Abstract
Patients with Parkinson's disease (PD) and REM sleep behavior disorder (RBD) show mostly unimpaired motor behavior during REM sleep, which contrasts strongly to coexistent nocturnal bradykinesia. The reason for this sudden amelioration of motor control in REM sleep is unknown, however. We set out to determine whether movements during REM sleep are processed by different motor networks than movements in the waking state. We recorded local field potentials in the subthalamic nucleus (STN) and scalp EEG (modified 10/20 montage) during sleep in humans with PD and RBD. Time-locked event-related β band oscillations were calculated during movements in REM sleep compared with movements in the waking state and during NREM sleep. Spectral analysis of STN local field potentials revealed elevated β power during REM sleep compared with NREM sleep and β power in REM sleep reached levels similar as in the waking state. Event-related analysis showed time-locked β desynchronization during WAKE movements. In contrast, we found significantly elevated β activity before and during movements in REM sleep and NREM sleep. Corticosubthalamic coherence was reduced during REM and NREM movements. We conclude that sleep-related movements are not processed by the same corticobasal ganglia network as movements in the waking state. Therefore, the well-known seemingly normal motor performance during RBD in PD patients might be generated by activating alternative motor networks for movement initiation. These findings support the hypothesis that pathological movement-inhibiting basal ganglia networks in PD patients are bypassed during sleep.
SIGNIFICANCE STATEMENT This study provides evidence that nocturnal movements during REM sleep in Parkinson's disease (PD) patients are not processed by the same corticobasal ganglia network as movements in the waking state. This implicates the existence of an alternative motor network that does not depend directly on the availability of l-Dopa in the basal ganglia. These findings further indicate that some PD patients are able to perform movements in the dopamine depleted state, possibly by bypassing the pathological basal ganglia network. The existence and direct activation of such alternative motor networks might finally have potential therapeutic effects for PD patients.
Keywords: β oscillations, Parkinson's disease, REM sleep behavior disorder, sleep, subthalamic nucleus
Introduction
Slowness of movement (bradykinesia) is the fundamental and most characteristic deficit in patients with Parkinson's disease (PD) (Marsden, 1989). Motor impairment in PD is linked to a complex dysfunction of the basal ganglia network, predominantly caused by progressive loss of nigrostriatal neurons (Obeso et al., 2000; Del Tredici et al., 2002). The subthalamic nucleus (STN) has been identified as a key structure for movement control, and many studies have linked hypersynchronous neuronal activity in the low β band (12–20 Hz) of subthalamic neurons with motor impairment (Brown et al., 2001; Quiroga-Varela et al., 2013). Within this framework, STN deep brain stimulation (DBS) is thought to counteract the pathologically elevated β activity, leading to significant motor improvement (Kumar et al., 2002; Kühn et al., 2008; Benabid et al., 2009). However, in addition to dopaminergic or neuromodulative interventions, also the external context of motor initiation modulates motor control. For example, PD patients with freezing of gait are typically able to switch from severe immobility to almost normal gait by use of external cues (Thaut et al., 1996; Burleigh-Jacobs et al., 1997; Nieuwboer et al., 2007). Similarly, strong emotions might lead to complete restoration of motor control, the extreme example being the anecdotal report of a PD patient with severe bradykinesia, who was able to escape rapidly from a house in a fire (Souques, 1921). Similarly, some PD patients show unimpaired motor control during REM sleep, a phenomenon known as REM sleep behavior disorder (RBD). These patients often perform surprisingly fast movements that are usually in relation to dream content (enacted dreams) (Schenck et al., 1986; De Cock et al., 2007). RBD episodes typically show strong emotional or violent characteristics (Comella et al., 1998); but also nonviolent behaviors, such as laughing or singing, have been reported (Oudiette et al., 2009; Siclari et al., 2011). Intriguingly, the seemingly unimpaired motor control in RBD patients stands in strong contrast to severe nocturnal bradykinesia due to reduced dopaminergic treatment during the night (De Cock et al., 2007).
However, little is known about the source of increased locomotor drive during REM sleep in RBD patients. Based on the observation that RBD movements include complex learned behavior, show predominance to the upper limbs (with larger cortical representations), and consist typically of sudden jerky (“unfiltered”) movements, De Cock et al. (2007) proposed that basal ganglia networks are bypassed during REM sleep. This hypothesis was further supported by the finding that parkinsonism also disappeared during REM sleep in patients with multiple system atrophy who were not sensitive to l-Dopa (De Cock et al., 2011). In this line, a recent ictal-SPECT study revealed activation of premotor areas, but no involvement of the basal ganglia during RBD (Mayer et al., 2015). Together, these findings strongly support the hypothesis of basal ganglia being bypassed during RBD (Arnulf, 2012).
