Abstract
Objective
To assess the effectiveness of behavioral parent-only (PO) and family-based (FB) interventions on child weight, dietary intake, glycated hemoglobin, and quality of life in rural settings.
Methods
This study was a three-armed, randomized controlled trial. Participants were children (age 8–12 years) with overweight or obesity and their parents. A FB (n = 88), a PO (n = 78) and a health education condition (HEC) (n = 83) each included 20 group contacts over 1 year. Assessment and treatment contacts occurred at Cooperative Extension Service offices. The main outcome was change in child body mass index z-score (BMIz) from baseline to year 2.
Results
Parents in all conditions reported high treatment satisfaction (mean of 3.5 or higher on a 4-point scale). A linear mixed model analysis of change in child BMIz from baseline to year 1 and year 2 found that there were no significant group by time differences in child BMIz (year 2 change in BMIz for FB = −0.03 [−0.1, 0.04], PO = −0.01 [−0.08, 0.06], and HEC = −0.09 [−0.15, −0.02]). While mean attendance across conditions was satisfactory during months 1–4 (69%), it dropped during the maintenance phase (42%). High attendance for the PO intervention was related to greater changes in child BMIz (p < .02). Numerous barriers to participation were reported.
Conclusion
Many barriers exist that inhibit regular attendance at in-person contacts for many families. Innovative delivery strategies are needed that balance treatment intensity with feasibility and acceptability to families and providers to facilitate broad dissemination in underserved rural settings.
ClinicalTrials.gov Identifier: NCT01820338.
Keywords: children, obesity, rural, randomized controlled trial, treatment
Introduction
Childhood obesity continues to be a critical public health issue associated with alarming consequences (Freedman, Ogden, & Kit, 2015; Schwimmer, Burwinkle, & Varni, 2003; Trasande & Chatterjee, 2009). Behavioral family-based (FB) interventions addressing childhood obesity are the most studied intervention for pediatric obesity, producing the best short-term and long-term outcomes for weight loss (Janicke et al., 2014; Kitzmann et al., 2010; Whitlock, O'Connor, Williams, Beil, & Lutz, 2010). In a series of studies, Epstein et al. demonstrated that behavioral FB interventions including separate but simultaneous groups for children and parents, and addressing dietary intake and physical activity, lead to greater improvement in child weight outcomes relative to controls (Epstein, Paluch, Roemmich, & Beecher, 2007; Epstein, Valoski, Wing, & McCurley, 1994). However, the generalizability of these interventions is less clear. Many of these programs have been developed and tested in well-controlled, well-resourced clinic settings with middle to high-income families, and have not been adequately tested in community-based settings. One of the greatest challenges facing health promotion is translating research findings from efficacy studies into evidence-based public health and community settings (Kerner, Rimer, & Emmons, 2005). Randomized controlled trials (RCT) testing the effectiveness of childhood obesity interventions in real-world community-based settings are needed to bridge this gap (Robinson, 2008). Within this broad domain of “effectiveness” interventions for childhood obesity, there are a number of key issues that need to be addressed.
Recent research suggests that behavioral interventions exclusively targeting only the parent in child weight loss interventions may be an effective alternative to behavioral FB programs (Boutelle, Cafri, & Crow, 2011; Golan, Fainaru, & Weizman, 1998). These behavioral parent-only (PO) interventions may be less costly, and ultimately have the potential to be more cost-effective than FB interventions as these interventions can influence healthy lifestyle habits of multiple family members by treating only one parent (Wolf, 1998). PO programs may be particularly well suited for community-based, underserved settings with limited resources. However, little research has examined this issue in real-world community settings.
Children from rural communities represent an underserved population that is a high risk for overweight and obesity. While only 20% of the U.S. population lives in rural areas, rural areas account for close to 75% of the medically underserved areas (U.S. Department of Health & Human Services). Children living in rural areas are more likely to have obesity than their peers living in metropolitan areas (Johnson & Johnson, 2015). The level of resources needed to deliver effective interventions, as well as the limited access to health promotion programs, healthy food options, and potential stigma due to receiving treatment represent significant barriers to dissemination into rural settings (Lim & Janicke, 2013). Developing and evaluating interventions that positively impact children’s long-term weight status and related health parameters are critical to promoting improved health of youth, especially in rural settings. Unfortunately, few studies have examined behavioral interventions to address pediatric obesity in rural communities. Most research examining health promotion and obesity prevention programs for rural children have been implemented in schools (Canavera, Sharma, & Murnan, 2009; Williamson et al., 2008). However, the efficacy of these programs on child weight status is mixed at best, likely because there is limited involvement of parents in these programs. Davis et al. examined the impact of behavioral family interventions delivered in a clinical setting via telehealth to children with overweight and obesity living in rural settings in two studies. Changes in child weight outcomes were limited, with no differences relative to comparison conditions (Davis, Sampilo, Gallagher, Landrum, & Malone, 2013; Davis et al., 2016).
Previously, the authors conducted a pilot study examining the feasibility and preliminary effectiveness of a two behavioral family lifestyle intervention program delivered through four Cooperative Extension Service (CES) offices in rural communities (Janicke et al., 2008). The CES is a partnership among the U.S. Department of Agriculture, land-grant universities, and county government that delivers educational programs and research-based information to residents in almost every county in the US. The CES network offers infrastructure and resources to support widespread dissemination of prevention and treatment programming to families. In this previous feasibility study, child–parent dyads were randomized to one of two 4-month behavioral treatment programs or a waitlist control. The first was a behavioral FB behavior in which both the child and parent attended group meetings. The second was a behavioral PO intervention in which only the parent attended group meetings. Results showed that children randomized to FB and PO conditions experienced significant reductions in body mass index z-scores (BMIz) from baseline to month 10 (four months of treatment plus six months of no-contact) relative to controls; there were no differences in BMIz scores between the PO and FB conditions at 10-month follow-up assessment (Janicke et al., 2008).
Given that the American Psychological Association, the Obesity Society, and others researchers recommend a minimum of 26 contact hours for FB treatment of obesity (Coppock, Ridolfi, Hayes, Paul, & Wilfley, 2014; Llabre et al., 2018; Wilfley et al., 2017b), and that FB interventions with a longer duration and more treatment contacts are associated with better child weight outcomes (Janicke et al., 2014; Kitzmann et al., 2010; Wilfley et al., 2017a), there is a need to examine the feasibility and benefits of extended care interventions addressing pediatric obesity in real-world settings. The purpose of this study was to examine the effectiveness of two extended behavioral interventions implemented through CES offices in rural communities on a larger scale with longer follow-up. The primary hypothesis was that the PO and FB interventions would exhibit greater reductions in child BMIz than a health education condition (HEC) condition from baseline to posttreatment (month 12) and follow-up (month 24). Finally, research examining barriers to treatment of childhood obesity has typically focused on limited access to care and limited recognition of obesity as a health concern, or reasons that prevent families from initiating treatment (Lim & Janicke, 2013). Given families in this study were enrolled in treatment and likely recognized child obesity as an area to be addressed, we examined parent perceptions of barriers that may hinder long-term participation in treatment, including logistical barriers, perceptions of limited utility of intervention content and processes, perceived stigma, and perceived burden of homework activities.
