Abstract
Background:
No proven treatment exists for nonarteritic anterior ischemic optic neuropathy (NAION), either in the acute or late phase.
Objective:
To assess safety and changes in visual function and structure following RPh201/placebo treatment in participants with previous NAION.
Design & Setting:
Phase 2a, single-site, prospective, randomized, placebo-controlled, double-masked trial (registration NCT02045212).
Main Outcomes Measures:
ETDRS best-corrected visual acuity (BCVA), visual fields (VF), retinal nerve fiber layer (RNFL), and visual evoked potential (VEP) at weeks 13, 26, and after a 13 week wash-out (“off-drug”) period; and safety.
Study population:
Twenty-two participants ≥18 years of age with previous NAION.
Intervention(s):
RPh201 (20 mg) or placebo (cottonseed oil vehicle) administered subcutaneously twice weekly at the study site.
Results:
Thirteen men and 9 women were randomized, of which 20 completed all visits. The mean (± SD) age was 61.0 ± 7.6 years. In a post-hoc analysis, after 26 weeks of treatment, BCVA improved by ≥15 letters in 4/11 (36.4%) eyes with RPh201, compared to 1/8 (12.5%) eyes with placebo (p=0.24). Overall, 7/11 (63.6%) of participants on RPh201 showed some improvement in BCVA, compared to 3/8 (37.5%) on placebo (p=0.26). Improvement in BCVA from a calculated baseline was 14.8 ± 15.8 letters for RPh201 and 6.6 ± 15.3 for placebo (p=0.27). Of the 154 adverse effects (AE), 52 were considered related to the study procedures/treatment. Across the study and 1,017 injections, the most frequently reported AE was injection site pain (23 events in 5 participants). There were no clinically significant changes in vital signs or laboratory values.
Conclusions:
This Phase 2a was designed to assess safety, feasibility, and explore potential efficacy signals in treating previous NAION with RPh201. No safety concerns were raised. The results support a larger trial in patients with previous NAION.
The annual incidence of acute NAION in the United States is estimated to be between 2.3–10.3 per 100,000, with an annual incidence of in those 50 years or older about 6,000 (1–3). The mean age of onset is between 57 and 65 years, and men and women are equally affected (4, 5). The prevalence of NAION has only been reported in a small number of other countries. In China, the annual incidence of NAION is approximately 6.25 per 100,000 for subjects aged over 40 years (6). In Croatia, the annual incidence of NAION was estimated at 2.9 per 100,000 for men and 2.5 per 100,000 for women (7).
Multiple approaches to treating acute NAION have been investigated, but either proved unsuccessful, inadequately powered, or lacking in scientific rigor (8). These include lowering intraocular pressure in an attempt to improve blood flow to the optic disc (8, 9), neuroprotective strategies with brimonidine (10, 11) or L-DOPA (12), decreasing edema with corticosteroids or bevacizumab (13), or in the largest published study, fenestration of the optic nerve sheath (14). Currently, there is no approved or proven treatment for patients with NAION, either to improve the visual outcome or to decrease the likelihood of second eye involvement (15). There also is no treatment proven to improve visual function in patients who have had previous NAION.
RPh201, an extract of gum mastic, is being developed by Regenera Pharma for the treatment of various neurological diseases. In vitro and in vivo animal toxicology studies performed using RPh201 for up to 39 weeks have revealed no genotoxic effects or safety concerns (16, 17). RPh201 induces neuronal differentiation, synaptogenesis, immunomodulation and neuroprotective effects in vitro and in vivo (unpublished data). Specifically, in in vitro assays RPh201 was shown to induce trans-differentiation of using human retinal epithelial cells (ARPE-19) into neuronal cells. In various in vivo animal models, RPh201 was shown to promote neurogenesis and synaptogenesis, and to enhance functional recovery of cognition, memory and sensorimotor deficits.