Electrophysiological studies in rodents (Urbain et al., 2000) and PD patients (Urrestarazu et al., 2009) revealed increased firing rate in the STN during REM sleep movements, in contrast to the otherwise observed decreased activity of STN neurons in self-initiated waking movements (Cassidy et al., 2002; Priori et al., 2002; Kühn et al., 2004). Furthermore, these studies suggested a fluctuating pattern of neuronal activity in the STN during REM sleep. However, the temporal evolution of β oscillations with respect to REM sleep movements is unknown.
In this study, we set out to determine whether different motor networks are active during movements in RBD compared with motor control in the waking state and during NREM sleep. To test this hypothesis, we recorded local field potentials (LFPs) in the STN in patients with RBD and analyzed event-related potentials upon movement initiation in REM sleep and in the waking state. Specifically, we asked whether the well-known time-locked modulatory effect of β oscillatory activity in the STN is also observed during REM sleep movements.
Materials and Methods
Patient selection and surgery.
In the time period between January 1, 2013 and June 30, 2013, 13 PD patients were scheduled for implantation of deep brain electrodes in the STN in our clinic. Among those patients, we identified four patients (1 female and 3 males) with clinically manifest RBD. Based on clinical indication, bilateral DBS electrodes (model 3389, Medtronic) were implanted after MR-based direct targeting of the STN. Optimal electrode position was verified by microelectrode recordings, intraoperative test stimulation, and postoperative CT scan. For later analysis of LFPs in the STN, DBS wires were temporarily externalized before implantation of the impulse generator. The study protocol was approved by the local ethics review board (Kantonale Ethikkommission Zurich, KEK-ZH 2012–0327). All patients gave written informed consent for study participation.
Sleep recording and spectral analysis.
Sleep analysis was performed in the second postoperative night (12 h recording: 8 P.M. to 8 A.M.), control tasks during the day before sleep recordings. All analyses were performed in l-Dopa OFF and stimulation OFF condition. We recorded scalp EEG from a 12-channel subset of the 10–20 system (Fp1/Fp2, F3/F4, C3/C4, O1/O2, Fpz/Fz/Cz/Oz), 2-channel electro-oculography (EOG), chin surface electromyography (EMG), and digital infrared video-monitoring. Simultaneously, we acquired bilateral LFPs from the STN (Xltek Mobee 32 EEG Unit, Natus Medical). The sampling rate for EEG, EOG, EMG, and LFP was 200 Hz.
Sleep stage scoring was performed visually on 30 s epochs according to revised standard criteria (Kales and Rechtschaffen, 1968; Iber et al., 2007). Scoring of REM sleep was based primarily on REM sleep-specific EEG and EOG patterns because REM-sleep atonia can be absent in patients with RBD.
For postprocessing of the subthalamic LFP, the raw signal was first rereferenced to a bipolar montage between subsequent electrode contacts on both sides (0–1; 1–2; 2–3, with 0 being the lowest and 3 the most cranial electrode contact). According to postoperative reconstruction of the electrode placement, contact 1 or contact 2 was found to be located in the dorsolateral STN in all patients. Therefore, all further analysis was pursued using the inner bipolar derivation (contacts 1 − contact 2) on both sides. For spectral analysis of the LFP signal, each 30 s epoch was subdivided in epochs of 5 s length. Artifacts were rejected by a semiautomated algorithm based on spectral power in the γ band (35–50 Hz). Then, we applied a fast Fourier spectral analysis on artifact-free 5 s epochs after multiplication with a Hanning window to address edge discontinuities (e.g., Brockwell and Davis, 2013). Finally, we collapsed and averaged all data according to sleep behavioral state. For comparison between individuals, all spectra were normalized to the total power (1–100 Hz).
REM sleep movements and control tasks.
Two experienced sleep specialists (M.H. and E.W.) reviewed all nocturnal video-EEG recordings. Movements in REM sleep were defined as visually observable movements with simultaneously elevated EMG signal. Based on movement onset, we identified 20 s fragments of EEG and LFP (10 s before and 10 s after movement onset) for further analysis of event-related potentials.
As control tasks, all patients performed several self-initiated movements during wakefulness (repetitive self-paced shaking and pressing movements) measured by an inertial measurement unit as described previously (Imbach et al., 2015). As a second control experiment, we identified movements during NREM sleep. For both control conditions, we collected data fragments 10 s prior and 10 s after movement in the same way as for the REM sleep movements. Fragments with obvious movement artifacts were excluded after visual inspection of the raw data.
