Abstract
Operating with some finite quantity of processing resources, an animal would benefit from prioritizing the sensory modality expected to provide key information in a particular context. The present study investigated whether rats dedicate attentional resources to the sensory modality in which a near-threshold event is more likely to occur. We manipulated attention by controlling the likelihood with which a stimulus was presented from one of two modalities. In a whisker session, 80% of trials contained a brief vibration stimulus applied to whiskers and the remaining 20% of trials contained a brief change of luminance. These likelihoods were reversed in a visual session. When a stimulus was presented in the high-likelihood context, detection performance increased and was faster compared with the same stimulus presented in the low-likelihood context. Sensory prioritization was also reflected in neuronal activity in the vibrissal area of primary somatosensory cortex: single units responded differentially to the whisker vibration stimulus when presented with higher probability compared with lower probability. Neuronal activity in the vibrissal cortex displayed signatures of multiplicative gain control and enhanced response to vibration stimuli during the whisker session. In conclusion, rats allocate priority to the more likely stimulus modality and the primary sensory cortex may participate in the redistribution of resources.
SIGNIFICANCE STATEMENT Detection of low-amplitude events is critical to survival; for example, to warn prey of predators. To formulate a response, decision-making systems must extract minute neuronal signals from the sensory modality that provides key information. Here, we identify the behavioral and neuronal correlates of sensory prioritization in rats. Rats were trained to detect whisker vibrations or visual flickers. Stimuli were embedded in two contexts in which either visual or whisker modality was more likely to occur. When a stimulus was presented in the high-likelihood context, detection was faster and more reliable. Neuronal recording from the vibrissal cortex revealed enhanced representation of vibrations in the prioritized context. These results establish the rat as an alternative model organism to primates for studying attention.
Keywords: attention, awake-recording, barrel cortex, behavior, extracellular, sensory coding
Introduction
In a natural environment, animals need to assess when to initiate actions based on uncertain or weak sensory inputs such as small changes in luminance or vibrations induced by predators. In such scenarios, animals benefit from prioritizing sensitivity in the modality that is more likely to provide the key information. Contemporary models of attention largely focus on the primate visual system. Although this system is highly efficient (Thorpe et al., 1996; Bisley, 2011; Carrasco, 2011), the mechanisms are difficult to unravel due to the complexity of the neuronal pathways and the large number of dimensions in the stimulus space. Rodent whisker touch represents an expert sensory system with the ability to encode the environment in a fast and reliable manner (Diamond and Arabzadeh, 2013). In addition to its efficiency, this system is tractable and offers the chance to investigate the neuronal basis of object detection and identification. Here, we establish a detection paradigm to study the neuronal correlates of attention by controlling the likelihood with which sensory stimuli are presented in one of two modalities, vision and whisker touch. Our behavioral evidence shows that the stimulus in the more likely modality is better detected, an indication that the paradigm leads to sensory prioritization.
Rats and mice are frequently active in darkness, using their array of mobile whiskers to acquire sensory information. The system is structurally well characterized; the vibrissal area of the primary somatosensory cortex (vS1) contains a magnified topographic map of the whiskers in the form of distinct clusters of neurons known as barrels in layer IV (Woolsey and Van der Loos, 1970; Welker, 1971). Using its whiskers, a rat can quickly obtain sufficient information to complete complex behavioral tasks such as discriminating between textures (Heimendahl et al., 2007; Diamond et al., 2008; Morita et al., 2011; Kuruppath et al., 2014; Zuo et al., 2015), detecting and discriminating vibrations (Adibi and Arabzadeh, 2011; Miyashita and Feldman, 2013; Fassihi et al., 2014; McDonald et al., 2014), and localizing objects (Harris et al., 1999; Berg and Kleinfeld, 2003; Brecht, 2007; Mehta et al., 2007; Bagdasarian et al., 2013; O'Connor et al., 2013). The functional efficiency of the whisker pathway and its structural organization make it an ideal system in which to investigate how attention affects sensory processing.
At the behavioral level, attention has been shown to improve perceptual accuracy and shorten reaction times in primates (Posner, 1980; Cohen and Maunsell, 2009; Carrasco, 2011), with near-threshold stimuli gaining the strongest improvements (Reynolds et al., 2000; Herrmann et al., 2010). At the neuronal level, a number of signatures of attention have been identified in primates: an increase in stimulus-evoked firing rate in various visual areas [LGN (McAlonan et al., 2008), V1 (Herrero et al., 2008; Buffalo et al., 2010), V2 (Buffalo et al., 2010), and V4 (Moran and Desimone, 1985; McAdams and Maunsell, 1999)]; an increase in baseline activity [V1, V2 (Luck et al., 1997), and V4 (Luck et al., 1997; Reynolds et al., 2000)]; and anticipatory responses to stimuli (Chen and Seidemann, 2012; Rodgers and DeWeese, 2014). Are any of these neuronal correlates of attention applicable to other species and sensory areas such as the rodent somatosensory cortex?
Here, we manipulated attention by controlling the likelihood with which a stimulus was presented from one of two modalities. In a whisker session, 80% of trials contained a brief vibration applied to whiskers and the remaining 20% of trials contained a brief change of luminance (flicker trials). During such a session, given the limited capacity of the attentional system (Posner, 1980), rats would be expected to prioritize processing in the whisker pathway. The opposite prioritization would be expected for a visual session (80% flicker trials and 20% vibration trials). We establish how alternating between whisker and visual sessions affect the sensitivity and reaction time in detecting stimuli from each modality and how the likelihood of receiving stimuli in the whisker modality affects single-unit activity in the vS1 cortex.
Materials and Methods
Subjects
Subjects were seven adult, male Hooded Long–Evans rats with initial weights of 170–210 g. All procedures were approved by the Animal Care and Ethics Committee at the Australian National University. Rats were housed in independently ventilated and air-filtered transparent plastic boxes in a climate-controlled colony room on a 12/12 h light/dark cycle during which lights were turned off at 7:00 P.M. A combination of food and water restriction was used to motivate the rats to perform the detection task. Rats had abundant access to water except during the 2–3 h before sessions. A total of 25–30 g of rat chow was provided after the session. All rats gained weight at a normal rate throughout the entire duration of the experiment.
Apparatus
Rats were trained in a chamber measuring 24 × 32 × 11 cm. The front panel had an aperture with a diameter of 4 cm and was elevated 10 cm from the floor. A stepping platform was placed below the aperture 6 cm from the floor. Outside of the aperture was a nose-poke and reward spout, both of which had infrared sensors to detect the animal's presence. On the right side, an aluminum mesh (5 × 5 cm) was attached to a ceramic piezoelectric wafer (Morgan Matroc) to transmit a vibration stimulus. The mesh was placed at 45° from the center of the nose-poke sensor. To display the flicker stimulus, an LCD monitor (model #U2312HM, Dell; 60 Hz refresh rate, 510 cm × 290 cm) was placed at a distance of 35 cm behind the wafer, 45° from the center of the nose-poke sensor. The vibration stimulus was a sequence of discrete Gaussian deflections generated from MATLAB (MathWorks) and presented through the analog output of a data acquisition card (National Instruments) at a sampling rate of 44.1 kHz. Each Gaussian deflection had σ of 5 ms, lasted for 15 ms, and was followed by a 10 ms pause before the next deflection, yielding a frequency of 40 Hz. Although the rats' trajectory was highly stereotyped, the distance between the mesh and the follicle could vary from trial to trial due to head position and the curved surface of the snout. By examination of video records, our estimate of the median distance is ∼4 mm. The amplitude of the vibration was modulated depending on the stage of learning (see “Procedure” section). The flicker stimulus was a change in luminance from baseline at 0.68 cd/m2 (black screen). This was generated and displayed using MATLAB Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997). The percentage of change in luminance was also modulated depending on the stage of learning (see “Procedure” section). The nose-poke behavior was monitored by a high-speed camera (A3800; Balser) with a resolution of 22.68 pixels/mm through a Nikon Lens (AF 50 mm f/1.8) at 150 frames/s. The video provided a top view of the whiskers with illumination from below the nose-poke aperture using a 940 nm LED. For all video sequences, 1 s movies captured the period from the nose-poke onset.
