Abstract
Intact spores and submicrometer size fragments are released from moldy building materials during growth and sporulation. It is unclear whether all fragments originate from fungal growth or if small pieces of building materials are also aerosolized as a result of microbial decomposition. In addition, particles may be formed through nucleation from secondary metabolites of fungi, such as microbial volatile organic compounds (MVOCs). In this study, we used the elemental composition of particles to characterize the origin of submicrometer fragments released from materials contaminated by fungi.
Particles from three fungal species (Aspergillus versicolor, Cladosporium cladosporioides and Penicillium brevicompactum), grown on agar, wood and gypsum board were aerosolized using the Fungal Spore Source Strength Tester (FSSST) at three air velocities (5, 16 and 27 m/s). Released spores (optical size, dp ≥ 0.8 micrometers) and fragments (dp ≤ 0.8 micrometers) were counted using direct-reading optical aerosol instruments. Particles were also collected on filters, and their morphology and elemental composition analyzed using scanning electron microscopes (SEM) coupled with an Energy-Dispersive X-ray spectroscopy (EDX).
Among the studied factors, air velocity resulted in the most consistent trends in the release of fungal particles. Total concentrations of both fragments and spores increased with an increase in air velocity for all species whereas fragment-spore (F/S) ratios decreased. EDX analysis showed common elements, such as C, O, Mg and Ca, for blank material samples and fungal growth. However, N and P were exclusive to the fungal growth, and therefore were used to differentiate biological fragments from non-biological ones. Our results indicated that majority of fragments contained N and P.
Because we observed increased release of fragments with increased air velocities, nucleation of MVOCs was likely not a relevant process in the formation of fungal fragments. Based on elemental composition, most fragments originated from fungi, but also fragments from growth material were detected.
Keywords: Fragments, air velocity, elemental analysis, scanning electron microscope, energy dispersive x-ray spectroscopy
1. INTRODUCTION
Moisture-damaged building materials and other indoor furnishes are the major sources of indoor mold (Meklin et al., 2004). Mold growth in indoor environments due to water damage can cause deterioration of indoor air quality and be harmful for human health (Ettenauer et al., 2012; IOM, 2004; WHO, 2009). Until recently, assessment of mold exposure was focused on spores only. However, no clear association has so far been documented between fungal spore concentrations and adverse health effects in indoor environments (Mendell et al., 2011).
Released fungal particles have been shown to be heterogeneous in nature and comprise of intact spores and submicrometer size fragments (Górny et al., 2002; Gόrny, 2004; Kildesø et al., 2003; Madsen et al., 2006). Fragments may arise from breakdown of spores and hyphal materials (Cho et al., 2005; Madelin and Madelin, 1995). When fungus grows on building materials it decomposes the material by fungal enzymes and acids in order to release nutrients (Adan, 1994; Davis, 2001). Therefore, it can be hypothesized that fragment particles released during fungal growth and sporulation may not all be from the growth but also from the growth material. Fragment particles may also originate by nucleation of by-products of fungal metabolism, such as microbial volatile organic compounds (MVOCs) (Fiedler et al., 2001; Górny et al., 2002; Korpi et al., 1997; Pasanen et al., 1997).
There is increased interest in the role of aerosolized fungal fragments in adverse effects considering the strong association between numbers of fine particles and adverse health effects (Gold et al., 2000; Green et al., 2006; Magari et al., 2001; Pekkanen et al., 2002). The submicrometer fragments are of utmost importance, because they tend to stay longer in the air, are easily inhaled and the smallest fragments (<0.1 μm) can deposit deep in the respiratory tract having the potential for causing adverse health effects (Cho et al., 2005; Frankel et al., 2014; Seo et al., 2008). Furthermore, the large surface area of the fragments relative to their mass may evoke high biological activity (Frankel et al., 2014). The high number of released fungal fragments in combination with their potential to deliver harmful antigens and mycotoxins to the alveolar region of the lung suggests the need for their characterization. The characterization of fungal fragments is important to help us understand the potential health effects associated with the exposure (Cho et al., 2005; McGinnis, 2007).
Several laboratory studies have been conducted to characterize particle size of aerosolized fungal fragments. These studies utilized direct-reading aerosol instruments, such as particle counters or aerodynamic particle sizers (Brandl et al., 2008; Cho et al., 2005; Górny et al., 2002; Seo et al., 2007). The disadvantage of these devices is that they do not distinguish biological particles from non-biological ones. The Ultra Violet Aerodynamic Particle Sizer (UVAPS) has been used to detect and count fungal particles from aerosol mixtures (Kanaani et al., 2008; Lee et al., 2010). This method employs the presence of fluorescence materials (nicotine adenine dinucleotide phosphate (NADPH) and riboflavin) in the biological propagules. Despite its ability to distinguish biological from non-biological materials, UVAPS may not be useful for characterizing fungal fragments because submicrometer size fragments give off very little or no fluorescence compared to larger spores (Kanaani et al., 2008; Saari et al., 2014).
Fungal fragments have also been characterized microscopically using morphology or chemically analyzing specific components such as (1→3)-β-D-glucan and β-N-acetylhexosaminidase (NAHA) (Adhikari et al., 2013; Afanou et al., 2014, 2015; Górny and Ławniczek-Wałczyk, 2012; Górny et al., 2002; Kanaani et al., 2008; Kildesø et al., 2003; Madsen et al., 2005; Seo et al., 2009). Other studies using immunochemical methods have also attempted to characterize fungal particles of various sizes to determine their biological activity (Górny et al., 2002; Schmechel et al., 2003). Also microscopic methods have been combined with immunostaining to increase the specificity (Green et al., 2005). Cascade impactors, e.g., Andersen impactor (Górny et al., 2002), and electrical low pressure impactor (ELPI) (Cho et al., 2005) have been used in other studies to characterize fungal fragments by aerodynamic sizes. These studies are informative but did not attempt to distinguish biological and non-biological fragments.
Bioparticles can be identified by microscopic methods. However, the different sources and similarities in particle appearance with particles from other sources may lead to difficulties in quantification (Wittmaack et al., 2005). Matthias-Maser and Jaenicke (1991, 1994) developed criteria for determining primary biogenic organic aerosols (PBOA) in atmospheric samples. PBOAs were determined based on the particle elemental composition, the particle morphology and the particle behavior during EDX analysis. Morphology of the biological particles ranged from rod shaped, through elongated shaped to curved particles, while behavior of the samples was mainly changes in shape of particle either by shrinking or disappearing during EDX. They found that PBOAs contain minor amounts of K, P, S, Na and Ca (usually <10% of relative element of x-ray intensity of the particle) and thereby EDX analysis can be used to separate PBOA from other types of particulate matter (PM) in ambient air samples (Matthias-Maser and Jaenicke, 1994; Matthias-Maser et al., 2000). This criterion was recently adopted by Coz et al. (2010) to characterize PBOA in the atmosphere.
To our knowledge, there are no previous studies characterizing the origin of submicrometer fragments released from mold contaminated building materials based on their elemental composition. The aim of this study was to characterize the origin of fungal fragments released from contaminated building materials using their chemical composition. The amount of spores and fragments was measured with direct-reading aerosol instruments to determine the optimal experimental conditions for the collection of EDX samples.
2. MATERIALS AND METHODS
2.1. Test microorganisms
Three fungal species, Aspergillus versicolor (Culture collection of the Institute for Health and Welfare, Finland: HT31), Cladosporium cladosporioides (German collection of Microorganisms and Cell Cultures: DSMZ 62121), and Penicillium brevicompactum, (American Type Cell Collection: ATCC 58606) were used in the experiments. These species are common in indoor air worldwide (Hyvärinen et al., 2002; Méheust et al., 2013; Reponen et al., 2012). A. versicolor strain used was previously isolated from indoor air samples collected in Finnish homes.
