Abstract
Background:
Depression is prevalent among persons living with HIV (PLWH). This study investigated the relationships between depressive symptomatology, health-related quality of life (HRQoL), and positive psychological factors in PLWH and age-matched HIV− individuals.
Methods:
One hundred twenty-two PLWH and 94 HIV− individuals, recruited in three age cohorts (36-45, 46-55, 56-65 years old), completed self-report questionnaires on depressive symptoms (CES-D), HRQoL, and positive psychological factors (resilience, grit, and self-rated successful aging [SRSA]). Participants were classified based on HIV status (H+ vs H−) and elevated depressive symptoms (D+ vs D−) into four groups (H+/D+; H−/D+; H+/D−; H−/D−).
Results:
Fifty-eight percent of PLWH had elevated depressive scores, compared to 33% of HIV− individuals (p < 0.001). The proportion of individuals reporting elevated depressive symptoms only differed among individuals 36-45 years old (H+: 61.5%; H−: 17.9%; p < 0.001). Individuals in the H+/D+ group reported the lowest HRQoL, resilience, grit, and SRSA across age cohorts. However, there were no differences on HRQoL or positive psychological factors between H+/D− and H−/D− groups; in fact, individuals 56-65 years in the H+/D− group endorsed aging the most successfully.
Limitations:
Small sample size within the groups and the cross-sectional nature of the analysis limit the ability to address onset of depressive symptoms in relation to HRQoL or positive psychological factors.
Conclusions:
Among PLWH depressive symptoms show a strong association with HRQoL and positive psychological factors compared to HIV− individuals. In the absence of elevated depressive symptoms, however, PLWH report similar HRQoL and positive psychological factors to HIV− individuals.
Introduction
Due to the success of antiretroviral therapy (ART) and an increase in the incidence of HIV infection among older adults, the proportion of older persons living with HIV (PLWH) in the United States is rapidly growing (Centers for Disease Control and Prevention, 2016; High et al., 2012; United States Special Committee on Aging, 2013). Therefore, it is important to evaluate physical and emotional health among the changing demographics of PLWH. One of the most prevalent psychiatric conditions among PLWH is major depressive disorder (MDD), with PLWH at a two- to seven-fold greater risk for depressive disorders compared to the general population (Satz et al., 1997). PLWH have a higher prevalence of both MDD and subsyndromal depression symptomatology than HIV− individuals of the same age or the general population (Hinkin, Castellon, Atkinson, & Goodkin, 2001; Milanini et al., 2017). A multi-site cohort study of over 1,500 PLWH found lifetime depressive symptom rates of 63% and across multiple studies diagnosis of lifetime MDD ranges from 22-54% in PLWH, compared to 4.9-17.1% lifetime MDD diagnosis in the general U.S. population (Badiee et al., 2012; Kamat et al., 2015; Rabkin, 2008; Rabkin, McElhiney, & Ferrando, 2004; Vance, Mugavero, Willig, Raper, & Saag, 2011).
These high rates of depression among PLWH represent a major public health concern, as depression has been linked to worse psychological and medical outcomes in PLWH, including lower reported quality of life, increased viral load, and a higher likelihood of mortality (Evans et al., 2002; Leserman, 2008; Millar, Starks, Gurung, & Parsons, 2017). Untreated depression in PLWH has also been related to increased cognitive complaints and worse reported daily functioning compared to PLWH without depression (Coleman, 2017; Leserman, 2008). These medical and psychological factors may be exacerbated in older PLWH who are often burdened to a higher degree with HIV-related medical and psychological factors, in conjunction with aging related problems (Grov, Golub, Parsons, Brennan, & Karpiak, 2010). Despite the high prevalence rates of depressive disorders among PLWH, depression is often under diagnosed and inadequately treated within this population, though (R. C. Moore, Marquine, et al., 2017; Zanjani, Saboe, & Oslin, 2007).
Given the prevalence of depression among PLWH, it is vital to evaluate other co-occurring factors that may be associated with elevated depressive symptoms. Multiple studies have found an association between higher depressive symptoms and worse quality of life (QOL), even after controlling for demographic factors (i.e., age and education) (Amini Lari, Faramarzi, Shams, Marzban, & Joulaei, 2013; Arseniou, Arvaniti, & Samakouri, 2014; Millar et al., 2017). PLWH with elevated depressive symptoms report lower mental and physical health-related quality of life (HRQoL), supporting the idea that depression affects multiple aspects of quality of life (Coleman, 2017; Elliott, Russo, & Roy-Byrne, 2002). However, there is a dearth of research regarding the association between depression and positive psychological factors, e.g. resilience, grit, and self-rated successful aging (SRSA) among PLWH. Two studies have found an association between higher resilience and lower depressive symptoms among PLWH (Dale et al., 2015; McGowan et al., 2018). Similarly, in PLWH greater grit (i.e., the perseverance and passion for long term goals; (Duckworth, Peterson, Matthews, & Kelly, 2007)) has been negatively associated with major depression (D. J. Moore et al., 2018; R. C. Moore et al., 2018). In older adult persons without HIV, lower levels of depressive symptoms have been associated with increased self-rated successful aging (Jeste et al., 2013); however, few studies have been conducted to evaluate positive psychological factors and quality of life in relation to depressive symptomatology in PLWH compared to control participants.
Given there is an increase in the population of older PLWH and that depression is a highly comorbid condition among PLWH, assessing the relationship between depressive symptoms and other psychological factors across different age decades may provide insights for clinical interventions. Therefore, we hypothesized that: 1) PLWH aged 56-65 would have the highest proportion of elevated depressive symptoms compared to HIV− participants; and 2) elevated depressive symptoms would be associated with lower ratings of HRQoL and positive psychological factors across groups, with strongest associations in the oldest PLWH.
