Abstract
Fibroblasts are key participants in wound healing and inflammation, and are capable of driving the progression of tissue repair to fully functional tissue or pathologic scar, or fibrosis, depending on the specific mechanical and biochemical cues with which they are presented. Thus, understanding and modulating the fibroblastic response to implanted materials is paramount to achieving desirable outcomes, such as long-term implant function or tissue regeneration. However, fibroblasts are remarkably heterogeneous and can differ vastly in their contributions to regeneration and fibrosis. This heterogeneity exists between tissues and within tissues, down to the level of individual cells. This review will discuss the role of fibroblasts, the pitfalls of describing them as a collective, the specifics of their function, and potential future directions to better understand and organize their highly variable biology.
Keywords: fibroblast, fibrosis, biomaterials, wound healing, inflammation, mechanobiology, mechanotransduction, review
Graphical abstract
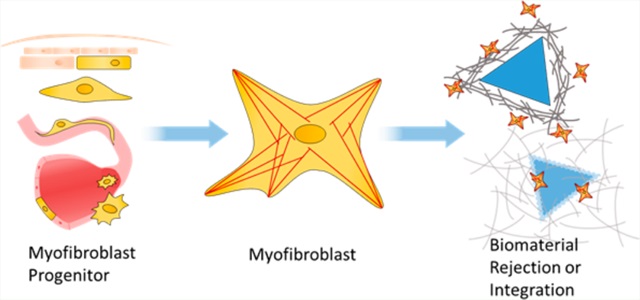
WOUND HEALING RESPONSE IN BIOMATERIALS
The body’s response to foreign material can be described as a modified process of wound healing. Insofar as the regenerative response is concerned, an implanted material is often treated as a chronic wound, with expectably deleterious consequences. A large focus of biomaterials science aims to develop strategies for integrating the material with the host, while avoiding the scarring and fibrotic response generated by recruited fibroblasts during wound healing.
The canonical process of wound healing is characterized by four progressing phases of hemostasis, inflammation, proliferation, and remodeling, as described in Figure 1. In the realm of biomaterials, this is collectively given the term, “foreign body response”. The process is a delicate orchestration of signaling by numerous cell types along a myriad axes. Immune cells, platelets, endothelial and associated perivascular cells, epithelium, and fibroblasts must all participate in the appropriate spatial and temporal arrangement to restore functional tissue and integrate with the material. An overloading or imbalance of these factors can cascade into fibrotic tissue. When a remodeling fibroblast, known as a myofibroblast, continues to receive activation cues long after it is no longer needed or experiences epigenetic alterations that inhibit its normal programmed apoptosis or dedifferentiation, the result is fibrosis and loss of function of both host tissue and implant.
Figure 1.
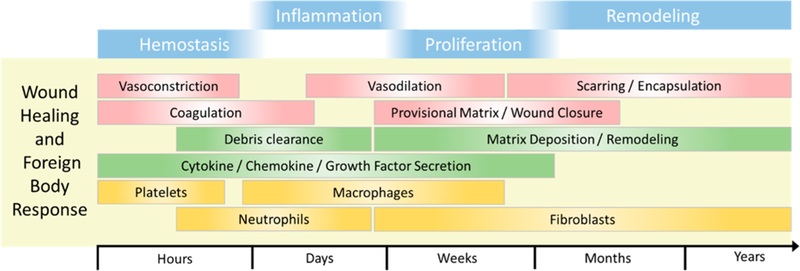
Timeline of wound healing and the foreign body response, broken into stages progressing from the initial response to the years beyond. Red, gross-scale tissue phenomena; green, cellular activity; gold, prominent cells.
Disruption of vascular endothelial integrity begins the hemostasis phase. Exposed matrix and pooling vascular contents activate blood platelets, which begin to form a plug of rapidly polymerizing fibrin at the wound site. These activated platelets and mechanically deformed extracellular matrix (ECM) recruit inflammatory cells1 and effect vasoconstriction.2,3 The fibrin–platelet plug, referred to in matrix biology as the “early provisional matrix”, leads to the cessation of bloodletting and maintains hemostasis. Concurrent with the resolution of the hemostatic phase is the inflammatory phase. This phase is characterized by massive cellular recruitment initiated by active platelets that release their contents from α-granules, damaged cells, and activation of the immune complement system.3
Inflammatory cells include a variety of monocytes and neutrophils, which clear debris and invading pathogens. Monocytes also secrete a large variety of cytokines which activate fibroblasts. Interleukin 1 beta (IL-1β), platelet-derived growth factor (PDGF) transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), and others are released and propagate further recruitment, clearance, and remodeling.4,5 This deluge of molecules activates both the canonical interstitial fibroblast as well as other fibroblast progenitors we will discuss below and begins the “repair” phase of wound healing. This effect is especially pronounced in the foreign body response, where macrophages condense into multinucleated giant cells, in an attempt to encircle, isolate, and destroy the foreign body. This mass accumulation of activated immune cells increases the levels of subsequent fibroblast recruitment.6 Directing inflammation toward pro-repair phenotypes and away from pro-inflammatory phenotypes remains a key scientific focus in biomaterials research, yet complete elimination of inflammation is not productive.6–8 For example, an interesting consequence of the burst of pro-inflammatory cytokines from macrophages and neutrophils is the transient “activation” of resident, quiescent fibroblasts through shedding of Thy-1 from their cell surface.9 The role of Thy-1 in fibroblast biology will be expanded later.
The proliferation (or repair) phase of wound healing is characterized by wound contraction, deposition of ECM, angiogenesis, and re-epithelialization in relevant tissue. Recruited fibroblasts stimulated with key growth factors, such as TGF-β, undergo a necessary transition to an activated myofibroblast (named for their expression of certain muscle proteins including α smooth muscle actin or αSMA) and deposit the so-called “late provisional matrix”, which provides a scaffold upon which revascularization and epithelialization occur. This late provisional matrix is rich in fibronectin10 and serves as the template for more permanent ECM comprising collagens.11 Fibroblasts and other adherent cells migrate across and mechanically interact with fibronectin and other proteoglycans in the wound via integrins, which facilitate cellular interactions with ECM and are elaborated below.
Over the course of weeks to years, the processes of epithelization and angiogenesis conclude, but fibroblasts remain in the healed wound. The late provisional matrix, composed primarily of fibronectin, is converted into a mature matrix comprising collagen III-rich ECM and is then slowly replaced during remodeling with collagen I. Collagen I comprises 80% of adult dermal collagen and is its most abundant molecular component.12 A balance of degradation via matrix metalloproteinases (MMPs) and deposition of new collagen is required for healthy maturation of tissue and the avoidance of excessive scarring, characteristic of collagen I-rich ECM.13 As the collagen turns over, the tensile strength of the regenerated dermis increases from 40% up to 70% of uninjured tissue.14 The myofibroblasts in the healed tissue are meant to eventually reach an equilibrium with their local ECM and will undergo apoptosis or dedifferentiate into a quiescent cell, as the net change in tissue composition trends toward zero.15
In the context of an implanted device, the development of thick, acellular ECM around the implant site is a strong indicator of poor biomaterial integration with the host. This “terminal” stage of biomaterial integration is characterized by a fibrotic capsule, which isolates implanted material from host tissue, save in certain contexts wherein it is desireable for the implant to become anchored and isolated, such as in implant-based breast reconstruction6
Fibrosis is not unique to implanted biomaterials; pathologic fibrosis is defined as an excessive, deleterious deposition of ECM, and is found wherever fibroblasts become “over-enthusiastic” remodelers and when myofibroblasts, the primary wound repair cell, are unable to undergo timely apoptosis or otherwise become inactive.