Electrophysiology
After animals were trained in the behavioral task, microelectrodes supported by microdrives were surgically implanted into vS1. Two types of microdrives and microelectrodes were used. Rats were implanted with either a custom built microdrive that supported a 16-channel array (Tucker-Davis Technology) or an Axona Versa-Drive (Axona Systems) that supported independent movement of four custom-made tetrodes. The array was arranged in a two × eight configuration with 250 μm spacing between shanks and 375 μm spacing between the two rows. Tetrodes were made from 4 7 μm platinum iridium microwires that were twisted together and plated with platinum black plating solution (Neuralynx) and gold plating solution (SIFCO). Spacing between tetrodes was 200 μm center to center.
Animals were given food and water ad libitum at least 24 h before surgery and for at least 5 d after surgery. Anesthesia was induced with 3% isoflurane in O2 and maintained with 2–3% isoflurane provided through a breathing mask throughout surgery. Depth of anesthesia was monitored by tail and hind-paw pinch responses. Body temperature was maintained at 37°C using a heating pad (Physitemp Instruments). Craniotomies were made through which electrodes were lowered at coordinates of 2.5 mm anterior to bregma and 4.3 mm from midline. In two of five brains, recording sites were verified histologically by comparing Nissl-stained 60 μm coronal brain sections with reference anatomical planes (Paxinos and Watson, 2007). The array positions indicated that recordings were made in the supragranular layers of the vibrissal area of the primary somatosensory cortex (see Fig. 6B). A multineuronal acquisition processor (16 channels; Axona Systems) was used to amplify and record signals. Single-units were filtered at 300–7000 Hz (Butterworth) and extrapolated by using Offline Sorter 3.2.4 (Plexon) according to the following criteria: (1) <0.1% of interspike intervals smaller than 1.0 ms and (2) spike waveform shapes as determined by a waveform template algorithm and principal component analysis.
Figure 6.
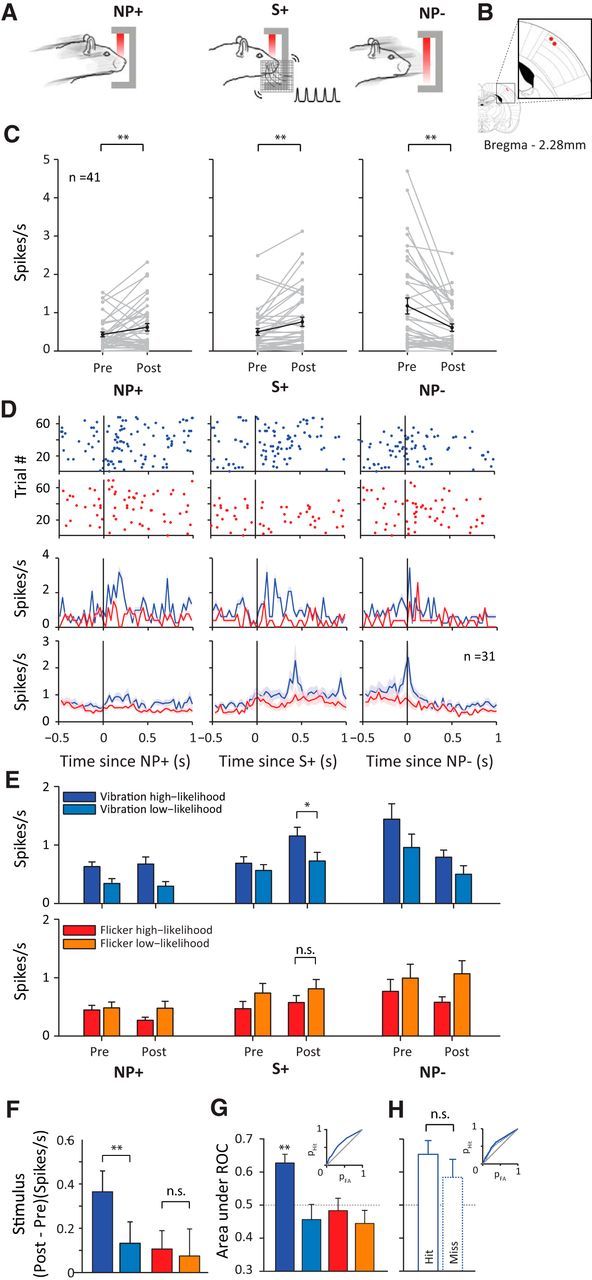
Single-unit activity in vS1. A, Schematic representation of behavioral time points of interest: NP+, nose-poke onset; S+, stimulus onset; NP−, nose-poke offset. B, Location of final recording sites in two rats on the rat brain atlas (Paxinos and Watson, 2007). C, Single-unit (n = 41) activity at each behavioral time period. Pre and Post represent average spike rate in a 200 ms window before and after each discrete behavioral event, respectively. Each gray line represents a neuron. Black line represents the average across all neurons. Error bar indicates SEM. D, Top, Raster plots showing spiking activity of the example neuron aligned to each behavioral event. Red dots represent spikes during visual session; blue dots represent spikes during whisker session. Middle, Perievent time histogram of the example neuron binned at 30 ms. Shaded areas represent SEM across trials. Bottom, Averaged perievent time histogram of all recorded neurons (n = 31) isolated in both a whisker and visual session. Shaded areas represent SEM across neurons. E, Average spike rate during before and after the event (nose-poke onset, stimulus onset, nose-poke offset). Trials are separated based on the type of stimulus (vibration or flicker) and the session type (whisker or visual) in which it was presented. Dark blue represents vibration trials in a whisker session; light blue represents vibration trials in a visual session; red represents flicker trials in a visual session; orange represents flicker trials in a whisker session. Error bars represent the SEM across neurons (n = 31). F, Analysis of stimulus response. Color notations are the same as in E. Each bar represents the mean difference in spike rate across neurons, calculated as the difference of poststimulus spike rate from prestimulus spike rate. G, Analysis of stimulus detectability. Color notations are the same as in E. Each bar represents the mean area under ROC across neurons. Inset shows the ROC curve for detection of vibration high-likelihood stimulus. H, Area under ROC from G plotted separately for the hit versus miss trials of vibration high-likelihood stimulus. Inset shows the ROC curves for hit trials (solid blue line) and for miss trials (dotted blue line).
Task
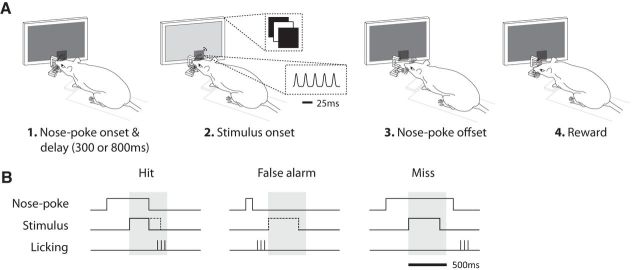
Figure 1, A and B, shows the behavioral setup and the training paradigm. Rats initiated a trial by performing a nose-poke. As they maintained the nose-poke, either a flicker or a vibration stimulus was presented at one of two delays (300 or 800 ms). The delays were pseudorandomized independently from the presentation order of stimulus modality. The randomization was such that, on every session, half of the trials were short delay (300 ms) and the other half were long delay (800 ms). The stimulus had a maximum duration of 400 ms and was terminated if the rat left the nose-poke earlier. Upon detecting the presence of the stimulus, rats were required to respond by leaving the nose-poke and entering the reward spout within 500 ms after the onset of the stimulus; correct actions were rewarded with 0.08 ml of 7% sucrose. We discouraged the rats from leaving nose-poke prematurely—that is, before detecting the stimulus—by setting the length of each trial to a fixed duration of 2.5 s. This was done by adjusting the intertrial interval on a trial-by-trial basis: when rats left the nose-poke before the stimulus onset, the intertrial interval was proportionately longer, thus acting as a time-out punishment.
Figure 1.