Methods
Participants
One hundred twenty-two PLWH and 94 HIV− individuals from the Multi-Dimensional Successful Aging Among HIV-Infected Adults study conducted at the University of California, San Diego (UCSD) HIV Neurobehavioral Research Program and the UCSD Stein Institute for Research on Aging participated in this study (R. C. Moore et al., 2018; R. C. Moore, Paolillo, et al., 2017). The study was approved by the UCSD Institutional Review Board, and all participants provided written informed consent after the study was explained to them by a trained staff member. In order to enroll a representative cohort of participants, minimal exclusion criteria were applied and included: 1) neurologic condition other than HIV known to impact cognitive functioning (e.g., Alzheimer’s disease), 2) psychotic disorders (e.g., schizophrenia), and 3) positive urine toxicology on the day of testing for illicit substances other than cannabis. Inclusion criteria were: 1) aged 36-65 years, 2) fluent in English, and 3) ability to provide informed consent.
Multi-Cohort Longitudinal Design
Participants were recruited to fill three age cohorts (36-45, 46-55, 56-65 years) with balanced recruitment providing approximately 40 PLWH and 30 HIV− participants per decade in a longitudinal study. The resulting participant cohorts: 1) 36-45 years old: 39 PLWH and 28 HIV−; 2) 46-55 years old: 43 PLWH and 34 HIV−; 3) 56-65 years old: 40 PLWH and 32 HIV−. Baseline data were used in the present analyses.
Measures
Demographic and Clinical Variables
HIV Disease Characteristics
All participants had confirmation testing for HIV status at the time of the visit (Abbott RealTime HIV-1 Test; HIV-1 and HIV-2 Antibody Antigen Evaluation). Lor PLWH CD4+ count and plasma HIV viral load were determined from blood specimens taken at the time of the study visit. Plasma HIV viral loads were deemed “undetectable” at 50 copies/mL or less. Nadir CD4+ count was self-reported unless the study lab value was determined to be lower than the self-report. AIDS diagnosis was made using the CDC classification of 3 or C (Centers for Disease Control and Prevention, 1993). Estimated duration of HIV infection and antiretroviral medication (ART) use were self-reported by the participant at the time of the visit.
Lifetime Substance Use Diagnoses and Major Depressive Disorder
Lifetime diagnosis of substance dependence (i.e., alcohol, cannabis, or any other substance) and history of Major Depressive Disorder (MDD) were assessed via the Composite International Diagnostic Interview (CIDI), v2.1 (Wittchen, 1994; World Health Organization, 1990). The CIDI is a lay-administered diagnostic tool, which follows DSM-IV diagnostic criteria and has exceptional interrater reliability across studies (Wittchen, 1994). Additionally, prior studies have demonstrated the utility of the CIDI to diagnose MDD and substance use disorders in PLWH (Badiee et al., 2012; D. J. Moore et al., 2012).
Variables of Interest
Current Depressive Symptoms
Depressive symptoms were measured using the Center for Epidemiological Studies-Depression Questionnaire (CES-D 20 item, (Randolff, 1977)). The CES-D is a 20-item measure that asks individuals to report how often over the past week they have experienced symptoms associated with depression (e.g., restless sleep, or feeling lonely). Response options range from 0 = Rarely or None of the Time to 3 = Much or Almost All of the Time for each item. Total scores range from 0 to 60, with higher scores indicating greater depressive symptoms (Randolff, 1977). Elevated depressive symptoms were defined as a score of 16 or greater given that this cutoff score has shown good sensitivity and specificity in identifying individuals at risk for clinical depression and to accurately identify depressive symptoms among PLWH (Braitstein et al., 2005; Cockram, Judd, Mijch, & Norman, 1999; Coleman, 2017; Cook et al., 2006; Dale et al., 2015; Lewinsohn, Seeley, Roberts, & Allen, 1997).
Health-Related Quality of Life (HRQoL)
Participants completed the Medical Outcome Study Short-Form Health Survey (MOS SF-36), which has high levels of reliability and validity in multiple patient populations, including in PLWH (Franchi & Wenzel, 1998; Ware & Sherbourne, 1992; Wu et al., 1991). The MOS SF-36 is a measure of health-related quality of life (HRQoL) that assesses eight health concepts comprised of multi item sub-scales. Scores are combined to create two aggregate measures, the physical (PCS-36) and mental health (MCS-36) summary scores (Preau et al., 2004; Ware et al., 1995). Scores range from 0-100, with higher scores indicating better health-related quality of life.
Positive Psychological Factors
Individuals also completed questionnaires relating to positive psychological factors. On a one-item measure of Self-Rated Successful Aging (SRSA), participants were asked to rate to what extent they thought they had aged successfully, on a 10-point Likert-scale, ranging from 1 (least successful) to 10 (most successful) (Montross et al., 2006). Subjects were instructed to use their own conceptualization of successful aging when responding to the questionnaire. The SRSA has been used in large cohort studies as a measure of subjective overall aging and functioning (Jeste et al., 2013).
Additionally, individuals filled out the Grit Scale, which is a 17-item questionnaire that measures perseverance and successful adaptation to adversity. Participants rate questions (e.g., I am diligent, Setbacks don’t discourage me) on a 5-point Likert-type scale ranging from 1 = very much like me to 5 = not like me at all (Duckworth et al., 2007). The 12-item overall grit average was utilized (Duckworth & Quinn, 2009), with higher scores indicating greater self-reported grittiness, has been previously published on in PLWH (R. C. Moore et al., 2018). Finally, participants completed the 10-item Connor-Davidson Resilience Scale (CD-RISC10), which evaluates positive adaption to adversity. Individuals rate items (e.g., I am not easily discouraged by failure) from 0 = not true at all to 4 = true nearly all of the time (Campbell-Sills & Stein, 2007) with higher scores indicating more resilience. The CD-RISC10 has been validated in multiple populations, including older adults and in PLWH (Dale et al., 2015; Lamond et al., 2008; Montross et al., 2006).
