Abstract
The dopamine transporter (DAT) is a plasma membrane phosphoprotein that actively translocates extracellular dopamine (DA) into presynaptic neurons. The transporter is the primary mechanism for control of DA levels and subsequent neurotransmission, and is the target for abused and therapeutic drugs that exert their effects by suppressing reuptake. The transport capacity of DAT is acutely regulated by signaling systems and drug exposure, providing neurons the ability to fine-tune DA clearance in response to specific conditions. Kinase pathways play major roles in these mechanisms, and this review summarizes the current status of DAT phosphorylation characteristics and the evidence linking transporter phosphorylation to control of reuptake and other functions. Greater understanding of these processes may aid in elucidation of their possible contributions to DA disease states and suggest specific phosphorylation sites as targets for therapeutic manipulation of reuptake.
Keywords: Protein Kinase C, Extracellular Signal Regulated Kinase, Protein Phosphatase 1/2A, PIN1, Amphetamine, Phospho-specific antibody
1. Introduction.
In the central nervous system the neurotransmitter dopamine (DA) mediates control of numerous functions including motor activity, mood, and cognition, and imbalances in DA levels are associated with disorders such as Parkinson’s disease, attention deficit hyperactivity disorder (ADHD), bipolar disorder, and drug addiction (Kristensen et al., 2011; Pramod et al., 2013). The primary mechanism for spatial and temporal control of free transmitter levels is the dopamine transporter (DAT), which actively transports DA from the extracellular space into the presynaptic neuron. Many drugs interact with DAT to suppress reuptake, including cocaine and amphetamine, which induce psychomotor stimulation and addiction, and other categories of inhibitors such as methylphenidate and bupropion that are used therapeutically to treat ADHD and other DA disorders (Iversen, 2006). The transport capacity of DAT is acutely regulated by a variety of conditions and signaling pathways that function to rapidly modulate DA clearance in response to physiological demands, and dysregulation of these responses is hypothesized to contribute to long-lasting transmitter imbalances in DA pathologies (Blakely and Bauman, 2000; German et al., 2015).
DAT is an integral protein expressed primarily on the plasma membrane where it can interact with extracellular transmitter (Nirenberg et al., 1996). It is composed of 12 transmembrane (TM) spanning helices that form the core of the substrate translocation pathway and large N- and C-terminal domains that are oriented toward the cytoplasm (Fig. 1). Translocation of substrate against its concentration gradient is driven by co-transport of Na+ and Cl− down their concentration gradients and occurs by an alternating access mechanism in which the protein cycles through inwardly and outwardly facing conformations that bind and release DA on opposite sides of the membrane (Forrest and Rudnick, 2009). Cocaine and other inhibitors bind to the outwardly facing form and prevent these movements, whereas substrates such as amphetamine (AMPH) and methamphetamine (METH) are carried into the cell and stimulate release of intracellular DA via reversal of the transport mechanism (Sitte and Freissmuth, 2015; Sulzer, 2011). The balance between uptake and efflux not only regulates free DA levels but is crucial for DA homeostasis, as inward transport is necessary for re-loading synaptic vesicles and transmitter recycling (Jones et al., 1998), and efflux leads to depletion of vesicular DA which contributes to abuse liability and neurotoxicity (Sulzer, 2011), and may mediate dopaminergic excitability (Falkenburger et al., 2001). The core structure of DAT and many of its transport mechanisms have been elucidated by homology to crystallized bacterial and Drosophila transporters (Beuming et al., 2006; Dahal et al., 2014; Wang et al., 2015), but the N- and C-terminal domains are not conserved across phylogeny and their structures have not been solved. In mammalian transporters the cytoplasmic domains interact with regulatory binding partners and contain sites for post-translational modifications including phosphorylation and palmitoylation (Bermingham and Blakely, 2016; Kristensen et al., 2011; Vaughan and Foster, 2013) (Fig. 1), and here we discuss role of transporter phosphorylation in kinase-mediated regulatory events.
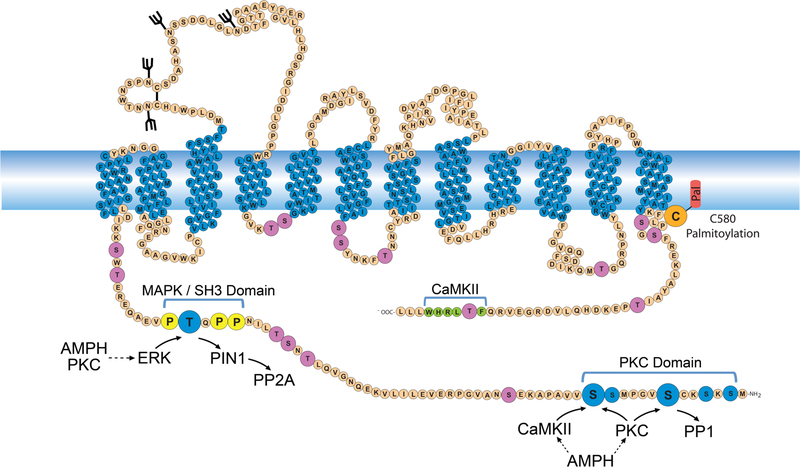
Figure 1. Phosphorylation characteristics of DAT.
Schematic diagram of rDAT showing PKC and MAPK domain phosphorylation sites in blue, with Ser7, Ser13, and Thr53 highlighted with large circles. Other intracellular Ser and Thr residues are shown in mauve, prolines flanking Thr53 that constitute an SH3 binding domain are shown in yellow, the CAMKII binding domain in the C-terminus is shown in green, and palmitoylation site Cys580 is shown in orange. Known and suspected kinase, phosphatase, and PIN1 inputs into Ser7, Ser13, and Thr53 are indicated with arrows, and dashed lines show indirect AMPH and PKC inputs into each site.
2. Kinase-Regulated Functions of DAT.
Numerous properties of DAT including forward transport, reverse transport, and cell surface expression are regulated by signaling pathways and psychostimulant drug exposure (German et al., 2015; Vaughan and Foster, 2013). Acute and long-term alteration of these properties could thus impact neurotransmission by affecting overall DA clearance capacity. Multiple kinases have been implicated in these processes, with the most well-studied including protein kinase C (PKC), calcium-calmodulin dependent kinase II (CAMKII), extracellular signal-regulated protein kinase (ERK) (German et al., 2015; Vaughan and Foster, 2013) Other signaling systems such as protein kinase A (PKA) (Batchelor and Schenk, 1998), tyrosine kinases (Hoover et al., 2007), arachidonic acid (Chen et al., 2003), Akt (Speed et al., 2010 phosphatidyl inositol 3-kinase (Carvelli et al., 2002), and nitric oxide (Pogun et al., 1994) also affect transporter functions, but have been less extensively characterized. Major issues regarding kinase effects include identifying the underlying mechanisms and determining whether actions are mediated through phosphorylation of transporter, phosphorylation of other proteins, or a combination of both.