Methods
Participants
The study protocol was approved by the governing institutional review board (IRB). Participants were 249 children and their parent(s) from 10 rural counties (Ricketts, Johnson-Webb, & Taylor, 1998) in north-central Florida. Power calculations were based on effect sizes from a previous pilot trial (Janicke et al., 2008). The goal was to randomize 80 dyads per intervention arm for a total of 240 dyads. Anticipating a dropout rate of 30% after two years, 56 children per arm were expected to complete the study through 24-month follow-up. This would give us sufficient power to assess the main study hypotheses.
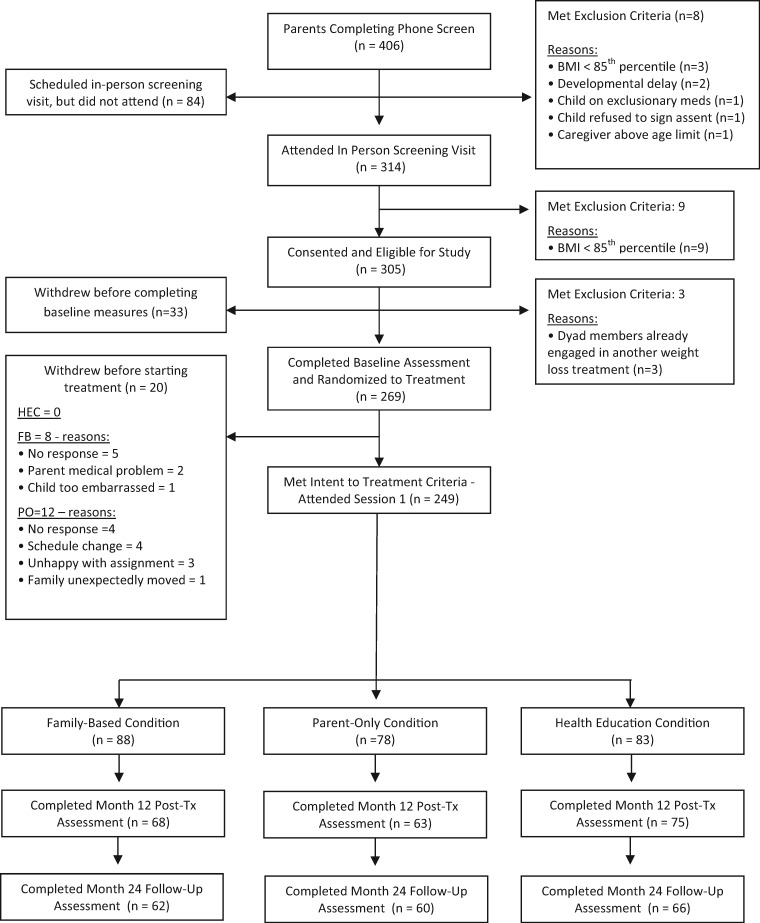
Children were between the ages of 8 and 12 years, with a BMI at or above the 85th percentile for age and sex (Kuczmarski et al., 2000). Families were excluded if the child had a developmental delay, the caregiver was above 75 years of age, or the child or parent was using prescription weight loss drugs or was enrolled in another weight loss program. Participant flow throughout the study is presented in Figure I. The study was conducted between 2010 and 2015.
Figure I.
CONSORT flow diagram.
Procedures
This study was a three-armed, RCT. Families were recruited through direct mailings, distribution of brochures through local schools and physician offices, and newspaper press releases. Interested parents were invited to call to learn about the study, complete a telephone screen, and schedule a screening visit. At the in-person screening child–parent dyads completed consent forms and were measured for height and weight by a trained study nurse. Families who met eligibility criteria were scheduled for baseline assessment. Study-related adverse events were assessed on a regular basis; when these events occurred, they were reported as required to the governing IRB.
Assessments
All assessment and intervention sessions took place at CES offices in participating counties. Baseline assessments were held 1–2 weeks before the start of treatment. Children and parents were again measured for height and weight and completed outcome measures.
All families completed posttreatment assessment at month 12 and follow-up assessment at month 24. Families received $50 as compensation for completing each assessment. Thus, families could receive a total of $100 for completing the month 12 and month 24 assessments. There were separate assessment teams for each county that were not involved in treatment delivery for that same county, and were kept blind to intervention condition. Individuals on the assessment team completed enrollment procedures and informed participants of assignment to intervention condition. Each dyad’s assignment to treatment condition was kept in a sealed envelope that was prepared by the study statistician and project director; the baseline assessment team was not aware of the content of each randomization envelope. At the end of the baseline assessment, one team member met with the family in private to open up the envelope and share the assignment to treatment condition with the dyad. Intervention group sizes ranged between four and nine dyads, with a mean of roughly seven dyads per group across conditions.
Randomization
The three interventions were held on different weeknights in each county. During the initial telephone screen, families were informed as to which evenings the group meetings would be held in their county, and then families indicated which of the 3 weekday evenings they could attend meetings. A majority of families (66%) were available to be randomized to two or three of the weekday evenings, while some families (33%) were available to attend just one of the three weekday evenings. To increase study feasibility, within each county families were randomized via computer assignment, based on availability, to one of the three weeknights on which groups would be held on a 1 to 1 to 1 basis. After all families were assigned to a weeknight, the interventions were randomly assigned by the study statistician to specific weeknights separately for each county. After completing the baseline assessment families were informed of their randomized assignment to treatment condition.
Interventions
For all three conditions, weekly group sessions were held for the first eight weeks, then every two weeks for the next eight weeks, and then monthly for the last eight months. Sessions occurred weekday evenings starting at 6 p.m. and lasted 90 min. Families were provided with $10 per treatment session attended as compensation for travel. Childcare was available at meetings for all three intervention conditions.