Statistical Analysis
All statistical analyses were performed using JMP Pro (JMP®, Version 12.0.1 (SAS Institute Inc., 1989-2007). To analyze the relationships between HIV serostatus, depressive symptoms, and additional questionnaires, individuals were categorized into four groups based on HIV status and meeting criteria for elevated CES-D scores: 1) PLWH/elevated depressive symptoms (H+/D+), 2) PLWH/no elevated depressive symptoms (H+/D−), 3) HIV-/elevated depressive symptoms (H−/D+), 4) HIV− /no elevated depressive symptoms (H−/D−). To explore how the above relationships differ by age, the four groups were also compared within each age cohort (i.e., 36-45, 46-55, and 56-65 years). To evaluate demographic factors, Welch’s t-tests assuming unequal variances were used for continuous dependent variables, while Pearson Chi-square or Fisher’s Exact Test when appropriate were used for categorical variables. Welch’s analysis of variance (ANOVA) was used to assess group differences for continuous outcome variables (e.g., HRQoL measures). Welch’s t-tests assuming unequal variances were used for post-hoc comparisons. Bonferroni adjustments for multiple comparisons were applied to each pairwise t-test of the groups for post-hoc comparisons (alpha=0.008).
Results
Demographic and Clinical Characteristics
Demographic and clinical characteristics are provided in Table 1. The H−/D− group had more years of formal education in comparison to the other three groups, while the H+/D+ group had the greatest proportion of individuals with a lifetime diagnosis of MDD and lifetime cannabis dependence. In regards to HIV disease characteristics, only nadir CD4+ was significantly different between the two groups of PLWH, with the H+/D+ group having higher nadir CD4+ counts than the H+/D− group (see Table 1).
Table 1.
Demographic, Clinical Characteristics and Questionnaires among PLWH and HIV− by Depressive Symptom Status
| A H−/D+ (n=31) |
B H−/D− (n=63) |
C H+/D+ (n=71) |
D H+/D− (n=51) |
p- value |
Test Statistic |
Comparison | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age (yrs), Mean (SD) | 53.1 (6.6) | 50.2 (8.0) | 50.1 (8.5) | 51.6 (8.2) | 0.214 | F = 1.5, df = 3, 102.8 | |
| 36-45 (yrs), % (n) | 16.1% (5) | 36.5% (23) | 33.8% (24) | 29.4% (15) | |||
| 46-55 (yrs), % (n) | 51.6% (16) | 28.6% (18) | 38.0% (27) | 31.4% (16) | |||
| 56-65 (yrs), % (n) | 32.3% (10) | 34.9% (22) | 28.2% (20) | 39.2% (20) | |||
| Education (yrs), Mean (SD) | 14.4 (2.3) | 15.4 (2.2) | 14.0 (2.6) | 14.0 (2.2) | 0.003 | F = 4.9, df = 3, 99.7 | B > All |
| Sex, % Male, (n) | 71.0% (22) | 69.8% (44) | 80.3% (57) | 88.2% (45) | 0.085 | Chi2 = 6.6 | |
| Race/Ethnicity, % White, (n) (vs. Non-white) | 74.2% (23) | 66.7% (42) | 56.3% (40) | 69.2% (65) | 0.117 | Chi2 = 5.9 | |
| % Black, (n) | 9.7% (3) | 15.9% (10) | 19.7% (14) | 13.8% (13) | -- | -- | |
| % Hispanic, (n) | 9.7% (3) | 17.5% (11) | 14.1% (10) | 14.9% (14) | -- | -- | |
| % Other, (n) | 6.5% (2) | 0.0% (0) | 10.0% (7) | 2.1% (2) | -- | -- | |
| HIV Disease Characteristics | |||||||
| Est. Duration of Infection (yrs), mean (SD) | -- | -- | 16.9 (8.8) | 17.6 (8.7) | 0.495 | t = 0.5 | |
| Current CD4a, Median [IQR] | -- | -- | 669.5 [495.0, 858.8] | 564 [351.8, 821.0] | 0.216 | t = −1.3 | |
| Nadir CD4b, Median [IQR] | -- | -- | 200 [60, 411] | 105 [19.8, 255.0] | 0.004 | t = −2.9 | |
| Det. Plasma VLc, % with (n) | -- | -- | 11.4% (8) | 2.1% (1) | 0.081 | FET | |
| AIDS Status, % with (n) | -- | -- | 54.9% (39) | 68.6% (35) | 0.127 | Chi2 = 2.3 | |
| On ARTc, % on (n) | -- | -- | 94.3% (66) | 98.0% (49) | 0.316 | Chi2 = 1.0 | |
| Mood/Substanced, % with (n) | |||||||
| LT MDD dx | 29.0% (9) | 16.4% (10) | 51.8% (42) | 42.0% (21) | <0.001 | Chi2 = 29.4 | C > All; D > B |
| LT Alcohol dependence dx | 22.6% (7) | 3.3% (2) | 31.4% (22) | 30.0% (15) | <0.001 | Chi2 = 18.1 | B < All |
| LT Cannabis dependence dx | 0.0% (0) | 1.6% (1) | 20.0% (14) | 4.0% (2) | <0.001 | Chi2 = 20.8 | C > All |
| LT Any Other dependence dx | 19.4% (6) | 4.9% (3) | 50.0% (35) | 40.0% (20) | <0.001 | Chi2 = 35.5 | B < C, D |
| Questionnaires, Mean (SD) | |||||||
| Health-Related Quality of Life (HRQoL) | |||||||
| MOS PCS-36d | 76.4 (21.8) | 87.9 (15.5) | 56.3 (27.2) | 80.3 (17.4) | <0.001 | F = 22.9, df =3, 90.1 | C < All |
| MOS MCS-36e | 75.0 (15.9) | 85.6 (11.6) | 53.5 (24.8) | 84.7 (12.1) | <0.001 | F = 34.2, df =3, 94.4 | C < All; A < B, D |
| Positive Psychological Factors | |||||||
| Self-Rated Successful Agingf | 7.6 (0.9) | 7.8 (1.8) | 6.4 (2.3) | 8.4 (1.4) | <0.001 | F = 12.3, df =3, 111.2 | C < All; A < D |
| Resiliencef | 30.8 (5.5) | 33.6 (6.5) | 27.2 (8.3) | 33.0 (5.3) | <0.001 | F = 10.1, df = 3, 101.4 | C < B, D |
| Gritf | 3.7 (0.4) | 4.0 (0.4) | 3.3 (0.6) | 3.9 (0.5) | <0.001 | F = 20.4, df = 3, 98.5 | C < All; A < B |
Note: Welch’s ANOVA run for variables reporting means (SD); Welch’s t-test run for continuous post-hoc tests; Chi2 = Pearson Chi2 Test; FET = Fisher’s Exact; For D+ groups, cutoff scores ≥16 for high depressive symptoms on the Center for Epidemiological Studies-Depression Questionnaire; MDD = Major Depressive Disorder; LT = Lifetime; Det. Plasma VL = Detectable Plasma Viral Load; MOS PCS 36 = Medical Outcome Study Short-Form Health Survey, PCS = Physical Health Summary Score; MCS = Mental Health Summary Score; high values = better scores for all questionnaires; significant post-hoc comparisons p < 0.008
n = 116;
n = 121;
n = 118;
n = 212;
n = 210;
n = 214.
