Abstract
Extinction of aversive memories has been a major concern in neuropsychiatric disorders, such as anxiety disorders and drug addiction. However, the mechanisms underlying extinction of aversive memories are not fully understood. Here, we report that extinction of conditioned place aversion (CPA) to naloxone-precipitated opiate withdrawal in male rats activates Rho GTPase Rac1 in the ventromedial prefrontal cortex (vmPFC) in a BDNF-dependent manner, which determines GABAA receptor (GABAAR) endocytosis via triggering synaptic translocation of activity-regulated cytoskeleton-associated protein (Arc) through facilitating actin polymerization. Active Rac1 is essential and sufficient for GABAAR endocytosis and CPA extinction. Knockdown of Rac1 expression within the vmPFC of rats using Rac1-shRNA suppressed GABAAR endocytosis and CPA extinction, whereas expression of a constitutively active form of Rac1 accelerated GABAAR endocytosis and CPA extinction. The crucial role of GABAAR endocytosis in the LTP induction and CPA extinction is evinced by the findings that blockade of GABAAR endocytosis by a dynamin function-blocking peptide (Myr-P4) abolishes LTP induction and CPA extinction. Thus, the present study provides first evidence that Rac1-dependent GABAAR endocytosis plays a crucial role in extinction of aversive memories and reveals the sequence of molecular events that contribute to learning experience modulation of synaptic GABAAR endocytosis.
SIGNIFICANCE STATEMENT This study reveals that Rac1-dependent GABAAR endocytosis plays a crucial role in extinction of aversive memories associated with drug withdrawal and identifies Arc as a downstream effector of Rac1 regulations of synaptic plasticity as well as learning and memory, thereby suggesting therapeutic targets to promote extinction of the unwanted memories.
Keywords: aversive memory, drug withdrawal, endocytosis, extinction, GABAA receptor, Rac1
Introduction
The aversive memory associated with drug withdrawal can evoke motivational and/or emotional states that lead to compulsive drug taking (Koob, 2000; Hutcheson et al., 2001). Extinction of such memory has been proposed as a therapeutic strategy for the treatment of drug addiction (Barad, 2005; Davis et al., 2006; Hofmann et al., 2006). Our previous study demonstrates that epigenetic regulation within the ventromedial prefrontal cortex (vmPFC) of BDNF transcription is required for the extinction of conditioned place aversion (CPA) to naloxone-precipitated opiate withdrawal (Wang et al., 2012). However, the mechanisms by which BDNF in the mPFC contribute to the extinction of aversive memory are unknown.
Extinction of classical fear conditioning is an active learning process requiring synaptic structural plasticity (Lai et al., 2012). Dendritic spines, which are central to synaptic structural plasticity (Lüscher et al., 2000), undergo activity-dependent structural remodeling that has been proposed to be a cellular basis of LTP (Engert and Bonhoeffer, 1999; Toni et al., 1999) as well as learning and memory (Matsuzaki et al., 2004; Yang et al., 2009; Fu et al., 2012). Activity-dependent changes in spine structure rely on rearrangements of the actin cytoskeleton. One of the best-characterized pathways for regulation of actin dynamics involves the Rho family of small GTPases, Rac1, Cdc42, and Rho (Hall, 1998, 2005). Rac1 has been shown to play a crucial role in synapse formation and plasticity (Luo, 2000; Nakayama et al., 2000). Studies of the role of Rac1 in spine morphogenesis in vivo and vitro have suggested that Rac1 plays an important role in regulating the size and density of spines in neurons (Luo et al., 1996; Zhang et al., 2003; Tolias et al., 2005; Xie et al., 2007). Although substantial evidence demonstrates that Rac1 is essential for diverse forms of learning, little is known whether Rac1 participates in an opposing form of learning (extinction) and how Rac1 contributes to extinction of an established memory.
Information processing in brain is controlled by a dynamic interaction between excitatory and inhibitory neurotransmission. GAGAA receptors (GABAARs) constitute the major inhibitory synaptic transmission network in the brain and are essential for maintaining the excitatory/inhibitory balance of neuronal circuits (Lüscher et al., 2011). Modulating the strength of GABAergic inhibitory transmission has important implications for synaptic plasticity and information processing in the brain (Smith and Kittler, 2010). It has been shown that inhibiting GABAergic transmission facilitates LTP induction in excitatory synapses in many brain regions, including mPFC (Wigström and Gustafsson, 1983; Mott and Lewis, 1991; Lu et al., 2010) and is implicated in the extinction of some forms of aversive memories (McGaugh et al., 1990; Berlau and McGaugh, 2006; Akirav, 2007; Hart et al., 2009; Makkar et al., 2010). In contrast to the extensive efforts made to understand the role of the insertion and removal of AMPARs at the postsynaptic membrane in synaptic plasticity (Collingridge et al., 2004), few studies have probed the molecular mechanisms and regulatory signaling pathways that locally control GABAAR trafficking.
GABAARs enter the endocytic pathway by a clathrin-mediated dynamin-dependent mechanism (Kittler et al., 2000; Herry and Garcia, 2003; Kittler et al., 2005). Substantial evidence demonstrates that the clathrin-mediated endocytosis of membrane receptors is dependent on ADF/cofilin-mediated actin dynamics (Schafer et al., 2002; Yarar et al., 2005; Gu et al., 2010; Liu et al., 2012). Given a crucial role of Rac1 for actin dynamics, we hypothesized that it may be crucially implicated in activity-dependent GABAAR endocytosis. Therefore, we tested this hypothesis using the CPA model. We demonstrated that GABAAR endocytosis was necessary and sufficient for LTP expression in the vmPFC and extinction of drug withdrawal memory. We further show that GABAAR endocytosis is dependent on Rac1-mediated actin polymerization, and we establish Arc as a critical component in the rodent vmPFC to link activity-triggered enhancement of Rac1 activity to endocytosis of GABAARs. These new data provide basic insight into the regulation of GABAAR trafficking during synaptic plasticity and learning and reveal a mechanism of extinction of drug withdrawal memory.
Materials and Methods
Animals
Sprague Dawley male rats weighing 220–250 g were obtained from the Laboratory Animal Center, the Chinese Academy of Sciences, or Kunming Medical University. Rats were housed 3 per cage and maintained on a 12 h light/dark cycle with access to food and water ad libitum. Rac1 transgenic mice were purchased from The Jackson Laboratory (stock #012361) and reproduced and bred until 2 months old at the Animal Center of TongJi University. All experimental procedures were in strict accordance with the National Institutes of Health Guide for the care and use of laboratory animals.
Drugs and antibodies
Morphine hydrochloride was purchased from Qinghai Pharmaceutical General Factory with a permission license for its use in experiments from local government (Shanghai and Kunming). Naloxone hydrochloride was supplied by Sigma-Aldrich (catalog #N7758). Latrunculin A was obtained from Merck/Millipore (catalog #428021) and dissolved in 25% DMSO to a final concentration of 0.5 μg/μl; BDNF was obtained from R&D Systems (catalog #248-BD-025/CF) and dissolved in PBS to 1.5 μg/μl; TrkB/FC was obtained from Sigma-Aldrich (catalog #T8694) and dissolved in PBS to 1.3 μg/μl; NSC23766 (catalog #2161), Myr-P4 (catalog #1776), and Myr-S (control) were obtained from Tocris Bioscience and dissolved in PBS to 10 μg/μl, 60 pmol/μl, and 60 pmol/μl, respectively. The antibodies of anti-BDNF (catalog #sc-546, RRID: AB_630940) and anti-Arc/Arg3.1 (catalog #sc-17839, RRID: AB_626696) were purchased from Santa Cruz Biotechnology. The antibodies of anti-pPak1 (catalog #2601S, RRID: AB_330220), anti-pCofilin (catalog #3311S, RRID: AB_330238), and anti-Rac1/cdc42 (catalog #4651S, RRID: AB_10612265) were purchased from Cell Signaling Technology. The antibodies of anti-GABAA β3 (catalog #05-474, RRID: AB_11212228) were purchased from Merck/Millipore. The antibodies of anti-actin (catalog #A5441, RRID: AB_476744) were purchased from Sigma-Aldrich.
Intra-vmPFC microinjection
Surgery.
Rats (weighing 220–280 g when surgery began) or Rac1 transgenic mice (weighing 25–28 g) were anesthetized with sodium pentobarbital (55 mg/kg, i.p. or 7 mg/kg, i.p.), treated with atropine sulfate (0.2 mg/kg, i.p.) and then placed in a stereotaxic apparatus (Narishige). Rats were implanted bilaterally with guide cannulae in the vmPFC (anteroposterior 2.8 mm; mediolateral ±0.6 mm; dorsoventral −3.5 mm). The cannulae were anchored to the skull with stainless-steel screws and dental cement. A stainless-steel blocker was inserted into each cannula before and after microinjection.
Intra-vmPFC microinjection.
Each infusion was 0.5 μl per side, infused at a rate of 0.25 μl/min. Bilateral microinfusions were made through 31 gauge injector (1.0 mm beyond the tip of guide cannulae, anteroposterior 2.8 mm; mediolateral ±0.6 mm; dorsoventral −4.5 mm) that was connected to a 10 μl microsyringe mounted in a microinfusion pump (Harvard Apparatus), and the drugs were infused into the vmPFC over 2 min and given an additional 2 min to diffuse. TrkB-FC and NSC23766 were bilaterally microinjected into the vmPFC 30 min before extinction training. Myr-P4 and Myr-S were bilaterally injected into the vmPFC 60 min before extinction training. Latrunculin A was bilaterally injected into the vmPFC 10 min before extinction training. The doses of TrkB-FC, NSC23766, latrunculin A, and Myr-P4 were chosen based on pilot experiments or previous studies (Peters et al., 2010; Liu et al., 2012; Wang et al., 2012; Ding et al., 2013).
Histology
After behavior test, rats were deeply anesthetized with sodium pentobarbital and perfused transcardially with 0.9% saline, followed by 4% PFA in PBS. The brains were removed and stored in 4% PFA for postfixation and then transferred in a 30% sucrose solution (w/v in PBS) solution for 3–5 d. Coronal sections (30 μm thick) were cut on a cryostat (Leica), stained with cresyl violet, and then examined by light microscopy to determine the injection sites.
Behavioral procedures
Apparatus.
The behavioral apparatus [62 cm (length) × 24 cm (width) × 24 cm (height)] was divided into two equal-sized compartments and separated by a removable board (10 × 10 cm), which allowed the rat free access to each compartment. The two compartments were distinguished by visual and tactile cues: one was a black wall with a smooth floor, whereas the other was a white wall with a textured floor. There was a camera above the middle of the apparatus to record rat activity, and the data were stored in a computer.
Procedures.
CPA procedures were performed as described by our previous study (Hou et al., 2009). Briefly, CPA consists of three phases: preconditioning, conditioning, and testing. In the preconditioning phase, rats were allowed to freely explore the entire apparatus for 15 min. Time spent in each compartment was recorded. Conditioning took place over the next 2 d. On the first day, the rats were injected with saline (1 ml/kg, s.c.). Four hours later, they were again given saline and then confined to either compartment in a counterbalanced manner for 30 min. On the second day, the rats were injected with either morphine (10 mg/kg, s.c) or saline. Four hours later, they were injected with naloxone (0.3 mg/kg, s.c) or saline and confined to the drug-paired side for 30 min. This compartment will be referred to as the “drug treatment-paired compartment.” Testing phase took place 24 h after the conditioning trial, and all rats were allowed to freely explore the entire apparatus for 15 min; the amount of time spent in each compartment was recorded. The CPA score represents the time in the drug treatment-paired compartment during the testing phase minus that during the preconditioning phase.
