Abstract
Holoprosencephaly (HPE) is a major structural birth defect of the brain that occurs in approximately 1 in 10,000 live births. Although some genetic causes of HPE are known, a substantial proportion of cases have an unknown etiology. Due to the low birth prevalence and rarity of exposure to many potential risk factors for HPE, few epidemiologic studies have had sufficient sample size to examine risk factors. A 2010 review of the literature identified several risk factors that had been consistently identified as occurring more frequently among cases of HPE, including maternal diabetes, twinning, and a predominance of females, while also identifying a number of potential risk factors that had been less widely studied. In this article, we summarize a systematic literature review conducted to update the evidence for nongenetic risk factors for HPE.
Keywords: cyclopia, diabetes, holoprosencephaly, sex ratio, twinning
1 |. INTRODUCTION
Holoprosencephaly (HPE) is a major structural birth defect of the brain and is considered one of the most frequently occurring brain abnormalities, with a prevalence of approximately 1 in 250 conceptions (Matsunaga & Shiota, 1977). However, the birth prevalence of HPE is much lower at about 1 in 10,000 live births, indicating a high rate of fetal death, which poses a challenge for epidemiologic study of possible risk factors (Orioli & Castilla, 2010; Rasmussen, Moore, Khoury, & Cordero, 1996). Case classification and ascertainment of HPE is also challenging because there is a continuum of phenotypic variation, including mild or asymptomatic presentations that are not always classified as HPE (Hahn & Barnes, 2010), and a spectrum of accompanying facial characteristics, such as cyclopia, the most severe form of HPE (Orioli & Castilla, 2010). Although some genetic causes of HPE have been identified (i.e., single gene disorders and chromosome abnormalities) (Roessler & Muenke, 2010), the etiology of many cases of HPE is unknown.
The vast majority of papers in the scientific literature regarding nongenetic risk factors for HPE in humans are case reports and small case series, which are important contributions to the literature in that they can generate hypotheses for future research. For example, recent case reports have described infants with HPE born to mothers with diabetes and a mother with alcoholism (Chen et al., 2012; Goswami & Kusre, 2015; Pallangyo et al., 2016). However, little inference can be made regarding whether the exposure-birth defect correlation observed is due to chance alone because these reports lack an appropriate comparison group and cannot account for the background rate of birth defects (Rasmussen, Hayes, Jamieson, & O’Leary, 2007).
Likewise, animal studies also provide important contributions to our understanding of teratogens. Recent studies have found associations between HPE, alcohol consumption and synthetic cannabinoids and evidence of gene-environment interactions with exposure to retinoic acid in animal models (Billington et al., 2015; Gilbert et al., 2016; Hong & Krauss, 2017). Although animal models allow experimental manipulation of exposures and provide an opportunity to investigate gene-environment interactions, findings may not be relevant to humans (Scialli et al., 2004).
Epidemiologic studies, including case-control studies, cohort studies, and reports from birth defects surveillance systems or pregnancy registries, can provide opportunities to evaluate risk factors for HPE in humans over time and with appropriate comparison groups. However, given the low birth prevalence of HPE and rarity of many exposures of interest, such as medication use or infections during pregnancy, few epidemiologic studies have adequate sample size, and statistical power to evaluate many potential risk factors. These epidemiologic studies are typically retrospective case-control studies that investigate a spectrum of risk factors for HPE or are studies of specific risk factors that have included HPE as one of many defects studied.
A 2010 review identified four large case-control studies that focused on risk factors for HPE and a few other studies that examined factors associated with a range of birth defects including HPE (Johnson & Rasmussen, 2010). The most extensively studied risk factor identified by the review was maternal diabetes, with numerous studies observing a prevalence of pre-existing and gestational diabetes at least twice as high among mothers of children with HPE as mothers of controls (Anderson et al., 2005; Correa et al., 2008; Croen, Shaw, & Lammer, 2000; Martínez-Frías, Bermejo, Rodríguez-Pinilla, Prieto, & Frías, 1998; Orioli & Castilla, 2007). Sex ratio has also been widely studied, with many studies finding an excess of females with HPE (Croen et al., 2000; Kallen et al., 1992; Olsen, Hughes, Youngblood, & Sharpe-Stimac, 1997; Orioli & Castilla, 2007; Rasmussen et al., 1996; Whiteford & Tolmie, 1996). HPE has also consistently been observed to occur more frequently among twins or other multiple births than singletons (Bullen, Rankin, & Robson, 2001; Miller, Rasmussen, Siega-Riz, Frias, & Honein, 2010; Odent, Le Marec, Munnich, Le Merrer, & Bonaïti-Pellie, 1998).
According to the 2010 review, a number of other maternal demographic characteristics and exposures have been identified as potential risk factors for HPE, but there was less evidence to support these. Epidemiologic studies assessing the use of aspirin and salicylates have had inconsistent results, with two studies finding an association with HPE and one that did not (Croen et al., 2000; Miller et al., 2010; Orioli & Castilla, 2007). Other factors such as use of assisted reproductive technologies and maternal sexually transmitted infections may increase the risk of HPE and warrant future study (Croen et al., 2000; Miller et al., 2010).
In the current manuscript, we update the evidence for nongenetic risk factors for HPE based on a systematic review of the literature published since the 2010 review was conducted (Johnson & Rasmussen, 2010). Given the limited conclusions that can be drawn from case reports and animal studies, we have elected to restrict this review to epidemiologic studies.
2 |. METHODS
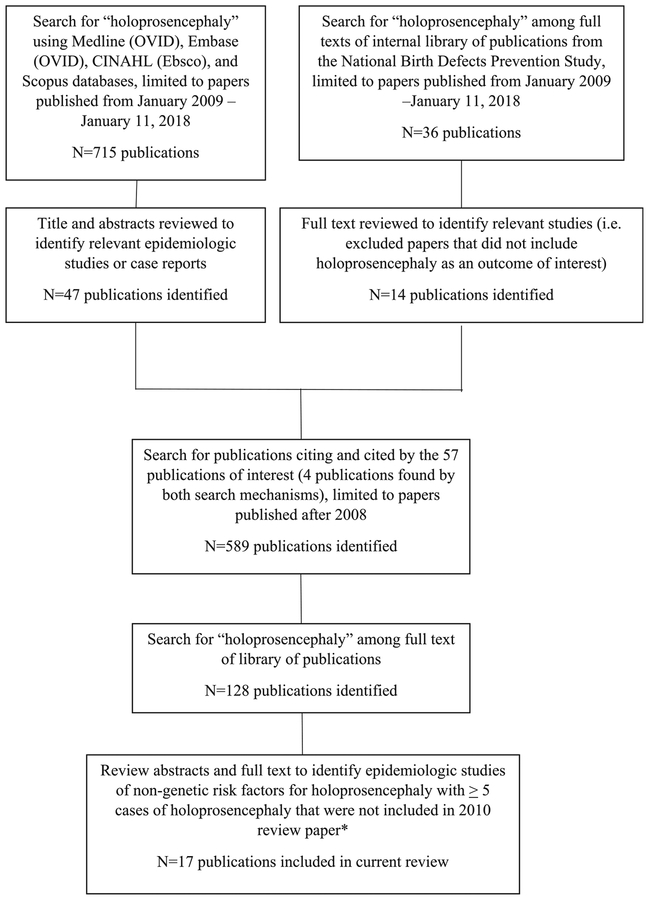
Because studies in which HPE was included as one of many defects under investigation for a particular risk factor might be missed in a traditional systematic literature review, we conducted our review in multiple phases (Figure 1). We first searched for “holoprosencephaly” using Medline (OVID), Embase (OVID), CINAHL (Ebsco), and Scopus databases (see Appendix A for full search strategy). We supplemented this with a search of full texts in an internal library of publications from the National Birth Defects Prevention Study (NBDPS), a case-control study that included rare defects such as HPE. We included publications from January 2009 through January 2018. After this first phase identified 57 epidemiologic studies and case reports that were potentially of interest, we searched for “holoprosencephaly” among the full text of the publications citing and cited by these publications. We limited the publications included in this review to those with at least five cases of HPE that were not included in the 2010 review paper. The full texts of these publications were reviewed and 17 publications were included in this review.
