Abstract
Objective
To test whether patients with MS on disease-modifying treatments (DMTs) are at a higher risk of acute or chronic hepatitis E virus (HEV) infections or extrahepatic manifestations, we monitored approximately 1,100 persons with MS (pwMS) during 3 years for HEV infection.
Methods
This is an observational case series study. All pwMS were followed in our MS center between January 2016 and December 2018 with at least annual standardized clinical and laboratory assessments. Patients with unexplained liver enzyme elevations were routinely screened for HEV infection.
Results
Four cases of acute HEV under DMT (fingolimod [n = 3]; dimethyl fumarate [n = 1]) were identified. Two presented with fulminant icteric hepatitis and one with a HEV-associated neurologic manifestation (neuralgic amyotrophy). No chronic HEV courses were observed. DMT was continued after clearing of HEV or normalization of liver function tests in all cases.
Conclusion
HEV infection is an important differential diagnosis of drug-induced liver injury in pwMS under DMT. Our data do not suggest an increased incidence of acute HEV infections or chronification in pwMS. However, epidemiologic studies in immunomodulatory-treated patients are needed to further investigate HEV disease courses and extrahepatic manifestations.
Hepatitis E virus (HEV) is the most common cause of hepatitis worldwide. Whereas HEV genotypes 1 and 2 are usually responsible for water-born outbreaks in developing countries, genotype 3 is endemic in Europe and North America and typically transmitted via the consumption of raw or undercooked pork meat and contaminated blood products. HEV IgG seroprevalence increases with age, and high rates have been reported in developed countries (IgG 20.4% in Switzerland) including hyperendemic regions such as southwest France, suggesting underestimation of the real impact of this disease in high-risk populations.1,2 Most HEV genotype 3 infections (>90%) are asymptomatic or show acute self-limiting courses, but chronic hepatitis and extrahepatic manifestations can occur. Chronic HEV infections, defined as persistence of positive HEV PCR for >6 months, are more frequent in immunocompromised patients. Patients with chronic HEV infection can develop rapid progression to liver cirrhosis.3
To date, more than 10 disease-modifying treatments (DMTs) are available for immunomodulation of MS. The use of highly effective DMTs may be associated with increasing risks for the development of infectious side effects. Whether persons with MS (pwMS) under DMTs are more susceptible for acute or chronic HEV infections or extrahepatic manifestations is unknown. Here, we describe the first case series of HEV infection in immunomodulatory-treated pwMS and discuss potential implications for the clinical management.
Methods
Between January 2016 and December 2018, on average, 1,084 pwMS per year were regularly followed with standardized clinical and laboratory assessments at the MS outpatient center at the University Hospital Basel, Switzerland. Patients with unexplained liver enzyme elevations were routinely screened for HEV infection. We identified 4 pwMS with symptomatic or asymptomatic HEV infection (identified by simultaneously elevated HEV IgG and IgM and/or viremia4) in our regularly followed MS cohort (figure 1).
