Abstract
Objective
To report the induction of anti–Ma2 antibody–associated paraneoplastic neurologic syndrome (Ma2-PNS) in 6 patients after treatment with immune checkpoint inhibitors (ICIs). We also analyzed (1) patient clinical features compared with a cohort of 44 patients who developed Ma2-PNS without receiving ICI treatment and (2) the frequency of neuronal antibody detection before and after ICI implementation.
Methods
Retrospective nationwide study of all patients with Ma2-PNS developed during ICI treatment between 2017 and 2018.
Results
Our series of patients included 5 men and 1 woman (median age, 63 years). The patients were receiving nivolumab (n = 3), pembrolizumab (n = 2), or a combination of nivolumab and ipilimumab (n = 1) for treatment of neoplasms that included lung (n = 4) and kidney (n = 1) cancers and pleural mesothelioma (n = 1). Clinical syndromes comprised a combination of limbic encephalitis and diencephalitis (n = 3), isolated limbic encephalitis (n = 2), and a syndrome characterized by ophthalmoplegia and head drop (n = 1). No significant clinical difference was observed between our 6 patients and the overall cohort of Ma2-PNS cases. Post-ICI Ma2-PNS accounted for 35% of the total 17 Ma2-PNS diagnosed in our center over the 2017–2018 biennium. Eight cases had been detected in the preceding biennium 2015–2016, corresponding to a 112% increase of Ma2-PNS frequency since the implementation of ICIs in France. Despite ICI withdrawal and immunotherapy, 4/6 patients died, and the remaining 2 showed a moderate to severe disability.
Conclusions
We show a clear association between ICI use and increased diagnosis of Ma2-PNS. Physicians need to be aware that ICIs can trigger Ma2-PNS because clinical presentation can be challenging.
Therapy with monoclonal antibodies (Abs) targeting immune checkpoints, including cytotoxic T lymphocyte–associated antigen 4 (CTLA-4), the programmed death-1 receptor (PD-1), and its ligand PD-L1, has led to a paradigm shift in the treatment of numerous types of cancer.1 Their unprecedented results in controlling tumors at a metastatic stage have come at the expense of an increased risk of developing immune-related adverse events (irAEs), including severe neurologic complications.2–6 Given their mechanism of action, a possible association with the development of paraneoplastic neurologic syndromes (PNSs) has been predicted.3,7 Recently, the emergence of individual cases and small series of patients developing encephalitis and other neurologic manifestations has caused growing concern.4,5,8 Because an increasing number of patients will be exposed to immune checkpoint inhibitors (ICIs) in the forthcoming future, it is crucial to identify the main features of neurologic irAEs.
PNS with Abs targeting the intracellular Ma2 antigen characterizes a peculiar form of encephalitis with prominent involvement of limbic, brainstem, and diencephalic structures, usually in association with testicular or lung cancer.9,10 Atypical manifestations including narcolepsy-cataplexy, weight gain, sexual dysfunction, and motor neuron syndrome were described and account for the difficulty in diagnosing anti–Ma2 antibody–associated PNS (Ma2-PNS).9–12
We herein report 6 patients who developed autoimmune encephalitis with anti-Ma2 Abs during treatment with ICIs. To assess the relevance of our findings, we analyzed (1) their clinical features compared with a cohort of 44 patients who had developed Ma2-PNS without receiving any ICI and (2) the impact of ICI use on the frequency of Ma2 detection in a national reference center.
Methods
Patient selection
This is a retrospective study of all patients with anti-Ma2-PNS observed after treatment with ICIs and diagnosed at the French National Reference Center for Paraneoplastic Neurological Syndromes in Lyon, France, between January 1, 2017, and December 31, 2018. All patients underwent a comprehensive laboratory examination for suspected PNS as recommended,13 including an initial assessment with immunohistochemistry on rat brain sections, followed by a second confirmatory test represented by dot blot analysis on recombinant proteins (Euroimmun, Lübeck, Germany, and/or RAVO Diagnostika, Freiburg, Germany) and/or cell-based assays (CBAs) (in-house techniques) for the presence of onconeuronal Abs. We systematically tested: anti-Hu, Yo, CV2/CRMP5, Ri, Ma2, amphiphysin, GAD65, AK5, NMDA receptor (NMDAR), AMPAR, GABAAR, GABABR, IgLON5, CASPR2, LGI1, and DPPX. Anti-Ma2 specificities14 were confirmed using in-house CBA and commercial dot blots (Euroimmun, Lubeck, Germany). Clinical and ancillary data were obtained by telephone or email at the time of diagnosis, based on the biological sample, and at least twice a year to assess clinical evolution. Immunotherapy treatment modalities and oncologic therapy were recorded. Outcomes were assessed using the modified Ranking Scale (mRS). The scale ranges from 0 (no symptoms) to 6 (death).
