Abstract
Cytomegaloviruses (CMVs) are highly prevalent herpesviruses, characterized by strict species specificity and the ability to establish non-productive latent infection from which reactivation can occur. Reactivation of latent human CMV (HCMV) represents one of the most important clinical challenges in transplant recipients secondary to the strong immunosuppression. In addition, HCMV is the major viral cause of congenital infection with severe sequelae including brain damage. The accumulated evidence clearly shows that cellular immunity plays a major role in the control of primary CMV infection as well as establishment and maintenance of latency. However, the efficiency of antiviral antibodies in virus control, particularly in prevention of congenital infection and virus reactivation from latency in immunosuppressed hosts, is much less understood. Because of a strict species specificity of HCMV, the role of antibodies in controlling CMV disease has been addressed by using murine CMV (MCMV) as a model. Here, we review and discuss the role played by the antiviral antibody response during CMV infections with emphasis on latency and reactivation not only in the MCMV model, but also in relevant clinical settings. We provide evidence to conclude that antiviral antibodies do not prevent the initiating molecular event of virus reactivation from latency but operate by preventing intra-organ spread and inter-organ dissemination of recurrent virus.
Keywords: antibodies, cytomegalovirus, immunotherapy, latency, passive immunization, reactivation, recurrence, serum transfer, viral entry complexes, virus dissemination, virus spread
Introduction
Cytomegalovirus (CMV) is the prototypical member of the beta-herpesvirus subfamily, characterized by strict species specificity, broad tissue tropism, slow growth, and the ability to establish latency. CMV latency can be defined as a lack of infectious virus but preserved viral genomes from which some triggers can initiate the productive cycle of viral gene expression that can proceed to virus recurrence ([1, 2], see also [3, 4] in this issue of MMIM). Human CMV (HCMV) persists in the majority of the adult population worldwide. Acute infection is usually subclinical in immunocompetent hosts, but in immunocompromised individuals it can cause a broad spectrum of diseases, including pneumonia, gastroenteritis, retinitis, graft rejection and organ failure [5, 6]. Moreover, HCMV is the most frequent viral cause of congenital infection and the leading infectious cause of mental retardation and hearing loss in children [7, 8]. HCMV reactivation and subsequent disease continue to remain a huge clinical problem in immunosuppressed transplant recipients and in AIDS patients [5, 6, 9].
Multiple mechanisms of innate and adaptive immunity are involved in the control of primary CMV infection. The principal mechanisms of innate immunity involved in the early control of CMV are type I interferons and various subsets of innate immune cells, including natural killer (NK) cells. The long-term control of CMV infection depends mainly on adaptive immunity, primarily on CD8 T cells that recognize viral peptides presented by MHC class I molecules. Millions of years of co-evolution of CMVs and their animal hosts, and continuous challenge to overwhelm each other, resulted in creation of many viral strategies to cope with immune host mechanisms. One can hardly name a single immune function that in some way is not subject to the regulation through CMV immunoevasion. Hence, the capacity of different immune mechanisms to control CMV infection is defined by the functions of viral immunoevasins [10]. For this reason, the efficacy of various approaches in immune intervention to contain CMV infection can only be predicted in the context of complete understanding of viral immunoevasion strategies.
Although antiviral antibodies are indispensable in the control of different viral infections, there have been many inconsistencies in results from studies that have attempted to describe their role during CMV infection. Here, we will review our own work, in part published decades ago, but also more recent findings on the role of antiviral antibodies during MCMV latency and reactivation. We will also discuss the current views on the role of antiviral antibodies in the prevention of congenital CMV infection as well as CMV reactivation following hematopoietic stem cell transplantation (HSCT) and solid organ transplantation (SOT) with emphasis on the potential contribution of viral strain specific antibody responses in various clinical settings.
In the model world of mice
Identification of protective immune cell subsets: the issue of immune system homeostasis and its stable remodeling after constitutive deletion of a specific subset
Because of the strict species specificity of cytomegaloviruses, large part of our understanding of immunobiology of CMV infection is based on studies in animal models, among which the mouse model of infection with murine CMV (MCMV) has been most extensively used and has proven its validity for predictions on HCMV disease and immune control (for more recent reviews, see [11, 12]). As in humans, primary MCMV infection in immunocompetent mice is efficiently contained, whereas infection of immunologically immature and immunocompromised mice leads to multiorgan disease with high mortality [13, 14]. Early studies using adoptive transfer of virus specific T cells into immunodeficient syngeneic recipients demonstrated that CD8 T cells are capable of controlling virus infection, whereas CD4 T cells were neither protective nor required for the protection by CD8 T cells [13, 15]. Moreover, CD8 T cells were found to be operative when administered prophylactically as well as therapeutically to MCMV infected, immunocompromised recipients [13]. Even though CD4 T cells derived from infected immunocompetent mice turned out to be inefficient in systemic virus control upon adoptive cell transfer, their function proved to be mandatory for virus control in the salivary glands. This was concluded from the finding that mice depleted long-term of CD4 T cells established a prolonged “persistent” productive infection of a specific cell type in salivary gland tissue, namely the glandular epithelial cell that accounts for most of the virus production at this mucosal site [16]. Since helper CD4 T cells are needed for the formation of an antibody response, the inability of CD4 depleted mice to terminate productive infection in the salivary glands was initially attributed to compromised antibody response. Yet, later studies showed that IFN-γ was essential for virus clearance from the salivary glands [17]. Furthermore, it was demonstrated that MCMV control in salivary gland tissue depends on CD4 T cells due to exclusive presentation of MCMV-derived antigens by MHC class II molecules on bystander antigen-presenting cells (APCs), resulting in IFN-γ secretion [18].
In ostensible contradiction to the early adoptive cell transfer studies that had predicted an indispensable role for CD8 T cells in containing CMV infection [13, 15], as discussed above, mice lacking CD8 T cells either genetically by gene-knockout or due to long-term depletion were able to control primary MCMV infection, and even the kinetics of virus clearance and establishment of latency was like in fully immunocompetent mice [19, 20]. This brings us to the most important yet most neglected topic in immunology discussed recently by Reddehase and Lemmermann [11], namely immune system homeostasis and its remodeling by constitutive removal of immune cell subsets. While remodeling compensates for the function of missing CD8 T cells by gain of function in the remaining part of the immune system, the physiological T cell response to CMVs is based on CD8 T cells. This became impressively evident from T cell-subset depletions during ongoing immune reconstitution in a murine model of HSCT and MCMV infection. Whereas depletion of CD4 T cells did not notably interfere with control of the infection, depletion of CD8 T cells resulted in an inevitably lethal multiple-organ failure caused by viral histopathology ([21, 22], reviewed in [12, 23]). So, no other innate or adaptive immune cell subset was timely educated and recruited to functionally compensate and prevent death from viral pathology. In clinical HSCT, human transplant donors and recipients do not usually have a genetic disorder that constitutively deprives them of CD8 T cells. So, for “model building” to predict a clinical correlate, mouse mutants with an altered immune system homeostasis may not be the best choice [11].
Antiviral antibodies are not essential for control of primary MCMV infection and establishment of latency
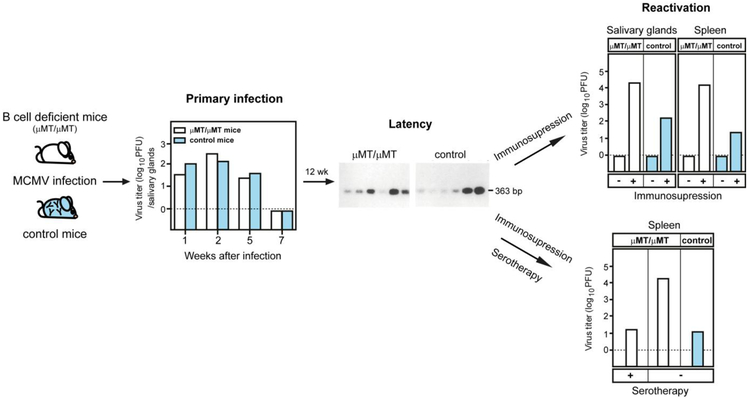
Although early studies have demonstrated the capacity of adoptive serotherapy to control primary CMV infection [24, 25], it remained unclear if antiviral antibodies generated in the course of primary infection are required for efficient control of virus spread and establishment of latency. It is generally believed that neutralizing antibodies block viral infection of cells and cell-to-cell spread, but once the virus enters the cell and begins to replicate, it becomes inaccessible to antibodies. At the time when an effective antibody response is formed during primary CMV infection, the majority of virus has entered susceptible cells and thus evades recognition by antibodies. Over two decades ago, we used mice homozygous for a deletion of the transmembrane exon of the Ig μ chain (μMT/μMT), which are devoid of B cells and therefore agammaglobulinemic [26], to address the question of whether antiviral antibodies are essential in virus control of primary CMV infection [27]. The results demonstrated that mice lacking antibodies not only resolved the primary infection but also established latent infection (Fig. 1). Furthermore, the kinetics of virus clearance was indistinguishable from wild-type control mice. Importantly, antibody-deficient mice and their seropositive controls showed similar levels of latent viral genome load, further supporting the notion that lack of antibodies does not impact on the capacity of the remaining immune system in these mice to control the kinetics of CMV infection or the magnitude of latency that was established. This turned out to be the case for salivary glands as well, thereby confirming that the requirement of CD4 T helper cells for termination of productive infection in this organ was independent of the activity of antiviral antibodies. Thus, antiviral antibodies are not essential for resolution of primary CMV infection or the prevention of horizontal virus spread.
Fig. 1.