FIGURE 1.
Search strategy for identifying epidemiologic studies of nongenetic risk factors of holoprosencephaly since the 2010 review was conducted. * Johnson and Rasmussen (2010)
3 |. RESULTS
3.1 |. National Birth Defects Prevention Study
Between 2009 and January 2018, 14 publications using data from the NBDPS included HPE as an outcome of interest (Table 1); however, one previous study of nongenetic risk factors for HPE published during this time period was included in the 2010 review and excluded from the current review (Miller et al., 2010). The NBDPS was a population-based case-control study of major birth defects that was conducted for births in 10 U.S. states between October 1997 and December 2011. NBDPS methods for recruitment of participants and case classification have been described in detail elsewhere (Rasmussen et al., 2003; Reefhuis et al., 2015; Yoon et al., 2001). Briefly, NBDPS cases include infants or fetuses with one or more major structural birth defect identified through population-based active case-finding surveillance systems in Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, and Utah. Cases were reviewed by a clinical geneticist, classified as isolated or multiple (>1 major unrelated birth defect in separate organ systems), and those with single-gene disorders or recognized chromosomal syndromes were excluded (Rasmussen et al., 2003). For inclusion, diagnoses of HPE in cases needed to be confirmed postnatally, either by imaging studies or post-mortem examination. Liveborn infants without major birth defects (controls) were randomly selected from the same ascertainment areas as cases using birth certificates or hospital birth records.
TABLE 1.
Analyses of risk factors for holoprosencephaly from the National Birth Defects Prevention Study
| Primary exposure of interest | Years included | Holoprosencephaly cases | Controls | Adjusted odds ratio (aOR) (95% confidence interval) | |
|---|---|---|---|---|---|
| Crider et al. (2009) | Maternal antibacterial medication use | 1997–2003 | Any antibacterial exposure: 10 (16.4%) Penicillin exposure: 4 (6.6%) Total: 61 |
Any antibacterial exposure: 641 (13.0%) Penicillin exposure: 293 (5.9%) Total: 4,941 |
Any antibacterial: aORa 1.5 (0.7–3.0) Penicillin: aORa 1.3 (0.5–3.7) |
| Browne et al. (2009) | Maternal thyroid disease | 1997–2004 | All cases: Exposed: 3 (5.9%) Total: 51 Isolated cases: Exposed: 3 Total: 73 |
Exposed: 111 (1.9%) Total: 5,875 |
All cases: unadjusted OR (uOR) 2.2 (0.4–6.9) Isolated cases: uOR 3.2 (0.6–10.3) |
| Carter et al. (2011) | Maternal genital tract infections | 1997–2004 | Exposed: 2 (4%) Total: 50 |
Exposed:139 (2.4%) Total:5913 |
aORb 1.79 (0.42–7.56) |
| Correa et al. (2012) | Joint effects of maternal diabetes mellitus and use of folic acid-containing multivitamins | 1997–2004 | Diabetic, took vitamins: 2 Nondiabetic, no use of vitamins: 13 Nondiabetic, took vitamins: 48 Total: 63 |
Diabetic, no use of vitamins: 2 Diabetic, took vitamins: 27 Nondiabetic, no use of vitamins: 671 Nondiabetic, took vitamins: 4,737 Total: 5,437 |
Ref: Nondiabetic, took vitamins Nondiabetic, no use of vitamins: aORc 1.52 (0.69–3.39) Diabetic, took vitamins: aORc 9.06 (1.97–41.77) |
| Tinker et al. (2011) | Maternal injury | 1997–2005 | Exposed: 4 (5.2%) Total: 77 |
Exposed: 166 (2.6%) Total: 6,328 |
aORd 2.6 (0.9–7.3) |
| Gill et al. (2012) | Maternal age | 1997–2007 | Total: 100 | Total: 8,169 | Ref: 25–29 years <20 years: aORe 1.0 (0.5–2.1) 20–24 years: aORe 1.2 (0.7–2.1) 30–34 years: aORe 1.0 (0.6–1.8) 35–39 years: aORe 1.2 (0.6–2.5) |
| Parker et al. (2012) | High dietary glycemic index | 1997–2007 | Total: 94 | Total: 7,505 | aORf 2.17 (0.66–7.15) |
| Dawson et al. (2016) | Twinning | 1997–2007 | Exposed: 7 (6.2%) Total: 113 |
Exposed: 218 (2.8%) Total: 7,872 |
aORg 2.7 (1.2–5.8) |
| Srisukhumbowornchai et al. (2012) | Maternal smoking | 2003–2007 | Exposed: 1 Total: not reported |
Exposed: 48 (7.9%) Total: 610 |
aORh 0.59 (0.06–5.36) |
| Michalski et al. (2015) | Sex ratio | 1997–2009 | Male: 53 Female: 83 |
Male: 5,034 Female: 4,859 |
Sex ratio M/F: 0.62 (0.44–0.87) |
| Howley et al. (2016) | Maternal autoimmune disease | 1997–2009 | Exposed: 1 (0.7%) Total 134 |
Exposed: 94 (1.0%) Total: 9,803 |
Not calculated |
| Hoyt et al. (2016) | Maternal exposure to secondhand tobacco smoke | 1997–2009 | Exposed: 9 (13.6%) Total: 66 |
Exposed: 1,012 (15.6%) Total: 6,480 |
aORi 0.72 (0.34–1.51) |
| Howley et al. (2017) | Maternal thyroid hormone use | 1997–2011 | Exposed: 7 (4.1%) Total: 172 | Exposed: 237 (2.1%) Total: 11,527 | aORj 2.48 (1.13–5.44) |
Model adjusted for maternal age, race, education, prepregnancy body mass index, time from the estimated date of delivery to the interview, use of folic acid or multivitamins containing folic acid from 1 month before pregnancy through the first month of pregnancy, and any periconceptional smoking or alcohol use.
Model adjusted for maternal age, race/ethnicity, education, parity, prepregnancy obesity, family history of a similar birth defect in a first-degree relative, use of folic acid supplements, smoking, alcohol use, and study center.
Models adjusted for maternal age, race, and ethnicity, entry into prenatal care, prepregnancy body mass index, parity, and household income.
Model adjusted for age, race and ethnicity, educational attainment, household income, alcohol use during pregnancy, smoking during pregnancy, prepregnancy body mass index, occupational status, and study site.
Models adjusted for race/ethnicity, body mass index, folic acid use, gravidity, education, smoking, and parental age difference; mothers with missing covariate data are excluded from these analyses.
Model adjusted for site, maternal age, maternal education, maternal race, and folic acid use in the month prior to or after conception.
Model adjusted for maternal age at delivery (continuous), race, parity, obesity, education, smoking, use of a folic acid-containing multivitamins, and study site.
Model adjusted for maternal age, race, education, and body mass index.
Model adjusted for maternal age, education, race, body mass index, nativity, alcohol intake one month prior to conception through the first trimester, folic acid intake in multivitamins or alone one month prior to conception through the first month of pregnancy, dietary folate equivalent, previous live births, pregnancy intention, household income divided by no. of people supported by this income, study center, and time to interview.
Model adjusted for maternal age, maternal race/ethnicity, and state of residence at the time of birth.