Extinction procedures were performed as described previously (Myers and Carlezon, 2010; Wang et al., 2012). Briefly, extinction training began 24 h after the post-training test and consisted of 30 min placement in the previously naloxone-paired boxes. Three sessions of extinction training were conducted with a 24 h interval between each, and a 15 min extinction test was performed 24 h after each extinction training. In each session of extinction training, the order of exposure was counterbalanced across rats and reversed relative to the preceding session. Saline was administered immediately before each extinction training. For examining the effects of dynamin function-blocking peptide (Myr-P4), Rac1 inhibitor NSC23766 and actin polymerization inhibitor latrunculin A on GABAAR endocytosis and extinction of CPA, Myr-P4, NSC23766, and latrunculin A were bilaterally infused into the vmPFC of conditioned rats before extinction training. For Western blotting analysis, the rats were decapitated after last extinction training.
Tissue sample preparations
Brains were rapidly removed, frozen in liquid nitrogen, and stored in a −80°C freezer before dissection. Coronal brain sections (1 mm thick) were obtained by using a rat brain slicer (Braintree Scientific). The infralimbic subdivisions of the vmPFC from both hemispheres of rats were punched from brain slices by using a blunt-end, 17 gauge syringe needle (1 mm inner diameter). In all subsequent procedures, the tissues were maintained at 4°C. Briefly, homogenate of the tissue in the 0.32 m sucrose buffer was centrifuged at 1000 × g for 10 min. The pellet was discarded, and the supernatant was centrifuged at 17,000 × g for 30 min to obtain a crude synaptosomal fraction. The resultant pellet was washed with 0.32 m sucrose buffer and then centrifuged at 17,000 × g for another 30 min. The synaptosomal membrane and subcellular fractionations were prepared as described previously (Hou et al., 2009; Liu et al., 2012). Briefly, the crude synaptosomal fraction was dissolved hypo-osmotically and centrifuged at 25,000 × g for 25 min to precipitate a synaptosomal membrane fraction. To separate F-actin and G-actin, synaptosomal membrane fraction was lysed in buffer A (1% Triton X-100, 20 mm HEPES, 100 mm NaCl, 2 mm EDTA, 5 mm NaF, 1 mm Na3VO4, 1 mm aprotinin, 1 mm leupeptin, 1 mm PMSF, pH 7.2) for 1 h and centrifuged at 10,000 × g for 20 min. Pellets were dissolved in buffer B (15 mm HEPES, 0.15 mm NaCl, 1% SDS, 10 mm EDTA, 1 mm DTT, 5 mm NaF, 1 mm Na3VO4, 1 mm aprotinin, 1 mm leupeptin, 1 mm PMSF, pH 7.5) for 1 h and centrifuged at 10,000 × g for 20 min. The G-actin fraction (the first supernatant) and the F-actin fraction (the second supernatant) were collected.
Western blotting
Briefly, loading buffer was added to each protein sample and boiled at 100°C for 10 min. Then the protein samples were cooled and loaded in each lane, separated in 10%–15% SDS-PAGE, and blotted onto PVDF membrane. The membranes were blocked for 1 h at room temperature in 5% BSA, followed by incubation overnight at 4°C with various primary antibodies that included the following: anti-BDNF at a dilution of 1:500; anti-GABAA β3, anti-pPak1, anti-pCofilin, anti-Rac1/cdc42 at a dilution of 1:2000; anti-Arc/Arg3.1 at a dilution of 1:1000; anti-actin at a dilution of 1:10,000. Then membranes were rinsed with TBST (Tris-buffered saline plus 0.05% Tween 20, pH 7.4) and incubated for 1 h with peroxidase-conjugated goat anti-rabbit or anti-mouse IgG. Membranes were rinsed with TBST again. Then chemiluminescent detection was performed with the ECL kit (GE Healthcare, catalog #RPN2232) and exposed against x-ray film (Eastman Kodak) for 30 s to 2 min. The immunopositive signals were quantified by Quantity One software (Bio-Rad).
Immunohistochemistry of Arc
Immunohistochemical assay was performed as described by our previous study (Li et al., 2009). Briefly, the brain slices from different groups were cut at a thickness of 30 μm and washed with 0.1 mol/L phosphate buffer. The brain slices were blocked with 10% normal goat serum for 2 h at room temperature and then incubated overnight with primary antibody (mouse anti-Arc antibody, 1:500 dilution in 10% normal goat serum) at 4°C. Next, the brain slices were incubated with secondary antibody (biotinylated goat anti-mouse IgG, 1:200 dilution in 10% normal goat serum) for 2 h at room temperature. Arc-positive sites were visualized using a streptavidin-ABC kit and a DAB kit using 0.1% DAB as the chromogen. In control slices in which the primary antibodies were omitted or replaced by nonimmune rabbit or goat serum, no stained cells were seen. The brain slices were subsequently dehydrated in alcohol and xylene, coverslipped, and imaged on an Olympus IX51 microscope.
Assay for GTPase activity
Active Rac1 and Cdc42 pull-downs were performed as described by the commercial active Rac1/Cdc42 Pull-Down and Detection Kit protocol (Pierce, catalog #16118). Briefly, lysates of the rat vmPFC tissue was centrifuged at 16,000 × g at 4°C for 15 min, and then the supernatants were transferred to a new tube and were added with GTPγS or GDP to incubate at 30°C for 15 min under the condition of constant agitation. Then the mixtures were incubated with glutathione resin beads and glutathione S-transferase-fused Rac/Cdc42-binding domain of p21-activated kinase (Pak) at 4°C for 1 h; beads had been washed several times previously to remove nonspecific binding. The beads and proteins bound to the fusion protein were washed three times with wash buffer at 4°C, eluted in SDS sample buffer, and analyzed for bound Cdc42 and Rac1 by Western blotting using antibodies against Cdc42 or antibodies against Rac1.
Surface receptor cross-linking with bis (sulfosuccinimidyl) suberate (BS3)
Surface and intracellular levels of the β3 subunit of the GABAAR levels were determined by using a protein cross-linking assay as previously described (Ives et al., 2002; Liu et al., 2012), with minor modifications. Briefly, rat brain tissue was incubated with the protein cross-linking reagent BS3 (Pierce Biotechnology, catalog #21580) to determine S (surface) and I (intracellular) levels of receptor subunit proteins. After the last extinction training, rats were decapitated. Brains were rapidly removed, and coronal brain sections (0.5 mm thick) containing the vmPFC were obtained by using a rat brain slicer (Braintree Scientific). Slices were then inserted into 2 ml Eppendorf tubes containing 1 ml of ACSF spiked with 2 mm BS3. The ACSF contained the following (in mm): NaCl 120, KCl 2.5, NaHCO3 26, NaH2PO4 1.25, CaCl2 2, MgSO4 2, and d-glucose 10, and was bubbled with the gas mixture of 95% O2 and 5% CO2 for at least 1 h. Incubation with gentle agitation proceeded for 30 min at 4°C. Cross-linking was terminated by quenching the reaction with 100 mm glycine (10 min, 4°C). The slices were pelleted for 2 min at 14,000 rpm, and the supernatant was discarded. Pellets were resuspended in ice-cold lysis buffer containing protease inhibitors (Roche, catalog #11836170001) and phosphatase inhibitors (Roche, catalog #4906837001) and homogenized rapidly by sonicating, samples were centrifuged at 14,000 rpm for 2 min, and the supernatant fractions were collected for Western blotting. Samples were liquated and stored at −20°C for Western blotting analysis.
Lentivirus construction and infection
Lentivirus construction and production.
The Lentivirus plasmid pSicoR was purchased from Addgene, and oligos coding for the various shRNAs were annealed and cloned into HpaI-XhoI-digested pSicoR vectors. The target shRNA regions were chosen as follows: Arc, GCTGATGGCTACGACTACA; Rac1, GCAAACAGACGTGTTCTTAAT; negative control, TTCTCCGAACGTGTCACGT. Lentiviruses were generated as described below. Briefly, 5 μg of Lentivirus vector and 2.5 μg of each packaging vector were cotransfected in HEK 293T cells by using the FuGENE 6 reagent (Roche Diagnostics). Supernatants were collected 36–48 h after transfection, filtered through a 0.4 μm filter. High-titer stocks were prepared by an initial ultracentrifugation for 1 h at 23,000 rpm (SW-28 rotor; Beckman Coulter) and a secondary tabletop centrifugation at 13,000 × g for 30 min. Viral pellets were resuspended in 1% BSA/PBS and stored at −80°C. Viral titers were determined by infection of HEK293T cells, and GFP-positive cells were visualized by fluorescent microscopy. After concentration, viral titers were 5 × 108 to 2 × 109 transducing units (TU)/ml. Lentivirus expressing Arc/Arg3.1-shRNAs or control shRNAs was bilaterally infused into the vmPFC of rats that underwent place aversion conditioning. Two weeks after virus infusion, the rats were subjected to extinction training.
Stereotaxic injections of lentivirus into the vmPFC
Rats were anesthetized with sodium pentobarbital (55 mg/kg, i.p. or 7 mg/kg, i.p.), treated with atropine sulfate (0.2 mg/kg, i.p.). Lentiviruses (5 × 108 to 1 × 109 TU/ml) were stereotaxically injected into the vmPFC (3 μl/site) over 5 min using a 31 gauge injector (1.0 mm beyond the tip of guide cannula), which was connected to a 10 μl microsyringe mounted in a microinfusion pump (Harvard Apparatus). The injector was retained in place for another 5 min before being withdrawn at 1 mm/min. The injections were performed bilaterally at the following coordinates, as calculated from bregma and the dura mater: anteroposterior 2.8 mm; mediolateral ±0.6 mm; dorsoventral −4.5 mm.
Assay for Rac1 function using Rac1 transgenic mice
For adeno-associated virus (AAV) construction and infection, the Cre recombinase was cloned into the pSB1844-CMV-GFP vector. The AAV2-based vector pseudotyped with AAV8 serotype capsid (AAV2/8) was purchased and supplied in titers 2–3 × 1013 (vg/ml) genomic copies per milliliters (Obio Technology). Rac1 transgenic mice (weighing 25–28 g when surgery began) were anesthetized with sodium pentobarbital anesthesia (7 mg/kg, i.p.) and then placed in the same stereotaxic apparatus. During the surgery, mice were bilaterally microinjection of AAV-Cre-GFP or control AAV-GFP into the vmPFC (anteroposterior 1.7 mm; mediolateral ±0.3 mm; dorsoventral −2.5 mm). The mice were allowed to recover from surgery for at least 1 week, during which they were injected with norfloxacin to protect them from infection.
In vitro electrophysiological recording
Adult male rats weighing 220–280 g were anesthetized with diethyl ether and decapitated. Brains were rapidly transferred into ice-cold ACSF containing the following (in mm): NaCl 120, KCl 2.5, NaHCO3 26, NaH2PO4 1.25, CaCl2 2.0, MgSO4 2.0, and d-glucose 10, continuously bubbled with a gas mixture of 95% O2. Coronal vmPFC slices (400 μm) were prepared with a vibratome (VT 1000S, Leica). Slices were left to recover for 20 min in an incubation chamber containing ACSF heated to 36 ± 1°C and then maintained at room temperature (22°C-25°C). Slices were placed in a recording chamber and perfused by oxygen saturated ACSF with a flow rate of 4–5 ml/min. The field EPSPs (fEPSPs) were recorded in layer 5, and a stimulating electrode was placed in layer 2/3 of the vmPFC. The fEPSPs were evoked using a bipolar platinum-iridium stimulating electrode (75 μm outside diameter) and recorded through a glass micropipette (3–4 mΩ, filled with ACSF). The stimulation was adjusted for each slice to produce reliable field potential that was ∼50% of the maximal response. LTP was elicited by high-frequency stimuli (50 Hz, 50 pulses, 1 train) after a baseline was stably recorded for at least 20 min at a frequency of 0.033 Hz.
