Abstract
Patient: Female, 75
Final Diagnosis: Intravascular lerge B-cell lymphoma
Symptoms: Dyspnea
Medication: —
Clinical Procedure: —
Specialty: Hematology
Objective:
Rare disease
Background:
Intravascular large B-cell lymphoma (IVLBCL) is characteristically diagnosed by histological examination of biopsies of bone marrow or randomly harvested skin specimens in the absence of any diagnostic abnormalities on imaging studies, including computed tomography (CT). In particular, diagnosis of IVLBCL with pulmonary manifestations is challenging, because even in patients with severe respiratory failure, there are rarely abnormalities on standard imaging studies.
Case Report:
A 75-year-old female presented with fatigue, weight loss, and high fever with chills for 3 months. Blood examinations on her initial visit to her primary physician showed high concentrations of C-reactive protein, lactate dehydrogenase, and soluble interleukin-2 receptor. There were no abnormalities on imaging studies. She was subsequently admitted to our hospital because of development of dyspnea over time (4 months after symptom onset). Although she was suspected of having IVLBCL, repeated biopsies from bone marrow, skin, liver, and lung did not result in a diagnosis. Finally, a lung biopsy obtained by video-associated thoracic surgery (VATS) from the right lung base, where fluorine-18 fluorodeoxyglucose positron emission tomography had shown high uptake, resulted in a definite diagnosis of IVLBCL.
Conclusions:
Highly invasive procedures such as thoracoscopic lung resection may be required to diagnose IVLBCL with pulmonary manifestations which can cause severe respiratory failure in the absence of any abnormalities on standard imaging studies.
MeSH Keywords: Anoxia; Lymphoma, B-Cell; Positron-Emission Tomography; Thoracic Surgery, Video-Assisted; Thoracoscopy
Background
Intravascular large B-cell lymphoma (IVLBCL) is a rare type of extra-nodal lymphoma, the neoplastic lymphocytes of which grow mainly within small blood vessels. IVLBCL is usually classified as a diffuse large B-cell lymphoma, most being lymphomas of B-cell origin [1]. Patients with IVLBCL rarely have abnormal findings on imaging studies, including brain or whole body computed tomography (CT), because their abnormal lymphocytes rarely infiltrate lymph nodes or form mass lesions [2,3]. Making a diagnosis of IVLBCL therefore often requires histological examination of multiple bone marrow or randomly harvested skin specimens [4,5]. However, even this strategy can fail to result in the correct diagnosis [4,5].
Manifestations of IVLBCL are extremely wide-ranging, depending on which organs are involved [4–6]. In Japan, more than half of patients with IVLBCL have atypical manifestations such as fever, hepatosplenomegaly, hemophagocytosis, bone marrow infiltration, and thrombopenia [4,6], whereas in Europe and the USA, IVLBCL reportedly tends to mainly involve the central nervous system or skin [4]. Although patients with IVLBCL may have respiratory symptoms and signs such as dyspnea or manifestations of severe respiratory failure, many have no abnormalities on standard imaging studies, including thoracic CT [2,3,7–10].
Making a diagnosis of IVLBCL is challenging without abnormalities on standard imaging studies and histological examinations by random biopsy. On that occasion, more invasive biopsy is required. Furthermore, fluorine-18 fluorodeoxyglucose (18-FDG) positron emission tomography (PET-CT) could be a crucial study to indicate the site of biopsy [3]. We herein report a patient with IVLBCL who required a lung biopsy supported by video-associated thoracic surgery (VATS) to make a diagnosis, no useful findings having been revealed on thoracic CT or by histological examination of multiple and random transbronchial lung biopsies (TBLBs).
Case Report
A 75-year-old female with a pacemaker for bradycardia started to develop fatigue, loss of appetite, and fever with evening chills 2 months prior to her first visit to our institution. Although she had visited her primary physician 1 month after symptom onset, blood examinations and imaging tests, including abdominal CT, and upper gastrointestinal endoscopy failed to reveal any abnormal findings. She remained symptomatic and a month later was found to have a high C-reactive protein (CRP) concentration (4.97 mg/dL), erythrocyte sedimentation rate of 112 mm/hour, and mildly increased concentrations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, and γ-glutamyl transpeptidase (γ-GT) of 74 U/L, 30 U/L, 228 U/L, and 78 U/L, respectively. A second abdominal CT did not reveal any abnormalities in her biliary system. At this point (defined as Day 0), she was referred to our outpatient clinic with a weight loss of 10 kg in 2 months as well as the aforementioned manifestations. There were no marked abnormalities on the initial physical examination. Blood examinations showed red blood cell count of 3.26×106/µL, hemoglobin (Hb) 10.0 g/dL, hematocrit (Ht) 30.0%, platelet count 16.4×104/µL, albumin 2.9 g/dL, AST 71 U/L, ALT 25 U/L, lactase dehydrogenase (LDH) 570 U/L, γ-GT 73 U/L, CRP 4.70 mg/dL, ferritin 1,842 ng/mL, and soluble interleukin 2 receptor (sIL-2R) 2,911 U/mL (Table 1). Her urinalysis showed 2+ protein. Because she still had dyspnea and an oxygen saturation of 88% in room air course, she was admitted to our hospital on Day 21.
