed to visual stimuli located close to the other hand. These effects cannot be attributed to changes in eye gaze, sensory stimulation, or the behavioral relevance of the plasticity-inducing events. Nor can the effects be attributed to a nonspecific change in neural excitability due to attention, as MEPs recorded from an adjacent hand muscle not targeted by PAS did not differ across the attention conditions. Thus, the present results provide compelling evidence that visual spatial attention has opposite effects on LTP- and LTD-like plasticity in the human motor cortex.
Visual spatial attention augments LTP-like plasticity
Experiment 1 demonstrated that LTP-like effects were larger when attention was directed to visual stimuli located near the hand receiving PAS than when attention was directed instead to a location near the other hand. This effect did not depend on visibility of the hands during PAS. A previous study found a similar result when the (occluded) hand undergoing PAS was directly monitored for electrical stimuli (Stefan et al., 2004). Methodological limitations in that study, however, preclude an unequivocal attribution of those effects to attention: the electrical stimuli were delivered to different hands across the attention conditions; there was no requirement for attention to be sustained on the hand, as stimuli were few in number and could have captured attention automatically; moreover, only participants who showed the expected plasticity effects at baseline were recruited. The current paradigm avoided these limitations and, by using a visual attention task that was orthogonal to the plasticity-inducing procedure, was also able to demonstrate that spatial attention exerts a cross-modal influence on LTP-like plasticity.
Based on the current results, it is not possible to determine whether the influence of spatial attention on plasticity operates on a continuum across the visual field or acts discretely within a hemifield. Specifically, MEPs in the attend-neither condition (Experiment 1A) did not differ from the other attention conditions. Importantly, because attention was not controlled in the attend-neither condition, we cannot rule out the possibility that (spontaneous) changes in the focus of attention or in attentional demands influenced those results (Kamke et al., 2012). For these reasons and because the center condition was not critical to the hypothesis under question, the attend-neither condition was not included in the remaining experiments. Nonetheless, when attention was controlled, shifts in the focus of attention influenced LTP-like effects, demonstrating that visual spatial attention can indeed modulate plasticity. Thus, one way in which increased attentional demands may reduce LTP-like plasticity (Kamke et al., 2012) is by restricting the spread of spatial attention toward the hand being targeted by the plasticity procedure (Lavie, 2005).
Visual spatial attention attenuates LTD-like plasticity
A key question in the present study was whether spatial attention influences LTD-like plasticity and whether this effect differs from that found for LTP-like plasticity. In contrast to the LTP-like effects, it was found that LTD-like plasticity was reduced when spatial attention was directed near the hand targeted by PAS, relative to when attention was directed near to the other hand. It is generally accepted that LTP and LTD act in concert to allow changes in the behavior of neural circuits (Malenka and Bear, 2004), but the interaction between these induction processes is only beginning to be elucidated. LTP is typically described as a process for strengthening neural networks that represent important information, such as a memory or new skill, whereas LTD is more commonly associated with sensory deprivation, uncorrelated inputs, or weakening unnecessary connections as an adjunct to LTP (Malenka and Bear, 2004; Massey and Bashir, 2007; Feldman, 2009). It has been suggested that attention acts to highlight which stimuli are important and, in so doing, which neural circuits should undergo modification (Roelfsema et al., 2010). The observation of increased LTP-like effects and reduced LTD-like effects is consistent with this notion, suggesting that attention acts both to enhance the strengthening, and suppress the weakening, of neural connections representing events that fall within the focus of attention. In this context, attention may be one factor that influences the sliding scale of bidirectional plasticity (Bear, 2003), influencing not only the magnitude of plasticity but also the direction in which it manifests.
PAS induces changes in excitability that resemble spike-timing-dependent plasticity (Müller-Dahlhaus et al., 2010), which is a multifactor process whose rules vary across synapses and physiological state (Feldman, 2009). The putative influence of attention on such plasticity rules may be realized through attention-related neuromodulators, such as dopamine. Dopamine is critical for reward-based learning (Schultz, 1997) and attentional modulation of neural responses (Moore, 2006). It has a major, dose-dependent, nonlinear influence on LTP/LTD (Seamans and Yang, 2004) and on the LTP/LTD-like effects induced by noninvasive brain stimulation (Kuo et al., 2008; Monte-Silva et al., 2010; Thirugnanasambandam et al., 2011). Application of dopamine in vitro facilitates spike-timing-dependent LTP of hippocampal synapses (Zhang et al., 2009) and can even reverse LTD to LTP (Zhang et al., 2009; Pawlak et al., 2010). Interestingly, dopamine has similar effects in vivo, augmenting LTP-like PAS-induced plasticity and abolishing LTD-like effects (Ilić et al., 2012). Thus, one potential mechanism through which spatial attention might have facilitated LTP-like plasticity and reduced LTD-like effects when focused nearer to the targeted hand is by upregulating dopamine.
In conclusion, we have shown that visual spatial attention exerts an opposite influence on LTP- and LTD-like plasticity in the human brain, at least for PAS targeting the right motor cortex. This effect of spatial attention does not, however, necessarily demonstrate an opposing influence on functional reorganization: Both augmenting LTP and attenuating LTD should act to enhance synaptic efficacy in neural networks representing relevant (attended) stimuli. These results extend the well-established function of spatial attention in transiently altering neural responses, suggesting that attention can also act on a more prolonged time scale to influence bidirectional changes in synaptic efficacy.