2.1. Protein Kinase C.
Multiple functions of DAT are regulated by PKC, with activation of the enzyme leading to reduced transport Vmax, elevated efflux Vmax, and enhanced transporter internalization (German et al., 2015; Vaughan and Foster, 2013 Each of these actions would result in increased extracellular DA levels, indicating PKC as a positive regulator of DA neurotransmission. Dephosphorylation mechanisms factor into these processes, as phosphatase inhibitors potentiate PKC effects and induce transport down-regulation in the absence of exogenous PKC activation (Bauman et al., 2000; Vaughan et al., 1997).
2.2. Amphetamines.
DAT down-regulation, efflux, and endocytosis responses are also stimulated by pretreatment of cells or striatal tissue with AMPH or METH, with many (Cervinski et al., 2005; Chen et al., 2009; Fog et al., 2006; Johnson et al., 2005; Richards and Zahniser, 2009), although not all (Boudanova et al., 2008), studies showing dependence of these properties on PKC and/or CAMKII. AMPH-induced trafficking and efflux effects have been linked to PKCβ (Johnson et al., 2005) and CAMKII effects are mediated by αCAMKII (Steinkellner et al., 2014). In the absence of AMPH, PKC and CAMKII activators stimulate DA efflux (Cowell et al., 2000; Fog et al., 2006), and AMPH-induced stimulation of these events is suppressed by pharmacological or genetic inhibition of the enzymes (Kantor et al., 1999; Steinkellner et al., 2014; Steinkellner et al., 2012). Importantly, kinase effects on AMPH-induced trafficking and efflux are not only observed in vitro, but support AMPH neurochemical and behavioral responses (Chen et al., 2009; Pizzo et al., 2014; Pizzo et al., 2013; Steinkellner et al., 2014), indicating their involvement in vivo.
2.3. Extracellular Signal Regulated Kinase.
contrast to down-regulation responses mediated by PKC, ERK pathways upregulate DAT surface levels and transport capacity (Bolan et al., 2007; Moron et al., 2003). This would function to reduce extracellular DA and dampen dopaminergic neurotransmission, indicating ERK as a negative regulator of DA signaling. Together these findings support the ability of DAT to undergo bidirectional regulation in response to distinct signaling systems and suggest that the tonic level of uptake is established by the integration of information from multiple inputs.
3. DAT phosphorylation characteristics.
Initial studies demonstrating the ability of of rat (r), mouse (m), and human (h) DATs to undergo phosphorylation were performed by 32P metabolic labeling of the proteins in heterologous expression systems (Granas et al., 2003; Huff et al., 1997) and in rat (Vaughan et al., 1997; Foster et al., 2002) and mouse (Vaughan and Foster, unpublished data) striatal tissue. Transporters undergo basal 32P labeling that reflects the tonic level of phosphate turnover, and 32P incorporation is rapidly elevated by activation of PKC with phorbol 12-myristate, 13-acetate (PMA), diacylglycerol analogs, or Gq receptor agonists (Granas et al., 2003; Huff et al., 1997; Vaughan et al., 1997). These effects are blocked by PKC inhibitors, supporting direct or indirect control of transporter phosphorylation by PKC (Fig. 1). In the absence of exogenous kinase activation, DAT 32P labeling is also strongly increased by okadaic acid (OA) and other inhibitors of protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) (Fig. 1), indicating that transporters are subject to robust tonic dephosphorylation that suppresses the steady-state phosphorylation level (Huff et al., 1997; Vaughan et al., 1997). OA dose-response and peptide inhibitor experiments implicate PP1 as the primary phosphatase regulating 32P labeling (Foster et al., 2003b; Gorentla et al., 2009; Vaughan et al., 1997), but the catalytic subunit of PP2A has been identified in complexes with DAT (Bauman et al., 2000), suggesting that the protein may be acted on by multiple phosphatases.
AMPH and METH also strongly increase DAT 32P labeling, whereas uptake blockers including cocaine, GBR 12909, mazindol, and methylphenidate are without effect (Cervinski et al., 2005; Gorentla and Vaughan, 2005). The AMPH and METH effects are cocaine sensitive, indicating a requirement for binding or transport by DAT, and are blocked by PKC inhibitors (Cervinski et al., 2005), suggesting that the drugs act upstream of the kinase (Fig. 1). Importantly, AMPH stimulation of 32P labeling is not only induced in model cell systems and synaptosomes, but occurs after injection of drug into animals, demonstrating that the responses occur in the brain (Cervinski et al., 2005).
32P labeling of DAT is thus stimulated by many of the pharmacological agents that induce PKC- or phosphatase-dependent regulation of transport and efflux, consistent with a mechanistic relationship. Whether this holds for other signaling pathways remains an open question, as attempts in our lab to demonstrate changes in DAT 32P labeling by modulation of other kinases including PKA (Vaughan et al., 1997) and CAMKII (Vaughan and Foster, unpublished data) have been unsuccessful, and to the best of our knowledge have not been reported elsewhere. However, it is not known if these findings represent true negatives regarding the involvement of these kinases in DAT phosphorylation or reflect insufficient sensitivity of 32P labeling to detect inputs from these pathways.
DAT phosphorylation is also impacted through mechanisms other than direct kinase or phosphatase modulation, indicating that the subcellular status of the transporter can affect enzymatic outcomes. For example, treatment of rat striatal tissue with the syntaxin 1A (syn 1A) protease Botulinum Neurotoxin C leads to reduced 32P labeling of DAT (Cervinski et al., 2010). This suggests that syn 1A, which binds to the transporter N-terminus, stabilizes tonic phosphorylation, possibly by affecting its access to kinases or phosphatases. In addition, DAT phosphorylation is affected by its palmitoylation state, as 32P labeling is increased by suppression of transporter palmitoylation and decreased by enhancement of palmitoylation (Moritz et al., 2015). Whether this occurs by steric hindrance between the modifications or by other mechanisms such as subcellular localization of modified transporters remains unknown. Similar to results obtained with kinase and phosphatase modulators, however, in each of these conditions transport activity was decreased with increased phosphorylation and increased with decreased phosphorylation, consistent with a mechanistic relationship.
4. Phosphorylation Sites.
Understanding the mechanism for phosphorylation-mediated control of transporter function requires identification of the modified residues. Phosphoamino acid analysis of expressed and striatal rDAT showed that ~90% of 32P labeling occurred on serine (Ser) with ~10% occurring on threonine (Thr) (Foster et al., 2002). Numerous Ser and Thr residues that could serve as phosphorylation sites are present throughout the N- and C-terminal domains and intracellular loops (Fig. 1). Many of these residues are conserved in mouse and human transporters, but there are also some non-conserved sites (Gorentla et al., 2009), suggesting the potential for similarities and differences in phosphorylation profiles across species. DAT also contains four intracellularly oriented tyrosine residues, but to date there is no evidence supporting the presence of phosphotyrosine on the protein.