Table 1.
Results of blood examination on admission.
| WBC | 4,300 | /μL | TP | 5.9 | g/dL | Na | 136 | mEq/L |
| Neu | 76.6 | % | Alb | 2.9 | g/dL | K | 4.2 | mEq/L |
| Lym | 14.1 | % | BUN | 19.2 | mg/dL | Cl | 103 | mEq/L |
| Mono | 8.9 | % | Cr | 0.84 | mg/dL | Ca | 8.5 | mg/dL |
| RBC | 3.26×106 | /μL | T-bil | 1.1 | mg/dL | Ferritin | 1,842 | ng/mL |
| MCV | 92 | fL | Glu | 110 | mg/dL | IgG | 1,362 | mg/dL |
| Hb | 10 | g/dL | AST | 71 | U/L | IgA | 268 | mg/dL |
| Ht | 30 | % | ALT | 25 | U/L | IgM | 50 | mg/dL |
| Platelet | 16.4×104 | /μL | LDH | 570 | U/L | RF | 5 | IU/mL |
| D-dimer | 4.91 | μg/mL | ALP | 192 | U/L | ANA | 40 | |
| ESR | 84 | mm/H | γ-GT | 73 | U/L | sIL-2R | 2,911 | U/mL |
| CK | 28 | U/L | PR3-ANCA | <1.0 | U/mL | |||
| CRP | 4.7 | mg/dL | MPO-ANCA | <1.0 | U/mL |
Neu – neutrophill; Lym – lymphocyte; Mono – monocyte; RF – rheumatoid factor; ANA – antinuclear antibody; sIL-2R – soluble Interleukin 2 receptor; ANCA – antineutrophilic cytoplasmic antibody.
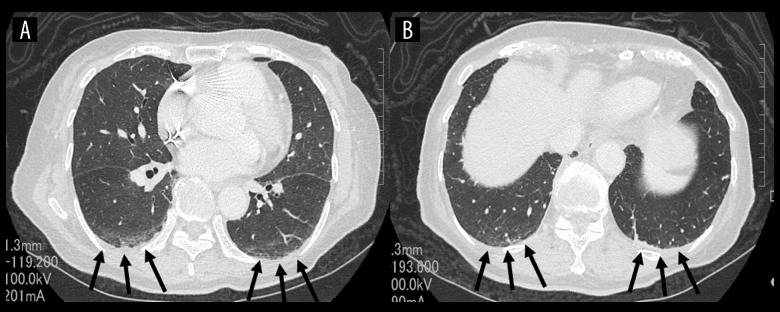
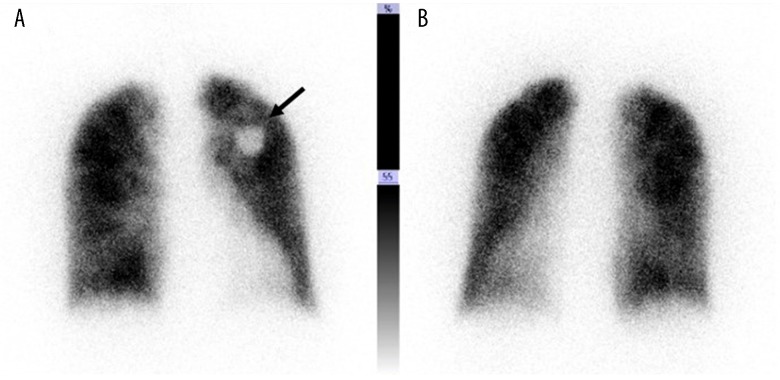
On admission, her temperature was 36.0°C, blood pressure 140/90 mmHg, pulse rate 70 beats per minute, and oxygen saturation 98% while breathing 2 L/minute of oxygen via a nasal cannula. No anemia, jaundice, abnormal heart or breath sounds, hepatosplenomegaly, abdominal mass, or palpable lymph nodes were detected on physical examination. No lymphoblasts or abnormal lymphocytes were observed in a peripheral blood film. Various autoantibodies, including rheumatoid factor, were all negative and her serum complement concentrations were normal. D-dimer was 4.91 µg/mL with normal prothrombin and activated partial thromboplastin time. Arterial blood gas analysis while breathing 2 L/minute of oxygen via a nasal cannula showed pH of 7.447, PaO2 101.0 mmHg, PaCO2 31.5 mmHg, HCO3−21.4 mmol/L, standard base excess (sBE) −2.0, and alveolar-to-arterial oxygen difference (A-aDO2) 56 mmHg. Various bacteriological cultures, including 2 