Experimental design and statistical analysis
All experimental male rats and mice were used at the ages indicated in the text. Rac1 transgenic mice were purchased from The Jackson Laboratory (stock #012361). Data analysis was performed using Clampfit 10.2 (Axon Instruments), Quantity One software (Bio-Rad Laboratories), Image-pro plus (Media Cybernetics), Conditioned place aversion software (Anilab Software and Instruments), and Prism 5 (GraphPad Software). Unless stated, all values are presented as mean ± SEM for the number of experiments (n) indicated in the text and in the figure legends. Data were analyzed by unpaired Student's t test, one-way or two-way ANOVA with Bonferroni post hoc test. Statistical significance was set at p < 0.05. The magnitude of LTP is the average of the last 5 min recordings expressed as percentage of the baseline fEPSP.
Results
Extinction training results in LTP induction and CPA extinction by dynamin-dependent GABAAR endocytosis
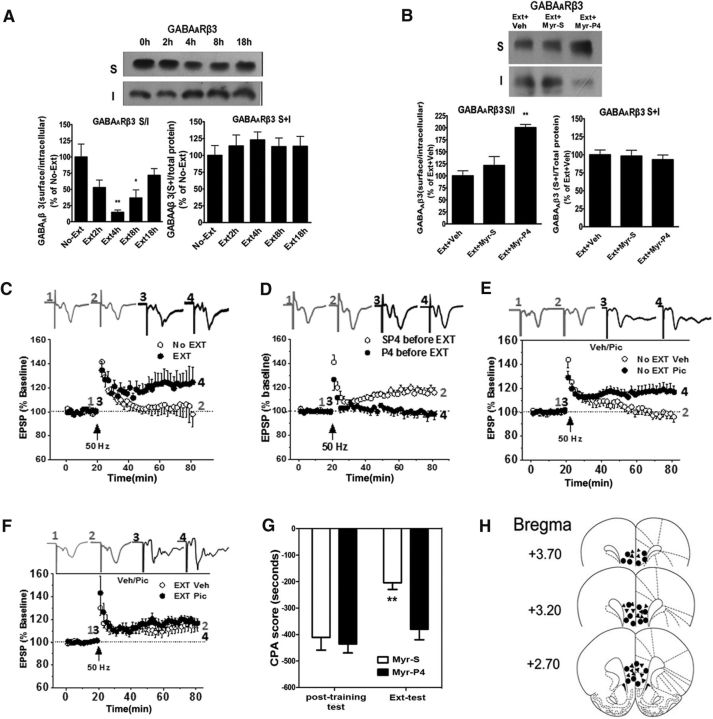
CPA can be extinguished by extinction training (Myers and Carlezon et al., 2010; Wang et al., 2012). Inhibiting GABAergic transmission is implicated in the extinction of several forms of aversive memories (Makkar et al., 2010). To elucidate the mechanisms underlying CPA extinction, we examined the effect of extinction training on GABAAR endocytosis. GABAAR β3 subunit is a major site for phosphorylation that determines receptor endocytosis in neurons (Kittler et al., 2005; Jacob et al., 2009). Thus, we tested whether extinction training produced GABAAR β3 subunit endocytosis using BS3 cross-linking assay (Ives et al., 2002). Rats were killed at different time points after extinction training. The vmPFC tissues were isolated quickly and treated with BS3. Immunoblot analysis of GABAARs in cross-linked tissue showed that the S/I ratio of the β3 subunit of the GABAARs was significantly decreased at 4 and 8 h after extinction training (4 h, 14.6 ± 3.62% of control, n = 6; 8 h, 36.7 ± 12.56% of control, n = 6; F(4,25) = 6.645, p = 0.0009; one-way ANOVA; Fig. 1A). Post hoc test revealed that there was significant decrease of the surface GABAAR β3 subunit at 4 and 8 h after extinction training compared with no-extinction groups (p < 0.01, p < 0.05). No significant differences in the levels of GABAAR total protein were found between any of the experimental groups, quantified by summing the optical densities of surface and intracellular bands and normalizing to total protein in the lanes (F(4,25) = 0.3332, p = 0.9677). Regulation of AMPAR trafficking is also known to be critical for synaptic plasticity and memory (Malinow and Malenka, 2002; Anggono and Huganir, 2012; Liu et al., 2012). Next, we determined whether extinction training also exerted an effect on AMPAR trafficking. It was found that extinction training had no significant effect on AMPAR endocytosis (Fig. 1-1).
Figure 1.
Extinction training results in LTP induction and CPA extinction by dynamin-dependent GABAAR endocytosis. A, Extinction training resulted in endocytosis of GABAAR β3 subunits (Fig. 1-1). B, Endocytosis of GABAAR β3 subunits was prevented by bilateral infusion of dynamin function-blocking peptide (Myr-P4, 30 pmol/0.5 μl/side) but not scramble inactive peptide (Myr-S) into the vmPFC 60 min before extinction training (Fig. 1-2). C, The 50 Hz stimulus induced reliable LTP in slices from rats that underwent extinction training but not in those that did not undergo extinction training. D, HFS at 50 Hz failed to induce LTP in slices from rats that were pretreated with bilateral infusion of Myr-P4 (30 pmol/0.5 μl/side) into the vmPFC 60 min before extinction training, but it normally induced reliable LTP in slices from rats that were pretreated with Myr-S before extinction training. E, In the presence of GABAAR blocker picrotocxin (100 μm), HFS at 50 Hz was able to elicit LTP induction in slices from rats that did not undergo extinction training. F, The same picrotoxin application did not induce significant change in the facilitated LTP induction found in slices from rats that underwent extinction training. G, CPA extinction was impaired by intra-vmPFC infusions of Myr-P4 (30 pmol/0.5 μl/side) but not Myr-S before extinction training. H, Schematic representation of injection sites in the vmPFC for rats used in the experiments. A, B, Top, Representative blots of surface (S) and internalized (I) GABAAR β3 subunits from the vmPFC tissues of rats prepared at different time points after extinction training (A: 2, 4, 8, and 18 h) or (B: 4 h). Bottom, Quantification of the S/I ratio of GABAAR β3 subunit levels from Western blot data. Error bars indicate mean ± SEM (n = 6–8). *p < 0.05, compared with the no-extinction control or vehicle control groups (one-way or two-way ANOVA followed by Bonferroni's post hoc test). **p < 0.01, compared with the no-extinction control or vehicle control groups (one-way or two-way ANOVA followed by Bonferroni's post hoc test). The magnitude of LTP was the average of the last 5 min recordings expressed as the mean ± SEM percentage of the baseline fEPSP. Data were analyzed with two-tailed Student's t tests.
Extinction training has no effect on endocytosis of surface GluR1- and GluR2-containing AMPARs. Top panels show representative blots of surface (S) and internalized (I) GluR1- (A) and GluR2- (B) containing AMPARs from vmPFC tissues prepared from rats 4 h after extinction training. Bottom panels show quantification of surface/internal GluR1- and GluR2-containing AMPARs levels from Western blot data. Download Figure 1-1, TIF file (1.7MB, tif)
Intra-vmPFC infusion of dynamin function-blocking peptide (Myr-P4) before extinction training has no effect on endocytosis of GABAAR β3 subunit in no-extinction training rats. Top panels show representative blots of surface (S) and internalized (I) GABAAR β3 subunit from vmPFC tissues prepared from No-Ext rats. Bottom panels show quantification of surface/internal GABAAR β3 subunit levels from Western blot data. Download Figure 1-2, TIF file (1.5MB, tif)
Dynamin-dependent endocytosis is important in the regulation of cell surface levels of GABAARs (Kittler et al., 2000; Herry and Garcia, 2003). Next, we examined the effect of Myr-P4, a dynamin function-blocking peptide, on extinction training-induced GABAAR β3 subunit endocytosis. As shown in Figure 1B, bilateral intra-vmPFC infusions of Myr-P4 but not inactive control peptide Myr-S (30 pmol/0.5 μl/side) 60 min before extinction training resulted in a higher level of the S/I ratio of the β3 subunit of GABAARs (vehicle, 100 ± 25.67%, n = 6; Myr-S, 121.55 ± 45.46%; Myr-P4, 200.44 ± 16.06%, n = 6). One-way ANOVA of the S/I ratio of β3 subunits of the GABAARs revealed a main effect of group (F(2,15) = 16.87, p = 0.001). Post hoc analysis confirmed that Myr-P4-infused rats exhibited significantly higher S/I ratios of the β3 subunit of GABAARs relative to Myr-S or vehicle-infused rats (p < 0.01), indicating that Myr-P4 abolished extinction training-induced GABAAR endocytosis, whereas Myr-P4 had no significant effect on GABAAR trafficking in no-extinction rats (Fig. 1-2). No significant difference was observed in the levels of GABAAR total protein between any of the experimental groups.
LTP is widely thought to be a key mechanism underlying formation of new memory (Cooke and Bliss, 2006). Given that inhibiting GABAergic transmission facilitates LTP induction in excitatory synapses in many brain regions, we tested whether extinction training could affect LTP induction at the excitatory synapses of the vmPFC. Here, a weak protocol in brain slices (i.e., high-frequency stimulation [HFS]) at 50 Hz rather than 100 or 200 Hz, was applied to study whether LTP induction in the vmPFC was facilitated by extinction training. As shown in Figure 1C, HFS at 50 Hz was unable to induce LTP in no-extinction rats (103 ± 6.56%), but it induced a reliable LTP in extinction rats (124.19 ± 12.29%, t(10) = 2.27, p = 0.046, compared with no-extinction rats). To determine whether LTP facilitation by extinction training was mediated by GABAAR endocytosis, we assessed whether 50 Hz-induced LTP in the rats with extinction training was prevented by intra-vmPFC infusion of dynamin function-blocking peptide (Myr-P4) (30 pmol/0.5 μl/side) 60 min before extinction training. As shown in Figure 1D, HFS at 50 Hz failed to induce LTP in the rats pretreated with Myr-P4 (97.72 ± 4.33%), but it induced a reliable LTP in the rats pretreated with scramble inactive peptide (Myr-S) (116.86 ± 4.10%, t(13) = 3.19, p = 0.006, compared with rats pretreated with Myr-P4), indicating that extinction training-facilitated LTP induction was due to dynamin-dependent GABAAR endocytosis. To further confirm that a reduced GABAergic inhibition by GABAAR endocytosis was involved in LTP facilitation at the excitatory synapses of the vmPFC neurons after extinction training, we further tested whether application of the GABAAR chloride channel blocker picrotoxin could be sufficient for LTP facilitation even without extinction training. HFS at 50 Hz failed to induce LTP in nonextinction rats (96.94 ± 4.26%), but it induced a reliable LTP in the condition with the presence of 100 μm picrotoxin (118.21 ± 6.21%, t(23) = 2.17, p = 0.04; Fig. 1E). Importantly, the same picrotoxin application did not cause further LTP facilitation in the rats that underwent extinction training (vehicle, 116.87 ± 7.55%; picrotoxin, 116.79 ± 4.34, t(20) = 0.23, p = 0.818; Fig. 2F), indicating that the effect of extinction had occluded that caused by reducing GABA inhibition with picrotoxin. This suggests that extinction training and picrotoxin application share the same mechanism by reducing GABA inhibition in facilitating LTP.