Schematic representation of the detection task. A, The rat initiated a trial by nose poking into the aperture while touching the mesh plate with its whiskers (1). After a delay of either 300 or 800 ms, during which nose poke was continually maintained, the rat received either a vibration (a sequence of Gaussian wavelet pulses) or visual stimulus (a change in luminance from a black display) (2). The rat expressed detection of the stimulus by leaving the nose-poke (3) and entering the reward spout (4). Correct detection was rewarded by 7% sucrose water. B, Schematic representation of the three trial types that arose from the animal's behavior. Shaded gray area defines the 500 ms window of opportunity. Nose-poke steps represent entrance and exit from nose-poke. Stimulus steps represent onset and offset of stimulus presentation. Licking bars represent the first few licks at the reward spout. Trial types were defined as the following: hit, which was leaving the nose-poke within the window of opportunity and entering the reward spout; false alarm, leaving the nose-poke before the onset of the stimulus and entering the reward spout; or miss, leaving the nose-poke after the window of opportunity. The stimulus was aborted upon exit from the nose-poke (see “hit”), and was not presented at all in the case of “false alarm.” The dashed line represents the stimulus profile if it was not aborted or cancelled.
Sessions were categorized as either whisker or visual. In a whisker session, 80% of trials consisted of a vibration stimulus and 20% of trials consisted of a flicker stimulus. These frequencies were reversed for a visual session. Each session contained 180 trials, with two low-likelihood trials inserted in random order within every 10 trial block. In 1 group of rats (n = 4), 2 sessions were conducted each day (3 h break between each session; no food or water was provided during this break) and the order of session type was counterbalanced daily. To facilitate tracking neurons across the two session types (visual and whisker), in a second group of rats (n = 3), the break between the 2 sessions was removed and the number of trials in each session was reduced to 100, effectively forming a single session with a continuous series of 200 trials and a modality likelihood switch at the midpoint. The order of the switch was counterbalanced and no cues were provided to the animal as to the occurrence of the switch in likelihoods. This protocol allowed us to quantify the temporal profile of prioritization after the switch in likelihoods on trial 101 (see Fig. 5B).
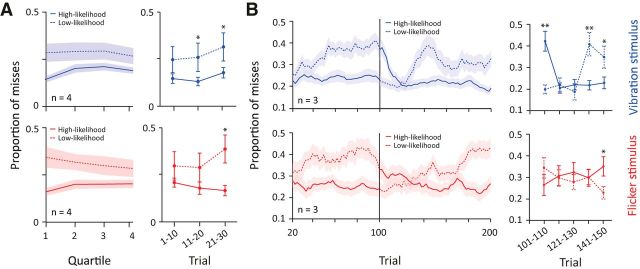
Figure 5.
Time course of sensory prioritization. A, Left, Proportion of misses as a function of trial quartiles, pooled across rats (n = 4) and sessions. Top (blue), Proportion of misses of vibration trials presented in high-likelihood condition (solid line) and low-likelihood condition (dashed line). Same notations are retained for bottom panel (red) representing flicker trials. Shaded areas indicate SEM. Right, Proportion of misses for the first 30 trials divided into nonoverlapping windows of 10 trials. Asterisks indicate statistically significant differences, p < 0.05, Student's t test. B, Left, Proportion of misses as a function of trials, pooled across rats (n = 3) in the within-session likelihood switching paradigm. Proportion of misses was calculated in windows of 20 trials at steps of two trials. The vertical line represents the switch in likelihood after trial 100. Top (blue), Proportion of misses when vibration stimulus was presented in a high–likelihood condition (solid) and low-likelihood condition (dashed). Note the line changes in notation as likelihood proportions are switched. The same notation is retained for bottom panel (red) representing flicker trials. Shaded areas indicate SEM. Right, Proportion of misses for the first 50 trials after the onset of the switch in likelihood. Each data point represents nonoverlapping windows of 10 trials. Asterisks indicate statistically significant differences, *p < 0.05, **p < 0.01, Student's t test.
Procedure
Shaping to go to spout.
Rats were placed in the experimental chamber and reward was freely available from the reward spout for 100 trials. The nose-poke area was blocked at this stage. When rats arrived at the reward spout to receive sucrose, both the visual stimulus (100% change in luminance) and the whisker stimulus (a series of deflections at 50 μm amplitude) were presented simultaneously.
Shaping to perform nose poking.
Rats were rewarded only after performing a nose poke. Two delays were imposed from the first nose-poke shaping day, with 80% of trials with a short delay (starting at 100 ms) and 20% with the longer delay (starting with 200 ms). The required duration of nose poke gradually increased to reach the final delays of 300 and 800 ms and the proportion of short and long delay was gradually shifted to 50%. At this stage, the two session types were also introduced.
Adjustment of the stimulus intensity.
When animals reached a performance >75% and a false alarm rate <15%, four separate levels of stimulus intensity were presented in an intermixed fashion on each session. Vibration intensity was manipulated by adjusting the amplitude of the Gaussian wavelets. Note that a change in amplitude causes a linearly related change in speed of the two phases (rise and fall) of the wavelet; previous work (Arabzadeh et al., 2003, 2004) indicates that whisker deflection speed is encoded by cortical neurons. The starting deflection amplitude was 25 μm and was reduced with steps of 3 μm to generate lower intensities. Flicker intensity was adjusted by the percentage change from baseline (0.68 cd/m2). The starting change in luminance was 47% and was reduced with steps of 7.8% to generate flickers of progressively lower intensity. At each level of difficulty, detection performance was indexed by the proportion of misses, defined as the number of miss trials divided by the sum of miss and hit trials. The stimulus difficulty increased until miss proportions grew to 30%. At the final stage, the stimulus difficulty was 3–8 μm for the vibration stimulus across rats and a 4–12% change in contrast for the flicker stimulus.
Data analysis
As shown in Figure 1B, trials were categorized as follows: (1) hit, in which the rat successfully waited in the nose-poke for the stimulus and then left the nose-poke and licked the reward spout within 500 ms of the onset of the stimulus; (2) false alarm, in which the rat left the nose-poke before stimulus presentation; and (3) miss, in which the rat successfully waited in the nose-poke for the stimulus but failed to leave in response to stimulus presentation (i.e., it left the nose-poke >500 ms after stimulus onset).
To quantify “onset” reaction times, we applied signal detection theory (Green and Swets, 1966) to determine perceptual accuracy, d′, for each stimulus modality and session type (visual or whisker). For this analysis, we compared nose-poke leaving times (nose-poke offset minus nose-poke onset) between short- and long-delay trials. Perceptual accuracy was calculated as follows:
where Z is the inverse of the cumulative Gaussian distribution function. The hit rate distribution was derived from the nose-poke leaving times of short-delay trials (300 ms) and false alarm rates were derived from the nose-poke leaving times of long-delay trials (800 ms). The d′ values were calculated as a function of time at a resolution of 5 ms. This was compared against a shuffled distribution where 1000 d′ traces were calculated by sampling from a randomized distribution of hits and false alarms. The first point of deviation of the observed d′ from the shuffled distribution was taken as the onset reaction time.
Stimulus detectability was computed from distributions of spike counts occurring 200 ms before and after each stimulus onset. A criterion shifted in steps of one spike across the two distributions was used to determine the hit and false alarms of the neuron, thus forming a receiver operating characteristic (ROC) curve. Detectability was expressed as the area under the ROC and significance testing was performed by bootstrapping spike counts across the two distributions.
Results
Learning of the detection task
Rats were trained to report the presence of either a vibration applied to their whiskers or a luminance flicker on the display monitor (Fig. 1A). They initiated a trial by performing a nose poke, which was registered by an optic sensor. On each trial, the stimulus was presented at one of two fixed delays after nose-poke: half of trials had an early stimulus onset (300 ms) and half had a late stimulus onset (800 ms). The stimulus had a maximum duration of 400 ms or was terminated when the rat left the nose-poke for both vibration and flicker trials. Every trial was classified as a hit (leaving the nose-poke within the 500 ms window of opportunity and entering the reward spout), a false alarm (leaving the nose-poke before the onset of the stimulus and entering the reward spout), or a miss (not leaving the nose-poke for that trial or leaving the nose-poke after the window of opportunity) (Fig. 1B). Because every trial except those aborted by false alarm contained a stimulus presentation, the usual class of “correct rejection” did not apply.