sets of blood cultures, were negative. Respiratory function tests demonstrated an obstructive impairment pattern and low diffusion capacity with FEV1.0 of 66%,% vital capacity (VC) of 94% and carbon monoxide diffusing capacity (DLCO) of 49.8%. Chest x-ray films showed neither cardiac enlargement nor any abnormal shadows in the lung fields. Thoracic CT with contrast enhancement showed mild interstitial patterns in both lower lung fields with no evidence suggestive of the presence of pulmonary thromboembolism (Figure 1). Furthermore, neither enlarged lymph nodes nor mass lesions were detected on whole body CT from the neck to pelvis. Upper gastrointestinal and large intestine endoscopies were normal. Transthoracic echocardiography was also normal, with no evidence of valvular disease, vegetations, or right heart strain pattern. Pulmonary perfusion scintigraphy showed no definite perfusion deficits and only slightly heterogeneous distribution (Figure 2).
Figure 1.
Thoracic computed tomography with contrast enhancement images on admission. Transaxial plane at the cardiac level (A) and diaphragmatic level (B). There are mild interstitial patterns in both lower lung fields (arrows), but no findings suggesting definite causes of hypoxemia such as pulmonary thromboembolism.
Figure 2.
Frontal (A) and dorsal (B) lung perfusion scintigraphy images showing no definite perfusion deficits and only slightly heterogeneous distribution. The dense area in the left upper lung field represents her implanted pacemaker (arrow).
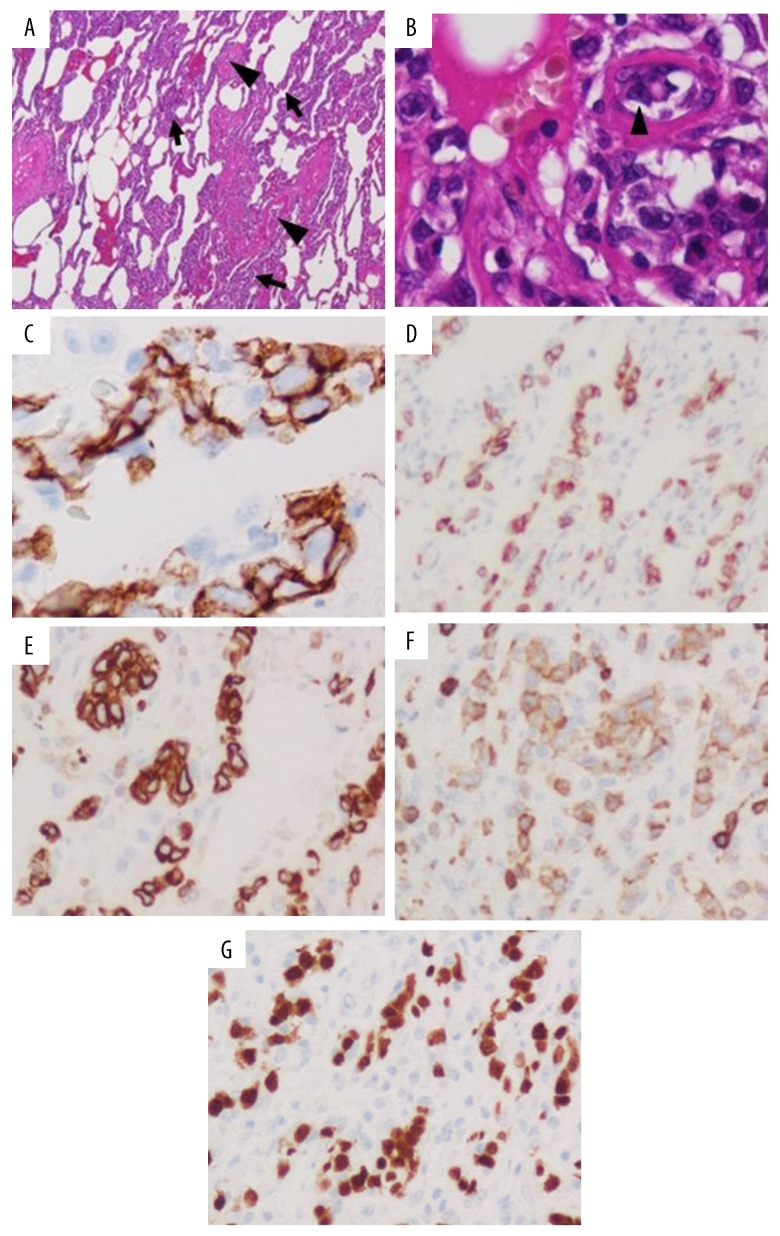
Obstruction of the peripheral pulmonary circulation by tumor cells, most likely IVLBCL, was strongly suspected at this point because of the presence of hypoxemia without definite abnormalities on imaging studies, including thoracic CT, or pulmonary ventilation–perfusion imbalance on pulmonary scintigraphy, as reported above. Two random skin biopsies, a bone marrow biopsy, a liver biopsy and even 2 TBLBs failed to reveal abnormal