The mechanisms by which RPh201 enhances recovery are not known, and may include reduction of inhibitory pathways for vision, facilitation and strengthening of synaptic connections, increasing the size of receptive fields from afferent neurons in the retina or efferent targets in visual centers, activating the retinal ganglion cell (RGC) intrinsic growth state (18, 19), or other mechanisms.
The safety and tolerability of RPh201 was demonstrated in a Phase 1 study, where doses of up to 20 mg of RPh201 were administered twice weekly to 36 healthy volunteers (submitted). Based on these data, a prospective, randomized placebo-controlled Phase 2a safety and efficacy study was initiated in participants with previous NAION (visual loss due to NAION occurring at least 6 months prior to screening) at a dose of 20 mg twice weekly. This study was not powered to detect small or moderate effects, but rather to assess the safety of RPh201, the feasibility of carrying out a trial testing the drug in patients with a previous episode of NAION, and to explore potential efficacy signals with RPh201 compared to vehicle control.
Method
Study Design
The study was designed as a Phase 2a, prospective, single-center, double-masked, placebo-controlled, randomized trial. It was performed in compliance with the ethical principles of the Declaration of Helsinki (1964) and Good Clinical Practice (GCP), as outlined in the International Conference on Harmonisation (ICH, 1997). Approval for the study was granted by the Israeli Ministry of Health and Western Galilee Hospital Nahariya Independent Review Board. Written informed consent was obtained from each participant before any study procedures were conducted. The ClinicalTrial.gov identifier is NCT02045212.
Participants
Participants were screened and, if eligible, treated at the Western Galilee Hospital Nahariya, Israel. Eligible participants were ≥ 18 years of age; diagnosed with NAION (diagnosed for a maximum of 3 years and at least 6 months without treatment following event and were stable) or traumatic neuropathy; had a best-corrected visual acuity (BCVA) worse than 20/200 and/or visual field (VF) < 15 degrees. Pregnant or nursing women, or those of child-bearing age not taking adequate contraception, were not eligible for inclusion. Participants were excluded if they had allergies to cottonseed oil; pre-existing ocular disease affecting the optic nerve; diabetic retinopathy; optic neuropathy caused by infection or tumor; active infection; clinically significant and/or uncontrolled conditions, or any other significant medical disease. Participants who were taking/had received corticosteroids, immunosuppressive drugs, cytotoxic agents, radiation therapy and chemotherapy within one month of screening or those with a history of alcohol/drug abuse in the last two years also were excluded from the study.
Changes in Trial Design
When it became clear that no participants with traumatic neuropathy had been enrolled, a protocol amendment was implemented to only include participants diagnosed with NAION.
Randomization and Treatment
Randomization codes were generated by Regenera Pharma at a ratio of 1.5:1 (RPh201:placebo) and distributed in advance to the unmasked pharmacist at the site. The pharmacist at the site used the codes sequentially to allocate each participant to either the RPh201 or placebo arms. During the trial, only the pharmacist was aware of the allocation of each participant; all other study team members, including the investigator, were masked.