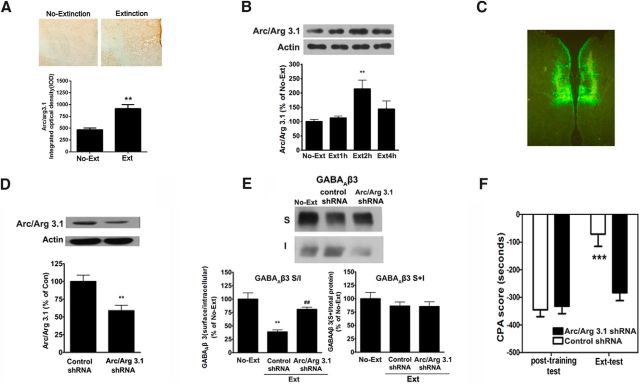
Figure 2.
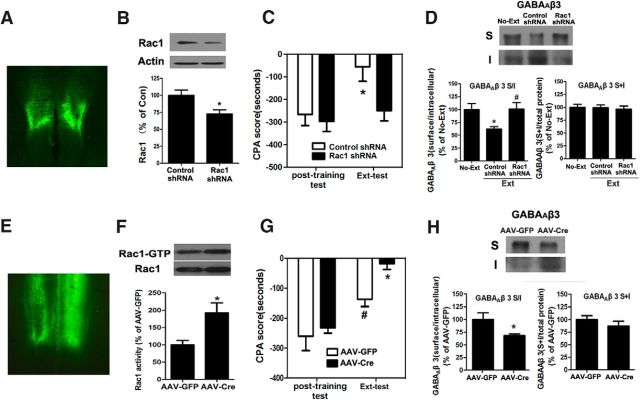
Extinction training increases synaptic localization of Arc to regulate GABAAR endocytosis and CPA extinction. A, CPA rats at 1 h after extinction training showed elevation of Arc protein expression in the vmPFC, detected by using immunohistochemical analysis. B, Increase of synaptic Arc protein levels in the vmPFC was detected at 2 h after extinction training by immunobloting. C, Lentivirus-infected regions in the vmPFC were visualized by fluorescence microscope. D, Infection of the vmPFC with lentivirus expressing Arc/Arg3.1-shRNA attenuated synaptic Arc protein expression within the vmPFC. E, Infection of the vmPFC with lentivirus expressing Arc/Arg3.1-shRNA inhibited GABAAR β3 subunit endocytosis. F, Infection of the vmPFC with lentivirus expressing Arc/Arg3.1-shRNA impaired CPA extinction. Error bars indicate mean ± SEM (n = 6–8). **p < 0.01, compared with no-extinction or control shRNA groups. ***p < 0.001 compared with no-extinction or control shRNA groups. ##p < 0.01, compared with control shRNA groups (two-tailed Student's t test, or one-way or two-way ANOVA followed by Bonferroni's post hoc test).
To determine whether GABAAR endocytosis was required for CPA extinction, we next tested the effect of intra-vmPFC infusion of Myr-P4 on the behavior. Bilateral intra-vmPFC infusions of Myr-P4 (30 pmol/0.5 μl/side) 60 min before extinction training impaired CPA extinction (main effect of Myr-P4, F(1,12) = 4.832, p < 0.05, two-way ANOVA, post hoc, p < 0.01 compared with Myr-S-infused control groups; Fig. 1G). Figure 1H showed the location of microinjection tips in the vmPFC. Together, these data suggest that LTP facilitation and CPA extinction by extinction training are both attributable to dynamin-dependent GABAAR endocytosis in the vmPFC.
Increase of synaptic Arc protein levels in the vmPFC is required for GABAAR endocytosis and CPA extinction
Several studies report that Arc interacts with dynamin and endophilin to modulate AMPA receptor endocytosis in primary neuronal cultures and in the amygdala (Chowdhury et al., 2006; Rial Verde et al., 2006; Liu et al., 2012). Given that GABAARs underwent endocytosis via a dynamin-dependent mechanism in the vmPFC in response to extinction training (Fig. 1B), we asked whether Arc played a role in extinction training-induced GABAAR endocytosis and CPA extinction. To this end, we first examined the effects of extinction training on Arc protein expression and synaptic localization in the vmPFC using immunohistochemistry and immunoblotting. As shown in Figure 2A, extinction training induced a robust increase in the staining of Arc/Arg3.1 in the vmPFC, indicative of an enhancement of Arc expression, which is consistent with a previous study that extinction of contextual fear memory induces Arc protein expression in the mPFC (Mamiya et al., 2009). Arc protein levels in the synapses were further analyzed by subcellular fractionation of lysates of the vmPFC, followed by immunoblotting using Arc/Arg3.1 antibody (Liu et al., 2012). As shown in Figure 2B, a significant increase in Arc protein levels in the synaptosomal fractions of the vmPFC was detected at 2 h after extinction training (F(3,12) = 5.72, p < 0.01, one-way ANOVA followed by post hoc test), indicating that extinction training increases synaptic Arc protein expression.
Next, we determined whether the increased synaptic Arc was involved in extinction training-induced endocytosis of the GABAAR β3 subunit. To do this, we used a lentivirus expressing short-hairpin (sh) RNA (Arc/Arg3.1-shRNA) to knock down Arc/Arg3.1 mRNA within the vmPFC of rats that underwent place aversion conditioning. The conditioned rats received bilateral infusions of lentivirus expressing Arc/Arg3.1-shRNAs with high-titer stocks (5 × 108 to 1 × 109 TU/ml) or control shRNAs into the vmPFC. Two weeks after virus infusion, the rats were subjected to extinction training. After extinction training, rats were killed and their brains were isolated and sectioned into slices to detect the sites infected by the virus via visualization of the tagged GFP under a fluorescence microscope. As shown in Figure 2C, strong GFP fluorescence was observed in the vmPFC, indicative of an effective infection of lentivirus expressing Arc/Arg3.1-shRNAsA in the vmPFC. Next, we examined whether Arc expression was interfered by Arc/Arg3.1-shRNA. As shown in Figure 2D, intra-vmPFC infusion of Arc/Arg3.1-shRNA before extinction training significantly attenuated extinction training-induced synaptic Arc protein expression (t(1,10) = 3.52, p < 0.01, Student's t test). Then, we examined the effect of in vivo knockdown of synaptic Arc protein expression using Arc/Arg3.1-shRNA on extinction training-induced endocytosis of GABAAR β3 subunits. As shown in Figure 2E, Arc/Arg3.1-shRNA-infected rats exhibited a significant increase in the S/I ratio of the β3 subunit of the GABAAR (control shRNA, 38.74 ± 9.20% of control, n = 6; Arc/Arg3.1 shRNA, 80.89 ± 9.85% of control, n = 6; F(2,15) = 17.67, p < 0.01, one-way ANOVA). Post hoc analysis showed that there was a significant difference between control shRNA and Arc/Arg3.1-shRNA groups (p < 0.01). No significant difference was observed in the levels of GABAARs total protein between any of the experimental groups. These results demonstrate that the knockdown of synaptic Arc expression with Arc/Arg3.1-shRNAs reduces GABAAR endocytosis, supporting that synaptic Arc indeed plays an essential role in regulating GABAAR endocytosis in response to extinction training.
We also examined the effect of synaptic Arc knockdown on CPA extinction. As shown in Figure 2F, Arc/Arg3.1-shRNAs-infected rats displayed significantly higher CPA scores than control shRNAs-infected rats (main effect of Arc/Arg3.1-shRNA F(1,23) = 10.25, p = 0.004, two-way ANOVA, post hoc, p < 0.01), indicating that knockdown of synaptic Arc expression in the vmPFC impaired CPA extinction. Together, these results suggest that enhancement of synaptic Arc levels contributes to extinction training-induced GABAAR endocytosis and CPA extinction.
Rac1 activity in the vmPFC contributes to increase of synaptic Arc protein levels following extinction training
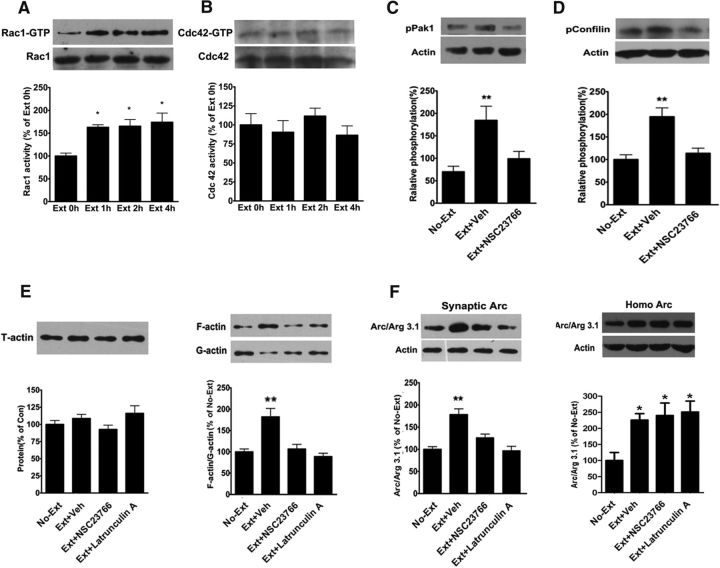
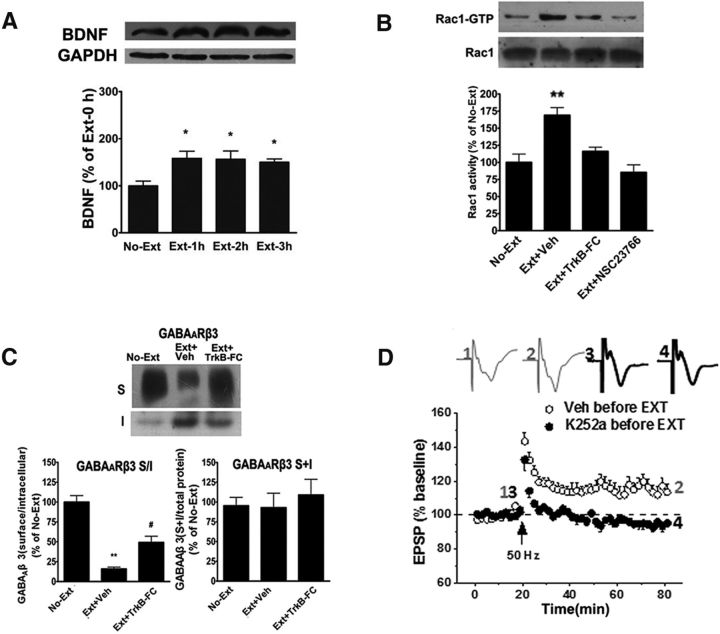
Our and other previous studies have suggested that actin polymerization is involved in synaptic translocation of Arc mRNA in response to LTP induction and behavioral learning (Huang et al., 2007; Liu et al., 2012). Next, we asked whether actin dynamics in the vmPFC was required for synaptic localization of Arc in response to extinction training. We first examined the activity of small GTPase Rac1 and cdc42 in the vmPFC following extinction training. As shown in Figure 3A, a significant increase in the levels of active Rac1 (Rac1-GTP) was detected in the vmPFC at 1, 2, and 4 h after extinction training (F(3,20) = 7.89, p < 0.01, one-way ANOVA followed by Bonferroni post hoc test). However, extinction training had no significant effect on the levels of active Cdc42 (Cdc42-GTP) (F(3,20) = 0.71, p > 0.05; Fig. 3B). Next, we examined the role of Rac1 in extinction training-induced actin polymerization. As shown in Figure 3C, D, extinction training resulted in activation (phosphorylation) of Pak1 (F(2,20) = 6.69, p < 0.01) and inactivation (phosphorylation) of cofilin (F(2,20) = 8.13, p < 0.01). Accordingly, extinction training also induced actin polymerization (F(3,14) = 7.89, p < 0.05; Fig. 4E). All these effects were blocked by bilateral intra-vmPFC infusions of Rac1 inhibitor NSC23766 (0.5 μg/ 0.5 μl/side) 30 min before extinction training (p < 0.01 vs vehicle-infused rats); however, NSC had no significant effect on cofilin and Pak1 phosphorylation in no-extinction rats (Fig. 3-1). In addition, actin polymerization was also abolished by bilateral intra-vmPFC infusions of the actin polymerization inhibitor latrunculin A (250 ng/0.5 μl/side) 10 min before extinction training (p < 0.01 compared with vehicle-infused rats; Fig. 3E). Together, these results support that extinction training induces actin polymerization within the vmPFC by activation of the Rac1/Pak1/cofilin signaling pathway.