2.2. Substrate and inoculation
Prior to inoculation on growth media (agar, gypsum boards and wood), A. versicolor and C. cladosporioides were grown on malt extract agar (ME) (LabM, Lancashire, UK) while P. brevicompactum was grown on dichloran glycerol 18% agar (DG18) (Merck, Darmstadt, Germany). The plates were incubated at room temperature for up to 2 weeks to obtain spores. The spores were harvested from the growth medium by applying one gram of glass beads (Ø = 425 – 600 μm; Sigma-Aldrich Co., Saint Louis, MO) to each plate and shaking the plates gently back and forth to aid the attachment of spores to the beads (Schmechel et al., 2003). Thereafter, the beads were transferred into a tube containing 15 ml 0.05 % Tween 80. The spores were suspended from the beads by shaking the tube and decanting the spore suspension. The spores were counted with hemacytometer (Fuchs-Rosenthal: Hirschmann EM Technicolor) and concentration was adjusted to about 1 × 106 spores/ml.
Altogether, 27 agar plates (11cm x 11cm) (3 fungal species x 3 time points x 3 replicates) were prepared to study the effect of age on the release of particles. Another set of 9 gypsum board and 9 wood samples (11 cm x 11 cm) (3 species x 3 replicates) were prepared to study the effect of material, air velocity and species on the release of particles. The materials were sterilized by gamma radiation (25kGy) for 50 – 60 hours (Scandinavian Clinics, Estonia OU, Harjumaa, Estonia). Prior to inoculation, the building materials were allowed to absorb about 50 ml of sterile distilled water to moisten them and also for easy spreading of the fungal suspension on the material surfaces. All building material samples and agar were inoculated by uniformly spreading 0.5 ml of the spore suspension onto their surfaces using a glass rod. Blank building material and agar plates inoculated with 0.05% Tween 80 solution without microbial load were used as controls.
Results from preliminary experiments showed that gypsum dust released from the sides of the cut materials contributed to total fragment concentrations. To prevent the contribution of gypsum dust to the total particle concentration during aerosolization, the edges of all cut gypsum boards were sealed with carpenters’ glue (Eri Keeper, Akzo Nobel Coating Oy, Vantaa). Visual observation showed that the treatment of edges did not inhibit fungal growth on top of the gypsum board.
Inoculated building materials and agar plates were incubated at room temperature (21 ± 2 ° C) in conditioned chambers (24 L). Relative humidity of the chamber was kept between 95 – 97 % by placing about 1L saturated K2SO4 (150 g/l) on the bottom of the chamber (Korpi et al., 1997). Inoculated agar plates were then incubated for 1 week, 1 month and 4 months while wood and gypsum boards were incubated for 1 month. Temperature and humidity were measured once a week with a thermohygrometer (Vaisala Oyj, Helsinki, Finland).
2.3. Aerosolization experiments and SEM-EDX analysis
The experimental setup for aerosolization of the fungal particles is shown in Fig. S1, in Supplementary Materials. The fungal particles were aerosolized using the Fungal Spore Source Strength Tester (FSSST) (Sivasubramani et al., 2004) directly from the agar plates, gypsum boards and wood samples. Three air flow rates (5, 15 and 25 lpm) corresponding to air velocities of 5, 16, 27 m/s, respectively, were used at the nozzle orifices above the growth surfaces in the FSSST. The same material sample was subjected to the three air velocities for three minutes each from lowest to highest velocity. All aerosolization experiments were done in triplicates. Before aerosolization experiments, the building materials and agar were kept in dry chambers (relative humidity of 18 ± 2%) overnight to dry the fungal growth for easier aerosolization. During all the aerosolization experiments, the temperature and relative humidity as measured at the end of sampling tubes with a thermohygrometer (Vaisala Oyj, Helsinki, Finland) were 18 – 22°C and 20 – 30%, respectively. In between the aerosolization experiments, the FSSST was first cleaned with 70% ethanol and then the experimental system was purged by passing clean air though it until the measuring devices (OPC and LAS-X) showed zero concentrations.
The total concentrations of spores and fragments were measured to determine variations in particles concentrations and to determine the optimal experimental conditions for the collection of SEM-EDX samples. Concentrations of released fungal particles were measured with an optical particle sizer (OPS, TSI Model 3330; TSI Inc., Shoreview, MN) in the size range of 0.3 – 10 μm and a laser aerosol spectrometer (LAS-X II; Particle Measuring Systems, Inc., Boulder, CO) in the size range of 0.1 – 1 μm.
Fungal particles were collected onto 25 mm diameter, 0.2 μm pore size black polycarbonate filters (Whatman Nuclepore, Clifton, NJ, USA) for SEM-EDX analysis. Particles released from one material sample at all three air velocities were collected onto one filter in order to have enough particles for SEM-EDX analysis.
About 5 mm x 5 mm of fungal growth on wood and gypsum board as well as blank surfaces of these materials were cut out using clean scalpel blades and mounted on aluminum stubs for EDX analysis. Approximately one fourth of the polycarbonate membrane filters with fungal particles were cut and coated with gold (Au) using an Agar Auto Sputter Coater (Model B7341, Agar Scientific Ltd., Stansted, UK) in order to obtain electrical conductivity of the sample surfaces and minimize surface charging. A sputter time of 60 s and a gold anode was used resulting in a coating thickness of approx. 20–30 nm. The micrographs were taken using an Everhart-Thornley type secondary electron detector with 300 V grid bias in order to gain surface topographic contrast. The morphology of spores and fragments was observed with SigmaHD VP field emission SEM (Carl Zeiss Microscopy Ltd, Cambridge, UK) with beam acceleration voltage between 6kV and 10kV and the variable chamber mode within low vacuum condition (P < 40 Pa). Elemental composition of spores and fragments was measured using two 60 mm2 SDD type EDX detector (Noran system 7, Thermo Scientific, Madison, WI, USA) setup at a working distance of 10.6 – 11.7 mm. EDX counts were collected from spotted particles under electron bombardment for 30 seconds per each analysis. Qualitative EDX analysis was made in order to recognize elemental components of interest. For quality control purposes, preliminary quantitative analysis was carried out for the spectra. Standardless analysis with Proza (Phi-Rho-Z) correction was used to determine weight percentage (wt %) of each element. Proza correction is typically used for lighter elements. The standardless analysis estimates the background (continuum X-ray signal) and subtracts it from the spectrum. Our analysis showed that A. versicolor spores contained 9.04 ± 1.25 % nitrogen and 1.09 ± 0.49 % phosphorus (N = 13) whereas fragments contained 4.11 ± 0.64 % nitrogen and 0.9 ± 0.3 % phosphorus (N = 6). Likewise, P. brevicompactum spores contained 8.43 ± 0.29 % nitrogen and 0.9 ± 0.43 % phosphorus (N = 4) and fragments contained 3.61 ± 0.97 % nitrogen and 0.7 ± 0.15 % phosphorus (N = 5). The detection limit for all elements was 0.05%. Although the elemental results were qualitative, the accuracy was sufficient to identify different particle types and to compare the differences in element detection of the same particle type in different samples.
2.4. Data Analysis
Ratios of released fragments to spores (F/S-ratio) were calculated to determine how much fragments were released per spore (Seo et al., 2009). Particle concentrations and F/S-ratio values were log-normally distributed; therefore, geometric mean (GM) and geometric standard deviation (GSD) were calculated. The effects of all the factors (fungal species, material type, air velocity, and age of culture for agar samples only) on the concentration of spores and fragments as well as on the F/S ratios were assessed from log-transformed data using 3-way ANOVA model with interactions. Post-hoc means from the combined materials, species and air velocity were further compared under the ANOVA model and adjusted for multiple comparisons using Tukey’s method. Differences of p<0.05 were considered statistically significant. All analyses were done with SPSS for Windows v21 (2014, Armonk, NY, USA) or GraphPad prism v6 (GraphPad Software, Inc., La Jolla, CA, USA).