Clinical comparison between ICI-induced Ma2-PNS vs classic Ma2-PNS
Demographic and clinical features of patients with Ma2-PNS triggered by ICIs were compared with those of the overall cohort of patients with Ma2-PNS unrelated to ICI treatment diagnosed in our center between 2002 and 2018 (n = 44).
Frequency of ICI-related PNS
To assess the impact of ICI use on the development of PNS at a national level, we compared the frequency of the different Ab detections in our Reference Center in the biennium 2017–2018 to the biennium 2015–2016 when the use of ICIs in France was at its starting point. The proportion of Ab-positive cases that developed after ICI use was calculated for each Ab specificity.
Statistical analysis
Descriptive analysis is presented as frequencies and percentages for categorical variables and as the median and range for continuous variables. Categorical data were analyzed with the Fisher exact test (2 tailed) and numerical data with the Mann-Whitney U test. Statistical analyses were performed using IBM SPSS Statistics Software (Version 25.0; IBM Corp, Armonk, NY). p Values <0.05 were considered significant.
Standard protocol approvals, registrations, and patient consents
Written consent was obtained from all patients, and the study was approved by the Institutional Review Board of the University Claude Bernard Lyon 1 and Hospices Civils de Lyon.
Data availability
Data reported in this manuscript are available within the article or its supplementary materials. More information regarding the data is available from the corresponding author on reasonable request.
Results
Patients with ICI-associated anti-Ma2 syndromes
Between 2002 and 2018, we identified 50 patients with Ma2-PNS in our center, 6 of which developed the syndrome after ICI treatment in the biennium 2017–2018. All the information on clinical and paraclinical results, together with associated treatments and outcomes of these 6 patients, is summarized in the table. Most of the patients were male (83%), with a median age of 63 years (range: 47–79 years). All were Caucasians. Four of them had an associated non–small-cell lung cancer, 1 a pleural mesothelioma, and the last one a renal clear cell carcinoma. At the time of ICI introduction—a median of 6.5 months (range: 0.5–25) after cancer diagnosis—all the patients except 1 (patient 2) had a metastatic disease, which included brain involvement in 2 cases (patients 1 and 3). ICIs used comprised nivolumab (3 cases), pembrolizumab (2 cases), and a combination of nivolumab and ipilimumab in 1 case. Median delay between ICI introduction and onset of the neurologic syndrome was 4 months (range: 2–8). When the neurologic syndrome ensued, the 2 patients with cerebral metastasis had stable or improved lesions on brain MRI, whereas the others showed no evidence of cancer dissemination in the CNS. Clinical syndromes included a combination of limbic encephalitis and diencephalitis (patients 1, 2, and 5), isolated limbic encephalitis (patients 3 and 6), and a syndrome characterized by ophthalmoplegia and motor neuron involvement (head drop) in patient 4. Onset of the neurologic symptoms was usually subacute (3 patients), whereas patient 5 had an acute onset and patient 1 a chronic/progressive course. All patients fulfilled the criteria for definite PNS.15 No statistically significant clinicodemographic differences (including sex, age at onset, cancer type, and main neurologic syndrome) were observed between our 6 patients and the overall cohort of 44 patients with anti-Ma2-PNS diagnosed in our center. Testicular cancer was present in 11/44 (25%) of the patients with “classic” anti-Ma2-PNS and in none of the post-ICI cases. It is noteworthy that patients with Ma2-PNS associated with testicular cancer (n = 11) were significantly younger than patients with ICI-induced Ma2 Ab syndrome (p = 0.003). Importantly, the timing of onset of the neurologic syndrome in relation to the discovery of cancer was clearly different in the cases triggered by ICIs (p = 0.004). Indeed, 77% of the patients in the overall Ma2 cohort manifested their neurologic syndrome before the oncologic diagnosis. On the contrary, all patients in the ICI group have, by definition, a cancer at the time of PNS onset. It is, however, interesting to note that the symptoms appear long after cancer diagnosis, a median of 10 months later (range: 5.5–28 months).