Biomaterial-associated fibrosis is similar to physiologic fibrosis in that the final “scar” is a highly fibrous and acellular matrix composed of a collagen I/III ratio that characterizes physiologic fibrosis.16 There is little comparative research on the two types of scar, which leaves an opening for both sides to collaborate using deliberately engineered biomaterial and pathologic contexts to explore and learn from unique yet congruent expertise.
The exact progression which drives fibroblasts into a fibrotic state is unknown; multivariable systems such as these are difficult to tackle wholly, but we now know many ways by which the fibroblast is driven to a pro-fibrotic phenotype. Fibroblast reciprocity in signaling between the cell and its local chemo-mechanical environment can result in dangerous and pathologic signaling loops. Fibroblasts respond to cytokines released from immune cells and damaged tissue, including the interleukins, latent TGF-β and PDGF-β, by increasing α-SMA expression, focal adhesion (FA) assembly, internal contractility, and synthesis of matrix proteins. The increase in contractile machinery and cell-matrix contact allows for more force to be generated on the matrix. The increased strain on local ECM releases further latent TGF-β, in addition to TGF-β being secreted by the fibroblast itself. Fibroblast activation can continue in a devastating positive feedback loop, which in concert with other factors drives excessive deposition of ECM.17–20 This alters the mechanical attributes of the tissue with consequences ranging from minor to fatal.21,22 For a biomaterial, this fibrosis prevents the material from properly integrating into the host, which can at best negate any intended benefit in materials not designed to take advantage of this phenomena. In this way, fibroblasts are the final arbiter of success or failure in biomaterial—host integration and understanding their biology and pathology is essential for biomaterials science.
Stiffness sensing is driving the biomaterials field away from stiff, smooth materials. Implants with textured or irregular topology,23 composed of hydrogel or other soft materials,24 and displaying endogenous ECM epitopes24–26 produce less severe fibrotic responses and integrate more thoroughly with tissue.
One thread of research dissecting the progression of fibrosis revolves around the outer leaflet glycoprotein Thy-1, introduced earlier. Thy-1 (also known as CD90), often used as a marker for mesenchymal stem cells, is a GPI-linked cell-surface protein found in a subset of fibroblasts. It was originally noted to differentiate fibroblast sensitivity toward PDGF-AA over PDGF-BB27 and is found in an inverse proportion to myofibroblast markers.9,28 The involvement of Thy-1 in fibroblast mechanobiology has since expanded, and it is now considered a major factor driving fibrosis. It is thought to differentiate environmentally responsive fibroblasts from non-responsive cells through its involvement in integrin-mediated mechanotransduction.29 Thy-1− cells are apoptitically resistant and are found in fibrotic foci in diseased tissue, which are areas of active fibroblast proliferation and fibrosis.9,29–31 The heterogeneity of Thy-1 expression in the fibrosing lung is caused by epigenetic silencing.30,32 Thy-1 may also play a critical role in the necessary transient activation of fibroblasts at the initial stages of wound repair. Specifically, inflammatory cytokines such as IL-1β and tumor necrosis factor α (TNFα) are known to induce a transient shedding of Thy-1 from the fibroblast surface through exosomal shedding. This event results in only a short-term loss of Thy-1, as opposed to its epigenetic silencing in fibrotic disorders, and could represent a mechanism linking inflammation to fibroblast recruitment.9
Since fibroblasts determine the final outcome of implanted biomaterials, they must be a priority consideration in biomaterial development. Designing around this constant hazard requires understanding fibroblast function, their origins, and their heterogeneity.
WHAT IS A FIBROBLAST?
The fibroblast in literature is a seemingly amorphous cell type, meeting a variety of indicative criteria. The most prevalent definition of a fibroblast is one based on in situ morphology. These fibroblasts are interstitial cells with ECM contacts. They are characteristically spindle-shaped with cellular processes extending from each tip33 and are easily isolated in culture via several passages of most tissues on plastic. This definition is simple and workable, with an easily identifiable in situ phenotype and a no-questions-asked system for cell culture.
However, fibroblasts by this definition exhibit high degrees of heterogeneity in expression and phenotype between tissues28,34,35 and even within the same tissue.28,36 The list of nonspecific fibroblast markers is long: collagen I, intermediate filament (IF) proteins, discoidin domain receptor 2 (DDR2), platelet-derived growth factor receptors (PDGFR-α and β), fibroblast growth factors (FGF10), periostin, transcription factor 21 (Tcf21), and Thy-1. Many of these markers are expressed only transiently or exclusively in the quiescent (or fibrotic) context.
Because of the remarkably heterogeneous nature of the fibroblast, there have recently been directed efforts to find a universal, fibroblast-specific marker. Those efforts have met with mixed success. For example, fibroblast specific protein 1 (FSP1) labels interstitial fibroblasts in studies of renal37 and pulmonary38 tissues, and shows some involvement in late developmental epithelial to mesenchymal transition (EMT),39 the putative source for most adult quiescent fibroblasts.40 However, FSP1 in recent years has garnered controversy after being found in a variety of other cell contexts, including inflammatory macrophages41 and vascular smooth muscle cells,42 among others.43,44
There are many other endogenous and engineered targets used to identify putative fibroblasts, all of which have their caveats. See the review from Tallquist45 for a more thorough exploration of genetic fibroblast-tracking tools and their controversies. The struggle to find a consistent fibroblast marker is summarized in her review in Table 2.
It is important to question the contributions of fibroblast heterogeneities at various scales. Understanding why one fibroblast displays one surface protein while another does not provides insight into basic biology, development, and the contributions fibroblasts make toward both tissue maintenance and fibrosis, including in response to biomaterials (and how those states differ between tissues).
For example, Thy-1, in addition to its biologic role in the progression of fibrosis, is an excellent example of the highly heterogeneous nature of a classically defined fibroblast. As discussed above, fibroblasts can be Thy-1+ or Thy-1−, with demonstrated phenotypic differences known between the subtypes: proliferation, apoptosis, response to growth factors, mechanotransduction, ECM synthesis, etc. However, further heterogeneity also exists within the Thy-1+ fibroblast population, as measured by liquid chromatography—mass spectrometry (LC-MS). Analysis of nuclear, cytoplasmic, and secreted protein fractions, gathered from quiescent primary, activated primary, and cancer-associated primary fibroblasts, showed extensive variability in expression. Thy-1 was just one of many proteins found to demonstrate differential expression profiles between tissues. Dermal fibroblasts expressed PDGFR-β in every tissue examined, as did myeloma-associated fibroblasts, while the remaining tissues showed inconsistent fibroblast PDGFR-β expression. Similar heterogeneity was found with MMP-1, proteoglycan 4, EGFR components, fibrillin, and CTGF, among others. Additionally, the density of procollagens, Thy-1, and other peptides in nuclear, cytoplasmic, and secreted fractions varied based on tissue origin.28
Moreover, even within a single tissue there exists additional sources of heterogeneity between fibroblasts. As an example, dermal fibroblast cDNA can be binned into discrete, local tissue-specific clusters of expression. This coordination is found across multiple gene families: ECM synthesis (fibronectin and fibrillin), growth factors including those involved in TGF-β and Wnt β-catenin signaling, migration, lipid metabolism, and developmental/differentiation genes. Forkhead box genes, as well as the hox family, correspond to topographic distribution of dermal interstitial fibroblasts.36 This topographic tissue heterogeneity can be resolved into a minimum of three anatomic divisions (anterior—posterior, proximal—distal, and dermal—nondermal) based on gene expression patterns.46 Further research into this differential expression elucidated the epigenetic mechanisms (in scalp and dura mater) which constitute of persistent site and age-specific fibroblast “memories.”47 This hierarchical progression of heterogeneity through pan-tissue markers, between tissues, and within tissues has been observed consistently through decades of fibroblast research and remains generally unaddressed, frustrating attempts to characterize fibroblasts as a generic, homogeneous population.