Once performance was stable, we modulated stimulus difficulty to yield similar performance across rats and for both modalities (see Materials and Methods). To characterize the overall detection performances for the vibration and flicker stimuli, we first combined the trials of a given modality across both high- and low-likelihood sessions (Fig. 2A). False alarm rates were low and consistent across all rats (vibration: 5.72 ± 2.78%, mean ± SD; flicker: 5.30 ± 3.76% across rats). Vibration and flicker stimuli showed similar hit rates (Student's t test, Rat 1: p = 0.07, Rat 2: p = 0.53, Rat 3: p = 0.86, Rat 4: p = 0.13) and miss rates (Student's t test, Rat 1: p = 0.19, Rat 2: p = 0.94, Rat 3: p = 0.98, Rat 4: p = 0.17). To establish that rats' behavior was based on stimulus detection rather than a nonspecific strategy such as timing, we quantified performance by comparing nose-poke durations between short (300 ms) and long (800 ms) delay trials. If rats left the nose-poke consequent to stimulus detection, the time spent in the nose-poke would depend on the duration of the delay preceding stimulus presentation. During vibration trials, rats showed significantly longer nose-poke durations for the long-delay trials (967 ± 11 ms, mean ± SD) than for the short-delay trials (539 ± 8 ms) (Wilcoxon rank-sum test, p < 0.01) and this stimulus onset dependence was consistent across rats (Fig. 2B). In flicker trials, just as in vibration trials, the time spent in the nose-poke depended on stimulus timing (Fig. 2B: long-delay: 976 ± 27 ms; short-delay: 593 ± 25 ms; Wilcoxon rank-sum test, p < 0.01). In sum, early and late stimulus onset trials led to distinct response-timing profiles.
Figure 2.
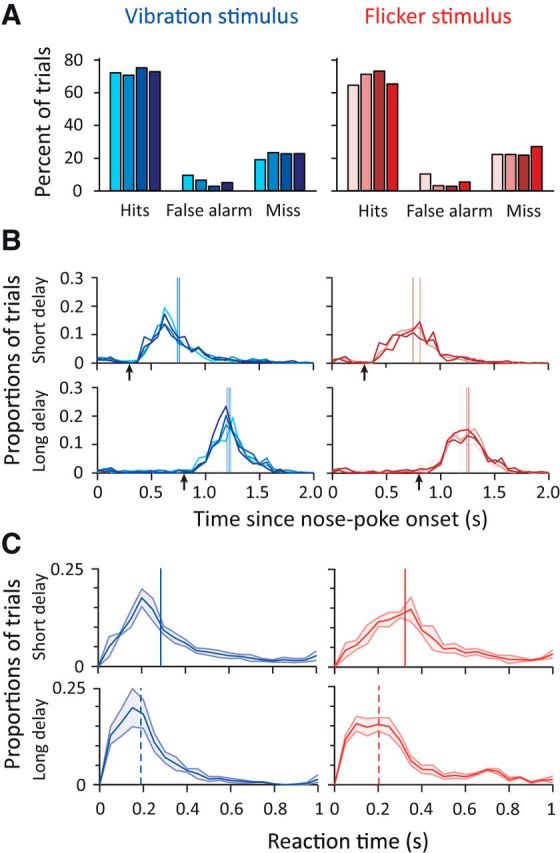
Rats showed high levels of behavioral detection performance. A, Detection performance when a vibration stimulus was presented (blue) and when a visual stimulus was presented (red). Each shade represents the performance of a single rat. B, Distribution of nose-poke durations (nose-poke offset − nose-poke onset). Top, Short-delay trials (300 ms). Bottom, Long-delay trials (800 ms). Arrows represent stimulus onset. Vertical lines represent median nose-poke duration for each rat. Miss trials in which the rat did not leave the nose-poke were excluded. Each shade and color represents a single rat and stimulus as in A. C, Distribution of reaction times (nose-poke offset minus stimulus onset) between short- and long-delay trials averaged across all four rats. Shaded area represents 1 SD. Top, Short-delay trials (300 ms). Bottom, Long-delay trials (800 ms). Vertical line represents median reaction time. Miss trials in which the rat did not leave the nose-poke were excluded. Color notations are the same as previous panels.
Given that a trial was equally likely to have an early or late stimulus, at the onset of nose poke, the probability of stimulus presentation at 300 ms was 0.5. However, if the rat detected no stimulus at 300 ms, then the probability of stimulus presentation at 800 ms was ∼83% (calculated as 1 divided by 1 plus the probability of having failed to detect a true stimulus presentation at 300 ms, the average miss probability across all rats and modality being 20 ± 5.4%). We investigated whether the expectation of a late stimulus (based on not sensing the early stimulus) resulted in faster reaction times and found it to be the case for both modalities: the distribution of reaction times, the time interval between stimulus onset and nose-poke exit, showed a faster rise and a lower median for the long-delay trials compared with the short-delay trials (Fig. 2C; vibration, p < 0.01; flicker, p < 0.01, Wilcoxon rank-sum test).
Reaction times to vibration and flicker stimuli
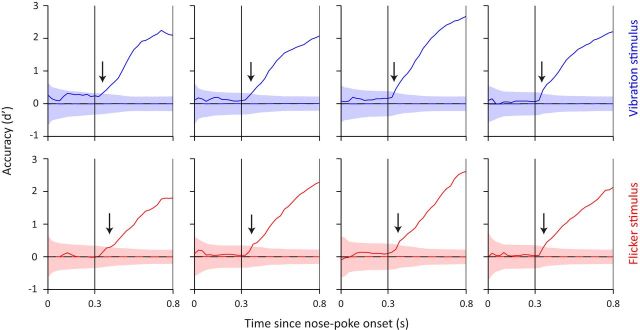
For the interval between 300 and 800 ms after nose poke, all trials can be divided into two categories: stimulus-present and stimulus-absent, with equal number of trials in each category (stimulus-absent trials in this first interval correspond to late stimulus trials). This design allowed us to calculate hit rate and false alarm rate and combine them to estimate sensitivity (d′) at each time point during the 300–800 ms interval (Fig. 3). The first point in time where d′ values deviated from chance (chance estimated by bootstrapping; see Materials and Methods) revealed remarkably fast reaction times. These onset reaction times capture the earliest reliable behavioral manifestation of stimulus detection and were consistent across rats. Averaged across all sessions and all rats, vibration and flicker trials gave a first reaction of 48 and 56 ms, respectively (Table 1).
Figure 3.
Rats showed fast onset reaction times as revealed by a change in detection accuracy (d′) as a function of trial time course. Each rat's detection accuracy is shown across panels. Shaded areas represent d′ chance level as calculated from bootstrapping. Dashed horizontal line represents the mean value of shaded area. Arrows indicate the first point in time d′ reached above chance level. Color notations are the same as in Figure 2.
Table 1.
Onset reaction times (ms)
| Rat 1 | Rat 2 | Rat 3 | Rat 4 | |
|---|---|---|---|---|
| Vibration | 61 | 50 | 40 | 39 |
| Flicker | 55 | 60 | 58 | 51 |
Effects of attention on performance and reaction time
Does attention modulate the speed and accuracy of stimulus detection? To address this question, we manipulated the likelihood with which a stimulus was presented within each modality. In a whisker session, 80% of trials required detection of a vibration and the remaining 20% of trials required detection of a flicker. These likelihoods were reversed in the visual session. In the preceding sections, we pooled the two session types, but we now consider the sessions separately to determine whether manipulation of stimulus likelihood resulted in systematic differences in behavioral performance. Trials in which the presented stimulus was in the modality corresponding to the session type were denoted as high-likelihood trials (i.e., vibration trials in whisker sessions and flicker trials in visual sessions), whereas trials in which the stimulus modality did not correspond to the session type were denoted low-likelihood trials (i.e., vibration trials in visual sessions and flicker trials in whisker sessions). When a vibration stimulus was presented in a whisker session (high-likelihood), all 4 rats were less likely to miss the stimulus compared with when it was presented in a visual session (low-likelihood) (Fig. 4A; Rat 1, p < 0.05; Rat 2, p < 0.01; Rat 3, p < 0.05; Rat 4, p < 0.01). This change in sensitivity was consistent across rats: on average, miss rates for vibration were 0.18 ± 0.01 and 0.34 ± 0.03 in whisker and visual sessions, respectively. Similar improvements in detection were observed for flicker stimuli when presented in a visual session (Fig. 4A; Rats 1–4, all p < 0.01; miss rates for flicker stimuli were 0.19 ± 0.02 and 0.32 ± 0.29 in visual and whisker sessions, respectively).