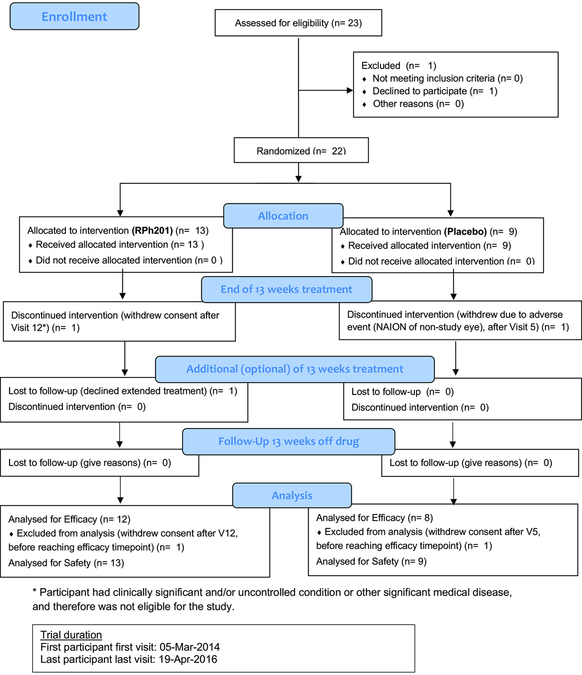
Eligible participants allocated to the RPh201 arm received 20 mg of RPh201 in 400 μL cottonseed oil vehicle. Participants allocated to the placebo arm received cottonseed oil alone. The appearance of the syringes was identical. Treatments were administered subcutaneously twice weekly by a study nurse masked to the treatment assignment. The first injection was administered at the study site. Subsequently, injections alternated between the study site and the participant’s home or place of work. The duration of the treatment was 13 weeks. After 13 weeks, an optional additional 13 weeks of treatment was offered to all participants, to allow the collection of additional safety and efficacy data for RPh201. Participants continued to receive the same treatment (RPh201 or vehicle alone) during the additional 13 weeks (Fig 1), After this treatment period, participants were followed in a wash-out period, where the drug was no longer given and allowed wash away from the body.
Figure 1.
Flow chart displaying participation in the study.
Study Objectives
The multiple primary endpoints were changes from baseline in Early Treatment Diabetic Retinopathy Study (ETDRS) best-corrected visual acuity (BCVA), automated visual fields (VF), visual evoked potentials (VEP), and retinal nerve fiber layer (RNFL) thickness by optical coherence tomography (OCT). The secondary endpoint was to assess safety.
Assessments
Study assessments were conducted by trained clinic staff.
Efficacy Assessments
BCVA was measured using the ETDRS chart at 4 m. Count fingers acuity was converted to 1.80 logMAR units (−5 letters) and hand motions acuity was converted to 1.90 logMAR units (−10 letters) based on the methodology for off-chart visual acuity from the Vitrase for Vitreous Hemorrhage trials (20). The clinically significant endpoint used was the proportion of subjects improving by ≥3 lines (≥15 letters) in ETDRS BCVA after 26 weeks of treatment, compared to baseline, with the screening examination used as the baseline visit. When a participant was enrolled with previous unilateral NAION, the affected eye (the “study eye”) was analyzed for safety and efficacy, and the other eye (the “non-study eye”) analyzed for safety. When there was previous bilateral NAION, the worse eye was the study eye.
Automated VF was performed with the Humphrey 24–2 SITA-Fast program using the size III stimulus, unless the participant could not perform the test because of poor acuity, in which case the 24–2 Fastpac program with the size V stimulus was used. VEP was measured with flash, pattern-shift, and multifocal stimuli. RNFL thickness was measured with Opko optical coherence tomography.
BCVA was tested weekly. VF, VEP, and RNFL were assessed at the screening/baseline visit and at weeks 13, 26, and after the 13 week off-drug wash-out period (i.e. at 39 weeks).
Safety Assessments
Physical examination, body mass index (BMI), blood testing, and urinalysis were conducted at weeks 13, 26, and after the 13 week off-drug wash-out period. Vital signs and AEs were recorded at each treatment visit and after the 13 week off-drug wash-out period.
Statistical Analysis
The sample size for the study was not powered for statistical significance. Descriptive statistical analysis was performed using SAS (Statistical Analysis Software). Changes between measurements at different time points were analyzed using the T-test for paired differences. Contingency tables were analyzed with chi-square without Yates’s correction. No corrections were made for multiple comparisons because of the exploratory nature of the analyses.
