Abstract
Objective:
Studies of neurocognitively elite older adults, termed SuperAgers, have identified clinical predictors and neurobiological indicators of resilience against age-related neurocognitive decline. Despite rising rates of older persons living with HIV (PLWH), SuperAging (SA) in PLWH remains undefined. We aimed to establish neuropsychological criteria for SA in PLWH and examined clinically-relevant correlates of SA.
Methods:
734 PLWH and 123 HIV-uninfected participants between 50 and 64 years of age underwent neuropsychological and neuromedical evaluations. SA was defined as demographically-corrected (i.e., sex, race/ethnicity, education) global neurocognitive performance within normal range for 25-year-olds. Remaining participants were labeled cognitively normal (CN) or impaired (CI) based on actual age. Chi-square and ANOVA tests examined HIV group differences on neurocognitive status and demographics. Within PLWH, neurocognitive status differences were tested on HIV disease characteristics, medical comorbidities, and everyday functioning. Multinomial logistic regression explored independent predictors of neurocognitive status.
Results:
Neurocognitive status rates and demographic characteristics differed between PLWH (SA=17%; CN=38%; CI=45%) and HIV-uninfected participants (SA=35%; CN=55%; CI=11%). In PLWH, neurocognitive groups were comparable on demographic and HIV disease characteristics. Younger age, higher verbal IQ, absence of diabetes, fewer depressive symptoms, and lifetime cannabis use disorder increased likelihood of SA. SA reported increased independence in everyday functioning, employment, and health-related quality of life than non-SA.
Conclusion:
Despite combined neurological risk of aging and HIV, youthful neurocognitive performance is possible for older PLWH. SA relates to improved real-world functioning and may be better explained by cognitive reserve and maintenance of cardiometabolic and mental health than HIV disease severity. Future research investigating biomarker and lifestyle (e.g., physical activity) correlates of SA may help identify modifiable neuroprotective factors against HIV-related neurobiological aging.
Keywords: neuropsychology, cognitive reserve, cognitive decline, diabetes, cannabis, Acquired Immunodeficiency Syndrome
Introduction
Antiretroviral therapy (ART) has facilitated increased life expectancy for people living with HIV (PLWH; Wing, 2016). In 2014, 45% of PLWH in the United States were over the age of 50 (Centers for Disease Control and Prevention, 2018) and this proportion is expected to increase (Smit et al., 2015). HIV-associated neurocognitive disorder (HAND) affects approximately half of PLWH (Heaton et al., 2010; Norman et al., 2011; Saloner & Cysique, 2017), and older PLWH are at three times higher risk for HAND compared to younger PLWH (Valcour et al., 2004). Furthermore, there is evidence to suggest that HIV accelerates and accentuates neurocognitive aging (Pathai, Bajillan, Landay, & High, 2014; Sheppard et al., 2017). Older PLWH are at increased risk for functional decline (Thames et al., 2011; Vance, Fazeli, & Gakumo, 2013), which is not only costly, but also negatively affects quality of life (Morgan et al., 2012). Identifying factors that promote successful cognitive aging with HIV and developing interventions to sustain or enhance them may avoid or reverse the adverse effects of aging.
While definitions of successful cognitive aging in PLWH differ slightly, all definitions require individuals to be neurocognitively unimpaired and functionally independent (Malaspina et al., 2011; Moore et al., 2017). Successful cognitive aging rates in older PLWH range from 19–32%, and translates into real-world benefits, including greater success in managing medication and medical appointments, less decline in activities of daily living, and better psychological health and health-related quality of life (Malaspina et al., 2011; Moore et al., 2017; Moore et al., 2014). Given that the neuropsychological criteria for successful cognitive aging solely requires the absence of neurocognitive impairment, taking into consideration age, there likely remains considerable heterogeneity in neurocognitive performance (e.g., low average to superior) among the successful cognitive aging group. Thus, distinguishing older PLWH with superior neurocognitive abilities from those with average neurocognitive abilities may explain additional variance in everyday functioning outcomes.
Older adults with preserved cognition appear to resist “normal” age-related decline. The term “SuperAger” refers to older adults that perform equivalently to young or middle-aged adults on episodic memory tests (Harrison, Maass, Baker, & Jagust, 2018; Rogalski et al., 2013; Sun et al., 2016). Alternatively, others have researched “SuperNormals” or “Optimal Memory Performers” – older adults who demonstrate above-average episodic memory performance in comparison to average older adults (Dekhtyar et al., 2017; Lin et al., 2017; Mapstone et al., 2017; Wang et al., 2017). Both definitions provide evidence that older adults with superior memory perform better on other cognitive domains, particularly executive functioning (Dekhtyar et al., 2017; Gefen et al., 2015) and processing speed (Dekhtyar et al., 2017; Harrison et al., 2018). Additionally, SuperAgers have larger volumes of the cerebral cortex, hippocampus, and cingulate cortex (Dekhtyar et al., 2017; Harrison et al., 2018; Lin et al., 2017; Rogalski et al., 2013; Sun et al., 2016; Wang et al., 2017) as well as slower rates of cortical atrophy (Cook et al., 2017). Furthermore, SuperAgers display lower levels of biomarkers of neurodegeneration such as oxidative stress (Mapstone et al., 2017), inflammation (Bott et al., 2017), and amyloid (Lin et al., 2017; Rogalski et al., 2013) and tau deposition (Gefen et al., 2015).
Despite not having a gold-standard definition of SuperAging (SA) or preserved cognition, commonalities exist among the definitions. Most studies have classified SuperAgers based on superior memory performance alone and only required either average age-adjusted performance for a few other neuropsychological measures (Harrison et al., 2018; Rogalski et al., 2013). Some have required that they be otherwise neurocognitively normal (Dekhtyar et al., 2017; Lin et al., 2017). Thus, SA studies predominantly focus on superior memory performance rather than superior global neurocognitive performance. The majority of these studies, which consist of primarily septua- and octogenarians, require SuperAgers to perform equivalent to or better than those in their mid-50’s; however, most neurocognitive abilities peak in the mid-20’s and then begin to decline (Hartshorne & Germine, 2015; Heaton, Taylor, & Manly, 2003; Salthouse, 2003, 2009). Although SA is typically evaluated in healthy adults who are at least 60 years old, the aging population of PLWH is younger with 50 years old serving as a cut-off for defining a medically advanced age (Blanco et al., 2012). Nevertheless, neurocognitive aging studies have demonstrated substantial inter-individual variability in neurocognition for healthy adult cohorts below the age of 60 (Lachman, Teshale, & Agrigoroaei, 2015; Martin & Zimprich, 2005; Schaie & Willis, 2010). Importantly, this heterogeneity in neurocognition tracks with variation in biopsychosocial factors such that high neurocognitive performance correlates with high cognitive reserve and low comorbidity burden (Anstey, Sargent-Cox, Garde, Cherbuin, & Butterworth, 2014; Ferreira et al., 2017).
While current definitions of SA may be appropriate for studying healthy older adults resistant to the clinical expressions of biological aging and Alzheimer’s disease, SA criteria should be tailored for study in older PLWH who are younger and at greater risk for multi-domain neurocognitive decline rather than focal memory deficits. Thus, we aimed to: 1) establish neuropsychological criteria for neurocognitive SA in PLWH; 2) identify clinical predictors of SA in PLWH; 3) assess the everyday functioning correlates of SA status.
Methods
Participants
Participants included 734 PLWH and 123 HIV-uninfected controls aged 50–64 years. 340 PLWH were enrolled in the NIH-funded CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) study, consisting of six participating university centers: Johns Hopkins University (Baltimore, MD, n=51); Mt. Sinai School of Medicine (New York, NY, n=92); University of California at San Diego (San Diego, CA, n=32); University of Texas Medical Branch (Galveston, TX, n=73); University of Washington (Seattle, WA, n=38); and Washington University (St. Louis, MO, n=54). The remaining 394 PLWH and 123 HIV-uninfected participants were enrolled in other NIH-funded research studies at the University of California, San Diego’s HIV Neurobehavioral Research Program (HNRP). All participant visits for the present study took place between 2002 and 2017. All studies were approved by local Human Subjects Protection Committees and all participants provided written informed consent. All PLWH were required to have ≥5 years of estimated duration of HIV disease to be considered for inclusion. Exclusion criteria were: 1) diagnosis of psychotic or mood disorder with psychotic features, neurological, or medical condition that may impair neurocognitive functioning, such as traumatic brain injury, stroke, epilepsy, or advanced liver disease; 2) low verbal IQ of <70 as estimated by the reading subtest of the Wide Range Achievement Test (WRAT; Wilkinson & Robertson, 2006); or 3) evidence of intoxication by illicit drugs (except marijuana) or Breathalyzer test for alcohol on the day of testing by positive urine toxicology.