In the context of wound healing, there is yet another example of heterogeneity in the fetal fibroblast. It is noted that fetal wounds rarely scar.48 The drivers of this regenerative phenotype and its potential applications in the realms of inflammation and wound healing are only just now being explored.
Somewhat paradoxically, fetal fibroblasts display a constant α-SMA+ phenotype that does not change in response to any TGF-β isoform,49,50 in contrast to adult fibroblasts, which differentiate from quiescent α-SMA− to myofibroblastic α-SMA+ cells upon treatment with TGF-β. Additionally, fetal and adult fibroblasts develop a diverging integrin composition when treated with TGF-β.50
Fetal fibroblasts have been shown to be efficacious when used as a transplant in tendon repair, demonstrating a reduced capacity for unwanted ossification of the regenerating tendon.49 Expression analyses show these fetal fibroblasts have an increase in myofibroblastic markers and a decrease in inflammatory and osteogenic expression relative to adult fibroblasts.49 Fetal fibroblasts additionally secrete more collagen I and III than their adult counterparts and have a larger surface area.51
Few steps have been undertaken to understand the nature of a fetal fibroblast. We do not know, for example, if these fibroblasts are positive for popular markers such as FSP1 or if they are derived from a common developmental lineage. It is possible that these cells differentiate into less regenerative adult fibroblasts or that they constitute a separate fibroblast family that dies out as development progresses. The efficacy of these fibroblasts in other healing contexts is unknown, but their seeming reluctance to participate in inflammation should make them attractive for biomaterials scientists. Therapeutic application and basic research into these fibroblasts could provide further insight into fibroblast heterogeneity and their potential utility in wound healing and biomaterial integration.
Acknowledging these heterogeneities in fibroblast populations can be uncomfortable; therefore, a popular approach within the biomaterials community has been to use immortalized cell lines of fibroblasts, including 3T3 and HFF cells. If we accept that the population of fibroblasts is heterogeneous within and between tissues, we are making a risky assumption about the applicability of conclusions generated from culture experiments as they pertain to fibroblast biology writ large. Cells selected using the markers above may exclude a large portion of the phenotypically diverse fibroblast population, and these heterogeneities have stymied most attempts at settling on a robust molecular or genetic definition of the cell. Passable indicators have been found and are in widespread use, such as FSP1, but the use of such markers requires an understanding of their specific use cases.
Perhaps, then, the definition of a fibroblast as an easily isolated and cultured cell is not specific enough. Given the difficulty in isolating any truly unique molecular signature across tissues and disease contexts, do we need to revisit our definition of what constitutes a fibroblast? Asking this question is essential if we hope to engineer biomaterials that have the goal of accounting for and/or manipulating fibroblast behaviors. The prerogative of biomaterials scientists and engineers is to control the cues received by fibroblasts and limit damaging inflammatory and scarring responses. In the pursuit of this goal, the field has developed an armamentarium of materials and techniques to drive phenotype in the implanted context. An immense opportunity exists for these same techniques to be applied to help distinguish, delineate, and define fibroblast identity.
ALTERNATIVE DEFINITIONS
Alternative approaches to defining the fibroblast categorize by remodeling potential or by cellular or developmental lineage.
Remodeling Potential.
Myofibroblasts are identified in vitro and in vivo by the presence of α-SMA stress fibers and a contractile, secretory, and TGF-β/PDGF sensitive phenotype.35,52–54 These cells are derived from a bevy of progenitor lines, many outside the traditional interstitial fibroblast lineage. Perivascular cells (pericytes),18,55,56 endothelial57 and epithelial58,59 cells, as well as the circulating bone marrow derived fibrocyte59–63 all contribute toward fibroblast populations in inflammatory contexts, as illustrated in Figure 2. These cells, while more difficult to isolate, respond to similar cues as the traditionally defined fibroblast.
Figure 2.
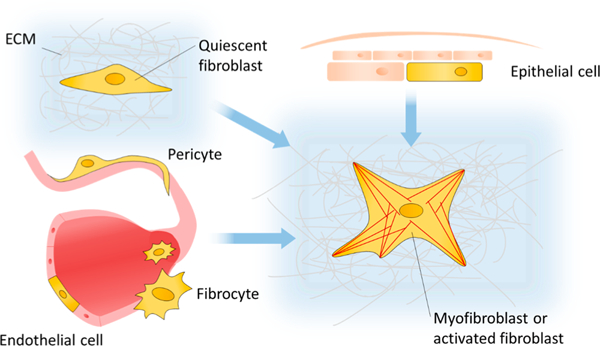
Range of cells which have been experimentally shown to become involved in fibrotic disease. Epithelial cells, tissue resident quiescent fibroblasts, microvasculature-associated pericytes, vascular endothelial cells, and circulating bone marrow derived fibrocytes can all differentiate into myofibroblasts and contribute toward fibrosis.
The prevailing hypothesis in the field is that a myofibroblast is a terminally differentiated cell which undergoes apoptosis upon resolution of inflammation, but isolated studies dispute this claim. It has been shown that nuclear factor erythroid 2-related factor 2 (Nrf2) is protective against pulmonary fibrosis.64–66 Expression of Nrf2 is depressed in pulmonary myofibroblasts relative to quiescent fibroblasts in the contexts of bleomycin-induced IPF or TGF-β/PDGF-BB treatment. Exogenous knockdown of Nrf2 drives a myofibroblast transition from lung fibroblasts in vitro. Interestingly, knocking in Nrf2 translocation into the nucleus via knockdown of inhibitor Kelch-like erythroid cell-derived protein CNC homology-associated protein 1 (Keap1) causes myofibroblasts to dedifferentiate as measured by reduced α-SMA and collagen production.67 Further examination of the mechanisms by which a myofibroblast becomes phenotypically “unstuck” is ongoing and includes factors such as MyoD and prostaglandin E2 68,69 This emerging body of evidence challenges the long-held assumption that myofibroblasts die and are cleared upon resolution of the wound healing response, and may simply be dedifferentiating into cells which are not myofibroblastic.
This consistency of remodeling potential is a strong contender for defining a fibroblast. It is not wholly unlike the current definition in that it relies upon a consistent phenotype but is superior in that it does not exclude cells based on extrinsic factors, such as the difficulty of isolation and culturing. However, there are still problems with specificity in this definition. Components of the myofibroblast phenotype are not exclusive to those cells. For example, many cell types remodel the extracellular matrix; osteoblasts,70 astrocytes,71 vascular endothelium,72 macrophages,73 and pericytes74,75 remodel ECM via MMP expression and/or matrix secretion. Perhaps these cells could also be classified as fibroblasts. Phenotypic behavior could be further clarified by a cell’s ability to remodel various biomaterials.
Cellular or Developmental Lineage.
The initial population of interstitial fibroblasts is generated during gestation, and these fibroblasts maintain an epigenetic “memory” of their origin even after multiple passages. 9,30,47 This memory has only been shown in the tissue resident fibroblast, but a similarly distinct epigenetic signature is entirely plausible for the more mobile fibroblasts/fibroblast progenitors discussed previously. This nascent field of fibroblast epigenomics could prove useful in identifying fibroblast subpopulations alone or in conjunction with more traditional systems of expression analysis.