Elevated Depressive Symptomology by HIV Status and Age
A significantly higher proportion of PLWH reported CES-D scores that met or exceeded the cut-off for risk of clinical depression (58%) compared to HIV− participants (33%) (see Table 2). This finding was driven by individuals in the youngest cohort aged 36-45, in which 61.5% of PLWH reported elevated depressive symptoms group compared to only 17.9% of the HIV− group (Fisher’s Exact, p) < 0.001). In contrast, there were no differences in proportions of individuals reporting elevated depressive symptoms within the 46-55 year-old and 56-65 year-old age cohorts (ps > 0.05).
Table 2.
Depressive Symptoms by HIV Status
| CES-D Questionnaire | PLWH (n=122) |
HIV− (n=94) |
p-value | Test Statistic |
|---|---|---|---|---|
| Continuous Score, Mean (SD)a | 19.3 (8.3) | 14.9 (4.8) | <0.001 | t = 4.9, df = 199.3 |
| Elevated Score (D+ groups), % (n) | 58.2% (71) | 33.0% (31) | <0.001 | Chi2 = 13.6 |
| Age 36-45 yrs | 61.5% (24) | 17.9% (5) | <0.001 | FET |
| Age 46-55 yrs | 62.8% (27) | 47.1% (16) | 0.167 | Chi2 = 1.9 |
| Age 56-65 yrs | 50.0% (20) | 31.3% (10) | 0.109 | Chi2 = 2.6 |
Note: Welch’s t-test run for variable reporting means (SD); Chi2 = Pearson Chi2 Test; FET = Fisher’s Exact; CES-D = Center for Epidemiological Studies-Depression Questionnaire;
Higher = more depressive symptoms.
Health-Related Quality of Life
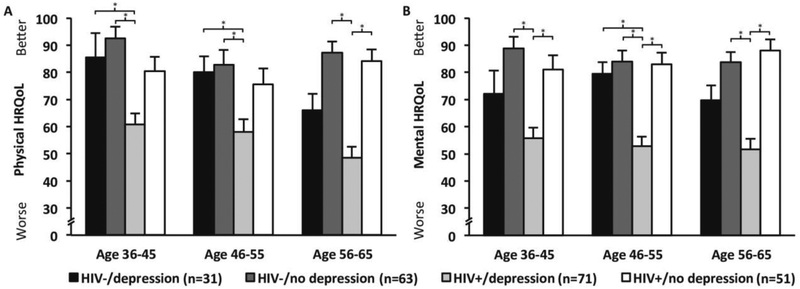
There were significant differences in MOS SF-36 Physical Health Summary (PCS-36) scores between the four groups (see Table 1). Overall, individuals in the H+/D+ group reported the lowest PCS-36 scores compared to the other three groups. Within each of the age cohorts, the H+/D+ group continued to have the lowest PCS-36 (Welch’s Anova, F = 9.54, df = 3, 16.76, youngest age cohort, p < 0.001; middle cohort, F = 3.99, df = 3, 38.72, p = 0.014; oldest cohort, F = 17.54, df = 3, 28.25, p < 0.001) (see Figure 1A). Post-hoc analyses indicated that, within the youngest age cohort, the H+/D+ group had markedly lower PCS-36 scores than both the HIV− groups (H−/D− [t = −5.09, df = 26.45 p < 0.001], and H−/D+ group [t = −3.32, df = 20.53, p = 0.003]). Similarly, in the middle age cohort, the H+/D+ group reported significantly worse PCS-36 scores than both the HIV− groups (H−/D− [t = −3.26, df = 41.95, p = 0.002] and H−/D+ [t = −3.01, df = 40.00, p = 0.005]). Within the oldest age cohort, however, the H+/D+ group had lower PCS-36 scores than the non-depressive symptom groups (H+/D− [t = 6.58, df= 32.31, p < 0.001], and H−/D− [t = −6.74, df= 35.75, p < 0.001]).
Fig. 1.
Note: For depression groups, cutoff scores ≥16 for high depressive symptoms on the Center for Epidemiological Studies-Depression Questionnaire; significant post-hoc comparisons shown by the bars, p < 0.008.