3. RESULTS
3.1. Size distributions
A typical size distribution of fragments and spores measured with both LAS-X II and OPC, respectively, is shown in Fig. S2. Two clear peaks, one below an optical size of 0.8 μm (fragments) and the other above this borderline (spores), were observed. Therefore, 0.8 μm was set as the cut-off size for fragments in this study.
3.2. Effect of culture age
The effect of cultivation time on the release of fungal particles is shown in Fig. S3. The 3-way ANOVA (adjusted for interactions and multiple comparisons) revealed a statistically significantly lower (p < 0.05) number of fragments aerosolized from 1 month old cultures than from 1 week old cultures. The decrease in fragment concentration was mainly driven by C. cladosporioides. Spore concentrations from 1 week old cultures were significantly higher than those from 1 month old cultures (p < 0.001) and also 4 months old cultures (p < 0.001). This was mainly driven by P. brevicompactum which had the highest spore release at 1 week. When ratios of fragments to spores were compared, 4 months old cultures had significantly higher (p < 0.001) average ratio compared to 1 week old cultures and 1 month old cultures (Fig. 1). There were no consistent trends in the release of both spores and fragments between species as a function of time. Therefore, as a compromise, 1 month old cultures were used in all the further experiments to study the effect of species, material and air velocity on the release of spores and fragments as well as for SEM-EDX analysis.
Fig. 1.
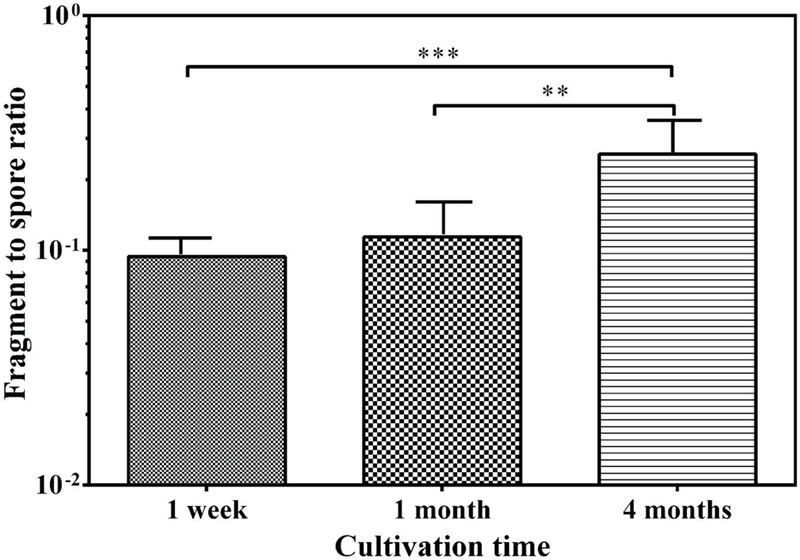
Fragment/spore-ratios obtained from agar at three cultivation time points. Bars represent the geometric means of data from all species inoculated on agar and released at all air velocities combined, n= 27. The error bars represent the geometric standard deviations. Asterisks indicate the level of statistical significance (**p < 0.01; *** p < 0.001).
3.3. ANOVA analysis on the effect of fungal species, material type and air velocity
The effects of the fungal species, material type and air velocity on spore and fragment concentrations as well as the F/S-ratios with interactions are shown in Table 1. All the factors had statistically significant effects. The ANOVA model revealed interaction among all the factors except for “air velocity * fungi” and “material * air velocity * fungi” for concentrations of both spores and fragments. Therefore, the post-hoc analyses presented below were adjusted for interaction effects.
Table 1.
Summary of a three-way ANOVA with interaction and significance levels of the effect of three factors (fungal species, material type and air velocity) on the concentration of spores and fragments as well as fragment/spore-ratios.
| Factor | Dependent Variable (Log concentration) | Type III Sum of Squares | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Material | Spores | .040 | .020 | .090 | .914 |
| Fragment | 13.620 | 6.810 | 17.809 | .000 | |
| Ratio FS | 14.364 | 7.182 | 36.645 | .000 | |
| Flow Rate | Spores | 50.270 | 25.135 | 112.005 | .000 |
| Fragment | 25.023 | 12.512 | 32.721 | .000 | |
| Ratio FS | 5.697 | 2.848 | 14.534 | .000 | |
| Fungi | Spores | 45.814 | 22.907 | 102.077 | .000 |
| Fragment | 10.432 | 5.216 | 13.642 | .000 | |
| Ratio FS | 13.915 | 6.958 | 35.501 | .000 | |
| Material * Flow Rate | Spores | 3.728 | .932 | 4.153 | .005 |
| Fragment | 6.318 | 1.580 | 4.131 | .006 | |
| Ratio FS | 17.640 | 4.410 | 22.501 | .000 | |
| Material * Fungi | Spores | 30.243 | 7.561 | 33.692 | .000 |
| Fragment | 20.996 | 5.249 | 13.727 | .000 | |
| Ratio FS | 2.265 | .566 | 2.889 | .031 | |
| Flow Rate * Fungi | Spores | .683 | .171 | .761 | .555 |
| Fragment | 1.556 | .389 | 1.018 | .407 | |
| Ratio FS | 4.251 | 1.063 | 5.422 | .001 | |
| Material * Flow Rate * Fungi | Spores | 1.760 | .220 | .980 | .462 |
| Fragment | 5.244 | .656 | 1.714 | .118 | |
| Ratio FS | 7.910 | .989 | 5.045 | .000 |
3.4. Effect of fungal species
Total concentration of both fragments and spores released from P. brevicompactum and C. cladosporioides were similar to each other, but significantly higher than those from A. versicolor (p<0.01) (Fig. S4). In contrast, the F/S-ratio of A. versicolor was significantly higher (p<0.001) compared to P. brevicompactum and C. cladosporioides (Fig. 2). Also significantly higher F/S-ratio (p<0.05) of C. cladosporioides was observed compared to that of P. brevicompactum. The higher concentrations of fragments released from P. brevicompactum as well as the higher F/S-ratio of A. versicolor prompted the use of these two species in the SEM-EDX analysis.
Fig. 2.
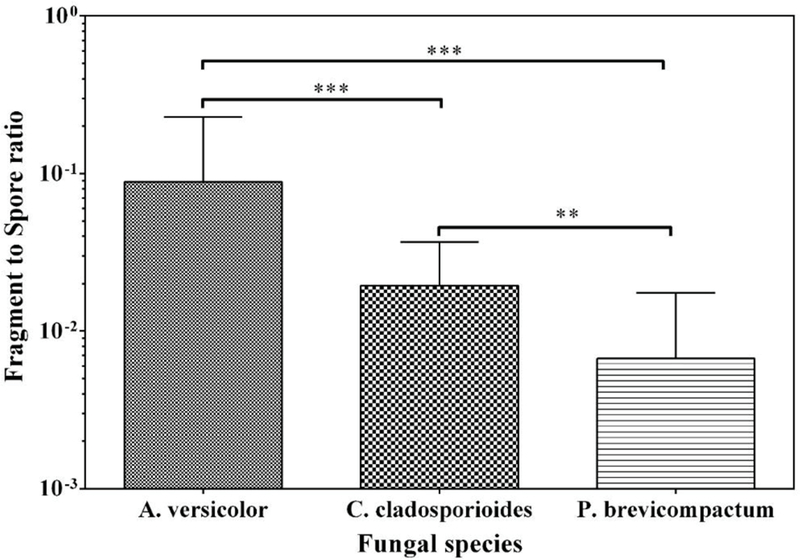
Fragment/spore-ratio obtained from three fungal species at 1 month of cultivation. Bars represent the geometric means of data from all materials, and all air velocities combined, n=9. The error bars represent the geometric standard deviations. Asterisks indicate the level of statistical significance (**p < 0.01; *** p < 0.001).