Recent developments in lineage tracing have enabled the study of fibroblast and myofibroblast generation in specific tissues,55,56,66,76,77 but few comprehensive studies exist investigating the differences between fibroblast sources. Given the heterogeneity in expression and phenotype described above, it follows that fibroblasts from two separate organ systems have a distinct lineage.
As it stands, the heterogeneous and tissue-specific definitions used across tissues and fields make comparisons between putative “fibroblasts” difficult, and there seems no simple answer with which to satisfy all definitions of a fibroblast. For the purposes of this review, we have defined any cell which has been shown to potentiate ECM remodeling and mechanical loading as a fibroblast. This common, participatory phenotype provides a more consistent classification based on function. Later, we will discuss methods by which the scientific community may be able to better understand and define a fibroblast, particularly as this definition pertains to the context of biomaterials design.
FIBROBLAST FUNCTION
Chemo-Mechanical Signal Integration.
Cells are highly responsive to their sensed chemo-mechanical environment, with mechano-dependent phenotypes ranging across all classifications of cellular behavior. Migration,78 proliferation,79,80 secretion,81 and cellular differentiation82–84 each have well-characterized relationships to their local environment. This recognition, binding, and interaction are facilitated by integrin binding to the ECM through complexes called focal adhesions (FA).
The extracellular matrix is the load bearing and buffering structure which supports cells and tissues. Composed of fibrous proteins, proteoglycans, and other bioactive saccharides, the ECM facilitates cell adhesion, migration, and directs proliferation and development. The fibrous proteins are collagens and elastins which provide the primary structure. The polysaccharide halyuronic acid forms a viscous gel with absorbed water, which provides space filling and compressive strength to the matrix, as well as a fluidity to matrix Other components include fibronectin and laminin which facilitate cellular interactions with the matrix and further modify the mechanical characteristics of the matrix.85 Given the influence the extracellular matrix has on cell fate and tissue integrity, biomaterials approaches must always consider its components as a core design objective. Fibronectin is the most highly studied extracellular matrix component given its ability to facilitate interactions between cells and their local matrix. Dysregulation of mechanosensing can drive pathologic ECM deposition and drives fibrotic disease.18,84
There are many soluble factors which can activate fibroblasts toward pro-healing and pro-fibrotic behaviors, and many of those factors are also secreted by the fibroblasts themselves. PDGF and TGF-β1 are the two most common factors used experimentally to activate fibroblasts. There are many more factors impacting fibroblasts that are outside the scope of this review, and a thorough review of these affectors can be found in the recent review from Kalluri.86 We diagram these factors in Figure 3.
Figure 3.
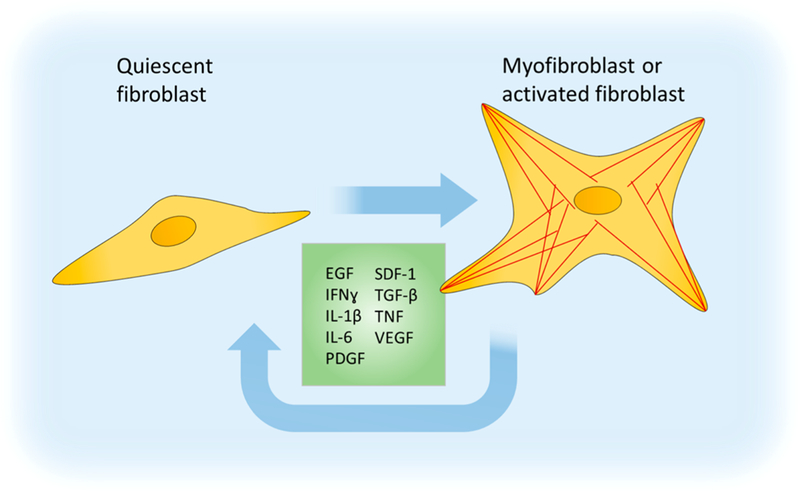
Soluble cues driving fibroblast activation into a proliferative, secretory, and remodeling phenotype. EGF, epidermal growth factor; IFNγ, interferon-γ; IL(s), interleukin; PDGF, platelet-derived growth factor; SDF-1, stromal cell-derived factor 1; TGF-β, transforming growth factor β; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Focal Adhesions and Stiffness Sensing.
Focal adhesions (FA) can be described as containing three regions along the z-axis across the membrane: the outermost integrins, the adhesome proximal to the intracellular integrin tails, and the final actin/myosin network.83,87,88
Integrins are heterodimeric transmembrane proteins with the ligand binding region composed of α and β subunits, which allow for binding to various ECM proteins. The subunits combine for a total of 24 identified receptors.89 These integrins bind to a variety of ECM ligands such as fibronectin, fibrinogen, and collagens. As such, the integrin composition of the FA determines the signals which are eventually integrated by the fibroblast. The multiple integrins for collagen and fibronectin have demonstrated distinct FA composition and signaling.90,91 One prolific integrin ligand is the Arg-Gly-Asp sequence, or RGD. Found in fibronectin, fibrinogen, osteopontin,89,92 and several laminins and collagens,89 RGDs have long been a popular target for the study of mechanobiology and the development of biomaterials as the sequence allows for a functionalized material to better integrate into its local tissue environment.
The integrins are linked to the actin cytoskeleton via linker proteins talin, vinculin, integrin-linked protein kinase complex, α-actinin, tensin, and filamin.87,93 These linker proteins, together with over 200 other associated components, are collectively referred to as the “integrin adhesome”. The adhesome undergoes conformational changes in response to strain and affect a signaling change in the cell. Cumulatively, the adhesome represents a systems level problem where wide genetic studies and large in silica analyses are being pursued. For a thorough review of the adhesome, see the 2014 review by Winograd-Katz et al.94
Lastly, the force-generating actomyosin network, the “stress fibers” referred to in myofibroblast literature, sense and generate mechanical loading within the cell, which is transferred through the FA onto bound ECM.95 This network is in a constant state of flux, striving toward dynamic equilibria of filament recruitment and degradation in response to sensed and generated tension. The precise mechanisms by which this network creates and transmits forces are still being elucidated, with research ongoing into transcription factors, such as myocardin-related transcription factor (MRTF). Upon polymerization of g-actin into f-actin (filaments), MRTF is unbound from g-actin and free to translocate to the nucleus where it forms a complex with serum response factor (SRF) to drive many genes that are considered to be in the fibrotic program.96,97
Understanding fibroblast function allows for targeting pro-healing and antifibrotic behavior. However, there is still a dearth of data regarding the function of these diverse cells and how they differ from one another.
LOOKING TOWARD THE FUTURE: TRENDS IN THE FIELD
We previously describe the difficulties in subjecting fibroblasts to rigorous pan-tissue definitions or molecular labels, elaborating on the controversies and unknowns facing the field regarding fibroblast origin, identification, and fate. Individual groups studying fibroblasts often generate islands of fibroblast characterization, each separated from one another by gulfs in methodologies and vernacular. This compartmental regime of study comes about from a lack of adequate tooling; the throughput to analyze cellular heterogeneity in multiple dimensions (expression, lineage, and microenvironment) has only recently come about. With these new and powerful methods exist a substantial opportunity within the field to thoroughly explore how we define a fibroblast; how fibroblasts from all tissues and lineages compare, and how those similarities and differences bring about cellular phenotype in the regenerative biomaterials context.
Fibroblast Origins and Tracing.
Presumably any fibroblast or remodeling cell will contain some indicator of its fibrotic potential, and discovering markers, if any exist, could allow us to truly constrain the definition of a fibroblast. As we become capable of observing the origin and development of a cell in addition to its immediate phenotype, we will potentially be able to settle on a workable definition of a fibroblast as one of remodeling phenotype, specific lineage, or some combination of both criteria.