Interventions were delivered by participating Family and Consumer Sciences (FCS) agents and 4-H Youth Development agents (n = 17) at each county CES office, in collaboration with members from the research team (postdoctoral psychologist and graduate students in psychology) (n = 10). Interventionists were allocated to an intervention group in each county based on weeknight availability. The FCS and 4-H agents had a Bachelor’s or Master’s degrees, often with a concentration in nutrition or youth development. Each parent group was led by two interventionists, while each child group was led by two separate interventionists. Interventionists received 12 hr of training before the intervention led by the study PI and participated in 30 min of weekly supervision. Training included discussion of session content, as well as practice and role-play exercises in goal setting, problem solving, addressing resistance to change and facilitating group discussion. Treatment manuals for the participants and group leaders were developed during the pilot study and updated and expanded for use in the current study. Sessions were recorded via audio tape to allow the investigator to monitor each interventionist’s performance and assess treatment fidelity.
FB and PO Behavioral Interventions
The FB and PO interventions and assessment methodology were very similar to those used in the previous pilot (Boutelle et al., 2011), with the main exception that additional sessions (sessions 13–20) were developed to focus on maintenance of behavior changes, and new measures were added to assess parent health behaviors and child glycated hemoglobin (HbA1c). The FB and PO interventions were grounded in social cognitive theory (Bandura 1998; Baranowski, Perry, & Parcel, 2002; Coppock et al., 2014). Consistent with this theory, the interventions helped families acquire behavioral weight management skills to better regulate their dietary intake and physical activity behaviors and create a healthier home environment. Additionally, parents were taught key behavior management skills to help them motivate and support their child to make healthy choices including shaping, differential reinforcement, restructuring the physical environment, self-monitoring of behavior, modeling, goal setting, feedback on behavior, problem solving, and social support. Changes in dietary habits were addressed via a modified version of the Stoplight Diet (Epstein & Squires, 1998). Child and parent participants in both treatment conditions were encouraged to monitor everything they ate, but were not required to record energy or macronutrient values. For families that struggled with completing these monitoring logs, an abbreviated log was provided in which they could track the number of servings of “red” foods (i.e., high-fat/high sugar foods) and “green” foods (i.e., fruits and vegetables) consumed per day. Parents and group leaders worked together to set individualized physical activity and dietary goals, which included limiting the consumption of “red” foods and increasing the consumption of “green” foods. Children and parents in both behavioral arms were provided with pedometers to wear daily and encouraged to gradually increase their daily steps. In the parent group, parents reviewed and discussed weekly progress implementing change strategies, and participated in knowledge and skill training related to nutrition, physical activity, and behavior management strategies.
In the FB intervention, parent and child dyads participated in simultaneous but separate groups. The child group sessions included review of progress during the previous week, a physical activity to demonstrate strategies to keep active, and preparation of a healthy snack. At the end of sessions, children and parents worked together to develop goals and action plans.
In the PO intervention, only the participating parent(s) attended group meetings. Steps and material covered were the same as those in the FB intervention. In addition, parents role-played setting goals with their children and were encouraged to work with their children at home to help them monitor health behaviors and set goals.
HEC Condition
Both the child and parent participants attended all meetings. Group sessions addressed a series of nutrition, physical activity, and health promotion topics. After the presentations, parent groups discussed how the information could be useful for their families. The families did not receive training in behavioral self-regulation or parenting strategies. Children participated in a group physical activity and were offered a healthy snack during each session. There was no discussion of implementing these activities or preparing healthy snacks in other settings.
Assessment of Treatment Fidelity
Audio recordings of 13 group meetings from each treatment condition (39 in total) were randomly selected via a computer-generated list and coded for treatment fidelity. A checklist of topics to address for each treatment session was developed across conditions using the group leader treatment manuals. Trained coders reviewed recordings to determine if each listed item was addressed. Ten (25%) of the 39 recordings were rated by a second coder to assess interrater agreement.
Outcome Measures
Height and weight were assessed for each child and parent by trained research team members. Height without shoes was measured to the nearest 0.1 cm using a Harpendon stadiometer. Weight was measured to the nearest 0.1 kg with one layer of clothing and without shoes using a Tanita BWB-800 digital scale. Height and weight were each measured three times, with the mean calculated and used for analysis. Child dietary intake was assessed via the Block Kids 2004. This 77-item questionnaire assessed children’s dietary intake over the past month (Berkeley, CA, 2004). The food list for this questionnaire was developed from the NHANES 1999 to 2002 dietary recall data. Each child and parent dyad worked together to complete the questionnaire. Parent dietary intake was assessed with the Block Brief 2000 Food Frequency Questionnaire (Harlan & Block, 1990). This is a revised version of a previously validated survey that asks respondents to estimate consumption of a wide variety of foods. Scoring yields estimates of macro- and micronutrient intake, as well as intake by specific food group. Child health-related quality of life was assessed via the Pediatric Quality of Life Inventory (PedsQL) (Varni et al., 2003). The PedsQL is a 23-item measure of health-related quality of life (QOL) in healthy children and those with acute and chronic conditions. The measure has been reported to have excellent internal consistency, clinical validity, and factor-analytic support for subscales. The total score was used in this analysis. There are both child and parent report forms; both were completed in this study. Coefficient alpha for the child version in this sample was .86, while for the adult version was .90. Child glycated hemoglobin (HbA1c) was assessed via a point-of-care Cholestech GDX Analyzer administered by the study nurse. At posttreatment, children and parents each completed a single item assessing overall program satisfaction, and parents also completed a 15-item measure developed by the research group and extension staff to assess barriers to attending group meetings.
Statistical Analysis
Descriptive statistics were calculated for outcomes and predictors by treatment and time. The outcomes of interest were child and parent BMI, child BMIz, child and parent-reported mean daily dietary intake, child-reported QOL, parent-reported child QOL, and child HbA1c. For each outcome, the change from baseline to 12 months and baseline to 24 months was examined. Two models were considered for each outcome using a modified intent-to-treat (ITT) approach for the analysis and comparison of treatment groups; the approach was considered a modified ITT analysis as dyads that dropped out prior to attending the first treatment session were not included in the analysis. Both models were linear mixed models of the change from baseline (e.g., BMIz) to the first and second follow-up; these models allow for dropout, with the assumption that the missingness was at random. The first was a basic model that considered time, treatment, and a time × treatment interaction and controlled for the baseline value of the outcome (e.g., BMIz). If the time × treatment interaction was not significant, it was removed from the model and the model was refit to examine for overall treatment effects. For this first model of overall treatment comparisons across time, any treatment effects were followed by pairwise comparisons among the groups (using a Tukey adjustment in determining statistical significance), either by time (if a time × treatment interaction) or overall (if no significant interaction). The second model was the same as the first, with the addition of a single demographic control and its interactions with time and treatment, in order to explore potential subgroups that benefited differentially from the treatment. If the three-way interaction demographic covariate × time × treatment was not significant, it was removed and the reduced model was evaluated. Given the exploratory nature of the covariate interaction models, significant results were not followed by pairwise comparison. However, with regards to the primary outcomes (child BMI or BMIz), any significant interactions were presented to provide data for future studies. In all cases, the change from baseline values was observed to be approximately normally distributed. If the baseline values were skewed, the natural log of the baseline value was used as the covariate in the models. For the model of child BMI, both age and gender were controlled in the analysis. In all cases, mean (95% confidence interval [CI]) changes from baseline at 12 months and 24 months are reported.