Procedures
Neurocognitive Assessment
Participants were classified as SA based on their performance on a comprehensive and standardized battery of neurocognitive tests, which has been described in detail elsewhere (Carey et al., 2004; Heaton et al., 2010). Briefly, the battery covers seven neurocognitive domains commonly impacted in HIV-infected persons: verbal fluency, executive functioning, processing speed, learning, delayed recall, attention/working memory, and motor skills (Heaton et al., 2010). Since some participants had been exposed to the test battery at prior research visits, raw scores for each test were converted to practice effect-adjusted scaled scores (M=10, SD=3; Heaton et al., 2001). These demographically-uncorrected scaled scores were converted to T scores (M=50, SD=10) that corrected for the effects of age, education, sex, and race/ethnicity on neurocognition (Heaton, Miller, Taylor, & Grant, 2004; Heaton et al., 2003; Norman et al., 2011).
In order to generate variables that reflect maximum neurocognitive performance at a younger age, a second set of adjusted T scores were computed in which the age of 25, instead of actual age, was entered into the demographic correction formulas along with actual education, sex, and race/ethnicity. These scores, referred to as “peak-age” T scores, consequently compare an individual’s neurocognitive performance to normative standards for 25 year-olds of the same education, sex, and race/ethnicity (Heaton, Miller, et al., 2004; Heaton et al., 2003; Norman et al., 2011). Both the actual-age and peak-age T scores for each measure were averaged to compute global and domain-specific T scores within each cognitive ability area. T scores were converted to actual-age and peak-age domain-specific deficit scores (DDS) that give differential weight to impaired, as opposed to normal scores, on a scale ranging from 0 (T ≥ 40; normal) to 5 (T < 20; severe impairment). DDS were then averaged to generate an actual-age and peak-age global deficit score (GDS). Consistent with prior studies, the presence of global impairment was defined by GDS ≥ 0.5 and domain-specific impairment by DDS ≥ 0.5 (Blackstone et al., 2012; Carey et al., 2004).
SuperAging Criteria
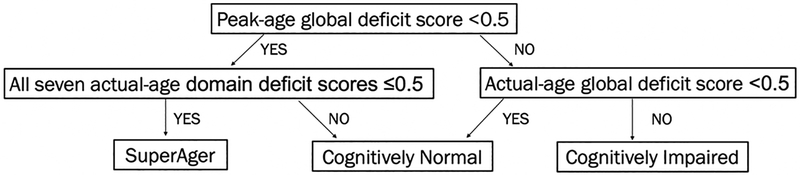
To estimate intact and peak neurocognitive functioning, SA status was operationally defined as: 1) peak-age GDS < 0.5; and 2) actual-age DDS ≤ 0.5 for all seven neurocognitive domains. Participants that did not meet SA criteria were classified as either cognitively normal (CN) or cognitively impaired (CI) using the standard actual-age GDS impairment cut-point of ≥ 0.5 (Figure1).
Figure 1. Neurocognitive status criteria.
SuperAging was operationalized as a peak-age global deficit score within normal limits (i.e., less than 0.5) and normal performance on all seven actual-age deficit scores (i.e., less or equal than 0.5).
Neuromedical and Laboratory Assessment
All participants underwent a comprehensive neuromedical assessment, including a medical history that included medications, Centers for Disease Control (CDC) staging, and blood draw. HIV infection was diagnosed by enzyme-linked immunosorbent assay with Western blot confirmation. Routine clinical chemistry panels, complete blood counts, rapid plasma reagin, hepatitis C virus antibody, and CD4+ T cells (flow cytometry) were performed at each site’s Clinical Laboratory Improvement Amendments (CLIA)–certified, or CLIA equivalent, medical center laboratory. Levels of HIV viral load in plasma were measured using reverse transcriptasepolymerase chain reaction (Amplicor, Roche Diagnostics, Indianapolis, IN, with a lower limit of quantitation 50 copies/ml).
Psychiatric Assessment
678 PLWH had available data from the Composite International Diagnostic Interview (CIDI), a fully-structured, computer-based interview, to determine DSM-IV diagnoses for current and lifetime mood and substance use disorders. (World Health Organization, 1998). Additionally, a subset of PLWH (n=712) completed the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996) to assess current symptoms of depressed mood.
Everyday Functioning and Quality of Life Assessment
Instrumental activities of daily living (IADL) dependence was assessed using a revised version of the Lawton and Brody (1969) self-report measure of everyday functioning (Heaton, Marcotte, et al., 2004; Woods et al., 2008), in which participants rated current abilities compared to previous abilities across 13 everyday functioning domains. Two outcome variables were generated: 1) A continuous variable of the number of declines in IADL; and 2) a dichotomous variable for IADL dependence, defined as ≥2 declines at least partially attributable to cognitive problems.
The Patient’s Assessment of Own Functioning Inventory (PAOFI) is a 33-item self-report measure used to measure perceived cognitive symptoms in everyday life (Chelune, Heaton, & Lehman, 1986). Items endorsed as fairly often or greater are considered clinically significant cognitive symptoms. A continuous variable for total number of clinically significant everyday cognitive symptoms and a dichotomous variable for employment status (i.e., employed/unemployed) were examined as outcome variables.
A subset of PLWH (n=490) completed the Medical Outcome Study 36 Item Short-Form version 1.0 (MOS-SF-36), which assesses health-related quality of life (HRQoL). The reliability and validity of the MOS-SF-36 has been extensively documented in PLWH (Henderson et al., 2010; Wu, Revicki, Jacobson, & Malitz, 1997). For this study, the physical and mental health composite scores were examined as primary outcome variables.
Statistical Analyses
HIV group differences on neurocognitive status and demographics were examined using ANOVAs or Kruskal-Wallis tests for continuous variables and Chi-square statistics for categorical variables. For the PLWH group only, the same statistical tests examined neurocognitive status group differences on demographics, HIV disease severity, medical and psychiatric characteristics, and everyday functioning outcomes. All pair-wise post-hoc comparisons (SA versus CN, SA versus CI, and CN versus CI) were conducted for any variable with at least an omnibus trend-level (i.e., p<0.10) difference across neurocognitive status groups. To control for multiple comparisons and limit Type I error, Tukey’s Honest Significant Difference (HSD) tests were conducted for continuous variables and Bonferroni-corrections were applied to Chi-square tests (MacDonald & Gardner, 2000). Cohen’s d statistics are presented for estimates of effect size for pair-wise comparisons. All group difference analyses were performed using JMP Pro version 12.0.1 (JMP®, Version <12.0.1>, SAS Institute Inc., Cary, NC, 1989–2007).
Next, any variable that displayed at least an omnibus trend-level difference was entered into a multinomial regression to determine the degree to which demographic and clinical characteristics segregate according to neurocognitive status. Race/ethnicity, sex, and education were not included in the model because the criteria for establishing neurocognitive status already adjusted for these factors. Actual age, however, was included in the model since the SA criteria adjusted each participant’s performance at peak age (i.e., 25) instead of actual age.
In order to determine the impact of age on global functioning within each neurocognitive status group (PLWH only), we conducted Pearson partial correlations between age and demographically-uncorrected global scaled scores stratified by group, co-varying for education, sex, and race/ethnicity. We calculated standardized Pearson partial r values that serve as effect sizes to enhance comparability and interpretability of the relationship between age and global neurocognitive performance across the neurocognitive status groups. Statistical differences in the magnitude of the Pearson partial correlations were compared using Fisher’s r-to-z transformations for independent correlations. Multinomial regression and Pearson’s partial correlations were performed using SPSS 24 (SPSS Inc., Chicago, IL).