Analysis of event-related potentials.
All selected data fragments were first bandpass filtered in a wide β range (13–35 Hz). Next, we calculated the Stockwell transform of each data fragment to obtain a high-resolution, time-frequency decomposition of the raw signal and averaged the time-frequency spectra among all movements (Stockwell et al., 1996). The temporal variation of β power before and after movement onset was then estimated by summarizing the total power in the β band (13–35 Hz) at each time point. Finally, we determined temporal synchronicity between the STN-LFP and the ipsilateral central EEG signal (C3/C4 electrode) by means of the phase locking value (Cohen, 2014). This approach was chosen for an accurate time-dependent analysis of synchronicity before and after movement onset. For this analysis, the instantaneous phase was estimated by first calculating the Hilbert transform of the raw signal. The phase locking value was then determined for each time point by summarizing phase differences in the complex plane between STN LFP and the ipsilateral central EEG signal over all trials (Cohen, 2014).
Statistical analysis.
Data postprocessing, spectral analyses, and calculation of phase locking value were performed with customized scripts written in MATLAB (The MathWorks; www.mathworks.com, RRID: SCR_001622). We calculated two-sided Student's t tests and one-way ANOVA for comparison of two or multiple groups as applicable. Statistical significance was established at p < 0.05.
Results
Sleep-related movements
In total, we identified 113 artifact-free REM sleep movements, 30 standardized self-paced movements during WAKE, and 99 NREM sleep movements for further analyses. REM sleep movements occurred primarily in the second half of the night. The observed predominant movement types during REM sleep were short-lasting unilateral and bilateral sudden jerks of the extremities (arms more than legs) and vocalizations. PD subtype (akinetic-rigid vs tremor dominant) had no influence on frequency and type of RBD movements. However, motor laterality of PD symptoms was linked to the predominant side of RBD movements, with more RBD movements on the predominant PD side. Patients' clinical characteristics and RBD movement types are summarized in Table 1.
Table 1.
Demographic data at the time of sleep EEG
| ID | Gender | Age (yr) | PD subtype | Predominancea | Disease duration (yr) | LED (mg) | Time in REM (min) | No. of REM sleep movements | Predominant motor phenomena |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 60 | Rigid akinetic | Left | 9 | 850 | 113 | 44 | Movement of left arm; jerks of whole body |
| 2 | Male | 63 | Tremor | Right | 12 | 1297 | 24 | 21 | Jerks and movements of both legs |
| 3 | Male | 68 | Rigid akinetic | Right | 11 | 1400 | 81 | 42 | Jerks of head, whole body, and right arm; vocalizations |
| 4 | Male | 72 | Tremor | Left | 11 | 760 | 44 | 6 | Jerks of whole body |
LED, Levodopa equivalent dose.
a PD symptom side predominance.
Increased β oscillatory activity during WAKE and REM sleep
Power spectral density of the subthalamic LFP signal differed between sleep behavioral states. The most prominent difference was observed in the wide β range (13–35 Hz). We found a significant increase of relative power spectral density in the β range during WAKE and REM sleep compared with slow-wave sleep. Total LFP β power did not differ significantly between WAKE and REM. In the α and θ range, we found no significant differences of spectral density between sleep behavioral states (Fig. 1).
Figure 1.