Mothers of cases and controls participated in a computer-assisted telephone interview, conducted in English or Spanish, between six weeks and 24 months after their expected date of delivery. The interview included detailed questions concerning pregnancy history, demographic information, and exposures, such as medication use and maternal infections, during the time period of three months before conception through the end of pregnancy. During the entire study period, the interview participation rate was approximately 65% for mothers of controls (n = 11,814) and 67% for mothers of children with HPE (n = 32,187) (Reefhuis et al., 2015). Between 1997 and 2011, 321 cases of HPE were ascertained and 181 mothers were interviewed; this includes cases of isolated HPE and cases with HPE and other major birth defects (multiple). Because analyses of NBDPS data were conducted throughout the study period and the inclusion criteria for each analysis varied, the number of cases and controls included varied. Unless otherwise noted, all NBDPS analyses described in this review included both cases of isolated and multiple HPE and exposures occurred during the periconceptional period, from one month prior to conception through the third month of pregnancy.
Of the 13 NBDPS studies included in this review, the first NBDPS analysis we describe included 1997–2003 data, was restricted to mothers without pre-existing diabetes, and found no significant association between periconceptional antibiotic use and HPE (Crider et al., 2009). In an analysis of 1997–2004 data, mothers of children with isolated HPE were more than three times more likely than mothers of controls to report a history of thyroid disease, although this finding was based on three exposed cases and was not statistically significant (Browne et al., 2009). A publication using data from 1997 to 2004 found that mothers of children with HPE were more likely than mothers of controls to have reported a genital tract infection during the first trimester, although this finding was based on two exposed cases and was not statistically significant (Carter et al., 2011). Among women who took a folic acid-containing multivitamin, maternal diabetes was associated with a ninefold increased risk of HPE (adjusted odds ratio (aOR) 9.06; 95% confidence interval (CI): 1.97–41.77); however, this finding was based on two exposed cases (Correa et al., 2012). Tinker and colleagues found that mothers of infants with HPE were more than twice as likely to report an injury than mothers of controls, although this finding was also not statistically significant (Tinker, Reefhuis, Dellinger, & Jamieson, 2011).
Three NBDPS analyses used data from 1997 to 2007 and a fourth included data from 2003 to 2007 from the Utah site only. After excluding women with diabetes and multiple births, Gill et al. (2012) found no association between HPE and maternal age. In a 2012 publication examining the association between birth defects and consumption of foods with a high glycemic index, among women without diabetes, an increased odds ratio was observed for HPE but was not statistically significant (Parker et al., 2012). In a 2016 publication, Dawson et al. (2016) found a nearly threefold increase in HPE prevalence among members of twins, compared to singletons (aOR 2.7; 95% CI: 1.2–5.8), which remained elevated in analyses restricted to women who did not use fertility treatments. In a study of data from the Utah NBDPS site, one mother of a child with HPE self-reported cigarette smoking at any time during pregnancy, which was not statistically different from mothers of control infants (Srisukhumbowornchai, Krikov, & Feldkamp, 2012).
Three later NBDPS analyses included data from 1997 to 2009 (Howley et al., 2016; Hoyt et al., 2016; Michalski et al., 2015), and one used data from the entire study period of 1997–2011 (Howley et al., 2017). In a 2015 study of male/female sex ratios, a significant predominance of females was found among the cases with HPE compared to controls (0.62; 95% CI: 0.44–0.87) (Michalski et al., 2015). This finding remained significant in a sub-analysis restricted to isolated cases. In an analysis examining the association between maternal autoimmune disease and birth defects (excluding mothers with pregestational diabetes), one exposed case of HPE was reported among 134 total cases, a slightly lower proportion of exposure than in the controls (Howley et al., 2016). In a study of maternal periconceptional secondhand tobacco smoke exposure in the home, workplace or school, exposure was slightly less common among mothers of children with HPE than controls, after excluding cases and controls from multiple births and mothers reporting active smoking or pregestational diabetes (Hoyt et al., 2016). Lastly, in a 2017 publication, use of thyroid hormones was significantly higher among mothers of children with HPE than among mothers of controls (aOR 2.48; 95% CI 1.13–5.44), based on seven exposed cases; the analysis remained statistically significant when restricted to isolated cases of HPE (Howley et al., 2017).
3.2 |. Other epidemiologic studies of HPE
One of the largest studies of HPE to date compared cases with isolated cyclopia to cases with associated malformations and those with chromosomal syndromes (Table 2) (Orioli et al., 2011). This study included data from 1968 to 2006 from 20 surveillance systems in 25 countries that were part of the International Clearinghouse of Birth Defects Surveillance and Research. Information on phenotype, genetic testing, and selected demographic and prenatal characteristics was provided. All diagnostic information were reported as verbatim descriptions and were centrally classified. The authors classified 97 isolated cases of cyclopia, 79 cases as having chromosomal syndromes, and 81 cases with malformations not usually considered part of the HPE spectrum. Of the isolated cases, 60.8% were female, a higher percentage than among cases with other malformations (53.1%) or those with chromosomal syndromes (51.9%), although these differences were not statistically significant. The authors also observed a higher birth prevalence of cyclopia among older mothers, but this was not statistically significant. Other characteristics such as parity, history of spontaneous abortion, plurality, and parental age difference were not significantly different for isolated cases compared to cases with other malformations or chromosomal syndromes.
TABLE 2.
Other epidemiologic studies of holoprosencephaly
| Primary exposure(s) of interest | Study design | Holoprosencephaly cases | Comparison group | Results | Comments | |
|---|---|---|---|---|---|---|
| Orioli et al. (2011) | Spectrum of fetal/infant, maternal, and paternal characteristics | Birth defects registries | Total cases: n = 257 Isolated cases: n = 97 Cases with associated malformations: n = 81 Chromosomal syndromes: n = 79 |
None | Females predominated for all subgroups (55.6% of total); No significant differences in demographic characteristics between subgroups | Data from International Clearinghouse for Birth Defects Surveillance and research from 1968 to 2006; diagnosis restricted to cyclopia |
| Tennant et al. (2011) | Sex ratio | Birth defects registry | Male: 14 Female: 22 |
Singleton births, not reported | Sex ratio M/F: 0.60 (0.31–1.18) | Data from Northern Congenital Abnormality Survey, UK, 1985–2003; Diagnosis included arhinencephaly/holoprosencephaly |
| Vaz et al. (2012) | Spectrum of maternal and paternal characteristics | Hospital-based case-control study | N = 23 | Control matched on gender and birthdate (n = 47) | Aboriginal mother: unadjusted OR (uOR) 3.5 (1.1–11.1); Aboriginal father: uOR 12.8 (3.0–55.1); Two aboriginal parents: uOR 8.8 (2.0–37.8); Family history midline facial defects: uOR 8.2 (1.5–45.2); Low socioeconomic status: uOR 3.0 (1.0–9.1); Other factors not statistically significant |
Data from Winnipeg Children's Hospital Section of Genetics and Metabolism records from 1990 to 2001 |
| Sokal et al. (2014) | Sex ratio | Case-control study | Not reported | Total population: males (n = 408,184), females (n = 385,985) | Sex ratio M/F: 0.47 (0.09–2.58) | The Health Improvement Network, anonymized primary care records covering 6% of UK population from 1990 to 2010 |
Two additional publications since 2009 have examined the association between HPE and sex ratio (Sokal, Tata, & Fleming, 2014; Tennant, Samarasekera, Pless-Mulloli, & Rankin, 2011). A 2011 publication used 1985–2003 data from the Northern Congenital Abnormality Survey, which collected diagnostic and demographic data for all birth defects, including live births and fetal deaths, in several counties in the North of England, United Kingdom (Tennant et al., 2011). Multiple births and cases associated with a known teratogen were excluded. Cases of HPE (including arhinencephaly) included 14 males and 22 females. The relative risk of HPE for males compared to females was not statistically significant when compared to the general population. A second U.K.-based study used data from 1990 to 2010 from The Health Improvement Network, an anonymized database of primary care records covering approximately 6% of the U.K. population (Sokal et al., 2014). The study population included all children in the database born during the study period who were registered with their general practice before their first birthday (n = 794,169). This study also found a predominance of females among cases of arhinencephaly/HPE, but that finding was also not statistically significant.