Antibodies are not required for control of primary MCMV infection and establishment of latency. (Left panel) Kinetics of MCMV clearance after primary infection in B cell deficient and control mice. (Center panels) Twelve weeks after infection, both groups of mice were in latency and showed no differences in load of latent viral genome. (Upper right panel) Latently infected mice were subjected to immunosupression by sub-lethal-γ-irradiation combined with cytolytic antibodies against T cells and NK cells. (Lower right panel) In addition, a group of latently infected and immunodepleted B cell deficient mice received hyperimmune anti-MCMV sera. Adapted from ©1994 JONJIĆ et al. Originally published in JOURNAL OF EXPERIMENTAL MEDICINE. https://doi.org/10.1084/JEM.179.5.1713 [27].
The finding that antibodies are not essential for controlling primary CMV infection does not rule out the ability of preformed antibodies to contain the infection. The early studies have shown that immunotherapy by immune serum obtained from mice infected with MCMV protected recipient mice from a challenge infection [24, 25]. Bootz and colleagues recently showed that combination of gB-specific neutralizing monoclonal antibodies (MAbs) are as potent in protection as is a polyvalent serum from immune animals and more potent than immunotherapy with individual antibodies [28]. Interestingly, both neutralizing and non-neutralizing antibodies showed comparable protection when given prophylactically, while when applied therapeutically neutralizing antibodies were superior. Moreover, Klenovsek et al. [29] have shown that not only preformed antibodies, but also memory B cells adoptively transferred into immunodeficient hosts have a dramatic effect on MCMV control in that they prevent both morbidity and mortality in recipient mice. In addition to being protective in both prophylactic and therapeutic settings, protection provided by transferred B cells was also long-lasting and therefore may have advantage over antibodies in some settings.
In the mouse model of CMV infection of the developing central nervous system (CNS) that utilizes MCMV infection of newborn mice, immunotherapy of CMV-induced encephalitis with either sera from latently infected donor mice or gB specific monoclonal antibodies reduced the load of infectious virus in brain and reduced virus-induced brain pathology [30]. Mice that received antiviral antibodies exhibited fewer and less extended histopathological lesions and had improved postnatal development of cerebellum compared to untreated infected mice. Thus, antiviral antibodies can be effective in preventing virus-associated developmental abnormalities in the CNS, likely by reduction of the virus titer and the host inflammatory response. Similar to adoptive serotherapy, transplacentally transferred maternal antibodies are also protective in the mouse model of congenital CMV infection. Even vaccination of female mice with a highly attenuated virus, such as the mutant expressing a high-affinity ligand for the NKG2D receptor, was able to induce antibodies that could be transplacentally transferred to their offspring and that provided protection from MCMV disease [31, 32].
Reactivated CMV is held in check by redundant and hierarchical contributions from T cell subsets and NK cells
Reactivation of latent HCMV is one of the most important clinical challenges in the field of transplantation (see below) secondary to the strong immunosuppression, for instance hemato-ablative leukemia therapy and prophylaxis against graft-versus-host disease (GVHD) in patients undergoing HSCT or prevention of graft rejection in patients undergoing SOT. Latent HCMV can reactivate either from the transplant or from recipients’ tissues and cause severe disease (see also the article by Reddehase and Lemmermann in this issue MMIM [4]). Better understanding of immune mechanisms required for prevention of CMV reactivation from latency, as well as its spread to different tissues and organs, is of crucial interest for prophylactic and immunotherapeutic interventions aimed at preventing and/or reducing recurrent CMV infection and recrudescent CMV disease in immunocompromised patients. Data obtained in mice latently infected with MCMV followed by immunosuppression nicely correspond to studies on HCMV recurrence in humans undergoing immunosuppression. Primary MCMV infection of mice subjected to hemato-ablative treatment by a sub-lethal dose of total-body-γ-irradiation is characterized by virus spread to all organs and tissues resulting in multiorgan disease and very high mortality [8]. MCMV recurrence in latently infected mice has been demonstrated by various modes of immunosuppression, including anti-lymphocyte serum and corticosteroids [33, 34], cyclophosphamide [35], γ-irradiation [36-38] and also sepsis [39].
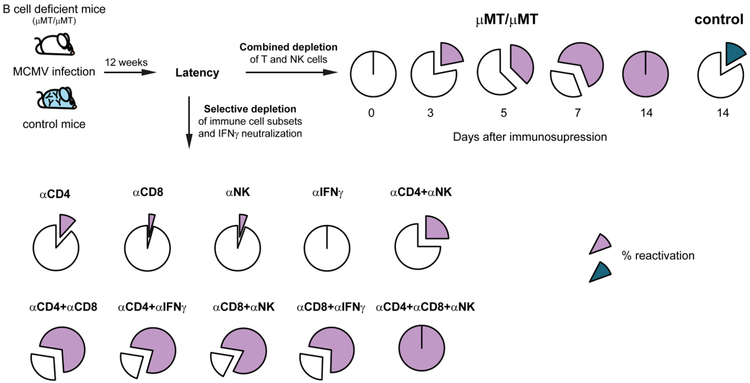
As mentioned above, adoptive cytoimmunotherapy in total-body-γ-irradiated mice demonstrated that reconstitution of antiviral CD8 T cells is critical for survival of MCMV-infected recipients after experimental HSCT [40]. However, dissection of the roles of individual subsets of cellular immunity (CD4 T cells, CD8 T cells, and NK cells) in prevention of recurrent CMV infection on the level of the whole organism turned out to be a challenging task because antiviral antibodies, normally present in latently infected mice in very high titers, would not only neutralize naturally released virions in situ, but can also interfere with detection of virus in tissue homogenates in the in vitro infectivity assay. This technical challenge was resolved by employing latently infected B cell deficient, and thus antibody deficient, μMT/μMT (μ−μ−) mice [41]. Absence of antiviral antibodies in these mice significantly facilitated detectability of recurrent MCMV, thus allowing us for the first time to accurately determine the individual contributions by CD8 and CD4 T cells, NK cells, and cytokines to the control of recurrent CMV infection (Fig. 2). We were actually the first to show that both T cell subsets, but also NK cells, are involved in prevention of CMV recurrence at the level of the whole organism, since depletion of any of these three subsets resulted in virus recurrence in a proportion of latently infected mice. Only the combined depletion of all three subsets resulted in the maximal viral recurrence with high titers in all tested mice (Fig. 2). In addition, it was shown that IFN-γ is also involved in prevention of virus recurrence, as its neutralization combined with CD4 or CD8 T cell depletion resulted in enhanced recurrence compared to single depletion of CD4 or CD8 T cells [41, 42]. In essence, this study demonstrated hierarchical and redundant immune control of latency, with selective contributions made by CD8 T cells, CD4 T cells, and NK cells. The redundant control of latent CMV must be very important in the complex biology of the host-CMV balance, because redundancy suggests that on the level of the whole organism a minimum of preserved cellular immune reactivity suffices for preventing deleterious recurrence.
Fig. 2.
Cellular immunity and IFN-γ prevent CMV reactivation while antibodies limit hematogenic spread of recurrent virus. (Upper right) Latently infected B cell deficient and control mice were depleted of CD4 T, CD8 T and NK cells and virus titers in their organs were determined on different time points after immunodepletion. (Lower left) Latently infected B cell deficient mice were depleted of CD4, CD8 T cells, NK cells and/or IFN-γ was blocked alone or in different combinations as indicated. Two weeks later, organs were analyzed for infectious virus. Adapted from ©1998 POLIĆ et al. Originally published in JOURNAL OF EXPERIMENTAL MEDICINE. https://doi.org/10.1084/jem.188.6.1047 [41].
Antiviral antibodies do not prevent virus reactivation but limit hematogenic dissemination and intra-tissue spread of recurrent virus
The studies discussed so far used B-cell/antibody-deficient μMT/μMT (μ−μ−) mice to improve the sensitivity of detecting reactivated virus. In clinical research, the terms “reactivation” and “recurrence” are mostly used as synonyms. Much of the misunderstanding between basic and clinical scientists results from a different “language”. In a molecular view, latency means that the viral genome is replicatively silenced and that limited gene expression does not initiate the productive gene expression cascade, so that no infectious virions are generated. “Reactivation” means that the productive cycle is re-initiated, which can be triggered by cytokine signaling to the major immediate-early (MIE) enhancer element and which is associated with opening of the otherwise closed chromatin-like structure of latent viral genomes. Finally “recurrence” describes the completion of the productive viral cycle, resulting in the release of infectious virions. There is evidence to propose that expression of epitope-encoding viral genes can lead to recognition of cells by T cells even before the productive cycle is completed by the release of infectious virions, so that indeed T cells might terminate the reactivation event as such ([38, 43], for more recent reviews see [3, 44, 45]).
A role for antiviral antibodies in preventing the intra-host dissemination of recurrent virus was first indicated by inducing virus reactivation in B cell-sufficient mice that were latently infected after resolution of a prolonged neonatal primary infection [37]. These mice revealed a high viral genome load in all organs tested, and upon strong suppression of cellular immunity stochastic “yes-no” patterns of virus recurrence in salivary glands, spleen, or lungs were observed (cumulative recurrence incidence: 19 of 30 mice) with apparently no hematogenic virus dissemination from a recurrence-negative to a recurrence-positive organ. The interpretation that antiviral antibodies prevented the dissemination of recurrent virus between the organs was corroborated by joint publications demonstrating strong reduction in hematogenic virus dissemination by passive immunization with serum from infected B-cell sufficient C57BL/6 (μ+μ+) or heterozygous μMT/+ (μ−μ+) mice as opposed to serum from infected B-cell-deficient μMT/μMT (μ−μ−) mutant mice [27,37]. It is worth to be emphasized that this approach of using sera from infected mice, sufficient or deficient in the B cell response, proved that the protective principle is indeed antiviral antibody. This excluded the alternative explanation that serum from infected mice may protect due to antiviral cytokines, of which a plethora is induced by infection [46]. A distinction between these alternatives is often missing in the literature.