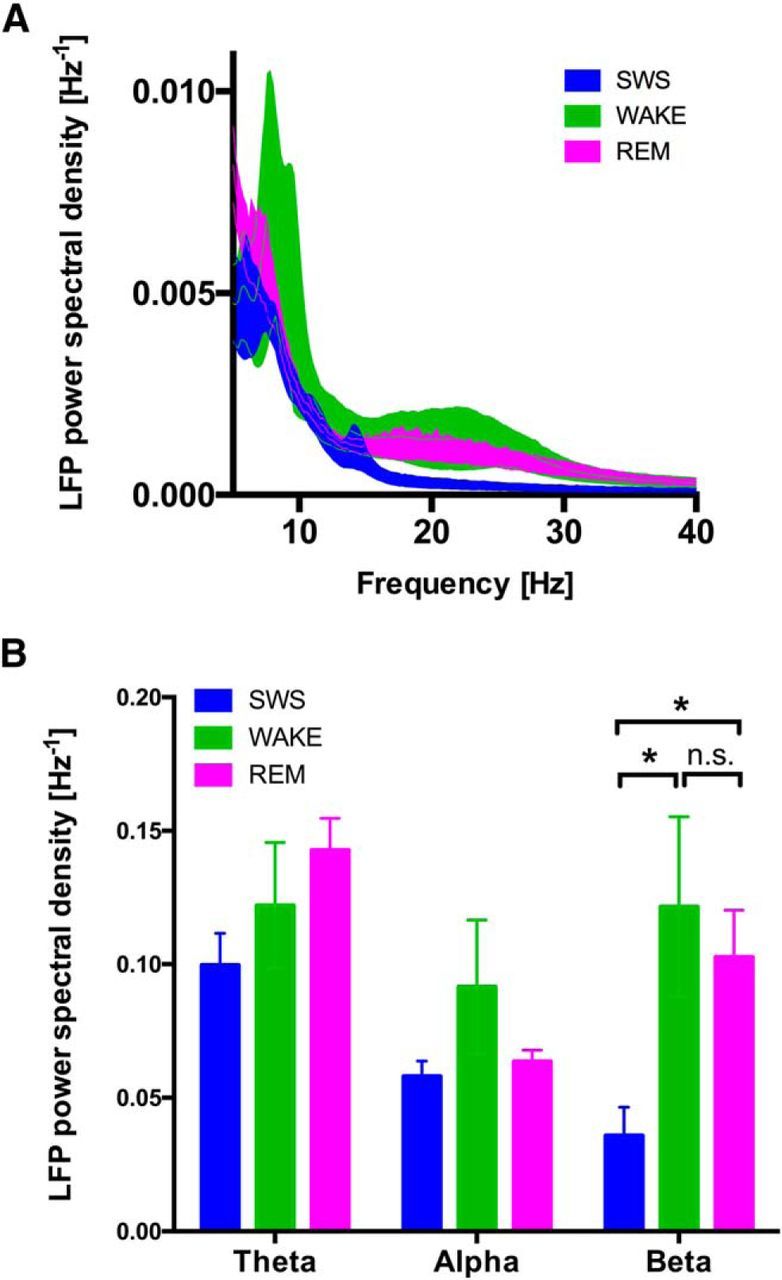
Power spectral density of subthalamic neurons according to behavioral state. A, Power spectral density of LFPs in the STN are shown in a 40 Hz spectrogram. REM sleep (magenta) and WAKE (green) show elevated β power compared with NREM sleep (blue). Ribbon represents SEM. B, Cumulative power in different frequency bands shows selective increase of β power during REM sleep and WAKE. No differences are observed in the α and θ range. Frequency bands: θ, 4–8 Hz; α, 8–13 Hz; β, 13–35 Hz. *p < 0.05.
Paradoxical β synchronization in REM sleep movements
Considering the selectively elevated power spectral density in the β range during REM sleep and WAKE (Fig. 1), all signals were β bandpass filtered (13–35 Hz) for further analysis of event-related potentials. In agreement with previous studies, we found a significant β desynchronization in the STN during movements in the off-medication waking state (Fig. 2A). β power was significantly different from baseline condition 2.5 s after movement onset (Fig. 2B). In contrast, during movements in REM sleep, we observed marked β synchronization with onset before the observed movement initiation. β synchronization reached a significant level 3.3 s before visually observable movements (Fig. 2C,D). During NREM sleep movements, we observed a similar pattern of time-locked β synchronization with significant increase 2.7 s before movement onset (Fig. 2E,F). Pairwise comparison of β power before and after movement onset revealed a significant decrease in β power during WAKE movements (p < 0.05) and even more pronounced increase during REM and NREM sleep movements (p < 0.005; Fig. 2B,D,F).
Figure 2.