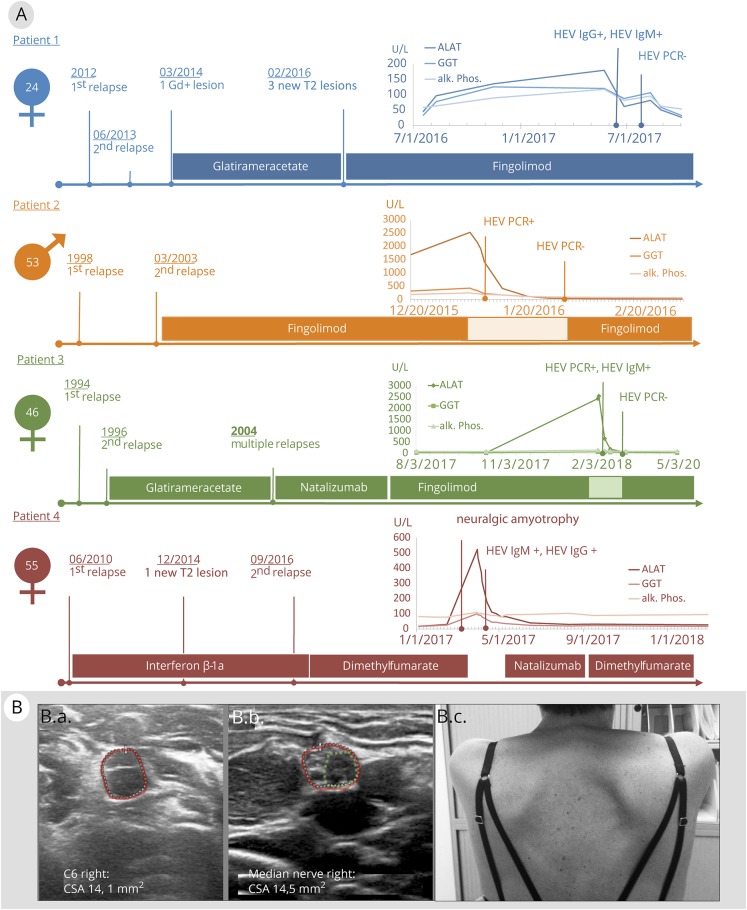
Figure 1. (A) Clinical and laboratory courses of MS and hepatitis E virus (HEV) infection.
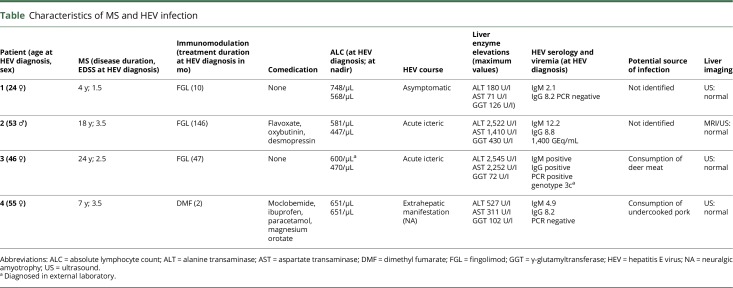
Time course of liver test results, key clinical features, and DMT in 4 persons with MS. Laboratory graphs depict courses of alanine transaminase, gamma-glutamyltransferase, and alkaline phosphatase (all shown in units/L). B, Extrahepatic HEV manifestation with neuralgic amyotrophy of the right shoulder (patient 4). High-resolution nerve ultrasound (Philips Affiniti 50G, linear 5–18 MHz probe) shows significant enlargements of C6 ventral nerve root (14.1 mm2 [B.a] right vs 11.1 mm2 left [not shown]) and proximal median nerve cross-sectional area (14.5 mm2 right [B.b] vs 11.5 mm2 left [not shown]) on the affected right side. Of note, the right median nerve further exhibited an enlarged hypoechoic fascicle (green circle B.b). A winged scapula was observed on clinical examination (B.c). ALAT = alanine transaminase; alk. phos. = alkaline phosphatase; GGT = gamma-glutamyltransferase.
Data availability
All 4 HEV patients signed an informed consent. Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Patient 1
A 21-year-old woman was diagnosed with relapsing-remitting MS (RRMS) and treated with glatiramer acetate (GAA) for 23 months. Ten months after switching to fingolimod (FGL) due to disease activity on MRI, she developed asymptomatic elevation of liver enzymes, and an acute HEV infection was diagnosed by positive serology (see the table for patient details). FGL was not discontinued because the trend of liver enzymes suggested recovery from HEV. The patient remained stable at Expanded Disability Status Scale (EDSS) 1.5.
Table.
Characteristics of MS and HEV infection
Patient 2
A 40-year-old man was diagnosed with RRMS and treated with FGL for 12 years without signs of clinical or MRI disease activity. He then presented with an acute icteric hepatic syndrome and significantly elevated liver enzymes. PCR and positive serology confirmed the diagnosis of an acute HEV infection. FGL was stopped and restarted 4 weeks after HEV PCR became negative. The patient remained stable at EDSS 3.5.