In direct comparison, B cell-sufficient mice, which became latently infected after a rapidly terminated primary infection at adult age, revealed a low viral genome load in organs associated with a low cumulative recurrence incidence (2 of 30 mice and in lungs only) upon the same degree of immunosuppression. Notably, neutralizing antibody titers in serum also positively correlated with recurrence incidence, instead of negatively as one would have expected [37]. These findings showed that latent virus genome load is a positive predictor for the risk of virus reactivation and recurrence, whereas neutralizing antibodies do not notably interfere with the reactivation events, which are reflected by the incidences, but rather act beyond that stage by preventing the dissemination of recurrent virus.
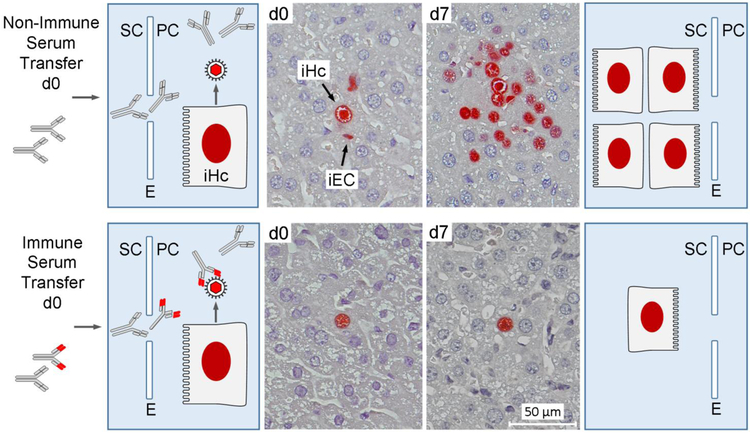
A later study by Wirtz and colleagues [47] addressed the question if antiviral antibodies can also limit the intra-tissue spread of virus from an infected cell to neighboring cells. For this, they modeled a reactivation event in liver tissue by choosing a time after primary infection when the virus has just entered the tissue and infected first-hit, single cells. At that time, defined as day 0 based on earliest tissue colonization, passive immunization was performed by transfer of immune serum compared to non-immune serum from infected or uninfected B cell-sufficient donor mice, respectively (Fig. 3). This model fits the reactivation situation pretty well, since liver sinusoidal endothelial cells (LSECs) are a recognized cellular site of MCMV latency ([48], reviewed in [4, 45]) and since virus spread from LSECs, which are very poor virus producers, to hepatocytes, which are very strong virus producers, has been documented with floxed “pseudo-latent” virus that was “pseudo-reactivated” by recombination in LSECs of Tie2-cre mice [49, 50]. Thus, upon virus reactivation in latently infected LSECs, minimal numbers of released virions can infect neighboring hepatocytes for a quantitative virus recurrence, except if this is prevented by antiviral antibody. The result was adamantly clear: while in the absence of immune serum virus spread to neigboring cells resulted in an extended focus of infection associated with histopathology (Fig. 3, upper panel), immune serum prevented intra-tissue spread and thus also prevented histopathology (Fig. 3, lower panel). That the protective principle is indeed antiviral antibody was verified in this model by transfer of serum from infected B cell-deficient μMT/μMT (μ−μ−) or B cell-sufficient μMT/+ (μ−μ+) mice allowing or preventing virus spread, respectively [47].
Fig. 3.
Virus-specific antibodies prevent cell-to-cell spread within host tissue. (Far left and far right panels) Explanatory charts of liver tissue compartments. SC sinusoidal compartment. E liver sinusoidal endothelium. PC parenchymal compartment. iHc infected hepatocyte. Unspecific and virus-specific (red-marked variable region) antibodies present in transferred sera enter liver parenchyma via fenestrae. Only virus-specific antibodies intercept released virions (Center images) Immunohistological images of liver tissue sections taken on day 0 (d0) and day 7 (d7) after serum transfer. iEC infected endothelial cell, a verified cellular site of MCMV latency [48]. Infected cells were identified by red staining of intranuclear IE1 protein. Conceptionally based on Wirtz et al. 2008, MMIM 197:151-158, with new tissue sections from stored embedded organs used for the original publication [47].
Interestingly, as we know only since recently, first entry of hematogenic/viremic MCMV into liver tissue strictly depends on the viral entry complex gH/gL/gO present in the virion envelope [51, 52], whereas subsequent cell-to-cell spread within liver tissue is redundantly mediated by gH/gL/gO and the alternative complex gH/gL/MCK-2 [52, 53], which is considered to be the MCMV analog of the HCMV pentameric entry receptor gH/gL/pUL(128,130,131A) (referenced in [52, 53], see also below in the sections on HCMV). Redundance in entry receptor usage for intra-tissue spread was concluded in that study from the finding that only double-deletion mutant ΔgOΔMCK-2-gOtrans, in which gO was transcomplemented for first tissue entry, failed to subsequently spread within the liver. It was predicted that “interventional strategies targeting only gO might be efficient in preventing organ manifestations after a primary viremia, whereas both gH/gL complexes need to be targeted for preventing intra-tissue spread of virus reactivated from latency within tissues” [49]. In retrospect, we thus conclude that the immune serum used in the study by Wirtz and colleagues [47] must have contained antibodies directed against both entry complexes.
Altogether, the murine model has unequivocally demonstrated a protective antiviral function of antibodies.
In the clinical world of humans
Clinical data versus animal models
Studies in murine models of CMV infection that have utilized well defined MAbs in passive transfer experiments have demonstrated that frequently used correlates of in vitro antiviral antibody activity, such as virus neutralization, do not uniformly translate into in vivo protective antibody activity and that informative surrogates of in vivo protection by antiviral antibodies remain to be defined [28]. This observation has provided at least one potential explanation for the failure of a number of different human MAbs and intravenous immunoglobulin (IVIG) preparations to modify the course of HCMV infections in clinical trials [54-57]. Despite the limitation inherent in modeling HCMV infections in small animals as well as in non-human primates, studies in animal models have identified potential targets of protective antiviral antibodies, provided insights into the pharmacokinetics of protective antibodies, and perhaps most importantly, have suggested specific mechanisms of protective antiviral antibodies that could be modeled in vitro and thus, identify useful surrogates of in vivo protective activity.
Clinical relevance of CMV infection including various immuno-deficiencies and the role of antibodies
In contrast to findings from more well defined and more readily controlled studies in animal model systems, the role of antiviral antibodies in the natural history of HCMV infections and the identification of protective antiviral antibody responses in different types of clinical HCMV infections are unanswered questions in current research and continue to generate considerable interest from both academic investigators and pharma. Very early studies in SOT recipients argued that the kinetics of anti-CMV antibody responses in high risk recipients (Donor+/Recipient−; D+/R−) was delayed and could contribute to the increased likelihood of clinical disease in HCMV infected patients [58]. Likewise observations from natural history studies of congenital HCMV (cCMV) infections and blood transfusion acquired HCMV infections in newborn infants have argued that HCMV antibodies play an important role in limiting disease but not in the prevention of infection [59]. Thus, there are a significant number of clinical observations that together with correlative laboratory findings have suggested that anti-HCMV antibodies can modify the course of HCMV infections. Studies in different human populations have failed to definitively demonstrate that anti-HCMV antibodies can prevent infection, particularly in community settings that often include repeated exposures to different sources of HCMV.
The early findings in SOT recipients that suggested that antiviral antibodies could provide some protection against severe HCMV infections in the posttransplant period prompted the development and testing of high titer HCMV IVIG in SOT recipients. The source of the early IVIG preparations included pooled immunoglobulins from individuals with high anti-HCMV antibody titers as measured in antigen binding assays that often utilized viral antigens from infected fibroblasts resulting in preparations that were enriched in anti-HCMV antibodies directed at non-envelope HCMV antigens [60]. As a result, antibodies reactive with proteins from the virion envelope that have been correlated with functional antiviral activities such as virus neutralization were present but not enriched in comparison to other HCMV antibody specificities. This property of IVIG preparations must be considered when evaluating the potential efficacy of different preparations in clinical trials. Nonetheless, early studies utilizing HCMV IVIG preparations in SOT, specifically renal allograft recipients, demonstrated modification of HCMV associated clinical disease in transplant recipients at high risk (D+/R−) for HCMV disease in the posttransplant period [61-63]. However, the potential value of IVIG in most SOT recipients was never fully realized as subsequent improvements in antiviral chemotherapy and diagnostic methodologies dramatically advanced clinical management of SOT recipients in the posttransplant period. Both antiviral prophylaxis and preemptive therapeutic approaches have demonstrated remarkable success in the early posttransplant period resulting in only sporadic use of IVIG in most transplant centers. One exception is the use of IVIG preparations in selective groups of high risk transplant recipients, including those receiving heart-lung or lung allografts [64]. Unfortunately, a clear understanding of the mechanism(s) of antiviral antibodies that accounted for protection from severe disease in recipients of IVIG was not determined in these early studies. Similarly, IVIG preparations were utilized to modify HCMV disease in hematopoietic transplants but failed to demonstrate significant clinical efficacy with the exception that course of acute GVHD appeared to be modified by IVIG [65, 66]. The impact of IVIG on GVHD has been attributed to the immune-modulatory effects of IVIG. However, it should be noted that utilization of IVIG in the posttransplant period to correct deficiencies in immunoglobulin deficiencies appears to be an accepted use of these preparations in some transplant centers [67-72].