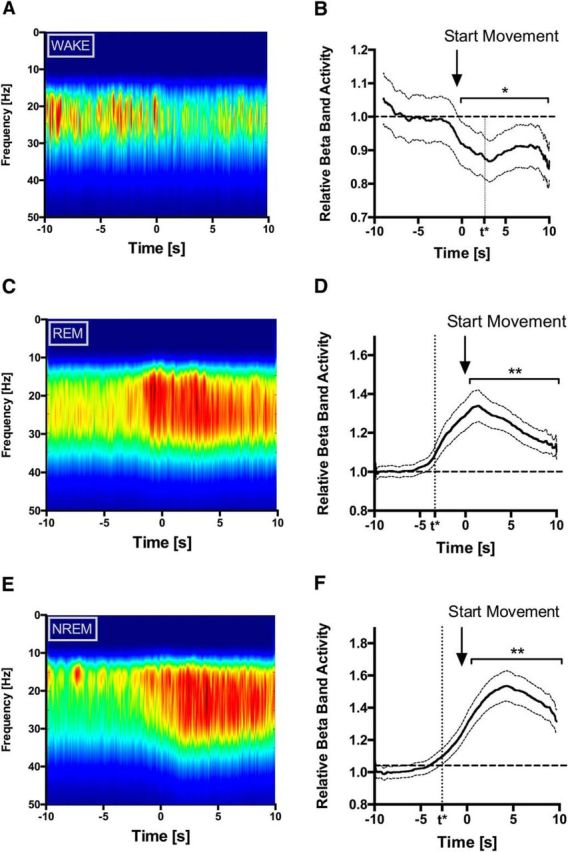
β modulation in the STN during WAKE and REM sleep movements. Time-frequency spectra of β filtered STN LFP in the period 5 s before and after movement initiation show β desynchronization during WAKE movements (A, WAKE) and increased β activity during REM sleep movements (C, REM) and NREM sleep movements (E, NREM). Time-frequency spectra show averaged S-transform values over all patients and all movements: WAKE, n = 30; REM, n = 113; NREM, n = 99. Right panels, Mean β activity as normalized to the 5 s period before movement initiation for WAKE (B), REM sleep (D), and NREM sleep (F). Pairwise comparison of β power before and after movement onset showed significant decrease during WAKE and increase during REM and NREM (horizontal line). B, D, F, *p < 0.05; **p < 0.005. Time points for the first significant difference (defined as a difference >2 × SD) of β power compared with baseline are shown as additional time points on the x-axis (t*).
Reduced corticosubthalamic synchronicity during REM sleep movements
Comparing the phase locking value between the STN and the ipsilateral motor cortex in the perimovement period 5 s before and after movement initiation, we found no relevant modulation of phase locking for WAKE movements (Fig. 3A). In contrast, during REM sleep, corticosubthalamic synchronicity (as measured by phase locking) in the β range was reduced after movement onset, indicating a decoupling of the basal ganglia from cortical neurons. Similarly, pairwise comparison of phase locking before and after movement initiation for all individuals showed significantly decreased synchronicity in the β range during ongoing movements in REM sleep compared with the average phase locking value before the movement. Again, for WAKE movements, we found no significant differences comparing the phase locking value before and after movement initiation (Fig. 3B). Phase locking analysis of NREM sleep movements revealed a higher baseline level of synchronicity during NREM sleep compared with REM sleep and WAKE. During NREM sleep movements, we found reduced corticosubthalamic synchronicity after movement onset, as observed during REM sleep movements (Fig. 3).
Figure 3.
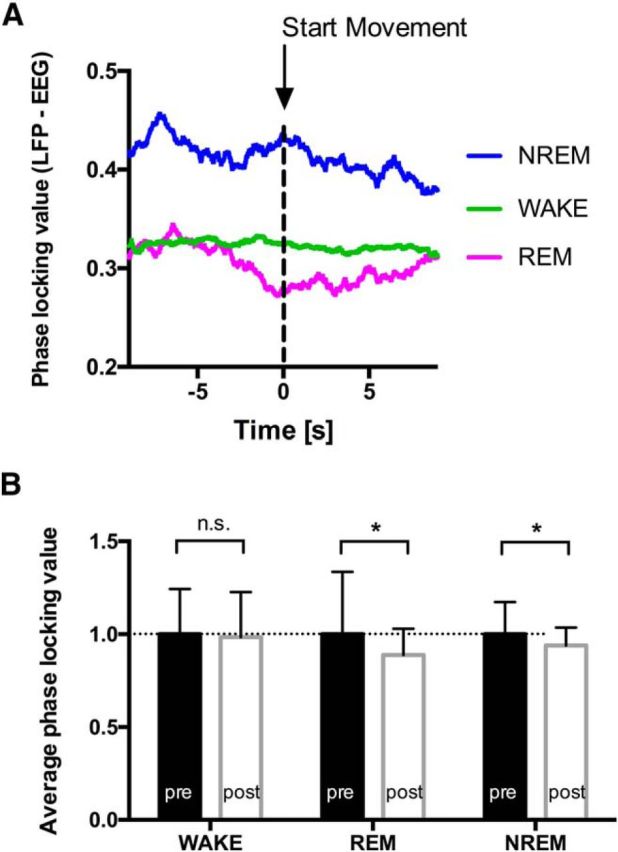
Synchronicity between STN and motor cortex during WAKE and REM sleep movements. A, The temporal evolution of the phase locking value is shown for the period 5 s before and after movement onset. During REM sleep movements, a reduced synchronicity was observed, whereas no modulation of the phase locking was found during WAKE. NREM sleep showed a higher baseline synchronicity with reduction upon movement initiation. B, Pairwise comparison of normalized phase locking values of the period prior (pre) and after (post) movement onset showed a significant difference only during REM and NREM sleep movements: WAKE, n = 30; REM, n = 113; NREM, n = 99. *p < 0.05.