3.5. Effect of material
Comparing total spore concentrations, no statistically significant differences were observed between the materials (Fig S5). Total concentration of fragments released from agar was significantly higher than that from wood or gypsum board. However, no significant difference was observed between wood and gypsum board. Comparing the F/S-ratios released from the different materials, agar had significantly higher F/S-ratio (p < 0.001) compared to gypsum board and wood (Fig. 3).
Fig. 3.
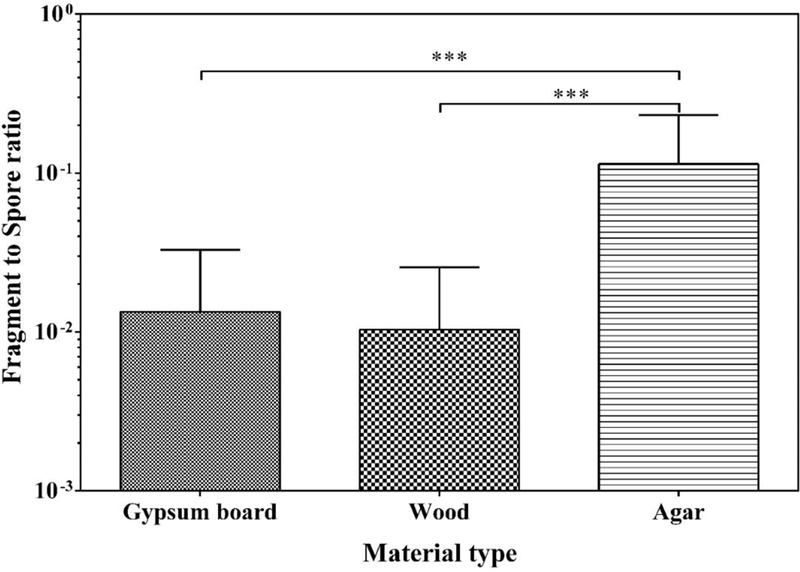
Fragment/spore-ratio obtained from gypsum board, wood and agar at 1 month of cultivation. The bars represent the geometric means of data from all species and air velocities combined, n = 9. Error bars represent the geometric standard deviations. Asterisks indicate the statistical significance (***, p < 0.001).
As highest number of fragments was released from agar, we attempted to use agar samples for the elemental analysis. However, we could not get signals from blank agar, whereas good signals were obtained from blank gypsum board and wood. Therefore, samples from gypsum board and wood were used for the SEM-EDX experiments.
3.6. Effect of air velocity
Among the studied factors, air velocity resulted in most consistent trends in the release of fungal particles. Total concentrations of both fragments and spores increased with increase in air velocity for all species. Total concentrations were significantly higher (p<0.001) when growths were subjected to air velocities of 16 and 27 m/s compared to 5 m/s (Fig. 4). Furthermore, total concentrations were significantly higher at 27 m/s than at 16 m/s (p<0.001). Interestingly, a reverse trend of the effect of air velocity was observed when the F/S-ratios were compared: the ratio was significantly higher with 5 m/s air velocity (p<0.01) than with 16 m/s or 27 m/s (Fig. 5). To maximize the particle yield on membrane filter samples for SEM-EDX analysis, all particles released from the same material sample at all three air velocities were collected onto one filter.
Fig. 4.
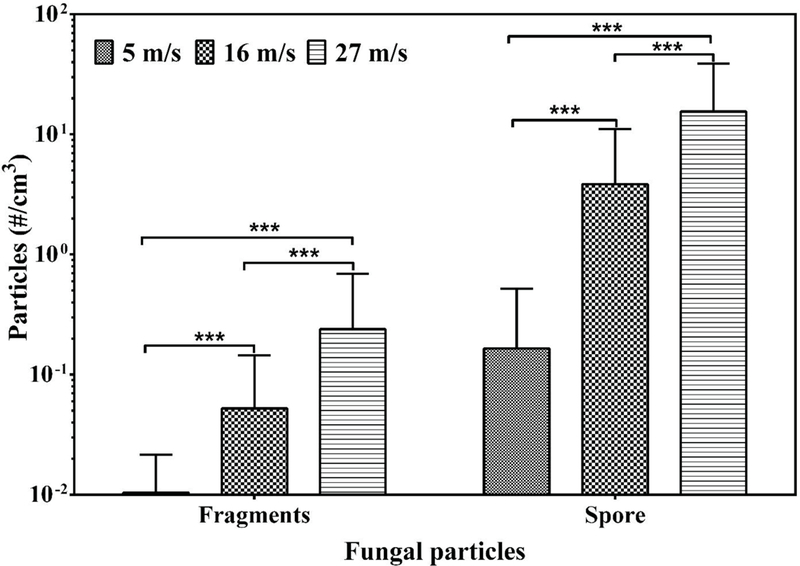
Total concentration of fragments and spores released at three air velocities at 1 month of cultivation. Bars are the geometric means of all data obtained from all fungal species and materials, n = 9. Error bars represent geometric standard deviation. Asterisks indicate the level of statistical significance (*, p < 0.05; ***, p < 0.001).
Fig 5.
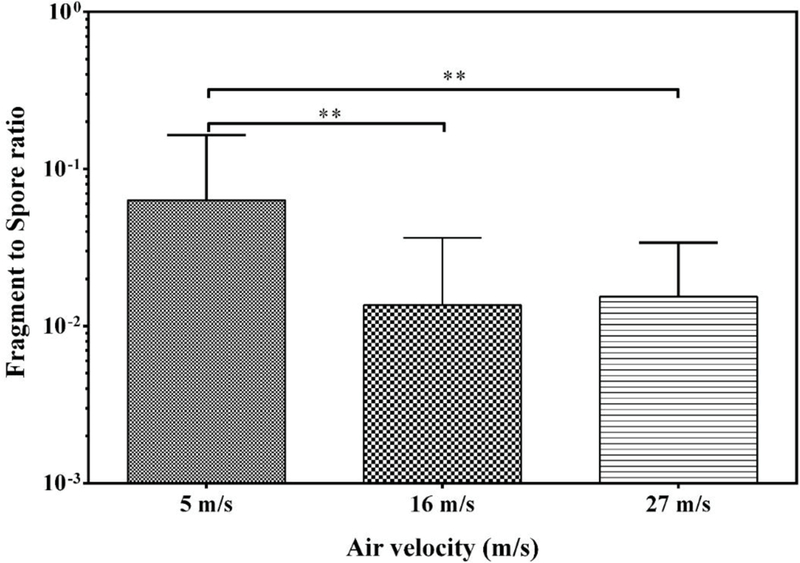
Fragment/spore-ratio obtained at three air velocities at 1 month of cultivation. The bars represent the geometric means of data from all species and materials combined, n= 9. The error bars represent the geometric standard deviations. Asterisks indicate the level of statistical significance (**, p < 0.01).
3.7. SEM-EDX Analysis
Based on the above results on the effects of culture age, species and materials on the release of fungal particles, one month old cultures of A. versicolor and P. brevicompactum on wood and gypsum board were used for the SEM-EDX experiments.