Figure 3.
Synaptic localization of Arc depends on actin polymerization induced by Rac1-mediated phosphorylation of Pak1 and cofilin. A, B, Extinction training resulted in activation of Rac1, but not Cdc42, in the vmPFC. C, D, Extinction training led to phosphorylation of Pak1 and cofilin, and both were blocked by bilateral intra-vmPFC infusions of Rac1 inhibitor NSC23766 (Fig. 3-1). E, Extinction training-induced actin polymerization could be inhibited by NSC23766 (0.5 μg/ 0.5 μl/side) and actin polymerization inhibitor latrunculin A (250 ng/0.5 μl/side), whereas they have no effects on total actin (left). F, Blockade of actin polymerization by NSC23766 and latrunculin A disrupted extinction training-induced enhancement of Arc protein levels at synaptic membrane preparations (left) but had no effects on extinction training-induced Arc protein levels in the vmPFC homogenates (right). Error bars indicate mean ± SEM (n = 8). *p < 0.05, compared with no-extinction control groups (one-way ANOVA followed by Bonferroni's post hoc test). **p < 0.01, compared with no-extinction control groups (one-way ANOVA followed by Bonferroni's post hoc test).
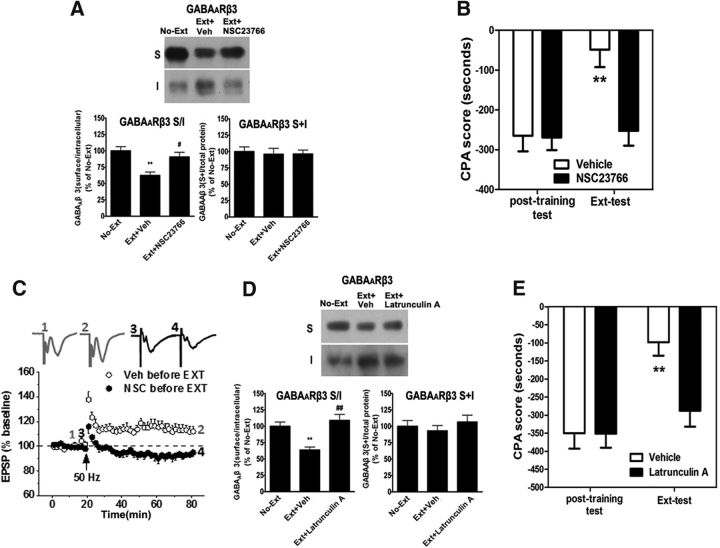
Figure 4.
Suppression of actin polymerization by NSC23766 and latrunculin A inhibits GABAAR endocytosis, LTP facilitation, and CPA extinction. A, B, Bilateral intra-vmPFC infusion of NSC23766 (0.5 μg/0.5 μl/side) 30 min before extinction training inhibited the endocytosis of GABAAR β3 subunits and impaired CPA extinction. C, HFS at 50 Hz failed to elicit LTP induction in slices from rats that were pretreated with Rac1 inhibitor NSC23766 before extinction training. D, E, Bilateral intra-vmPFC infusion of latrunculin A (250 ng/0.5 μl/side) 10 min before extinction training inhibited the endocytosis of GABAAR β3 subunits and impaired CPA extinction. Error bars indicate mean ± SEM (n = 6–8). **p < 0.01, compared with no-extinction control groups. #p < 0.05, compared with vehicle control groups (one-way ANOVA followed by Bonferroni's post hoc test). ##p < 0.01, compared with vehicle control groups (one-way ANOVA followed by Bonferroni's post hoc test). The magnitude of LTP was the average of the last 5 min recordings expressed as the mean ± SEM percentage of the baseline fEPSP. Data were analyzed with two-tailed Student's t tests.
Intra-vmPFC infusions of Rac1 inhibitor NSC23766 has no effect on the phosphorylation of Cofilin (A) or Pak1 (B) in the vmPFC of no-extinction rats. Download Figure 3-1, TIF file (1.4MB, tif)
Thereafter, we examined whether actin polymerization was required for elevation of synaptic Arc protein levels. Because NSC23766 and latrunculin A both blocked extinction training-induced actin polymerization, we examined the effects of these two agents on extinction training-induced increases in synaptic Arc protein levels. As shown in Figure 3F, extinction training-induced enhancement of synaptic Arc protein in the vmPFC can be blocked by both NSC23766 and latrunculin A (F(3,12) = 5.72, p < 0.01, one-way ANOVA followed by post hoc test; Fig. 3F, left). However, bilateral intra-vmPFC infusions of these two blocking reagents had no effects on total Arc protein levels in the vmPFC homogenates (p > 0.05, compared with vehicle-infused rats; Fig. 3F, right), indicating that actin polymerization is required for increased Arc protein levels in synapses. Overall, these results suggest that extinction training yields enhancement of synaptic Arc protein levels by Rac1-Pak1-cofilin signaling-dependent actin dynamics in the vmPFC.
Rac1 inhibitor NSC23766 and actin polymerization inhibitor latrunculin A suppress extinction training-induced GABAAR endocytosis extinction memory
Given the important role of activity-associated actin polymerization in synaptic localization of Arc, it was predicted that inhibition of actin polymerization could suppress extinction training-induced GABAAR endocytosis. To confirm this, we assessed the effect of actin polymerization inhibition using NSC23766 and latrunculin A on the extinction training-induced GABAAR β3 endocytosis, LTP facilitation, and CPA extinction. As shown in Figure 5A, bilateral intra-vmPFC injections of NSC23766 (0.5 μg/0.5 μl/side) 30 min before extinction training significantly inhibited the endocytosis of GABAAR β3 subunits (vehicle, 62.40 ± 12.55% of control; NSC23766, 90.57 ± 17.45% of control; F(2,17) = 9.89, p = 0.0018, one-way ANOVA, post hoc, p < 0.01 compared with vehicle-treated rats). Accordingly, bilateral intra-vmPFC injections of NSC23766 (0.5 μg/0.5 μl/side) 30 min before extinction training also impaired CPA extinction (main effect of drug, F(1,45) = 21.7, p < 0.001, two-way ANOVA, post hoc, p < 0.01 compared with vehicle-treated rats) (Fig. 5B). Next, we examined the effect of HFS at 50 Hz on LTP induction in vmPFC slices from rats that were pretreated with NSC23766 before extinction training. We found that LTP could not be induced anymore by the stimuli in the vmPFC from rats pretreated with NSC23766 (vehicle, 111.89 ± 4.19%, NSC23766, 94.03 ± 2.86%, t(16) = 3.90, p = 0.001; Fig. 5C). Likewise, bilateral intra-vmPFC injections of the latrunculin A (250 ng/0.5 μl/side) 10 min before extinction training also resulted in significant inhibition of endocytosis of GABAAR β3 subunits (vehicle, 63.79 ± 10.76% of control; latrunculin A, 98.87 ± 12.29% of control; F(2,17) = 14.77, p = 0.0003, one-way ANOVA, post hoc, p < 0.01 compared with vehicle-treated rats; Fig. 5D) and also impaired CPA extinction (main effect of drug, F(1,45) = 21.7, p < 0.001, two-way ANOVA, post hoc, p < 0.01 compared with vehicle-treated rats; Fig. 5E). These results further support that Rac1 activity-associated actin polymerization plays critical roles in GABAAR endocytosis, LTP facilitation, and CPA extinction.
Figure 5.
Genetically manipulating Rac1 activity in the vmPFC bidirectionally regulates drug withdrawal memory extinction and endocytosis of GABAARs. A–D, Knockdown of the Rac1 expression by Rac1-shRNA impaired CPA extinction and suppressed endocytosis of GABAAR β3 subunits. A, Infection of the vmPFC with lentivirus expressing Rac1-shRNA was visualized by fluorescence microscope. B, Infection of lentivirus expressing Rac1-shRNA attenuated Rac1 expression within the vmPFC. C, Knockdown of the Rac1 expression in the vmPFC with Rac1-shRNA impaired CPA extinction. D, Knockdown of the Rac1 expression in the vmPFC with Rac1-shRNA suppressed endocytosis of GABAAR β3 subunits. *p < 0.05, compared with control shRNA or no-extinction control groups. #p < 0.05, compared with control shRNA groups. E–H, Facilitation of CPA extinction and endocytosis of GABAAR β3 subunits were observed in floxed mice to express constitutively active form of Rac1 induced by adeno-associated virus carrying Cre recombinase (AAV-Cre). E, Infection of the vmPFC with AAV-Cre was visualized by fluorescence microscope. B, Elevation of Rac1 activity was induced by expressing constitutively active Rac1 in the vmPFC through AAV-Cre. G, H, The constitutively active Rac1 mice displayed acceleration of CPA extinction and augmentation of endocytosis of GABAAR β3 subunits. *p < 0.05, compared with AAV-GFP control groups (two-tailed Student's t test or two-way ANOVA followed by Bonferroni's post hoc test). #p < 0.05, compared with no-extinction control groups (two-tailed Student's t test or two-way ANOVA followed by Bonferroni's post hoc test). Error bars indicate mean ± SEM (n = 6).