Results
SuperAging Prevalence
Of the 734 PLWH, 124 (17%) met criteria for SA. Of the remaining 610 non-SA participants, 279 (38%) were CN and 331 (45%) were CI. Figure 2 displays differences in actual-age T and peak-age T scores within and across SA and CN PLWH with Cohen’s d effect size estimates for actual-age T scores. The prevalence of SA and CN were significantly higher, and prevalence of CI was significantly lower, in the HIV-uninfected group (χ2 =63.7, p<.0001). Of the 123 HIV-uninfected participants, 43 (35%) were SA, 67 (55%) were CN and 13 (11%) were CI.
Figure 2. SuperAger (SA) versus cognitively normal (CN) differences in neurocognitive performance.
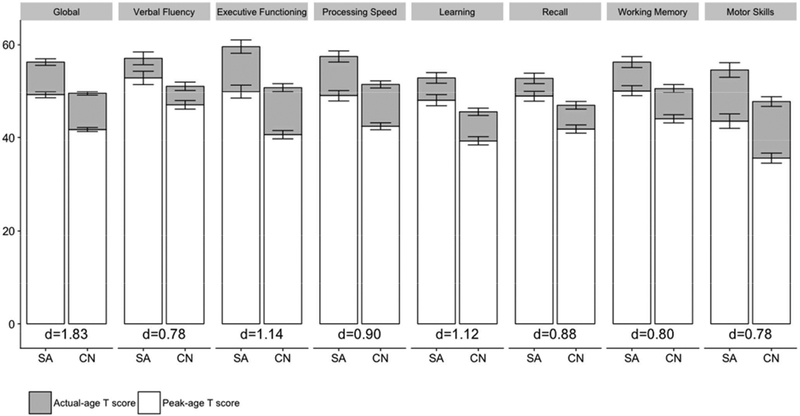
Cohen’s d effect size estimates reflect differences in actual-age T scores.
Demographics
Table 2 displays PLWH neurocognitive status group differences in demographic, clinical, and neuromedical variables. Only percent non-Hispanic white differed significantly among demographic factors. Although the CN group exhibited the lowest proportion of non-Hispanic white, no significant pairwise differences were found. SA individuals were on average a year younger than their CN and CI counterparts and this difference approached significance, but this did not result in significant pairwise differences. Although groups did not differ with respect to education, SA displayed significantly higher WRAT scores than CN (d=0.43) and CI (d=0.61) participants.
Table 2.
Demographic and clinical characteristics by neurocognitive status in people living with HIV
| Variable | SA (n=124) | CN (n=279) | CI (n=331) | p | Pair-wise comparisonsa |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years)b | 54.3 (3.97) | 55.2 (3.91) | 55.3 (4.08) | .06 | |
| Gender (male) | 107 (86.3) | 235 (84.2) | 276 (83.4) | .75 | |
| Race/ethnicity (non- hispanic white) | 79 (63.7) | 142 (50.9) | 203 (61.3) | .01 | |
| Cognitive Reserve | |||||
| Education (years) | 13.4 (2.58) | 13.4 (2.64) | 13.8 (2.69) | .14 | |
| Estimated premorbid verbal IQ (WRAT) | 103.5 (11.82) | 98.1 (13.32) | 96 (12.83) | <.001 | SA > CN, CI |
| HIV Disease Characteristics | |||||
| History of AIDS | 90 (72.6) | 211 (75.6) | 259 (78.3) | .43 | |
| Detectable virusc | 27 (21.8) | 71 (25.5) | 101 (30.5) | .13 | |
| Current CD4 count | 507 [367 – 700] | 491 [333 – 691] | 488 [275 – 674] | .16 | |
| Current CD4 <200 | 9 (7.3) | 31 (11.1) | 52 (15.7) | .03 | SA < CI |
| Nadir CD4 count | 112 [18 – 258] | 96 [19 – 225] | 97 [21 – 209] | .72 | |
| Nadir CD4 <200 | 79 (63.7) | 196 (70.3) | 236 (71.3) | .29 | |
| Years of known HIV-infection | |||||
| Mean (SD) | 18 (6.27) | 17.9 (6.6) | 17.7 (6.54) | .90 | |
| Min - Max | 5.6–31.1 | 5.4–31.0 | 5.2–33.7 | - | |
| On ART | 107 (86.3) | 249 (89.3) | 297 (89.7) | .59 | |
| Medical Comorbidities | |||||
| Hypertension | 48 (38.7) | 124 (44.4) | 148 (44.7) | .48 | |
| Hyperlipidemia | 42 (33.9) | 90 (32.3) | 108 (32.6) | .95 | |
| BMI | 26.5 (5.14) | 26.6 (5.03) | 26 (5.08) | .31 | |
| Diabetes | 12 (9.7) | 56 (20.1) | 62 (18.7) | .02 | SA < CN, CI |
| HCV | 37 (29.8) | 119 (42.7) | 124 (37.5) | .05 | SA < CN |
Note. Values are presented as mean (SD), median [IQR], or N (%); WRAT = Wide-Range Achievement reading subtest version 3 or 4; ART = antiretroviral therapy; BMI= body mass index; HCV= hepatitis C virus
Pair-wise comparisons were examined using Tukey’s HSD (α = 0.05) for continuous outcomes and Bonferroni-adjustments (α = 0.05/3 = 0.0167) for dichotomous outcomes
Range of study sample restricted to 50–64 years for each neurocognitive status group
Defined as >50 copies/mL
Compared to PLWH, the HIV-uninfected comparison group had significantly higher rates of non-Hispanic white participants (81% vs. 58%; p<.0001), females (38% vs. 16%; p<.0001), mean years of education (14.4 vs. 13.6; p<.001), and higher mean WRAT scores (106 vs. 98; p<.0001). By design, the HIV-uninfected group did not significantly differ from PLWH in mean age (55.5 vs. 55.1; p=.87).
HIV Disease Characteristics
A stair-step pattern of indicators of HIV disease severity was commonly observed such that SA displayed the lowest amount of HIV disease burden followed by CN then CI individuals. Although this stair-step pattern occurred for history of AIDS diagnosis, detectable plasma HIV, current CD4 count, and nadir CD4<200; only omnibus group differences in current CD4<200 were significant. Post-hoc comparisons indicated that the SA group had significantly lower rates of participants with current CD4<200 than the CI group. In the full sample, participants with current CD4<200 were more likely to be off ART (19.6%) compared to those with current CD4≥200 (9.8%; χ2 =6.7, p=.01). No noteworthy group differences were found for estimated duration of HIV disease or receipt of ART.
Medical Comorbidities
Examination of medical comorbidities revealed significant group differences for rates of HCV seropositivity and diabetes. Post-hoc comparisons indicated that SA had significantly lower rates of HCV than the CN group and lower rates of diabetes than both the CN and CI groups. No significant group differences were found for other markers of metabolic syndrome (i.e., hypertension, hyperlipidemia, BMI).
Psychiatric and Substance Use Characteristics
Significant group differences were observed for rates of lifetime cannabis use disorder and cocaine use disorder. SA had significantly higher rates of cannabis use disorder than CI individuals and CN individuals displayed higher rates of cocaine use disorder than the CI group (Table 3). Although lifetime and current diagnoses of major depressive disorder (MDD) did not differ across groups, SA endorsed significantly fewer depressive symptoms on the BDI-II than both the CN (d=−0.35) and CI (d=−0.46) groups.
Table 3.
Neuropsychiatric characteristics by neurocognitive status in people living with HIV
| Variable | SA (n=119) | CN (n=264) | CI (n=295) | p | Pair-wise Comparisonsa |
|---|---|---|---|---|---|
| Lifetime Substance Use Disorders | |||||
| Alcohol | 72 (60.5) | 145 (54.9) | 164 (55.6) | .57 | |
| Cannabis | 48 (40.3) | 91 (34.5) | 76 (25.8) | .01 | SA > CI |
| Cocaine | 46 (38.7) | 114 (43.2) | 96 (32.5) | .03 | CN > CI |
| Methamphetamine | 31 (26.1) | 57 (21.6) | 70 (23.7) | .62 | |
| Opioid | 15 (12.6) | 43 (16.3) | 52 (17.6) | .44 | |
| Depression | |||||
| Lifetime MDD | 66 (55.5) | 160 (60.6) | 182 (61.7) | .50 | |
| Current MDD (n=651) | 12 (10.4) | 30 (11.6) | 34 (12.2) | .88 | |
| BDI-II (n=714) | 8.7 (8.06) | 11.9 (10.33) | 13.0 (10.36) | <.001 | SA < CN, CI |
Note. Values are presented as mean (SD) or N (%); MDD= major depressive disorder; BDI-II= Beck Depression Inventory-II total score
Pair-wise comparisons were examined using Tukey’s HSD. (α = 0.05) for BDI-II and Bonferroni-adjustment (α = 0.05/3 = 0.0167) for diagnosis variables.
