More than 2000 years ago, Hippocrates reported that a severed nerve neither grows nor unites (Hippocrates and Lloyd, 1978), but ∼100 years ago evidence of CNS neuroregeneration began to see the light of day with the discovery by Tello and Ramón y Cajal (1913) that an injured optic nerve can extend its axon into an adjoining peripheral nerve graft. This work revealed that CNS axons can regenerate if provided with a permissive environment. Nonetheless, more recent studies have confirmed that in attempting to block unbridled inflammation by secluding injured neurons from surrounding tissue (Faulkner et al., 2004), the CNS of higher vertebrates provides an environment that stifles axon growth (Davies et al., 1997). In particular, inhibitory myelin-associated factors contribute to the nonpermissive environment of adult CNS injury (Caroni and Schwab, 1988).
While great strides have been made in promoting axonal growth by neutralizing extrinsic, myelin-associated inhibition (Caroni and Schwab, 1988), these successes have revealed a new hurdle: CNS neurons shut down their intrinsic growth program soon after the animal is born (Goldberg et al., 2002). Although such growth repression enables proper synaptic development (Tedeschi et al., 2016), it comes at a price: CNS neurons resist external growth-promoting strategies. Hence, to achieve full functional recovery, prospective therapies will likely need to overcome the intrinsic resistance to growth of CNS neurons, as well as to suppress the inhibitory nature of the growth environment.
Attempts to overcome the intrinsic inhibition of axonal growth have identified several pathways that may be manipulated to promote regeneration. Vitreal inflammation can trigger retinal ganglion cells (RGCs) to adopt a growth state similar to that in embryonic development (Yin et al., 2003). In addition, PTEN expression, which has been shown to begin in the mouse brain on the day of birth, limits regeneration by inhibiting PI3K-mediated promotion of protein synthesis, which is required for growth (Lachyankar et al., 2000). Levels of cAMP, another molecule that regulates protein expression, also fluctuate in parallel with the developmental switch in CNS regenerative capacity: cAMP levels are high in rat neurons on postnatal day 1, but they decline on postnatal days 3–4. Notably, cAMP also blocks the inhibitory role of CNS myelin in utero, enabling myelin to be a permissive growth substrate during embryonic life. The waning levels of cAMP by postnatal days 3–4 coincide with the onset of inhibitory CNS myelin (Cai et al., 2001). Importantly, combining PTEN deletion with cAMP elevation and vitreal inflammation can boost RGC regeneration ∼10-fold versus single-agent therapy (Kurimoto et al., 2010).
Although attempts to stimulate axonal regeneration have met with increasing success, whether regenerating CNS neurons can reestablish their axon initial segment (AIS) and nodes of Ranvier has been unclear. This is important because the AIS, in addition to being the site of action potential initiation, acts as a selective filter to ensure that somatodendritic and axonal proteins remain in their respective domains, thus maintaining neuronal polarity (Hedstrom et al., 2008). The nodes of Ranvier are important because they ensure efficient action potential transmission with minimal energy taxation. Previous work suggests that whether the AIS is damaged is an important determinant of axonal regeneration: in vitro axotomy within 35 μm of the soma (i.e., in the region of the AIS) led to axonal transformation of a nearby dendrite, while lesions beyond 35 μm induced normal regeneration of the original axon (Gomis-Rüth et al., 2008).
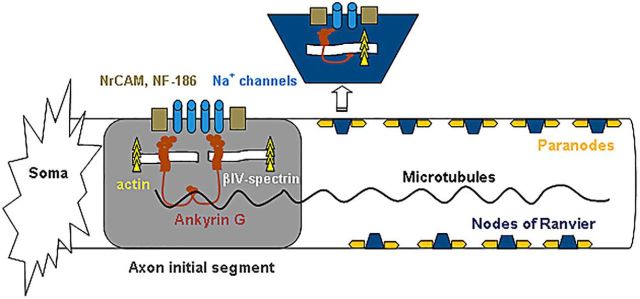
Formation of the AIS and nodes of Ranvier is linked to the establishment of neuronal polarity, because the function of the AIS and nodes depends on an appropriately positioned molecular scaffold for clustering ion channels (Fig. 1). Interestingly, in addition to regulating axon growth, cAMP and the PI3K pathway are involved in neuronal polarity (Barnes and Polleux, 2009; Muñoz-Llancao et al., 2015). Consequently, boosting these signaling pathways may help to reestablish the AIS and nodes of Ranvier as they stimulate the growth of regenerating axons.