Genetically manipulating Rac1 activity in the vmPFC bidirectionally regulates drug withdrawal memory extinction and endocytosis of GABAARs
The above results suggest that Rac1 activity can use cofilin to modulate actin dynamics, which in turn enables synaptic localization of Arc that is necessary for GABAAR endocytosis, LTP facilitation, and CPA extinction. Thus, we further confirmed the crucial role of Rac1 activity in GABAAR endocytosis and CPA extinction by genetically manipulating Rac1 levels within the vmPFC. We first examined the necessity of in vivo knockdown of Rac1 expression using Rac1-shRNA for GABAAR endocytosis and CPA extinction. The rats that underwent CPA received bilateral injections of lentivirus expressing Rac1-shRNA with high-titer stocks (5 × 108 to 1 × 109 TU/ml) or control shRNAs into the vmPFC. Two weeks later, the rats were subjected to extinction training. Figure 6A showed that bilateral intra-vmPFC infections of lentivirus resulted in effective infection with Rac1-shRNA in the vmPFC, as indicated by the tagged GFP staining. As shown in Figure 6B, C, Rac1-shRNA-infected rats displayed a significant reduction of Rac1 protein expression compared with the control shRNA group (control shRNA, 100 ± 7.882%, n = 8; Rac1-shRNA, 72.66 ± 6.168%, n = 6; t(1,12) = 2.58, p = 0.0241, Student's t test) and impaired CPA extinction (main effect of gene, F(1,22) = 4.967, p < 0.05, two-way ANOVA, post hoc, p < 0.05 compared with control shRNA groups). Accordingly, Rac1-shRNA-infected rats also showed a significant increase in the S/I ratio for GABAARs (control shRNA, 62.49 ± 4.399% of control, n = 7; Rac1-shRNA, 101.1 ± 12.31% of control, n = 7; F(2,18) = 4.786, p = 0.0215, one-way ANOVA, post hoc, p < 0.05 compared with control shRNA groups; Fig. 6D). No significant differences in the levels of total GABAAR protein were found between any of the experimental groups. These results further demonstrate that Rac1 within the vmPFC is necessary and for extinction training-induced GABAAR endocytosis and CPA extinction.
Figure 6.
BDNF scavenger TrkB-FC suppresses extinction training-induced activation of Rac1, endocytosis of GABAARs, and induction of LTP. A, Extinction training increased BDNF protein expression in the vmPFC. B, Extinction training induced Rac1 activation was blocked by intra-vmPFC infusion of BDNF scavenger TrkB-FC (0.65 μg/0.5 μl/side) and Rac1 inhibitor NSC23766 before extinction training. C, Endocytosis of GABAAR β3 subunits was suppressed by intra-vmPFC infusion of BDNF scavenger TrkB-FC before extinction training. D, HFS at 50 Hz failed to elicit LTP induction in slices from rats that were pretreated with TrkB receptor antagonist k252a (35.7 μm/μl/side), given by intra-vmPFC infusion bilaterally before extinction training. *p < 0.05, compared with no-extinction control groups. **p < 0.01, compared with no-extinction control groups. #p < 0.05, compared with vehicle control groups (one-way ANOVA followed by Bonferroni's post hoc test). The magnitude of LTP was the average of the last 5 min recordings expressed as the mean ± SEM percentage of the baseline fEPSP. Data were analyzed with two-tailed Student's t tests.
Next, we examined the sufficiency of Rac1 activity within the vmPFC for GABAAR endocytosis and CPA extinction using Rac1 transgenic mice, which have a loxP-flanked Neo-STOP cassette preventing transcription of the constitutively active form of Rac1. Once microinjected with the virus to express Cre recombinase, the mice would have the STOP cassette deleted and subsequently expressed a constitutively active form of Rac1. Figure 6E showed that bilateral intra-vmPFC infusions of Cre recombinase virus infected the vmPFC region effectively 2 weeks after the injection. As shown in Figure 6F, G, Cre recombinase virus-infected rats displayed a significant upregulation of Rac1 activity (control virus, 100 ± 12.86%, n = 6; Cre virus, 192.9 ± 28.04%, n = 6; t(1,10) = 3.01 p < 0.05, Student's t test) and facilitated CPA extinction (main effect of gene, F(1,34) = 29.63, p < 0.001, two-way ANOVA, post hoc, p < 0.05 compared with control virus groups). Accordingly, Cre recombinase virus-infected rats also showed a significant decrease in the S/I ratio for GABAARs (control virus, 100 ± 13.29%, n = 9; Cre virus, 70.58 + 3.285%, n = 9; t(1,12) = 2.331, p < 0.05, Student's t test; Fig. 6H). No significant difference in the levels of total GABAAR protein was found between any of the experimental groups. These results clearly demonstrate that Rac1 activity within the vmPFC is sufficient for extinction training-induced GABAAR endocytosis and CPA extinction.
Increased BDNF expression in the vmPFC is required for Rac1-mediated endocytosis of GABAARs and induction of LTP
Our previous study demonstrates that BDNF in the vmPFC is required for extinction of drug withdrawal-associated memory (Wang et al., 2012), although the mechanisms underlying BDNF that contribute to extinction of such memory are unclear. We hypothesized that the molecular events occurred following extinction training might be BDNF-dependent. To test this hypothesis, we first examined the effect of extinction training on BDNF expression in the vmPFC. As shown in Figure 6A, extinction training significantly increased synaptic BDNF protein levels 1, 2, and 3 h after training (p < 0.05, one-way ANOVA followed by Bonferroni post hoc test), consistent with our previous study (Wang et al., 2012). Next, we determined whether increased BDNF expression was involved in activation of Rac1, endocytosis of GABAAR, and facilitation of LTP. We found that the activation of Rac1 induced by extinction training was blocked significantly by bilateral intra-vmPFC infusions of BDNF scavenger TrkB-FC (0.65 μg/0.5 μl/side) and Rac1 inhibitor NSC23766 (0.5 μg/0.5 μl/side) 30 min before extinction training (F(3,20) = 7.89, p < 0.01). To further confirm whether GABAAR β3 subunit endocytosis is linked to the elevated BDNF expression in the vmPFC following extinction training, we assessed the effect of the BDNF scavenger TrkB-FC on extinction training-induced endocytosis of GABAAR β3 subunits at synapses. As shown in Figure 6C, bilateral infusions of TrkB-FC (0.65 μg/0.5 μl/side) into the vmPFC 30 min before extinction training resulted in a significant increase in the S/I ratio of the β3 subunit of the GABAARs (vehicle, 15.87 ± 5.02% of control; TrkB-FC: 49.23 ± 17.38% of control; F(2,14) = 41.22, p < 0.001, one-way ANOVA). Post hoc analysis revealed a significant difference between vehicle and TrkB-FC groups (p < 0.05). Thus, reduction of GABAAR endocytosis by using TrkB-FC indicates that BDNF participates in dynamin-dependent GABAAR endocytosis in the vmPFC.
To further determine whether the increase of BDNF expression was essential for LTP facilitation after extinction training, we examined LTP induction in extinction-trained rats that were pretreated with the TrkB receptor antagonist K252a (35.7 μm/μl/per side) before extinction training. We found that LTP was no longer induced in the extinction trained rats pretreated with K252a (vehicle, 115.32 ± 4.46%, K252a, 94.44 ± 2.44%, t(16) = 4.16, p = 0.0007; Fig. 6D). Together, these results clearly demonstrate that increased BDNF expression was linked to activation of Rac1, endocytosis of GABAAR, and facilitation of LTP.
Discussion
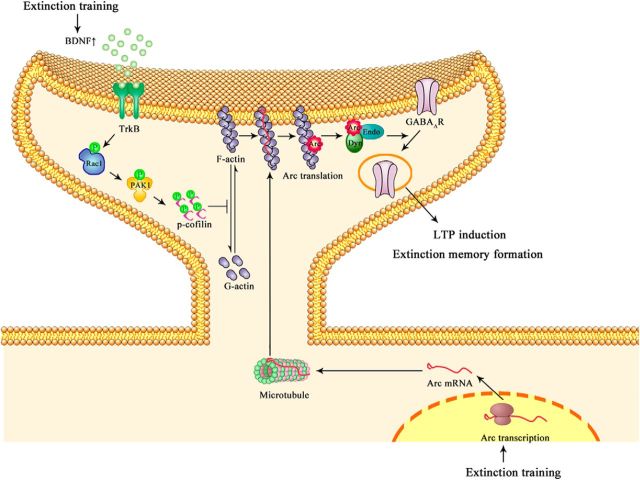
CPA is a highly sensitive animal model for measurement of the aversive memories of opiate withdrawal (Stinus et al., 2000; Azar et al., 2003). CPA, once formed, is long-lasting (Stinus et al., 2000) but can be extinguished by extinction training (Myers and Carlezon et al., 2010; Wang et al., 2012). The present study reveals that extinction training activates Rac1 in a BDNF-dependent manner, which determines the extinction of CPA to naloxone-precipitated opiate withdrawal by facilitating GABAAR endocytosis through triggering synaptic translocation of Arc via regulation of actin polymerization (Fig. 7). Therefore, our findings provide new insight into the molecular mechanisms by which a previously established memory could be extinguished, thereby suggesting therapeutic targets to promote extinction of the unwanted memory.
Figure 7.
A synaptic model proposed for extinction of memory. Extinction training activates Rac1 in a BDNF-dependent manner in the vmPFC. Active Rac1 induces synaptic actin polymerization via activating Pak1-cofilin signal pathway. Meanwhile, extinction training also results in Arc mRNA transcription. Arc mRNA is then translocated at dendritic spines through actin polymerization-dependent machinery. Within the dendritic spines, Arc proteins are rapidly translated and then form a complex with dynamin (Dyn) to facilitate GABAAR endocytosis, thereby leading to LTP induction and CPA extinction.
Important role of mPFC GABAAR endocytosis in extinction of drug withdrawal memory
The vmPFC is a key component of the brain's extinction circuitry (Quirk et al., 2006; Sotres-Bayon et al., 2006). Studies based on lesion and pharmacological manipulation show that the vmPFC is required for long-term extinction of fear memory (Quirk et al., 2000; Milad and Quirk, 2002; Milad et al., 2004; Santini et al., 2004; Sotres-Bayon et al., 2006). Our recent study also demonstrates that the vmPFC is required for extinction of drug withdrawal memory (Wang et al., 2012). GABAARs are the main mediators of inhibitory transmission in the brain. Activity-dependent regulation of GABAergic transmission is essential for synaptic plasticity and information processing in the brain (Davies et al., 1991; Paulsen and Moser, 1998; Smith and Kittler, 2010). In the present study, we found that extinction training resulted in suppressing the efficacy of inhibitory synaptic transmission in the vmPFC by increasing of GABAAR endocytosis, which was required for LTP induction and extinction of drug withdrawal memory, because blockade of GABAAR endocytosis by a dynamin function-blocking peptide (Myr-P4) abolished LTP induction and extinction of drug withdrawal memory. The essential role of the diminishing GABAergic transmission for vmPFC LTP induction is further demonstrated by using the GABAAR antagonist picrotoxin. Suppressing GABAergic transmission with picrotoxin facilitates LTP induction in no-extinction control rats, the same picrotoxin application produces no additional LTP facilitation in extinction rats. This occlusion of LTP induction suggests that extinction training and picrotoxin application share the same mechanism in facilitating LTP induction by reducing GABAergic transmission, and indicates that GABAAR-mediated GABAergic transmission normally suppresses LTP induction in vmPFC excitatory neurons, and extinction training facilitates LTP induction by reducing GABAergic inhibition through the endocytosis of GABAARs.
Arc serves as a critical regulator for GABAAR endocytosis
GABAARs enter the endocytosis pathway by a clathrin-mediated and dynamin-dependent mechanism (Kittler et al., 2000; Herry and Garcia, 2003; van Rijnsoever et al., 2005). In neurons, endocytosis of GABAARs occurs primarily by interactions of the GABAAR β3 subunits with the clathrin adaptor protein AP2 (Kittler et al., 2000; Jovanovic et al., 2004; Jacob et al., 2009; Lu et al., 2010), a process that is essential for the targeting of receptors into clathrin-coated pits. Receptors delivered into clathrin-coated pits by AP2 are then internalized by dynamin, a component of the endocytic machinery, mediated scission of clathrin-coated pits from the plasma membrane (Hill et al., 2001; Merrifield et al., 2002). Blockade of the effect of dynamin by a dynamin function blocking peptide can prevent GABAAR endocytosis in the hippocampus, amygdala, and nucleus accumbens (Kittler et al., 2000; Chen et al., 2006; Terunuma et al., 2008; Lin et al., 2011). Consistent with these studies, we found that LTP induction and CPA extinction induced by extinction training were abolished by blocking GABAAR endocytosis using a dynamin function-blocking peptide Myr-P4 (Fig. 4B), indicating that dynamin is involved in the endocytosis of GABAARs induced by extinction training. More importantly, this study reveals that the activity-regulated cytoskeleton-associated protein Arc is response for triggering dynamin-dependent GABAAR endocytosis.