Lastly, a case-control study from 2012 examined a number of potential risk factors for nonsyndromic HPE using data from the Winnipeg Children’s Hospital Section of Genetics and Metabolism in Manitoba, Canada (Vaz et al., 2012). Cases were live births, terminations, and stillbirths between January 1990 and September 2001 that had radiologic or autopsy confirmation or strongly suggestive clinical sequence of HPE. Controls were from the same database and matched on gender and nearest birthdate. If the control selected had (a) a midline craniofacial or structural central nervous system anomaly or (b) multiple birth defects and a strongly suspected structural central nervous system anomaly, they were excluded and the next eligible person was selected as the control. In total, 47 patients with HPE were identified and 47 matched controls were selected; 24 of the cases with HPE were determined to be part of a syndrome. The final analysis included 23 cases of nonsyndromic HPE and all 47 controls. Significant positive associations with HPE were observed for having an Aboriginal parent, either mother or father, and having a family history of a midline facial defect. While the authors reported that mothers of children with HPE were nearly seven times more likely than mothers of controls to report pre-existing diabetes, the study only included three exposed cases and one exposed control and the finding did not reach statistical significance. Other factors that were observed to have elevated odds ratios were based on small numbers and were not statistically significant; these included low socioeconomic status and having had a previous stillbirth or a previous neonatal death.
4 |. DISCUSSION
The majority of the studies examining risk factors for nonsyndromic HPE that have been published since 2009 have used data from the NBDPS, which was well positioned to provide data for these analyses as a result of the large sample size and diversity of topics included in the maternal interview. However, even in the NBDPS, most analyses included small numbers of exposed cases, and findings were often not statistically significant. NBDPS publications added evidence to support previous findings of an association between HPE and diabetes, twinning, and sex ratio.
When considering potential risk factors for birth defects, assessment of the biologic plausibility of the findings is important. Maternal diabetes is a risk factor for a large number of birth defects (Gabbay-Benziv, Reece, Wang, & Yang, 2015), including HPE. Several mechanisms have been proposed to explain the effects of maternal hyperglycemia as a cause of birth defects, including increased oxidative stress, hypoxia, apoptosis, and epigenetic changes (Ornoy, Reece, Pavlinkova, Kappen, & Miller, 2015). Multivitamin supplements might attenuate the risk for some diabetes-associated birth defects (Correa, Botto, Liu, Mulinare, & Erickson, 2003; Correa et al., 2012); however, diabetes was a strong risk factor for HPE, even among women who were taking folic acid containing supplements (Correa et al., 2012). A better understanding of these mechanisms could lead to future measures to prevent birth defects among women with diabetes.
Twinning has also been associated with several different types of birth defects (Weber & Sebire, 2010), including HPE. It has been hypothesized that early structural defects (such as HPE) in monozygotic twins might represent cleavage disorders of the midline field (Opitz & Gilbert, 1982; Suslak, Mimms, Desposito, Opitz, & Reynolds, 1987).
The reason for the female predominance among HPE cases is unclear. Female embryos might be more susceptible to perturbations that cause HPE or male embryos might be more likely to be lost through spontaneous abortion. A large proportion of embryos with HPE are lost prenatally (Matsunaga & Shiota, 1977); however, an equal sex ratio among fetuses identified prenatally at 16–36 weeks’ gestation (Berry, Gosden, Snijders, & Nicolaides, 1990) suggests that losses at this later stage in pregnancy do not occur more frequently among males.
The association between thyroid hormone use and HPE observed in data from the NBDPS needs to be replicated in other studies. Whether the observed association is related to use of thyroid hormone, or to the underlying condition for which thyroid hormone treatment is used, or is due to chance is unknown. However, thyroid hormone is necessary for development of the fetal nervous system (Schroeder & Privalsky, 2014), so the association could be biologically plausible.
HPE is a rare and serious birth defect that often results in pregnancy loss, which makes it challenging to identify nongenetic risk factors. Studies with a small number of exposed cases result in unstable effect estimates and wide confidence intervals, making it difficult to distinguish true risk factors from findings observed by chance. To better understand risk factors for HPE, data sets with large numbers of cases and sufficient statistical power are needed. Because HPE has a wide spectrum of phenotypes and is often associated with a chromosomal abnormality or single gene disorder, careful clinical review and classification are also essential to create well-defined outcome categories. In addition, incorporation of information on genetic factors is important to permit the study of gene-environment interaction.
AUTHOR BIOGRAPHIES

D. SUMMERS is an epidemiologist in the Division of Congenital and Developmental Disorders at the Centers for Disease Control and Prevention. She received her MPH in Epidemiology from Emory University.

J. REEFHUIS is a senior epidemiologist in the Division of Congenital and Developmental Disorders at the Centers for Disease Control and Prevention and the lead epidemiologist for the National Birth Defects Prevention Study. She received her PhD in Epidemiology from the University of Groningen in the Netherlands.

J. TALIANO is a research librarian working in the Stephen B. Thacker CDC Library. She has over 10 years of experience working as a librarian in the field of Public Health at the Centers for Disease Control and Prevention in Atlanta, GA. She holds a Master Degree in Library Science and a Master Degree in English Literature from the State University of New York.

S. A. RASMUSSEN is a pediatrician and clinical geneticist at the Centers for Disease Control and Prevention. She received her MD with honors from the University of Florida and completed a residency in pediatrics at Massachusetts General Hospital and fellowships in clinical genetics at Johns Hopkins and the University of Florida.
APPENDIX A
Search Strategy and Results of Systematic Review
| Database | Strategy | Run date | Records |
|---|---|---|---|
| Medline (OVID) 1946- | Holoprosencephaly | 2/15/2018 | 59 |
| AND | |||