More recently, renewed interest has developed in the application of potent human anti-HCMV MAbs in prophylaxis and treatment of HCMV infections in immunocompromised hosts. Thus far, all of these preparations include antibodies that target envelope components of the HCMV virion, including gB, gH, and the gH/gL/UL 128-131 (pentamer complex). The results from early studies utilizing an anti-gH MAb in HIV infected individuals were disappointing and only very limited data was generated from very early studies of an anti-gB MAb [55, 73]. More recently, investigators have tested a combination of two antibodies, an anti-gH and anti-pentamer (gH/gL/UL128-131) MAb in a prophylactic trial in high risk renal allograft recipients [74]. The results of this study failed to demonstrate an effect of the MAb on the primary outcome of the trial, a reduction in HCMV viremia at 12 weeks posttransplant; however, additional analysis of the findings from this study indicated that treatment with the MAb decreased the incidence of manifestations of HCMV associated disease as well as delayed the development of viremia after 12 weeks [74]. Results from this clinical trial were encouraging and suggested that anti-envelope MAb could potentially modify the natural history of HCMV infections in SOT. Similarly, results from a clinical trial of a subunit gB vaccine in SOT recipients suggested that vaccine induced antiviral antibodies provided some protection as measured by a reduction in the need for antiviral preemptive therapy [75]. Although consistent with the potentially protective activity of IVIG in SOT recipients described above, the correlates of antiviral antibody activity in this study was also confounded by the potential contribution of HCMV specific T lymphocyte responses in this study population. Lastly and in contrast to promising results from passive anti-HCMV antibody therapy in SOT, the role of passively acquired antibodies in the control of HCMV infections in HSCT has not been convincingly demonstrated in clinical trials [76, 77]. In contrast to limited interest in passively transferred anti-HCMV antibodies in this population, there continues to be active research in adoptive HCMV specific T lymphocyte therapies for HSCT recipients at high risk for HCMV infection and disease, an approach first described nearly 25 years ago [78, 79].
Antiviral antibodies and the natural history of congenital HCMV infections
In the past there has been a near universal acceptance that maternal anti-HCMV antibody status determined much of the natural history of cCMV. This dogma was conventional in terms of the existing view that antiviral antibodies provided some level of protection in most viral diseases and most importantly, agreed with existing observations derived from studies of other congenital infections such as those following maternal rubella virus infection [80, 81]. Even though findings from studies of allograft transplant recipients have repeatedly demonstrated an essential role of HCMV specific T lymphocytes but not antiviral antibodies in control of HCMV infections in immunocompromised populations, the role of antiviral antibody responses, including those responses induced by prophylactic vaccines, has and continues to remain a central theme in studies of protective adaptive immunity in cCMV infections that follow maternal HCMV infections during pregnancy. Decades of studies have described characteristics of maternal anti-HCMV antibody responses including the kinetics of antiviral antibody development, specificities of the antiviral antibody response, and quantities of maternal anti-HCMV antibodies [82-90]. Although in some cases results from these studies were correlated with decreased rates of maternal to fetal transmission and the incidence of severe cCMV infections, the variability of responses between individual women in these studies suggests that a direct and perhaps quantitative relationship between anti-HCMV antibody responses and the outcome of maternal infection for an individual woman will be difficult to define. Thus, it is likely that undefined parameters of maternal infections such as source, amount of virus in the inoculum, frequency of exposure, and genetic complexity of viral exposures will contribute independently to the phenotype of maternal infections. The complexity of maternal HCMV infections will almost certainly continue to confound the identification of correlates of the activity of antiviral antibodies measured in conventional assays. Such an explanation could explain the differences between findings from studies in animal models under controlled laboratory settings and those from observational studies in humans. This variability in the outcome of HCMV infection in pregnant women is illustrated by the well documented finding that following community acquired primary maternal infection, only 20-30% of women transmit virus to their offspring and less than 10% of these infected infants exhibit any long term morbidity associated with this intrauterine infection. More recently, there has been a renewed appreciation in older findings that maternal adaptive immunity does not prevent transmission of HCMV to the developing fetus nor does it prevent neurodevelopmental sequelae in cCMV infected infants born to women with preexisting HCMV immunity prior to conception [91]. In fact, in some highly immune maternal populations such as women in Brazil who have a >96% rate of HCMV serological immunity, over 90% of infants with cCMV infections are born to women with documented HCMV antibody reactivity prior to pregnancy [92]. This finding is consistent with the results from several smaller studies from other parts of the world and with calculations that the vast majority of all infants born with cCMV infections are born to women who were immune to HCMV and therefore have antiviral antibodies prior to pregnancy [93-95]. Interestingly, when anti-HCMV antibodies were assayed in the group of Brazilan women described above there was little or no correlation between the levels, specificities, or functional activities of anti-HCMV antibodies and protection from intrauterine transmission [88]. In contrast to this interpretation of existing data, some investigators have argued, based on data from observational studies, that maternal antibodies can limit intrauterine transmission and perhaps severe fetal infection [96]. Although the significance of anti-HCMV antibodies and the outcome of maternal infections during pregnancy remains contentious, the biological impact of anti-HCMV antibodies in the natural history of cCMV infection likely ranges from a significant role in individual women to little if any biological importance in larger populations. However, even this oversimplification must be tempered by the paucity of definitive data supporting the use of in vitro assays of anti-HCMV antibody activity as informative surrogates of in vivo protective activity of anti-HCMV antibodies in maternal HCMV infections. If informative and predictive assays become available, application of these assays to maternal populations could provide some clarity in the interpretation of the myriad of results from observational studies in HCMV infections during pregnancy.
Understanding the significance of anti-HCMV antibodies in modifications of HCMV infection in a non-immune women during pregnancy (primary maternal infection) is confounded by several parameters including; (i) heterogeneity in the source of virus as well as the types and frequency of exposure to infectious virus in susceptible women, (ii) heterogeneity of the magnitude and breadth of the adaptive response to HCMV infection, particularly the contribution of T lymphocyte responses to the control of HCMV infections in pregnant women, (iii) the efficiency of transmission of HCMV and maternal antibodies to the fetus as a function of placental development, and finally (iv) the contribution of fetal immunity to the outcome of the fetal infection. At a population level, the contribution of anti-HCMV antibodies to prevention of intrauterine transmission and the severity of fetal infection has frequently been estimated by the comparison of results from studies of the natural history of cCMV in women undergoing primary infection and those undergoing non-primary infection. Such comparisons are difficult to control and confounded by the lack of laboratory assays that can definitively identify re-infections or reactivations of latent infections in individual women infected with HCMV long before pregnancy. Thus, results of studies in populations of pregnant women in which all immune women are assigned to the same risk of reinfection and/or reactivation of latent infection and then compared to non-immune women undergoing primary infections who are often identified by laboratory assays such as measure of antiviral antibody avidity that infer a recent infection and not by demonstration of a de-novo IgG seroconversion [91]. Such comparisons can be unknowingly biased and to date have not permitted a definitive estimate of the protection afforded by anti-HCMV antibodies in either maternal-to-fetal transmission or the severity of cCMV.
Attempts to modify the rate of intrauterine HCMV transmission and the severity of cCMV infections by passive immunization with CMV IVIG have thus far provided little convincing evidence that HCMV specific antibodies in these IVIG preparations provide protective activity [54, 56]. However, it is important to note that results from a recent study utilizing early treatment (1st trimester gestational age) and repeated infusion of an HCMV IVIG suggested that treatment could decrease the intrauterine transmission rate as compared to historical controls [97]. This study raised the possibility that earlier and repeated prophylaxis of women undergoing primary infection during pregnancy with HCMV IVIG could be efficacious and offers some evidence that anti-HCMV antibodies can modify the natural history of cCMV infections. In contrast to these findings, a recent study utilizing serum from women with non-primary infections demonstrated no differences in titers of neutralizing antibodies reactive with the pentamer (gH/gL/UL128-131), trimer (gH/gL/gO), or gH/gL complexes between women who transmitted virus to their fetuses versus controls who did not transmit virus [88]. This latter finding is consistent with several previous studies that failed to demonstrate a difference in the quantity or quality of the anti-HCMV specific antibody responses in women with non-primary infections during pregnancy regardless if they transmitted or did not transmit HCMV to their fetuses.
Virus strain specific antibody response and its potential impact on protection
Although the role of anti-HCMV antibodies in limiting infection and/or dissemination can be inferred from studies in animal models and from correlative findings in humans, the lack of consistent and definitive evidence demonstrating protective anti-HCMV antibodies in humans remains difficult to explain. The variability of individual responses such as the kinetics of the development of high avidity of antibody responses, restricted antibody responses resulting in limited in vivo activity perhaps secondary to IgG subclass bias, generation of antibodies directed at highly immunogenic but non-protective epitopes, and potentially incomplete responses to protective viral antigens that are present in heterogeneous mixtures of viruses could limit the induction of protective anti-HCMV antibody responses. In addition to these parameters that are inherent in the host response, several characteristics of the virus itself could limit the activity of potentially protective antibodies. These include induction of blocking antibodies that are reactive with targets of functional antibodies such as virus neutralizing antibodies [98-100]. Extensive glycosylation of envelope glycoproteins, including gB, gO, and gN, could present a barrier to virus neutralizing antibodies as has been described in the HIV literature as a glycan shield [101]. Genotypic variability, particularly in components of the virion envelope, could induce strain dependent anti-HCMV antibody responses that could limit recognition and therefore activity of antiviral antibodies against genetically unrelated strains of HCMV [102-104]. Such considerations cannot be considered as novel, as differences in serological recognition of individual HCMV isolates in conventional assays of antibody activity were noted over 3 decades ago [105]. Strain dependent virus neutralizing antibody responses have been described in studies of human antibody responses against gB, gH, and gN [106-110]. Moreover, variability in epitopes within regions of gB that are targets of functional antibodies have been demonstrated, suggesting that reinfection with new strains of HCMV could explain the acquisition and spread of virus in individuals with strain dependent antibody activities [111, 112]. Reinfection by new strains of virus has been demonstrated to occur relatively frequently in community settings, including children in group care, individuals attending STI clinics, and HIV infected individuals [113-116]. Moreover, reinfection with new viral genotypes is the rule in HCMV immune and non-immune SOT recipients of organs from HCMV infected donors (D+/R− ;D+/R+) [117]. Lastly, reinfection of pregnant women with HCMV serological immunity prior to conception has been linked to intrauterine transmission of the new strain of virus, including intrauterine transmission of the reinfecting strain of HCMV [118, 119]. Thus, HCMV presents several mechanisms that can limit effective control by antiviral antibodies and together these mechanisms appear to contribute to infection (reinfection) and can lead to disease in the immunocompromised host.