Figure 1.
Ankyrin G, the molecular glue of the axon initial segment and nodes of Ranvier. AnkG binds to the sodium channels and cell adhesion molecules (NF-186, NrCAM) in the membrane, and links them to the cytoskeletal actin–βIV-spectrin complex below. Marin et al. (2016) found that knocking out AnkG disrupted nodal structure and the axon initial segment but did not affect regeneration.
In an article published in The Journal of Neuroscience, Marin et al. (2016) studied the triple combination of Pten deletion, cAMP analog administration, and inflammation on AIS and nodal reassembly in mouse RGCs after optic nerve crush, and asked whether the reassembly of these structures is necessary for axonal regeneration. In control adult Pten knock-out mice, optic nerve crush caused a loss of axons, a decrease in nodal density, and disassembly of the AIS that increased between 0.5 and 30 d postlesion (dpl). Degeneration progressed centrifugally from the injury site, with a greater loss in regions close to the injury. This pattern of degeneration is similar to what occurs in acute axonal degeneration (Knöferle et al., 2010). Because calcium influx occurs early in acute axonal degeneration, and because calcium channel inhibitors can double the number of regenerating axons within ∼400 μm of the crush site (Ribas et al., 2017), axonal and nodal degeneration described by Marin et al. (2016) might be attributable to activation of calcium-dependent calpain proteases near the injury site, as a result of calcium influx in that region. Because calpain catalyzes cleavage of α-spectrin (Ribas et al., 2017), a protein found in paranodal regions flanking the nodes, immunostaining for calpain-mediated cleavage products of α-spectrin to determine whether fluorescence intensity is greatest in regions flanking the injury site could shed light on the mechanism of axonal disintegration in this region.
In mice lacking Pten and treated intravitreally with a cAMP analog and zymosan (which induces inflammation), regeneration was detected via neurofilament-M immunostaining within 14 dpl. Regeneration was absent in saline-injected controls with Pten knockout. A possible confound for these experiments was the presence of degenerating axons, which retain neurofilament-M staining for 2 weeks after injury. To discriminate between degenerating and regenerating axons, and thus to enable accurate quantification of regenerating axons, it might be helpful to inject a cell-impermeant fluorescent nucleic acid dye to identify dying axons (Tsuda et al., 2016). Nevertheless, a difference between treated and control nerves was evident after 6 and 12 weeks (Marin et al., 2016, their Fig. 4). It is worth noting that the optic nerve was injured at some distance from the soma; hence, it is unclear whether injuring the axon in the vicinity of the AIS, as was done by Gomis-Rüth et al. (2008), will affect regeneration differently.
To investigate the ability of treated RGCs to reform paranodes (which implies remyelination) and nodes, Marin et al. (2016) used Caspr and βIV-spectrin immunostaining, respectively. As an essential cell adhesion molecule of paranodes, Caspr forms part of the axoglial junction with the myelin sheath. Unlike axon regeneration, which was seen within 14 dpl, remyelination and nodal reassembly were lacking after 2 weeks. With time, however, remyelination and nodal reassembly were clearly detected. The pattern of remyelination and nodal reassembly appeared to progress proximodistally because nodes and remyelination were detected after 6 weeks both at the injury site and proximal to the site, while neither was found distal to the injury after 6 weeks. After 12 weeks, however, both nodes and paranodes were detected distally, albeit at a lower frequency than in more proximal regions. Because neurons undergo axon–dendrite specification during migration in early development (Zolessi et al., 2006; Barnes and Polleux, 2009), and this migration responds to axon guidance cues, such as Netrins, Slits, and Ephrins, which have been found to constitute gradients along the migratory path (Kennedy et al., 2006), these guidance molecules might play a role in the proximodistal pattern of nodal reassembly and remyelination found by Marin et al. (2016, their Figs. 5, 6).