Lineage tracing techniques are increasing the diversity of fibroblasts which are available to study and identifying previously unknown subsets of fibroblasts by their developmental markers. Inducible lineage tracing models include labeling developmental genes such as the forkhead box (FOX) group, which has been used to identify a subset of perivascular fibroblasts in kidney55,77 and lung.56 Tcf2198 and Wt176 in cardiac tissue are also being used to track fibroblast generation and phenotype. Fibrocyte lineages are more simple, with a collagen reporter bone marrow transplant into WT mice allowing for visualization of marrow-derived fibroblasts.61
Another method to understand the lineage of a fibroblast, and its developmental environmental context, is to examine epigenetic markers of the cell. It has been shown that fibroblasts retain an epigenetic memory of a pathologically stiff environment for 2 weeks after removal.99,100 What may not be detectable at the lineage or transcript level could in fact be epigenetic drivers of fibroblast phenotype. For example, the promoter region of Thy-1 has been shown to display hypermethylation resulting in a permanent Thy-1− phenotype,30 driving the progression of fibrotic disease and preventing the fibroblast from returning to quiescence or undergoing apoptosis.
Further techniques are being brought to bear on evaluating heterogeneous phenotype and identifying targets for study, such as cellular barcoding using multiplexed mass cytometry. Mass cytometry combines the high throughput of flow cytometry with the spectrographic ability to discern between dozens of unique mass markers, offering unprecedented throughput and efficiency in collecting data about individual cells.101 Mass cytometry currently offers over 40102 distinct mass tags, allowing for rapid and simultaneous quantitation of transcript and peptide levels within single cells.
Approaches from other fields which are embracing similar heterogeneities could be adapted to describing the heterogeneous fibroblast; similarly to how the macrophage M1 to M2 paradigm is being supplanted by the radial color wheel of fluid phenotype,103 an inclusive model of fibroblast lineage and functional markers might be applied to a multitissue analysis of fibroblasts. Dimensionality along the axes of lineage, mechanosensitivity, expression, and epigenetic profiles would condense and contextualize the diverse data we collect on various fibroblasts.
Cues, Metabolism, and Networks.
Computational models are a potential solution to the multidimensional quandary of inputs and outputs of fibroblast signaling. Simple, substantiated molecular events and interactions can be fed into a simulation of cellular behavior and reveal undiscovered relationships between phenotype and the cellular, chemical, and mechanical environment. This process is used to explore hypotheses and inform further research.104–106 Current fibroblast modeling is trending toward larger, multiscale modeling techniques which incorporate -omics and drug data into more complex and exhaustive systems. These systems allow for rapid assessment of cell-cell, cell-material, and cell-factor interactions and output genes, receptors, and signaling pathways which merit further study. In silico studies of fibroblast dynamics in pulmonary,107,108 liver,109 and kidney110 tissues demonstrate the increasing complexity and accuracy of these model systems. A comprehensive discussion of cardiac-centric fibroblast modeling by the Saucerman group111 is recommended for further reading into fibroblast modeling.
To accurately parametrize these models requires massive amounts of phenotypic data. In addition to mass cytometry and traditional -omics approaches, powerful new methods, such as stochastic profiling allow researchers to measure gene expression at the level of individual cells, providing insight into the high variability of cellular pathways within cells in a superficially homogeneous tissue or culture context.112 These profiles allow models to much more accurately simulate the behaviors of heterogeneous cells in vitro and in vivo.
DISCUSSION
The disparity in phenotype between fibroblasts found across the body is a vital consideration for those seeking to control wound healing, inflammation, and the foreign body response/biomaterial-associated fibrosis. Heterogeneity can be seen across tissues and within tissues, and even found in the expression of “pan-fibroblast” markers, such as FSP1. Cells not traditionally considered fibroblasts have demonstrated the ability to contribute to inflammation and fibrosis. Taken together, these difficulties may justify reevaluating what we choose to define as a fibroblast.
By whatever classification, these remodeling and mechanically active cells are vital to homeostasis. Their ability to sense and respond to cues both soluble and physical make them indispensable components of wound healing and regeneration. However, disruption of these systems can result in disaster, with out-of-control deposition of ECM resulting in scarring and loss of biomaterial function.
A consequence of fibroblasts being so heterogeneous is the disclaimer in the introduction of many fibroblast papers, wherein the author claims his or her work should not be taken as representative of fibroblasts as a whole. We believe that these statements speak to an untapped opportunity for thorough, systems-level approaches to understand fibroblasts across tissues and bridge these disconnected islands of understanding through new technologies and approaches.
We think that biomaterials science is uniquely suited to approach these problems, for two reasons. First, out of necessity: biocompatibility requires mastering of the inflammation and scarring environment in order to maximize integration. Second: biomaterials are invariably a simplified, constrained approximation of some physiologic feature. This constraint reduces variables and allows for the asking and answering of questions which may be intractable in a more complex experimental model. Collaborative efforts between biomaterials scientists and those studying fibrosis will yield dividends in both our basic understanding of fibroblast biology and the effectiveness of biomaterial—host integration.
Acknowledgments
Funding
This work was supported by the following funding sources to S.M.P. and T.H.B: The Hartwell Foundation, NIH R01HL127283, NIH R01HL132585, and NIH U01AR069393.
Footnotes
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Wietecha MS; DiPietro LA Therapeutic Approaches to the Regulation of Wound Angiogenesis. Adv. Wound Care 2013, 2 (3), 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Atluri P The Surgical Review: An Integrated Basic and Clinical Science Study Guide; Lippincott Williams & Wilkins, 2005; p 300. [Google Scholar]
- (3).Sinno H; Prakash S Complements and the wound healing cascade: an updated review. Plast. Surg. Int. 2013, 2013, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Robbins S; Cotran RS Acute and Chronic Inflammation In Pathologic Basis of Disease; Saunders: Philadelphia, PA, 1989; pp 47–86. [Google Scholar]
- (5).Simpson DM; Ross R The neutrophilic leukocyte in wound repair: A study with antineutrophil serum. J. Clin. Invest. 1972, 51 (8), 2009–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Major MR; Wong VW; Nelson ER; Longaker MT; Gurtner GC The foreign body response: at the interface of surgery and bioengineering. Plast. Reconstr. Surg. 2015, 135 (5), 1489–1498. [DOI] [PubMed] [Google Scholar]