Arc is known to be important for the stabilization of synaptic plasticity and long-term memory formation (Steward et al., 1998; Guzowski et al., 1999, 2000; Cooke and Bliss, 2006; Plath et al., 2006). Its expression is dynamically regulated by learning experiences and stimulus protocols generating LTP (Link et al., 1995; Steward et al., 1998; McIntyre et al., 2005; Ploski et al., 2008; Mamiya et al., 2009; Liu et al., 2012). In the present study, we found that extinction training resulted in robust increase of synaptic Arc expression in the vmPFC. We further demonstrated that knockdown of synaptic Arc expression in the vmPFC using Arc/Arg3.1-shRNA suppressed GABAAR endocytosis and extinction of drug withdrawal memory (Fig. 2E,F), suggesting that Arc plays an important role in GABAAR endocytosis and extinction of drug withdrawal memory. Given the evidence that extinction training-induced GABAAR endocytosis could be blocked by the dynamin function-interfering peptide (Fig. 1B) and that Arc has been shown to interact with components of the endocytic machinery dynamin (Chowdhury et al., 2006), the present study suggests that Arc may trigger GABAAR endocytosis via interaction with dynamin. Previous studies have shown that Arc interacts with dynamin to modulate AMPAR endocytosis, thus allowing it to mediate LTD induction (Chowdhury et al., 2006; Rial Verde et al., 2006; Wang and Kriegstein, 2008; Liu et al., 2012). The present study extends previous findings by showing that Arc can mediate LTP induction by facilitation of GABAAR endocytosis, and identifies Arc as a downstream effector of Rac1 regulations of synaptic plasticity as well as learning and memory.
Activation of Rac1 in a BDNF-dependent manner is critical for synaptic localization of Arc through actin polymerization
Localization of Arc mRNA at active synapses is one of the critical events that are required for its regulatory effect on synaptic plasticity (Tzingounis and Nicoll, 2006). Synaptic actin polymerization may be a possible mechanism underlying Arc localization at active synapse because the actin filaments within the dendritic spines can serve as a path for mRNA and protein trafficking (Langford and Molyneaux, 1998; Kaech et al., 2001) or act as an anchor for mRNA and protein docking (Allison et al., 1998; Lisman and Zhabotinsky, 2001). One of the major regulators of synaptic actin dynamics is the actin depolymerizing protein cofilin; its phosphorylation and dephosphorylation are important for actin polymerization and depolymerization (Bamburg, 1999). Cofilin-regulated actin dynamics has been associated with insertion and endocytosis of AMPA receptors (Gu et al., 2010; Liu et al., 2012; Wang et al., 2013).
Cofilin phosphorylation is regulated by parallel signaling pathways through the Rho GTPase effectors RhoA kinase or Pak (Rex et al., 2009). Pak is a critical regulator of actin dynamics in the cortex (Hayashi et al., 2004), is concentrated in spines of mature cortical neurons, and has been shown to promote synaptic actin dynamics via phosphorylation of cofilin through activation of LIM kinase (Edwards et al., 1999; Hayashi et al., 2004). Pak activity is largely controlled by Rac1 and Cdc42 (Manser et al., 1994; Edwards et al., 1999). The present study demonstrated that extinction training activated Rac1, but not Cdc42, phosphorylated Pak1 and cofilin, and induced actin polymerization, which was required for the synaptic localization of Arc, because inhibition of actin polymerization with the Rac1 inhibitor NSC23766 or the actin polymerization inhibitor latrunculin A abolished the enhancement of synaptic Arc localization (Fig. 3F). Accordingly, the inhibition of actin polymerization with NSC23766 or latrunculin A also suppressed GABAAR endocytosis and extinction of drug withdrawal memory (Fig. 5A,D). These results indicate a critical role of Rac1 activation for extinction training-induced GABAAR endocytosis and extinction of drug withdrawal memory. This conclusion is further supported by the results obtained from genetically manipulating Rac1 expression within the vmPFC. Knockdown of Rac1 expression by using Rac1-shRNA suppressed GABAAR endocytosis and extinction of drug withdrawal memory, whereas expression of a constitutively active form of Rac1 accelerated GABAAR endocytosis and extinction of drug withdrawal memory (Fig. 6). Furthermore, we found that activation of Rac1 induced by extinction training was BDNF-dependent because infusion of the BDNF scavenger TrkB-FC into the vmPFC before extinction training abolished extinction training-induced activation of Rac1, phosphorylation of Pak1 and cofilin, and polymerization of actin (Fig. 3) as well as endocytosis of GABAARs and induction of LTP (Fig. 6). BDNF is the most studied neurotrophin involved in learning and memory (Minichiello, 2009). Our results support that BDNF plays a crucial role in synaptic plasticity and diverse forms of learning and memory, and provides first evidence that BDNF can contribute to synaptic and behavioral plasticity by facilitating GABAAR endocytosis through Rac1-dependent synaptic localization of Arc.
Footnotes
This work was supported by Ministry of Science and Technology of China Grants 2013CB835100 and 2015CB553502 to J.-G.L., Ministry of Science and Technology of China Grant 2013CB8351003 to L.X., Foundation of National Natural Science of China Grants 81130087 and 91232716 to J.-G.L., and Committee of Science and Technology of Shanghai Grant 13JC140680 to J.-G.L.
The authors declare no competing financial interests.
References
- Akirav I. (2007) NMDA partial agonist reverses blocking of extinction of aversive memory by GABA(A) agonist in the amygdala. Neuropsychopharmacology 32:542–550. 10.1038/sj.npp.1301050 [DOI] [PubMed] [Google Scholar]
- Allison DW, Gelfand VI, Spector I, Craig AM (1998) Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci 18:2423–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anggono V, Huganir RL (2012) Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol 22:461–469. 10.1016/j.conb.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar MR, Jones BC, Schulteis G (2003) Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology (Berl) 170:42–50. 10.1007/s00213-003-1514-y [DOI] [PubMed] [Google Scholar]
- Bamburg JR. (1999) Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol 15:185–230. 10.1146/annurev.cellbio.15.1.185 [DOI] [PubMed] [Google Scholar]
- Barad M. (2005) Fear extinction in rodents: basic insight to clinical promise. Curr Opin Neurobiol 15:710–715. 10.1016/j.conb.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL (2006) Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem 86:123–132. 10.1016/j.nlm.2005.12.008 [DOI] [PubMed] [Google Scholar]
- Chen G, Kittler JT, Moss SJ, Yan Z (2006) Dopamine D3 receptors regulate GABAA receptor function through a phospho-dependent endocytosis mechanism in nucleus accumbens. J Neurosci 26:2513–2521. 10.1523/JNEUROSCI.4712-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF (2006) Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron 52:445–459. 10.1016/j.neuron.2006.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT (2004) Receptor trafficking and synaptic plasticity. Nat Rev Neurosci 5:952–962. 10.1038/nrn1556 [DOI] [PubMed] [Google Scholar]
- Cooke SF, Bliss TV (2006) Plasticity in the human central nervous system. Brain 129:1659–1673. 10.1093/brain/awl082 [DOI] [PubMed] [Google Scholar]
- Davies CH, Starkey SJ, Pozza MF, Collingridge GL (1991) GABA autoreceptors regulate the induction of LTP. Nature 349:609–611. 10.1038/349609a0 [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R (2006) Effects of d-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry 60:369–375. 10.1016/j.biopsych.2006.03.084 [DOI] [PubMed] [Google Scholar]
- Ding ZB, Wu P, Luo YX, Shi HS, Shen HW, Wang SJ, Lu L (2013) Region-specific role of Rac in nucleus accumbens core and basolateral amygdala in consolidation and reconsolidation of cocaine-associated cue memory in rats. Psychopharmacology (Berl) 228:427–437. 10.1007/s00213-013-3050-8 [DOI] [PubMed] [Google Scholar]
- Edwards DC, Sanders LC, Bokoch GM, Gill GN (1999) Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol 1:253–259. 10.1038/12963 [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T (1999) Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399:66–70. 10.1038/19978 [DOI] [PubMed] [Google Scholar]
- Fu M, Yu X, Lu J, Zuo Y (2012) Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature 483:92–95. 10.1038/nature10844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, Hartzell HC, Chen G, Bamburg JR, Zheng JQ (2010) ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat Neurosci 13:1208–1215. 10.1038/nn.2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF (1999) Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci 2:1120–1124. 10.1038/16046 [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA (2000) Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci 20:3993–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. (1998) G proteins and small GTPases: distant relatives keep in touch. Science 280:2074–2075. 10.1126/science.280.5372.2074 [DOI] [PubMed] [Google Scholar]
- Hall A. (2005) Rho GTPases and the control of cell behaviour. Biochem Soc Trans 33:891–895. 10.1042/BST20050891 [DOI] [PubMed] [Google Scholar]
- Hart G, Harris JA, Westbrook RF (2009) Systemic or intra-amygdala injection of a benzodiazepine (midazolam) impairs extinction but spares re-extinction of conditioned fear responses. Learn Mem 16:53–61. 10.1101/lm.1154409 [DOI] [PubMed] [Google Scholar]
- Hayashi ML, Choi SY, Rao BS, Jung HY, Lee HK, Zhang D, Chattarji S, Kirkwood A, Tonegawa S (2004) Altered cortical synaptic morphology and impaired memory consolidation in forebrain-specific dominant-negative PAK transgenic mice. Neuron 42:773–787. 10.1016/j.neuron.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Herry C, Garcia R (2003) Behavioral and paired-pulse facilitation analyses of long-lasting depression at excitatory synapses in the medial prefrontal cortex in mice. Behav Brain Res 146:89–96. 10.1016/j.bbr.2003.09.017 [DOI] [PubMed] [Google Scholar]
- Hill E, van Der Kaay J, Downes CP, Smythe E (2001) The role of dynamin and its binding partners in coated pit invagination and scission. J Cell Biol 152:309–323. 10.1083/jcb.152.2.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW (2006) Augmentation of exposure therapy with d-cycloserine for social anxiety disorder. Arch Gen Psychiatry 63:298–304. 10.1001/archpsyc.63.3.298 [DOI] [PubMed] [Google Scholar]
- Hou YY, Lu B, Li M, Liu Y, Chen J, Chi ZQ, Liu JG (2009) Involvement of actin rearrangements within the amygdala and the dorsal hippocampus in aversive memories of drug withdrawal in acute morphine-dependent rats. J Neurosci 29:12244–12254. 10.1523/jneurosci.1970-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Chotiner JK, Steward O (2007) Actin polymerization and ERK phosphorylation are required for Arc/Arg3.1 mRNA targeting to activated synaptic sites on dendrites. J Neurosci 27:9054–9067. 10.1523/JNEUROSCI.2410-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A (2001) The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci 4:943–947. 10.1038/nn0901-943 [DOI] [PubMed] [Google Scholar]
- Ives JH, Drewery DL, Thompson CL (2002) Differential cell surface expression of GABAA receptor alpha1, alpha6, beta2 and beta3 subunits in cultured mouse cerebellar granule cells influence of cAMP-activated signalling. J Neurochem 80:317–327. 10.1046/j.0022-3042.2001.00700.x [DOI] [PubMed] [Google Scholar]