Patient 3
A 25-year-old woman was diagnosed with RRMS and received GAA for 8 years and natalizumab (NTZ) for 7 years. When her John Cunningham virus antibody status became positive, she was switched to FGL. After 4 years of FGL treatment, she developed an acute icteric hepatic syndrome with elevated liver enzymes. Positive PCR and serology confirmed the diagnosis of an acute HEV infection. FGL was discontinued for 6 weeks until HEV PCR was negative. The patient remained stable at EDSS 2.5.
Patient 4
A 48-year-old woman was initially diagnosed with a clinically isolated syndrome and treated with interferon-ß 1a intramuscular for 79 months. MS was diagnosed due to imaging and clinical disease activity, and she switched to dimethyl fumarate (DMF) at EDSS 3.5. After 2 months, she developed a painful shoulder syndrome with a subtle scapula alata on the right side (figure 2, B.c) accompanied by moderate liver enzyme elevations and a short-term drop of 45% in lymphocyte count (from 1,196 to 651 per μL). After detection of an acute HEV infection by positive serology, DMF treatment was suspended. Nerve ultrasound showed significant enlargements of the ventral nerve root C6 (figure 2, B.a) and the proximal median nerve with an enlarged hypoechoic fascicle (figure 2, B.b) on the affected side, suggesting a “forme fruste” of HEV-associated neuralgic amyotrophy (NA).5
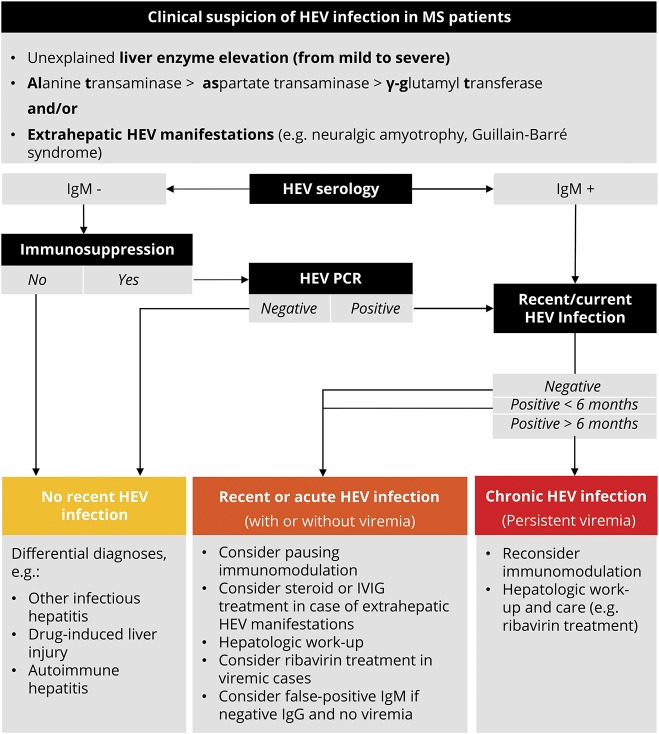
Figure 2. Diagnosis of HEV infection and practical considerations in patients with MS.
Adapted from EASL Clinical Practice Guidelines on hepatitis E virus infection 2018.4 HEV = hepatitis E virus. IgM = anti-HEV immunoglobulin M; IVIG = intravenous immunoglobulins; MS = multiple sclerosis.
After normalization of liver enzymes, the patient was treated temporarily with NTZ and DMF before she stopped both treatments for personal reasons. She recovered well from NA, but developed new MS-associated symptoms with EDSS 4.0 in the subsequent year.