Results
Patient Disposition and Baseline Characteristics
The first participant was enrolled into the study on March 4, 2014 and the last participant visit was on April 19, 2016. Twenty-two participants with previous NAION were randomized, 13 to RPh201 and 9 to placebo (Fig 1). Table E1 displays the demographic data for the study. The mean (± SD) age of participants was 61.0 ± 7.6 years), with 13 men and 9 women. Four participants had bilateral NAION, 3 in the RPh201 group and one in the placebo group. Two participants withdrew prior to completing 13 weeks of treatment: one participant in the RPh201 group withdrew consent, and another participant in the placebo arm developed NAION in the fellow eye. Twenty participants completed 13 weeks of treatment, at which point they were given the option to continue with an additional 13 weeks of treatment (i.e. 26 weeks of treatment in total) in order to collect additional safety and efficacy data. Nineteen of the 20 participants chose to continue with the additional 13 weeks of treatment, 11 of whom received RPh201 and 8 who received placebo. All 20 participants had a follow-up assessment conducted after a 13 week off-drug wash-out period, i.e. at 26 weeks for the one participant who did not continue with the additional 13 weeks of treatment, and at 39 weeks for the 19 participants who did have an additional 13 weeks of treatment.
Treatment Exposure
A total 1,017 injections were administered. Eleven participants each received 52 twice-weekly doses of RPh201, one received 26 doses of RPh201, and 8 each received 52 doses of placebo. For the two participants that withdrew prematurely (i.e. before the 13 weeks of treatment), one received 12 doses of RPh201 and the other 5 doses of placebo.
Intention-to-treat analysis
The trial protocol did not have a separate baseline visit from the screening visit, and, therefore the intention-to-treat analysis used the screening visual acuity and visual field for the baseline values. Following 26 weeks of treatment, those participants who received RPh201 demonstrated similar improvement in BCVA as with placebo, with a change from screening of 19.8 ± 4.6 and 23.0 ± 6.9 letters, respectively, p = 0.69 (Table E2). Seven of 11 (63.6%) eyes treated with RPh201 improved BCVA by ≥15 letters (3 lines) after 26 weeks of treatment, compared to 5 of 8 (62.5%) eyes given placebo (p = 0.96). This contrasted with the data from earlier time points, with less improvement in BCVA in the RPh201 group compared to placebo from baseline to week 13. After a 13-week off-drug wash out period (i.e. at 39 weeks), 4 out of 12 eyes (33.3%) previously treated with RPh201 improved by ≥15 letters of BCVA, compared with 5 out of 8 (62.5%; p = 0.20) previously treated with placebo.
For VF, the outcome measure for the size III test stimulus was mean deviation (MD). In participants who were unable to perform the test reliably, the size V test stimulus was used, for which MD was not calculated, and instead the mean of the sensitivity values at each field location was calculated. An improvement in VF was observed in the RPh201 group compared to the placebo group when the size III test stimulus was used (Table E3). After 26 weeks, participants treated with RPh201 had a MD improvement of 7 dB compared to placebo (7.85 ± 5.90 vs. 0.84 ± 0.73, p=0.22). VF measurements using the size V test stimulus at 26 weeks did not demonstrate improvement in mean sensitivity in the RPh201 group compared to the placebo group (0.41 ± 1.66 vs. 0.49 ± 2.59, p = 0.98; Table E3).
There was no difference between RPh201 and placebo groups in change in total OCT RNFL thickness at 26 weeks compared to baseline (−4.6 ± 1.6 vs. −1.8 ± 2.1; p=0.29) or change in size V stimulus mean sensitivity compared to baseline (0.41 ± 1.66 vs. 0.49 ± 2.59; p=0.98). VEP was assessed qualitatively, and no differences were observable.
There were no safety concerns thinning of RNFL or abnormalities of the VEP following RPh201 treatment in either the study or non-study eye.