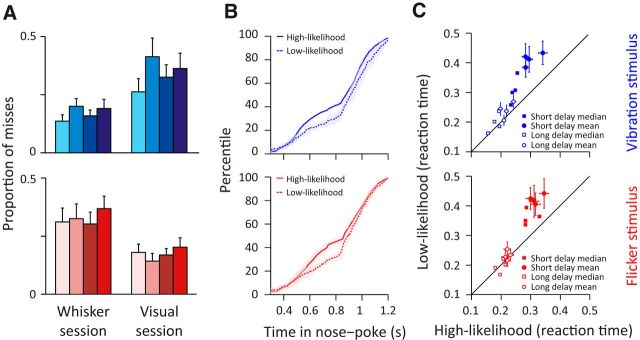
Figure 4.
Increased detection performance and speed when stimulus was presented in a high-likelihood versus low-likelihood session. A, Proportion of misses calculated as the number of miss trials divided by the sum of miss and hit trials. Top (blue), Miss proportion when vibration stimulus was presented in a whisker (high-likelihood trials) and visual session (low-likelihood trials). Bottom (red), Miss proportion when flicker stimulus was presented in a visual (high-likelihood trials) and whisker session (low-likelihood trials). Error bars indicate SDs for each rat. Color notations are the same as in Figure 2. B, Cumulative distribution frequency of nose-poke duration of hit and miss trials. Top (blue), Cumulative distribution frequency of vibration trials presented in high- (solid line) and low-likelihood session (dashed line). Bottom (red), Cumulative distribution frequency of flicker trials presented in high–likelihood session (solid line) and low-likelihood session (dashed line). Shaded area represents SD across rats. C, Scatter plot comparing reaction time between stimuli presented in high- and low-likelihood sessions for each rat. Trials were separated by short-delay stimulus (filled markers) and long-delay stimulus (open markers). Circles represent mean values. Squares represent median values. Error bars indicate SEMs.
We next investigated whether stimulus likelihood affected the speed of detection. Figure 4B plots the cumulative distribution of nose-poke duration for high-likelihood (solid line) and low-likelihood (dashed line) stimuli. Across rats, the high-likelihood stimulus resulted in significantly shorter nose-poke duration compared with the low-likelihood stimulus for both vibration and flicker trials (vibration and flicker, Rats 1–4: p < 0.01, Wilcoxon rank-sum). Although reaction times were on the whole (Fig. 2C) longer on short-delay trials (300 ms stimulus onset) compared with long-delay trials (800 ms stimulus onset), the short-delay trials showed a more prominent benefit in detection speed on high-likelihood versus low-likelihood trials (filled symbols in Fig. 4C) than did the long-delay trials (open symbols in Fig. 4C). On short-delay trials, rats reacted significantly faster to the vibration stimulus when presented in a whisker session (filled circles, 301 ± 27 ms, mean ± SD across rats) compared with a visual session (430 ± 17 ms, mean ± SD, Wilcoxon rank-sum, p < 0.01). The context-dependent improvement in reaction time was also the case for flicker stimulus (in visual session: 318 ± 19 ms; in whisker session: 438 ± 48 ms, p < 0.01). To determine whether the improvement in the overall reaction time was due to miss trials with long reaction times, we measured the median reaction times to minimize the effect of outliers. For short-delay trials, the median values also replicated the enhanced reaction time (filled squares in Fig. 4C; high-likelihood vibration trials: 244 ms; low-likelihood vibration trials: 308 ms; high-likelihood flicker: 295 ms; low-likelihood flicker: 360 ms; all values averaged across rats). Unlike short-delay trials, the long-delay trials did not exhibit a robust difference in reaction times: the difference between high- and low-likelihood trials was significant for vibration trials (218 ± 18 ms vs 239 ± 26 ms, p = 0.04), but not for flicker trials (221 ± 7 ms vs 232 ± 16 ms, p = 0.95).
To investigate how rapidly the enhanced sensitivity for the high-likelihood stimulus emerged, we divided each 180-trial session into four 45-trial quartiles (Fig. 5A, left) and found that the differential performance was present from the first quartile. During the first quartile, the vibration stimulus gave a miss rate of 0.15 when presented in a whisker session (solid line) and 0.28 when presented in a visual session (dashed line); the difference in miss rate was significant across rats (p < 0.01). Enhanced sensitivity was also observed for the flicker stimulus when its likelihood was elevated, giving a miss rate of 0.17 (first quartile of visual session) versus 0.34 (first quartile of whisker session), a significant difference (p < 0.01). As the session progressed, the performances in the high- versus low-likelihood conditions appear to converge (Fig. 5A, left); however, this trend was not statistically significant (difference in miss performance over time, r (30) = −0.02, p = 0.91, Pearson correlation test). Figure 5A, right, demonstrates in more detail how prioritization developed at the beginning of the session by plotting the proportion of misses for high- and low-likelihood trials in nonoverlapping windows of 10 trials (trials 1–10, 11–20, and 21–30). The trend of increased performance for the high-likelihood stimulus was present in the first 10 trials and reached statistical significance at 20–30 trials (vibration: p < 0.05; flicker: p < 0.05; Fig. 5A).
To further quantify the temporal profile of prioritization, three rats were trained in a modified version of the paradigm: a 200-trial session that contained an uncued switch in likelihoods after 100 trials. Rats detected the switch in likelihood and shifted their performance accordingly (Fig. 5B, left). Again, we investigated in more detail how prioritization developed after the switch by plotting the proportion of misses for high- and low-likelihood trials in nonoverlapping windows of 10 trials (Fig. 5B, right). By 20 trials after the switch in likelihoods, the previous prioritization was no longer expressed (i.e., miss rates were approximately equivalent for the high- and low-likelihood trials) and, within 20–30 additional trials, the rats expressed prioritization in relation to the new likelihoods (Fig. 5B). The first change in performance appears to be a decrease in miss rate for the high-likelihood stimuli.
Effects of sensory prioritization on neuronal activity in vS1
In five rats, we implanted electrodes into vS1 cortex contralateral to the side of whisker stimulation and recorded single-unit activity while the animals performed the detection task. One of the signatures of activity in vS1 is the response to whisker movement (Peron et al., 2015) typically found during exploration (Diamond and Arabzadeh, 2013). We identified three key time points: nose-poke entry, stimulus presentation, and nose-poke exit, as depicted in Figure 6A. The recording site was verified functionally based on the activity during each behavioral time point and was histologically confirmed for two rats (Fig. 6B). Overall, vS1 units (n = 41) showed low firing rates (<5 spikes/s), typical of supragranular neurons. As expected, neurons showed changes in firing rate during entry into (NP+) and exit from (NP−) the nose-poke. Firing rate increased after nose poke as the whiskers came in contact with the mesh (Wilcoxon rank-sum, 200 ms before and after nose-poke onset: p < 0.01; Fig. 6C, left). The presentation of the vibration stimulus produced a significant increase in firing rate (Wilcoxon rank-sum, 200 ms before and after nose-poke onset: p < 0.01; Fig. 6C, middle). Finally, withdrawal from the nose-poke was accompanied by a significant decrease in firing rate (Wilcoxon rank-sum, 200 ms before and after nose-poke offset: p < 0.01; Fig. 6C, right).