- (7).Anderson JM; Rodriguez A; Chang DT FOREIGN BODY REACTION TO BIOMATERIALS. Semin. Immunol. 2008, 20 (2), 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Tang L; Eaton JW Inflammatory responses to biomaterials. Am. J. Clin. Pathol. 1995, 103 (4), 466–471. [DOI] [PubMed] [Google Scholar]
- (9).Hagood JS; Prabhakaran P; Kumbla P; Salazar L; MacEwen MW; Barker TH; Ortiz LA; Schoeb T; Siegal GP; Alexander CB; et al. Loss of Fibroblast Thy-1 Expression Correlates with Lung Fibrogenesis. Am. J. Pathol. 2005, 167 (2), 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Clark RA; Lanigan JM; DellaPelle P; Manseau E; Dvorak HF; Colvin RB Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J. Invest. Dermatol. 1982, 79 (5), 264–269. [DOI] [PubMed] [Google Scholar]
- (11).Sottile J; Hocking DC Fibronectin Polymerization Regulates the Composition and Stability of Extracellular Matrix Fibrils and Cell-Matrix Adhesions. Mol. Biol. Cell 2002, 13 (10), 3546–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Booth BA; Polak KL; Uitto J Collagen biosynthesis by human skin fibroblasts. I. Optimization of the culture conditions for synthesis of type I and type III procollagens. Biochim. Biophys. Acta, Nucleic Acids Protein Synth. 1980, 607 (1), 145–160. [DOI] [PubMed] [Google Scholar]
- (13).Visse R; Nagase H Matrix metalloproteinases and tissue inhibitors of metalloproteinases structure, function, and biochemistry. Circ. Res. 2003, 92 (8), 827–839. [DOI] [PubMed] [Google Scholar]
- (14).Abercrombie M; Flint M; James D Wound contraction in relation to collagen formation in scorbutic guinea-pigs. Development 1956, 4 (2), 167–175. [Google Scholar]
- (15).Desmouliere A; Redard M; Darby I; Gabbiani G Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am. J. Pathol. 1995, 146 (1), 56–66. [PMC free article] [PubMed] [Google Scholar]
- (16).Akilbekova D; Bratlie KM Quantitative Characterization of Collagen in the Fibrotic Capsule Surrounding Implanted Polymeric Microparticles through Second Harmonic Generation Imaging. PLoS One 2015, 10 (6), e0130386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Leask A; Abraham DJ TGF-beta signaling and the fibrotic response. FASEB J. 2004, 18, 816–827. [DOI] [PubMed] [Google Scholar]
- (18).Liu F; Mih JD; Shea BS; Kho AT; Sharif AS; Tager AM; Tschumperlin DJ Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 2010, 190 (4), 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Aarabi S; Bhatt KA; Shi Y; Paterno J; Chang EI; Loh SA; Holmes JW; Longaker MT; Yee H; Gurtner GC Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007, 21 (12), 3250–3261. [DOI] [PubMed] [Google Scholar]
- (20).Wilgus TA; Ferreira AM; Oberyszyn TM; Bergdall VK; DiPietro LA Regulation of scar formation by vascular endothelial growth factor. Lab. Invest. 2008, 88 (6), 579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Travers JG; Kamal FA; Robbins J; Yutzey KE; Blaxall BC Cardiac Fibrosis. Circ. Res. 2016, 118 (6), 1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Datta A; Scotton CJ; Chambers RC Novel therapeutic approaches for pulmonary fibrosis. Br. J. Pharmacol. 2011, 163 (1), 141–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Prasad BR; Brook MA; Smith T; Zhao S; Chen Y; Sheardown H; D’souza R; Rochev Y Controlling cellular activity by manipulating silicone surface roughness. Colloids Surf. B 2010, 78 (2), 237–242. [DOI] [PubMed] [Google Scholar]
- (24).Janmey PA; Winer JP; Weisel JW Fibrin gels and their clinical and bioengineering applications. J. R. Soc.j Interface 2009, 6 (30), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Beale EW; Hoxworth RE; Livingston EH; Trussler AP The role of biologic mesh in abdominal wall reconstruction: a systematic review of the current literature. Am. J. Surg. 2012, 204 (4), 510–517. [DOI] [PubMed] [Google Scholar]
- (26).Cheng A; Lakhiani C; Saint-Cyr M Treatment of capsular contracture using complete implant coverage by acellular dermal matrix: a novel technique. Plast. Reconstr. Surg. 2013, 132 (3), 519–529. [DOI] [PubMed] [Google Scholar]
- (27).Hagood JS; Miller PJ; Lasky JA; Tousson A; Guo B; Fuller GM; McIntosh JC Differential expression of platelet-derived growth factor-α receptor by Thy-1− and Thy-1+ lung fibroblasts. Am. J. Physiol.-Lung Cell. Mol Physiol. 1999, 277 (1), L218. [DOI] [PubMed] [Google Scholar]
- (28).Slany A; Meshcheryakova A; Beer A; Ankersmit HJ; Paulitschke V; Gerner C Plasticity of fibroblasts demonstrated by tissue-specific and function-related proteome profiling. Clin. Proteomics 2014, 11 (1), 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Fiore VF; Strane PW; Bryksin AV; White ES; Hagood JS; Barker TH Conformational coupling of integrin and Thy-1 regulates Fyn priming and fibroblast mechanotransduction. J. Cell Biol. 2015, 211 (1), 173–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Sanders YY; Pardo A; Selman M; Nuovo GJ; Tollefsbol TO; Siegal GP; Hagood JS Thy-1 Promoter Hypermethylation: A Novel Epigenetic Pathogenic Mechanism in Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2008, 39 (5), 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Koumas L; Smith TJ; Feldon S; Blumberg N; Phipps RP Thy-1 Expression in Human Fibroblast Subsets Defines Myofibro-blastic or Lipofibroblastic Phenotypes. Am. J. Pathol. 2003, 163 (4), 1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Sanders YY; Tollefsbol TO; Varisco BM; Hagood JS Epigenetic Regulation of Thy-1 by Histone Deacetylase Inhibitor in Rat Lung Fibroblasts. Am. J. Respir. Cell Mol. Biol. 2011, 45 (1), 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Tarin D; Croft C Ultrastructural studies of wound healing in mouse skin. II. Dermo-epidermal interrelationships. J. Anat. 1970, 106 (1), 79. [PMC free article] [PubMed] [Google Scholar]
- (34).Fries KM; Blieden T; Looney RJ; Sempowski GD; Silvera MR; Willis RA; Phipps RP Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin. Immunol. Immunopathol. 1994, 72 (3), 283–292. [DOI] [PubMed] [Google Scholar]
- (35).Hinz B; Phan SH; Thannickal VJ; Galli A; Bochaton-Piallat M-L; Gabbiani G The Myofibroblast: One Function, Multiple Origins. Am. J. Pathol. 2007, 170 (6), 1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Chang HY; Chi J-T; Dudoit S; Bondre C; van de Rijn M; Botstein D; Brown PO Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 2002, 99 (20), 12877–12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Hay E An overview of epithelio-mesenchymal transformation. Cells Tissues Organs 1995, 154 (1), 8–20. [DOI] [PubMed] [Google Scholar]
- (38).Lawson WE; Polosukhin VV; Zoia O; Stathopoulos GT; Han W; Plieth D; Loyd JE; Neilson EG; Blackwell TS Characterization of Fibroblast-specific Protein 1 in Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2005, 171 (8), 899–907. [DOI] [PubMed] [Google Scholar]
- (39).Okada H; Danoff TM; Kalluri R; Neilson EG Early role of Fsp1 in epithelial-mesenchymal transformation. Am. J. Physiol.-Ren. Physiol. 1997, 273 (4), F563. [DOI] [PubMed] [Google Scholar]
- (40).Komuro T Re-evaluation of fibroblasts and fibroblast-like cells. Anat. Embryol. 1990, 182 (2), 103–112. [DOI] [PubMed] [Google Scholar]
- (41).Österreicher CH; Penz-Osterreicher M; Grivennikov SI; Guma M; Koltsova EK; Datz C; Sasik R; Hardiman G; Karin M; Brenner DA Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (1), 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Brisset AC; Hao H; Camenzind E; Bacchetta M; Geinoz A; Sanchez J-C; Chaponnier C; Gabbiani G; Bochaton-Piallat ML Intimal Smooth Muscle Cells of Porcine and Human Coronary Artery Express S100A4, a Marker of the Rhomboid Phenotype In Vitro. Circ. Res. 2007, 100 (7), 1055. [DOI] [PubMed] [Google Scholar]