Post-hoc analyses
In the intention-to-treat analysis, there was no separate baseline visit, and therefore the screening visit had to be used for the baseline values. This raises concern about regression to the mean, which could occur if participants with varying visual acuity were entered when they varied worse than 20/200, yet varied back to their true baseline during the trial. The data from the first few weeks also demonstrated a rapid and sizeable improvement in BCVA in both the RPh201 and placebo groups (31.8 ± 9.9 and 44.0 ± 12.7 letters at 3 weeks, respectively). The rapid improvement BCVA in both groups suggested a training effect, which is commonly seen when patients with eccentric fixation are asked to read visual acuity charts under training situations. To address the potential confounding training factor, the analysis was repeated using a modified baseline, calculated for each eye as the maximum number of letters read at the first 3 visits.
Following 26 weeks of treatment, those participants who received RPh201 demonstrated more improvement in BCVA compared to placebo, with a change from modified baseline of 14.8 ± 15.8 and 6.6 ± 15.3 letters, respectively, p = 0.27 (Table E4). Four of 11 (36%) eyes treated with RPh201 improved BCVA by ≥15 letters (3 lines) after 26 weeks of treatment, compared to 1 of 8 (12%) eyes given placebo, (p = 0.24). This contrasted with the data from earlier time points, with less improvement in BCVA in the RPh201 group compared to placebo from modified baseline to week 13. Seven of 11 (63.6%) of eyes on RPh201 showed at least one line improvement in BCVA, compared to 3/8 (37.5%) on placebo, p=0.26. After a 13-week off-drug wash out period (i.e. at 39 weeks), 3 out of 12 eyes (25%) previously treated with RPh201 improved by ≥15 letters of BCVA, compared with 1 out of 8 (12%; p = 0.49) previously treated with placebo.
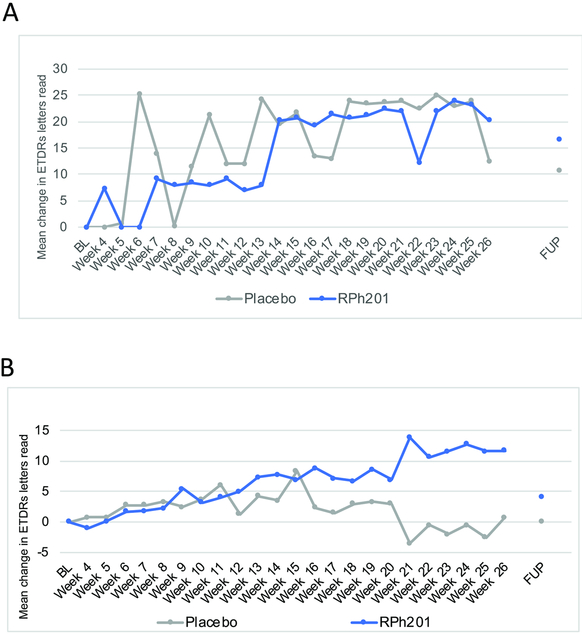
Nine participants (5 in the RPh201 and 3 in the placebo group) at screening could not read any letters on the ETDRS chart, i.e. had hand motions or count fingers visual acuity. These off-chart participants exhibited highly variable acuity readings between visits, which alternated from being off chart to being on chart (Fig 2A). An analysis was therefore conducted for the participants with on-chart BCVA. For the on-chart participants, 2 out of 7 eyes (29%) treated with RPh201 showed an improvement of ≥15 letters (3 lines) after 26 weeks treatment, compared to 0 of 5 (0%) of the eyes in the placebo arm (p = 0.19). Figure 2B depicts the changes in VA for on-chart participants over time.
Figure 2.
Weekly BCVA assessments for participants with on-chart visual acuity at screening. Graph reflects mean change in the number of ETDRS letters read by participants treated with RPh201 or placebo. A. Mean change in EDTRS letters from baseline for off-chart participants. B. Mean change in EDTRS letters from baseline for on-chart participants. L = baseline; FUP = follow-up after 13 weeks off-drug wash out period (39 weeks).