A similar pattern existed for mental health, such that the H+/D+ group displayed the lowest MCS-36 scores compared to the other three groups; however, the H−/D+ group also had lower scores than the non-depressive symptom groups (see Table 1). When broken down by age cohort there remained differences among each of the four groups (Welch’s Anova, F = 11.34, df = 3, 14.62, youngest age cohort, p < 0.001; middle cohort, F = 11.54, df = 3, 37.62, p < 0.001; oldest cohort, F = 13.80, df = 3, 28.29, p < 0.001) (see Figure 1B). Post-hoc analyses indicated that within the youngest cohort, the H+/D+ group had significantly worse MCS-36 scores than either of the non-depressive groups (H−/D− [t = −5.76, df = 26.06 p < 0.001] and H+/D− [t = 3.81, df = 35.44, p = 0.001]). In the middle cohort, the H+/D+ individuals reported significantly worse mental health than the other three groups (H+/D− [t = 5.39, df= 39.91, p < 0.001]; H−/D+ [t = −5.20, df = 34.21, p < 0.001]; and H−/D− [t = −5.42, df = 40.68, p < 0.001]). Finally, within the oldest cohort, there were significant differences in the MCS-36 score between the H+/D+ group and both non-depressive symptom groups (H+/D− [t = 6.23, df = 24.24, p < 0.001] and H−/D− [t = −5.22, df = 28.33, p < 0.001]). On both the PCS-36 and MCS-36 scores there were no differences between the two non-depressed groups (H+/D− and H−/D−) in any of the three age cohorts (ps > 0.05).
Positive Psychological Factors
Similar to HRQoL (PCS-36 and MCS-36), the H+/D+ group had the lowest scores on all positive psychological questionnaires (see Table 1). Examining resilience, there were significant differences in total resilience score between the four H/D groups within the youngest and oldest age cohorts such that the H+/D+ group reported the lowest resilience (Welch’s Anova, youngest cohort, F = 5.24, df = 3, 14.87, p = 0.011; oldest cohort, F = 4.02, df = 3, 30.89, p = 0.016).
There were no differences in resilience within the middle age cohort (see Figure 2A). There were also significant group differences on the grit scale within all three age cohorts, with the H+/D+ group reporting the least amount of grit at each age (Welch’s Anova, youngest cohort, F = 6.23, df = 3, 16.32, p = 0.005; middle, F = 8.48, df = 3, 38.61, p < 0.001; oldest cohort, F = 8.55, df = 3, 31.59, p < 0.001) (see Figure 2B). Notably, there were no differences in grit score between the H+/D− group and the two HIV− groups within any of the age cohorts.
Fig. 2.
Note: For depression groups, cutoff scores ≥16 for high depressive symptoms on the Center for Epidemiological Studies-Depression Questionnaire; SRSA = Self-Rated Successful Aging; significant post-hoc comparisons shown by the bars, p < 0.008.
Similarly, the H+/D+ group reported aging less successfully than the other three groups (see Table 1). Within the youngest and middle-age cohorts, there was no difference in SRSA score between the four groups. Notably, in the oldest age cohort, the H+/D− group reported the highest successful aging scores (Welch’s Anova, F = 9.78, df = 3, 34.09, p < 0.001, and significantly higher than the H+/D+ t = 5.10, df = 28.44, p < 0.001) (see Figure 2C).
Discussion
The present study provides unique findings on the interplay of depression, HRQoL, and positive psychological factors among middle-aged and older PLWH and HIV− individuals in a multi-cohort design structure. In our sample, PLWH were significantly more likely to report elevated depressive scores compared to HIV− individuals. This finding supports prior studies that have found PLWH endorse more depressive symptoms than HIV− individuals (Milanini et al., 2017; Zanjani et al., 2007). Contrary to our hypothesis, the youngest cohort (aged 36-45) seemed to drive this finding, with a significantly larger proportion of PLWH reporting elevated depressive symptoms compared to HIV-individuals within this age group. That is, proportion of elevated depressive symptoms did not differ by HIV status among the middle-aged and older age cohorts. This difference highlights the importance of age in relation to depressive symptoms. For example, rates of elevated depression among PLWH were similarly high in all age groups. In contrast, only the youngest HIV− group had relatively low rates of elevated depressive symptomology, with higher rates in older cohorts. This is consistent with research estimating high prevalence of subsyndromal depression among middle-aged to older adults, especially those with greater medical burden, disability, and lower social support (Meeks et al., 2011). Overall the H+/D+ group reported the lowest physical and mental HRQoL; however, the relationships between the four groups differed depending on age cohort. While depressive symptoms in PLWH consistently related to lower mental HRQoL across ages, elevated depressive symptoms most prominently impacted physical HRQoL in the oldest H+/D+ group. These findings are consistent with prior studies that have reported a correlation between worse HRQoL and depression among PLWH (Amini Lari et al., 2013; Mekuria, Sprangers, Prins, Yalew, & Nieuwkerk, 2015). However, our novel findings highlight that the relationship between depression, age and HRQoL differs for mental components compared to physical components.
Importantly, there were no differences on HRQoL or positive psychological factors between the two non-elevated depressive symptom groups (H+/D− and H−/D−). Similar to prior research, the H+/D− group reported comparable grittiness, resilience, and successful aging to the H−/D− group, which indicates that in the absence of elevated depressive symptoms PLWH rate themselves as having favorable positive psychological factors (D. J. Moore et al., 2018). In the oldest age decade, the H+/D− group had the highest positive psychological factors, suggesting an important relationship between these positive psychological factors and being able to live a relatively long, non-depressed life as a person living with HIV. Hence, positive psychological factors may be protective for PLWH. Individuals’ subjective health ratings may provide valuable insight to their overall well-being, as previous studies have shown an association between reported worse health ratings and an increased risk of mortality (DeSalvo, Bloser, Reynolds, He, & Muntner, 2006). This finding may also reflect a potential “survivor effect” given that these older individuals have had HIV for longer and as long-term survivors, may view living with HIV more positively (e.g. aging more successfully) compared to prior expectations.
This study has strengths in its multi-cohort design methodology that allows us to examine the combined effects of HIV and depression on HRQoL across age cohorts; there are also some limitations, however. For example, we were not able to address questions regarding the onset of depressive symptoms in relation to HRQoL or the positive psychological factors. The cross-sectional nature of the current data analyses prevents any causal attributions. For instance, depression may lead to less resilience and grit or vice versa. Like prior studies (Hinkin et al., 2001; Milanini et al., 2017), we found a higher proportion of elevated depressive symptoms among PLWH, and individuals with elevated depressive symptoms reported lower HRQoL and positive psychological factors. There may be other factors related to depression and acquiring HIV (e.g., social stigma) not captured by our present variables that may account for the difference in depressive symptoms by HIV status. Another limitation is the small sample size per group, especially within the H−/D+ group. Furthermore, the sample, particularly the within the PLWH groups, was predominantly male and these results may not be generalizable to females. However, within the United Sates the majority of middle-aged to older PLWH are male; thus, our study cohort is similar to the broader characteristics of PLWH in the U.S. (Centers for Disease Control and Prevention, 2016).