lymphocytes; however, a bone marrow biopsy showed hemophagocytosis. Next, PET-CT was performed, and this showed slightly increased uptake in both peripheral lower and dorsal lung fields, which is compatible with the finding of IVLBCL (Figure 3). A lung biopsy was therefore obtained with support by video-assisted thoracic surgery (VATS). Microscopic examination of this biopsy showed aggregations of large abnormal lymphocytes in pulmonary microvessels despite normal macroscopic findings. Immunohistological studies showed monoclonality of those lymphocytes with B cell markers, CD20, SD79a, PAX5, bcl-2, and CD5. The patient was therefore diagnosed as having IVLBCL (non-germinal center B-cell like) (Figure 4). Because her LDH and soluble IL-2R concentrations started to increase steeply, chemotherapy was started with prednisolone 1 mg/kg/day on Day 57 and R-THP-COP (rituximab-pirarubicin-cyclophosphamide, vincristine, prednisolone) on Day 59. After 1 course of chemotherapy, her respiratory condition improved, and she no longer required oxygen. She was discharged on Day 76 and had not developed any recurrence with continuing chemotherapy in an ambulatory setting by 7 months after her discharge.
Figure 3.
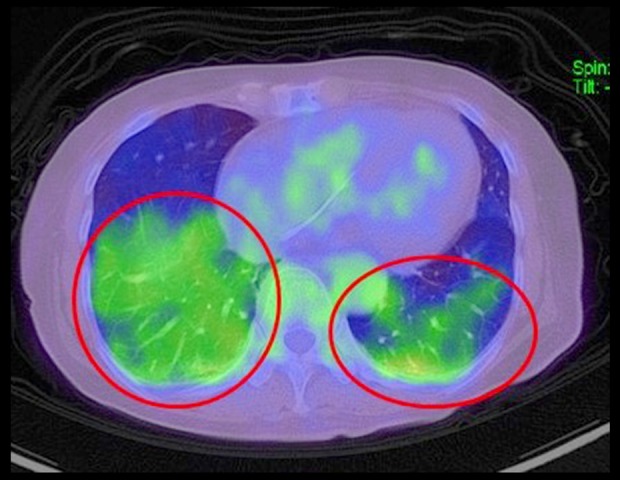
Positron emission tomography-computed tomography image. There is slightly high uptake in both peripheral lower and dorsal lung fields (circles) with no abnormal findings in other sites.
Figure 4.
Photomicrographs of lung biopsy specimens acquired with support of video-associated thoracic surgery. Hematoxylin and eosin stain; 40× (A) and 400× (B). The alveolar walls are thick (A, arrows). Numerous abnormal lymphocytes are aggregated in pulmonary microvessels (A, B, arrowhead).
Immunohistological stains: 400× (C–G). Aggregated abnormal lymphocytes in microvessels are stained by markers of B cell differentiation, CD20 (C), CD79a (D), bcl-2 (E), CD5 (F), and PAX5 (G). The findings are consistent with a diagnosis of intravascular large B cell lymphoma.