We then investigated how sensory prioritization affected neuronal activity in vS1. Figure 6D, raster plots and upper PSTHs, shows the activity of an example neuron aligned to the three behavioral time points during a visual (red) and whisker (blue) session. During the whisker session, the neuron's firing rate increased at nose-poke entry, after stimulus presentation, and before nose-poke exit. The neuron's firing rate was reduced during the visual session and the modulations of activity when aligned to the three behavioral events were either absent or reduced. These findings were replicated averaging across 31 single units that were maintained in both a visual and a whisker session (Fig. 6D, bottom PSTHs). For every trial, we measured the average firing rate from 0.5 s before nose-poke entry to 0.5 s after nose-poke exit. Across neurons and trials, the average firing rate was 24.6% lower during the visual sessions compared with the whisker sessions and this difference was statistically significant (Wilcoxon rank-sum test, p < 0.05). To better quantify the differences between the two sessions, we characterized spike rates across these behavioral time windows. Figure 6E separates trials into four discrete categories based on their stimulus type (vibration or flicker) and likelihood (high or low): a whisker session is composed of vibration high-likelihood and flicker low-likelihood trials (dark blue and orange bars). A visual session is composed of flicker high-likelihood and vibration low-likelihood trials (light blue and red bars). Across neurons, vS1 firing rates were generally enhanced during whisker sessions compared with visual sessions (dark blue larger than light blue and orange larger than red) and this gain modulation was statistically significant (pooled across all behavioral windows, p < 0.01, Wilcoxon rank-sum). We also applied the Wilcoxon rank-sum test to examine separately the gain modulation at each behavioral time window: before nose-poke entry, p = 0.19; after nose-poke entry, p = 0.45; before stimulus onset, p = 0.09; after vibration onset, p = 0.03; after flicker onset, p = 0.15; before nose-poke exit, p = 0.06; and after nose-poke exit, p = 0.04.
What is the effect of prioritization on stimulus detectability? We quantified the change in neuronal activity with respect to stimulus presentation in a whisker and visual session. The evoked response was quantified as the difference in firing rate around stimulus onset (the firing rate 200 ms poststimulus onset minus the firing rate 200 ms prestimulus onset). Vibration stimuli produced an evoked response that was statistically higher during the whisker session compared with the visual session (p < 0.01, Student's t test; Fig. 6F). The modulation in firing rate around flicker presentation was not affected by session type (p = 0.901; Fig. 6F). An ROC analysis (see Materials and Methods) revealed that an ideal observer of neuronal activity in vS1 could reliably detect the vibration stimulus only when it was presented in the whisker session (p < 0.01; Fig. 6G). Z-score normalization during the stimulus period (firing rate 200 ms prestimulus and poststimulus onset) revealed the same relationship, with neuronal activity in vS1 being significantly higher than chance only during vibration presentation in whisker sessions (p < 0.01, Student's t test), which was also significantly higher compared with vibration presentation in visual sessions (p < 0.01, Student's t test).
In the preceding section, we reported that sensory prioritization increased the overall firing of vS1 neurons and, specifically, boosted the neuronal response to vibration stimuli. Next, we investigated whether the neuronal encoding of the vibration stimulus correlated with the rat's behavioral performance (hit vs miss). To address this question, we compared the vibration-evoked activity (the firing rate 200 ms poststimulus onset minus the firing rate 200 ms prestimulus onset) between the hit and miss trials and found no significant difference (p = 0.318; Student's t test). In fact, on miss trials, an ideal observer of neuronal activity in vS1 could still detect the vibration stimulus better than chance (area under ROC of 0.58; p < 0.05; Fig. 5H; see Materials and Methods).
Discussion
Animals need to assess when to initiate actions based on uncertain sensory evidence. This is most evident when dealing with weak sensory inputs such as small changes in luminance or vibrations induced by an approaching predator. Operating with some finite quantity of attentional resources, an animal would benefit from prioritizing the modality expected to provide key information. For instance, in a dark burrow, the signal is likely to come from the tactile domain, but upon leaving the burrow at daybreak, visual events would be more relevant.
To better understand how animals delegate attentional resources, we devised a paradigm that encouraged rats to prioritize processing in one sensory modality. Prioritization led to a drop in miss rates (Fig. 4A) and faster reaction times (Fig. 4B,C), which is consistent with previous findings where attention improved performance (Posner, 1980; Cohen and Maunsell, 2009; Carrasco, 2011) and reduced reaction time (Eriksen and Hoffman, 1972; Henderson and Macquistan, 1993). In every 10-trial block, two trials presented the low-likelihood stimulus. Therefore, an ideal observer would be able to identify the session type after as few as three trials of the same stimulus modality. The first group of rats was tested with just one likelihood condition per session and required a small number of trials to identify the session type: they improved detection for the high-likelihood stimulus as early as 10 trials into the session, reaching statistical significance after 20–30 trials (Fig. 5A). A second group of rats was tested in a modified version of the paradigm that contained an uncued switch in likelihoods in the middle of the recording session. Within 20 trials after the switch, rats no longer expressed the previous prioritization and, within 20–30 more trials, they prioritized modalities in accordance with the new likelihoods (Fig. 5B).
Detection paradigms involving whiskers typically use a variable delay with uniform distribution to increase uncertainty (go-no-go tasks: Sachidhanandam et al., 2013; Stüttgen and Schwarz, 2010; Guo et al., 2014; two-alternative forced-choice tasks: Adibi et al., 2012; Miyashita and Feldman, 2013; McDonald et al., 2014). The current study used only two discrete delays, providing the temporal precision and statistical power to quantify the earliest withdrawal from nose-poke. The d′ analysis revealed that rats reacted to vibrations as early as 39 ms (Fig. 3, Table 1). Electrophysiological and imaging studies uncover touch-evoked signals in vS1 as early as 4–6 ms after whisker deflection (Petersen and Diamond, 2000; Matyas et al., 2010), whereas signals arrive in the V1 as early as 42–44 ms after presentation of 100% contrast flash (Wang et al., 2014). The fast reaction times in our task suggest that the perceptual/motor system operates close to threshold: small differences in sensory cortex are amplified in a cascade that recruits motor outputs already primed for action execution (de Lafuente and Romo, 2006). The use of only two discrete delays would allow rats to prepare the motor response before the expected stimulus onset times. Motor preparation is likely to have contributed to the fast reaction times observed in our study. Consistent with this hypothesis, during 800-ms-delay trials, a brief head bob is visible at ∼300 ms in video records, which may correspond to a preparatory action that would be transformed to a complete nose-poke withdrawal in the event that a flicker or vibration stimulus were sensed (Movie 1).
Movie 1.
Example hit trials of a well trained rat performing the detection prioritization task. Four example trial types with stimulus presentation at 800 ms are shown: vibration high-likelihood, vibration low-likelihood, flicker high-likelihood, and flicker low-likelihood. The rat places its snout in the nose-poke to initiate the trial. After the prestimulus delay, the stimulus (vibration or flicker) is presented. The rat then leaves the nose-poke and enters the reward spout (below the nose-poke) to receive reward. Infrared lighting is used to illuminate the video sequence, which was not visible to the animal. The video playback is at 20× reduced speed.
The use of two discrete delays also creates nonstationary demands on attention. If rats detected no stimulus at 300 ms, then the probability of stimulus presentation at 800 ms was ∼83% (see Results). This reduction in temporal uncertainty of stimulus onset timing in long-delay trials resulted in significantly faster reaction times (Fig. 2C). Temporal uncertainty interacted with modality uncertainty; as shown in Figure 4C, improvements in reaction time to the high-likelihood stimulus with respect to the low-likelihood stimulus were strongest for short-delay trials. We speculate that, on 800 ms presentations, the temporal certainty masked the advantage of being able to predict modality (with ∼80% certainty), leading to a diminished reaction time difference between high- and low-likelihood trials.
Neuronal recordings revealed changes in firing rate during nose-poke entry and exit (Fig. 6C). This can reflect the two modes in which rats use their whiskers to interact with the environment (Diamond and Arabzadeh, 2013). In the generative mode, rats “whisk” to actively seek and palpate objects (Berg and Kleinfeld, 2003; Mehta et al., 2007; Morita et al., 2011; Bagdasarian et al., 2013; Kuruppath et al., 2014). In the receptive mode, when self-generated movement would produce noise and reduce detectability of external events, rats immobilize whiskers to capture mechanical energy from their environment (Miyashita and Feldman, 2013; Fassihi et al., 2014). The self-generated whisker movements would allow rats to enter and exit the nose-poke with precision, meanwhile evoking activity in vS1 neurons. As they remain in the nose-poke, the switch to receptive mode would enhance the encoding of the vibration stimulus.