Discussion
Parkinson patients with RBD show relatively unimpaired motor function during REM sleep. However, the mechanism allowing for this temporary normalization of motor control is unknown. Considering the overwhelming data on impaired basal ganglia function in PD patients in the l-Dopa off state, the question arises how rapid motor output is possible despite the motor-inhibiting network state of basal ganglia during sleep. At least two explanations can be discussed for this phenomenon: First, REM sleep could have a direct influence on pathological basal ganglia networking in an analogous way as l-Dopa administration or chronic electrical stimulation of the STN. In this view, the modified global brain state during REM sleep would normalize basal ganglia function, mimicking an instantaneous dopaminergic disinhibition of the basal ganglia, eventually leading to unimpaired motor output. Alternatively, the pathological inhibitory corticobasal ganglia network could be bypassed during REM sleep, as suggested by others previously (De Cock et al., 2011; Arnulf, 2012). In this model, REM sleep movements are not processed by dopamine-depleted basal ganglia, but by an alternative (yet unknown) central motor control network without direct interaction with the basal ganglia. In other words, we set out to determine whether the basal ganglia networks are modulated or merely bypassed during RBD.
Considering the outstanding role and well-described modulatory effects of β oscillations in the STN, the measurement of STN LFPs during REM sleep movements provides a direct possibility to test these hypotheses: β oscillations of subthalamic neurons are known to be desychronized during WAKE movements, imaginary movements, l-Dopa administration, or chronic DBS (Priori et al., 2002; Kühn et al., 2004, 2006; López-Azcárate et al., 2010). Now, if REM sleep movements are also processed by the basal ganglia, one could expect a similar β desynchronization before and during movements in REM sleep. Thus, our problem simplifies to the question: Are β oscillations desychronized during paradoxical REM sleep movements or do we observe a different network activity in the basal ganglia during REM sleep?
This study provides compelling further evidence that REM sleep movements are processed by an alternative network showing different patterns of β modulation compared with WAKE movements. In contrast to the well-known β desynchronization during WAKE movements, we found β power to be significantly enhanced during REM sleep movements. In a simplified model for basal ganglia function in the waking state, β oscillations in the STN can be interpreted as an alternating go/no-go signaling (with β desynchronization corresponding to “go” and β synchronization signifying “no-go”). In this analogy, our findings suggest that the functional state of the basal ganglia translates to a motor inhibitory signal (no-go), exactly during ongoing REM sleep movements. Therefore, the observed paradoxical β synchronization in the STN supports the previous hypothesis that pathological basal ganglia signaling might be bypassed during REM sleep (De Cock et al., 2007).
Our findings are in agreement with earlier human (Urrestarazu et al., 2009) and rodent (Urbain et al., 2000) studies showing intermittent increased β activity in relation to REM sleep movements. However, in addition to these previous studies, the observed event-related β synchronization upon movement initiation in REM sleep provides further evidence for a direct interplay between RBD movements and β synchronization in the STN.
The observed analogous temporal modulation of β activity in NREM and REM sleep may indicate a common alternative pathway for all sleep-related movements (NREM and REM). Therefore, the question arises whether the observed synchronization reflects physiologically altered motor activation during sleep in general. However, in this study, we only examined patients with definitive RBD; therefore, the observed synchronization in NREM sleep movements might still represent a specific effect of altered motor control in RBD patients. Nevertheless, further studies might address these issues (e.g., by performing analogous analyses in a comparative approach in PD patients with and without RBD).
As a limitation of our study, motor behavior during REM sleep was significantly different from waking movements (sudden jerky RBD movements vs smooth repetitive movements in WAKE), and this difference in motor output might directly influence β oscillatory activity in the STN. However, as many previous studies generally showed β desynchronization upon movement initiation in the waking state (Priori et al., 2002; Kühn et al., 2004; López-Azcárate et al., 2010), we consider our finding not to be fully explained by the different characteristic of motor output alone.
Corticobasal ganglia coherence is a measure to quantify the synchronicity of neuronal activity between the STN and the motor cortex. We found that, during REM sleep, cortico-STN coherence was significantly reduced compared with WAKE movements. Again, this finding supports the hypothesis that REM sleep movements are not processed by the “conventional” corticobasal ganglia pathway, but by other (possibly subcortical) networks.