Table.
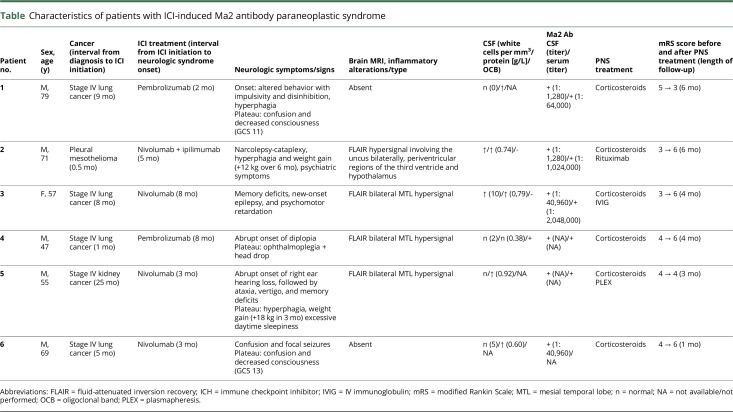
Characteristics of patients with ICI-induced Ma2 antibody paraneoplastic syndrome
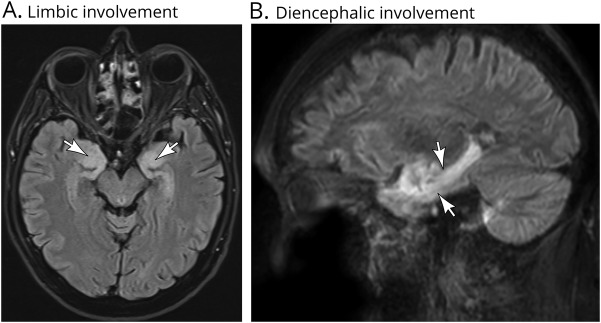
All the patients in the present study were investigated using brain MRI, which showed bilateral fluid-attenuated inversion recovery hyperintensity involving the mesial temporal lobes in 4 cases, including 1 with coexisting hyperintensity of the periventricular regions of the third ventricle and hypothalamus (figure 1). Contrast enhancement was not detected in any patient at the level of inflammatory lesions on T1-weighted sequences. CSF analysis revealed inflammatory alterations in all cases, with the most common abnormality being an increased protein content (5 cases), followed by pleocytosis (2 cases) and presence of CSF-exclusive oligoclonal bands in 1 patient. Patient 5 showed additional anti-Ma1 positivity; no other onconeural Abs were detected in the remainder of the patients. The neurologic syndrome was moderately severe with a median pretreatment mRS score of 4 (range: 0–6), characteristic of a patient unable to walk unassisted and to attend to own bodily needs. Treatment included ICI withdrawal and corticosteroids in all patients. Additional treatment was adopted in 4 patients: 2 received IV immunoglobulin, 1 was treated with plasmapheresis, and 1 with rituximab. Median follow-up was 4 months (range: 1–6 months). Despite all these measures, 4/6 patients died, and the remaining 2 showed a moderate to severe disability (mRS score: 3 and 4, respectively). The cause of death was directly related to the neurologic involvement or its associated complications in 3 patients, whereas in 1 case, it was attributed to the tumor progression. Of note, before ICI withdrawal, all patients except 1 (patient 4) showed a good response of cancer to immunotherapy, with stabilization or reduction of the neoplastic lesions. Intriguingly, for 1 case (patient 2), a serum sample was taken before ICI initiation and then stored in a biobank. The retrospective analysis of this sample revealed the presence of Ma2 Abs even before cancer immunotherapy onset. No antecedent serum sample was available for the other patients.
Figure 1. Results of paraclinical studies in patients with anti-Ma2 encephalitis triggered by immune checkpoint inhibitors [ICIs].
Brain MRI (fluid-attenuated inversion recovery sequences) in 2 patients with anti-Ma2 encephalitis triggered by ICIs. Note the prominent limbic (A, axial view) and diencephalic (B, sagittal view) involvement (arrowheads).