To address whether neurons require neuronal polarity for regeneration, Marin et al. (2016) used a conditional knockout of Ank3 [the Ankyrin G (AnkG) gene] combined with treatment with Curdlan, a strong inducer of inflammation (to avoid the need to simultaneously delete Pten) to test whether Ank3 deletion affects RGC regeneration. AnkG is the “molecular glue” that holds together the scaffold of the AIS and nodes (Fig. 1). AnkG in the AIS is essential for maintaining neuronal polarity, as indicated by the fact that AnkG loss leads axons to assume dendritic features (Hedstrom et al., 2008). Marin et al. (2016) found that regeneration, measured by growth-associated protein 43 immunostaining, was similar in treated mice with or without Ank3 deletion 2 weeks after injury, although AnkG was missing in the nodes of treated knock-out mice. This is in contrast to the treated Pten knock-out mouse, where AIS reassembly (and evident regeneration) was detected (using βIV-spectrin immunostaining of the reassembled AIS) after 6 and 12 weeks. Thus, despite AnkG loss in the AIS, treated axons maintained regenerative ability. An important caveat, however, comes from a recent article (Ho et al., 2014) showing that if AnkG is lost in adult RGCs, ion channel clustering and nodal structure could still be salvaged due to the presence of a preexisting cellular pool of AnkR. AnkR binds to ion channels of the nodes at a lower affinity than AnkG yet, in the absence of AnkG, can replace its “glue” function. Thus, testing whether axonal regeneration in treated mice occurs in the absence of both AnkG and AnkR would be valuable in dissecting any compensatory role played by AnkR.
In summary, Marin et al. (2016) provided answers to two critical queries pertaining to CNS regeneration. By demonstrating that regenerating neurons, under controlled treatment, reassemble their excitable domains, which are indispensable to action potential generation and propagation, and that regeneration is independent of the preservation of AnkG-dependent neuronal polarity, they have brought the neural regeneration community two steps closer to the realization of functional recovery in the CNS after devastating injuries.
Footnotes
Editor's Note: These short reviews of recent JNeurosci articles, written exclusively by students or postdoctoral fellows, summarize the important findings of the paper and provide additional insight and commentary. If the authors of the highlighted article have written a response to the Journal Club, the response can be found by viewing the Journal Club at www.jneurosci.org. For more information on the format, review process, and purpose of Journal Club articles, please see http://jneurosci.org/content/preparing-manuscript#journalclub.
The author declares no competing financial interests.