4.1. PKC domain phosphorylation.
Peptide mapping and mutagenesis studies of expressed and native rDATs identified the distal end of the cytoplasmic N-terminus as the major site of basal, PKC- and AMPH-stimulated phosphorylation (Cervinski et al., 2005; Foster et al., 2002). This domain contains a cluster of five closely-spaced serines (Ser2, Ser4, Ser7, Ser12, and Ser13) that are conserved in mDAT and hDAT, as well as nearby Ser21 which is not conserved (Fig. 1). This domain is the primary region detected by 32P metabolic phosphorylation, as truncation of the first 21/22 residues in rDAT/hDAT eliminates the majority of basal, PKC-stimulated, and AMPH-stimulated 32P incorporation (Cervinski et al., 2005; Granas et al., 2003). Significant levels of 32P labeling are retained in individual S→A mutants, however (Foster et al., 2003a), indicating that multiple sites in this domain are modified. While the vast majority of basal, PKC- stimulated, and AMPH-stimulated metabolic phosphorylation occurs in this domain, a small amount of labeling may remain on the Δ21/22 transporters, suggesting that sites elsewhere on the protein could be modified at lower stoichiometric levels.
Due to the large number of possible phosphorylation combinations in the PKC domain and the difficulties of pinpointing specific sites via partial reductions in 32P labeling after S→A mutagenesis, we adopted an in vitro approach to characterize phosphorylation properties of recombinant rDAT N- and C-terminal tail peptides (NDAT and CDAT). We found that NDAT was an excellent substrate for numerous kinases including PKC, PKA, CAMK, and ERK1/2 (Gorentla et al., 2009), demonstrating the ability of these enzymes to directly act on the N-terminal domain sequence. Phosphorylation of NDAT by PKC showed strong similarities to the in vivo pattern, with in vitro phosphorylation occurring at multiple residues, primarily Ser4, Ser7, and Ser13, in the PKC domain. Other kinases also catalyzed phosphorylation of this domain, however, with PKA phosphorylation occurring solely on Ser7 and CAMK phosphorylation occurring soley on Ser13. If these findings reflect in vivo kinase usage and specificity, the overlapping but distinct phosphorylation patterns suggest the potential for both specific and integrative regulatory inputs into this domain. We also found that CDAT can be phosphorylated by PKC and CAMK, but the site(s) have not been mapped or investigated in vivo.
Based on our NDAT phosphorylation profiles we focused on Ser7 and Ser13 as likely candidates for in vivo phosphorylation (Fig. 1). We have now obtained strong evidence in support of Ser7 as a PKC-dependent phosphorylation site in both expressed and native protein, as Ser7→Ala mutation reduced basal and PKC-stimulated 32P labeling of rDAT by ~50%; phospho (p) Ser7 was identified in expressed hDAT by mass spectrometry; and rat striatal DAT phosphopeptides co-migrate through 2-dimensional thin layer chromatography with NDAT Ser7 phosphopeptides (Moritz et al., 2013). We have not yet mapped the AMPH-stimulated phosphorylation sites in this domain and do not know if the pattern follows or differs from that of PKC. The retention of significant 32P labeling in S7A DAT indicates the presence of one or more additional phosphorylation sites in this domain, and as tonic and AMPH-stimulated efflux are regulated by both PKC and CAMKII, our findings that Ser13 is phosphorylated in vitro by both of these kinases makes this an attractive candidate for this function (Fig. 1). However, we have not yet been able to demonstrate the usage of Ser13 in vivo, and the additional phosphorylation site(s) in this domain currently remain unknown.
4.2. Functions regulated by PKC domain phosphorylation.
Important mechanistic issues for understanding regulation of DAT and for potential use of this information for interventional purposes are whether kinase effects on function occur by single or multiple mechanisms and whether they are mediated directly via phosphorylation of the transporter or indirectly via phosphorylation of regulatory partners. The results described below are beginning to suggest that DAT is regulated by distinct kinetic and endocytotic mechanisms that differ in their phosphorylation inputs and may provide multiple targets for therapeutic manipulation of reuptake.
4.2.1. Transport.
With respect to regulation of transport, many studies initially equated PKC-induced down-regulation with transporter endocytosis and reduction of surface transporters available to mediate uptake. An early study that examined the phosphorylation dependence of these processes showed that PMA-stimulated endocytosis and reductions in transport were not lost in Δ22 hDAT, which lacks the PKC domain (Granas et al., 2003), leading to the paradigm that these functions are independent of transporter phosphorylation. Further studies in both exogenous and native systems however, showed that transport down-regulation was reduced, although not eliminated, when DAT internalization was blocked (Foster et al., 2008; Foster and Vaughan, 2011). These findings demonstrate the presence of a kinetic regulatory mechanism that operates in parallel with endocytosis, and are consistent with earlier reports of altered PKC regulation of a trafficking-impaired DAT mutant (Mazei-Robison and Blakely, 2005) and with trafficking-independent down-regulation induced by AMPH (Richards and Zahniser, 2009).
Several lines of evidence now indicate that this kinetic mechanism is driven by phosphorylation of Ser7, as PKC-dependent down-regulation of S7A DAT is blunted relative to the WT protein and is essentially eliminated when endocytosis is blocked; S7A DAT possesses higher steady state transport capacity than the WT protein; and DA transport is suppressed by reduction of palmitoylation on cysteine (Cys) 580, which elevates Ser7 phosphorylation in the absence of exogenous PKC activation (Moritz et al., 2013; Moritz et al., 2015) (Table 1). However, PKC-induced endocytosis of S7A rDAT does not appear to be substantially impaired (Moritz et al., 2015), similar to results obtained with Δ22 hDAT, indicating that the level of down-regulation retained in these mutants results from internalization. Together these findings support a model in which PKC-induced reductions in transport occur by a combination of kinetic regulation driven by phosphorylation of Ser7 and endocytotic regulation that is independent of PKC domain phosphorylation.
Table 1.
Regulatory inputs and functional responses of demonstrated DAT phosphorylation sites.
| In vivo site | Increased phosphorylation | Outcome | Decreased phosphorylation | Outcome |
|---|---|---|---|---|
| PKC domain | PKC activators | ↓DA uptake Vmax(g,h,l,n) | PKC inhibitors | ↑DA uptake Vmax(b,l,m) |
| AMPH, METH | ↑DA efflux Vmax(e,i,j) | Δ21/22 truncation | ↓PKC-induced down-regulation(f,n) | |
| PP1 inhibitors | ↑PKC-induced down-regulation(f) | Palmitoylation enhancers | ↓AMPH-induced efflux(h,i,m) | |
| Palmitoylation inhibitors | ↑CFT affinity(k) | Botulinum neurotoxin C | ↓AMPH-induced down-regulation(a) | |
| Ser7 | PKC activators | ↓DA uptake Vmax(l) | S7A mutation | ↑DA uptake Vmax(l) |
| C580A mutation | ↓Cys580 palmitoylation(l) | ↓AMPH-induced efflux(j,n,o,p) | ||
| Palmitoylation inhibitors | ↑CFT affinity(l) | ↓PKC-induced down-regulation(f) | ||
| ↑Cys580 palmitoylation(l) | ||||
| ↓CFT affinity(l) | ||||
| Thr53 | PIN1 inhibitors | ↑DA efflux(d) | T53A mutation | ↓DA uptake Vmax(c,f) |
| PKC activators | ↓AMPH-induced MPP+ efflux(f) | |||
| AMPH, METH | ↑AMPH-induced DA efflux(c) | |||
| PP1/2A inhibitors | ↓CFT affinity(c) |
Superscripts denote references for studies that link the indicated outcomes to one or more of the conditions that increase or decrease phosphorylation of specified sites:
Granas et al., 1995
4.2.2. Efflux.
Links between reverse transport and PKC domain phosphorylation were first identified in studies showing that AMPH-stimulated DA efflux is reduced in hDAT Δ22 or PKC domain S→A mutants, with retention of responsiveness upon substitution of Ser7 and Ser12 with aspartic acid (D) to potentially mimic the phosphorylation state (Khoshbouei et al., 2004). Similar but not identical results that point to roles for Ser4, Ser7 and/or Ser13 in efflux have been obtained by our lab (Zhen et al., 2012) and by others (Wang et al., 2016) (Table 1). As AMPH-stimulated efflux is PKC-dependent, these results are consistent with efflux being enhanced by PKC-induced phosphorylation of these residues. Although efflux responses could result from AMPH-induced transporter trafficking (Chen et al., 2010), in our hands DAT surface levels showed no alteration during the efflux procedures (Moritz et al., 2013), again linking PKC domain phosphorylation to transporter kinetics.
While CAMKII also plays a role in AMPH-induced efflux, its mechanism remains to be clarified. CAMKII interacts with DAT via a site on the distal end of the C-terminus (Fig. 1), and disruption of this interaction reduces efflux in vitro and in vivo (Fog et al., 2006; Steinkellner et al., 2014). Because CAMKII-dependent efflux and behaviors are reduced by PKC domain S→A mutations and maintained by phosphomimetic mutations (Fog et al., 2006; Pizzo et al., 2014) it has been assumed that this indicates CAMKII phosphorylation of these residues. However, formal demonstration that this occurs remains lacking, leaving open the possibility that the kinase effect is mediated by an alernative mechanism.
4.2.3. Cocaine analog binding.
Alterations in forward and reverse transport kinetics suggest that phosphorylation of PKC domain residues affects the transporter conformational equilibrium, which alters the rate at which the protein transitions through the substrate translocation cycle. As one method to assess this possibility we examined Ser7 mutants for effects on binding of the cocaine analog 2β - carbomethoxy- 3β- (4-fluorophenyl) tropane (CFT), which is thought to be favored by the outwardly facing transporter conformation (Dahal et al., 2014; Wang et al., 2015; Yamashita et al., 2005), and for Zn2+ stimulation of CFT binding, which is thought to occur by stabilization of the outward conformation (Norregaard et al., 1998). We found that S7A and S7D mutants possess reduced CFT affinity and altered CFT responses to Zn2+ and also showed that phosphorylation conditions increased the transporter CFT affinity (Moritz et al., 2013) (Table 1). This is consistent with the ability of Ser7 phosphorylation to regulate the transporter conformational equilibrium and suggests its potential to impact transporter responsiveness to cocaine.
4.2.4. Palmitoylation.
Ser7 phosphorylation also regulates palmitoylation of DAT on Cys580, with increased phosphorylation leading to reduced palmitoylation and reduced phosphorylation leading to increased palmitoylation (Moritz et al., 2015; Rastedt et al., 2015) (Table 1). The mechanisms underlying the concerted regulation of these events are unknown, but the findings indicate complex interplay between these modifications and communication between N- and C-terminal domains.
4.3. Thr53 phosphorylation.
Our phosphorylation analyses using recombinant cytoplasmic domains were also instrumental in guiding the discovery of a second phosphorylation site on DAT, as phosphorylation of NDAT was catalyzed by several mitogen-activated protein kinases (MAPKs) including ERK, JNK, and p38 (Gorentla et al., 2009). ERK-catalyzed phosphorylation of NDAT was mapped to Thr53, and the usage of this site was subsequently confirmed in vivo (Foster et al., 2012). This may be the sole site of Thr phosphorylation on rDAT as Thr53→Ala mutation led to the apparent loss of all 32P-labeled pThr (Gorentla et al., 2009). Thr53 is present in the membrane proximal region of the N-terminus and is followed by a proline (Pro) (Fig. 1), which serves as a targeting signal for MAPKs and other Pro-directed kinases, and is inhibitory for AGC and related kinases including PKC, PKA, and CAMK (Ubersax and Ferrell, 2007). The Pro-rich region flanking Thr53 also constitutes an SH3 binding domain that may drive binding partner interactions (Fig. 1).
To study phosphorylation of Thr53 against the backdrop of the larger amount of PKC domain phosphorylation, we developed a phospho-specific antibody that recognizes pThr53 in rDAT and mDAT, but does not react with non-phosphorylated Thr53 or with hDAT, which possesses a Ser-Pro motif at the homologous site (Foster et al., 2012). Although no published studies have reported metabolic phosphorylation of hDAT Ser53, the likelihood for this occurring is supported by our findings that ERK catalyzes robust in vitro phosphorylation of this site in the human version of NDAT (Foster and Stanislowski, unpublished data).
Initial characterization of rDAT with this antibody showed increased Thr53 phosphorylation in response to PMA and OA (Foster et al., 2012), suggesting potential involvement of the site in PKC mechanisms. However, as PKC cannot directly phosphorylate Pro-directed sites, this is likely due to cross-talk of PKC and MAPK pathways (Rozengurt, 2007). More recently we have determined that Thr53 phosphorylation is stimulated in vitro and in vivo by AMPH and METH (Challasivakanaka etal., 2012), also implicating this site in physiological mechanisms of these drugs. Whether the drug effects on this site are mediated by PKC or MAPKs has not been determined.
These findings have major structure-function implications for DAT, as phosphorylation of Pro-directed sites promotes cis-isomerization of S/T-P peptide bonds (Lu and Zhou, 2007). This configuration is far less common in proteins than the more energetically favorable trans configuration, and can have significant impact on protein function. In addition, the cis conformer must be returned to the trans state before the residue can be dephosphorylated (Lu, 2004). For Pro-directed phosphorylation sites this is catalyzed by the phosphorylation-specific protein prolyl isomerase Peptidylprolyl Cis/Trans Isomerase, NIMA-Interacting 1 (PIN1), which thus functions to control the duration of the phosphorylated state in addition to the backbone configuration (Lu and Zhou, 2007).
DAT is likely to be subject to these mechanisms, as ERK-phosphorylated NDAT displays reduced electrophoretic mobility on SDS-PAGE gels that could be caused by Thr53-Pro54 cis-isomerization (Gorentla et al., 2009), and PIN1 inhibitors induce robust increases in Thr53 phosphorylation (Challasivakanaka et al., 2014) (Table 1), consistent with suppression of dephosphorylation in the absence of cis→trans conversion. PIN1 levels and activity are under tight control, and its dysregulation has been implicated in many diseases including cancer, diabetes, and Alzheimer’s disease (Lu, 2004), suggesting this enzyme as a novel DAT input whose dysregulation could affect Thr53 functions. Dephosphorylation properties of pThr53 have not been extensively investigated, but many proline-directed sites are acted on by PP2A (Lu et al., 1999), suggesting this as the enzymatic input into this step (Fig. 1). PP2A is also subject to complex regulation by signaling pathways, targeting subunits, and inhibitors, with many diseases associated with its dysregulation (Brautigan, 2013).