Results
Overall, 249 dyads accepted randomization and started treatment. Across groups, participants attended 69% of core treatment sessions (during the first four months), and 42.6% of maintenance sessions (during months 5–12). There was a significant difference in attendance across conditions for both core sessions (FB = 65.1%, PO = 66.2%, HEC = 75.6%; p = .035) and maintenance sessions (FB = 37.1%, PO = 37.0, HEC = 52.9%; p = .011), with higher attendance in the HEC relative to the behavioral treatments. A total of 83% of dyads completed year 1 posttreatment assessment and 75% completed follow-up assessment at year 2. There was no difference in baseline demographic or outcome variables between assessment completers and noncompleters. Baseline demographic and outcome variables are listed in Table I.
Table I.
Participant Characteristics and Outcome at Baseline, 12 and 24 months by Treatment Group in E-FLIP for Kids Intervention Study
| Treatment |
Health education (control) (N = 83) |
Parent only (N = 78) |
Family based (N = 88) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%)/M (SD) | Baseline | 12 months | 24 months | Baseline | 12 months | 24 months | Baseline | 12 months | 24 months |
| Baseline demographics | |||||||||
| Male (%) | 39 (47.0) | 40 (51.3) | 34 (38.6) | ||||||
| White (%) | 55 (66.3) | 48 (61.5) | 61 (69.3) | ||||||
| Child age | 10.4 (1.4) | 10.3 (1.3) | 10.4 (1.5) | ||||||
| Outcomes | |||||||||
| Child BMI z-score (kg/m2) | 2.2 (0.3) | 2.1 (0.4) | 2.1 (0.4) | 2.2 (0.4) | 2.2 (0.4) | 2.2 (0.4) | 2.1 (0.4) | 2.1 (0.5) | 2.1 (0.5) |
| Child dietary intake (kcal) | 1,327 (770) | 1,169 (745) | 1,127 (837) | 1,235 (555) | 1,099 (675) | 1,000 (720) | 1,280 (714) | 1,133 (608) | 1,009 (493) |
| Parent dietary intake (kcal) | 1,762 (1,091) | 1,442 (699) | 1,444 (766) | 1,556 (658) | 1,119 (454) | 1,222 (534) | 1,566 (735) | 1,196 (459) | 1,227 (634) |
| Child-reported QOL | 75.1 (12.1) | 79.7 (14.2) | 80.6 (14.0) | 76.2 (12.6) | 81.9 (12.4) | 80.0 (12.1) | 76.2 (16.2) | 78.1 (15.8) | 80.5 (14.2) |
| Parent-reported child QOL | 74.0 (14.9) | 76.9 (15.6) | 78.0 (13.7) | 72.1 (15.7) | 75.2 (14.5) | 74.2 (13.6) | 73.3 (15.9) | 73.7 (15.5) | 77.3 (16.9) |
| Child HbA1c (%) | 5.5 (0.2) | 5.4 (0.2) | 5.4 (0.3) | 5.5 (0.2) | 5.4 (0.3) | 5.5 (0.3) | 5.5 (0.3) | 5.4 (0.3) | 5.4 (0.3) |
| Parent BMI (kg/m2) | 34.3 (7.6) | 34.7 (8.0) | 34.5 (8.7) | 33.7 (7.1) | 33.8 (7.9) | 35.3 (7.9) | 36.7 (8.8) | 35.1 (8.3) | 35.5 (8.4) |
Note. BMI = body mass index; HbA1c = health behaviors and child glycated hemoglobin; QOL = quality of life.
There were no significant differences between groups on baseline characteristics.
Study outcomes are listed in Table II. In the basic linear mixed model, there was not a significant time by treatment interaction for any child health outcomes. When the interaction was removed, a significant overall treatment effect for child HbA1c measures was observed. Pairwise comparisons show that children who received the HEC or FB interventions had a greater decrease in HbA1c in the first year than children in the PO intervention, while the difference between the children in the FB and PO was not significant (Table II). This decrease relative to baseline was observed at two years as well with no significant time effect. Fifty percent of children completing assessments showed decreases in BMIz at posttreatment and follow-up assessments in each of the treatment conditions. However, only 16% in the FB treatment, 13% in the PO treatment, and 5% in the HEC experienced clinically significant BMIz reductions at two year follow-up, based on guidelines from Wilfley et al. (2017b).
Table II.