Impact of ICI use on the frequency of Ma2-PNS
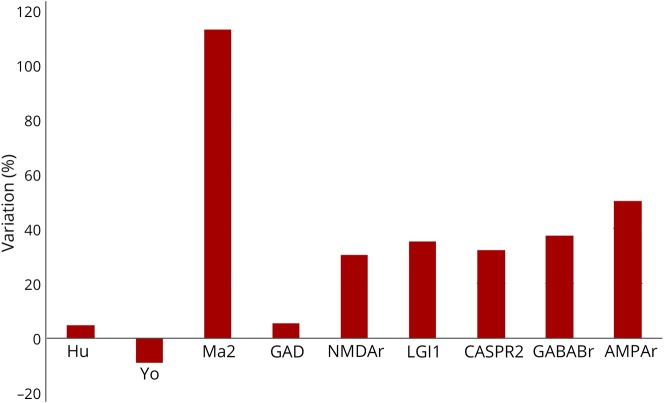
We questioned whether ICI use had an impact on the frequency of Ma2-PNS in our center. During the study period 2017–2018, a total of 17 patients with Ma2 Abs were diagnosed in our reference center. The 6 Ma2-PNS described herein representing 35% of all the cases. During the biennium 2015–2016, when the use of ICIs in France was at its starting point, we observed only 8 Ma2-PNS cases, meaning that a 112.5% increase was observed since ICI implementation in France. As a matter of fact, the annual number of anti-Ma2 positivities diagnosed in our national reference center was relatively stable over the last decade, with a median of 4 cases per year (range: 1–6 cases), and this observation is therefore unprecedented. No other onconeural Ab targeting intracellular antigens demonstrated a similar increment (figure 2). A lower increment was demonstrated for the recently implemented neural surface Abs (such as NMDAR, Lgi1, CASPR2, GABABR, and AMPAR), ranging from 30 to 50%, probably reflecting their relatively novel adoption in clinical practice if compared with the former group. Remarkably, only 1 case associated with neural surface antibody (CASPR2 positivity) developed after ICI initiation, accounting for 3% of all CASPR2 patients diagnosed in our center in the same period.
Figure 2. Proportion of variation in antibody detection between 2017 and 2018 vs 2015 and 2016 at the French Reference Center for Paraneoplastic Neurological Syndromes.
Note the 112% increase in Ma2-associated paraneoplastic neurologic syndrome detection observed after immune checkpoint inhibitor introduction. No other onconeural antibody (Ab) targeting intracellular antigens demonstrated a similar increment. A lower increment is observed for the recently implemented neural surface Abs, ranging from 30% to 50%, probably reflecting their relatively novel adoption in clinical practice if compared with the former group.
Discussion
We describe here 6 patients who developed anti-Ma2-PNS after receiving ICI treatment. Their demographic, clinical, and paraclinical features were remarkably uniform. Most of them were male, middle-age/elderly adults who developed a neurologic syndrome characterized by prominent limbic and diencephalic involvement, mainly associated with lung cancer. This clinical presentation is in line with both the original descriptions of the Ma2 syndrome9,10,16,17 and the clinical features from the 44 remaining patients of our overall cohort. We, however, note 3 notable differences: (1) in the classic, paraneoplastic form, the neurologic syndrome is known to precede cancer diagnosis by several weeks to months. When the tumor is eventually found, it is usually detected at a limited disease stage. On the contrary, when the disease appeared as a complication of ICIs, patients already presented with metastasis and the neurologic syndrome manifested several months after cancer discovery. (2) Testicular cancer is a frequently associated neoplasia in Ma2-PNS, and patients with Ma2 autoimmunity in the context of testicular tumors tend to be younger. Because the current treatment of testicular cancer does not include ICIs, this could explain the older age at onset and higher percentage of lung cancer association observed herein. (3) Contrast enhancement of inflammatory brain alterations is usually detected in up to one-third of Ma2-PNS cases. However, this pattern was not observed in the cases elicited by ICI treatment.6
Despite these differences, the inflammatory alterations detected by CSF analysis, the presence of well-characterized Abs, and the selective brain MRI involvement of the mesial temporal lobe and diencephalon structures strongly suggest an immune-mediated pathogenesis.18 We therefore consider that the ICI treatment elicited the autoimmune encephalitis in our patients.