Morphology
Qualitative SEM analysis of the aerosols released from gypsum board and wood samples revealed four types of fragment components. A proportion of fragments were from broken down spores (6A) surface structures of spores (6B), hyphal breakdown (6C), fungal growth (6D and 6E) and potentially from building materials (Fig. 6F). All the fragments were irregular in shape. We could not use similarities in surface structures (rodlets) between the spores and fragments to determine fragments from spore breakdown as posited by Afanou et al. (2014) due to partial covering of the rodlets by gold coating (Figs. S6A and S6B). . This made fragments from both fungal spores and hyphae as well as building materials have similar morphology and therefore, differences in the elemental composition were used to differentiate them.
Fig 6.
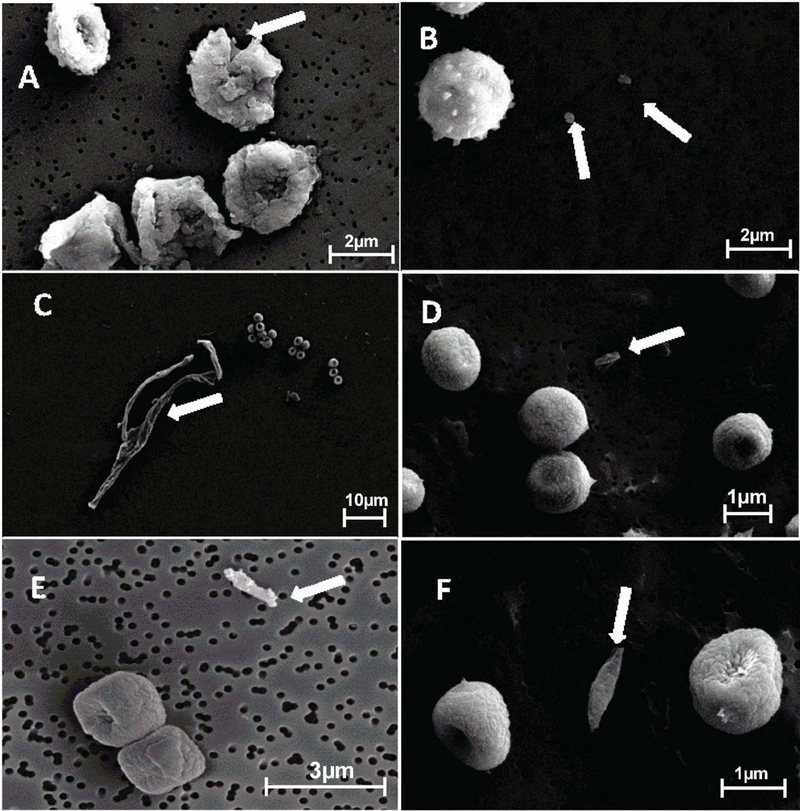
SEM micrographs of gold coated spores and fragments originating from A. versicolor spore breakdown (A), spiny structures on A. versicolor spore surface (B), P. brevicompactum hyphal breakdown (C), C. cladosporioides and P. brevicompactum spores and fragments (D, E respectively) and P. brevicompactum spores with possible fragment from gypsum board (F) (Arrowed).
Chemical composition
Figures 7 and 8 show the SEM images and EDX spectra of elements detected in blank membrane filters, building materials and in fungi grown on the respective materials. The blank membrane filters showed mainly C, O and Al mainly from the aluminium stub on which the filters were mounted. Carbon (C) and O were detected in abundance (usually above 50% of relative element x-ray intensity of the particle) in all types of samples except for the inner core of the gypsum board which had less than 10% of C. On the top of blank gypsum board, we additionally detected Al, Si, S and Ca (usually below 10% of relative element x-ray intensity of the particle). The inner core of gypsum had the same elements as the top, except that S and Ca were more dominant (usually above 50%). From wood blanks, S, K, Na, Mg and Ca (below 10%) were detected in addition to C and O. In general, N and P (below 10%) were only detected in the fungal growth and fungal spores but not in any of the blank building materials. These elements were therefore selected as candidate indicators used to differentiate biological fragments from non-biological ones.
Fig. 7.
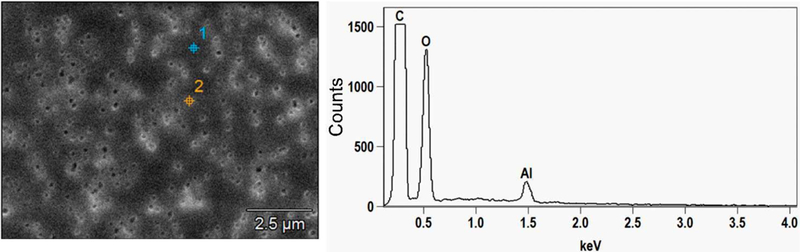
SEM image and elemental composition (EDX spectra) of non gold coated blank polycarbonate membrane filter.
Fig. 8.
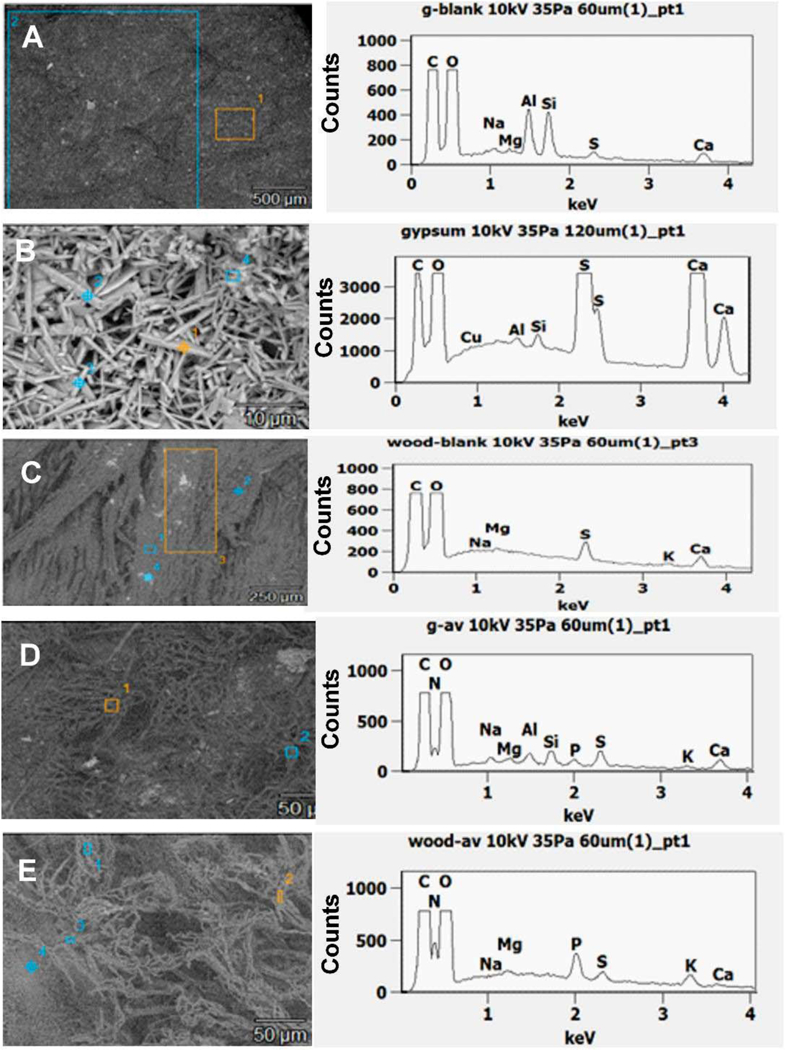
SEM images and elemental composition (SEM-EDX spectra) of non gold coated top of blank gypsum board (A), blank inner core of gypsum (B), top of blank wood (C), A. versicolor growth on gypsum board (D) and A. versicolor growth on wood (E). Figures D and E have the same scale.