4.4. Functions regulated by Thr53 phosphorylation.
Because PKC, AMPH/METH, and PP1/PP2A also regulate phosphorylation of the PKC domain, we currently do not understand their specific contribution to MAPK domain functions. At present we have few tools for selective activation of Thr53 phosphorylation, and are in the process of identifying inputs into this site that do not stimulate phosphorylation of PKC domain residues. However, some suggestion of Thr53 regulatory functions have been revealed by studies involving mutagenesis and PIN1 inhibition.
4.4.1. Transport.
T53A and T53D DATs possess lower DA transport Vmax values than the WT protein (Foster et al., 2012), suggesting that tonic phosphorylation of the site supports steady-state uptake capacity. Inputs that stimulate phosphorylation of the site may thus function to increase transmitter clearance and serve as negative regulators of DA signaling. This could be consistent with Thr53 serving as a mechanism for ERK-mediated regulation of transport, as ERK inhibitors suppress tonic transport activity (Moron et al., 2003), and activation of DA and kappa opioid receptors increase DA uptake through ERK-sensitive mechanisms (Bolan et al., 2007; Thompson et al., 2000; Zapata et al., 2007). While upregulation of DAT surface levels may contribute to some of these effects, the reduced steady state transport in the phosphorylation-null mutants suggests the potential for kinetic effects related to this site as well. Kappa receptor agonists oppose neurochemical and behavioral effects of cocaine (Thompson et al., 2000), which could potentially occur via ERK-mediated upregulation of transport, suggesting Thr53 phosphorylation as a potential target for therapeutic manipulation of cocaine neurochemical endpoints.
4.4.2. Efflux and cocaine analog binding.
T53A and T53D mutants also show loss of AMPH-stimulated [3H]MPP+ (1-methyl 4-phenylpyridinium) efflux (Foster et al., 2012), implicating a role for the site in reverse transport. Consistent with this idea, we have found in rat striatal synaptosomes that PIN1 that inhibitors th stimulate Thr53 phosphorylation also stimulate [3H]DA efflux (Challasivakanaka et al., 2014) (Table 1), although further work is needed to determine if this result is specific to the modification or occurs indirectly. In contrast to our findings with MPP+, however, more recent work from our lab indicates that DA efflux is moderately elevated in T53A DAT (Challasivakanaka et al., 2012), suggesting that the residue is not required per se for reverse transport but rather performs a regulatory function. The pronounced differences between effects of Thr53 mutations on MPP+ efflux relative to DA efflux likely arise from the different structures of the ligands, but suggest the potential for the Thr53 phosphorylation status to affect outcomes of neurotoxic substrates by impacting their ability to be extruded from the cell. For both MPP+ and DA, efflux differences in Thr53 mutants relative to the WT protein were not accompanied by altered transporter surface levels, indicating that they result from kinetic effects. Consistent with this idea, T53A DAT shows reduced CFT affinity and loss of Zn2+ stimulation of CFT binding (Challasivakanaka et al., 2012), indicative of kinetic alterations, and supporting a role for Thr53 phosphorylation in regulation of cocaine functions.
5. Potential Phosphorylation Mechanisms in Kinetic Regulation of Transport.
The changes in substrate and inhibitor kinetics associated with Ser7 and Thr53 phosphorylation states indicate that modification of these sites impacts the transporter conformational equilibrium to alter forward and reverse transport velocities and affect ligand binding. Structural transitions of the protein during the transport cycle involve suspected movements of the inner segment of TM1 and the membrane proximal region of the C-terminus (Krishnamurthy and Gouaux, 2012; Wang et al., 2015) that are controlled by extracellular and intracellular gating networks driven by ionic, H-bond, and aromatic side chain interactions (Kniazeff et al., 2008). One potential mechanism for kinetic regulation of transport is thus that negative phosphoryl charges and/or N-terminal backbone isomerization status affects transport velocity via impacts on these functions. The proximity of Thr53 to intracellular gate residues and the cytoplasmic end of TM1 is consistent with this idea, and recent evidence indicates that the distal end of the N-terminus interacts with membrane phospholipids (Khelashvili et al., 2015a; Khelashvili et al., 2015b; Khelashvili and Weinstein, 2015), which could also place PKC domain serines near these elements. A non-exclusive alternative possibility is that phosphorylation of PKC or MAPK domains affects transport kinetics by controlling interactions with DAT regulatory binding partners that impact these processses (Eriksen et al., 2010; Sager and Torres, 2011). Although to date there has been no demonstration of a binding partner interaction affected by the transporter phosphorylation state, hints of such a mechanism have been seen with syn 1A, which regulates DAT uptake and efflux kinetics, interacts with the distal end of the N-terminus via a CAMK-dependent mechanism, (Binda et al., 2008; Carvelli et al., 2008; Cervinski et al., 2010), and stabilizes the transporter phosphorylation level (Cervinski et al., 2010).
6. Subcellular aspects of phosphorylation
Kinetic regulation of transport by phosphorylation requires that modified transporters are present on the plasma membrane. Subcellular fractionation and surface biotinylation studies indicate that both 32P-labeled (i.e., PKC domain) and pThr53 DATs are present in plasma membrane fractions (Moritz et al., 2015, Foster and Vaughan, unpublished data), fulfilling this requirement. DAT phosphorylation is also related to its localization in cholesterol-rich membrane rafts, which represent preferred sites for PKC-dependent phosphorylation (Foster et al., 2008). Mechanisms that regulate raft partitioning of DAT thus have the potential to indirectly affect transporter phosphorylation and associated functions. DAT kinetic properties are affected by membrane cholesterol levels, either through effects on raft partitioning or direct interaction (Foster et al., 2008; Hong and Amara, 2010; Jones et al., 2012), consistent with interplay of these mechanisms. Raft localization of many proteins is also driven by lipid modifications, suggesting this as a possible mechanism underlying the concerted regulation of DAT palmitoylation and phosphorylation (Rastedt et al., 2016 this issue). DAT membrane raft partitioning and PKC-dependent endocytosis are also driven by the raft protein Flotillin 1. Mutation of a conserved Flotillin 1 phosphorylation site suppresses DAT internalization (Cremona et al., 2011), demonstrating this as an example of indirect regulation of the transporter via phosphorylation of a binding partner.