Résumé from both worlds
Although the protective potential of antiviral antibodies is undoubted from mouse models and clinical investigation, it appears that the reductionistic approach in mouse models that uses well-controlled experimental variables, a defined host genetics, as well as defined virus strains and genetically designed virus mutants, underestimates the multivariate reality in a genetically polymorphic human population that faces virus strain variance with frequent co- and superinfection and also a highly dynamic intra-host selection [120]. Intra-host selection favors evasion of antibodies. In addition, the individual infection history that results in “private” populations of virus variants likely also impacts on the antibody composition of human immune sera in quantitative and qualitative terms. Such mostly unknown variables in human infection may explain the often disappointingly low efficacy of antibody-based vaccination and of sero-therapies in clinical trials. This gives a challenge to the “mouse modelers” to stepwise increase model complexity to better approach the clinical correlate [12].
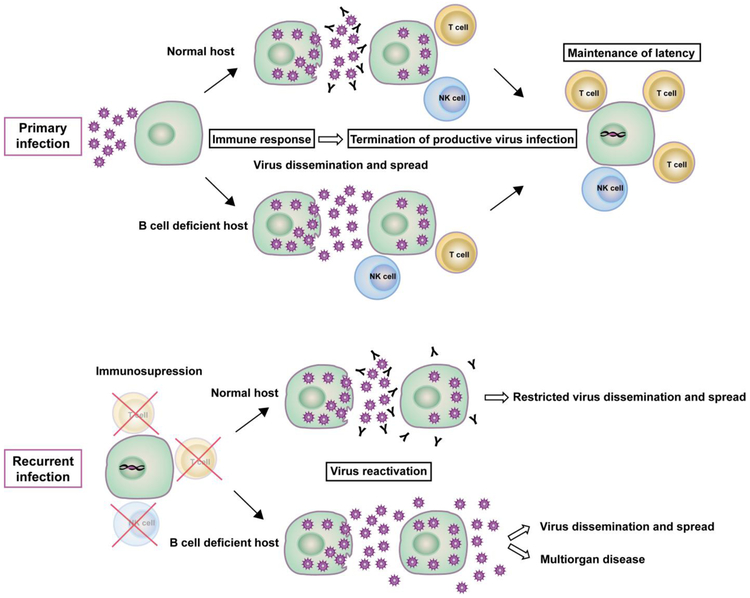
Notwithstanding this recognized limitation in fully recapitulating the clinical situation, the models can identify basic principles. Regarding the mode of action in containing virus recurrence, the murine model has revealed that antibodies do not prevent the initiating molecular event of virus reactivation from latency but operate by preventing intra-organ spread and inter-organ dissemination of recurrent virus (Fig. 4).
Fig. 4.
Graphical synopsis
Acknowledgements
S.J. has been supported by the grant “Strengthening the capacity of CerVirVac for research in virus immunology and vaccinology“, KK.01.1.1.01.0006, awarded to the Scientific Centre of Excellence for Virus Immunology and Vaccines and co-financed by the European Regional Development Fund. W.J.B. has been supported by NIH R01 DC015980-01A1 and AI089956. A.K. has been supported by Croatian Science Foundation under the project IP-2018-01-9086. M.J.R. receives funding from the Deutsche Forschungsgemeinschaft, SFB1292, individual project TP11.
Footnotes
Conflict of interest The authors declare they have no conflict of interest.
This article is part of the Special Issue on Immunological Imprinting during Chronic Viral Infection.
References
- 1.Roizman B, Sears AE (1987) An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol 41:543–571. doi: 10.1146/annurev.mi.41.100187.002551 [DOI] [PubMed] [Google Scholar]
- 2.Reddehase MJ, Podlech J, Grzimek NK (2002) Mouse models of cytomegalovirus latency: overview. J Clin Virol 25 Suppl 2:S23–36 [DOI] [PubMed] [Google Scholar]
- 3.Elder E, Sinclair J (2019) HCMV latency: what regulates the regulators? Med Microbiol Immunol, doi: 10.1007/s00430-019-00581-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddehase MJ, Lemmermann NA (2019) Cellular reservoirs of latent cytomegaloviruses. Med Microbiol Immunol. (MMIM-D-19-00049, accepted and at the publication office) [DOI] [PubMed] [Google Scholar]
- 5.Boppana SB, Britt WJ (2013) Synopsis of clinical aspects of human cytomegalovirus disease In: Reddehase MJ (ed) Cytomegaloviruses: From molecular pathogenesis to intervention, vol II Caister Academic Press, Norfolk, UK, pp 1–26 [Google Scholar]
- 6.Ho M (2008) The history of cytomegalovirus and its diseases. Med Microbiol Immunol 197:65–73. doi: 10.1007/s00430-007-0066-x [DOI] [PubMed] [Google Scholar]
- 7.Cannon MJ (2009) Congenital cytomegalovirus (CMV) epidemiology and awareness. J Clin Virol 46 Suppl 4:S6–10. doi: 10.1016/j.jcv.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 8.Britt WJ (2017) Congenital human cytomegalovirus infection and the enigma of maternal immunity. J Virol 91:e02392–16. doi: 10.1128/JVI.02392-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sissons JG, Wills MR (2015) How understanding immunology contributes to managing CMV disease in immunosuppressed patients: now and in future. Med Microbiol Immunol 204:307–316. doi: 10.1007/s00430-015-0415-0 [DOI] [PubMed] [Google Scholar]
- 10.Reddehase MJ (2002) Antigens and immunoevasins: opponents in cytomegalovirus immune surveillance. Nat Rev Immunol 2:831–844. doi 10.1038/nri932 [DOI] [PubMed] [Google Scholar]
- 11.Reddehase MJ (2016) Mutual interference between cytomegalovirus and reconstitution of protective immunity after hematopoietic cell transplantation. Front Immunol 7:294. doi: 10.3389/fimmu.2016.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddehase MJ, Lemmermann NAW (2018) Mouse model of cytomegalovirus disease and immunotherapy in the immunocompromised host: predictions for medical translation that survived the "test of time". Viruses 10 (12). doi: 10.3390/v10120693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddehase MJ, Weiland F, Myünch K, Jonjic S, Lüske A, Koszinowski UH (1985) Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol 55:264–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Britt WJ, Cekinovic D, Jonjic S (2013) Murine model of neonatal cytomegalovirus infection In: Reddehase MJ (ed) Cytomegaloviruses From Molecular Pathogenesis to Intervention, vol 1 Caister Academic Press, Norfolk, UK, pp 119–141 [Google Scholar]
- 15.Reddehase MJ, Jonjic S, Weiland F, Mutter W, Koszinowski UH (1988) Adoptive immunotherapy of murine cytomegalovirus adrenalitis in the immunocompromised host: CD4-helper-independent antiviral function of CD8-positive memory T lymphocytes derived from latently infected donors. J Virol 62:1061–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonjić S, Mutter W, Weiland F, Reddehase MJ, Koszinowski UH (1989) Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J Exp Med 169:1199–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucin P, Pavic I, Polic B, Jonjic S, Koszinowski UH (1992) Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J Virol 66:1977–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walton SM, Mandaric S, Torti N, Zimmermann A, Hengel H, Oxenius A (2011) Absence of cross-presenting cells in the salivary gland and viral immune evasion confine cytomegalovirus immune control to effector CD4 T cells. PLoS Pathog 7 (8):e1002214. doi: 10.1371/journal.ppat.1002214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonjic S, Pavic I, Lucin P, Rukavina D, Koszinowski UH (1990) Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J Virol 64:5457–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polić B, Jonjić S, Pavić I, Crnković I, Zorica I, Hengel H, Lucin P, Koszinowski UH (1996) Lack of MHC class I complex expression has no effect on spread and control of cytomegalovirus infection in vivo. J Gen Virol 77:217–25. doi: 10.1099/0022-1317-77-2-217 [DOI] [PubMed] [Google Scholar]
- 21.Podlech J, Holtappels R, Wirtz N, Steffens HP, Reddehase MJ (1998) Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J Gen Virol 79:2099–104. doi: 10.1099/0022-1317-79-9-2099 [DOI] [PubMed] [Google Scholar]
- 22.Podlech J, Holtappels R, Pahl-Seibert MF, Steffens HP, Reddehase MJ (2000) Murine model of interstitial cytomegalovirus pneumonia in syngeneic bone marrow transplantation: persistence of protective pulmonary CD8-T-cell infiltrates after clearance of acute infection. J Virol 74:7496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holtappels R, Ebert S, Podlech J, Fink A, Böhm V, Lemmermann NAW, Freitag K, Renzaho A, Thomas D, Reddehase MJ (2013) Murine model for cytoimmunotherapy of CMV disease after hematopoietic cell transplantation In: Reddehase MJ (ed) Cytomegaloviruses: from molecular pathogenesis to intervention, vol II Caister Academic Press, Norfolk, pp 353–381 [Google Scholar]
- 24.Farrell HE, Shellam GR (1991) Protection against murine cytomegalovirus infection by passive transfer of neutralizing and non-neutralizing monoclonal antibodies. J Gen Virol 72:149–156. doi: 10.1099/0022-1317-72-1-149 [DOI] [PubMed] [Google Scholar]
- 25.Shanley JD, Jordan MC, Stevens JG (1981) Modification by adoptive humoral immunity of murine cytomegalovirus infection. J Infect Dis 143:231–237 [DOI] [PubMed] [Google Scholar]
- 26.Kitamura D, Roes J, Kuhn R, Rajewsky K(1991) A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423–426. doi: 10.1038/350423a0 [DOI] [PubMed] [Google Scholar]