| Case* OR epidemiolog* OR report* OR inciden* OR occurrence OR surveillance OR Diabetes OR diabetic* OR drug* OR substance abuse* OR medication* OR prescription* OR prescribe* OR nutrition* OR maternal health OR (mother* ADJ2 health) OR side effect* OR adverse reaction* OR adverse effect* OR adverse event* OR hypertension OR blood pressure OR complication* OR exposure* OR expose* OR risk* OR factor* OR non-genetic OR nongenetic OR non-syndromic OR nonsyndromic OR environmental OR (maternal ADJ2 (illness* OR disease*)) OR sociodemographic* OR demographic* OR alcohol* OR ethanol OR teratogen* OR hyperglycemi* OR infection* OR CMV OR cytomegalovirus OR rubella OR herpes OR virus* OR syphilis OR influenza OR fever OR STI* OR STD* OR chlamydia OR anemia OR supplement* OR salicylate* OR sulfasalazine OR aspirin OR acetaminophen OR advanced age OR smoking OR smoked OR tobacco OR antibiotic* OR penicillin* OR ampicillin OR trimethoprim OR sulfa-methoxazole OR hormone* OR progesterone OR contraceptive* OR progesto-gen* OR birth control OR assisted reproduct* OR cholesterol OR statin* OR lovastatin OR methotrexate OR tranexamic acid OR chloriazepoxide OR imipramine OR lithium OR dimenhydrinate OR fertility OR obesity OR BMI OR diet* OR education* OR income OR socioeconomic* Or cancer* OR epilepsy OR epileptic OR antiepileptic* OR anticonvulsant* OR acne OR retinoic acid | |||
| Limit 2009 ; | |||
| Embase (OVID) 1947- | Holoprosencephaly | 2/15/2018 | 116 – 58 duplicates = 58 unique items |
| AND | |||
| Case* OR epidemiolog* OR report* OR inciden* OR occurrence OR surveillance OR Diabetes OR diabetic* OR drug* OR substance abuse* OR medication* OR prescription* OR prescribe* OR nutrition* OR maternal health OR (mother* ADJ2 health) OR side effect* OR adverse reaction* OR adverse effect* OR adverse event* OR hypertension OR blood pressure OR complication* OR exposure* OR expose* OR risk* OR factor* OR non-genetic OR nongenetic OR nonsyndromic OR nonsyndromic OR environmental OR (maternal ADJ2 (illness* OR disease*)) OR sociodemographic* OR demographic* OR alcohol* OR ethanol OR teratogen* OR hyperglycemi* OR infection* OR CMV OR cytomegalovirus OR rubella OR herpes OR virus* OR syphilis OR influenza OR fever OR STI* OR STD* OR chlamydia OR anemia OR supplement* OR salicylate* OR sulfasalazine OR aspirin OR acetaminophen OR advanced age OR smoking OR smoked OR tobacco OR antibiotic* OR penicillin* OR ampicillin OR trimethoprim OR sulfa-methoxazole OR hormone* OR progesterone OR contraceptive* OR progesto-gen* OR birth control OR assisted reproduct* OR cholesterol OR statin* OR lovastatin OR methotrexate OR tranexamic acid OR chloriazepoxide OR imipr-amine OR lithium OR dimenhydrinate OR fertility OR obesity OR BMI OR diet* OR education* OR income OR socioeconomic* Or cancer* OR epilepsy OR epileptic OR antiepileptic* OR anticonvulsant* OR acne OR retinoic acid | |||
| Limit 2009 ; | |||
| CINAHL (Ebsco) | Holoprosencephaly | 02/15/2018 | 8–6 duplicates = 2 unique items |
| AND | |||
| Case* OR epidemiolog* OR report* OR inciden* OR occurrence OR surveillance OR Diabetes OR diabetic* OR drug* OR “substance abuse*” OR medication* OR prescription* OR prescribe* OR nutrition* OR “maternal health” OR (mother* N2 health) OR “side effect*” OR “adverse reaction*” OR “adverse effect*” OR “adverse event*” OR hypertension OR blood pressure OR complication* OR exposure* OR expose* OR risk* OR factor* OR non-genetic OR nonge-netic OR non-syndromic OR nonsyndromic OR environmental OR (maternal N2 (illness* OR disease*)) OR sociodemographic* OR demographic* OR alcohol* OR ethanol OR teratogen* OR hyperglycemi* OR infection* OR CMV OR cytomega-lovirus OR rubella OR herpes OR virus* OR syphilis OR influenza OR fever OR STI* OR STD* OR chlamydia OR anemia OR supplement* OR salicylate* OR sul-fasalazine OR aspirin OR acetaminophen OR advanced age OR smoking OR smoked OR tobacco OR antibiotic* OR penicillin* OR ampicillin OR trimethoprim OR sulfamethoxazole OR hormone* OR progesterone OR contraceptive* OR progestogen* OR “birth control” OR “assisted reproduct*” OR cholesterol OR statin* OR lovastatin OR methotrexate OR tranexamic acid OR chloriazepoxide OR imipramine OR lithium OR dimenhydrinate OR fertility OR obesity OR BMI OR diet* OR education* OR income OR socioeconomic* Or cancer* OR epilepsy OR epileptic OR antiepileptic* OR anticonvulsant* OR acne OR “retinoic acid” | |||
| Limit 2009 ; exclude Medline records | |||
| Cochrane library | Holoprosencephaly:ti,ab | 02/15/2018 | 0 |
| AND | |||
| (Case* OR epidemiolog* OR report* OR inciden* OR occurrence OR surveillance OR Diabetes OR diabetic* OR drug* OR “substance abuse*” OR medication* OR prescription* OR prescribe* OR nutrition* OR “maternal health” OR (mother* NEAR/2 health) OR “side effect*” OR “adverse reaction*” OR “adverse effect*” OR “adverse event*” OR hypertension OR blood pressure OR complication* OR exposure* OR expose* OR risk* OR factor* OR non-genetic OR nonge-netic OR non-syndromic OR nonsyndromic OR environmental OR (maternal NEAR/2 (illness* OR disease*)) OR sociodemographic* OR demographic* OR alcohol* OR ethanol OR teratogen* OR hyperglycemi* OR infection* OR CMV OR cytomegalovirus OR rubella OR herpes OR virus* OR syphilis OR influenza OR fever OR STI* OR STD* OR chlamydia OR anemia OR supplement* OR salicylate* OR sulfasalazine OR aspirin OR acetaminophen OR advanced age OR smoking OR smoked OR tobacco OR antibiotic* OR penicillin* OR ampicillin OR trimethoprim OR sulfamethoxazole OR hormone* OR progesterone OR contraceptive* OR progestogen* OR “birth control” OR “assisted reproduct*” OR cholesterol OR statin* OR lovastatin OR methotrexate OR tranexamic acid OR chloriazepoxide OR imipramine OR lithium OR dimenhydrinate OR fertility OR obesity OR BMI OR diet* OR education* OR income OR socioeconomic* Or cancer* OR epilepsy OR epileptic OR antiepileptic* OR anticonvulsant* OR acne OR “retinoic acid”):ti,ab | |||
| Limit 2009 ; | |||
| Scopus | Non-genetic risk factors for holoprosencephaly cited by | 02/15/2018 | 0 |
| OR | |||
| TITLE-ABS-KEY(Holoprosencephaly AND (Case* OR epidemiolog* OR report* OR inciden* OR occurrence OR surveillance OR Diabetes OR diabetic* OR drug* OR “substance abuse*” OR medication* OR prescription* OR prescribe* OR nutrition* OR “maternal health” OR (mother* W/2 health) OR “side effect*” OR “adverse reaction*” OR “adverse effect*” OR “adverse event*” OR hypertension OR “blood pressure” OR complication* OR exposure* OR expose* OR risk* OR factor* OR non-genetic OR nongenetic OR non-syndromic OR nonsyndromic OR environmental OR (maternal W/2 illness*) OR (maternal W/2 disease*) OR sociodemographic* OR demographic* OR alcohol* OR ethanol OR teratogen* OR hyperglycemi* OR infection* OR CMV OR cytomegalovirus OR rubella OR herpes OR virus* OR syphilis OR influenza OR fever OR STI* OR STD* OR chlamydia OR anemia OR supplement* OR salicylate* OR sulfasalazine OR aspirin OR acetaminophen OR advanced age OR smoking OR smoked OR tobacco OR antibiotic* OR penicillin* OR ampicillin OR trimethoprim OR sulfamethoxazole OR hormone* OR progesterone OR contraceptive* OR progestogen* OR “birth control” OR “assisted reproduct*” OR cholesterol OR statin* OR lovastatin OR methotrexate OR tranexamic acid OR chloriazepoxide OR imipramine OR lithium OR dimenhydrinate OR fertility OR obesity OR BMI OR diet* OR education* OR income OR socioeconomic* Or cancer* OR epilepsy OR epileptic OR antiepileptic* OR anticonvulsant* OR acne OR “reti-noic acid”)) AND NOT INDEX(medline) AND NOT INDEX(embase) | |||
| Limit 2009 ; | |||
| Medline (OVID) 1946- | Holoprosencephaly | 1/11/2018 | 465 |
| AND | |||