Change in Outcome Variables with Confidence Intervals at 12 and 24 months for Participants in E-FLIP for Kids Intervention Study
| Predictors |
Outcome (change from baseline) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Child BMI | Child BMI z-score | Child dietary intake (kcal) | Parent dietary intake (kcal) | Child-reported QOL | Parent-reported child QOL | Child HbA1c | Parent BMI | ||
| Treatment | Time (month) | Change from baseline: model-based estimatesa | |||||||
|
| |||||||||
| Health education | 12 | 1.11 | −0.06 | −142.80 | −244.71 | 4.41 | 2.15 | −0.16 | −0.19 |
| (0.70, 1.51) | (−0.10, −0.02) | (−294.97, 9.36) | (−342.75, −146.67) | (1.73, 7.08) | (−0.66, 4.97) | (−0.22, −0.11) | (−0.66, 0.28) | ||
| 24 | 2.28 | −0.09 | −188.13 | −276.92 | 5.72 | 3.99 | −0.15 | 0.11 | |
| (1.62, 2.94) | (−0.15, −0.02) | (−355.85, −20.40) | (−405.19, −148.65) | (3.08, 8.35) | (0.67, 7.31) | (−0.23, −0.07) | (−0.54, 0.76) | ||
| Parent only | 12 | 1.25 | −0.03 | −160.18 | −399.55 | 6.33 | 1.66 | −0.05 | −0.43 |
| (0.81, 1.69) | (−0.08, 0.01) | (−325.87, 5.51) | (−505.47, −293.64) | (3.43, 9.23) | (−1.41, 4.74) | (−0.11, 0.01) | (−0.94, 0.08) | ||
| 24 | 2.86 | −0.01 | −265.80 | −323.99 | 4.48 | 1.06 | −0.03 | 0.55 | |
| (2.15, 3.56) | (−0.08, 0.06) | (−441.39, −90.22) | (−458.57, −189.41) | (1.70, 7.27) | (−2.44, 4.56) | (−0.10, 0.03) | (−0.13, 1.23) | ||
| Family based | 12 | 1.41 | −0.02 | −157.13 | −366.22 | 3.08 | −0.15 | −0.10 | −0.69 |
| (0.98, 1.84) | (−0.06, 0.02) | (−316.84, 2.57) | (−468.11, −264.32) | (0.29, 5.88) | (−3.15, 2.85) | (−0.15, −0.04) | (−1.19, −0.20) | ||
| 24 | 2.91 | −0.03 | −281.72 | −320.95 | 4.47 | 2.76 | −0.13 | −0.96 | |
| (2.22, 3.60) | (−0.10, 0.04) | (−454.01, −109.43) | (−451.80, −190.11) | (1.75, 7.19) | (−0.67, 6.20) | (−0.20, −0.06) | (−1.63, −0.29) | ||
| Time × treatment p-value | .4522 | .3349 | .8040 | .4081 | .2096 | .4332 | .6701 | .0042 b | |
| Treatment p-value (if time interaction NS) | .3953 | .3115 | .8359 | .3359 | .6015 | .5398 | .0037 c | ||
|
| |||||||||
| Covariate | Interaction | Change from baseline with covariates: interaction p-valuesd | |||||||
|
| |||||||||
| Gender | Time × treatment × gender | 0.7757 | 0.7225 | 0.5655 | 0.4543 | 0.0888 | 0.6260 | 0.5974 | 0.1951 |
| Treatment × gender | 0.7008 | 0.8152 | 0.0588 | 0.6039 | 0.5006 | 0.6076 | 0.2255 | 0.2360 | |
| Adult BMIe | Time × treatment × adult BMI | 0.2166 | 0.1293 | 0.2627 | 0.0177 | 0.3355 | 0.9128 | 0.6821 | |
| Treatment × adult BMI | 0.5702 | 0.2637 | 0.7038 | 0.1897 | 0.9102 | 0.0472 | |||
| Child age | Time × treatment × child age | 0.9607 | 0.9863 | 0.4747 | 0.6145 | 0.6886 | 0.6099 | 0.7288 | 0.0930 |
| Treatment × child age | 0.7130 | 0.6164 | 0.6201 | 0.9316 | 0.0898 | 0.1386 | 0.2834 | 0.4768 | |
| Child race/ethnicity | Time × treatment × child race/ethnicity | 0.0307 | 0.1618 | 0.8179 | 0.1925 | 0.8893 | 0.4149 | 0.8947 | 0.5153 |
| Treatment × child race/ethnicity | 0.9629 | 0.0567 | 0.6526 | 0.1870 | 0.6349 | 0.7638 | 0.3856 | ||
Note. BMI = body mass index; HbA1c = health behaviors and child glycated hemoglobin; QOL = quality of life.
Estimates come from least square means, evaluated at average baseline value for the outcome. A negative value indicates a decrease from baseline and a positive value indicates an increase from baseline.
Subsequent significant pairwise comparisons (adjusted p < 0.05 using Tukey adjustment): FB versus PO at 24 months (p = .0059).
Subsequent significant pairwise comparisons (across time since no interaction, using Tukey adjustment).
If the three-way interaction was not significant it was removed and the pairwise interactions with the covariate were examined.
Categorical BMI (obese and not obese).Bold and italics indicate p < .05
For parent outcomes, only change in parent BMI had a significant time by treatment effect after controlling for baseline BMI. All three conditions had a decrease in parent’s BMI from baseline to year 1. However, pairwise comparisons show that the parents in the FB condition experienced a greater decrease in BMI at year 2 compared to those in the PO condition (Table II). At two year follow-up, 23% of parents in the FB and HEC conditions, and 16.7% of parents in the PO condition, experienced a clinically significant decrease in weight of 5%.
For the models with covariates, there was a significant time by treatment by race/ethnicity interaction for child BMI. For all three treatment groups, there was an increase of approximately 1 kg/m2 in child BMI from baseline to year 1 for non-Hispanic White children and about 1.5 kg/m2 for non-White children. Non-White children overall experienced greater increases in BMI across the two years compared to White children.
There were no differences across treatment conditions in parent program satisfaction, with mean parent satisfaction ratings equal to 3.5 or higher on a 4-point Likert scale. Child ratings of program satisfaction showed that children in the FB (M = 3.81, SD = 0.47) and HEC (M = 3.74, SD = 0.62) reported higher satisfaction relative to the PO condition (M = 3.52, SD = 0.86; p =.03). Treatment fidelity was rated above 95% for all conditions; agreement across coders was 98.7%.
Table III lists the parent-reported barriers to attending group meetings. Over 60% of families reported experiencing an ongoing family scheduling conflict that hindered regular attendance at group meetings. Mean number of barriers reported by parents did not differ across groups (FB [M = 2.1, SD = 1.3] and HEC [M = 1.6; SD = 1.3; p = .06]); however, there was an effect size of 0.4. Across groups, the total number of barriers reported was negatively correlated with total attendance (r = −.35, p < .001), while the number of total scheduling conflicts was negatively correlated with change in child BMIz at posttreatment (r = −.19, p = .02). Exploratory analyses were conducted to determine if child BMIz outcomes were related to meeting attendance. For the PO condition, children of parents who attended nine or more of the 12 core group treatment meetings experienced great improvements in weight status relative to children of parents who attended less than nine sessions at both year 1 (mean BMIz Δ of 0.078 vs. −0.012; p < .02) and year 2 (mean BMIz Δ of 0.064 vs. −0.042; p < .05). Fifty percent of parents in the PO condition met this 75% attendance threshold. There was no such relationship for participants in the FB intervention. There were no differences across groups in the types of treatment barriers reported.
Table III.
Parent Identified Barriers to Attending Group Meetings (Parents Allowed to Check All That Apply)
| 1. Participating child started an activity (e.g., sports, clubs, church group) 34% that conflicted with groups | 34% |
| 2. I, or a member of our family, participated in an activity that conflicted with groups (e.g., sports, PTO, church groups, club) | 28.7% |
| 3. Changes in work schedule or change in job | 22% |
| 4. I, my child, or member of my family became ill, injured, or required hospitalization that conflicted with groups. | 20.7% |
| 5. Participating child had too much homework | 18% |
| 6. Car problems or unreliable transportation | 10% |
| 7. It too long for us to drive or travel to group sessions | 9.3% |
| 8. It took too much time to complete the various homework assignments or food logs in the study | 8% |
| 9. Changes in family income made it too difficult to attend groups | 6.7% |
| 10. My child was embarrassed or did not like participating in groups | 5.3% |
| 11. There was death in the family/close family friend | 4.7% |
| 12. I did not feel like information given in the groups was helpful for me or my family | 3.3% |
| 13. I was embarrassed or did not like participating in groups | 2.7% |
| 14. I, or my spouse, lost our job | 2% |
| 15. My spouse did not like my family participating in groups | 0.7% |
Note. PTO = parent–teacher organization.