- 27.Jonjic S, Pavic I, Polic B, Crnkovic I, Lucin P, Koszinowski UH (1994) Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J Exp Med 179:1713–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bootz A, Karbach A, Spindler J, Kropff B, Reuter N, Sticht H, Winkler TH, Britt WJ, Mach M (2017) Protective capacity of neutralizing and non-neutralizing antibodies against glycoprotein B of cytomegalovirus. PLoS Pathog 13:e1006601. doi: 10.1371/journal.ppat.1006601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klenovsek K, Weisel F, Schneider A, Appelt U, Jonjic S, Messerle M, Bradel-Tretheway B, Winkler TH, Mach M (2007) Protection from CMV infection in immunodeficient hosts by adoptive transfer of memory B cells. Blood 110:3472–3479. doi: 10.1182/blood-2007-06-095414 [DOI] [PubMed] [Google Scholar]
- 30.Cekinovic D, Golemac M, Pugel EP, Tomac J, Cicin-Sain L, Slavuljica I, Bradford R, Misch S, Winkler TH, Mach M, Britt WJ, Jonjic S (2008) Passive immunization reduces murine cytomegalovirus-induced brain pathology in newborn mice. J Virol 82:12172–12180. doi: 10.1128/JVI.01214-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slavuljica I, Busche A, Babić M, Mitrović M, Gašparović I, Cekinović D, Markova Car E, Pernjak Pugel E, Ciković A, Lisnić VJ, Britt WJ, Koszinowski U, Messerle M, Krmpotić A, Jonjić S (2010) Recombinant mouse cytomegalovirus expressing a ligand for the NKG2D receptor is attenuated and has improved vaccine properties. J Clin Invest 120:4532–4545. doi: 10.1172/JCI43961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirsl L, Brizic I, Jenus T, Juranic Lisnic V, Reichel JJ, Jurkovic S, Krmpotic A, Jonjic S (2018) Murine CMV expressing the high affinity NKG2D ligand MULT-1: A model for the development of cytomegalovirus-based vaccines. Front Immunol 9:991. doi: 10.3389/fimmu.2018.00991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardner MB, Officer JE, Parker J, Estes JD, Rongey RW (1974) Induction of disseminated virulent cytomegalovirus infection by immunosuppression of naturally chronically infected wild mice. Infect Immun 10:966–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordan MC, Shanley JD, Stevens JG (1977) Immunosuppression reactivates and disseminates latent murine cytomegalovirus. J Gen Virol 37:419–423. doi: 10.1099/0022-1317-37-2-419 [DOI] [PubMed] [Google Scholar]
- 35.Mayo DR, Armstrong JA, Ho M (1977) Reactivation of murine cytomegalovirus by cyclophosphamide. Nature 267:721–723 [DOI] [PubMed] [Google Scholar]
- 36.Balthesen M, Messerle M, Reddehase MJ (1993) Lungs are a major organ site of cytomegalovirus latency and recurrence. J Virol 67:5360–5366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddehase MJ, Balthesen M, Rapp M, Jonjic S, Pavic I, Koszinowski UH (1994) The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J Exp Med 179:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurz SK, Reddehase MJ (1999) Patchwork pattern of transcriptional reactivation in the lungs indicates sequential checkpoints in the transition from murine cytomegalovirus latency to recurrence. J Virol. 73:8612–8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forster MR, Trgovcich J, Zimmerman P, Chang A, Miller C, Klenerman P, Cook CH (2010) Antiviral prevention of sepsis induced cytomegalovirus reactivation in immunocompetent mice. Antiviral Res 85:496–503. doi: 10.1016/j.antiviral.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steffens HP, Kurz S, Holtappels R, Reddehase MJ (1998) Preemptive CD8 T-cell immunotherapy of acute cytomegalovirus infection prevents lethal disease, limits the burden of latent viral genomes, and reduces the risk of virus recurrence. J Virol 72:1797–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polic B, Hengel H, Krmpotic A, Trgovcich J, Pavic I, Luccaronin P, Jonjic S, Koszinowski UH (1998) Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J Exp Med 188:1047–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S (2003) Pathogenesis of murine cytomegalovirus infection. Microbes Infect 5:1263–1277 [DOI] [PubMed] [Google Scholar]
- 43.Simon CO, Holtappels R, Tervo HM, Böhm V, Däubner T, Oehrlein-Karpi SA, Kühnapfel B, Renzaho A, Strand D, Podlech J, Reddehase MJ, Grzimek NK (2006) CD8 T cells control cytomegalovirus latency by epitope-specific sensing of transcriptional reactivation. J Virol 80:10436–10456. doi: 10.1128/JVI.01248-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddehase MJ, Simon CO, Seckert CK, Lemmermann N, Grzimek NK (2008) Murine model of cytomegalovirus latency and reactivation. Curr Top Microbiol Immunol 325:315–331. [DOI] [PubMed] [Google Scholar]
- 45.Seckert CK, Griessl M, Büttner JK, Scheller S, Simon CO, Kropp KA, Renzaho A, Kühnapfel B, Grzimek NK, Reddehase MJ (2012) Viral latency drives 'memory inflation': a unifying hypothesis linking two hallmarks of cytomegalovirus infection. Med Microbiol Immunol 201:551–566. doi: 10.1007/s00430-012-0273-y [DOI] [PubMed] [Google Scholar]
- 46.Biron CA, Tarrio ML (2015) Immunoregulatory cytokine networks: 60 years of learning from murine cytomegalovirus. Med Microbiol Immunol. 204:345–354. doi: 10.1007/s00430-015-0412-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wirtz N, Schader SI, Holtappels R, Simon CO, Lemmermann NA, Reddehase MJ, Podlech J (2008) Polyclonal cytomegalovirus-specific antibodies not only prevent virus dissemination from the portal of entry but also inhibit focal virus spread within target tissues. Med Microbiol Immunol 197:151–158. doi: 10.1007/s00430-008-0095-0 [DOI] [PubMed] [Google Scholar]
- 48.Seckert CK, Renzaho A, Tervo HM, Krause C, Deegen P, Kühnapfel B, Reddehase MJ, Grzimek NK (2009) Liver sinusoidal endothelial cells are a site of murine cytomegalovirus latency and reactivation. J Virol. 83:8869–84. doi: 10.1128/JVI.00870-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sacher T, Podlech J, Mohr CA, Jordan S, Ruzsics Z, Reddehase MJ, Koszinowski UH (2008) The major virus-producing cell type during murine cytomegalovirus infection, the hepatocyte, is not the source of virus dissemination in the host. Cell Host Microbe. 3:263–72. doi: 10.1016/j.chom.2008.02.014 [DOI] [PubMed] [Google Scholar]
- 50.Sacher T, Andrassy J, Kalnins A, Dölken L, Jordan S, Podlech J, Ruzsics Z, Jauch KW, Reddehase MJ, Koszinowski UH (2011) Shedding light on the elusive role of endothelial cells in cytomegalovirus dissemination. PLoS Pathog. 7:e1002366. doi: 10.1371/journal.ppat.1002366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Podlech J, Reddehase MJ, Adler B, Lemmermann NA (2015) Principles for studying in vivo attenuation of virus mutants: defining the role of the cytomegalovirus gH/gL/gO complex as a paradigm. Med Microbiol Immunol. 204:295–305. doi: 10.1007/s00430-015-0405-2 [DOI] [PubMed] [Google Scholar]
- 52.Lemmermann NA, Krmpotic A, Podlech J, Brizic I, Prager A, Adler H, Karbach A, Wu Y, Jonjic S, Reddehase MJ, Adler B (2015) Non-redundant and redundant roles of cytomegalovirus gH/gL complexes in host organ entry and intra-tissue spread. PLoS Pathog. 11:e1004640. doi: 10.1371/journal.ppat.1004640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner FM, Brizic I, Prager A, Trsan T, Arapovic M, Lemmermann NA, Podlech J, Reddehase MJ, Lemnitzer F, Bosse JB, Gimpfl M, Marcinowski L, MacDonald M, Adler H, Koszinowski UH, Adler B (2013) The viral chemokine MCK-2 of murine cytomegalovirus promotes infection as part of a gH/gL/MCK-2 complex. PLoS Pathog. 9:e1003493. doi: 10.1371/journal.ppat.1003493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blazquez-Gamero D, Galindo Izquierdo A, Del Rosal T, Baquero-Artigao F, Izquierdo Mendez N, Soriano-Ramos M, Rojo Conejo P, Gonzalez-Tome MI, Garcia-Burguillo A, Perez Perez N, Sanchez V, Ramos-Amador JT, De la Calle M (2019) Prevention and treatment of fetal cytomegalovirus infection with cytomegalovirus hyperimmune globulin: a multicenter study in Madrid. J Matern Fetal Neonatal Med. 32 (4):617–625. doi: 10.1080/14767058.2017.1387890 [DOI] [PubMed] [Google Scholar]
- 55.Boeckh M, Bowden RA, Storer B, Chao NJ, Spielberger R, Tierney DK, Gallez-Hawkins G, Cunningham T, Blume KG, Levitt D, Zaia JA (2001) Randomized, placebo-controlled, double-blind study of a cytomegalovirus-specific monoclonal antibody (MSL-109) for prevention of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 7:343–351 [DOI] [PubMed] [Google Scholar]
- 56.Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, Guaschino S, Vergani P, Todros T, Frusca T, Arossa A, Furione M, Rognoni V, Rizzo N, Gabrielli L, Klersy C, Gerna G, Group CS (2014) A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 370:1316–1326. doi: 10.1056/NEJMoa1310214 [DOI] [PubMed] [Google Scholar]
- 57.Winston DJ, Ho WG, Lin CH, Bartoni K, Budinger MD, Gale RP, Champlin RE (1987) Intravenous immune globulin for prevention of cytomegalovirus infection and interstitial pneumonia after bone marrow transplantation. Ann Intern Med 106:12–18 [DOI] [PubMed] [Google Scholar]