Because the anti–Ma2-associated syndrome is characterized by atypical manifestations such as increased daytime sleepiness, hyperphagia, and weight gain,10–12 it is important for the clinician to recognize the prominent features of this disease to avoid diagnostic pitfalls. These symptoms are related to the diencephalic involvement and need to be promptly differentiated from the clinical correlate of primary hypothyroidism, which is a much more common irAE that shares a similar presentation.19–21 The latter misdiagnosis occurred in 1 patient that we present (patient 2). Clinical worsening despite thyroid hormone therapy prompted further investigations until a final diagnosis of polysomnography-proven narcolepsy-cataplexy was finally made, together with the discovery of low hypocretin levels in the CSF. Patients treated with cancer immunotherapy are also at an increased risk of developing hypophysitis, which is less frequent than primary hypothyroidism and more difficult to diagnose, presenting mainly with fatigue, hormonal disturbances, and headache.20
Diagnostic delay could result in inappropriate continuation of ICI therapy and late introduction of immunosuppressants, with obvious repercussions on patients' status. Indeed, the clinical outcome of patients with Ma2 Ab was poor, with most of the patients dying due to the neurologic involvement, and the remainder being left severely disabled. To this matter, we would like to underline that (1) contrary to previous reports,2,8 we demonstrate that ICI-related encephalitis can develop beyond the first 4–8 weeks of treatment and (2) ICI withdrawal and administration of corticosteroids, which is the recommended course of treatment in this situation,19 is not sufficient for all patients; (3) the adoption of second-line immunosuppressants is probably warranted for refractory cases.22 As such, the use of drugs—such as natalizumab—that can act on brain inflammatory processes without hampering the immune reaction against systemic localizations of cancer, was recently suggested.23
The pathogenesis of neurologic irAEs due to ICI use remains to be elucidated, although several lines of evidence suggest that (1) ICIs act by blocking the signaling from certain molecules—CTLA-4, PD-1, and its ligands—that exert inhibitory regulatory effects on T-cell activation, thus promoting antitumor immunity1,3; (2) the antitumor immune response might in turn cross-react with CNS autoantigens leading to a PNS, as demonstrated in a preclinical model using CTLA-4 blockade24; and (3) CD8+ T cells, activated by ICIs, were found to play a major effector role in neuronal death in PNS.24 In agreement with this model, it has been previously shown that the pathologic substrate of post-ICI encephalitis is characterized by prominent CD8+ lymphocytic perivascular infiltration.25
Two previous cases of ICI-induced anti-Ma2 encephalitis have been described. One concerned a patient with pleural mesothelioma treated with the anti-CTLA-4 Ab tremelimumab,26 and the second was in a patient with kidney cancer treated with nivolumab.25 Including the present series, this brings the total of cases reported in the literature up to 8. The reason for the increase in susceptibility to anti-Ma2 autoimmune response among all other Ab-associated PNS remains unclear. We propose that it reflects the fact that non–small-cell lung cancer is one of the cancers in which ICIs are most extensively used,1 and this tumor is known to associate with Ma2-PNS.9,10,17 We therefore hypothesize that an analogous increment of anti-Yo and anti-Ri syndrome will be seen after the adoption of ICIs in breast cancer.24,27 The same will probably occur for anti-Hu or anti-CV2/CRMP5 PNS if their use is extended to small-cell lung cancer.28,29
The retrospective detection of Ma2 Ab in the serum of one of our patients taken before ICI administration is an intriguing finding that deserves further discussion. First, it should be considered that at the time, the sample was taken and stored in a biobank, and the patient was asymptomatic. Neurologic symptoms appeared only 5 months later, following treatment with the combination of ipilimumab-nivolumab. Second, the finding of a confirmed Ma2 positivity in a patient without neurologic syndrome is exceedingly rare,15 whereas other onconeural Abs, such as anti-Hu and anti-CV2/CRMP5, are detected in 16% and 9% of neurologically asymptomatic patients with SCLC, respectively.15 These Abs are known to be reliable biomarkers of an underlying cancer but are not pathogenic because a T cell–mediated response is advocated as the cause of the neurologic syndrome.15,30 We therefore hypothesize, similarly to what we have observed in patients with ovary cancer with Yo-Abs and paraneoplastic cerebellar ataxia,31 that the tumor (a pleural mesothelioma in this case) expressed aberrantly the Ma2 antigens and triggered the systemic Ab production. This event per se was not sufficient to elicit a PNS, but required a loss of self-tolerance as permitted by the use of ICIs. This hypothesis needs to be verified in prospective studies assessing the presence and titer of onconeural Abs over time, their relation to immunotherapy, and the development of an overt neurologic syndrome. Our practical recommendation is to test patients undergoing ICI treatment for onconeural Abs before initiation of immunotherapy, and to closely follow those with an Ab positivity, with special caution for Ma2-positive cases. Patients with preexisting Abs are probably at an increased risk of developing irAEs, as demonstrated for anti-acetylcholine receptor autoantibodies and subsequent myositis in patients treated with avelumab.32
The present study is limited by its retrospective nature, small sample size, and, possibly, referral bias toward more complex and/or treatment-refractory cases. Nevertheless, it represents the 2-year experience of a national reference center focused on the diagnosis and treatment of PNS.