Discussion
This is the first large RCT examining the effectiveness of behavioral lifestyle interventions to address obesity for children in real-world rural community settings. Interventions were implemented through CES offices. The E-FLIP program received strong support from CES staff and administrators. While over a half of child participants completing assessments showed decreases in BMIz, roughly only one in six children in the two behavior conditions experienced clinically significant reductions in BMIz at month 12 follow-up, and there were no significant differences in BMIz and child health outcomes across conditions at either posttreatment or follow-up assessment. Thus, the main hypotheses were not supported. Decreases in child dietary intake and increases in child QOL were in the hypothesized directions, but roughly similar across conditions. Moreover, there was a significant difference for change in parent BMI favoring the FB intervention.
These results are in contrast to recent efficacy studies demonstrating positive weight outcomes associated with behavioral interventions for children with obesity delivered in clinical settings (Boutelle et al., 2011; Wilfley et al., 2017a). Notably, in contrast to the design of E-FLIP for Kids, these efficacy studies were conducted with participants from primary metropolitan areas and interventions were delivered in clinical settings by multiple medical professionals with expertise in obesity. Alternatively, our results are similar to a recent behavioral family intervention implemented to children with obesity and their parents in rural settings using telehealth by Davis et al., which showed limited changes in weight status over time subsequent to eight months, 14 session intervention.
Notably, the child weight change outcomes in the current study contrast with those found in our previous pilot study with children in rural settings, in which children assigned to behavioral PO and FB interventions delivered through CES offices, on average, each showed significant decreases in mean BMIz relative to children assigned to the waitlist control condition (Janicke et al., 2008). One likely reason for this difference was the large scope of the current study. In each of the 10 participating counties, treatment groups were conducted on three different weeknights. Given two to three counties were included in each cohort, up to nine treatment groups were run each week across the length of the study. This required a large number of interventionists, which impacted time allotted for weekly supervision for each interventionist. While the measure of treatment integrity in this study showed that the interventionists were implementing the treatment components as specified in the treatment manuals, it is possible that skill and sensitivity with which goal setting and problem solving were implemented in the behavior groups was not optimal. Notably, CES agents acknowledged that learning group facilitation, goal setting, problem solving, and implementation of behavioral management skills with parents were new skill sets for them that required a number of months of actual “real-world use” before feeling comfortable independently utilizing the skills in group formats. This suggests longer and on-going training is likely necessary to optimize group leader performance.
Less than optimal participant attendance was a major challenge. Session attendance dropped dramatically during the maintenance phase. Many families reported difficulty balancing attending group meetings for such an extended period with family and work responsibilities, most commonly due to different types of scheduling conflicts or transportation barriers that hindered regular attendance at meetings. Notably, perceptions of stigma, limited usefulness of intervention, and a burdensome level of assigned homework tasks were endorsed relatively infrequently. These scheduling conflicts and transportation barriers are consistent with limited previous research in this area (Lim & Janicke, 2013). This is a particular challenge in rural areas, where there is a lack of available public transportation. These barriers are notable given that more reported barriers were associated with poorer attendance, and that more scheduling conflicts were related to poorer child weight outcomes. Such issues speak to the need for alternative delivery methods that can be more convenient for children and families.
The timing of the outcome assessment could have impacted the results. Posttreatment assessment was conducted at month 12 after the completion of the maintenance sessions, as opposed to at the completion of the core four month treatment as was done in the previous feasibility study (Janicke et al., 2008). Maintenance of behavior and weight change after treatment is a significant challenge in adult and pediatric obesity research (MacLean et al., 2015; Wilfley et al., 2007) and could partially explain why the findings are not consistent with recent RCTs (Janicke et al., 2014), as well as the previous feasibility study.
In reflecting on these results, one should consider the potential positive aspects of the HEC condition, given the favorable changes in BMIz, as well as the higher attendance and strong satisfaction ratings. Notably, no parents withdrew from the study prior to starting treatment after being informed of their assignment to the HEC condition. Anecdotally, many parents in the HEC condition reported satisfaction with their group during and following treatment, specifically noting that they valued the support offered by the group format, and also felt less burdened by not being asked to monitor dietary intake and physical activity on a daily basis. Future research may want to consider this model, or a hybrid model combining the current HEC condition with the addition of more education on parent and behavioral weight management strategies without inclusion of the perceived burdensome daily monitoring or weekly goal setting requirements. This could be a low-resource, low-cost model for engaging families of children with obesity in weight management efforts that may be more feasible for delivery in settings like those included in the current trial, given community and staff resources.
It is notable that across treatment conditions children from racial and ethnic minority backgrounds evidenced more weight gain over the course of the program compared to non-Hispanic White youth. These differences occurred despite no significant differences in reported barriers to participation, attendance or program satisfaction. These results support continued efforts to better understand factors impacting differential outcomes among minority youth and develop culturally sensitive interventions that help address health disparities (Wilson, 2009).
While some key study limitations have been discussed above, three additional limitations should be noted. First, resources varied across CES sites, with some counties having large, high-quality facilities, and multiple staff available to lead groups, while other counties had limited facilities and staff available to optimally support intervention programs. These differences have implications for potential dissemination quality. Second, as part of the assessment, children were asked to wear accelerometers to objectively measure physical activity. However, adherence to wearing the accelerometers was poor and did not allow for meaningful analysis on changes in physical activity over time. Third, self-report measures of dietary intake, including food frequency questionnaires, often underreport caloric intake and thus may not have adequately captured changes in actual dietary intake. Third, it is clear that the length of the intervention was a limiting factor for many families. However, substantial research shows that more treatment contacts are associated with better outcomes (Janicke et al., 2014; Kitzmann et al., 2010; Wilfley et al., 2017a), and multiple sources recommend a minimum of 26 hr of behavioral family contacts (Coppock et al., 2014; Llabre et al., 2018; Wilfley et al., 2017b). Balancing these key issues will clearly be a challenge for families and providers operating in real-world community settings. Finally, we did not capture data on which behavioral weight loss and parenting strategies were used most often by participants and how these were related to health outcomes. Further our barriers measure may have missed some important barriers that hinder families’ abilities to make effective lifestyle changes that live in rural locations. This information can be helpful in redesigning interventions for future examination.
There are a number of lessons that can guide future research. First, 249 families were recruited and enrolled in this study, which demonstrates recognition of the health issue by parents, as well as need and desire for treatment addressing pediatric obesity in rural settings.