Footnotes
This work was supported by the National Health and Medical Research Council of Australia (Project Grant APP1028210) and by a bequest made to the University of Queensland by the Estate of Dr. Salvatore Vitale. J.B.M. was supported by an Australian Research Council Laureate Fellowship (FL110100103). M.V.S. was supported by a National Health and Medical Research Council Training Fellowship (APP1012153). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding bodies. We thank Dr David Lloyd for technical assistance.
The authors declare no competing financial interests.
References
- Baker JT, Donoghue JP, Sanes JN. Gaze direction modulates finger movement activation patterns in human cerebral cortex. J Neurosci. 1999;19:10044–10052. doi: 10.1523/JNEUROSCI.19-22-10044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF. Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond B Biol Sci. 2003;358:649–655. doi: 10.1098/rstb.2002.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardini F, Longo MR, Driver J, Haggard P. Rapid enhancement of touch from non-informative vision of the hand. Neuropsychologia. 2012;50:1954–1960. doi: 10.1016/j.neuropsychologia.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousineau D. Confidence intervals in within-subject designs: a simpler solution to Loftus and Masson's method. Tutorials Quant Meth Psychol. 2005;1:42–45. [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster B, Eimer M. Vision and gaze direction modulate tactile processing in somatosensory cortex: evidence from event-related brain potentials. Exp Brain Res. 2005;165:8–18. doi: 10.1007/s00221-005-2274-1. [DOI] [PubMed] [Google Scholar]
- Iliæ NV, Petroviæ I, Grajiæ M, Iliæ TV. Effects of diazepam and levodopa single doses on motor cortex plasticity modulation in healthy human subjects: a TMS study. Srp Arh Celok Lek. 2012;140:14–21. doi: 10.2298/SARH1202014I. [DOI] [PubMed] [Google Scholar]
- Kamke MR, Hall MG, Lye HF, Sale MV, Fenlon LR, Carroll TJ, Riek S, Mattingley JB. Visual attentional load influences plasticity in the human motor cortex. J Neurosci. 2012;32:7001–7008. doi: 10.1523/JNEUROSCI.1028-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MF, Paulus W, Nitsche MA. Boosting focally-induced brain plasticity by Dopamine. Cereb Cortex. 2008;18:648–651. doi: 10.1093/cercor/bhm098. [DOI] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused? Selective attention under load. Trends Cogn Sci. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Macaluso E. Orienting of spatial attention and the interplay between the senses. Cortex. 2010;46:282–297. doi: 10.1016/j.cortex.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Massey PV, Bashir ZI. Long-term depression: multiple forms and implications for brain function. Trends Neurosci. 2007;30:176–184. doi: 10.1016/j.tins.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Monte-Silva K, Liebetanz D, Grundey J, Paulus W, Nitsche MA. Dosage-dependent non-linear effect of l-dopa on human motor cortex plasticity. J Physiol. 2010;588:3415–3424. doi: 10.1113/jphysiol.2010.190181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. The neurobiology of visual attention: finding sources. Curr Opin Neurobiol. 2006;16:159–165. doi: 10.1016/j.conb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Müller-Dahlhaus F, Ziemann U, Classen J. Plasticity resembling spike-timing dependent synaptic plasticity: the evidence in human cortex. Front Synaptic Neurosci. 2010;2:34. doi: 10.3389/fnsyn.2010.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak V, Wickens JR, Kirkwood A, Kerr JN. Timing is not everything: neuromodulation opens the STDP gate. Front Synaptic Neurosci. 2010;2:146. doi: 10.3389/fnsyn.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema PR, van Ooyen A, Watanabe T. Perceptual learning rules based on reinforcers and attention. Trends Cogn Sci. 2010;14:64–71. doi: 10.1016/j.tics.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, Nordstrom MA. Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci. 2008;28:8285–8293. doi: 10.1523/JNEUROSCI.1963-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–197. doi: 10.1016/S0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol. 2004;92:66–72. doi: 10.1152/jn.00383.2003. [DOI] [PubMed] [Google Scholar]
- Taylor-Clarke M, Kennett S, Haggard P. Vision modulates somatosensory cortical processing. Curr Biol. 2002;12:233–236. doi: 10.1016/S0960-9822(01)00681-9. [DOI] [PubMed] [Google Scholar]
- Thirugnanasambandam N, Grundey J, Paulus W, Nitsche MA. Dose-dependent nonlinear effect of l-DOPA on paired associative stimulation-induced neuroplasticity in humans. J Neurosci. 2011;31:5294–5299. doi: 10.1523/JNEUROSCI.6258-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Lau PM, Bi GQ. Gain in sensitivity and loss in temporal contrast of STDP by dopaminergic modulation at hippocampal synapses. Proc Natl Acad Sci U S A. 2009;106:13028–13033. doi: 10.1073/pnas.0900546106. [DOI] [PMC free article] [PubMed] [Google Scholar]