Discussion
We showed a clear association between ICI use in France and an increased frequency of anti-Ma2-PNS. Although final arguments proving a causal relationship between ICI and PNS development are lacking, there are several findings suggesting that this syndrome is related to ICI. Middle-aged/elderly men with lung cancer appeared to be at particular risk of developing post-ICI anti-Ma2-PNS. Given the anticipated rise in the use of immunotherapy for oncologic practices, we highlight the importance of early detection of these immune-mediated neurotoxic effects, which can be severe or even fatal.
Acknowledgment
The authors thank NeuroBioTec Hospices Civils de Lyon BRC (France, AC-2013-1867, NFS96-900) for banking sera and CSF samples. They also thank Dr. Pauline De L'Estang Du Rusquec, Dr. Youcef Douadi, Dr. Aurore Jourdain, Dr. Justin Le Tallec, and Dr Jerome Meunier who provided additional clinical data for the study. They gratefully acknowledge Véréna Landel, PhD, for English language editing (Direction de la Recherche Clinique, Hospices Civils de Lyon).
Glossary
- CBA
cell-based assay
- CTLA-4
cytotoxic T lymphocyte–associated antigen 4
- ICI
immune checkpoint inhibitor
- irAE
immune-related adverse event
- Ma2-PNS
Ma2 antibody–associated paraneoplastic neurologic syndrome
- mRS
modified Ranking Scale
- NMDAR
NMDA receptor
- PD-1
programmed death-1 receptor
Appendix. Authors

Study funding
This study is supported by research grants from Agence Nationale de la Recherche (ANR-14-CE15-0001-MECANO), Fondation pour la recherche médicale (DQ20170336751).
Disclosure
The authors have no conflicts of interest to disclose. Go to Neurology.org/NN for full disclosures.
References
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalakas MC. Neurological complications of immune checkpoint inhibitors: what happens when you ‘take the brakes off’ the immune system. Ther Adv Neurol Disord 2018;11:1756286418799864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yshii LM, Hohlfeld R, Liblau RS. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives. Nat Rev Neurol 2017;13:755–763. [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, Chmielowski B, Lao CD, et al. Neurologic serious adverse events associated with nivolumab plus ipilimumab or nivolumab alone in advanced melanoma, including a case series of encephalitis. Oncologist 2017;22:709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kao JC, Liao B, Markovic SN, et al. Neurological complications associated with anti–programmed death 1 (PD-1) antibodies. JAMA Neurol 2017;74:1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Touat M, Talmasov D, Ricard D, Psimaras D. Neurological toxicities associated with immune-checkpoint inhibitors. Curr Opin Neurol 2017;30:659–668. [DOI] [PubMed] [Google Scholar]
- 7.Graus F, Dalmau J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat Rev Clin Oncol Epub 2019 Mar 12. [DOI] [PubMed]
- 8.Williams TJ, Benavides DR, Patrice KA, et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol 2016;73:928. [DOI] [PubMed] [Google Scholar]
- 9.Dalmau J. Clinical analysis of anti-Ma2-associated encephalitis. Brain 2004;127:1831–1844. [DOI] [PubMed] [Google Scholar]
- 10.Vogrig A, Joubert B, Maureille A, et al. Motor neuron involvement in anti-Ma2-associated paraneoplastic neurological syndrome. J Neurol 2019;266:398–410. [DOI] [PubMed] [Google Scholar]
- 11.Dauvilliers Y, Bauer J, Rigau V, et al. Hypothalamic immunopathology in anti-Ma-associated diencephalitis with narcolepsy-cataplexy. JAMA Neurol 2013;70:1305–1310. [DOI] [PubMed] [Google Scholar]