7. Phosphorylation mechanisms in disease.
In addition to involvement with psychostimulant drug mechanisms, increasing evidence supports dysregulation of phosphorylation-related DAT properties in other disorders. Multiple rare polymorphisms of DAT that result in amino acid substitutions have been identified by genetic screening of patients diagnosed with dopaminergic disorders (Hahn and Blakely, 2007). Two that have been associated with ADHD and bipolar disorder are Ala559Val, a residue on the extracellular end of TM12 (Mazei-Robison et al., 2008) and Arg615Cys, a residue in the C-terminal CAMKII binding site (Sakrikar et al., 2012). In vitro analyses of these proteins revealed alterations in multiple regulatory properties including anomalous DA efflux, which could result from transporter hyperphosphorylation, and reductions in membrane raft targeting, which could indirectly affect phosphorylation levels. Another polymorphism that affects transporter regulation is Val328Ala, (Mazei-Robison and Blakely, 2005), a residue in extracellular loop four, which functions to stabilize closure of the extracellular gate during the inward phase of transport. This polymorphism was identified in screens of patients with alcohol dependence or Tourette’s syndrome, although it was not reported if the sample derived from disease or control individuals. The protein shows enhanced trafficking-independent down-regulation that could follow from PKC domain hyperphosphorylation and result in hyperdopaminergia (Mazei-Robison and Blakely, 2005). A hypodopaminergic condition with potential links to DAT phosphorylation is Angelman syndrome, a genetic disorder caused by reduction in CAMKII activity (Dichter et al., 2012; Mabb et al., 2011; Steinkellner et al., 2012). In a mouse model of this disease, loss of CAMKII interaction with DAT results in reduced efflux capacity that could follow from PKC domain hypophosphorylation (Steinkellner et al., 2012). These findings support the potential for alterations in phosphorylation-mediated regulatory events to contribute to DA imbalances and phenotypic manifestations through effects on uptake or efflux, and further dysfunctions in regulatory mechanisms associated with disease may be revealed as increasing numbers of DAT genetic conditions are identified (Marecos et al., 2014).
8. Summary
The studies described here indicate the ability of multiple enzymatic and physiological factors to regulate DA reuptake capacity by mechanisms mediated by transporter phosphorylation. These findings suggest that genetic, physiological, or drug-induced alterations in enzymes (kinases, phosphatases, PIN1), signaling components (receptors, second messengers, targeting subunits), or other factors (cholesterol levels, membrane raft partitioning) that modulate DAT phosphorylation could alter clearance and lead to dysregulated neurotransmission. Impacts of transporter phosphorylation on cocaine analog affinity also suggest its potential to affect in vivo responses of cocaine or other addictive or therapeutic ligands.
Our current evidence indicates that PKC and MAPK domain phosphorylation affect uptake kinetics, likely in different directions, and that both domains regulate efflux and cocaine analog binding. Important issues that remain to be determined include how this information is integrated and transmitted to the protein, whether other kinases or phosphatases feed into these or additional sites, and identification of the endogenous signaling pathways that regulate these events. In addition, most DAT regulatory studies to date have focused on transport, efflux, and trafficking, but many other transporter functionalities such as biosynthesis, stability, oligomerization, and ionic properties remain to be investigated with respect to impacts of phosphorylation. Improved understanding of these processes will guide the elucidation of potential strategies for utilizing these modifications as therapeutic targets in DA disorders.
Acknowledgements.
Work from our labs was supported by grants DA13147 and 5P20 GM104360 (RAV), and DA 031991 (JDF). We thank our students and collaborators whose hard work resulted in generation of many of the discussed findings.
References
- Batchelor M, Schenk JO, 1998. Protein kinase A activity may kinetically upregulate the striatal transporter for dopamine. J Neurosci 18, 10304–10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman AL, Apparsundaram S, Ramamoorthy S, Wadzinski BE, Vaughan RA, Blakely RD, 2000. Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J Neurosci 20, 7571–7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham DP, Blakely RD, 2016. Kinase-dependent Regulation of Monoamine Neurotransmitter Transporters. Pharmacol Rev 68, 888–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuming T, Shi L, Javitch JA, Weinstein H, 2006. A comprehensive structure-based alignment of prokaryotic and eukaryotic neurotransmitter/Na+ symporters (NSS) aids in the use of the LeuT structure to probe NSS structure and function. Mol Pharmacol 70, 1630–1642. [DOI] [PubMed] [Google Scholar]
- Binda F, Dipace C, Bowton E, Robertson SD, Lute BJ, Fog JU, Zhang M, Sen N, Colbran RJ, Gnegy ME, Gether U, Javitch JA, Erreger K, Galli A, 2008. Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux. Mol Pharmacol 74, 1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely RD, Bauman AL, 2000. Biogenic amine transporters: regulation in flux. Curr Opin Neurobiol 10, 328–336. [DOI] [PubMed] [Google Scholar]
- Bolan EA, Kivell B, Jaligam V, Oz M, Jayanthi LD, Han Y, Sen N, Urizar E, Gomes I, Devi LA, Ramamoorthy S, Javitch JA, Zapata A, Shippenberg TS, 2007. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol 71, 1222–1232. [DOI] [PubMed] [Google Scholar]
- Boudanova E, Navaroli DM, Melikian HE, 2008. Amphetamine-induced decreases in dopamine transporter surface expression are protein kinase C-independent. Neuropharmacology 54, 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautigan DL, 2013. Protein Ser/Thr phosphatases--the ugly ducklings of cell signalling. FEBS J 280, 324–345. [DOI] [PubMed] [Google Scholar]
- Carvelli L, Blakely RD, DeFelice LJ, 2008. Dopamine transporter/syntaxin 1A interactions regulate transporter channel activity and dopaminergic synaptic transmission. Proc Natl Acad Sci U S A 105, 14192–14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, Moron JA, Kahlig KM, Ferrer JV, Sen N, Lechleiter JD, Leeb-Lundberg LM, Merrill G, Lafer EM, Ballou LM, Shippenberg TS, Javitch JA, Lin RZ, Galli A, 2002. PI 3-kinase regulation of dopamine uptake. J Neurochem 81, 859–869. [DOI] [PubMed] [Google Scholar]
- Cervinski MA, Foster JD, Vaughan RA, 2005. Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. J Biol Chem 280, 40442–40449. [DOI] [PubMed] [Google Scholar]
- Cervinski MA, Foster JD, Vaughan RA, 2010. Syntaxin 1A regulates dopamine transporter activity, phosphorylation and surface expression. Neuroscience 170, 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challasivakanaka S, Foster JD, Vaughan RA, 2012. Endogenous and psychostimulant substrates but not blockers stimulate dopamine transporter phosphorylation at a proline-directed site. FASEB J 26, 763.763. [Google Scholar]
- Challasivakanaka S, Smith MA, Foster JD, Vaughan RA, 2014. Post-phosphorylation control of dopamine transporter by peptidyl prolyl cis-trans isomerase PIN1. Faseb J 28, 803.806. [Google Scholar]
- Chen N, Appell M, Berfield JL, Reith ME, 2003. Inhibition by arachidonic acid and other fatty acids of dopamine uptake at the human dopamine transporter. Eur J Pharmacol 478, 89–95. [DOI] [PubMed] [Google Scholar]