Responses of neurons recorded during whisker sessions were consistently greater than during visual sessions (Fig. 6E). Sensory prioritization may act via sensory amplification or “multiplicative gain control” (McAdams and Maunsell, 2000; Olsen et al., 2012; Zhang et al., 2014) in the cortex. Specifically, prioritization of the whisker pathway is achieved by increasing overall excitability in the vS1 cortex, whereby evoked activity is amplified disproportionately more than is spontaneous activity. This increase in gain would allow deflections to be more reliably detected by subsequent stages in processing. Therefore, unit recordings showed enhanced response to vibration stimuli during whisker sessions compared with visual sessions (Fig. 6F). A consequence of the gain modulation was improved signal detectability: in our sample of neurons, an ideal observer of neuronal activity in vS1 could decode the presence of vibration only in whisker sessions (Fig. 6G). This increased stimulus-evoked response accompanying prioritization is consistent with attention studies in the visual pathway (McAdams and Maunsell, 1999; Herrero et al., 2008; McAlonan et al., 2008). An alternative explanation of the increased detectability and enhanced firing may be bottom-up differences such as alterations in head position or whisking behavior across the conditions. However, inspection of high-speed videos (e.g., Movie 1) revealed stereotyped behavior across trials with no evident systematic differences between session types in head and whisker motion or position. However, our data do not exclude the possibility of minute differences outside our resolution.
The absence of significant choice probability, a correlation between single-trial activity and the animal's decision on that trial, in single-unit firing in sensory cortex is consistent with observations of sensory cortex in monkeys (de Lafuente and Romo, 2005, 2006). Single-unit firing in the monkey primary somatosensory cortex did not covary with the monkeys' perceptual reports to near threshold vibration stimuli. In our recordings, on vibration miss trials, an ideal observer of neuronal activity in vS1 could still detect the stimulus better than chance. This indicates that differences between hits and misses may be due to fluctuation in the state of the networks in higher-order brain areas that “read out” vS1 activity; for example, variation in their receptivity to inputs from sensory cortex.
Attention is thought to arise through two possible routes (Corbetta and Shulman, 2002). The bottom-up route is activated by salient or unexpected stimuli (Kayser et al., 2005). A second route is activated by expectation, a top-down process of selection. In our experiment, because the stimuli in all sessions were of equivalent intensity, attention cannot be due to a bottom-up saliency effect. In the same light, investigations of adaptation indicate that repeated whisker stimulation decreases vS1 activity, but produces a net effect of increased total information (Adibi et al., 2013a, 2013b). However, our results indicate that, in whisker sessions, where whisker stimuli were more frequent, neuronal signals in vS1 were amplified, arguing against adaptation as a detection enhancement mechanism. More likely is a top-down process of expectation, which in humans involves intraparietal cortex and superior frontal cortex and might involve analogous regions in rat cortex. The vibrissal motor cortex, also known as the premotor cortex, is a candidate area (Leonard, 1969). This area is considered analogous to the primate frontal eye fields (FEF) that are critical in the voluntary control of visual attention. Similar to FEF, the premotor area in rat has strong reciprocal projections to prefrontal cortex (Condé et al., 1995) and direct brainstem projections to areas involving orienting response (Stuesse and Newman, 1990). Unilateral lesions in this area produce contralateral neglect in both primates and rats (Erlich et al., 2011). A recent study found that prefrontal cortex exerts its top-down modulation through a circuit involving the thalamic reticular nucleus (TRN) rather than directly on sensory cortex (Wimmer et al., 2015). The dynamic control of sensory gain through feedforward inhibition from the TRN could underlie the observed gain modulations of cortical activity in our study.
Recently, a number of studies have used rodents to investigate aspects of visual (Carli et al., 1983; Marote and Xavier, 2011; Wang et al., 2014; Wimmer et al., 2015) and auditory (Jaramillo and Zador, 2011; Rodgers and DeWeese, 2014) attention. Our results provide evidence for sensory selection in rodents and a potential neuronal correlate in the primary somatosensory cortex.
Footnotes
This work was supported by the Australian Research Council (ARC Discovery Project DP130101364, Future Fellowship to E.A., and the ARC Centre of Excellence for Integrative Brain Function CE140100007), the Human Frontier Science Program (http://www.hfsp.org; Project RG0015/2013), the European Research Council (Advanced Grant CONCEPT (http://erc.europa.eu; Project 294498), the European Commission Seventh Framework Programme (Project CORONET; http://cordis.europa.eu/fp7/ict/home_en.html; Project 269459), and Italian Ministero dell'Istruzione dell'Universita e della Ricerca (MIUR Grant HANDBOT; http://hubmiur.pubblica.istruzione.it/web/ricerca/home; Project GA 280778).
References
- Adibi M, Arabzadeh E. A comparison of neuronal and behavioral detection and discrimination performances in rat whisker system. J Neurophysiol. 2011;105:356–365. doi: 10.1152/jn.00794.2010. [DOI] [PubMed] [Google Scholar]
- Adibi M, Diamond ME, Arabzadeh E. Behavioral study of whisker-mediated vibration sensation in rats. Proc Natl Acad Sci USA. 2012;109:971–976. doi: 10.1073/pnas.1116726109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi M, Clifford CW, Arabzadeh E. Informational basis of sensory adaptation: entropy and single-spike efficiency in rat barrel cortex. J Neurosci. 2013a;33:14921–14926. doi: 10.1523/JNEUROSCI.1313-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi M, McDonald JS, Clifford CW, Arabzadeh E. Adaptation improves neural coding efficiency despite increasing correlations in variability. J Neurosci. 2013b;33:2108–2120. doi: 10.1523/JNEUROSCI.3449-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabzadeh E, Petersen RS, Diamond ME. Encoding of whisker vibration by rat barrel cortex neurons: implications for texture discrimination. J Neurosci. 2003;23:9146–9154. doi: 10.1523/JNEUROSCI.23-27-09146.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabzadeh E, Panzeri S, Diamond ME. Whisker vibration information carried by rat barrel cortex neurons. J Neurosci. 2004;24:6011–6020. doi: 10.1523/JNEUROSCI.1389-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian K, Szwed M, Knutsen PM, Deutsch D, Derdikman D, Pietr M, Simony E, Ahissar E. Pre-neuronal morphological processing of object location by individual whiskers. Nat Neusci. 2013;16:622–631. doi: 10.1038/nn.3378. [DOI] [PubMed] [Google Scholar]
- Berg RW, Kleinfeld D. Rhythmic whisking by rat: retraction as well as protraction of the vibrissae is under active muscular control. J Neurophysiol. 2003;89:104–117. doi: 10.1152/jn.00600.2002. [DOI] [PubMed] [Google Scholar]
- Bisley JW. The neural basis of visual attention. J Physiol. 2011;589:49–57. doi: 10.1113/jphysiol.2010.192666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Brecht M. Barrel cortex and whisker-mediated behaviors. Curr Opin Neurobiol. 2007;17:408–416. doi: 10.1016/j.conb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Fries P, Landman R, Liang H, Desimone R. A backward progression of attentional effects in the ventral stream. Proc Natl Acad Sci USA. 2010;107:361–365. doi: 10.1073/pnas.0907658106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Carrasco M. Visual attention: the past 25 years. Vis Res. 2011;51:1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Seidemann E. Attentional modulations related to spatial gating but not to allocation of limited resources in primate V1. Neuron. 2012;74:557–566. doi: 10.1016/j.neuron.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009;12:1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condé F, Maire-Lepoivre E, Audinat E, Crépel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- de Lafuente V, Romo R. Neuronal correlates of subjective sensory experience. Nat Neurosci. 2005;8:1698–1703. doi: 10.1038/nn1587. [DOI] [PubMed] [Google Scholar]