Multinomial Regression Predicting Neurocognitive Status
A multinomial logistic regression was performed with the three neurocognitive groups in PLWH as the dependent variable. Predictors were all outcome variables from Tables 2 and 3 with a trend-level omnibus effect (excluding race/ethnicity, i.e., age, WRAT, current CD4<200, HCV, diabetes, cannabis use disorder, and BDI-II). Based on available data, the sample size for this model included 113 SA, 259 CN, and 287 CI participants. Overall, the model was significant (χ2(14,659)=83.73; p<0.001; Nagelkerke pseudo-R2=0.137). Likelihood ratio tests indicated that older age, lower WRAT scores, diagnosis of diabetes, and higher BDI-II scores all increased the likelihood of classification as either CN or CI compared to SA (Table 4). Furthermore, a lifetime diagnosis of cannabis use disorder decreased the likelihood of classification as CI compared to SA.
Table 4.
Multinomial logistic regression predicting neurocognitive status in people living with HIV
| Outcome: Classification as CN (reference: SA) | Outcome: Classification as CI (reference: SA) | ||||||
|---|---|---|---|---|---|---|---|
| Predictor | OR | 95% CI | p | Predictor | OR | 95% CI | p |
| Age | 1.10 | [1.03, 1.17] | <.001 | Age | 1.11 | [1.05, 1.19] | .001 |
| WRAT | 0.96 | [0.94, 0.98] | <.001 | WRAT | 0.95 | [0.93, 0.97] | <.001 |
| CD4<200 | 1.40 | [0.60, 3.26] | .63 | CD4<200 | 1.98 | [0.86, 4.51] | .11 |
| HCV | 1.32 | [0.80, 2.18] | .28 | HCV | 0.97 | [0.59, 1.62] | .92 |
| Diabetes | 2.23 | [1.11, 4.47] | .02 | Diabetes | 2.14 | [1.06, 4.33] | .03 |
| Cannabis | 0.74 | [0.46, 1.20] | .22 | Cannabis | 0.46 | [0.28, 0.75] | .002 |
| BDI-II | 1.04 | [1.02, 1.07] | <.001 | BDI-II | 1.06 | [1.03, 1.09] | <.001 |
Note. OR= Odds Ratio; 95% CI= 95% Confidence Interval; WRAT= Wide-Range Achievement reading subtest version 3 or 4; HCV= hepatitis C virus; Cannabis= lifetime cannabis use disorder; BDI-II= Beck Depression Inventory-II total score.
To focus on a clinically relevant subgroup, we reran the multinomial logistic regression among participants with undetectable levels of HIV plasma RNA. Of the 535 participants with an undetectable viral load, 97 (18%) were SA, 208 (39%) were CN, and 230 (43%) were CI. Age, WRAT, BDI-II, and diagnosis of lifetime cannabis use disorder remained significant predictors of neurocognitive status in this virally suppressed subgroup. Although diabetes increased likelihood of CN (OR=1.74, p=0.13) or CI (OR=1.63, p=0.19) compared to SA, these associations were no longer statistically significant.
Age and Global Performance Relationship by Neurocognitive Status
To examine the relationship between age and global neurocognitive performance within each neurocognitive status group in PLWH, we performed Pearson’s partial correlations between age and demographically-uncorrected global scaled scores, co-varying for education, sex, and race/ethnicity. Age negatively correlated with lower global scaled scores within the CN (partial r=−.24; p<.001) and CI (partial r=−.15; p<.001) groups. However, age did not significantly relate to global scaled scores among the SA group (partial r=−.11; p=.24). Despite this lack of significance, comparison of Fisher’s r-to-z transformed correlations indicated that the effect size of age on global scaled scores in SA did not significantly differ from the effect sizes of age on global scaled scores in CN (z=1.23; p=.22) and CI (z=.38; p=.70). Similarly, the magnitude of the relationship between age and global scaled scores did not differ between CN and CI (z=−1.15; p=.25).
Everyday Functioning and Health-Related Quality of Life Correlates of Neurocognitive Status
A stair-step pattern was observed for most outcomes from the PAOFI, IADL, and MOSSF-36 measures, with SA individuals endorsing the most favorable everyday functioning and HRQoL outcomes followed by CN then CI participants. SA individuals endorsed significantly fewer cognitive symptoms on the PAOFI than CN (d=−0.34, p<.001) and CI participants (d=−0.64, p<.0001) and fewer declines in IADLs than either CN (d=−0.42, p<.01) or CI participants (d=−0.70, p<.0001). The CN group also reported significantly fewer cognitive symptoms (d=−0.30, p<.05) and IADL declines (d=−0.33, p<.001) than the CI group. Figure 3 displays similar group differences on rates of unemployment and IADL dependence as well as the MOS-SF-36 physical and mental HRQoL composite scores.
Figure 3. Everyday functioning and health-related quality of life by neurocognitive status.
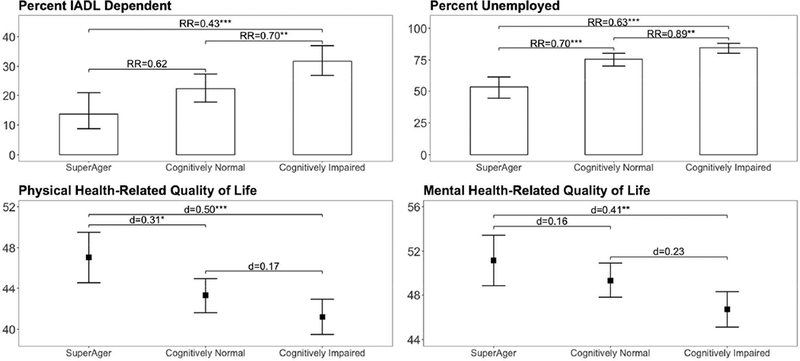
Risk ratio (RR) estimates represent the reduction in risk of IADL dependence or unemployment for each pair-wise comparison. Cohen’s d effect size estimates reflect differences in health-related quality of life for each pair-wise comparison. All p-values are significant after Bonferroni-adjustment or Tukey’s HSD.
***p<.001
**p<.01
*p<.05
Discussion
The emerging concept of neurocognitive SA has produced invaluable insights into age-related neurocognitive phenotypes and has undermined the widely-held assumption that age-related neurocognitive deterioration is inevitable. However, the prospect of maintaining intact neurocognitive capacities throughout the lifespan is highly daunting for PLWH. In our study sample with 17% meeting criteria for SA, we demonstrate that youthful neurocognitive performance is possible for older PLWH. Our findings suggest that SA status is independently related to diverse factors that reflect current physical and mental health as well as premorbid neurocognitive functioning. Furthermore, SA status is associated with better everyday functioning, supporting the ecological validity of distinguishing SA from CN and CI individuals.
Given the marked difference in average age between our cohort of older PLWH and previous SA cohorts of healthy elders, our SA criteria and study results cannot be directly linked to the extant SA literature. However, there are several strengths of our peak-age approach to defining neurocognitive SA in the context of HIV infection. First, we do not focus on one specific domain of neurocognitive functioning. Instead, our SA criteria are defined by absence of peak-age impairment in global neurocognitive functioning and absence of actual-age impairment in all domains assessed. PLWH are a heterogeneous group whose neurocognition may be impacted by HIV and demographic and clinical confounds, contributing to a neurocognitive profile that is not defined by deficits in any one neurocognitive domain. Thus, we demonstrate merit in defining SA by global performance to match what is known about neurocognitive functioning among PLWH. An important feature of our global estimates of neurocognitive functioning is that they are adjusted for practice effects, as some study participants had prior exposure to the neurocognitive testing battery. Practice, or learning, effects complicate assessment of SA because seemingly elite neurocognition can be an artifact of prior testing experience. By correcting for normal test-retest fluctuations, we reduce the likelihood of overestimating neurocognitive ability and enhance the stringency of our SA criteria. We compared neurocognitive functioning of our sample to normative standards for age 25 when neurocognitive functioning is maximal (Salthouse, 2009). The concept of SA (Rogalski et al., 2013) posits that within an individual’s adult life, aging does not necessitate neurocognitive decline. Rather, aging increases the likelihood of encountering adverse events that contribute to neuronal damage and decline in neurocognition. Defining SA in this way may facilitate understanding of the kinds of events or experiences that either support, or damage, neurocognitive functioning.