Safety
A total of 154 AEs were reported (95 for RPh201 and 59 for placebo), and the majority (98.7%) of were mild in severity (Table E5). Of the 154 AEs, 52 (33.8%) were considered related to the study procedures/treatment (45 events in 8 participants in the RPh201 group, and 7 events in 2 participants in the placebo group). Across the 1,017 subcutaneous injections, site pain (23 events in 5 subjects) and pruritus (8 events in 4 subjects) were the most common AEs recorded. None of the participants treated with RPh201 had worsening of VA and/or VFs in the fellow-eye over the 26 weeks treatment period or after the 13 weeks off-drug wash out period (i.e. at 39 weeks).
Five serious AEs (SAE) were reported, 4 in two RPh201 participants and one in a placebo participant. One participant (treated with RPh201) experienced concurrent symptoms of moderate vertigo, dizziness, and mild decrease in left eye BCVA, all of which were assessed as unrelated. A second participant (treated with RPh201 and who withdrew from the study after visit 12) developed severe brucellosis, also assessed as unrelated. A third placebo-treated participant was diagnosed with NAION in the non-study eye and was discontinued from the study after 5 weeks. All SAEs were considered unrelated. One participant (from the placebo group), developed NAION in the fellow-eye and withdrew from the study. The event was considered unrelated to the study procedure/treatment. There were no clinically significant changes in vital signs or clinical laboratory values. No deaths were reported during the study.
Discussion
This Phase 2a study enrolled small numbers of participants with previous NAION. It had multiple endpoints with post hoc analyses, and therefore was not powered to detect small or moderate effects. It was nonetheless encouraging that BCVA improved about twice as much in the participants receiving RPh201 than those receiving placebo, and three times as many receiving RPh201 improved by three lines or more, compared to placebo. These results were seen at 26 weeks, suggesting that the neuroenhancing effects of the drug or its metabolites took place over months. Consistent with this interpretation, the BCVA improvement in the RPh201 group was mostly progressive, with changes starting after 10–13 weeks of treatment and slowly increasing. This pattern was most apparent in the participants with on-chart visual acuity at enrollment (Fig 2).
Participants treated with RPh201 showed improvement in VF mean deviation measured using the size III stimulus, but this was not statistically significant when compared to placebo. VF using the size V test stimulus did not demonstrate improvement in mean sensitivity. This lack of change may have been due to insufficient sensitivity of the chosen testing procedures, lack of a biological effect, or chance. No improvements in RNFL were seen, as would be expected with a treatment that enhanced neural activity but did not regenerate axons. On the other hand, we cannot exclude regeneration of tens to hundreds of axons subserving the macula, which would not be detectable with OCT of the retinal nerve fiber layer. No improvements in VEP were seen as well, which is not surprising given that the VEP is a mass response, and a poor measure of changes in visual function. VEP also would be insensitive to changes in a small number of axons.
Over 1000 subcutaneous injections were administered. The safety profile of the drug was good. AEs considered to be related to the study/treatment were mainly injection site reactions (61.5%, Table E5). Injection site pain (23 events in 5 subjects) and pruritus (8 events in 4 subjects) were the highest related AEs. This is not surprising given the treatments were administered subcutaneously. There were no clinically significant changes in vital signs or clinical laboratory values. Overall, RPh201 was well-tolerated.
Patients who have NAION in one eye have a 15–19% risk of developing a similar event in the fellow-eye over the subsequent months to 5 years (15, 21–23). One participant in the placebo group developed NAION in the fellow-eye. None of the RPh201-treated participants exhibited worsening in VA or VF in the fellow-eye after 26 weeks of treatment or during the 13 weeks off-drug period. Given the small numbers, it is impossible to assess whether RPh201 affected the risk of developing NAION.
Interpretation of the results of this study is limited by several issues. It was a proof-of-concept trial and was underpowered to detect small and moderate effects on visual function, especially when multiple endpoints were examined. Second, participants with both on-chart and off-chart visual acuity were included, but based on the highly variable BCVA measurements seen in the latter group, their data were difficult to interpret. The reason for the variability likely reflects difficulties in using the ETDRS charts when visual acuity is poor.