- (43).Iwano M; Plieth D; Danoff TM; Xue C; Okada H; Neilson EG Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 2002, 110, 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Kong P; Christia P; Saxena A; Su Y; Frangogiannis NG Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am. J. Physiol.-Heart Circ. Physiol 2013, 305 (9), H1363–H1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Swonger JM; Liu JS; Ivey MJ; Tallquist MD Genetic tools for identifying and manipulating fibroblasts in the mouse. Differentiation 2016, 92 (3), 66–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Rinn JL; Bondre C; Gladstone HB; Brown PO; Chang HY Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006, 2, e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Ivanov NA; Tao R; Chenoweth JG; Brandtjen A; Mighdoll MI; Genova JD; McKay RD; Jia Y; Weinberger DR; Kleinman JE; et al. Strong Components of Epigenetic Memory in Cultured Human Fibroblasts Related to Site of Origin and Donor Age. PLoS Genet. 2016, 12 (2), e1005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Coolen NA; Schouten KC; Boekema BK; Middelkoop E; Ulrich MM Wound healing in a fetal, adult, and scar tissue model: a comparative study. Wound Repair Regen. 2010, 18 (3), 291–301. [DOI] [PubMed] [Google Scholar]
- (49).Fang Z; Zhu T; Shen WL; Tang QM; Chen JL; Yin Z; Ji JF; Heng BC; Ouyang HW; Chen X Transplantation of Fetal Instead of Adult Fibroblasts Reduces the Probability of Ectopic Ossification During Tendon Repair. Tissue Eng., Part A 2014, 20 (13–14), 1815–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Moulin V; Tam BYY; Castilloux G; Auger FA; O’Connor-McCourt MD; Philip A; Germain L Fetal and adult human skin fibroblasts display intrinsic differences in contractile capacity. J. Cell. Physiol. 2001, 188 (2), 211–222. [DOI] [PubMed] [Google Scholar]
- (51).Brink HE; Bernstein J; Nicoll SB Fetal dermal fibroblasts exhibit enhanced growth and collagen production in two-and three-dimensional culture in comparison to adult fibroblasts. J. Tissue Eng. Regener. Med. 2009, 3 (8), 623–633. [DOI] [PubMed] [Google Scholar]
- (52).Baum J; Duffy HS Fibroblasts and Myofibroblasts: What are we talking about? J. Cardiovasc. Pharmacol. 2011, 57 (4), 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Desmouliere A; Chaponnier C; Gabbiani G Perspective Article: Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005, 13 (1), 7–12. [DOI] [PubMed] [Google Scholar]
- (54).Gabbiani G; Hirschel BJ; Ryan GB; Statkov PR; Majno G GRANULATION TISSUE AS A CONTRACTILE ORGAN: A STUDY OF STRUCTURE AND FUNCTION. J. Exp. Med. 1972, 135 (4), 719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Humphreys BD; Lin S-L; Kobayashi A; Hudson TE; Nowlin BT; Bonventre JV; Valerius MT; McMahon AP; Duffield JS Fate Tracing Reveals the Pericyte and Not Epithelial Origin of Myofibroblasts in Kidney Fibrosis. Am. J. Pathol. 2010, 176 (1), 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Hung C; Linn G; Chow Y-H; Kobayashi A; Mittelsteadt K; Altemeier WA; Gharib SA; Schnapp LM; Duffield JS Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2013, 188 (7), 820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Hashimoto N; Phan SH; Imaizumi K; Matsuo M; Nakashima H; Kawabe T; Shimokata K; Hasegawa Y Endothelial-Mesenchymal Transition in Bleomycin-Induced Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2010, 43 (2), 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Kim KK; Kugler MC; Wolters PJ; Robillard L; Galvez MG; Brumwell AN; Sheppard D; Chapman HA Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl. Acad. Sci. U. S. A. 2006, 103 (35), 13180–13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Tanjore H; Xu XC; Polosukhin VV; Degryse AL; Li B; Han W; Sherrill TP; Plieth D; Neilson EG; Blackwell TS; et al. Contribution of Epithelial-derived Fibroblasts to Bleomycin-induced Lung Fibrosis. Am. J. Respir. Crit. Care Med. 2009, 180 (7), 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Haudek SB; Xia Y; Huebener P; Lee JM; Carlson S; Crawford JR; Pilling D; Gomer RH; Trial J; Frangogiannis NG; et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc. Natl. Acad. Sci. U. S. A. 2006, 103 (48), 18284–18289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Kisseleva T; Uchinami H; Feirt N; Quintana-Bustamante O; Segovia JC; Schwabe RF; Brenner, D. A. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J. Hepatol. 2006, 45 (3), 429–438. [DOI] [PubMed] [Google Scholar]
- (62).Phillips RJ; Burdick MD; Hong K; Lutz MA; Murray LA; Xue YY; Belperio JA; Keane MP; Strieter RM Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J. Clin. Invest. 2004, 114 (3), 438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Suga H; Rennert RC; Rodrigues M; Sorkin M; Glotzbach JP; Januszyk M; Fujiwara T; Longaker MT; Gurtner GC Tracking the Elusive Fibrocyte: Identification and Characterization of Collagen-Producing Hematopoietic Lineage Cells During Murine Wound Healing. Stem Cells 2014, 32 (5), 1347–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Chan K; Kan YW Nrf2 is essential for protection against acute pulmonary injury in mice. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Cho HY; Reddy SP; Yamamoto M; Kleeberger SR The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004, 18, 1258–1260. [DOI] [PubMed] [Google Scholar]
- (66).Kikuchi N; Ishii Y; Morishima Y; Yageta Y; Haraguchi N; Itoh K; Yamamoto M; Hizawa N Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance. Respir. Res. 2010, 11 (1), 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Artaud-Macari E; Goven D; Brayer S; Hamimi A; Besnard V; Marchal-Somme J; Ali ZE; Crestani B; Kerdine-Römer S; Boutten A Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid. Redox Signaling 2013, 18 (1), 66–79. [DOI] [PubMed] [Google Scholar]
- (68).Hecker L; Jagirdar R; Jin T; Thannickal VJ Reversible Differentiation of Myofibroblasts by MyoD. Exp. Cell Res. 2011, 317(13), 1914–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Garrison G; Huang SK; Okunishi K; Scott JP; Kumar Penke LR; Scruggs AM; Peters-Golden M Reversal of Myofibroblast Differentiation by Prostaglandin E(2). Am. J. Respir. Cell Mol. Biol. 2013, 48 (5), 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Ortega N; Behonick DJ; Werb Z Matrix remodeling during endochondral ossification. Trends Cell Biol. 2004, 14 (2), 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Hernandez MR The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog. Retinal Eye Res. 2000, 19 (3), 297–321. [DOI] [PubMed] [Google Scholar]
- (72).Davis GE; Senger DR Endothelial extracellular matrix. Circ. Res. 2005, 97 (11), 1093–1107. [DOI] [PubMed] [Google Scholar]
- (73).Shapiro SD; Senior RM Matrix metalloproteinases: matrix degradation and more. Am. J. Respir. Cell Mol. Biol. 1999, 20 (6), 1100–1102. [DOI] [PubMed] [Google Scholar]