Given the negative consequences of depression in PLWH, it is important to identify those in greatest need of treatment. Prior work has highlighted the usefulness of cognitive behavioral therapy for depression treatment among PLWH, even in those with advanced HIV disease (Wiles et al., 2013). Furthermore, meta-analytic work has shown psychotherapeutic interventions (e.g., cognitive behavioral therapy and cognitive behavioral stress management) reduce depressive symptoms in PLWH, which in turn may lead to improved psychiatric and medical outcomes (Sherr, Clucas, Harding, Sibley, & Catalan, 2011; Walkup, Wei, Sambamoorthi, & Crystal, 2008). With this said, older PLWH are less likely to be engaged in behavioral health treatment for depression than younger PLWH, highlighting the need to address underlying factors contributing to the lack of adequate mental health treatment among older PLWH (R. C. Moore, Marquine, et al., 2017; Zanjani et al., 2007). However, increasing or improving positive psychological factors may provide one potential avenue to mitigate depressive symptoms.
Conclusion
Overall, our findings suggest that PLWH aged 36-45 years may be especially vulnerable to elevated depression symptomatology as compared to age-matched persons without HIV. We also found that elevated depressive symptoms related to worse HRQoL and lower positive psychological factors, particularly among PLWH. Conversely, PLWH without elevated depressive symptoms reported comparable HRQoL and positive psychological factors to other HIV/depression status groups. In fact, older PLWH without elevated depressive symptoms had the highest self-rated successful aging compared to other groups. These results suggest that depressive symptoms may have a particularly negative impact on HRQoL and positive psychological factors among PLWH. The current work highlights the complexities of depression across the lifespan and the need for depression treatment to improve overall quality of life and well-being among PLWH.
Highlights.
Persons living with HIV (PLWH) are significantly more likely to report elevated depressive symptoms compared to persons without HIV.
PLWH with elevated depressive symptoms report lower health-related quality of life compared to PLWH without elevated depressive symptoms.
PLWH without elevated depressive symptoms report similar health-related quality of life and positive psychological factors compared to HIV− individuals without elevated depressive symptoms.
Older PLWH without depression (aged 56-65) reported aging most successfully compared to all other HIV/depression status groups.
Acknowledgments:
Funding/Support: This work was primarily supported by the National Institute of Mental Health (NIMH) grant R01 MH099987 (D.V. Jeste & D.J. Moore, MPI). Additional support was provided by the following National Institutes of Health (NIH) grants: P30 MH062512 (R.K. Heaton, PI) and K23 MH107260 (R.C. Moore, PI) and by the National Institutes of Health Ruth L. Kirschstein National Research Service Award (NRSA) T32 AA013525.
Appendix
The San Diego HIV Neurobehavioral Research Program group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Terry Jernigan, Ph.D., Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D., Ian Everall, FRCPsych., FRCPath., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Anya Umlauf, M.S., Christi Kao, M.S. The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
Footnotes
Ethical approval: This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Written informed consent was obtained from all individual participants included in the study after the nature of the study was explained to participants by a trained staff member.
Conflicts of Interest: Dr. R.C. Moore reports grants from Gilead Sciences, Inc., unrelated to the submitted work. Dr. Letendre reports grants from Gilead Sciences, personal fees from ViiV Healthcare, outside the submitted work. Ms. Rooney, Ms. Paolillo, Mr. Gouaux, Ms. Umlauf, Dr. Jeste, and Dr. D.J. Moore have nothing to disclose.
Declarations of Interest: Dr. R.C. Moore reports grants from Gilead Sciences, Inc., unrelated to the submitted work. Dr. Letendre reports grants from Gilead Sciences, personal fees from ViiV Healthcare, outside the submitted work. Ms. Rooney, Ms. Paolillo, Mr. Gouaux, Ms. Umlauf, Dr. Jeste, and Dr. D.J. Moore have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott RealTime HIV-1 Test. Abbott Laboratories; In. Illinois, USA. [Google Scholar]
- Amini Lari M, Faramarzi H, Shams M, Marzban M, & Joulaei H (2013). Sexual Dysfunction, Depression and Quality of Life in Patients With HIV Infection. Iran J Psychiatry Behav Sci, 7(1), 61–68. [PMC free article] [PubMed] [Google Scholar]
- Arseniou S, Arvaniti A, & Samakouri M (2014). HIV infection and depression. Psychiatry Clin Neurosci, 68(2), 96–109. doi: 10.1111/pcn.12097 [DOI] [PubMed] [Google Scholar]
- Badiee J, Moore DJ, Atkinson JH, Vaida F, Gerard M, Duarte NA, … Grant I (2012). Lifetime suicidal ideation and attempt are common among HIV+ individuals. J Affect Disord, 136(3), 993–999. doi: 10.1016/j.jad.2011.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitstein P, Montessori V, Chan K, Montaner JS, Schechter MT, O'Shaughnessy MV, & Hogg RS (2005). Quality of life, depression and fatigue among persons co-infected with HIV and hepatitis C: outcomes from a population-based cohort. AIDS Care, 17(4), 505–515. doi: 10.1080/09540120412331291733 [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, & Stein MB (2007). Psychometric analysis and refinement of the Connor-davidson Resilience Scale (CD-RISC): Validation of a 10-item measure of resilience. J Trauma Stress, 20(6), 1019–1028. doi: 10.1002/jts.20271 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (1993). 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA, 269(6), 729–730. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2016). HIV Surveillance Report. vol. 28, https://Www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published November 2017 Accessed [January 2nd 2018]. [Google Scholar]