SA had lower rates of unemployment and IADL dependence than the other neurocognitive status groups and higher self-reported physical and mental HRQoL. Thus, our method for defining SA appears to be concurrently valid with measures of everyday functioning and HRQoL. Importantly, CN and SA groups differ in real-world outcomes, indicating heterogeneity among neurocognitively unimpaired individuals. Unlike prior investigations of SA, our definition of SA did not require self-reported IADL independence as a criterion. Despite performance-based data indicating SA, a small proportion of the SA group endorsed IADL dependence. Among our SA group, self-reported declines in IADL may represent actual decline, such that SA individuals may have started at higher levels of functioning and experienced a decline that is not necessarily at an impaired level. To this point, our measure of IADL dependence may be overly sensitive in detecting decline and not specific in detecting whether this decline represents a shift from within normal functioning to impairment status. Given that most other studies rely on absence of IADL dependence or decline when defining SA, these studies may be potentially misidentifying SA individuals who perform at peak-age levels on neurocognitive tests. Thus, future investigations need to consider the appropriate use of performance-based versus self-reported deficits when classifying individuals as SA versus CN.
Consistent with prior research, the WRAT reading subtest, an estimate of premorbid verbal IQ that is relatively resistant to HIV-associated neurocognitive decline (Casaletto et al., 2014), was higher in SA and predicted SA status. Moore et al. (2014) demonstrated a positive correlation between a composite measure of cognitive reserve, including verbal IQ, and successful cognitive aging in older PLWH. The theory of cognitive reserve postulates that effects of neural insults, such as age and comorbidities, are buffered by robust brain networks (Stern, 2002). Although operational definitions and methods of quantifying cognitive reserve may vary across studies (Moore et al., 2014; Nucci, Mapelli, & Mondini, 2012; Reed et al., 2010; Selzam et al., 2017), cognitive reserve is considered to reflect a combination of genetically-driven intellectual capacity and cognitively stimulating life experiences that promote resilience against age-related neurocognitive decline (Daffner, 2010; Stern, 2012). Although SA displayed higher premorbid functioning on the WRAT, neurocognitive status groups did not differ on years of education. Thus, neuroprotective benefits measured by higher WRAT performance may be better explained by factors other than education, such as genetically-driven neurocognitive resilience. More granular methods of quantifying both the genetic (e.g., polygenic risk scores) and environmental (e.g., educational quality, socioeconomic factors) loadings of cognitive reserve are needed to thoroughly address questions regarding premorbid functioning and age-related neuroprotection.
Among HIV-uninfected individuals, diabetes is also strongly associated with neurocognitive impairment and is considered to be a predisposing factor for later development of vascular dementia and Alzheimer’s disease (Cheng, Huang, Deng, & Wang, 2012; Taguchi, 2009). Insulin resistance and diabetes are associated with MRI structural abnormalities and functional alterations of the blood brain barrier, resulting in processes that facilitate the pathogenesis and progression of neurocognitive impairment (Archibald et al., 2014; Mogi & Horiuchi, 2011; Prasad, Sajja, Naik, & Cucullo, 2014). We found a stair-step effect for the influence of diabetes on neurocognitive status such that CI individuals were characterized by the highest rates of diabetes, followed by CN, and then SA participants; associations between diabetes and neurocognitive status remained in multivariable analyses. Other studies have found similar increases in risk for HAND among HIV-infected persons with self-reported diabetes or elevated fasting insulin levels (McCutchan et al., 2012; Valcour et al., 2006; Valcour et al., 2005; Vance et al., 2014). Thus, for SA participants, their relatively low incidence of diabetes likely contributed to better neurocognitive functioning. However, the effect of diabetes was not significant when restricting our multinomial regression analysis to virally suppressed participants, underscoring the importance of other contributing factors to SA status.
SA had lower BDI-II scores than both CN and CI univariately and in the multinomial logistic regression. In contrast, rates of current and lifetime MDD diagnoses did not significantly differ by neurocognitive status group, indicating that among older PLWH, current subclinical depressive symptoms are associated with neurocognitive functioning more closely than active or remote clinical depression. This relationship may reflect known neurological consequences of depression, including neuroinflammation and associated neuronal damage, apoptosis, and reduced neurogenesis (Kubera, Obuchowicz, Goehler, Brzeszcz, & Maes, 2011; Maes et al., 2009). Behavioral mechanisms may also underlie the relationship between depression and neurocognition, as depressive symptoms (even those that are subclinical) negatively impact engagement in activities known to promote neurocognitive health, including exercise, healthy nutrition, and social activity (Jeste, Depp, & Vahia, 2010; Moore et al., 2018; Vahia et al., 2010).
SA displayed greater rates of lifetime cannabis use disorder in comparison to CI, and this pattern also remained significant in the multinomial logistic regression. This result is supported by evidence suggesting neuroprotective effects of cannabis use through activation of cannabinoid receptors (i.e., CB1 and CB2) in the central nervous system (Sanchez & Garcia-Merino, 2012). Specifically, CB1 agonists reduce excitotoxity in post-synaptic neurons (Marsicano et al., 2003) while CB2 agonists promote anti-inflammatory and immunomodulatory actions (Rom & Persidsky, 2013). Nevertheless, the relationship between cannabis use and brain integrity among PLWH and HIV-uninfected adults remains a controversial matter. While chronic cannabis use has been associated with neurometabolic abnormalities, reduced gray matter volumes, and memory deficits in cohorts comprised of PLWH and seronegative controls (Battistella et al., 2014; Chang, Cloak, Yakupov, & Ernst, 2006; Cristiani, Pukay-Martin, & Bornstein, 2004; Thames et al., 2017), emerging evidence suggests that active cannabis use may limit HIV viral replication and attenuate HIV-related immunosuppression, inflammation, and cerebral glutamate depletion (Chang et al., 2006; Rizzo et al., 2018; Thames, Mahmood, Burggren, Karimian, & Kuhn, 2016). These neuroprotective properties of the cannabinoid system are not referenced in the context of a cannabis use disorder, which may reflect problematic use or heavy exposure that could exceed therapeutic levels. Moreover, prior studies examining elite neurocognition in healthy elders have excluded participants with substance use histories that could influence neurocognition. Thus, our cannabis-related findings cannot be compared to prior SA studies and the relationship between cannabis use disorder and neuroprotection in HIV remains poorly characterized. Future research is needed to explore therapeutic levels of cannabis use and identify potential benefits of cannabinoid receptor activation on neurocognition among PLWH.
Despite stair-step patterns for HIV disease characteristics in SA individuals compared to CN and CI participants, only the proportion of participants with current CD4 counts below 200 was statistically significantly different among the neurocognitive status groups. Specifically, the SA group had a lower proportion with current CD4 counts below 200 than the CI group. However, this difference was not statistically significant when controlling for other clinical and demographic variables (e.g., age, WRAT, and depressive symptoms). Unexpectedly, the SA and CN groups had low nadir CD4 counts comparable to the CI group, possibly reflecting underlying resilience to the “legacy” effects of advanced immunosuppression. In a comparison of predictors of HAND before and during the era of ART, only low nadir CD4 was found to increase risk of neurocognitive impairment in both treatment eras (Heaton et al., 2011). However, when examining factors associated with decline to symptomatic HAND, current CD4 also predicted decline to symptomatic status (Grant et al., 2014). SA with current CD4 counts below 200 were more likely to be off ART. Furthermore, the higher proportion of participants with CD4 counts below 200 in the CI group may result from poorer ART adherence that is a consequence of their cognitive impairment. Given that the majority of participants were likely to begin ART after having advanced HIV, it is unclear whether similar relationships between HIV disease severity and neurocognitive status exist for modern era patients who typically start treatment at earlier stages.