- Jacob TC, Wan Q, Vithlani M, Saliba RS, Succol F, Pangalos MN, Moss SJ (2009) GABA(A) receptor membrane trafficking regulates spine maturity. Proc Natl Acad Sci U S A 106:12500–12505. 10.1073/pnas.0903943106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ (2004) Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cell-surface stability. J Neurosci 24:522–530. 10.1523/JNEUROSCI.3606-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S, Parmar H, Roelandse M, Bornmann C, Matus A (2001) Cytoskeletal microdifferentiation: a mechanism for organizing morphological plasticity in dendrites. Proc Natl Acad Sci U S A 98:7086–7092. 10.1073/pnas.111146798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ (2000) Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci 20:7972–7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Honing S, Bogdanov Y, McAinsh K, Arancibia-Carcamo IL, Jovanovic JN, Pangalos MN, Haucke V, Yan Z, Moss SJ (2005) Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci U S A 102:14871–14876. 10.1073/pnas.0506653102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. (2000) Neurobiology of addiction: toward the development of new therapies. Ann N Y Acad Sci 909:170–185. 10.1111/j.1749-6632.2000.tb06682.x [DOI] [PubMed] [Google Scholar]
- Lai CS, Franke TF, Gan WB (2012) Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature 483:87–91. 10.1038/nature10792 [DOI] [PubMed] [Google Scholar]
- Langford GM, Molyneaux BJ (1998) Myosin V in the brain: mutations lead to neurological defects. Brain Res Brain Res Rev 28:1–8. 10.1016/S0165-0173(98)00020-4 [DOI] [PubMed] [Google Scholar]
- Li M, Hou YY, Lu B, Chen J, Chi ZQ, Liu JG (2009) Expression pattern of neural synaptic plasticity marker-Arc in different brain regions induced by conditioned drug withdrawal from acute morphine-dependent rats. Acta Pharmacol Sin 30:282–290. 10.1038/aps.2009.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Tseng YC, Mao SC, Chen PS, Gean PW (2011) GABAA receptor endocytosis in the basolateral amygdala is critical to the reinstatement of fear memory measured by fear-potentiated startle. J Neurosci 31:8851–8861. 10.1523/JNEUROSCI.0979-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D (1995) Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci U S A 92:5734–5738. 10.1073/pnas.92.12.5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Zhabotinsky AM (2001) A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron 31:191–201. 10.1016/S0896-6273(01)00364-6 [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhou QX, Hou YY, Lu B, Yu C, Chen J, Ling QL, Cao J, Chi ZQ, Xu L, Liu JG (2012) Actin polymerization-dependent increase in synaptic Arc/Arg3.1 expression in the amygdala is crucial for the expression of aversive memory associated with drug withdrawal. J Neurosci 32:12005–12017. 10.1523/JNEUROSCI.0871-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Cheng PL, Lim BK, Khoshnevisrad N, Poo MM (2010) Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron 67:821–833. 10.1016/j.neuron.2010.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L. (2000) Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci 1:173–180. 10.1038/35044547 [DOI] [PubMed] [Google Scholar]
- Luo L, Hensch TK, Ackerman L, Barbel S, Jan LY, Jan YN (1996) Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature 379:837–840. 10.1038/379837a0 [DOI] [PubMed] [Google Scholar]
- Lüscher B, Fuchs T, Kilpatrick CL (2011) GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron 70:385–409. 10.1016/j.neuron.2011.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Nicoll RA, Malenka RC, Muller D (2000) Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci 3:545–550. 10.1038/75714 [DOI] [PubMed] [Google Scholar]
- Makkar SR, Zhang SQ, Cranney J (2010) Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology 35:1625–1652. 10.1038/npp.2010.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25:103–126. 10.1146/annurev.neuro.25.112701.142758 [DOI] [PubMed] [Google Scholar]
- Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, Kida S (2009) Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci 29:402–413. 10.1523/JNEUROSCI.4639-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L (1994) A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367:40–46. 10.1038/367040a0 [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H (2004) Structural basis of long-term potentiation in single dendritic spines. Nature 429:761–766. 10.1038/nature02617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Introini-Collison IB, Nagahara AH, Cahill L, Brioni JD, Castellano C (1990) Involvement of the amygdaloid complex in neuromodulatory influences on memory storage. Neurosci Biobehav Rev 14:425–431. 10.1016/S0149-7634(05)80065-X [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL (2005) Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc Natl Acad Sci U S A 102:10718–10723. 10.1073/pnas.0504436102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield CJ, Feldman ME, Wan L, Almers W (2002) Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat Cell Biol 4:691–698. 10.1038/ncb837 [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ (2002) Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420:70–74. 10.1038/nature01138 [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ (2004) Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci 118:389–394. 10.1037/0735-7044.118.2.389 [DOI] [PubMed] [Google Scholar]
- Minichiello L. (2009) TrkB signalling pathways in LTP and learning. Nat Rev Neurosci 10:850–860. 10.1038/nrn2738 [DOI] [PubMed] [Google Scholar]
- Mott DD, Lewis DV (1991) Facilitation of the induction of long-term potentiation by GABAB receptors. Science 252:1718–1720. 10.1126/science.1675489 [DOI] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA Jr (2010) D-cycloserine facilitates extinction of naloxone-induced conditioned place aversion in morphine-dependent rats. Biol Psychiatry 67:85–87. 10.1016/j.biopsych.2009.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama AY, Harms MB, Luo L (2000) Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci 20:5329–5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen O, Moser EI (1998) A model of hippocampal memory encoding and retrieval: GABAergic control of synaptic plasticity. Trends Neurosci 21:273–278. 10.1016/S0166-2236(97)01205-8 [DOI] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ (2010) Induction of fear extinction with hippocampal-infralimbic BDNF. Science 328:1288–1290. 10.1126/science.1186909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, et al. (2006) Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron 52:437–444. 10.1016/j.neuron.2006.08.024 [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE (2008) The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J Neurosci 28:12383–12395. 10.1523/JNEUROSCI.1662-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K (2000) The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci 20:6225–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, González-Lima F (2006) Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry 60:337–343. 10.1016/j.biopsych.2006.03.010 [DOI] [PubMed] [Google Scholar]
- Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, Lynch G (2009) Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol 186:85–97. 10.1083/jcb.200901084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT (2006) Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron 52:461–474. 10.1016/j.neuron.2006.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Peña de Ortiz S, Quirk GJ (2004) Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci 24:5704–5710. 10.1523/JNEUROSCI.0786-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DA, Weed SA, Binns D, Karginov AV, Parsons JT, Cooper JA (2002) Dynamin2 and cortactin regulate actin assembly and filament organization. Curr Biol 12:1852–1857. 10.1016/S0960-9822(02)01228-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Kittler JT (2010) The cell biology of synaptic inhibition in health and disease. Curr Opin Neurobiol 20:550–556. 10.1016/j.conb.2010.06.001 [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE (2006) Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry 60:329–336. 10.1016/j.biopsych.2005.10.012 [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF (1998) Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron 21:741–751. 10.1016/S0896-6273(00)80591-7 [DOI] [PubMed] [Google Scholar]
- Stinus L, Caille S, Koob GF (2000) Opiate withdrawal-induced place aversion lasts for up to 16 weeks. Psychopharmacology (Berl) 149:115–120. 10.1007/s002139900358 [DOI] [PubMed] [Google Scholar]
- Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, Haydon PG, Coulter DA, Moss SJ (2008) Deficits in phosphorylation of GABA(A) receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci 28:376–384. 10.1523/JNEUROSCI.4346-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias KF, Bikoff JB, Burette A, Paradis S, Harrar D, Tavazoie S, Weinberg RJ, Greenberg ME (2005) The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron 45:525–538. 10.1016/j.neuron.2005.01.024 [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D (1999) LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402:421–425. 10.1038/46574 [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Nicoll RA (2006) Arc/Arg3.1: linking gene expression to synaptic plasticity and memory. Neuron 52:403–407. 10.1016/j.neuron.2006.10.016 [DOI] [PubMed] [Google Scholar]
- van Rijnsoever C, Sidler C, Fritschy JM (2005) Internalized GABA-receptor subunits are transferred to an intracellular pool associated with the postsynaptic density. Eur J Neurosci 21:327–338. 10.1111/j.1460-9568.2005.03884.x [DOI] [PubMed] [Google Scholar]
- Wang DD, Kriegstein AR (2008) GABA regulates excitatory synapse formation in the neocortex via NMDA receptor activation. J Neurosci 28:5547–5558. 10.1523/JNEUROSCI.5599-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WS, Kang S, Liu WT, Li M, Liu Y, Yu C, Chen J, Chi ZQ, He L, Liu JG (2012) Extinction of aversive memories associated with morphine withdrawal requires ERK-mediated epigenetic regulation of brain-derived neurotrophic factor transcription in the rat ventromedial prefrontal cortex. J Neurosci 32:13763–13775. 10.1523/JNEUROSCI.1991-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dong Q, Xu XF, Feng X, Xin J, Wang DD, Yu H, Tian T, Chen ZY (2013) Phosphorylation of cofilin regulates extinction of conditioned aversive memory via AMPAR trafficking. J Neurosci 33:6423–6433. 10.1523/JNEUROSCI.5107-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigström H, Gustafsson B (1983) Facilitated induction of hippocampal long-lasting potentiation during blockade of inhibition. Nature 301:603–604. 10.1038/301603a0 [DOI] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P (2007) Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron 56:640–656. 10.1016/j.neuron.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB (2009) Stably maintained dendritic spines are associated with lifelong memories. Nature 462:920–924. 10.1038/nature08577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarar D, Waterman-Storer CM, Schmid SL (2005) A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol Biol Cell 16:964–975. 10.1091/mbc.E04-09-0774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Zhang Y, Shacter E (2003) Caspase 3-mediated inactivation of rac GTPases promotes drug-induced apoptosis in human lymphoma cells. Mol Cell Biol 23:5716–5725. 10.1128/MCB.23.16.5716-5725.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Extinction training has no effect on endocytosis of surface GluR1- and GluR2-containing AMPARs. Top panels show representative blots of surface (S) and internalized (I) GluR1- (A) and GluR2- (B) containing AMPARs from vmPFC tissues prepared from rats 4 h after extinction training. Bottom panels show quantification of surface/internal GluR1- and GluR2-containing AMPARs levels from Western blot data. Download Figure 1-1, TIF file (1.7MB, tif)
Intra-vmPFC infusion of dynamin function-blocking peptide (Myr-P4) before extinction training has no effect on endocytosis of GABAAR β3 subunit in no-extinction training rats. Top panels show representative blots of surface (S) and internalized (I) GABAAR β3 subunit from vmPFC tissues prepared from No-Ext rats. Bottom panels show quantification of surface/internal GABAAR β3 subunit levels from Western blot data. Download Figure 1-2, TIF file (1.5MB, tif)
Intra-vmPFC infusions of Rac1 inhibitor NSC23766 has no effect on the phosphorylation of Cofilin (A) or Pak1 (B) in the vmPFC of no-extinction rats. Download Figure 3-1, TIF file (1.4MB, tif)