Fig. 9 shows examples of SEM-EDX spectra obtained from spores and fragments differentiating their origin. As earlier established, indicator elements, N and P, were used to differentiate biological fragments from non-biological ones. Fragments that had N and P were considered biological and those without them were considered non-biological, hence coming from the growth materials. Most fragments, irrespective of shape or size, had elemental composition similar to those of the spores and the fungal growth (Fig. 9A), while minority of fragments contained elements that were similar to those of the growth materials (Fig. 9B). Relative element x-ray intensity obtained for the fragments was less than that of the blank materials, fungal growth and spores.
Fig 9.
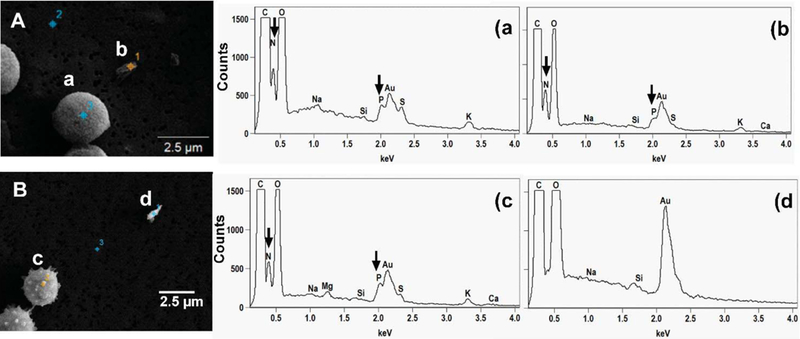
SEM images and EDX spectra of elemental composition of gold coated samples of (A) P. brevicompactum spore (a) and possible biological fragment (b) and (B) A. versicolor spore (c) and non-biological fragment (d) aerosolized from gypsum board. N and P detected in the samples are arrowed.
4. DISCUSSION
In this study, SEM-EDX analysis was employed to characterize biological and non-biological fragments. SEM images showed fragments generated from the tested fungal cultures with different shapes, sizes and origins. While some originated from breakups of spores and hyphae, others came off from surface structures of spores. Spores may break up when exposed to prolonged air currents. During the aerosolization process, dry air was used. Although the generation process lasted only for 3 minutes, it is possible that a decrease in humidity occurred causing desiccation of the fungal growth leading to ruptures in the fungal structures. It is also possible that as larger fungal particles were carried along the air stream in the sampling lines, they impacted on the inner walls of the lines and induced shear forces that led to fungal particle fragmentation (Afanou et al., 2014). However, we found higher F/S-ratio with lower air velocity when shear forces due to impaction are lower than at high flow rate.
During growth, fungi release acids and enzymes to degrade the growth materials in order to obtain nutrients (Adan, 1994) and this decomposition could potentially lead to aerosolization of small material pieces from contaminated surfaces. We attempted to use the criteria developed by Matthias-Maser and Jaenicke (1991, 1994) to determine if fragments had likely been aerosolized from building materials. Out of the three criteria – elemental composition, morphology, and behavior of the sample during EDX, only the first one was useful in our analysis. Fragments were too small to be classified using morphology and they behaved similarly to each other, irrespective of origin, during EDX. Scanning EM-EDX methods have been used in various studies to determine the origin of environmental particles differentiating biogenic particles from anthropogenic ones. In all the studies, C and O peaks above 70% and K, P, S, Si, Ca less than 10% were used (Coz et al., 2010, Pauchauri et al., 2013). None of these studies detected N probably due to the use of environmental samples.
To compare elemental composition of the substrates and the sampled fungal particles, EDX analysis of the blank building materials and the blank membrane filter were performed. EDX analysis of the blank building material samples showed C, O, Na, Mg, S, Ca and Si. The blank membrane filters contained mainly C, O and Al which came from the aluminium stub on which the membrane filters were mounted. In addition to these elements, the fungal growth distinctively had N and P. Among the indicator elements listed by Matthias-Maser and Jaenicke (1991, 1994), our results only agreed with their findings regarding the detection of P, whereas we detected K, S, and Ca also in the blank growth materials. This discrepancy may be due to the fact that our study was on indoor fungi grown on selected building materials, while their study dealt with outdoor particles. The detection of N in our study agrees with studies by Keszthelyi et al. (1984) who related the detection of N to the protein content of the sample. It should be noted that X-ray counts of elements detected in the fungal growth and spores were higher compared to those detected in fragments. This may be due to the small size and thinness of the submicrometer particles. Therefore, the results obtained from SEM-EDX analysis were qualitative in nature. Using the detection of N and P that were present only in fungal growth and spores, fragments in which these elements were detected were considered to be of fungal origin. Most of the fragments analyzed had N and P pointing to fungal origin. Minority of fragments had elemental composition similar to blank materials indicating that some fragments did originate from the building materials.
SEM-EDX has proved very effective in differentiating biogenic particles from anthropogenic particles in environmental samples (Coz et al., 2010; Pipal et al., 2011, 2014). This is because most of the particles were mainly stable particles with consistent chemical composition. Biological particles on the other hand, vary in thickness, texture and chemical composition. This may affect the reaction of the biological particles to electron bombardments resulting in variations in the types and amounts of elements detected in them (Niemi et al., 2006). Coating of samples is known to maintain the electrical conductivity of the sample and also improve image quality (Schwyzer et al., 2013), therefore, we used thin gold coating. Several studies have used gold and gold/platinum alloy (Srivastava et al., 2009; Pipal et al., 2011, 2014). The coating increases the ability of the method to correctly identify particles and perform EDX with appreciable detection of elements. The ability of the method to give consistent amount of elements is affected by the thickness of the coatings. However, from our assessment, the gold layer did not interfere with signals from the elements of interest. Since biological particles vary in nature, an optimum coating thickness needs to be determined for different biological particles. Also, since electron bombardments cause specimen deformation and damage, exposure to the electrons need to be minimized in order to reduce damage to the samples. These factors, to a large extent, results in large variability in EDX counts obtained from particles complicating their recognition. Overall, the analytical methods used to characterize fragments in this study, based on SEM-EDX techniques, can supply valuable data on the morphological characteristics of fragments and their elemental compositions.
Fungal growth typically results also in the emission of MVOCs (Fiedler et al., 2001; Gόrny, 2004; Korpi et al., 1997; Pasanen et al., 1997), which could potentially lead to formation of submicrometer particles by nucleation (Górny, 2004; Górny et al., 2002). If fragment particles were formed by nucleation of released vapors from fungal growth, the concentration of fragments should decrease with higher flow rates due to the increased dilution. Our results showed an opposite trend indicating that nucleation is not likely to be a relevant process for origin of fungal fragments.
An optical particle size of 0.8 μm was used in this study as the borderline between spores and fragments. It should be noted that >0.8 μm particles could include also large fungal fragments as direct reading aerosol instruments are not able to distinguish between spores and large fragments (Afanou et al., 2014; 2015). However, it is not known if the health effects of large fragments differ from those of spores of similar aerodynamic size. Our size distribution data are in agreement with findings from Cho et al. (2005) who used the ELPI to measure the aerodynamic size of fungal particles in a laboratory study. They also observed two peaks in the size distribution and minimum particle concentration at about 0.8 μm. They also reported that the concentration of particles smaller than 0.8 μm was comparable or higher than that of larger particles in the spore size range. They further confirmed these particles were truly fragments by microscopic counting methods. To our knowledge size distribution data from field studies are not available.