The observation that SA prevalence was twice as high in HIV-uninfected comparison participants as compared to PLWH provides important context to our findings. This difference, in addition to the higher prevalence of CN and lower prevalence of CI in HIV-uninfected controls, aligns with the known independent neurotoxic effects of HIV and potential synergistic effects of aging with HIV. Compared to their seronegative counterparts, older PLWH must withstand a greater amount of exposure to neural insults in order to sustain an elite level of neurocognitive performance. It is important to note that the HIV-uninfected group was demographically distinct from the PLWH group, as indicated by a higher prevalence of non-Hispanic Whites, more years of education, and better WRAT Reading scores. Thus, the estimated two-fold difference in SA prevalence may be partially confounded by potential socio-demographic advantages of the HIV-uninfected group.
Several limitations to the present study warrant discussion. Our peak-age corrected neurocognitive scores, based on a normative sample of 25 year-olds, serve as proxy measures for neurocognitive resilience and do not directly capture the true within-subject change in neurocognitive performance since age 25. Because our data is cross-sectional, we cannot rule out the possibility that members of the SA group have experienced considerable lifetime neurocognitive decline and that their SA status is an artifact of superior baseline neurocognitive capacity. Although our analysis demonstrating that older age was associated with lower global scaled scores in CN and CI groups, but not the SA group, preliminarily supports the validity of our SA criteria, the magnitude of these age effects were small and did not significantly differ across groups. In addition to other factors importantly contributing to variance in global neurocognitive performance, these small effect sizes are likely influenced by the narrow age range of our sample.
Our results highlight clinically informative predictors and benefits of neurocognitive resilience; yet, the racial/ethnic composition of our sample was predominantly non-Hispanic white men and may limit the generalizability of our findings to more socio-demographically diverse populations. Furthermore, our cohort of older PLWH is relatively young compared to the healthy adult cohorts studied in the extant SA literature of persons not living with HIV, but the age range is indicative of some of the oldest PLWH with a sufficient sample size to be studied. Although the inclusion of an age-matched HIV-uninfected comparison group provided an informative anchor point for SA prevalence in healthy adults, this comparison group was not comparable to the PLWH group on other important demographic factors. Consequently, important questions remain regarding the extent to which our definition of SA in PLWH reflects resilience to the effects of HIV and aging into late adulthood, which may only be adequately addressed with data from ideal comparison groups. As the proportion of PLWH older than 65 years of age increases, longitudinal cohort studies of PLWH will be better equipped to address critical questions related to the prevalence, stability, and impact of SA in PLWH compared to healthy seniors. Although we focused on evaluating the relationships between SA status and clinical correlates commonly assessed in PLWH, the absence of biomarker data indicative of central nervous system integrity (e.g., neuroimaging, cerebrospinal fluid assays) prevents us from determining the neurobiological correlates of SA status. Additionally, an assessment of modifiable behaviors (e.g., physical activity, neurocognitive activity, positive psychological outlook) that may mediate the relationships between SA status and psychosocial, medical, and everyday functioning correlates could help to prioritize research in clinical interventions to increase the fraction of SA in PLWH (Vance & Burrage, 2006).
Taken together, our results demonstrate that a substantial fraction of older, HIV-infected patients maintain their maximal neurocognitive abilities that confer real-world benefits even compared to patients with normal age-related cognitive decline. Although HIV disease negatively impacts the prevalence of SA, our findings highlight the clinical value in identifying neurocognitive resilience within PLWH and focus on the potential for positive outcomes despite aging with HIV. Examination of the stability of SA status through longitudinal analysis, exploration of biological and genetic markers of neuronal integrity, and assessment of modifiable lifestyle factors should enhance studies of future interventions to improve neurocognitive aging in older PLWH.
Table 1.
Neurocognitive tests administered by domain.
| Domain | Test |
|---|---|
| Verbal Fluency | Category Fluencya |
| Letter Fluencya | |
| Attention/Working Memory | Paced Auditory Serial Addition Taska |
| WAIS-III Letter-Number Sequencingbc | |
| WMS-III Spatial Spanbc | |
| Processing Speed | WAIS-III Digit Symbola |
| WAIS-III Symbol Searcha | |
| Trail Making Test Aa | |
| Stroop Color & Word Test Color Scorebd | |
| Executive Functioning | Wisconsin Card Sorting Test-64 Perseverative Errorsa |
| Trail Making Test Ba | |
| Stroop Color & Word Test Interference Scorebd | |
| Halstead Category Testb | |
| Learning | Hopkins Verbal Learning Test-Revised Total Learninga |
| Brief Visuospatial Memory Test-Revised Total Learninga | |
| Heaton Story Memory Test Learningbe | |
| Heaton Figure Memory Test Learningbe | |
| Delayed Recall | Hopkins Verbal Learning Test-Revised) Delayed Recalla |
| Brief Visuospatial Memory Test-Revised Delayed Recalla | |
| Heaton Story Memory Test Delayed Recallbe | |
| Heaton Figure Memory Test Delayed Recallbe | |
| Motor skills | Grooved Pegboard Test- Dominant Handa |
| Grooved Pegboard Test- Non-dominant Handa |
Core test administered across all studies with less than 5% missing data in the present sample.
Supplemental test administered in select studies.
Each study participant completed WAIS-III Letter-Number Sequencing (n=525) and/or WMS-III Spatial Span (n=224).
n=308.
Hopkins Verbal Learning Test-Revised and Brief Visuospatial Memory Test-Revised data were not used for learning and delayed recall domain scores for participants who also completed Heaton Story Memory Test and Heaton Figure Memory Test (n=138) data.
Bolded items have the greatest influence on global neurocognitive performance scores.
Acknowledgments
Data for this study was collected as part of six larger ongoing studies: 1) The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) study is supported by awards N01MH22005, HHSN271201000036C, and HHSN271201000030C from NIH; 2) the HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH; 3) the California NeuroAIDS Tissue Network (CNTN) is supported by awards U01MH083506, R24MH59745, and U24MH100928 from NIMH; 4) the Multi-Dimensional Successful Aging Among HIV-Infected Adults study is supported by award R01MH099987. Stipend support to RS is funded by NIAAA award T32AA013525. The authors declare no conflicts of interest.