Discussion
Even though we strongly suspected IVLBCL with obstruction of the peripheral pulmonary circulation on the basis of our patient’s respiratory failure, B symptoms, and high sIL-2R concentration in the absence of any significant findings on thoracic CT with contrast enhancement, making a diagnosis was extremely challenging, eluding us on multiple bone marrow and random skin biopsies and 2 random TBLBs. Widening of the A-aDO2 and a decrease in DLCO suggested that her respiratory failure was attributable to both ventilation–perfusion mismatch and diffusion impairment, indicating possible diagnoses of pulmonary diseases such as interstitial pneumonia, pulmonary thromboembolism, pulmonary edema, sarcoidosis, or pulmonary IVLBCL. Because there was no evidence of interstitial pneumonia or pulmonary thromboembolism on thoracic CT and pulmonary perfusion scintigraphy, we strongly suspected that she had pulmonary IVLBCL.
Although there are few reports of lung as the primary site affected by IVLBCL [7,8], Souza et al. reported that 60% of patients with IVLBCL are found to have lung lesions at autopsy [8]. One study found that 8 of 16 Japanese patients with IVLBCL had respiratory manifestations [6]. Although patients with pulmonary IVLBCL are usually reported to have only dyspnea, fever, and weight loss, severely affected individual cases sometimes have hypoxemia or pulmonary hypertension [7,11]. Although our patient did not have pulmonary hypertension, she did have dyspnea, fever, weight loss, and high LDH and sIL-2R concentrations, all of which are compatible with a diagnosis of pulmonary IVLBCL [7,11].
Respiratory manifestations such as dyspnea, hypoxemia, and pulmonary hypertension with B symptoms or high LDH concentration without abnormal findings on imaging studies can be clues to the diagnosis of IVLBCL; however, detection of abnormal lymphocytes by histological studies is required to make a definite diagnosis [1]. Although skin, generally as random skin specimens, and bone marrow are useful target tissues for biopsy to make a diagnosis of the usual type of IVLBCL [12,13], TBLB, including randomly performed TBLB without specific target sites, is often required to make a diagnosis of IVLBCL with pulmonary lesions [2,10,14]. However, even TBLB often fails to acquire useful specimens [9,15]. Although target sites of TBLB are usually identified according to the findings of imaging studies such as thoracic CT or pulmonary perfusion scintigraphy, in individuals with pulmonary IVLBCL these imaging studies usually show only subtle abnormalities such as nonspecific infiltration or interstitial shadows in both lung fields, necessitating random TBLBs [2,7–10]. Additionally, specimens acquired by TBLB are rather small. Although pulmonary perfusion scintigraphy can show diffuse presence of small deficits regardless of lung segments in individuals with IVLBCL, there are no established pathognomonic findings [2,10,14]. When TBLB fails, cytological study of blood specimens gathered from the pulmonary artery through a Swan-Ganz catheter can reportedly be useful [15,16]; however, we did not perform this procedure.
PET-CT has been used for making the initial diagnosis, staging, and assessment of effectiveness of treatments for IVLBCL because these patients usually show high uptake of 18-FDG in involved organs [3,17,18]. A few case reports have found PET-CT helpful in making a diagnosis of IVLBCL by identifying appropriate target sites for lung biopsy when other imaging modalities have failed to detect any abnormalities [3,18]. When PET-CT showed lesions in the peripheral parts of both lower lung fields after 2 random TBLBs had failed to achieve a diagnosis, we decided to obtain a lung biopsy with support of VATS, and this finally confirmed the suspected diagnosis of IVLBCL.
Conclusions
When making a diagnosis of pulmonary IVLBCL is challenging without any abnormality on standard imaging studies or random and multiple histological examinations of skin or bone marrow, VATS could play a crucial role. PET-CT should be performed before VATS, which could indicate appropriate sites of this invasive procedure.