Third, a single BCVA measurement at screening was used as the baseline. This could have both contributed to regression to the mean and would have failed to account for the training effect associated with learning to use eccentric fixation to read the ETDRS charts. Patients with NAION do not typically improve beyond the initial few months (24) yet remarkable improvement was seen in the placebo group, which was greatest during the first 13 weeks of the study. This initial improvement was most likely due to a combination of placebo and training effects. Some patients with NAION are often unable to see the vision chart during their first visit because the scotoma affects central vision. Over time, patients become more adept at using eccentric fixation, and the measured VA therefore improves. This is due to the patient’s improved ability to use this eccentric viewing technique, rather than physiological changes, and is, as such, referred to as a training effect. To limit this the training effect, future trials will include a training period.
In conclusion, subcutaneous administration of RPh201 twice weekly for 26 weeks at a dose of 20 mg to participants with previous NAION did not raise any safety concerns. Some improvements in visual function were observed in this small Phase 2a study. However, efficacy of RPh201 in improving visual function needs to be further investigated in adequately powered Phase 3 studies.
Supplementary Material
Acknowledgments
Independent medical writers Rukhsana Shaikh-Zaidi and Sally Tucker assisted with the drafting and editing of this manuscript, and were paid by Regenera for this task.
Ilana Fishman is Chief Executive Officer of Endpoint Contract Research Organization, which assisted in the performance of the trial.
Cathie Leister and John Conlon assisted with statistical analyses.
Funding: The study was funded by Regenera Pharma Limited. Leonard A. Levin received funding from the Canadian Foundation for Innovation, Canadian Research Chairs program, National Institutes of Health (R21 EY025074 and P30 EY016665), and is the Canada Research Chair in Translational Visual Science.
Footnotes
Conflict of interest: Zvi I. Segal, and Ethan Z. Rath were PI’s of the study. Zadik Hazan and Konstantin Adamsky are employees of Regenera Pharma Limited, which funded the study. Leonard A. Levin is a consultant to Regenera, as well as to Aerie, Eyevensys, Galimedix, and Quark.
References
- 1.Hattenhauer MG, Leavitt JA, Hodge DO, Grill R, Gray DT. Incidence of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1997;123:103–107. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LN, Arnold AC. Incidence of nonarteritic and arteritic anterior ischemic optic neuropathy. Population-based study in the state of Missouri and Los Angeles County, California. J Neuroophthalmol. 1994;14:38–44. [PubMed] [Google Scholar]
- 3.Cestari DM, Gaier ED, Bouzika P, Blachley TS, De Lott LB, Rizzo JF, Wiggs JL, Kang JH, Pasquale LR, Stein JD. Demographic, Systemic, and Ocular Factors Associated with Nonarteritic Anterior Ischemic Optic Neuropathy. Ophthalmology. 2016;123:2446–2455. [DOI] [PubMed] [Google Scholar]
- 4.Boghen DR, Glaser JS. Ischaemic optic neuropathy. The clinical profile and history. Brain. 1975;98:689–708. [DOI] [PubMed] [Google Scholar]
- 5.Repka MX, Savino PJ, Schatz NJ, Sergott RC. Clinical profile and long-term implications of anterior ischemic optic neuropathy. Am J Ophthalmol. 1983;96:478–483. [DOI] [PubMed] [Google Scholar]
- 6.Xu L, Cui T, Yang H, Hu A, Ma K, Zheng Y, Sun B, Li J, Fan G, Jonas JB. Prevalence of visual impairment among adults in China: the Beijing Eye Study. Am J Ophthalmol. 2006;141:591–593. [DOI] [PubMed] [Google Scholar]