- 58.Pass RF, Griffiths PD, August AM (1983) Antibody response to cytomegalovirus after renal transplantation: comparison of patients with primary and recurrent infections. J Infect Dis 147:40–46 [DOI] [PubMed] [Google Scholar]
- 59.Yeager AS, Grumet FC, Hafleigh EB, Arvin AM, Bradley JS, Prober CG (1981) Prevention of transfusion-acquired cytomegalovirus infections in newborn infants. J Pediatr 98:281–287 [DOI] [PubMed] [Google Scholar]
- 60.Kropff B, Landini MP, Mach M (1993) An ELISA using recombinant proteins for the detection of neutralizing antibodies against human cytomegalovirus. J Med Virol 39:187–195 [DOI] [PubMed] [Google Scholar]
- 61.Falagas ME, Snydman DR, Ruthazer R, Griffith J, Werner BG, Freeman R, Rohrer R (1997) Cytomegalovirus immune globulin (CMVIG) prophylaxis is associated with increased survival after orthotopic liver transplantation. The Boston Center for Liver Transplantation CMVIG Study Group. Clin Transplant 11:432–437 [PubMed] [Google Scholar]
- 62.Snydman DR (1990) Cytomegalovirus immunoglobulins in the prevention and treatment of cytomegalovirus disease. Rev Infect Dis 12:839–848 [DOI] [PubMed] [Google Scholar]
- 63.Snydman DR, Werner BG, Heinze-Lacey B, Berardi VP, Tilney NL, Kirkman RL, Milford EL, Cho SI, Bush HL Jr., Levey AS, et al. (1987) Use of cytomegalovirus immune globulin to prevent cytomegalovirus disease in renal-transplant recipients. N Engl J Med 317:1049–1054. doi: 10.1056/nejm198710223171703 [DOI] [PubMed] [Google Scholar]
- 64.Rea F, Potena L, Yonan N, Wagner F, Calabrese F (2016) Cytomegalovirus hyper immunoglobulin for CMV prophylaxis in thoracic transplantation. Transplantation 100:S19–26. doi: 10.1097/tp.0000000000001096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cordonnier C, Chevret S, Legrand M, Rafi H, Dhedin N, Lehmann B, Bassompierre F, Gluckman E (2003) Should immunoglobulin therapy be used in allogeneic stem-cell transplantation? A randomized, double-blind, dose effect, placebo-controlled, multicenter trial. Ann Intern Med 139:8–18 [DOI] [PubMed] [Google Scholar]
- 66.Winston DJ, Antin JH, Wolff SN, Bierer BE, Small T, Miller KB, Linker C, Kaizer H, Lazarus HM, Petersen FB, Cowan MJ, Ho WG, Wingard JR, Schiller GJ, Territo MC, Jiao J, Petrarca MA, Tonetta SA (2001) A multicenter, randomized, double-blind comparison of different doses of intravenous immunoglobulin for prevention of graft-versus-host disease and infection after allogeneic bone marrow transplantation. Bone Marrow Transplant 28:187–196. doi: 10.1038/sj.bmt.1703109 [DOI] [PubMed] [Google Scholar]
- 67.Abdel-Azim H, Elshoury A, Mahadeo KM, Parkman R, Kapoor N (2017) Humoral Immune Reconstitution Kinetics after Allogeneic Hematopoietic Stem Cell Transplantation in Children: A Maturation Block of IgM Memory B Cells May Lead to Impaired Antibody Immune Reconstitution. Biol Blood Marrow Transplant 23:1437–1446. doi: 10.1016/j.bbmt.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 68.Bourassa-Blanchette S, Knoll G, Tay J, Bredeson C, Cameron DW, Cowan J (2017) A national survey of screening and management of hypogammaglobulinemia in Canadian transplantation centers. Transpl Infect Dis 19 e12706. doi: 10.1111/tid.12706 [DOI] [PubMed] [Google Scholar]
- 69.D'Orsogna LJ, Wright MP, Krueger RG, McKinnon EJ, Buffery SI, Witt CS, Staples N, Loh R, Cannell PK, Christiansen FT, French MA (2009) Allogeneic hematopoietic stem cell transplantation recipients have defects of both switched and igm memory B cells. Biol Blood Marrow Transplant 15:795–803. doi: 10.1016/j.bbmt.2008.11.024 [DOI] [PubMed] [Google Scholar]
- 70.Heimall J, Logan BR, Cowan MJ, Notarangelo LD, Griffith LM, Puck JM, Kohn DB, Pulsipher MA, Parikh S, Martinez C, Kapoor N, O'Reilly R, Boyer M, Pai SY, Goldman F, Burroughs L, Chandra S, Kletzel M, Thakar M, Connelly J, Cuvelier G, Davila Saldana BJ, Shereck E, Knutsen A, Sullivan KE, DeSantes K, Gillio A, Haddad E, Petrovic A, Quigg T, Smith AR, Stenger E, Yin Z, Shearer WT, Fleisher T, Buckley RH, Dvorak CC (2017) Immune reconstitution and survival of 100 SCID patients post-hematopoietic cell transplant: a PIDTC natural history study. Blood 130:2718–2727. doi: 10.1182/blood-2017-05-781849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaplan B, Bonagura VR (2019) Secondary hypogammaglobulinemia: an increasingly recognized complication of treatment with immunomodulators and after solid organ transplantation. Immunol Allergy Clin North Am. 39:31–47. doi: 10.1016/j.iac.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 72.Yamazaki R, Kikuchi T, Kato J, Sakurai M, Koda Y, Hashida R, Yamane Y, Abe R, Hasegawa N, Okamoto S, Mori T (2018) Recurrent bacterial pneumonia due to immunoglobulin G2 subclass deficiency after allogeneic hematopoietic stem cell transplantation: Efficacy of immunoglobulin replacement. Transpl Infect Dis 20:e12863. doi: 10.1111/tid.12863 [DOI] [PubMed] [Google Scholar]
- 73.Anonymous (1997) MSL-109 adjuvant therapy for cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome: the Monoclonal Antibody Cytomegalovirus Retinitis Trial. The Studies of Ocular Complications of AIDS Research Group. AIDS Clinical Trials Group. Arch Ophthalmol 115:1528–1536 [PubMed] [Google Scholar]
- 74.Ishida JH, Patel A, Mehta AK, Gatault P, McBride JM, Burgess T, Derby MA, Snydman DR, Emu B, Feierbach B, Fouts AE, Maia M, Deng R, Rosenberger CM, Gennaro LA, Striano NS, Liao XC, Tavel JA (2017) Phase 2 randomized, double-blind, placebo-controlled trial of RG7667, a combination monoclonal antibody, for prevention of cytomegalovirus infection in high-risk kidney transplant recipients. Antimicrob Agents Chemother 61: e01794–16. doi: 10.1128/aac.01794-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Griffiths PD, Stanton A, McCarrell E, Smith C, Osman M, Harber M, Davenport A, Jones G, Wheeler DC, O'Beime J, Thorburn D, Patch D, Atkinson CE, Pichon S, Sweny P, Lanzman M, Woodford E, Rothwell E, Old N, Kinyanjui R, Haque T, Atabani S, Luck S, Prideaux S, Milne RS, Emery VC, Burroughs AK (2011) Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 377:1256–1263. doi: 10.1016/s0140-6736(11)60136-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O (2008) Immunoglobulin prophylaxis in hematological malignancies and hematopoietic stem cell transplantation. Cochrane Database Syst Rev. 4:CD006501. doi: 10.1002/14651858.CD006501.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O (2009) Immunoglobulin prophylaxis in hematopoietic stem cell transplantation: systematic review and meta-analysis. J Clin Oncol 27:770–781 [DOI] [PubMed] [Google Scholar]
- 78.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD (1992) Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 257:238–241 [DOI] [PubMed] [Google Scholar]
- 79.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR (1995) Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med 333:1038–1044 [DOI] [PubMed] [Google Scholar]
- 80.Cutts FT, Vynnycky E (1999) Modelling the incidence of congenital rubella syndrome in developing countries. International journal of epidemiology 28:1176–1184 [DOI] [PubMed] [Google Scholar]
- 81.Freij BJ, South MA, Sever JL (1988) Maternal rubella and the congenital rubella syndrome. Clin Perinatol 15:247–257 [PubMed] [Google Scholar]
- 82.Britt WJ, Vugler L (1989) Antiviral antibody responses in mothers and their newborn infants with clinical and subclinical congenital cytomegalovirus infections. J Infect Dis 161:214–219 [DOI] [PubMed] [Google Scholar]
- 83.Alford CA, Hayes K, Britt W (1988) Primary cytomegalovirus infection in pregnancy: comparison of antibody responses to virus-encoded proteins between women with and without intrauterine infection. J Infect Dis 158:917–924 [DOI] [PubMed] [Google Scholar]
- 84.Lilleri D, Kabanova A, Revello MG, Percivalle E, Sarasini A, Genini E, Sallusto F, Lanzavecchia A, Corti D, Gerna G (2013) Fetal human cytomegalovirus transmission correlates with delayed maternal antibodies to gH/gL/pUL128-130-131 complex during primary infection. PLoS One 8:e59863. doi: 10.1371/journal.pone.0059863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boppana SB, Britt WJ (1995) Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. The Journal of infectious diseases 171:1115–1121 [DOI] [PubMed] [Google Scholar]
- 86.Lilleri D, Gerna G (2017) Maternal immune correlates of protection from human cytomegalovirus transmission to the fetus after primary infection in pregnancy. Rev Med Virol 27 (2). doi: 10.1002/rmv.1921 [DOI] [PubMed] [Google Scholar]