References
- Anstey KJ, Sargent-Cox K, Garde E, Cherbuin N, & Butterworth P (2014). Cognitive development over 8 years in midlife and its association with cardiovascular risk factors. Neuropsychology, 28(4), 653–665. doi: 10.1037/neu0000044 [DOI] [PubMed] [Google Scholar]
- Archibald SL, McCutchan JA, Sanders C, Wolfson T, Jernigan TL, Ellis RJ, … Fennema-Notestine C (2014). Brain morphometric correlates of metabolic variables in HIV: the CHARTER study. J Neurovirol, 20(6), 603–611. doi: 10.1007/s13365-014-0284-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella G, Fornari E, Annoni JM, Chtioui H, Dao K, Fabritius M, … Giroud C (2014). Long-Term Effects of Cannabis on Brain Structure. Neuropsychopharmacology, 39(9), 2041–2048. doi: 10.1038/npp.2014.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Steer R, & Brown G (1996). Manual for Beck Depression Inventory II (BDI-II). San Antonio, TX, Psychology Corporation. [Google Scholar]
- Blackstone K, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, … Heaton RK (2012). Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol, 26(6), 894–908. doi: 10.1080/13854046.2012.694479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco JR, Jarrín I, Vallejo M, Berenguer J, Solera C, Rubio R, … Moreno S (2012). Definition of advanced age in HIV infection: looking for an age cut-off. AIDS Res Hum Retroviruses, 28(9), 1000–1006. doi: 10.1089/aid.2011.0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott NT, Bettcher BM, Yokoyama JS, Frazier DT, Wynn M, Karydas A, … Kramer JH (2017). Youthful Processing Speed in Older Adults: Genetic, Biological, and Behavioral Predictors of Cognitive Processing Speed Trajectories in Aging. Front Aging Neurosci, 9, 55. doi: 10.3389/fnagi.2017.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, & Heaton RK (2004). Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol, 26(3), 307–319. doi: 10.1080/13803390490510031 [DOI] [PubMed] [Google Scholar]
- Casaletto KB, Cattie J, Franklin DR, Moore DJ, Woods SP, Grant I, & Heaton RK (2014). The Wide Range Achievement Test-4 Reading Subtest “Holds” in HIV-infected Individuals. J Clin Exp Neuropsychol, 36(9), 992–1001. doi: 10.1080/13803395.2014.960370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2018). HIV Among People Aged 50 and Over. Retrieved from https://www.cdc.gov/hiv/group/age/olderamericans/index.html
- Chang L, Cloak C, Yakupov R, & Ernst T (2006). Combined and Independent Effects of Chronic Marijuana Use and HIV on Brain Metabolites. J Neuroimmune Pharmacol, 1(1), 65–76. doi: 10.1007/s11481-005-9005-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelune GJ, Heaton RK, & Lehman RAW (1986). Neuropsychological and personality correlates of patients’ complaints of disability In Advances in clinical neuropsychology, Vol. 3 (pp. 95–126). New York, NY, US: Plenum Press. [Google Scholar]
- Cheng G, Huang C, Deng H, & Wang H (2012). Diabetes as a risk factor for dementia and mild cognitive impairment: a meta‐analysis of longitudinal studies. Internal Medicine Journal, 42(5), 484–491. doi:doi: 10.1111/j.1445-5994.2012.02758.x [DOI] [PubMed] [Google Scholar]
- Cook AH, Sridhar J, Ohm D, Rademaker A, Mesulam M-M, Weintraub S, & Rogalski E (2017). Rates of cortical atrophy in adults 80 years and older with superior vs average episodic memory. Jama, 317(13), 1373–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook Maher A, Kielb S, Loyer E, Connelley M, Rademaker A, Mesulam MM, … Rogalski E (2017). Psychological well-being in elderly adults with extraordinary episodic memory. PLoS One, 12(10), e0186413. doi: 10.1371/journal.pone.0186413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristiani SA, Pukay-Martin ND, & Bornstein RA (2004). Marijuana use and cognitive function in HIV-infected people. J Neuropsychiatry Clin Neurosci, 16(3), 330–335. doi: 10.1176/jnp.16.3.330 [DOI] [PubMed] [Google Scholar]
- Daffner KR (2010). Promoting successful cognitive aging: a comprehensive review. J Alzheimers Dis, 19(4), 1101–1122. doi: 10.3233/jad-2010-1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekhtyar M, Papp KV, Buckley R, Jacobs HIL, Schultz AP, Johnson KA, … Rentz DM (2017). Neuroimaging markers associated with maintenance of optimal memory performance in late-life. Neuropsychologia, 100, 164–170. doi: 10.1016/j.neuropsychologia.2017.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira D, Machado A, Molina Y, Nieto A, Correia R, Westman E, & Barroso J (2017). Cognitive variability during middle-age: possible association with neurodegeneration and cognitive reserve. Frontiers in aging neuroscience, 9, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefen T, Peterson M, Papastefan ST, Martersteck A, Whitney K, Rademaker A, … Geula C (2015). Morphometric and histologic substrates of cingulate integrity in elders with exceptional memory capacity. J Neurosci, 35(4), 1781–1791. doi: 10.1523/JNEUROSCI.2998-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Franklin DR Jr., Deutsch R, Woods SP, Vaida F, Ellis RJ, … Group C. (2014). Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology, 82(23), 2055–2062. doi: 10.1212/WNL.0000000000000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TM, Maass A, Baker SL, & Jagust WJ (2018). Brain morphology, cognition, and β-amyloid in older adults with superior memory performance. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2018.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne JK, & Germine LT (2015). When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychol Sci, 26(4), 433–443. doi: 10.1177/0956797614567339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, … Grant I (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 75(23), 2087–2096. doi: 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, … Grant I (2011). HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol, 17(1), 3–16. doi: 10.1007/s13365-010-0006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, … Grant I (2004). The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc, 10(3), 317–331. doi: 10.1017/s1355617704102130 [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, & Grant I (2004). Revised Comprehensive Norms for an Expanded Halstead Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Heaton RK, Taylor MJ, & Manly J (2003). Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III In Clinical interpretation of the WAIS-III and WMS-III. (pp. 181–210). San Diego, CA, US: Academic Press. [Google Scholar]
- Heaton RK, Temkin N, Dikmen S, Avitable N, Taylor MJ, Marcotte TD, & Grant I (2001). Detecting change: A comparison of three neuropsychological methods, using normal and clinical samples. Arch Clin Neuropsychol, 16(1), 75–91. [PubMed] [Google Scholar]
- Henderson WA, Schlenk EA, Kim KH, Hadigan CM, Martino AC, Sereika SM, & Erlen JA (2010). Validation of the MOS-HIV as a measure of health-related quality of life in persons living with HIV and liver disease. AIDS Care, 22(4), 483–490. doi: 10.1080/09540120903207292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Depp CA, & Vahia IV (2010). Successful cognitive and emotional aging. World Psychiatry, 9(2), 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Savla GN, Thompson WK, Vahia IV, Glorioso DK, Martin AS, … Depp CA (2013). Association between older age and more successful aging: critical role of resilience and depression. Am J Psychiatry, 170(2), 188–196. doi: 10.1176/appi.ajp.2012.12030386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, & Maes M (2011). In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry, 35(3), 744–759. doi: 10.1016/j.pnpbp.2010.08.026 [DOI] [PubMed] [Google Scholar]
- Lachman ME, Teshale S, & Agrigoroaei S (2015). Midlife as a Pivotal Period in the Life Course: Balancing Growth and Decline at the Crossroads of Youth and Old Age. Int J Behav Dev, 39(1), 20–31. doi: 10.1177/0165025414533223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Ren P, Mapstone M, Meyers SP, Porsteinsson A, Baran TM, & Alzheimer’s Disease Neuroimaging I. (2017). The cingulate cortex of older adults with excellent memory capacity. Cortex, 86, 83–92. doi: 10.1016/j.cortex.2016.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PL, & Gardner RC (2000). Type I error rate comparisons of post hoc procedures for I j Chi-Square tables. Educational and Psychological Measurement, 60(5), 735–754. [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, … Maj M (2009). The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis, 24(1), 27–53. doi: 10.1007/s11011-008-9118-1 [DOI] [PubMed] [Google Scholar]
- Malaspina L, Woods SP, Moore DJ, Depp C, Letendre SL, Jeste D, … Group H. I. V. N. R. P. (2011). Successful cognitive aging in persons living with HIV infection. J Neurovirol, 17(1), 110–119. doi: 10.1007/s13365-010-0008-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapstone M, Lin F, Nalls MA, Cheema AK, Singleton AB, Fiandaca MS, & Federoff HJ (2017). What success can teach us about failure: the plasma metabolome of older adults with superior memory and lessons for Alzheimer’s disease. Neurobiol Aging, 51, 148–155. doi: 10.1016/j.neurobiolaging.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, … Lutz B (2003). CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science, 302(5642), 84–88. doi: 10.1126/science.1088208 [DOI] [PubMed] [Google Scholar]
- Martin M, & Zimprich D (2005). Cognitive development in midlife. Middle adulthood: A lifespan perspective, 179–206. [Google Scholar]