Discussion
Between 2016 and 2018, we identified 4 cases of HEV infections in our regularly followed monocentric cohort with approximately 1,100 pwMS. Although liver enzyme levels in immunosuppressed patients with acute HEV infections were reported to be lower than in immunocompetent patients,3,6 2/4 of our cases (both under FGL) developed fulminant icteric HEV courses with strongly elevated liver enzymes and evidence of viremia. On the other hand, patient 1 had a clinically asymptomatic HEV disease course under continued FGL and only minor liver function test elevations. Liver enzyme elevations in patient 4 were moderate, and HEV infection under DMF became symptomatic with a mild form of HEV-associated NA.5 In 3 cases, we temporarily discontinued and restarted a DMT after normalization of liver enzymes. Because drug-induced liver injury (DILI) is a common feature in a number of DMTs, our case series underlines the relevance of acute HEV infections as important differential diagnosis of DILI in pwMS and unexpected liver enzyme elevations (HEV diagnostic steps and practical considerations, figure 2). HEV infection in all 4 patients was not associated with concomitant signs of clinical or radiologic MS disease activity.
Little is known about the disease course—and eventual chronification—of HEV infections under immunomodulation. Bauer et al.7 described a series of 23 rheumatologic patients under immunosuppression without evidence of chronic HEV. However, immunocompromised solid organ transplant patients (especially under tacrolimus and with low lymphocyte counts) seem to have an elevated risk of persistent viremia and transition to chronic HEV infection.3 In our cases, duration of viremia, present in 2/4 cases, was short, and none developed a chronic HEV disease course.
The mechanism by which certain HEV strains cause extrahepatic and specifically neurologic injury, as NA in our case, remains elusive. Possible pathophysiologic concepts include direct affection of nervous tissue by the virus and immune-mediated damage to neural targets. PwMS under DMT might be more prone to direct neural infection and less susceptible for immune-mediated neurologic injury. In line with this hypothesis, in a recently published case-control study with 200 prospectively followed HEV infections, neurologic symptoms were observed in 22.6% of immunocompetent and only 3.2% of immunocompromised patients (p < 0.001).6
Currently, there are no data on prevalence, incidence, and disease course of HEV infections in pwMS. Although our data do not allow a precise epidemiologic evaluation of HEV in pwMS, in a cautious estimation, we may assume a minimal annual incidence of 0.12% in our patient population. The incidence of HEV infection varies geographically, and reliable data are scarce. Several studies in developed countries estimated annual incidence rates between 0.35% and 1.1% in healthy blood donors.8,9 The annual incidence in immunocompromised solid organ–transplanted patients was estimated to reach up to 3.2%.10 Even if oligo- or asymptomatic HEV infections may have remained undetected, our study does not suggest an increased overall risk for HEV infections in pwMS. With respect to a large proportion of oral treatments in our patient population, we cannot clarify whether certain DMTs or a treatment-associated lymphopenia may influence the susceptibility for symptomatic HEV infections. Systematic, prospective observations in larger MS cohorts may allow estimating an accurate prevalence of HEV-related liver and neurologic damage and their relation to specific DMTs.
Glossary
- DILI
drug-induced liver injury
- DMF
dimethyl fumarate
- FGL
fingolimod
- GAA
glatiramer acetate
- HEV
hepatitis E virus
- NA
neuralgic amyotrophy
- NTZ
natalizumab
- pwMS
persons with MS
- RRMS
relapsing-remitting MS
Appendix. Authors
Study funding
No targeted funding reported.