| Case* OR epidemiolog* OR report* OR inciden* OR occurrence OR surveillance OR Diabetes OR diabetic* OR drug* OR substance abuse* OR medication* OR prescription* OR prescribe* OR nutrition* OR maternal health OR (mother* ADJ2 health) OR side effect* OR adverse reaction* OR adverse effect* OR adverse event* OR hypertension OR blood pressure OR complication* OR exposure* OR expose* OR risk* OR factor* OR non-genetic OR nongenetic OR nonsyndromic OR nonsyndromic OR environmental OR (maternal ADJ2 (illness* OR disease*)) OR sociodemographic* OR demographic* OR alcohol* OR ethanol OR teratogen* OR hyperglycemi* OR infection* OR CMV OR cytomegalovirus OR rubella OR herpes OR virus* OR syphilis OR influenza OR fever OR STI* OR STD* OR chlamydia OR anemia OR supplement* OR salicylate* OR sulfasalazine OR aspirin OR acetaminophen OR advanced age OR smoking OR smoked OR tobacco OR antibiotic* OR penicillin* OR ampicillin OR trimethoprim OR sulfamethoxazole OR hormone* OR progesterone OR contraceptive* OR progestogen* OR birth control OR assisted reproduct* OR cholesterol OR statin* OR lovastatin OR methotrexate OR tranexamic acid OR chloriazepoxide OR imipramine OR lithium OR dimenhydrinate OR fertility OR obesity OR BMI OR diet* OR education* OR income OR socioeconomic* Or cancer* OR epilepsy OR epileptic OR antiepileptic* OR anticonvulsant* OR acne OR retinoic acid | |||
| Limit 2010- ; | |||
| Embase (OVID) 1947- | Holoprosencephaly | 1/11/2018 | 137 – 22 duplicates = 115 unique items |
| AND | |||
| Case* OR epidemiolog* OR report* OR inciden* OR occurrence OR surveillance OR Diabetes OR diabetic* OR drug* OR substance abuse* OR medication* OR prescription* OR prescribe* OR nutrition* OR maternal health OR (mother* ADJ2 health) OR side effect* OR adverse reaction* OR adverse effect* OR adverse event* OR hypertension OR blood pressure OR complication* OR exposure* OR expose* OR risk* OR factor* OR non-genetic OR nongenetic OR non-syndromic OR nonsyndromic OR environmental OR (maternal ADJ2 (illness* OR disease*)) OR sociodemographic* OR demographic* OR alcohol* OR ethanol OR teratogen* OR hyperglycemi* OR infection* OR CMV OR cytomegalovirus OR rubella OR herpes OR virus* OR syphilis OR influenza OR fever OR STI* OR STD* OR chlamydia OR anemia OR supplement* OR salicylate* OR sulfasalazine OR aspirin OR acetaminophen OR advanced age OR smoking OR smoked OR tobacco OR antibiotic* OR penicillin* OR ampicillin OR trimethoprim OR sulfa-methoxazole OR hormone* OR progesterone OR contraceptive* OR progesto-gen* OR birth control OR assisted reproduct* OR cholesterol OR statin* OR lovastatin OR methotrexate OR tranexamic acid OR chloriazepoxide OR imipr-amine OR lithium OR dimenhydrinate OR fertility OR obesity OR BMI OR diet* OR education* OR income OR socioeconomic* Or cancer* OR epilepsy OR epileptic OR antiepileptic* OR anticonvulsant* OR acne OR retinoic acid | |||
| Limit 2010- ; exclude Medline journals | |||
| CINAHL (Ebsco) | Holoprosencephaly | 1/11/2018 | 10–6 duplicates = 4 unique items |
| AND | |||
| Case* OR epidemiolog* OR report* OR inciden* OR occurrence OR surveillance OR Diabetes OR diabetic* OR drug* OR “substance abuse*” OR medication* OR prescription* OR prescribe* OR nutrition* OR “maternal health” OR (mother* N2 health) OR “side effect*” OR “adverse reaction*” OR “adverse effect*” OR “adverse event*” OR hypertension OR blood pressure OR complication* OR exposure* OR expose* OR risk* OR factor* OR non-genetic OR nongenetic OR non-syndromic OR nonsyndromic OR environmental OR (maternal N2 (illness* OR disease*)) OR sociodemographic* OR demographic* OR alcohol* OR ethanol OR teratogen* OR hyperglycemi* OR infection* OR CMV OR cytomegalovirus OR rubella OR herpes OR virus* OR syphilis OR influenza OR fever OR STI* OR STD* OR chlamydia OR anemia OR supplement* OR salicylate* OR sulfasalazine OR aspirin OR acetaminophen OR advanced age OR smoking OR smoked OR tobacco OR antibiotic* OR penicillin* OR ampicillin OR trimethoprim OR sulfamethoxazole OR hormone* OR progesterone OR contraceptive* OR progestogen* OR “birth control” OR “assisted reproduct*” OR cholesterol OR statin* OR lovastatin OR methotrexate OR tranexamic acid OR chloriazepoxide OR imipramine OR lithium OR dimenhydrinate OR fertility OR obesity OR BMI OR diet* OR education* OR income OR socioeconomic* Or cancer* OR epilepsy OR epileptic OR antiepileptic* OR anticonvulsant* OR acne OR “retinoic acid” | |||
| Limit 2010- ; exclude Medline records | |||
| Cochrane library | Holoprosencephaly:ti,ab | 1/11/2018 | 0 |
| AND | |||
| (Case* OR epidemiolog* OR report* OR inciden* OR occurrence OR surveillance OR Diabetes OR diabetic* OR drug* OR “substance abuse*” OR medication* OR prescription* OR prescribe* OR nutrition* OR “maternal health” OR (mother* NEAR/2 health) OR “side effect*” OR “adverse reaction*” OR “adverse effect*” OR “adverse event*” OR hypertension OR blood pressure OR complication* OR exposure* OR expose* OR risk* OR factor* OR non-genetic OR nongenetic OR non-syndromic OR nonsyndromic OR environmental OR (maternal NEAR/2 (illness* OR disease*)) OR sociodemographic* OR demographic* OR alcohol* OR ethanol OR teratogen* OR hyperglycemi* OR infection* OR CMV OR cytomegalovirus OR rubella OR herpes OR virus* OR syphilis OR influenza OR fever OR STI* OR STD* OR chlamydia OR anemia OR supplement* OR salic-ylate* OR sulfasalazine OR aspirin OR acetaminophen OR advanced age OR smoking OR smoked OR tobacco OR antibiotic* OR penicillin* OR ampicillin OR trimethoprim OR sulfamethoxazole OR hormone* OR progesterone OR contraceptive* OR progestogen* OR “birth control” OR “assisted reproduct*” OR cholesterol OR statin* OR lovastatin OR methotrexate OR tranexamic acid OR chloriazepoxide OR imipramine OR lithium OR dimenhydrinate OR fertility OR obesity OR BMI OR diet* OR education* OR income OR socioeconomic* Or cancer* OR epilepsy OR epileptic OR antiepileptic* OR anticonvulsant* OR acne OR “retinoic acid”):ti,ab | |||
| Limit 2010- ; | |||
| Scopus | Non-genetic risk factors for boloprosencephaly cited by | 1/11/2018 | 23–11 duplicates = 12 unique items |
| OR | |||
| TITLE-ABS-KEY(Holoprosencephaly AND (Case* OR epidemiolog* OR report* OR inciden* OR occurrence OR surveillance OR Diabetes OR diabetic* OR drug* OR “substance abuse*” OR medication* OR prescription* OR prescribe* OR nutrition* OR “maternal health” OR (mother* W/2 health) OR “side effect*” OR “adverse reaction*” OR “adverse effect*” OR “adverse event*” OR hypertension OR “blood pressure” OR complication* OR exposure* OR expose* OR risk* OR factor* OR non-genetic OR nongenetic OR non-syndromic OR non-syndromic OR environmental OR (maternal W/2 illness*) OR (maternal W/2 disease*) OR sociodemographic* OR demographic* OR alcohol* OR ethanol OR teratogen* OR hyperglycemi* OR infection* OR CMV OR cytomegalovirus OR rubella OR herpes OR virus* OR syphilis OR influenza OR fever OR STI* OR STD* OR chlamydia OR anemia OR supplement* OR salicylate* OR sulfasalazine OR aspirin OR acetaminophen OR advanced age OR smoking OR smoked OR tobacco OR antibiotic* OR penicillin* OR ampicillin OR trimethoprim OR sulfamethoxazole OR hormone* OR progesterone OR contraceptive* OR progestogen* OR “birth control” OR “assisted reproduct*” OR cholesterol OR statin* OR lovastatin OR methotrexate OR tranexamic acid OR chloriazepoxide OR imipramine OR lithium OR dimenhydrinate OR fertility OR obesity OR BMI OR diet* OR education* OR income OR socioeconomic* Or cancer* OR epilepsy OR epileptic OR antiepileptic* OR anticonvulsant* OR acne OR “retinoic acid”)) AND NOT INDEX(medline) AND NOT INDEX(embase) |
Notes. Duplicates were identified using the Endnote automated “find duplicates” function with preference set to match on title, author and year, and removed from your Endnote library. There will likely be additional duplicates found that Endnote was unable to detect.