References
- Barnes AP, Polleux F (2009) Establishment of axon-dendrite polarity in developing neurons. Annu Rev Neurosci 32:347–381. 10.1146/annurev.neuro.31.060407.125536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT (2001) Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci 21:4731–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P, Schwab ME (1988) Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron 1:85–96. 10.1016/0896-6273(88)90212-7 [DOI] [PubMed] [Google Scholar]
- Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J (1997) Regeneration of adult axons in white matter tracts of the central nervous system. Nature 390:680–683. [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24:2143–2155. 10.1523/JNEUROSCI.3547-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JL, Klassen MP, Hua Y, Barres BA (2002) Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science 296:1860–1864. 10.1126/science.1068428 [DOI] [PubMed] [Google Scholar]
- Gomis-Rüth S, Wierenga CJ, Bradke F (2008) Plasticity of polarization: changing dendrites into axons in neurons integrated in neuronal circuits. Curr Biol 18:992–1000. 10.1016/j.cub.2008.06.026 [DOI] [PubMed] [Google Scholar]
- Hedstrom KL, Ogawa Y, Rasband MN (2008) AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol 183:635–640. 10.1083/jcb.200806112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippocrates, Lloyd GER (1978) Hippocratic writings (Chadwick J, Mann WN, translators). Harmondsworth, UK: Penguin Books. [Google Scholar]
- Ho TS, Zollinger DR, Chang KJ, Xu M, Cooper EC, Stankewich MC, Bennett V, Rasband MN (2014) A hierarchy of ankyrin-spectrin complexes clusters sodium channels at nodes of Ranvier. Nat Neurosci 17:1664–1672. 10.1038/nn.3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy TE, Wang H, Marshall W, Tessier-Lavigne M (2006) Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord. J Neurosci 26:8866–8874. 10.1523/JNEUROSCI.5191-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöferle J, Koch JC, Ostendorf T, Michel U, Planchamp V, Vutova P, Tönges L, Stadelmann C, Brück W, Bähr M, Lingor P (2010) Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci U S A 107:6064–6069. 10.1073/pnas.0909794107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto T, Yin Y, Omura K, Gilbert HY, Kim D, Cen LP, Moko L, Kügler S, Benowitz LI (2010) Long-distance axon regeneration in the mature optic nerve: contributions of oncomodulin, cAMP, and Pten gene deletion. J Neurosci 30:15654–15663. 10.1523/JNEUROSCI.4340-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachyankar MB, Sultana N, Schonhoff CM, Mitra P, Poluha W, Lambert S, Quesenberry PJ, Litofsky NS, Recht LD, Nabi R, Miller SJ, Ohta S, Neel BG, Ross AH (2000) A role for nuclear PTEN in neuronal differentiation. J Neurosci 20:1404–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin MA, de Lima S, Gilbert HY, Giger RJ, Benowitz L, Rasband MN (2016) Reassembly of excitable domains after CNS axon regeneration. J Neurosci 36:9148–9160. 10.1523/JNEUROSCI.1747-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Llancao P, Henríquez DR, Wilson C, Bodaleo F, Boddeke EW, Lezoualc'h F, Schmidt M, González-Billault C (2015) Exchange protein directly activated by cAMP (EPAC) regulates neuronal polarization through Rap1B. J Neurosci 35:11315–11329. 10.1523/JNEUROSCI.3645-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. (1913–1914) Estudios sobre la degeneracion y regeneracion del sistema nervioso. Madrid: Moya. English translation: May RM (trans. and ed) (1928) Degeneration and regeneration of the nervous system. London: Oxford UP. [Google Scholar]
- Ribas VT, Koch JC, Michel U, Bähr M, Lingor P (2017) Attenuation of axonal degeneration by calcium channel inhibitors improves retinal ganglion cell survival and regeneration after optic nerve crush. Mol Neurobiol 54:72–86. 10.1007/s12035-015-9676-2 [DOI] [PubMed] [Google Scholar]
- Tedeschi A, Dupraz S, Laskowski CJ, Xue J, Ulas T, Beyer M, Schultze JL, Bradke F (2016) The calcium channel subunit alpha2delta2 suppresses axon regeneration in the adult CNS. Neuron 92:419–434. 10.1016/j.neuron.2016.09.026 [DOI] [PubMed] [Google Scholar]
- Tsuda S, Tanaka Y, Kunikata H, Yokoyama Y, Yasuda M, Ito A, Nakazawa T (2016) Real-time imaging of RGC death with a cell-impermeable nucleic acid dyeing compound after optic nerve crush in a murine model. Exp Eye Res 146:179–188. 10.1016/j.exer.2016.03.017 [DOI] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, Benowitz LI (2003) Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci 23:2284–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolessi FR, Poggi L, Wilkinson CJ, Chien CB, Harris WA (2006) Polarization and orientation of retinal ganglion cells in vivo. Neural Dev 1:2. 10.1186/1749-8104-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]