- Cockram A, Judd FK, Mijch A, & Norman T (1999). The evaluation of depression in inpatients with HIV disease. Aust N Z J Psychiatry, 33(3), 344–352. doi: 10.1046/j.1440-1614.1999.00579.x [DOI] [PubMed] [Google Scholar]
- Coleman CL (2017). Health related quality of life and depressive symptoms among seropositive African Americans. Appl Nurs Res, 33, 138–141. doi: 10.1016/j.apnr.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Cook JA, Grey D, Burke-Miller J, Cohen MH, Anastos K, Gandhi M, … Young M (2006). Effects of treated and untreated depressive symptoms on highly active antiretroviral therapy use in a US multi-site cohort of HIV-positive women. AIDS Care, 18(2), 93–100. doi: 10.1080/09540120500159284 [DOI] [PubMed] [Google Scholar]
- Dale SK, Weber KM, Cohen MH, Kelso GA, Cruise RC, & Brody LR (2015). Resilience Moderates the Association Between Childhood Sexual Abuse and Depressive Symptoms Among Women with and At-Risk for HIV. AIDS Behav, 19(8), 1379–1387. doi: 10.1007/s10461-014-0855-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSalvo KB, Bloser N, Reynolds K, He J, & Muntner P (2006). Mortality prediction with a single general self-rated health question. A meta-analysis. J Gen Intern Med, 21(3), 267–275. doi: 10.1111/j.1525-1497.2005.00291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth AL, Peterson C, Matthews MD, & Kelly DR (2007). Grit: perseverance and passion for long-term goals. J Pers Soc Psychol, 92(6), 1087–1101. doi: 10.1037/0022-3514.92.6.1087 [DOI] [PubMed] [Google Scholar]
- Duckworth AL, & Quinn PD (2009). Development and validation of the short grit scale (grit-s). J Pers Assess, 91(2), 166–174. doi: 10.1080/00223890802634290 [DOI] [PubMed] [Google Scholar]
- Elliott AJ, Russo J, & Roy-Byrne PP (2002). The effect of changes in depression on health related quality of life (HRQoL) in HIV infection. Gen Hosp Psychiatry, 24(1), 43–47. [DOI] [PubMed] [Google Scholar]
- Evans DL, Ten Have TR, Douglas SD, Gettes DR, Morrison M, Chiappini MS, … Petitto JM (2002). Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. Am J Psychiatry, 159(10), 1752–1759. doi: 10.1176/appi.ajp.159.10.1752 [DOI] [PubMed] [Google Scholar]
- Franchi D, & Wenzel RP (1998). Measuring health-related quality of life among patients infected with human immunodeficiency virus. Clin Infect Dis, 26(1), 20–26. [DOI] [PubMed] [Google Scholar]
- Grov C, Golub SA, Parsons JT, Brennan M, & Karpiak SE (2010). Loneliness and HIV-related stigma explain depression among older HIV-positive adults. AIDS Care, 22(5), 630–639. doi: 10.1080/09540120903280901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, … Aging. (2012). HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr, 60 Suppl 1, S1–18. doi: 10.1097/QAI.0b013e31825a3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Atkinson JH, & Goodkin K (2001). Neuropsychiatric aspects of HIV infection among older adults. J Clin Epidemiol, 54 Suppl 1, S44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIV-1 and HIV-2 Antibody Antigen Evaluation. Fourth Generation. Quest Diagnostics. In.
- Jeste DV, Savla GN, Thompson WK, Vahia IV, Glorioso DK, Martin AS, … Depp CA (2013). Association between older age and more successful aging: critical role of resilience and depression. Am J Psychiatry, 170(2), 188–196. doi: 10.1176/appi.ajp.2012.12030386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat R, Cattie JE, Marcotte TD, Woods SP, Franklin DR, Corkran SH,… Heaton RK (2015). Incident major depressive episodes increase the severity and risk of apathy in HIV infection. J Affect Disord, 175, 475–480. doi: 10.1016/j.jad.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond AJ, Depp CA, Allison M, Langer R, Reichstadt J, Moore DJ, … Jeste DV (2008). Measurement and predictors of resilience among community-dwelling older women. J Psychiatr Res, 43(2), 148–154. doi: 10.1016/j.jpsychires.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leserman J (2008). Role of depression, stress, and trauma in HIV disease progression. Psychosom Med, 70(5), 539–545. doi: 10.1097/PSY.0b013e3181777a5f [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Seeley JR, Roberts RE, & Allen NB (1997). Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging, 12(2), 277–287. [DOI] [PubMed] [Google Scholar]
- McGowan JA, Brown J, Lampe FC, Lipman M, Smith C, & Rodger A (2018). Resilience and Physical and Mental Well-Being in Adults with and Without HIV. AIDS Behav, 22(5), 1688–1698. doi: 10.1007/s10461-017-1980-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks TW, Vahia IV, Lavretsky H, Kulkarni G, & Jeste DV (2011). A tune in “a minor” can “b major”: a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. Journal of affective disorders, 129(1-3), 126–142. doi: 10.1016/j.jad.2010.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekuria LA, Sprangers MA, Prins JM, Yalew AW, & Nieuwkerk PT (2015). Health-related quality of life of HIV-infected adults receiving combination antiretroviral therapy in Addis Ababa. AIDS Care, 27(8), 934–945. doi: 10.1080/09540121.2015.1020748 [DOI] [PubMed] [Google Scholar]