Footnotes
Publisher's Disclaimer: DISCLAIMER
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
CONFLICT OF INTEREST
None.
REFERENCES
- Anderson JL, Waller DK, Canfield MA, Shaw GM, Watkins ML, & Werler MM (2005). Maternal obesity, gestational diabetes, and central nervous system birth defects. Epidemiology, 16(1), 87–92. 10.1097/01.ede.0000147122.97061.bb [DOI] [PubMed] [Google Scholar]
- Berry SM, Gosden C, Snijders RJ, & Nicolaides KH (1990). Fetal holoprosencephaly: Associated malformations and chromosomal defects. Fetal Diagnosis and Therapy, 5(2), 92–99. 10.1159/000263552 [DOI] [PubMed] [Google Scholar]
- Billington CJ Jr., Schmidt B, Marcucio RS, Hallgrimsson B, Gopalakrishnan R, & Petryk A (2015). Impact of retinoic acid exposure on midfacial shape variation and manifestation of holoprosencephaly in Twsg1 mutant mice. Disease Models & Mechanisms, 8(2), 139–146. 10.1242/dmm.018275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne ML, Rasmussen SA, Hoyt AT, Waller DK, Druschel CM, Caton AR, … Romitti PA (2009). Maternal thyroid disease, thyroid medication use, and selected birth defects in the National Birth Defects Prevention Study. Birth Defects Research Part A: Clinical and Molecular Teratology, 85(7), 621–628. 10.1002/bdra.20573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen PJ, Rankin JM, & Robson SC (2001). Investigation of the epidemiology and prenatal diagnosis of holoprosencephaly in the North of England. American Journal of Obstetrics and Gynecology, 184 (6), 1256–1262. 10.1067/mob.2001.111071 [DOI] [PubMed] [Google Scholar]
- Carter TC, Olney RS, Mitchell AA, Romitti PA, Bell EM, & Druschel CM & National Birth Defects Prevention Study (2011). Maternal self-reported genital tract infections during pregnancy and the risk of selected birth defects. Birth Defects Research Part A Clinical and Molecular Teratology, 91(2), 108–116. 10.1002/bdra.20749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CP, Su TH, Chern SR, Su JW, Lee CC, & Wang W (2012). Alobar holoprosencephaly, cebocephaly, and micropenis in a Klinefelter fetus of a diabetic mother. Taiwanese Journal of Obstetrics and Gynecology, 51(4), 630–634. 10.1016/j.tjog.2012.09.021 [DOI] [PubMed] [Google Scholar]
- Correa A, Botto L, Liu Y, Mulinare J, & Erickson JD (2003). Do multivitamin supplements attenuate the risk for diabetes-associated birth defects? Pediatrics, 111(5 Pt 2), 1146–1151. [PubMed] [Google Scholar]
- Correa A, Gilboa SM, Besser LM, Botto LD, Moore CA, Hobbs CA, … Reece EA (2008). Diabetes mellitus and birth defects. American Journal of Obstetrics and Gynecology, 199(3), 237.e1–237.e239. 10.1016/j.ajog.2008.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A, Gilboa SM, Botto LD, Moore CA, Hobbs CA, Cleves MA, … National Birth Defects Prevention Study (2012). Lack of periconceptional vitamins or supplements that contain folic acid and diabetes mellitus-associated birth defects. American Journal of Obstetrics and Gynecology, 206(3), 218.e1–213. 10.1016/j.ajog.2011.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, & Hu DJ (2009). Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Archives of Pediatrics & Adolescent Medicine, 163(11), 978–985. 10.1001/archpediatrics.2009.188 [DOI] [PubMed] [Google Scholar]
- Croen LA, Shaw GM, & Lammer EJ (2000). Risk factors for cytogenetically normal holoprosencephaly in California: A population-based case-control study. American Journal of Medical Genetics, 90(4), 320–325. [DOI] [PubMed] [Google Scholar]
- Dawson AL, Tinker SC, Jamieson DJ, Hobbs CA, Berry RJ, Rasmussen SA, … National Birth Defects Prevention Study (2016). Twinning and major birth defects, National Birth Defects Prevention Study, 1997–2007. Journal of Epidemiology and Community Health, 70(11), 1114–1121. 10.1136/jech-2015-206302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay-Benziv R, Reece EA, Wang F, & Yang P (2015). Birth defects in pregestational diabetes: Defect range, glycemic threshold and pathogenesis. World Journal of Diabetes, 6(3), 481–488. 10.4239/wjd,v6.i3.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MT, Sulik KK, Fish EW, Baker LK, Dehart DB, & Parnell SE (2016). Dose-dependent teratogenicity of the synthetic cannabinoid CP-55,940 in mice. Neurotoxicology and Teratology, 58, 15–22. 10.1016/j.ntt.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SK, Broussard C, Devine O, Green RF, Rasmussen SA, & Reefhuis J & National Birth Defects Prevention Study (2012). Association between maternal age and birth defects of unknown etiology: United States, 1997–2007. Birth Defects Research Part A Clinical and Molecular Teratology, 94(12), 1010–1018. 10.1002/bdra.23049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami D, & Kusre G (2015). Agnathia holoprosencephaly and situs inversus in a neonate born to an alcoholic mother. Journal of Clinical and Diagnostic Research, 9(5), AD01–AD02. 10.7860/JCDR/2015/12733.5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JS, & Barnes PD (2010). Neuroimaging advances in holoprosencephaly: Refining the spectrum of the midline malformation. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 154C(1), 120–132. 10.1002/ajmg.c.30238 [DOI] [PubMed] [Google Scholar]
- Hong M, & Krauss RS (2017). Ethanol itself is a holoprosencephaly-inducing teratogen. PLoS One, 12(4), e0176440 10.1371/journal.pone.0176440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley MM, Browne ML, Van Zutphen AR, Richardson SD, Blossom SJ, Broussard CS, … National Birth Defects Prevention Study (2016). Maternal autoimmune disease and birth defects in the National Birth Defects Prevention Study. Birth Defects Research Part A Clinical and Molecular Teratology, 106(11), 950–962. 10.1002/bdra.23527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley MM, Fisher SC, Van Zutphen AR, Waller DK, Carmichael SL, & Browne ML & National Birth Defects Prevention Study (2017). Thyroid medication use and birth defects in the National Birth Defects Prevention Study. Birth Defects Research, 109 (18), 1471–1481. 10.1002/bdr2.1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt AT, Canfield MA, Romitti PA, Botto LD, Anderka MT, Krikov SV, … Feldkamp ML (2016). Associations between maternal periconceptional exposure to secondhand tobacco smoke and major birth defects. American Journal of Obstetrics and Gynecology, 215(5), 613.e1–613.e11. 10.1016/j.ajog.2016.07.022 [DOI] [PubMed] [Google Scholar]
- Johnson CY, & Rasmussen SA (2010). Non-genetic risk factors for holoprosencephaly. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 154c(1), 73–85. 10.1002/ajmg.c.30242 [DOI] [PubMed] [Google Scholar]
- Kallen B, Castilla EE, Lancaster PA, Mutchinick O, Knudsen LB, Martinez-Frias ML, … Robert E (1992). The cyclops and the mermaid: An epidemiological study of two types of rare malformation. Journal of Medical Genetics, 29(1), 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Frías ML, Bermejo E, Rodríguez-Pinilla E, Prieto L, & Frías JL (1998). Epidemiological analysis of outcomes of pregnancy in gestational diabetic mothers. American Journal of Medical Genetics, 78 (2), 140–145. [DOI] [PubMed] [Google Scholar]