- 87.Furione M, Rognoni V, Sarasini A, Zavattoni M, Lilleri D, Gerna G, Revello MG (2013) Slow increase in IgG avidity correlates with prevention of human cytomegalovirus transmission to the fetus. J Med Virol 85:1960–1967. doi: 10.1002/jmv.23691 [DOI] [PubMed] [Google Scholar]
- 88.Vanarsdall AL, Chin AL, Liu J, Jardetzky TS, Mudd JO, Orloff SL, Streblow D, Mussi-Pinhata MM, Yamamoto AY, Duarte G, Britt WJ, Johnson DC (2019) HCMV trimer- and pentamer-specific antibodies synergize for virus neutralization but do not correlate with congenital transmission. Proc Natl Acad Sci USA 116:3728–3733 doi: 10.1073/pnas.1814835116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schoppel K, Kropff B, Schmidt C, Vornhagen R, Mach M (1997) The humoral immune response against human cytomegalovirus is characterized by a delayed synthesis of glycoprotein-specific antibodies. J Infect Dis 175:533–544 [DOI] [PubMed] [Google Scholar]
- 90.Dauby N, Sartori D, Kummert C, Lecomte S, Haelterman E, Delforge ML, Donner C, Mach M, Marchant A (2016) Limited Effector Memory B-Cell Response to Envelope Glycoprotein B During Primary Human Cytomegalovirus Infection. J Infect Dis 213:1642–1650. doi: 10.1093/infdis/jiv769 [DOI] [PubMed] [Google Scholar]
- 91.Britt WJ (2017) Congenital HCMV infection and the enigma of maternal immunity. J Virol 91:e02392–16. doi: 10.1128/jvi.02392-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mussi-Pinhata MM, Yamamoto AY, Aragon DC, Duarte G, Fowler KB, Boppana S, Britt WJ (2018) Seroconversion for Cytomegalovirus infection during pregnancy and fetal infection in a highly seropositive population: "The BraCHS Study". J Infect Dis 218:1200–1204. doi: 10.1093/infdis/jiy321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dar L, Pati SK, Patro AR, Deorari AK, Rai S, Kant S, Broor S, Fowler KB, Britt WJ, Boppana SB (2008) Congenital cytomegalovirus infection in a highly seropositive semi-urban population in India. Pediatr Infect Dis J 27:841–843. doi: 10.1097/INF.0b013e3181723d55 [doi] [DOI] [PubMed] [Google Scholar]
- 94.Wang C, Zhang X, Bialek S, Cannon MJ (2011) Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin Infect Dis 52:e11–13. doi: 10.1093/cid/ciq085 [DOI] [PubMed] [Google Scholar]
- 95.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK (2013) The "silent" global burden of congenital cytomegalovirus. Clin Microbiol Rev 26:86–102. doi: 10.1128/CMR.00062-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Permar SR, Schleiss MR, Plotkin SA (2018) Advancing our understanding of protective maternal immunity as a guide for development of vaccines to reduce congenital cytomegalovirus infections. J Virol 92: e00030–18.. doi: 10.1128/jvi.00030-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kagan KO, Enders M, Schampera MS, Baeumel E, Hoopmann M, Geipel A, Berg C, Goelz R, De Catte L, Wallwiener D, Brucker S, Adler SP, Jahn G, Hamprecht K (2018) Prevention of maternal-fetal transmission of CMV by hyperimmunoglobulin (HIG) administered after a primary maternal CMV infectionin early gestation. Ultrasound Obstet Gynecol 53:383–389. doi: 10.1002/uog.19164 [DOI] [PubMed] [Google Scholar]
- 98.Utz U, Britt W, Vugler L, Mach M (1989) Identification of a neutralizing epitope on glycoprotein gp58 of human cytomegalovirus. J Virol 63:1995–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schoppel K, Hassfurther E, Britt W, Ohlin M, Borrebaeck CA, Mach M (1996) Antibodies specific for the antigenic domain 1 of glycoprotein B (gpUL55) of human cytomegalovirus bind to different substructures. Virology 216:133–145 [DOI] [PubMed] [Google Scholar]
- 100.Speckner A, Glykofrydes D, Ohlin M, Mach M (1999) Antigenic domain 1 of human cytomegalovirus glycoprotein B induces a multitude of different antibodies which, when combined, results in incomplete virus neutralization. J Gen Virol 80:2183–2191 [DOI] [PubMed] [Google Scholar]
- 101.Kropff B, Burkhardt C, Schott J, Nentwich J, Fisch T, Britt W, Mach M (2012) Glycoprotein N of human cytomegalovirus protects the virus from neutralizing antibodies. PLoS Pathog 8:e1002999. doi: 10.1371/journal.ppat.1002999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chou S (1992) Comparative analysis of sequence variation in gp116 and gp55 components of glycoprotein B of human cytomegalovirus. Virology 188:388–390 [DOI] [PubMed] [Google Scholar]
- 103.Pignatelli S, Dal Monte P, Rossini G, Chou S, Gojobori T, Hanada K, Guo JJ, Rawlinson W, Britt W, Mach M, Landini MP (2003) Human cytomegalovirus glycoprotein N (gpUL73-gN) genomic variants: identification of a novel subgroup, geographical distribution and evidence of positive selective pressure. J Gen Virol 84:647–655 [DOI] [PubMed] [Google Scholar]
- 104.Rasmussen L, Geissler A, Cowan C, Chase A, Winters M (2002) The genes encoding the gCIII complex of human cytomegalovirus exist in highly diverse combinations in clinical isolates. J Virol 76:10841–10848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Faix RG (1985) Cytomegalovirus antigenic heterogeneity can cause false-negative results in indirect hemagglutination and complement fixation antibody assays. J Clin Microbiol 22:768–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Britt WJ (1991) Recent advances in the identification of significant human cytomegalovirus-encoded proteins. Transplant Proc 23:64–69 [PubMed] [Google Scholar]
- 107.Urban M, Britt W, Mach M (1992) The dominant linear neutralizing antibody-binding site of glycoprotein gp86 of human cytomegalovirus is strain specific. J Virol 66:1303–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schoppel MKK, Amvrossiadis N, Mach M (1999) Strain-specific neutralization of human cytomegalovirus isolates of human sera. J Virol 73:878–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pati SK, Novak Z, Purser M, Arora N, Mach M, Britt WJ, Boppana SB Strain-specific neutralizing antibody responses against human cytomegalovirus envelope glycoprotein N. Clin Vaccine Immunol 19:909–913. doi: 10.1128/CVI.00092-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Klein M, Schoppel K, Amvrossiadis N, Mach M (1999) Strain-specific neutralization of human cytomegalovirus isolates by human sera. J Virol 73:878–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Spindler N, Rucker P, Potzsch S, Diestel U, Sticht H, Martin-Parras L, Winkler TH, Mach M (2013) Characterization of a discontinuous neutralizing epitope on glycoprotein B of human cytomegalovirus. J Virol 87:8927–8939. doi: 10.1128/jvi.00434-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wiegers AK, Sticht H, Winkler TH, Britt WJ, Mach M (2015) Identification of a neutralizing epitope within antigenic domain 5 of glycoprotein B of human cytomegalovirus. J Virol 89:361–372. doi: 10.1128/jvi.02393-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bale JF Jr., Petheram SJ, Souza IE, Murph JR (1996) Cytomegalovirus reinfection in young children. J of Pediatr 128:347–352 [DOI] [PubMed] [Google Scholar]
- 114.Drew WL, Sweet ES, Miner RC, Mocarski ES (1984) Multiple infections by cytomegalovirus in patients with acquired immune deficiency syndrome: documentation by Southern blot hybridization. J Infect Dis 150:952–953 [DOI] [PubMed] [Google Scholar]
- 115.Collier AC, Chandler SH, Handsfield HH, Corey L, McDougall JK (1989) Identification of multiple strains of cytomegalovirus in homosexual men. J Infect Dis 159:123–126 [DOI] [PubMed] [Google Scholar]
- 116.Chandler SH, Handsfield HH, McDougall JK (1987) Isolation of multiple strains of cytomegalovirus from women attending a clinic for sexually transmitted disease. J Infect Dis 155:655–660 [DOI] [PubMed] [Google Scholar]
- 117.Rubin RH, Colvin RB (1986) Cytomegalovirus infection in renal transplantion: clinical importance and control In: Williams GM, Burdick JF, Solez K (ed) Kidney transplant rejection: diagnosis and treatment. Dekker, New York, [Google Scholar]
- 118.Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ (2001) Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med 344:1366–1371 [DOI] [PubMed] [Google Scholar]
- 119.Yamamoto AY, Mussi-Pinhata MM, Boppana SB, Novak Z, Wagatsuma VM, Oliviera PD, Duarte G, Britt WJ (2010) Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am J Obstet Gynecol 202:297.e291–e298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Renzette N; Gibson L; Jensen JD; Kowalik TF Human cytomegalovirus intrahost evolution—A new avenue for understanding and controlling herpesvirus infections. Curr. Opin. Virol 2014, 8, 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]