Disclosure
M. Diebold received grants from the University of Basel and speaker honoraria from Biogen Switzerland for the institution (University Hospital Basel) used exclusively for research purposes. B. Fischer-Barnicol and C. Tsagkas report no disclosures. J. Kuhle received speaker fees, research support, travel support, and/or served on advisory boards by ECTRIMS, Swiss MS Society, Swiss National Research Foundation (320030_160221), University of Basel, Bayer, Biogen, Genzyme, Merck, Novartis, Protagen AG, Roche, and Teva. L. Kappos' institution (University Hospital Basel) received and used exclusively for research support: steering committee, advisory board, and consultancy fees from Actelion, Addex, Bayer HealthCare, Biogen, Biotica, Celgene/Receptos, Genzyme, Lilly, Merck, Mitsubishi, Novartis, Ono Pharma, Pfizer, Sanofi, Santhera, Siemens, Teva, UCB, and Xenoport; speaker fees from Bayer HealthCare, Biogen, Merck, Novartis, Sanofi, and Teva; support of educational activities from Bayer HealthCare, Biogen, CSL Behring, Genzyme, Merck, Novartis, Sanofi, and Teva; and grants from Bayer HealthCare, Biogen, F. Hoffmann-La Roche Ltd, Merck, Novartis, the European Union, the Roche Research Foundations, the Swiss Multiple Sclerosis Society, and the Swiss National Research Foundation. T. Derfuss serves on scientific advisory boards/steering committees/data safety monitoring boards for Novartis Pharmaceuticals, Merck, MedDay, Biogen, Sanofi Genzyme, GeNeuro, Mitsubishi Pharma, Teva Pharmaceuticals, and Bayer Schering Pharma; has received funding for travel and/or speaker honoraria from Biogen, Sanofi Genzyme, Novartis, and Merck; and receives research support from Biogen, Novartis Pharma, the European Union (ABIRISK project under grant agreement no. 115303), the Swiss National Foundation, and the Swiss MS Society. B.F. Décard received travel support and/or fees for the institution (University Hospital Basel) from advisory boards or speaker fees from Almirall, Biogen, Genzyme, Roche, Teva, and Novartis that were used exclusively for research support and receives research support from the Swiss MS Society and the Bangerter-Rhyner Stiftung. Go to Neurology.org/NN for full disclosures.
References
- 1.Mansuy JM, Gallian P, Dimeglio C, et al. . A nationwide survey of hepatitis E viral infection in French blood donors. Hepatology 2016;63:1145–1154. [DOI] [PubMed] [Google Scholar]
- 2.Niederhauser C, Widmer N, Hotz M, et al. . Current hepatitis E virus seroprevalence in Swiss blood donors and apparent decline from 1997 to 2016. Euro Surveill 2018;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamar N, Garrouste C, Haagsma EB, et al. . Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011;140:1481–1489. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol 2018;68:1256–1271. [DOI] [PubMed] [Google Scholar]
- 5.van Eijk JJJ, Dalton HR, Ripellino P, et al. . Clinical phenotype and outcome of hepatitis E virus-associated neuralgic amyotrophy. Neurology 2017;89:909–917. [DOI] [PubMed] [Google Scholar]
- 6.Abravanel F, Pique J, Couturier E, et al. . Acute hepatitis E in French patients and neurological manifestations. J Infect 2018;77:220–226. [DOI] [PubMed] [Google Scholar]
- 7.Bauer H, Luxembourger C, Gottenberg JE, et al. . Outcome of hepatitis E virus infection in patients with inflammatory arthritides treated with immunosuppressants: a French retrospective multicenter study. Medicine (Baltimore) 2015;94:e675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juhl D, Baylis SA, Blümel J, Görg S, Hennig H. Seroprevalence and incidence of hepatitis E virus infection in German blood donors. Transfusion (Paris) 2014;54:49–56. [DOI] [PubMed] [Google Scholar]
- 9.Slot E, Hogema BM, Riezebos-Brilman A, Kok TM, Molier M, Zaaijer HL. Silent hepatitis E virus infection in Dutch blood donors, 2011 to 2012. Euro Surveill 2013;18:20550. [DOI] [PubMed] [Google Scholar]
- 10.Legrand-Abravanel F, Kamar N, Sandres-Saune K, et al. . Hepatitis E virus infection without reactivation in solid-organ transplant recipients, France. Emerg Infect Dis 2011;17:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All 4 HEV patients signed an informed consent. Anonymized data not published within this article will be made available by request from any qualified investigator.