We can only hypothesize upon the origin of the observed time-locked β synchronization during sleep-related movements. The hyperdirect pathway provides direct activating input from cortical areas (e.g., the presupplementary motor area) to the STN, leading eventually to movement inhibition, and can be interpreted as an early inhibitory signal during movement preparation to provide appropriate movement selection through the later to start activating direct pathway (Aron, 2011; Jahanshahi et al., 2015). In this line, we speculate that, during sleep-related movement, the hyperdirect pathway might be activated before the movement to prevent early movement initiation (possibly in a similar way as in the waking state); but due to the proposed basal ganglia bypassing during sleep, this early STN synchronization is not followed by β desynchronization by means of the direct pathway. This model could also explain why β synchronization was observed before visible movement onset during sleep.
Finally, the reason for the hypothesized bypassing of the basal ganglia in PD patients with RBD remains unknown. Considering the strong association of RBD with PD, one might speculate that pathologically reduced modulating activity in the extrapyramidal system results in compensatory overactivity of the direct pyramidal or another pathway that can apparently be unlinked from the basal ganglia during sleep by means of a yet unknown mechanism.
Footnotes
This work was supported by the Clinical Research Priority Program “Sleep and Health” of the University of Zurich and by the HSM-II Initiative of the Canton of Zurich.
The authors declare no competing financial interests.
References
- Arnulf I. REM sleep behavior disorder: motor manifestations and pathophysiology. Mov Disord. 2012;27:677–689. doi: 10.1002/mds.24957. [DOI] [PubMed] [Google Scholar]
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurol. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- Brockwell PJ, Davis RA. Time series: theory and methods. New York: Springer Science and Business Media; 2013. [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J Neurosci. 2001;21:1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson's disease: influence of levodopa and external sensory triggers. Mov Disord. 1997;12:206–215. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]
- Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Di Lazzaro V, Brown P. Movement-related changes in synchronization in the human basal ganglia. Brain. 2002;125:1235–1246. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- Cohen MX. Analyzing neural time series data: theory and practice. Cambridge, MA: Massachusetts Institute of Technology; 2014. [Google Scholar]
- Comella CL, Nardine TM, Diederich NJ, Stebbins GT. Sleep-related violence, injury, and REM sleep behavior disorder in Parkinson's disease. Neurology. 1998;51:526–529. doi: 10.1212/WNL.51.2.526. [DOI] [PubMed] [Google Scholar]
- De Cock VC, Vidailhet M, Leu S, Texeira A, Apartis E, Elbaz A, Roze E, Willer JC, Derenne JP, Agid Y, Arnulf I. Restoration of normal motor control in Parkinson's disease during REM sleep. Brain. 2007;130:450–456. doi: 10.1093/brain/awl363. [DOI] [PubMed] [Google Scholar]
- De Cock VC, Debs R, Oudiette D, Leu S, Radji F, Tiberge M, Yu H, Bayard S, Roze E, Vidailhet M, Dauvilliers Y, Rascol O, Arnulf I. The improvement of movement and speech during rapid eye movement sleep behaviour disorder in multiple system atrophy. Brain. 2011;134:856–862. doi: 10.1093/brain/awq379. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Rüb U, De Vos RA, Bohl JR, Braak H. Where does Parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- Iber C, Chesson A, Quan S, editors. The American Academy of Sleep Medicine manual for the scoring of sleep and associated events: rules, terminology, and technical specification. Darien, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Imbach LL, Baumann-Vogel H, Baumann CR, Sürücü O, Hermsdörfer J, Sarnthein J. Adaptive grip force is modulated by subthalamic beta activity in Parkinson's disease patients. Neuroimage Clin. 2015;9:450–457. doi: 10.1016/j.nicl.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Obeso I, Rothwell JC, Obeso JA. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat Rev Neurosci. 2015;16:719–732. doi: 10.1038/nrn4038. [DOI] [PubMed] [Google Scholar]
- Kales A, Rechtschaffen A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, DC: Department of Health, Education and Welfare; 1968. [Google Scholar]