Probably most important, given that logistic considerations and competing time demands were the main barriers to attending group intervention meetings, the research literature and current data show that more treatment contact is associated with better outcomes (Wilfley et al., 2017a; Wilson, 2009); balancing intensity and duration of treatment with family’s ability to attend ongoing sessions is critical. In this vein, developing and evaluating novel strategies or alternative delivery formats to limit barriers to participation, such as telehealth or mHealth strategies are clearly warranted. For example, phone counseling has shown promise in facilitating weight management in adults, with research also starting to accumulate with children (Pbert et al., 2016; Perri et al., 2008). The previous pilot study by this research team supported the feasibility of a four month in-person group intervention (Janicke et al., 2008). It may be that adding a telehealth maintenance component, instead of continuing in-person meetings, may be more feasible for families, while still providing the on-going support to facilitate behavior change. Further studies are clearly needed to examine what amount, type and frequency of contact are feasible for families and providers in real-word settings, and how different dosage and types of treatments may lead to different weight outcomes. Policy makers and community leaders should also consider augmenting these family interventions with broader community intervention efforts (Economos et al., 2007) that could provide greater support for maintenance of lifestyle behavior changes. Moreover, to expand the dissemination of obesity intervention programs, whether through CES offices or other community venues, stakeholder groups should consider developing and evaluating efficient training and supervisory protocols to increase treatment fidelity across diverse sites. Novel strategies, including technological approaches would also be important to consider as a way to facilitate training and monitor personnel delivering interventions in rural and high-risk communities. Finally, although no definitive conclusions can be drawn based on the post-hoc analysis, the data suggest that the PO intervention has the potential to positively impact child weight change, if providers and families can work together to facilitate regular and ongoing participation in treatment. Given the various limitations in the current study, further research examining gold standard intensive intervention formats relative to novel intervention formats (i.e., PO, different modes of patient contact) are warranted.
One of the greatest challenges facing health promotion efforts is translating research findings from efficacy studies into evidence-based public health and community interventions (Kerner et al., 2005), especially in underserved settings. RCTs testing the effectiveness of childhood obesity interventions in real-world community-based settings are needed to bridge this gap. Contrary to the hypothesis, the extended behavioral interventions implemented in CES offices across rural counties in this trial did not lead to greater changes in child weight status compared to an education control condition. This study pointed to a number of challenges that provider organizations may face when delivering health behavior change interventions for families in real-world community settings. However, results from this trial provided critical information to suggest what works and does not work, as well as identifying strategies to overcome the challenges of implementing programs addressing obesity in real-world settings.
Funding
This study was funded by National Institute of Diabetes and Digestive and Kidney Diseases [#R18DK082374].
Conflicts of interest: None declared.
References
- Bandura A. (1998). Health promotion from the perspective of social cognitive theory. Psychology & Health, 13, 623–649. doi:10.1080/08870449808407422 [Google Scholar]
- Berkeley C. A. (2004). Block Kids FFQ 2004. Berkeley, CA; Block Dietary Systems; 2004. [Google Scholar]
- Boutelle K. N., Cafri G., Crow S. J. (2011). Parent-only treatment for childhood obesity: a randomized controlled trial. Obesity, 19, 574–580. doi:10.1038/oby.2010.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavera M., Sharma M., Murnan J. (2009). Development and pilot testing a social cognitive theory-based intervention to prevent childhood obesity among elementary students in rural Kentucky. International Quarterly of Community Health Education, 29, 57–70. doi:10.2190/IQ.29.1.e [DOI] [PubMed] [Google Scholar]
- Coppock J. H., Ridolfi D. R., Hayes J. F., Paul M. S., Wilfley D. E. (2014). Current approaches to the management of pediatric overweight and obesity. Current Treatment Options in Cardiovascular Medicine, 16, 343..doi:10.1007/s11936-014-0343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. M., Sampilo M., Gallagher K. S., Dean K., Saroja M. B., Yu Q., Sporn N. (2016). Treating rural paediatric obesity through telemedicine vs. telephone: outcomes from a cluster randomized controlled trial. Journal of Telemedicine and Telecare, 22, 86–95. doi:10.1177/1357633X15586642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. M., Sampilo M., Gallagher K. S., Landrum Y., Malone B. (2013). Treating rural pediatric obesity through telemedicine: outcomes from a small randomized controlled trial. Journal of Pediatric Psychology, 38, 932–943. doi:10.1093/jpepsy/jst005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economos C. D., Hyatt R. R., Goldberg J. P., Must A., Naumova E. N., Collins J. J., Nelson M. E. (2007). A community intervention reduces BMI z-score in children: shape up Somerville first year results. Obesity, 15, 1325–1336. doi:10.1038/oby.2007.155 [DOI] [PubMed] [Google Scholar]
- Epstein L. H., Paluch R. A., Roemmich J. N., Beecher M. (2007). Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychology, 26, 381.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. H., Valoski A., Wing R. R., McCurley J. (1994). Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychology, 13, 373–383. doi:10.1037/0278-6133.13.5.373 [DOI] [PubMed] [Google Scholar]
- Epstein L., Squires S. (1998). The Stoplight Diet for Children: An Eight-Week Program for Parents and Children. Boston, MA: Little Brown and Compant. [Google Scholar]
- Freedman D. S., Ogden C. L., Kit B. K. (2015). Interrelationships between BMI, skinfold thicknesses, percent body fat, and cardiovascular disease risk factors among U.S. children and adolescents. BMC Pediatrics, 15, 1–9. doi:10.1186/s12887-015-0493-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan M., Fainaru M., Weizman A. (1998). Role of behaviour modification in the treatment of childhood obesity with the parents as the exclusive agents of change. International Journal of Obesity, 22, 1217–1224. doi:10.1038/sj.ijo.0800749 [DOI] [PubMed] [Google Scholar]