- de Lafuente V, Romo R. Neural correlate of subjective sensory experience gradually builds up across cortical areas. Proc Natl Acad Sci USA. 2006;103:14266–14271. doi: 10.1073/pnas.0605826103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, Arabzadeh E. Whisker sensory system: from receptor to decision. Prog Neurobiol. 2013;103:28–40. doi: 10.1016/j.pneurobio.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Diamond ME, von Heimendahl M, Knutsen PM, Kleinfeld D, Ahissar E. “Where” and “what” in the whisker sensorimotor system. Nat Rev Neurosci. 2008;9:601–612. doi: 10.1038/nrn2411. [DOI] [PubMed] [Google Scholar]
- Eriksen CW, Hoffman JE. Temporal and spatial characteristics of selective encoding from visual displays. Percept Psychophys. 1972;12:201–204. doi: 10.3758/BF03212870. [DOI] [Google Scholar]
- Erlich JC, Bialek M, Brody CD. A cortical substrate for memory-guided orienting in the rat. Neuron. 2011;72:330–343. doi: 10.1016/j.neuron.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassihi A, Akrami A, Esmaeili V, Diamond ME. Tactile perception and working memory in rats and humans. Proc Natl Acad Sci USA. 2014;111:2331–2336. doi: 10.1073/pnas.1315171111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D, Swets J. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Guo ZV, Hires SA, Li N, O'Connor DH, Komiyama T, Ophir E, Huber D, Bonardi C, Morandell K, Gutnisky D, Peron S, Xu NL, Cox J, Svoboda K. Procedures for behavioral experiments in head-fixed mice. PLoS One. 2014;9:e88678. doi: 10.1371/journal.pone.0088678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Petersen RS, Diamond ME. Distribution of tactile learning and its neural basis. Proc Natl Acad Sci USA. 1999;96:7587–7591. doi: 10.1073/pnas.96.13.7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JM, Macquistan AD. The spatial distribution of attention following an exogenous cue. Percept Psychophys. 1993;53:221–230. doi: 10.3758/BF03211732. [DOI] [PubMed] [Google Scholar]
- Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann K, Montaser-Kouhsari L, Carrasco M, Heeger DJ. When size matters: attention affects performance by contrast or response gain. Nat Neurosci. 2010;13:1554–1559. doi: 10.1038/nn.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo S, Zador AM. The auditory cortex mediates the perceptual effects of acoustic temporal expectation. Nat Neurosci. 2011;14:246–251. doi: 10.1038/nn.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C, Petkov CI, Lippert M, Logothetis NK. Mechanisms for allocating auditory attention: An auditory saliency map. Curr Biol. 2005;15:1943–1947. doi: 10.1016/j.cub.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Kuruppath P, Gugig E, Azouz R. Microvibrissae-based texture discrimination. J Neurosci. 2014;34:5115–5120. doi: 10.1523/JNEUROSCI.4217-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CM. The prefrontal cortex of the rat. I. Cortical projection of the mediodorsal nucleus. II. Efferent connections. Brain Res. 1969;12:321–343. doi: 10.1016/0006-8993(69)90003-1. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Marote CF, Xavier GF. Endogenous-like orienting of visual attention in rats. Anim Cogn. 2011;14:535–544. doi: 10.1007/s10071-011-0388-3. [DOI] [PubMed] [Google Scholar]
- Matyas F, Sreenivasan V, Marbach F, Wacongne C, Barsy B, Mateo C, Aronoff R, Petersen CC. Motor control by sensory cortex. Science. 2010;330:1240–1243. doi: 10.1126/science.1195797. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Attention to both space and feature modulates neuronal responses in macaque area V4. J Neurophysiol. 2000;83:1751–1755. doi: 10.1152/jn.2000.83.3.1751. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456:391–394. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JS, Adibi M, Clifford CW, Arabzadeh E. Sampling time and performance in rat whisker sensory system. PLoS One. 2014;9:e116357. doi: 10.1371/journal.pone.0116357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SB, Whitmer D, Figueroa R, Williams BA, Kleinfeld D. Active spatial perception in the vibrissa scanning sensorimotor system. PLoS Biol. 2007;5:e15. doi: 10.1371/journal.pbio.0050015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Feldman DE. Behavioral detection of passive whisker stimuli requires somatosensory cortex. Cereb Cortex. 2013;23:1655–1662. doi: 10.1093/cercor/bhs155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Morita T, Kang H, Wolfe J, Jadhav SP, Feldman DE. Psychometric curve and behavioral strategies for whisker-based texture discrimination in rats. PLoS One. 2011;6:e20437. doi: 10.1371/journal.pone.0020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DH, Hires SA, Guo ZV, Li N, Yu J, Sun QQ, Huber D, Svoboda K. Neural coding during active somatosensation revealed using illusory touch. Nat Neurosci. 2013;16:958–965. doi: 10.1038/nn.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Bortone DS, Adesnik H, Scanziani M. Gain control by layer six in cortical circuits of vision. Nature. 2012;483:47–52. doi: 10.1038/nature10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 6. New York: Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. doi: 10.1163/156856897X00366. [DOI] [PubMed] [Google Scholar]
- Peron SP, Freeman J, Iyer V, Guo C, Svoboda K. A cellular resolution map of barrel cortex activity during tactile behavior. Neuron. 2015;86:783–799. doi: 10.1016/j.neuron.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Petersen RS, Diamond ME. Spatial–temporal distribution of whisker-evoked activity in rat somatosensory cortex and the coding of stimulus location. J Neurosci. 2000;20:6135–6143. doi: 10.1523/JNEUROSCI.20-16-06135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron. 2000;26:703–714. doi: 10.1016/S0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- Rodgers CC, DeWeese MR. Neural correlates of task switching in prefrontal cortex and primary auditory cortex in a novel stimulus selection task for rodents. Neuron. 2014;82:1157–1170. doi: 10.1016/j.neuron.2014.04.031. [DOI] [PubMed] [Google Scholar]
- Sachidhanandam S, Sreenivasan V, Kyriakatos A, Kremer Y, Petersen CC. Membrane potential correlates of sensory perception in mouse barrel cortex. Nat Neurosci. 2013;16:1671–1677. doi: 10.1038/nn.3532. [DOI] [PubMed] [Google Scholar]
- Stuesse SL, Newman DB. Projections from the medial agranular cortex to brain stem visuomotor centers in rats. Exp Brain Res. 1990;80:532–544. doi: 10.1007/BF00227994. [DOI] [PubMed] [Google Scholar]
- Stüttgen MC, Schwarz C. Integration of vibrotactile signals for whisker-related perception in rats is governed by short time constants: comparison of neurometric and psychometric detection performance. J Neurosci. 2010;30:2060–2069. doi: 10.1523/JNEUROSCI.3943-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe S, Fize D, Marlot C. Speed of processing in the human visual system. Nature. 1996;381:520–522. doi: 10.1038/381520a0. [DOI] [PubMed] [Google Scholar]
- von Heimendahl M, Itskov PM, Arabzadeh E, Diamond ME. Neuronal activity in rat barrel cortex underlying texture discrimination. PLoS Biol. 2007;5:e305. doi: 10.1371/journal.pbio.0050305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Chen C, Zhang D, Yao H. Cumulative latency advance underlies fast visual processing in desynchronized brain state. Proc Natl Acad Sci USA. 2014;111:515–520. doi: 10.1073/pnas.1316166111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of SM1 cerebral neocortex in albino rat. Brain Res. 1971;26:259–275. [PubMed] [Google Scholar]
- Wimmer RD, Schmitt LI, Davidson TJ, Nakajima M, Deisseroth K, Halassa MM. Thalamic control of sensory selection in divided attention. Nature. 2015;526:705–709. doi: 10.1038/nature15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (S I) of mouse cerebral cortex: the description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-X. [DOI] [PubMed] [Google Scholar]
- Zhang S, Xu M, Kamigaki T, Phong Hoang Do JP, Chang WC, Jenvay S, Miyamichi K, Luo L, Dan Y. Selective attention: long-range and local circuits for top-down modulation of visual cortex processing. Science. 2014;345:660–665. doi: 10.1126/science.1254126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Safaai H, Notaro G, Mazzoni A, Panzeri S, Diamond ME. Report complementary contributions of spike timing and spike rate to perceptual decisions in rat S1 and S2 cortex complementary contributions of spike timing and spike rate to perceptual decisions in rat S1 and S2 cortex. Curr Biol. 2015;25:357–363. doi: 10.1016/j.cub.2014.11.065. [DOI] [PubMed] [Google Scholar]