- Chen R, Furman CA, Gnegy ME, 2010. Dopamine transporter trafficking: rapid response on demand. Future Neurol 5, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Furman CA, Zhang M, Kim MN, Gereau R.W.t., Leitges M, Gnegy ME, 2009. Protein kinase Cbeta is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. The Journal of pharmacology and experimental therapeutics 328, 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell RM, Kantor L, Hewlett GH, Frey KA, Gnegy ME, 2000. Dopamine transporter antagonists block phorbol ester-induced dopamine release and dopamine transporter phosphorylation in striatal synaptosomes. Eur J Pharmacol 389, 59–65. [DOI] [PubMed] [Google Scholar]
- Cremona ML, Matthies HJ, Pau K, Bowton E, Speed N, Lute BJ, Anderson M, Sen N, Robertson SD, Vaughan RA, Rothman JE, Galli A, Javitch JA, Yamamoto A, 2011. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nature neuroscience 14, 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal RA, Pramod AB, Sharma B, Krout D, Foster JD, Cha JH, Cao J, Newman AH, Lever JR, Vaughan RA, Henry LK, 2014. Computational and biochemical docking of the irreversible cocaine analog RTI 82 directly demonstrates ligand positioning in the dopamine transporter central substrate-binding site. J Biol Chem 289, 29712–29727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Damiano CA, Allen JA, 2012. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen J, Jorgensen TN, Gether U, 2010. Regulation of dopamine transporter function by protein-protein interactions: new discoveries and methodological challenges. J Neurochem 113, 27–41. [DOI] [PubMed] [Google Scholar]
- Falkenburger BH, Barstow KL, Mintz IM, 2001. Dendrodendritic inhibition through reversal of dopamine transport. Science 293, 2465–2470. [DOI] [PubMed] [Google Scholar]
- Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, Nikandrova Y, Bowton E, McMahon DG, Colbran RJ, Daws LC, Sitte HH, Javitch JA, Galli A, Gether U, 2006. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron 51, 417–429. [DOI] [PubMed] [Google Scholar]
- Forrest LR, Rudnick G, 2009. The rocking bundle: a mechanism for ion-coupled solute flux by symmetrical transporters. Physiology (Bethesda) 24, 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Adkins SD, Lever JR, Vaughan RA, 2008. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem 105, 1683–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Blakely RD, Vaughan RA, 2003a. Mutational analysis of potential phosphorylation sites in the N-terminal tail of the rat dopamine transporter. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience Program No. 16712. [Google Scholar]
- Foster JD, Pananusorn B, Cervinski MA, Holden HE, Vaughan RA, 2003b. Dopamine transporters are dephosphorylated in striatal homogenates and in vitro by protein phosphatase 1. Brain Res Mol Brain Res 110, 100–108. [DOI] [PubMed] [Google Scholar]
- Foster JD, Pananusorn B, Vaughan RA, 2002. Dopamine transporters are phosphorylated on N-terminal serines in rat striatum. J Biol Chem 277, 25178–25186. [DOI] [PubMed] [Google Scholar]
- Foster JD, Vaughan RA, 2011. Palmitoylation controls dopamine transporter kinetics, degradation, and protein kinase C-dependent regulation. J Biol Chem 286, 5175–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Yang JW, Moritz AE, Challasivakanaka S, Smith MA, Holy M, Wilebski K, Sitte HH, Vaughan RA, 2012. Dopamine transporter phosphorylation site threonine 53 regulates substrate reuptake and amphetamine-stimulated efflux. The Journal of biological chemistry 287, 29702–29712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German CL, Baladi MG, McFadden LM, Hanson GR, Fleckenstein AE, 2015. Regulation of the Dopamine and Vesicular Monoamine Transporters: Pharmacological Targets and Implications for Disease. Pharmacol Rev 67, 1005–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorentla BK, Moritz AE, Foster JD, Vaughan RA, 2009. Proline-directed phosphorylation of the dopamine transporter N-terminal domain. Biochemistry 48, 1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorentla BK, Vaughan RA, 2005. Differential effects of dopamine and psychoactive drugs on dopamine transporter phosphorylation and regulation. Neuropharmacology 49, 759–768. [DOI] [PubMed] [Google Scholar]
- Granas C, Ferrer J, Loland CJ, Javitch JA, Gether U, 2003. N-terminal truncation of the dopamine transporter abolishes phorbol ester- and substance P receptor-stimulated phosphorylation without impairing transporter internalization. J Biol Chem 278, 4990–5000. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Blakely RD, 2007. The functional impact of SLC6 transporter genetic variation. Annual review of pharmacology and toxicology 47, 401–441. [DOI] [PubMed] [Google Scholar]
- Hong WC, Amara SG, 2010. Membrane cholesterol modulates the outward facing conformation of the dopamine transporter and alters cocaine binding. J Biol Chem 285, 32616–32626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover BR, Everett CV, Sorkin A, Zahniser NR, 2007. Rapid regulation of dopamine transporters by tyrosine kinases in rat neuronal preparations. J Neurochem 101, 1258–1271. [DOI] [PubMed] [Google Scholar]
- Huff RA, Vaughan RA, Kuhar MJ, Uhl GR, 1997. Phorbol esters increase dopamine transporter phosphorylation and decrease transport Vmax. J Neurochem 68, 225–232. [DOI] [PubMed] [Google Scholar]
- Iversen L, 2006. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br J Pharmacol 147 Suppl 1, S82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME, 2005. Regulation of amphetamine-stimulated dopamine efflux by protein kinase C beta. J Biol Chem 280, 10914–10919. [DOI] [PubMed] [Google Scholar]
- Jones KT, Zhen J, Reith ME, 2012. Importance of cholesterol in dopamine transporter function. Journal of neurochemistry 123, 700–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG, 1998. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A 95, 4029–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor L, Hewlett GH, Gnegy ME, 1999. Enhanced amphetamine- and K+-mediated dopamine release in rat striatum after repeated amphetamine: differential requirements for Ca2+- and calmodulin-dependent phosphorylation and synaptic vesicles. J Neurosci 19, 3801–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelashvili G, Doktorova M, Sahai MA, Johner N, Shi L, Weinstein H, 2015a. Computational modeling of the N-terminus of the human dopamine transporter and its interaction with PIP2 -containing membranes. Proteins 83, 952–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelashvili G, Stanley N, Sahai MA, Medina J, LeVine MV, Shi L, De Fabritiis G, Weinstein H, 2015b. Spontaneous inward opening of the dopamine transporter is triggered by PIP2-regulated dynamics of the N-terminus. ACS Chem Neurosci 6, 1825–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelashvili G, Weinstein H, 2015. Functional mechanisms of neurotransmitter transporters regulated by lipid-protein interactions of their terminal loops. Biochim Biophys Acta 1848, 1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA, 2004. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol 2, E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeff J, Shi L, Loland CJ, Javitch JA, Weinstein H, Gether U, 2008. An intracellular interaction network regulates conformational transitions in the dopamine transporter. J Biol Chem 283, 17691–17701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy H, Gouaux E, 2012. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature 481, 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, Gether U, 2011. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev 63, 585–640. [DOI] [PubMed] [Google Scholar]