- Harlan L. C., Block G. (1990). Use of adjustment factors with a brief food frequency questionnaire to obtain nutrient values. Epidemiology (Cambridge, Mass.), 1, 224–231. [DOI] [PubMed] [Google Scholar]
- Janicke D. M., Sallinen B. J., Perri M. G., Lutes L. D., Huerta M., Silverstein J. H., Brumback B. (2008). Comparison of parent-only vs family-based interventions for overweight children in underserved rural settings: outcomes from Project STORY. Archives of Pediatrics & Adolescent Medicine, 162, 1119–1125. doi:10.1001/archpedi.162.12.1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke D. M., Steele R. G., Gayes L. A., Lim C. S., Clifford L. M., Schneider E. M., Westen S. (2014). Systematic review and meta-analysis of comprehensive behavioral family lifestyle interventions addressing pediatric obesity. Journal of Pediatric Psychology, 39, 809–825. doi:10.1093/jpepsy/jsu023 [DOI] [PubMed] [Google Scholar]
- Johnson J. A., Johnson A. M. (2015). Urban-rural differences in childhood and adolescent obesity in the United States: a systematic review and meta-analysis. Childhood Obesity, 11, 233–241. doi:10.1089/chi.2014.0085 [DOI] [PubMed] [Google Scholar]
- Kerner J., Rimer B., Emmons K. (2005). Introduction to the special section on dissemination: dissemination research and research dissemination: how can we close the gap? Health Psychology, 24, 443–446. doi:10.1037/0278-6133.24.5.443 [DOI] [PubMed] [Google Scholar]
- Kitzmann K. M., Dalton W. T., Stanley C. M., Beech B. M., Reeves T. P., Buscemi J., Midgett E. L. (2010). Lifestyle interventions for youth who are overweight: a meta-analytic review. Health Psychology, 29, 91–101. doi:10.1037/a0017437 [DOI] [PubMed] [Google Scholar]
- Kuczmarski R. J., Ogden C. L., Grummer-Strawn L. M., Flegal K. M., Guo S. S., Wei R., Johnson C. L. (2000). CDC growth charts: United States. Advance Data, 314, 1–27. [PubMed] [Google Scholar]
- Lim C. S., Janicke D. M. (2013). Barriers related to delivering pediatric weight management interventions to children and families from rural communities. Children’s Health Care, 42, 214–230. doi:10.1080/02739615.2013.816596 [Google Scholar]
- Llabre M. M., Ard J. D., Bennett G., Brantley P. J., Fiese B., Gray J., Wilfley D. (2018, March 10). Clinical practice guideline for multicomponent behavioral treatment of obesity and overweight in children and adolescents. Retrieved from https://www. apa.org/about/offices/directorates/guidelines/obesity-clinical-practice-guideline.pdf
- MacLean P. S., Wing R. R., Davidson T., Epstein L., Goodpaster B., Hall K. D., Ryan D. (2015). NIH working group report: innovative research to improve maintenance of weight loss. Obesity, 23, 7–15. doi:10.1002/oby.20967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pbert L., Druker S., Barton B., Olendzki B., Andersen V., Persuitte G., Geller A. C. (2016). Use of a FITLINE to support families of overweight and obese children in pediatric practices. Childhood Obesity, 12, 33–43. doi:10.1089/chi.2015.0101 [DOI] [PubMed] [Google Scholar]
- Perri M. G., Limacher M. C., Durning P. E., Janicke D. M., Lutes L. D., Bobroff L. B., Martin A. D. (2008). Treatment of Obesity in Underserved Rural Settings (TOURS): a randomized trial of extended-care programs for weight management. Archives of Internal Medicine, 168, 2347–2354. doi:10.1001/archinte.168.21.2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, C. L., Baranowski, T., & Parcel, G. S. (1990). How individuals, environments, and health behavior interact: Social learning theory. In K. Glanz, F. M. Lewis, & B. K. Rimer (Eds.), The Jossey-Bass health series. Health behavior and health education: Theory, research, and practice (pp. 161–186). San Francisco, CA, US: Jossey-Bass. [Google Scholar]
- Ricketts T. C., Johnson-Webb K. D., Taylor P. (1998). Definitions of Rural: A Handbook for Health Policy Makers and Researchers. Chapel Hill, NC: Federal Office of Rural Health Policy; Retrieved from http://www.shepscenter.unc.edu/rural/pubs/report/ruralit.pdf [Google Scholar]
- Robinson T. N. (2008). Treating pediatric obesity: generating the evidence. Archives of Pediatrics & Adolescent Medicine, 162, 1191–1192. doi:10.1001/archpedi.162.12.1191 [DOI] [PubMed] [Google Scholar]
- Schwimmer J. B., Burwinkle T. M., Varni J. W. (2003). Health-related quality of life of severely obese children & adolescents. JAMA, 289, 1813–1819. doi:10.1001/jama.289.14.1813 [DOI] [PubMed] [Google Scholar]
- Trasande L., Chatterjee S. (2009). The impact of obesity on health service utilization and costs in childhood. Obesity, 17, 1749–1754. doi:10.1038/oby.2009.67 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health & Human Services. Health Services Research Administration—Data Warehouse Retrieved from https://datawarehouse.hrsa.gov/topics/shortageAreas.aspx
- Varni J. W., Burwinkle T. M., Jacobs J. R., Gottschalk M., Kaufman F., Jones K. L. (2003). The PedsQL in type 1 and type 2 diabetes: reliability and validity of the pediatric quality of life inventory generic core scales and type 1 diabetes module. Diabetes Care, 26, 631–637. doi:10.2337/diacare.26.3.631 [DOI] [PubMed] [Google Scholar]
- Whitlock E. P., O'Connor E. A., Williams S. B., Beil T. L., Lutz K. W. (2010). Effectiveness of weight management interventions in children: a targeted systematic review for the USPSTF. Pediatrics, 125, e396–e418. doi:10.1542/peds.2009-1955 [DOI] [PubMed] [Google Scholar]
- Wilfley D. E., Saelens B. E., Stein R. I., Best J. R., Kolko R. P., Schechtman K. B., Epstein L. H. (2017a). Dose, content, and mediators of family-based treatment for childhood obesity. JAMA Pediatrics, 171, 1151–1159. doi:10.1001/jamapediatrics.2017.2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfley D. E., Staiano A. E., Altman M., Lindros J., Lima A., Hassink S. G., Cook S. (2017b). Improving access and systems of care for evidence-based childhood obesity treatment: conference key findings and next steps. Obesity, 25, 16–29. doi:10.1002/oby.21712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfley D. E., Stein R. I., Saelens B. E., Mockus D. S., Matt G. E., Hayden-Wade H. A., Epstein L. H. (2007). Efficacy of maintenance treatment approaches for childhood overweight: a randomized controlled trial. JAMA, 298, 1661–1673. doi:10.1001/jama.298.14.1661 [DOI] [PubMed] [Google Scholar]
- Williamson D. A., Champagne C. M., Harsha D., Han H., Martin C. K., Newton R., Ryan D. H. (2008). Louisiana Health: design and methods for a childhood obesity prevention program in rural schools. Contemporary Clinical Trials, 29, 783–795. doi:10.1016/j.cct.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. K. (2009). New perspectives on health disparities and obesity interventions in youth. Journal of Pediatric Psychology, 34, 231–244. doi:10.1093/jpepsy/jsn137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A. M. (1998). What is the economic case for treating obesity? Obesity Research, 6, 2S–7S. doi:10.1002/j.1550-8528.1998.tb00682 [DOI] [PubMed] [Google Scholar]