- 12.Adams C, McKeon A, Silber MH, Kumar R. Narcolepsy, REM sleep behavior disorder, and supranuclear gaze palsy associated with Ma1 and Ma2 antibodies and tonsillar carcinoma. Arch Neurol 2011;68:521–524. [DOI] [PubMed] [Google Scholar]
- 13.Waters P, Pettingill P, Lang B. Detection methods for neural autoantibodies. Handb Clin Neurol 2016;133:147–163. [DOI] [PubMed] [Google Scholar]
- 14.Rosenfeld MR, Eichen JG, Wade DF, Posner JB, Dalmau J. Molecular and clinical diversity in paraneoplastic immunity to Ma proteins. Ann Neurol 2001;50:339–348. [PubMed] [Google Scholar]
- 15.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004;75:1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voltz R, Gultekin SH, Rosenfeld MR, et al. A serologic marker of paraneoplastic limbic and brain-stem encephalitis in patients with testicular cancer. N Engl J Med 1999;340:1788–1795. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann LA, Jarius S, Pellkofer HL, et al. Anti-Ma and anti-Ta associated paraneoplastic neurological syndromes: 22 newly diagnosed patients and review of previous cases. J Neurol Neurosurg Psychiatry 2008;79:767–773. [DOI] [PubMed] [Google Scholar]
- 18.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2017;28:iv119–iv142. [DOI] [PubMed] [Google Scholar]
- 20.Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 2018;4:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldman AZ, Shrestha RT, Hennessey JV. Neuropsychiatric manifestations of thyroid disease. Endocrinol Metab Clin North Am 2013;42:453–476. [DOI] [PubMed] [Google Scholar]
- 22.Hottinger AF. Neurologic complications of immune checkpoint inhibitors. Curr Opin Neurol 2016;29:806–812. [DOI] [PubMed] [Google Scholar]
- 23.Hottinger AF, de Micheli R, Guido V, Karampera A, Hagmann P, Du Pasquier R. Natalizumab may control immune checkpoint inhibitor–induced limbic encephalitis. Neurol Neuroimmunol Neuroinflamm 2018;5:e439 doi: 10.1212/NXI.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yshii LM, Gebauer CM, Pignolet B, et al. CTLA4 blockade elicits paraneoplastic neurological disease in a mouse model. Brain 2016;139:2923–2934. [DOI] [PubMed] [Google Scholar]
- 25.Kopecký J, Kubeček O, Geryk T, et al. Nivolumab induced encephalopathy in a man with metastatic renal cell cancer: a case report. J Med Case Rep 2018;12:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogrig A, Ferrari S, Tinazzi M, Manganotti P, Vattemi G, Monaco S. Anti-Ma-associated encephalomyeloradiculopathy in a patient with pleural mesothelioma. J Neurol Sci 2015;350:105–106. [DOI] [PubMed] [Google Scholar]
- 27.Santa-Maria CA, Nanda R. Immune checkpoint inhibitor therapy in breast cancer. J Natl Compr Canc Netw 2018;16:1259–1268. [DOI] [PubMed] [Google Scholar]
- 28.Calles A, Aguado G, Sandoval C, Álvarez R. The role of immunotherapy in small cell lung cancer. Clin Transl Oncol Epub 2019 Jan 12. [DOI] [PubMed]
- 29.Graus F, Keime-Guibert F, Reñe R, et al. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain 2001;124:1138–1148. [DOI] [PubMed] [Google Scholar]
- 30.Honnorat J, Antoine JC. Paraneoplastic neurological syndromes. Orphanet J Rare Dis 2007;2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Small M, Treilleux I, Couillault C, et al. Genetic alterations and tumor immune attack in Yo paraneoplastic cerebellar degeneration. Acta Neuropathologica 2018;135:569–579. [DOI] [PubMed] [Google Scholar]
- 32.Mammen AL, Rajan A, Pak K, et al. Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Ann Rheum Dis 2019;78:150–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data reported in this manuscript are available within the article or its supplementary materials. More information regarding the data is available from the corresponding author on reasonable request.