- Milanini B, Catella S, Perkovich B, Esmaeili-Firidouni P, Wendelken L, Paul R, … Valcour V (2017). Psychiatric symptom burden in older people living with HIV with and without cognitive impairment: the UCSF HIV over 60 cohort study. AIDS Care, 1–8. doi: 10.1080/09540121.2017.1281877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar BM, Starks TJ, Gurung S, & Parsons JT (2017). The Impact of Comorbidities, Depression, and Substance Use Problems on Quality of Life Among Older Adults Living With HIV. AIDS Behav, 21(6), 1684–1690. doi: 10.1007/s10461-016-1613-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montross LP, Depp C, Daly J, Reichstadt J, Golshan S, Moore D, … Jeste DV (2006). Correlates of self-rated successful aging among community-dwelling older adults. Am J Geriatr Psychiatry, 14(1), 43–51. doi: 10.1097/01.JGP.0000192489.43179.31 [DOI] [PubMed] [Google Scholar]
- Moore DJ, Blackstone K, Woods SP, Ellis RJ, Atkinson JH, Heaton RK, … The Tmarc, G. (2012). Methamphetamine use and neuropsychiatric factors are associated with antiretroviral non-adherence. AIDS Care, 24(12), 1504–1513. doi: 10.1080/09540121.2012.672718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Fazeli PL, Moore RC, Woods SP, Letendre SL, Jeste DV, … Program, H. I. V. N. R. (2018). Positive Psychological Factors are Linked to Successful Cognitive Aging Among Older Persons Living with HIV/AIDS. AIDS Behav, 22(5), 1551–1561. doi: 10.1007/s10461-017-2001-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Hussain MA, Watson CW, Fazeli PL, Marquine MJ, Yarns BC, … Moore DJ (2018). Grit and Ambition are Associated with Better Neurocognitive and Everyday Functioning Among Adults Living with HIV. AIDS Behav. doi: 10.1007/s10461-018-2061-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Marquine MJ, Straus E, Depp CA, Moore DJ, Schiehser DM, … Eyler LT (2017). Predictors and Barriers to Mental Health Treatment Utilization Among Older Veterans Living With HIV. Prim Care Companion CNS Disord, 19(1). doi: 10.4088/PCC.16m02059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Paolillo EW, Heaton A, Fazeli PL, Jeste DV, & Moore DJ (2017). Clinical utility of the UCSD Performance-Based Skills Assessment-Brief (UPSA-B) in adults living with HIV: Associations with neuropsychological impairment and patient-reported everyday functioning difficulties. PLoS One, 12(8), e0183614. doi: 10.1371/journal.pone.0183614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preau M, Leport C, Salmon-Ceron D, Carrieri P, Portier H, Chene G, … Morin M (2004). Health-related quality of life and patient-provider relationships in HIV-infected patients during the first three years after starting PI-containing antiretroviral treatment. AIDS Care, 16(5), 649–661. doi: 10.1080/09540120410001716441 [DOI] [PubMed] [Google Scholar]
- Rabkin JG (2008). HIV and depression: 2008 review and update. Curr HIV/AIDS Rep, 5(4), 163–171. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, McElhiney MC, & Ferrando SJ (2004). Mood and substance use disorders in older adults with HIV/AIDS: methodological issues and preliminary evidence. AIDS, 18 Suppl 1, S43–48. [PubMed] [Google Scholar]
- Randolff LS (1977). The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas, 1(3), 385–401. [Google Scholar]
- SAS Institute Inc. (1989-2007). JMP® (Version 12.0.1). Cary, NC. [Google Scholar]
- Satz P, Myers HF, Maj M, Fawzy F, Forney DL, Bing EG, … Janssen R (1997). Depression, substance use, and sexual orientation as cofactors in HIV-1 infected men: cross-cultural comparisons. NIDA Res Monogr, 172, 130–155. [PubMed] [Google Scholar]
- Sherr L, Clucas C, Harding R, Sibley E, & Catalan J (2011). HIV and depression--a systematic review of interventions. Psychol Health Med, 16(5), 493–527. doi: 10.1080/13548506.2011.579990 [DOI] [PubMed] [Google Scholar]
- United States Special Committee on Aging. (2013). Hearing: Older Americans: The Changing Face of HIV/AIDS in America. Washington, D.C. [Google Scholar]
- Vance DE, Mugavero M, Willig J, Raper JL, & Saag MS (2011). Aging with HIV: a cross-sectional study of comorbidity prevalence and clinical characteristics across decades of life. J Assoc Nurses AIDS Care, 22(1), 17–25. doi: 10.1016/j.jana.2010.04.002 [DOI] [PubMed] [Google Scholar]
- Walkup J, Wei W, Sambamoorthi U, & Crystal S (2008). Antidepressant treatment and adherence to combination antiretroviral therapy among patients with AIDS and diagnosed depression. Psychiatr Q, 79(1), 43–53. doi: 10.1007/s11126-007-9055-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE Jr., Kosinski M, Bayliss MS, McHorney CA, Rogers WH, & Raczek A (1995). Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care, 33(4 Suppl), AS264–279. [PubMed] [Google Scholar]
- Ware JE Jr., & Sherbourne CD (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care, 30(6), 473–483. [PubMed] [Google Scholar]
- Wiles N, Thomas L, Abel A, Ridgway N, Turner N, Campbell J, … Lewis G (2013). Cognitive behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet, 381(9864), 375–384. doi: 10.1016/S0140-6736(12)61552-9 [DOI] [PubMed] [Google Scholar]
- Wittchen HU (1994). Reliability and validity studies of the WHO--Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res, 25(1), 57–84. [DOI] [PubMed] [Google Scholar]
- World Health Organization; (1990). Composite International Diagnostic Interview. Geneva, Switzerland. [Google Scholar]
- Wu AW, Rubin HR, Mathews WC, Ware JE Jr., Brysk LT, Hardy WD, … Richman DD (1991). A health status questionnaire using 30 items from the Medical Outcomes Study. Preliminary validation in persons with early HIV infection. Med Care, 29(8), 786–798. [DOI] [PubMed] [Google Scholar]
- Zanjani F, Saboe K, & Oslin D (2007). Age difference in rates of mental health/substance abuse and behavioral care in HIV-positive adults. AIDS Patient Care STDS, 21(5), 347–355. doi: 10.1089/apc.2006.0043. [DOI] [PubMed] [Google Scholar]