- Lu KP, 2004. Pinning down cell signaling, cancer and Alzheimer’s disease. Trends Biochem Sci 29, 200–209. [DOI] [PubMed] [Google Scholar]
- Lu KP, Zhou XZ, 2007. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol 8, 904–916. [DOI] [PubMed] [Google Scholar]
- Lu PJ, Wulf G, Zhou XZ, Davies P, Lu KP, 1999. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 399, 784–788. [DOI] [PubMed] [Google Scholar]
- Mabb AM, Judson MC, Zylka MJ, Philpot BD, 2011. Angelman syndrome: insights into genomic imprinting and neurodevelopmental phenotypes. Trends in neurosciences 34, 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marecos C, Ng J, Kurian MA, 2014. What is new for monoamine neurotransmitter disorders? Journal of inherited metabolic disease 37, 619–626. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Blakely RD, 2005. Expression studies of naturally occurring human dopamine transporter variants identifies a novel state of transporter inactivation associated with Val382Ala. Neuropharmacology 49, 737–749. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Bowton E, Holy M, Schmudermaier M, Freissmuth M, Sitte HH, Galli A, Blakely RD, 2008. Anomalous dopamine release associated with a human dopamine transporter coding variant. J Neurosci 28, 7040–7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz AE, Foster JD, Gorentla BK, Mazei-Robison MS, Yang JW, Sitte HH, Blakely RD, Vaughan RA, 2013. Phosphorylation of dopamine transporter serine 7 modulates cocaine analog binding. The Journal of biological chemistry 288, 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz AE, Rastedt DE, Stanislowski DJ, Shetty M, Smith MA, Vaughan RA, Foster JD, 2015. Reciprocal Phosphorylation and Palmitoylation Control Dopamine Transporter Kinetics. J Biol Chem 290, 29095–29105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moron JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, Shippenberg TS, 2003. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci 23, 8480–8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM, 1996. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci 16, 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norregaard L, Frederiksen D, Nielsen EO, Gether U, 1998. Delineation of an endogenous zinc-binding site in the human dopamine transporter. Embo J 17, 4266–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo AB, Karam CS, Zhang Y, Ma CL, McCabe BD, Javitch JA, 2014. Amphetamine-induced behavior requires CaMKII-dependent dopamine transporter phosphorylation. Mol Psychiatry 19, 279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo AB, Karam CS, Zhang Y, Yano H, Freyberg RJ, Karam DS, Freyberg Z, Yamamoto A, McCabe BD, Javitch JA, 2013. The membrane raft protein Flotillin-1 is essential in dopamine neurons for amphetamine-induced behavior in Drosophila. Mol Psychiatry 18, 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogun S, Baumann MH, Kuhar MJ, 1994. Nitric oxide inhibits [3H]dopamine uptake. Brain Res 641, 83–91. [DOI] [PubMed] [Google Scholar]
- Pramod AB, Foster J, Carvelli L, Henry LK, 2013. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Molecular aspects of medicine 34, 197–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastedt DE, Foster JD, Vaughan RA, 2015. Dopamine transporter expression and transport capacity is regulated by palmitoylation. FASEB J 29. [Google Scholar]
- Rastedt DE, Vaughan RA, Foster JD, 2016. Kinetic regulation of the dopamine transporter by palmitoylation. J Chem Neuroanat, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards TL, Zahniser NR, 2009. Rapid substrate-induced down-regulation in function and surface localization of dopamine transporters: rat dorsal striatum versus nucleus accumbens. J Neurochem 108, 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E, 2007. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol 213, 589–602. [DOI] [PubMed] [Google Scholar]
- Sager JJ, Torres GE, 2011. Proteins interacting with monoamine transporters: current state and future challenges. Biochemistry 50, 7295–7310. [DOI] [PubMed] [Google Scholar]
- Sakrikar D, Mazei-Robison MS, Mergy MA, Richtand NW, Han Q, Hamilton PJ, Bowton E, Galli A, Veenstra-Vanderweele J, Gill M, Blakely RD, 2012. Attention deficit/hyperactivity disorder-derived coding variation in the dopamine transporter disrupts microdomain targeting and trafficking regulation. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 5385–5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitte HH, Freissmuth M, 2015. Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends Pharmacol Sci 36, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed NK, Matthies HJ, Kennedy JP, Vaughan RA, Javitch JA, Russo SJ, Lindsley CW, Niswender K, Galli A, 2010. Akt-dependent and isoform-specific regulation of dopamine transporter cell surface expression. ACS Chem Neurosci 1, 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkellner T, Mus L, Eisenrauch B, Constantinescu A, Leo D, Konrad L, Rickhag M, Sorensen G, Efimova EV, Kong E, Willeit M, Sotnikova TD, Kudlacek O, Gether U, Freissmuth M, Pollak DD, Gainetdinov RR, Sitte HH, 2014. In vivo amphetamine action is contingent on alphaCaMKII. Neuropsychopharmacology 39, 2681–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkellner T, Yang JW, Montgomery TR, Chen WQ, Winkler MT, Sucic S, Lubec G, Freissmuth M, Elgersma Y, Sitte HH, Kudlacek O, 2012. Ca(2+)/calmodulin-dependent protein kinase IIalpha (alphaCaMKII) controls the activity of the dopamine transporter: implications for Angelman syndrome. The Journal of biological chemistry 287, 29627–29635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, 2011. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69, 628–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AC, Zapata A, Justice JB Jr., Vaughan RA, Sharpe LG, Shippenberg TS, 2000. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci 20, 9333–9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubersax JA, Ferrell JE Jr., 2007. Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol 8, 530–541. [DOI] [PubMed] [Google Scholar]
- Vaughan RA, Foster JD, 2013. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol Sci 34, 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan RA, Huff RA, Uhl GR, Kuhar MJ, 1997. Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J Biol Chem 272, 15541–15546. [DOI] [PubMed] [Google Scholar]
- Wang KH, Penmatsa A, Gouaux E, 2015. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 521, 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Bubula N, Brown J, Wang Y, Kondev V, Vezina P, 2016. PKC phosphorylates residues in the N-terminal of the DA transporter to regulate amphetamine-induced DA efflux. Neurosci Lett 622, 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E, 2005. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature 437, 215–223. [DOI] [PubMed] [Google Scholar]
- Zapata A, Kivell B, Han Y, Javitch JA, Bolan EA, Kuraguntla D, Jaligam V, Oz M, Jayanthi LD, Samuvel DJ, Ramamoorthy S, Shippenberg TS, 2007. Regulation of dopamine transporter function and cell surface expression by D3 dopamine receptors. J Biol Chem 282, 35842–35854. [DOI] [PubMed] [Google Scholar]
- Zhen J, Foster JD, Moritz AE, Vaughan RA, Reith MEA, 2012. Dopamine efflux and N-terminal dopamine transporter phosphorylation. Society for Neuroscience: Washington DC, Program No. 4209. [Google Scholar]