- (74).Birbrair A; Zhang T; Files DC; Mannava S; Smith T; Wang Z-M; Messi ML; Mintz A; Delbono O Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res. Ther. 2014, 5 (6), 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Underly RG; Levy M; Hartmann DA; Grant RI; Watson AN; Shih AY Pericytes as inducers of rapid, matrix metal-loproteinase-9 dependent capillary damage during ischemia. J. Neurosci. 2017, 37, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Wessels A; van den Hoff MJ; Adamo RF; Phelps AL; Lockhart MM; Sauls K; Briggs LE; Norris RA; van Wijk B; Perez-Pomares JM Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev. Biol. 2012, 366 (2), 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Gomez IG; Duffield JS The FOXD1 lineage of kidney perivascular cells and myofibroblasts: functions and responses to injury. Kidney Int. Suppl 2014, 4 (1), 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Lo C-M; Wang H-B; Dembo M; Wang Y Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000, 79 (1), 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Hadjipanayi E; Mudera V; Brown R Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J. Tissue Eng. Regener. Med. 2009, 3 (2), 77. [DOI] [PubMed] [Google Scholar]
- (80).Schrader J; Gordon-Walker TT; Aucott RL; van Deemter M; Quaas A; Walsh S; Benten D; Forbes SJ; Wells RG; Iredale JP Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology 2011, 53 (4), 1192–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Wipff P-J; Rifkin DB; Meister J-J; Hinz B Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J. Cell Biol. 2007, 179 (6), 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Engler AJ; Sen S; Sweeney HL; Discher DE Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126 (4), 677–689. [DOI] [PubMed] [Google Scholar]
- (83).Goffin JM; Pittet P; Csucs G; Lussi JW; Meister J-J; Hinz B Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibers. J. Cell Biol. 2006, 172 (2), 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Huang X; Yang N; Fiore VF; Barker TH; Sun Y; Morris SW; Ding Q; Thannickal VJ; Zhou Y Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am. J. Respir. Cell Mol. Biol. 2012, 47 (3), 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Lodish HF Molecular Cell Biology; W.H. Freeman and Co.: New York, 2013. [Google Scholar]
- (86).Kalluri R The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16 (9), 582–598. [DOI] [PubMed] [Google Scholar]
- (87).Ciobanasu C; Faivre B; Le Clainche C Integrating actin dynamics, mechanotransduction and integrin activation: The multiple functions of actin binding proteins in focal adhesions. Eur. J. Cell Biol. 2013, 92 (10–11), 339–348. [DOI] [PubMed] [Google Scholar]
- (88).Geiger B; Spatz JP; Bershadsky AD Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10 (1), 21–33. [DOI] [PubMed] [Google Scholar]
- (89).Plow EF; Haas TA; Zhang L; Loftus J; Smith JW Ligand Binding to Integrins. J. Biol. Chem. 2000, 275 (29), 21785–21788. [DOI] [PubMed] [Google Scholar]
- (90).Ivaska J; Reunanen H; Westermarck J; Koivisto L; Kahari V-M; Heino J Integrin α2β1 mediates isoform-specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the α2 cytoplasmic tail. J. Cell Biol. 1999, 147 (2), 401–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Schiller HB; Hermann M-R; Polleux J; Vignaud T; Zanivan S; Friedel CC; Sun Z; Raducanu A; Gottschalk K-E; Théry M β1-and αv-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat. Cell Biol. 2013, 15 (6), 625–636. [DOI] [PubMed] [Google Scholar]
- (92).Arnaout MA; Mahalingam B; Xiong J-P INTEGRIN STRUCTURE, ALLOSTERY, AND BIDIRECTIONAL SIGNALING. Annu. Rev. Cell Dev. Biol. 2005, 21 (1), 381–410. [DOI] [PubMed] [Google Scholar]
- (93).Tomasek JJ; Gabbiani G; Hinz B; Chaponnier C; Brown RA Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3 (5), 349–363. [DOI] [PubMed] [Google Scholar]
- (94).Winograd-Katz SE; Fassler R; Geiger B; Legate K R The integrin adhesome: from genes and proteins to human disease. Nat. Rev. Mol. Cell Biol. 2014, 15 (4), 273–288. [DOI] [PubMed] [Google Scholar]
- (95).Burridge K; Wittchen ES The tension mounts: stress fibers as force-generating mechanotransducers. J. Cell Biol. 2013, 200 (1), 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Creemers EE; Pinto YM Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc. Res. 2011, 89 (2), 265–272. [DOI] [PubMed] [Google Scholar]
- (97).Small EM; Thatcher JE; Sutherland LB; Kinoshita H; Gerard RD; Richardson JA; DiMaio JM; Sadek H; Kuwahara K; Olson EN. Myocardin-Related Transcription Factor-A Controls Myofibroblast Activation and Fibrosis in Response to Myocardial Infarction. Circ. Res. 2010, 107 (2), 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Acharya A; Baek ST; Huang G; Eskiocak B; Goetsch S; Sung CY; Banfi S; Sauer MF; Olsen GS; Duffield JS The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 2012, 139 (12), 2139–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Balestrini JL; Chaudhry S; Sarrazy V; Koehler A; Hinz B The mechanical memory of lung myofibroblasts. Integr. Biol. 2012, 4(4), 410–421. [DOI] [PubMed] [Google Scholar]
- (100).Hinz B; Phan SH; Thannickal VJ; Prunotto M; Desmouliere A; Varga J; De Wever O; Mareel M; Gabbiani G Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am. J. Pathol. 2012, 180, 1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Zunder ER; Finck R; Behbehani GK; Amir ED; Krishnaswamy S; Gonzalez VD; Lorang CG; Bjornson Z; Spitzer MH; Bodenmiller B; et al. Palladium-based mass tag cell barcoding with a doublet-filtering scheme and single-cell deconvolution algorithm. Nat. Protoc. 2015, 10 (2), 316–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Frei AP; Bava F-A; Zunder ER; Hsieh EW; Chen S-Y; Nolan GP; Gherardini PF Highly multiplexed simultaneous detection of RNAs and proteins in single cells. Nat. Methods 2016, 13, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Mosser DM; Edwards JP Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8 (12), 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Martin KS; Virgilio KM; Peirce SM; Blemker SS Computational Modeling of Muscle Regeneration and Adaptation to Advance Muscle Tissue Regeneration Strategies. Cells Tissues Organs 2015, 202 (3–4), 250–266. [DOI] [PubMed] [Google Scholar]
- (105).Martin KS; Kegelman CD; Virgilio KM; Passipieri JA; Christ GJ; Blemker SS; Peirce SM In Silico and In Vivo Experiments Reveal M-CSF Injections Accelerate Regeneration Following Muscle Laceration. Ann. Biomed. Eng. 2017, 45 (3), 747–760. [DOI] [PubMed] [Google Scholar]
- (106).Martin KS; Blemker SS; Peirce SM Agent-based computational model investigates muscle-specific responses to disuse-induced atrophy. J. Appl. Physiol. 2015, 118 (10), 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Brown BN; Price IM; Toapanta FR; DeAlmeida DR; Wiley CA; Ross TM; Oury TD; Vodovotz Y An agent-based model of inflammation and fibrosis following particulate exposure in the lung. Math. Biosci. 2011, 231 (2), 186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Voit EO Mesoscopic modeling as a starting point for computational analyses of cystic fibrosis as a systemic disease. Biochim. Biophys. Acta, Proteins Proteomics 2014, 1844 (1), 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Dutta-Moscato J; Solovyev A; Mi Q; Nishikawa T; Soto-Gutierrez A; Fox IJ; Vodovotz Y A Multiscale Agent-Based in silico Model of Liver Fibrosis Progression. Front. Bioeng. Biotechnol. 2014, 2, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Hao W; Rovin BH; Friedman A Mathematical model of renal interstitial fibrosis. Proc. Natl. Acad. Sci U. S. A. 2014, 111 (39), 14193–14198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Zeigler AC; Richardson WJ; Holmes JW; Saucerman JJ Computational modeling of cardiac fibroblasts and fibrosis. J. Mol. Cell. Cardiol. 2016, 93, 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Janes KA; Wang C-C; Holmberg KJ; Cabral K; Brugge JS Identifying single-cell molecular programs by stochastic profiling. Nat. Methods 2010, 7 (4), 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]