- Kühn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, Yarrow K, Brown P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127:735–746. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Doyle L, Pogosyan A, Yarrow K, Kupsch A, Schneider GH, Hariz MI, Trottenberg T, Brown P. Modulation of beta oscillations in the subthalamic area during motor imagery in Parkinson's disease. Brain. 2006;129:695–706. doi: 10.1093/brain/awh715. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Kempf F, Brücke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider GH, Hariz MI, Vandenberghe W, Nuttin B, Brown P. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory β activity in patients with Parkinson's disease in parallel with improvement in motor performance. J Neurosci. 2008;28:6165–6173. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Bhatia M, Behari M. Sleep disorders in Parkinson's disease. Mov Disord. 2002;17:775–781. doi: 10.1002/mds.10167. [DOI] [PubMed] [Google Scholar]
- López-Azcárate J, Tainta M, Rodríguez-Oroz MC, Valencia M, González R, Guridi J, Iriarte J, Obeso JA, Artieda J, Alegre M. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson's disease. J Neurosci. 2010;30:6667–6677. doi: 10.1523/JNEUROSCI.5459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD. Slowness of movement in Parkinson's disease. Mov Disord. 1989;4:S26–S37. doi: 10.1002/mds.870040505. [DOI] [PubMed] [Google Scholar]
- Mayer G, Bitterlich M, Kuwert T, Ritt P, Stefan H. Ictal SPECT in patients with rapid eye movement sleep behaviour disorder. Brain. 2015;138:1263–1270. doi: 10.1093/brain/awv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwboer A, Kwakkel G, Rochester L, Jones D, van Wegen E, Willems AM, Chavret F, Hetherington V, Baker K, Lim I. Cueing training in the home improves gait-related mobility in Parkinson's disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78:134–140. doi: 10.1136/jnnp.200X.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Lanciego JL, Artieda J, Gonzalo N, Olanow CW. Pathophysiology of the basal ganglia in Parkinson's disease. Trends Neurosci. 2000;23(Suppl 1):S8–S19. doi: 10.1016/s1471-1931(00)00028-8. [DOI] [PubMed] [Google Scholar]
- Oudiette D, De Cock VC, Lavault S, Leu S, Vidailhet M, Arnulf I. Nonviolent elaborate behaviors may also occur in REM sleep behavior disorder. Neurology. 2009;72:551–557. doi: 10.1212/01.wnl.0000341936.78678.3a. [DOI] [PubMed] [Google Scholar]
- Priori A, Foffani G, Pesenti A, Bianchi A, Chiesa V, Baselli G, Caputo E, Tamma F, Rampini P, Egidi M, Locatelli M, Barbieri S, Scarlato G. Movement-related modulation of neural activity in human basal ganglia and its l-DOPA dependency: recordings from deep brain stimulation electrodes in patients with Parkinson's disease. Neurol Sci. 2002;23(Suppl 2):S101–S102. doi: 10.1007/s100720200089. [DOI] [PubMed] [Google Scholar]
- Quiroga-Varela A, Walters JR, Brazhnik E, Marin C, Obeso JA. What basal ganglia changes underlie the parkinsonian state? The significance of neuronal oscillatory activity. Neurobiol Dis. 2013;58:242–248. doi: 10.1016/j.nbd.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- Siclari F, Wienecke M, Poryazova R, Bassetti CL, Baumann CR. Laughing as a manifestation of rapid eye movement sleep behavior disorder. Parkinsonism Relat Disord. 2011;17:382–385. doi: 10.1016/j.parkreldis.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Souques A. Older description of parkinsonian persons who can run much easier than walk. Revue Neurol (Paris) 37 (1921) 1921;37:559–560. [Google Scholar]
- Stockwell RG, Mansinha L, Lowe RP. Localization of the complex spectrum: the S transform. IEEE Trans Signal Processing. 1996;44:998–1001. doi: 10.1109/78.492555. [DOI] [Google Scholar]
- Thaut MH, McIntosh GC, Rice RR, Miller RA, Rathbun J, Brault JM. Rhythmic auditory stimulation in gait training for Parkinson's disease patients. Mov Disord. 1996;11:193–200. doi: 10.1002/mds.870110213. [DOI] [PubMed] [Google Scholar]
- Urbain N, Gervasoni D, Soulière F, Lobo L, Rentéro N, Windels F, Astier B, Savasta M, Fort P, Renaud B, Luppi PH, Chouvet G. Unrelated course of subthalamic nucleus and globus pallidus neuronal activities across vigilance states in the rat. Eur J Neurosci. 2000;12:3361–3374. doi: 10.1046/j.1460-9568.2000.00199.x. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Iriarte J, Alegre M, Clavero P, Rodríguez-Oroz MC, Guridi J, Obeso JA, Artieda J. Beta activity in the subthalamic nucleus during sleep in patients with Parkinson's disease. Mov Disord. 2009;24:254–260. doi: 10.1002/mds.22351. [DOI] [PubMed] [Google Scholar]