Acknowledgments
We thank Dr Trish Reynolds, MBBS, FRACP, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
References:
- 1.Jaffe ES, Harris NL, Stein H, et al. World Health Organization Classification of Tumors of Hematopoietic and Lymphoid Tissues. Geneva, Switzerland: WHO Press; International Agency for Research on Cancer; 2001. pp. 177–78. [Google Scholar]
- 2.Kaku N, Seki M, Doi S, et al. A case of intravascular large b-cell lymphoma (IVLBCL) with no abnormal findings on chest computed tomography diagnosed by random transbronchial lung biopsy. Intern Med. 2010;24:2697–701. doi: 10.2169/internalmedicine.49.3986. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita H, Suzuki A, Takahashi Y, et al. Intravascular large B-cell lymphoma with diffuse FDG uptake in the lung by 18-FDG-PET/CT without chest CT findings. Ann Nucl Med. 2012;26:515–21. doi: 10.1007/s12149-012-0600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponzoni M, Ferreri AJ, Campo E, et al. Definition, diagnosis, and management of intravascular large b-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol. 2007;21:3168–73. doi: 10.1200/JCO.2006.08.2313. [DOI] [PubMed] [Google Scholar]
- 5.Kanno M. Intravascular lymphoma (IVL) – overview for the diagnosis and treatment of a unique B-cell lymphoma. Journal of Nara Medical Association. 2006;57(4–5):95–103. [Google Scholar]
- 6.Masaki Y, Dong L, Nakajima A, et al. Intravascular large B cell lymphoma: Proposed of the strategy for early diagnosis and treatment of patients with rapid deteriorating condition. Int J Hematol. 2009;89:600–10. doi: 10.1007/s12185-009-0304-7. [DOI] [PubMed] [Google Scholar]
- 7.Souza CA, Quan K, Seely J, et al. Pulmonary intravascular lymphoma. J Thoracic Imaging. 2009;24:231–33. doi: 10.1097/RTI.0b013e31819724d9. [DOI] [PubMed] [Google Scholar]
- 8.Martusewicz-Boros M, Wiatr E, Radzikowska E, et al. Pulmonary intravascular large B-cell lymphoma as a cause of severe hypoxemia. J Clin Oncol. 2007;15:2137–39. doi: 10.1200/JCO.2007.10.7201. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Lai N, Zhou Y, et al. Intravascular large B-cell lymphoma confirmed by lung biopsy. Int J Clin Exp Pathol. 2014;7(9):6301–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Sawada M, Amano H. [A case of intravascular large B-cell lymphoma presented hypoxemia with normal chest CT findings] Nihon Kokyuki Gakkai Zasshi. 2015;4(4):293–97. [in Japanese] [Google Scholar]
- 11.Owa M, Koyama J, Asakawa K, et al. Intravascular lymphomatosis presenting as reversible severe pulmonary hypertension. Int J Cardiol. 2000;75:283–84. doi: 10.1016/s0167-5273(00)00311-9. [DOI] [PubMed] [Google Scholar]
- 12.Shimada K, Kinoshita T, Naoe T, Nakamura S. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10(9):895–902. doi: 10.1016/S1470-2045(09)70140-8. [DOI] [PubMed] [Google Scholar]
- 13.Spencer J, Dusing R, Yap W, et al. Intravascular large B-cell lymphoma presenting with diffusely increased pulmonary fluorodeoxyglucose uptake without corresponding CT abnormality. Radiol Case Rep. 2019;14:260–64. doi: 10.1016/j.radcr.2018.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okada A, Hirakawa R, Yamashita M, et al. [Two cases of intravascular diffuse large B-cell lymphoma diagnosed by transbronchial lung biopsy] Nihon Kokyuki Gakkai Zasshi. 2010;48(10):779–85. [in Japanese] [PubMed] [Google Scholar]
- 15.Yamakawa H, Yoshida M, Yabe M, et al. Useful strategy of pulmonary microvascular cytology in the early diagnosis of intravascular large B-cell lymphoma in a patient with hypoxemia: a case report and literature review. Intern Med. 2015;54:1403–6. doi: 10.2169/internalmedicine.54.4379. [DOI] [PubMed] [Google Scholar]
- 16.Ishiguro T, Takayanagi N, Baba Y, et al. Case series of pulmonary tumor embolism and intravascular lymphoma: Evaluation of the usefulness of pulmonary microvascular cytology. Intern Med. 2016;55:2679–84. doi: 10.2169/internalmedicine.55.6855. [DOI] [PubMed] [Google Scholar]
- 17.Odawara J, Asada N, Aoki T, et al. 18-F-Fluorodeoxyglucose positron emission tomography for evaluation of intravascular large B-cell lymphoma. British Journal Haematology. 2006;27:684. doi: 10.1111/j.1365-2141.2006.06414.x. [DOI] [PubMed] [Google Scholar]
- 18.Kohan AA, Paganini L, Biedak P, et al. Pulmonary intravascular lymphoma detected by FDG PET-CT: A case report. Rev Esp Med Nucl Imagen Mol. 2013;32(5):318–20. doi: 10.1016/j.remn.2012.11.002. [DOI] [PubMed] [Google Scholar]