- 7.Roscic V, Bojic L, Marovic T. [The incidence of nonarteritic ischemic optic neuropathy in the Split-Dalmatia County]. Acta Med Croatica. 2009;63:169–172. [PubMed] [Google Scholar]
- 8.Atkins EJ, Bruce BB, Newman NJ, Biousse V. Treatment of nonarteritic anterior ischemic optic neuropathy. Surv Ophthalmol. 2010;55:47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman NJ, Scherer R, Langenberg P, Kelman S, Feldon S, Kaufman D, Dickersin K, Ischemic Optic Neuropathy Decompression Trial Research G. The fellow eye in NAION: report from the ischemic optic neuropathy decompression trial follow-up study. Am J Ophthalmol. 2002;134:317–328. [DOI] [PubMed] [Google Scholar]
- 10.Fazzone HE, Kupersmith MJ, Leibmann J. Does topical brimonidine tartrate help NAION? Br J Ophthalmol. 2003;87:1193–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilhelm B, Ludtke H, Wilhelm H. Efficacy and tolerability of 0.2% brimonidine tartrate for the treatment of acute non-arteritic anterior ischemic optic neuropathy (NAION): a 3-month, double-masked, randomised, placebo-controlled trial. Graefes Arch Clin Exp Ophthalmol. 2006;244:551–558. [DOI] [PubMed] [Google Scholar]
- 12.Johnson LN, Guy ME, Krohel GB, Madsen RW. Levodopa may improve vision loss in recent-onset, nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 2000;107:521–526. [DOI] [PubMed] [Google Scholar]
- 13.Bennett JL, Thomas S, Olson JL, Mandava N. Treatment of nonarteritic anterior ischemic optic neuropathy with intravitreal bevacizumab. J Neuroophthalmol. 2007;27:238–240. [DOI] [PubMed] [Google Scholar]
- 14.The ischemic optic neuropathy decompression trial (IONDT): design and methods. Control Clin Trials. 1998;19:276–296. [DOI] [PubMed] [Google Scholar]
- 15.Miller NR, Arnold AC. Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischaemic optic neuropathy. Eye (Lond). 2015;29:65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramot Y, Hazan Z, Lucassen A, Adamsky K, Santhosh Kumar DP, Vijayasarathi SK, Krishnappa H, Seervi MS, Nyska A. Toxicity and toxicokinetic study of RPh201 in Sprague-Dawley rats. Food Chem Toxicol. 2018;112:168–177. [DOI] [PubMed] [Google Scholar]
- 17.Ramot Y, Hazan Z, Lucassen A, Adamsky K, Ross V, Young N, Saunders M, Ehall H, Nyska A. Toxicity and Toxicokinetic Study of Subcutaneously Administered RPh201 in Minipigs. Toxicol Pathol. 2018;46:693–705. [DOI] [PubMed] [Google Scholar]
- 18.Benowitz LI, Yin Y. Optic nerve regeneration. Arch Ophthalmol. 2010;128:1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner BA, He Z. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480:372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuppermann BD, Thomas EL, de Smet MD, Grillone LR. Pooled efficacy results from two multinational randomized controlled clinical trials of a single intravitreous injection of highly purified ovine hyaluronidase (Vitrase) for the management of vitreous hemorrhage. Am J Ophthalmol. 2005;140:573–584. [DOI] [PubMed] [Google Scholar]
- 21.Beck RW, Hayreh SS, Podhajsky PA, Tan ES, Moke PS. Aspirin therapy in nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1997;123:212–217. [DOI] [PubMed] [Google Scholar]
- 22.Kupersmith MJ, Frohman L, Sanderson M, Jacobs J, Hirschfeld J, Ku C, Warren FA. Aspirin reduces the incidence of second eye NAION: a retrospective study. J Neuroophthalmol. 1997;17:250–253. [PubMed] [Google Scholar]
- 23.Liu Y, Lee RK. Neuro-rejuvenation for neuronal function. Neural Regen Res. 2016;11:1560–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ischemic Optic Neuropathy Decompression Trial: twenty-four-month update. Arch Ophthalmol. 2000;118:793–798. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.