Interesting findings were that A. versicolor produced the higher F/S-ratio compared to C. cladosporioides and P. brevicompactum, agar produced the highest amount of fungal particles compared to wood and gypsum board, and the F/S-ratio decreased when air velocity increased. The higher F/S-ratio observed for A. versicolor could be due to the breaks of the spiny structures found on their surfaces yielding more fragments compared to the smooth surfaces of C. cladosporioides and P. brevicompactum. Our results are in agreement with (Afanou et al., 2015; Afanou et al., 2014), who found that more fragments were released from A. versicolor than from P. chrysogenum. The higher amount of fungal particles released from agar compared to gypsum board contradicts findings from Seo et al. (2009), who did not find any difference in the fungal particle release from these materials. The reason might be that their cultivation protocol differed from ours as they added nutrient broth on top of the gypsum board to simulate settled dust. Similar to our study, previous studies have found that increased air velocity increases spore release (Górny et al., 2001; Pasanen et al., 1991). However, increased F/S-ratios as a function of air velocity have not previously been reported. In our aerosolization experiments, the release of both fragments and spores increased, but the spore release increased more with increased air velocity, hence the decrease in F/S-ratio. This decrease agrees with the hypothesis that fungal fragments are already liberated from fungal growth before exposure to air currents, and therefore fragment release is high already at low air velocity (Górny et al., 2002). This implies that fragments and intact spores are released through different processes (Górny et al., 2001) and exposure to fungal fragments may occur in conditions that do not favor the release of spores, such as environments with low air currents.
5. CONCLUSIONS
Concentration of aerosolized fungal particles as well as F/S-ratios were affected by the fungal species, growth materials and air velocity. Most consistent trends were observed with air velocity: the concentrations of both fragments and spores increased with increasing air velocity. These results indicate that nucleation of MVOCs is not a likely process to form fungal fragments but mechanical processes dominate. In contrast to concentration values, the F/S-ratio decreased with increasing air velocity. This implies that exposure to fragments may be proportionally more important in situations that do not favor the release of spores.
SEM images showed fragments from various sources such as broken spores and hyphae, surface structures of spores as well as growth materials. EDX analysis gave information on the origin of the fragments. Although our study was qualitative in nature, N and P were detected as the indicator elements that could be used to distinguish biological from non-biological fragments. Based on these elements, most of the fragments were classified as biological, but also non-biological fragments with elemental composition similar to growth materials were detected. The fragments aerosolized from building materials could be a potential health hazard, depending on the composition of the material. However, more studies will be needed to quantitatively determine the total amounts fragments collected that are biological or non-biological and the health-relevance of both types of fragments. The present results will help to improve our knowledge on the origin of fungal fragments.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by Finnish Distinguished Professor (FiDiPro) program for the Development of Bioaerosol Testing Facilities (BiTeFa) project through TEKES (1391/31/2011).
ABBREVIATIONS
- MVOCs
Microbial Volatile Organic Compounds
- SEM
Scanning Electron Microscope
- FSSST
Fungal Spore Source Strength Tester
- EDX
Energy Dispersive X-Ray Spectroscopy
- OPS
Optical Particle Sizer
- PBOA
Primary Biogenic Organic Aerosol
- NADPH
Nicotine Adenine Dinucleotide Phosphate
- NAHA
β-N-acetylhexosaminidase
- UVAPS
Ultra Violet Aerodynamic Particle Sizer
- WHO
World Health Organization
- PM
Particulate Matter
- ME
Malt Extract Agar
- DG18
Dichloran Glycerol 18% Agar
- LAS-X
Laser Aerosol Spectrometer
REFERENCES
- Adan OCG. On the fungal defacement of interior furnishes 1994. [Google Scholar]
- Adhikari A, Reponen T, Rylander R. Airborne fungal cell fragments in homes in relation to total fungal biomass. Indoor Air 2013;23:142–7. [DOI] [PubMed] [Google Scholar]
- Afanou KA, Straumfors A, Skogstad A, Skaar I, Hjeljord L, Skare Ø et al. Profile and Morphology of Fungal Aerosols Characterized by Field Emission Scanning Electron Microscopy (FESEM). Aerosol Sci Tech 2015: 49:6, 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afanou KA, Straumfors A, Skogstad A, Nilsen T, Synnes O, Skaar I et al. Submicronic fungal bioaerosols: high-resolution microscopic characterization and quantification. Appl Environ Microbiol 2014;80:7122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl H, von Däniken A, Hitz C, Krebs W. Short-term dynamic patterns of bioaerosol generation and displacement in an indoor environment. Aerobiologia 2008;24:203–9. [Google Scholar]
- Cho S, Seo S, Schmechel D, Grinshpun SA, Reponen T. Aerodynamic characteristics and respiratory deposition of fungal fragments. Atmos Environ 2005;39:5454–65. [Google Scholar]
- Coz E, Artíñano B, Clark LM, Hernandez M, Robinson AL, Casuccio GS et al. Characterization of fine primary biogenic organic aerosol in an urban area in the northeastern United States. Atmos Environ 2010;44:3952–62. [Google Scholar]
- Davis PJ. Molds, toxic molds, and indoor air quality: California State Library, California Research Bureau; 2001. [Google Scholar]
- Ettenauer JD, Piñar G, Lopandic K, Spangl B, Ellersdorfer G, Voitl C et al. Microbes on building materials—Evaluation of DNA extraction protocols as common basis for molecular analysis. Sci Total Environ 2012;439:44–53. [DOI] [PubMed] [Google Scholar]
- Fiedler K, Schütz E, Geh S. Detection of microbial volatile organic compounds (MVOCs) produced by moulds on various materials. Int J Hyg Environ Health 2001;204:111–21. [DOI] [PubMed] [Google Scholar]
- Frankel M, Hansen EW, Madsen AM. Effect of relative humidity on the aerosolization and total inflammatory potential of fungal particles from dustǦinoculated gypsum boards. Indoor Air 2014;24:16–28. [DOI] [PubMed] [Google Scholar]
- Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B et al. Ambient pollution and heart rate variability. Circulation 2000;101:1267–73. [DOI] [PubMed] [Google Scholar]
- Górny RL, Ławniczek-Wałczyk A. Effect of two aerosolization methods on the release of fungal propagules from a contaminated agar surface. Ann Agric Environ Med 2012;19:279–84. [PubMed] [Google Scholar]
- Górny RL, Reponen T, Grinshpun SA, Willeke K. Source strength of fungal spore aerosolization from moldy building material. Atmos Environ 2001;35:4853–62. [Google Scholar]
- Górny R, Reponen T, Willeke K, Robine E, Boissier M, Grinshpun S. Release of fungal fragments from moldy surfaces. Appl Environ Microbiol 2002;68:3522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gόrny RL. Filamentous microorganisms and their fragments in indoor air–a review. Ann Agric Environ Med 2004;11:185–97. [PubMed] [Google Scholar]
- Green BJ, Tovey ER, Sercombe JK, Blachere FM, Beezhold DH, Schmechel D. Airborne fungal fragments and allergenicity. Med Mycol 2006;44:S245–55. [DOI] [PubMed] [Google Scholar]