- McCutchan JA, Marquie-Beck JA, Fitzsimons CA, Letendre SL, Ellis RJ, Heaton RK, … Grant I (2012). Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology, 78(7), 485–492. doi: 10.1212/WNL.0b013e3182478d64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, & Horiuchi M (2011). Neurovascular coupling in cognitive impairment associated with diabetes mellitus. Circulation Journal, 75(5), 1042–1048. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Fazeli PL, Moore RC, Woods SP, Letendre SL, Jeste DV, … HIV Neurobehavioral Research Program. (2017). Positive Psychological Factors are Linked to Successful Cognitive Aging Among Older Persons Living with HIV/AIDS. AIDS Behav. doi: 10.1007/s10461-017-2001-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Fazeli PL, Jeste DV, Moore DJ, Grant I, & Woods SP (2014). Successful Cognitive Aging and Health-Related Quality of Life in Younger and Older Adults Infected with HIV. AIDS Behav, 18(6), 1186–1197. doi: 10.1007/s10461-014-0743-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Hussain MA, Watson CW, Fazeli PL, Marquine MJ, Yarns BC, … Moore DJ (2018). Grit and Ambition are Associated with Better Neurocognitive and Everyday Functioning Among Adults Living with HIV. AIDS Behav. doi: 10.1007/s10461-018-2061-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Iudicello JE, Weber E, Duarte NA, Riggs PK, Delano-Wood L, … Group H. N. R. P. (2012). Synergistic effects of HIV infection and older age on daily functioning. Journal of acquired immune deficiency syndromes (1999), 61(3), 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MA, Moore DJ, Taylor M, Franklin D Jr., Cysique L, Ake C, … Group H. (2011). Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol, 33(7), 793–804. doi: 10.1080/13803395.2011.559157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucci M, Mapelli D, & Mondini S (2012). Cognitive Reserve Index questionnaire (CRIq): a new instrument for measuring cognitive reserve. Aging Clin Exp Res, 24(3), 218–226. doi: 10.3275/7800 [DOI] [PubMed] [Google Scholar]
- Pathai S, Bajillan H, Landay AL, & High KP (2014). Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci, 69(7), 833–842. doi: 10.1093/gerona/glt168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Sajja RK, Naik P, & Cucullo L (2014). Diabetes Mellitus and Blood-Brain Barrier Dysfunction: An Overview. Journal of pharmacovigilance, 2(2), 125. doi: 10.4172/2329-6887.1000125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BR, Mungas D, Farias ST, Harvey D, Beckett L, Widaman K, … DeCarli C (2010). Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain, 133(Pt 8), 2196–2209. doi: 10.1093/brain/awq154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo MD, Crawford RB, Henriquez JE, Aldhamen YA, Gulick P, Amalfitano A, & Kaminski NE (2018). HIV-infected cannabis users have lower circulating CD16+ monocytes and IFN-gamma-inducible protein 10 levels compared with nonusing HIV patients. Aids, 32(4), 419–429. doi: 10.1097/qad.0000000000001704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski EJ, Gefen T, Shi J, Samimi M, Bigio E, Weintraub S, … Mesulam MM (2013). Youthful memory capacity in old brains: anatomic and genetic clues from the Northwestern SuperAging Project. J Cogn Neurosci, 25(1), 29–36. doi: 10.1162/jocn_a_00300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom S, & Persidsky Y (2013). Cannabinoid receptor 2: potential role in immunomodulation and neuroinflammation. J Neuroimmune Pharmacol, 8(3), 608–620. doi: 10.1007/s11481-013-9445-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner R, & Cysique LA (2017). HIV-Associated Neurocognitive Disorders: A Global Perspective. Journal of the International Neuropsychological Society, 23(9–10), 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2003). Memory aging from 18 to 80. Alzheimer Dis Assoc Disord, 17(3), 162–167. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (2009). When does age-related cognitive decline begin? Neurobiol Aging, 30(4), 507–514. doi: 10.1016/j.neurobiolaging.2008.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AJ, & Garcia-Merino A (2012). Neuroprotective agents: cannabinoids. Clin Immunol, 142(1), 57–67. doi: 10.1016/j.clim.2011.02.010 [DOI] [PubMed] [Google Scholar]
- Schaie KW, & Willis SL (2010). The Seattle Longitudinal Study of Adult Cognitive Development. ISSBD Bull, 57(1), 24–29. [PMC free article] [PubMed] [Google Scholar]
- Selzam S, Krapohl E, von Stumm S, O’Reilly PF, Rimfeld K, Kovas Y, … Plomin R (2017). Predicting educational achievement from DNA. Mol Psychiatry, 22(2), 267–272. doi: 10.1038/mp.2016.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DP, Iudicello JE, Morgan EE, Kamat R, Clark LR, Avci G, … Woods SP (2017). Accelerated and accentuated neurocognitive aging in HIV infection. J Neurovirol, 23(3), 492–500. doi: 10.1007/s13365-017-0523-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, van Sighem A, … Hallett TB (2015). Future challenges for clinical care of an ageing population infected with HIV: a modelling study. The Lancet Infectious Diseases, 15(7), 810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y (2002). What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc, 8(3), 448–460. [PubMed] [Google Scholar]
- Stern Y (2012). Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol, 11(11), 1006–1012. doi: 10.1016/s1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FW, Stepanovic MR, Andreano J, Barrett LF, Touroutoglou A, & Dickerson BC (2016). Youthful Brains in Older Adults: Preserved Neuroanatomy in the Default Mode and Salience Networks Contributes to Youthful Memory in Superaging. J Neurosci, 36(37), 9659–9668. doi: 10.1523/JNEUROSCI.1492-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A (2009). Vascular factors in diabetes and Alzheimer’s disease. J Alzheimers Dis, 16(4), 859–864. doi: 10.3233/jad-2009-0975 [DOI] [PubMed] [Google Scholar]
- Thames AD, Kim MS, Becker BW, Foley JM, Hines LJ, Singer EJ, … Hinkin CH (2011). Medication and finance management among HIV-infected adults: The impact of age and cognition. J Clin Exp Neuropsychol, 33(2), 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Kuhn TP, Williamson TJ, Jones JD, Mahmood Z, & Hammond A (2017). Marijuana effects on changes in brain structure and cognitive function among HIV+ and HIV− adults. Drug Alcohol Depend, 170, 120–127. doi: 10.1016/j.drugalcdep.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Mahmood Z, Burggren AC, Karimian A, & Kuhn T (2016). Combined Effects of HIV and Marijuana Use on Neurocognitive Functioning and Immune Status. AIDS Care, 28(5), 628–632. doi: 10.1080/09540121.2015.1124983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahia IV, Meeks TW, Thompson WK, Depp CA, Zisook S, Allison M, … Jeste DV (2010). Subthreshold depression and successful aging in older women. Am J Geriatr Psychiatry, 18(3), 212–220. doi: 10.1097/JGP.0b013e3181b7f10e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, … Sacktor N (2004). Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology, 63(5), 822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour VG, Sacktor NC, Paul RH, Watters MR, Selnes OA, Shiramizu BT, … Shikuma CM (2006). Insulin resistance is associated with cognition among HIV-1-infected patients: the Hawaii Aging With HIV cohort. J Acquir Immune Defic Syndr, 43(4), 405–410. doi: 10.1097/01.qai.0000243119.67529.f5 [DOI] [PubMed] [Google Scholar]
- Valcour VG, Shikuma CM, Shiramizu BT, Williams AE, Watters MR, Poff PW, … Sacktor NC (2005). Diabetes, insulin resistance, and dementia among HIV-1-infected patients. J Acquir Immune Defic Syndr, 38(1), 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, & Burrage JW (2006). Promoting successful cognitive aging in adults with HIV: strategies for intervention. J Gerontol Nurs, 32(11), 34–41. [DOI] [PubMed] [Google Scholar]
- Vance DE, Fazeli PL, Dodson JE, Ackerman M, Talley M, & Appel SJ (2014). The Synergistic Effects of HIV, Diabetes, and Aging on Cognition: Implications for Practice and Research. The Journal of neuroscience nursing : journal of the American Association of Neuroscience Nurses, 46(5), 292–305. doi: 10.1097/JNN.0000000000000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Fazeli PL, & Gakumo CA (2013). The impact of neuropsychological performance on everyday functioning between older and younger adults with and without HIV. Journal of the Association of Nurses in AIDS Care, 24(2), 112–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ren P, Baran TM, Raizada RDS, Mapstone M, Lin F, & Alzheimer’s Disease Neuroimaging I. (2017). Longitudinal Functional Brain Mapping in Supernormals. Cereb Cortex, 1–11. doi: 10.1093/cercor/bhx322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G, & Robertson G (2006). Wide Range Achievement Test-4 (WRAT-4). Lutz, FL: Psychological Assessment Resources Inc. [Google Scholar]
- Wing EJ (2016). HIV and aging. Int J Infect Dis, 53, 61–68. doi: 10.1016/j.ijid.2016.10.004 [DOI] [PubMed] [Google Scholar]
- Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS, & Grant I (2008). HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology, 22(1), 110–117. doi: 10.1037/0894-4105.22.1.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (1998). Composite Diagnositic International Interview (CIDI, version 2.1). Geneva, Switzerland: World Health Organization. [Google Scholar]
- Wu AW, Revicki DA, Jacobson D, & Malitz FE (1997). Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV). Qual Life Res, 6(6), 481–493. [DOI] [PubMed] [Google Scholar]