- Matsunaga E, & Shiota K (1977). Holoprosencephaly in human embryos: Epidemiologic studies of 150 cases. Teratology, 16(3), 261–272. 10.1002/tera.1420160304 [DOI] [PubMed] [Google Scholar]
- Michalski AM, Richardson SD, Browne ML, Carmichael SL, Canfield MA, VanZutphen AR, … Druschel CM (2015). Sex ratios among infants with birth defects, National Birth Defects Prevention Study, 1997–2009. American Journal of Medical Genetics Part A, 167 (5), 1071–1081. 10.1002/ajmg.a.36865 [DOI] [PubMed] [Google Scholar]
- Miller EA, Rasmussen SA, Siega-Riz AM, Frias JL, & Honein MA (2010). Risk factors for non-syndromic holoprosencephaly in the National Birth Defects Prevention Study. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 154C(1), 62–72. 10.1002/ajmg.c.30244 [DOI] [PubMed] [Google Scholar]
- Odent S, Le Marec B, Munnich A, Le Merrer M, & Bonaïti-Pellie C (1998). Segregation analysis in nonsyndromic holoprosencephaly. American Journal of Medical Genetics, 77(2), 139–143. [DOI] [PubMed] [Google Scholar]
- Olsen CL, Hughes JP, Youngblood LG, & Sharpe-Stimac M (1997). Epidemiology of holoprosencephaly and phenotypic characteristics of affected children: New York State, 1984–1989. American Journal of Medical Genetics, 73(2), 217–226. [DOI] [PubMed] [Google Scholar]
- Opitz JM, & Gilbert EF (1982). Pathogenetic analysis of congenital anomalies in humans. Pathobiology Annual, 12, 301–349. [PubMed] [Google Scholar]
- Orioli IM, Amar E, Bakker MK, Bermejo-S anchez E, Bianchi F, Canfield MA, … Castilla EE (2011). Cyclopia: An epidemiologic study in a large dataset from the International Clearinghouse of Birth Defects Surveillance and Research. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 157(4), 344–357. 10.1002/ajmg.c.30323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orioli IM, & Castilla EE (2007). Clinical epidemiologic study of holoprosencephaly in South America. American Journal of Medical Genetics Part A, 143A(24), 3088–3099. 10.1002/ajmg.a.32104 [DOI] [PubMed] [Google Scholar]
- Orioli IM, & Castilla EE (2010). Epidemiology of holoprosencephaly: Prevalence and risk factors. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 154C(1), 13–21. 10.1002/ajmg.c.30233 [DOI] [PubMed] [Google Scholar]
- Ornoy A, Reece EA, Pavlinkova G, Kappen C, & Miller RK (2015). Effect of maternal diabetes on the embryo, fetus, and children: Congenital anomalies, genetic and epigenetic changes and developmental outcomes. Birth Defects Research Part C: Embryo Today: Reviews, 105 (1), 53–72. 10.1002/bdrc.21090 [DOI] [PubMed] [Google Scholar]
- Pallangyo P, Lyimo F, Nicholaus P, Makungu H, Mtolera M, & Mawenya I (2016). Semilobar holoprosencephaly in a 12-month-old baby boy born to a primigravida patient with type 1 diabetes mellitus: A case report. Journal of Medical Case Reports, 10(1), 10.1186/s13256-016-1141-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SE, Werler MM, Shaw GM, Anderka M, Yazdy MM & National Birth Defects Prevention Study (2012). Dietary glycemic index and the risk of birth defects. American Journal of Epidemiology, 176(12), 1110–1120. 10.1093/aje/kws201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SA, Hayes EB, Jamieson DJ, & O’Leary DR (2007). Emerging infections and pregnancy: Assessing the impact on the embryo or fetus. American Journal of Medical Genetics Part A, 143A (24), 2896–2903. 10.1002/ajmg.a.32077 [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Moore CA, Khoury MJ, & Cordero JF (1996). Descriptive epidemiology of holoprosencephaly and arhinencephaly in Metropolitan Atlanta, 1968–1992. American Journal of Medical Genetics, 66(3), 320–333. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, & Moore CA (2003). Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Research Part A: Clinical and Molecular Teratology, 67(3), 193–201. 10.1002/bdra.10012 [DOI] [PubMed] [Google Scholar]
- Reefhuis J, Gilboa SM, Anderka M, Browne ML, Feldkamp ML, Hobbs CA, … Honein MA (2015). The National Birth Defects Prevention Study: A review of the methods. Birth Defects Research Part A – Clinical and Molecular Teratology, 103(8), 656–669. 10.1002/bdra.23384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, & Muenke M (2010). The molecular genetics of holoprosencephaly. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 154C(1), 52–61. 10.1002/ajmg.c.30236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder AC, & Privalsky ML (2014). Thyroid hormones, T3 and T4, in the brain. Frontiers in Endocrinology, 5, 1–5. 10.3389/fendo.2014.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialli AR, Buelke-Sam JL, Chambers CD, Friedman JM, Kimmel CA, Polifka JE, & Tassinari MS (2004). Communicating risks during pregnancy: A workshop on the use of data from animal developmental toxicity studies in pregnancy labels for drugs. Birth Defects Research Part A: Clinical and Molecular Teratology, 70(1), 7–12. 10.1002/bdra.10150 [DOI] [PubMed] [Google Scholar]
- Sokal R, Tata LJ, & Fleming KM (2014). Sex prevalence of major congenital anomalies in the United Kingdom: A national population-based study and international comparison meta-analysis. Birth Defects Research Part A – Clinical and Molecular Teratology, 100(2), 79–91. 10.1002/bdra.23218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisukhumbowornchai S, Krikov S, & Feldkamp ML (2012). Self-reported maternal smoking during pregnancy by source in Utah, 2003–2007. Birth Defects Research Part A – Clinical and Molecular Teratology, 94(12), 996–1003. 10.1002/bdra.23058 [DOI] [PubMed] [Google Scholar]
- Suslak L, Mimms GM, Desposito F, Opitz JM, & Reynolds JF (1987). Monozygosity and holoprosencephaly: Cleavage disorders of the “midline field”. American Journal of Medical Genetics, 28(1), 99–102. 10.1002/ajmg.1320280114 [DOI] [PubMed] [Google Scholar]
- Tennant PW, Samarasekera SD, Pless-Mulloli T, & Rankin J (2011). Sex differences in the prevalence of congenital anomalies: A population-based study. Birth Defects Research Part A – Clinical and Molecular Teratology, 91(10), 894–901. 10.1002/bdra.22846 [DOI] [PubMed] [Google Scholar]
- Tinker SC, Reefhuis J, Dellinger AM, & Jamieson DJ (2011). Maternal injuries during the periconceptional period and the risk of birth defects, National Birth Defects Prevention Study, 1997–2005. Paediatric and Perinatal Epidemiology, 25(5), 487–496. 10.1111/j.1365-3016.2011.01215.x [DOI] [PubMed] [Google Scholar]
- Vaz SS, Chodirker B, Prasad C, Seabrook JA, Chudley AE, & Prasad AN (2012). Risk factors for nonsyndromic holoprosencephaly: A Manitoba case-control study. American Journal of Medical Genetics Part A, 158A(4), 751–758. 10.1002/ajmg.a.35240 [DOI] [PubMed] [Google Scholar]
- Weber MA, & Sebire NJ (2010). Genetics and developmental pathology of twinning. Seminars in Fetal & Neonatal Medicine, 15(6), 313–318. 10.1016/j.siny.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Whiteford ML, & Tolmie JL (1996). Holoprosencephaly in the West of Scotland 1975–1994. Journal of Medical Genetics, 33(7), 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon PW, Rasmussen SA, Lynberg MC, Moore CA, Anderka M, Carmichael SL, … Edmonds LD (2001). The National Birth Defects Prevention Study. Public Health Reports, 116(Suppl 1), 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]