- Green BJ, Schmechel D, Sercombe JK, Tovey ER. Enumeration and detection of aerosolized Aspergillus fumigatus and Penicillium chrysogenum conidia and hyphae using a novel double immunostaining technique. J Immunol Methods 2005;307:127–34. [DOI] [PubMed] [Google Scholar]
- Hyvärinen A, Meklin T, Vepsäläinena A, Nevalainena A. Fungi and actinobacteria in moisture-damaged building materials - concentrations and diversity. Int Biodeterior Biodegrad 2002;49:27–37. [Google Scholar]
- Institute of Medicine (US). Committee on Damp Indoor Spaces and Health. Damp indoor spaces and health: National Academies Press; 2004. [PubMed] [Google Scholar]
- Kanaani H, Hargreaves M, Smith J, Ristovski Z, Agranovski V, Morawska L. Performance of UVAPS with respect to detection of airborne fungi. J Aerosol Sci 2008;39:175–89. [Google Scholar]
- Keszthelyi L, Varga L, Demeter I, Hollós-Nagy K, Szõkefalvi-Nagy Z. Elemental analysis of samples of biological origin relative to their protein content by means of charged particle bombardment. Anal Biochem 1984;139:418–26. [DOI] [PubMed] [Google Scholar]
- Kildesø J, Würtz H, Nielsen KF, Kruse P, Wilkins K, Thrane U et al. Determination of fungal spore release from wet building materials. Indoor Air 2003;13:148–55. [DOI] [PubMed] [Google Scholar]
- Korpi A, Pasanen A, Pasanen P, Kalliokoski P. Microbial growth and metabolism in house dust. Int Biodeterior Biodegrad 1997;40:19–27. [Google Scholar]
- Lee JH, Hwang GB, Jung JH, Lee DH, Lee BU. Generation characteristics of fungal spore and fragment bioaerosols by airflow control over fungal cultures. J Aerosol Sci 2010;41:319–25. [Google Scholar]
- Madelin T, Madelin M. Biological analysis of fungi and associated molds. Bioaerosols Handbook 1995:361–86. [Google Scholar]
- Madsen AM, Kruse P, Schneider T. Characterization of microbial particle release from biomass and building material surfaces for inhalation exposure risk assessment. Ann Occup Hyg 2006;50:175–87. [DOI] [PubMed] [Google Scholar]
- Madsen AM, Wilkins CK, Poulsen OM. Micro-particles from fungi. In Johanning E, ed. Bioaerosols, fungi, bacteria, mycotoxins and human health: Pathophysiology, clinical effects, exposure assessment, prevention and control in indoor environments and work New York: 2005. Fungal Research Group Foundation, Inc. 276–91. [Google Scholar]
- Magari SR, Hauser R, Schwartz J, Williams PL, Smith TJ, Christiani DC. Association of heart rate variability with occupational and environmental exposure to particulate air pollution. Circulation 2001;104:986–91. [DOI] [PubMed] [Google Scholar]
- Matthias-Maser S, Jaenicke R. Examination of atmospheric bioaerosol particles with radii> 0.2 μm. J Aerosol Sci 1994;25:1605–13. [Google Scholar]
- Matthias-Maser S, Jaenicke R. A method to identify biological aerosol particles with radius> 0.3 μmfor the determination of their size distribution. J Aerosol Sci 1991;22:S849–52. [Google Scholar]
- Matthias-Maser S, Obolkin V, Khodzer T, Jaenicke R. Seasonal variation of primary biological aerosol particles in the remote continental region of Lake Baikal/Siberia. Atmos Environ 2000;34:3805–11. [Google Scholar]
- McGinnis MR. Indoor mould development and dispersal. Med Mycol 2007;45:1–9. [DOI] [PubMed] [Google Scholar]
- Méheust D, Le Cann P, Reponen T, Wakefield J, Vesper S, Gangneux J. Possible application of the Environmental Relative Moldiness Index in France: A pilot study in Brittany. Int J Hyg Environ Health 2013;216:333–40. [DOI] [PubMed] [Google Scholar]
- Meklin T, Haugland RA, Reponen T, Varma M, Lummus Z, Bernstein D et al. Quantitative PCR analysis of house dust can reveal abnormal mold conditions. J Environ Monitor 2004;6:615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ Health Perspect 2011;119:748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi JV, Saarikoski S, Tervahattu H, Makela T, Hillamo R, Vehkamaki H, Sogacheva L, Kulmala M. Changes in Background Aerosol Composition in Finland during Polluted and Clean Periods Studied by TEM/EDX Individual Particle Analysis. Atmos. Chem. Phys 2006; 6: 5049–5066. [Google Scholar]
- Pachauri T, Singla V, Satsangi A, Lakhani A, Kumari KM. SEM-EDX characterization of individual coarse particles in Agra, India. Aerosol Air Qual Res 2013;13: 523–536. [Google Scholar]
- Pasanen A, Pasanen P, Jantunen M, Kalliokoski P. Significance of air humidity and air velocity for fungal spore release into the air. Atmospheric Environment. Part A.General Topics 1991;25:459–62. [Google Scholar]
- Pasanen P, Korpi A, Kalliokoski P, Pasanen A. Growth and volatile metabolite production of Aspergillus versicolor in house dust. Environ Int 1997;23:425–32. [Google Scholar]
- Pekkanen J, Peters A, Hoek G, Tiittanen P, Brunekreef B, de Hartog J et al. Particulate air pollution and risk of ST-segment depression during repeated submaximal exercise tests among subjects with coronary heart disease: the Exposure and Risk Assessment for Fine and Ultrafine Particles in Ambient Air (ULTRA) study. Circulation 2002;106:933–8. [DOI] [PubMed] [Google Scholar]
- Pipal AS, Kulshrestha A, Taneja A. Characterization and morphological analysis of airborne PM 2.5 and PM 10 in Agra located in north central India. Atmos Environ 2011; 45: 3621–3630. [Google Scholar]
- Pipal AS, Jan R, Satsangi PG, Tiwari S, Taneja A. Study of surface morphology, elemental composition and origin of atmospheric aerosols (PM2. 5 and PM10) over Agra, India. Aerosol Air Qual Res 2014;14:1685–1700. [Google Scholar]
- Reponen T, Lockey J, Bernstein DI, Vesper SJ, Levin L, Hershey GKK et al. Infant origins of childhood asthma associated with specific molds. J Allergy Clin Immunol 2012;130:639,644. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari S, Reponen T, Keskinen J. Performance of two fluorescence-based real-time bioaerosol detectors: BioScout vs. UVAPS. Aerosol Sci Tech 2014;48:371–8. [Google Scholar]
- Schmechel D, Górny RL, Simpson JP, Reponen T, Grinshpun SA, Lewis DM. Limitations of monoclonal antibodies for monitoring of fungal aerosols using Penicillium brevicompactum as a model fungus. J Immunol Methods 2003;283:235–45. [DOI] [PubMed] [Google Scholar]
- Schwyzer I, Kaegi R, Sigg L, Nowack B. Colloidal stability of suspended and agglomerate structures of settled carbon nanotubes in different aqueous matrices. Water research, 2013;47: 3910–3920. [DOI] [PubMed] [Google Scholar]
- Seo S, Grinshpun SA, Iossifova Y, Schmechel D, Rao CY, Reponen T. A new field-compatible methodology for the collection and analysis of fungal fragments. Aerosol Sci Tech 2007;41:794–803. [Google Scholar]
- Seo S, Reponen T, Levin L, Grinshpun SA. Size-fractionated (1→ 3)-β-D-glucan concentrations aerosolized from different moldy building materials. Sci Total Environ 2009;407:806–14. [DOI] [PubMed] [Google Scholar]
- Seo SC, Reponen T, Levin L, Borchelt T, Grinshpun SA. Aerosolization of particulate (1-->3)-beta-D-glucan from moldy materials. Appl Environ Microbiol 2008;74:585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasubramani SK, Niemeier RT, Reponen T, Grinshpun SA. Fungal spore source strength tester: laboratory evaluation of a new concept. Sci Total Environ 2004;329:75–86. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Jain VK, Srivastava A.. SEM-EDX analysis of various sizes aerosols in Delhi India. Environ Monit Assess 2009;50:405–416. [DOI] [PubMed] [Google Scholar]
- WHO Regional Office for Europe. WHO Guidelines for Indoor Air Quality: Dampness and Mould 2009. [PubMed] [Google Scholar]
- Wittmaack K, Wehnes H, Heinzmann U, Agerer R. An overview on bioaerosols viewed by scanning electron microscopy. Sci Total Environ 2005;346:244–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


