The presence of fluid in the brain ventricles has been known since the ancient Egyptians. However, the first mention of CSF circulation did not occur until 1532, when the Italian anatomist Niccolò Massa first described the movement of a “watery excess” through brain foramina (Herbowski, 2013). Three centuries later, thanks to the meticulous work of the French physician François Magendie, CSF circulation became a notion widely accepted by the scientific community (Herbowski, 2013). In the classical representation, CSF produced in the lateral ventricles flows through the brain foramina to the spinal canal before flowing back upward to the brain subarachnoid space, where it is finally reabsorbed mostly by the arachnoid granulations, along the superior sagittal sinus.
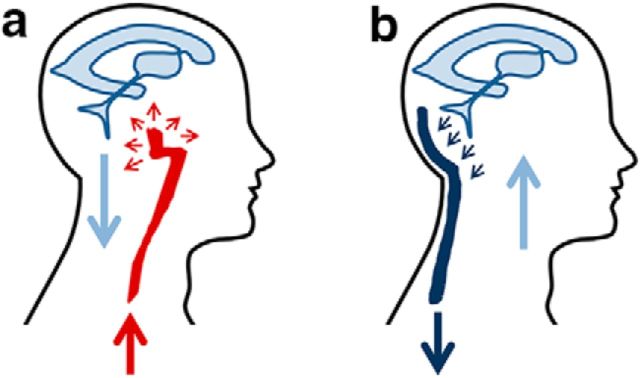
It was not until 1943 that one of the fathers of modern neurosurgery, John E.A. O'Connell, suggested that CSF movement from the skull into the spinal cord is induced by the transmission of intracranial arterial pulsations (O'Connell, 1943). The idea was based on CSF pressure measurements and observations during open skull surgeries, as well as on the so called Monro–Kellie hypothesis (Mokri, 2001). According to this hypothesis, the cranium is not a compressible structure; therefore, the constituents of its volume (blood, CSF, and brain parenchyma) create a state of equilibrium. When the volume of one of the three components increases, the volume of another must decrease to compensate. According to this hypothesis, the arrival in the skull of the arterial pulse wave will cause CSF outflow into the spinal canal (Fig. 1a). The pulsatile nature of CSF movement was later demonstrated in vivo by magnetic resonance imaging (MRI; Greitz et al., 1992).
Figure 1.
The two models for CSF flow. a, In the classical view (O'Connell, 1943), the arrival of the arterial pulse wave (red) into the skull causes a temporary pressure increase in the incompressible intracranial compartment, resulting in CSF outflow (light blue) into the spinal canal. b, In the model proposed by Dreha-Kulaczewski et al. (2017), the negative venous pressure (dark blue) produced during inspiration causes a temporary pressure decrease in the intracranial compartment, resulting in CSF flow into the head.
Recent studies have challenged the textbook model of CSF flow. Applying in vivo two-photon imaging techniques, Iliff et al. (2012) reported that in rodents a large portion of the subarachnoid CSF flows along arteries to enter the brain interstitium, from which it is cleared by the venous pathways. Using a high-speed MRI technique, Dreha-Kulaczewski et al. (2015) recently identified the respiratory cycle, instead of arterial pulsations, as the main force responsible for CSF flow in humans. In another article that appeared in a recent issue of the Journal of Neuroscience, (Dreha-Kulaczewski et al. (2017) extended their findings, reporting that during the initial phase of inspiration, CSF flows from the spine toward the head. Together, these innovative findings seem to draw a picture of CSF flow that differs from the classical model previously outlined.
Dreha-Kulaczewski et al. (2017) took advantage of a novel real-time phase-contrast MRI, which allowed them to simultaneously measure CSF and venous flow in healthy human subjects. Dreha-Kulaczewski et al. (2017) quantified CSF flow at the following four levels: in the cerebral aqueduct, and in the spinal canal at cervical level 3 (C3) and thoracic levels 2 (Th2) and 5 (Th5). In apparent disagreement with the hypothesis that CSF produced from the choroid plexus in the lateral ventricles flows out of the head to the spinal canal, Dreha-Kulaczewski et al. (2017) observed that CSF flow was mainly directed into the head during forced inspiration. Concomitantly with CSF flow, they measured venous flux in the internal venous plexus of the upper cervical spine, the most important venous drainage pathway for the brainstem. The synopsis of their findings is the following: with every cycle of forced inspiration, there was an increase in the venous outflow from the skull and an influx of CSF into the skull. Conversely, with every forced expiration there was a corresponding reduction in venous outflow and an efflux of CSF from the skull. Of note, arterial pulse-related CSF flow was still observed, but this represented a very minor contribution.
The excellent work by Dreha-Kulaczewski et al. (2017) is of crucial importance for our understanding of CSF dynamics. The negative intrathoracic venous pressure generated during inspiration is one of the main forces promoting venous return to the heart. It has now been shown that this negative pressure is also responsible for CSF movement from the spinal canal to the head. The observations confirm the previously outlined Monro–Kellie hypothesis on the relationship among the three brain compartments, with the only distinction being that CSF flow is induced by volume variations in the venous compartment rather than in the arterial compartment (Fig. 1b). The study also reinforces the recent findings by Iliff et al. (2012), supporting the idea that the brain venous system has a prominent role in CSF clearance from the brain interstitium. In summary, it seems that a new paradigm for CSF flow is emerging.
A minor consideration on the study regards the nonphysiological position in which MRI was performed on the study subjects. Although MRI in orthostasis is a very uncommon technique, the supine position in which MRI is commonly performed lowers the spinal CSF pressure due to a reduction of hydrostatic pressure from the cranial compartment. In this way, CSF flow from the spine to the head will be promoted. In other words, the observation that forced inspiration causes CSF flow toward the head might be valid only in the supine position, not in the physiological orthostasis. For this reason, the findings by Dreha-Kulaczewski et al. (2017) should be referred to as “retrograde movement of CSF” rather than upward movement.
The clinical implications of identifying respiration and venous return as the main drivers of CSF flow are nevertheless various and important. First, it has been reported that conditions associated with impaired venous return to the heart can result in an accumulation of CSF within the brain, a dangerous pathology known as hydrocephalus. Specifically, hydrocephalus has been reported as a consequence of high venous pressure, such as that occurring in vein of Galen malformations (Meila et al., 2016) or arteriovenous malformations (Lobato et al., 1980; Geibprasert et al., 2009), or reduced venous oncotic pressure, such as that occurring in severe liver of kidney failure (Caplan, 2006). The underlying mechanism for these types of hydrocephalus has been poorly understood. It now appears, however, that if respiration and negative venous pressure are the main drivers for CSF flow, any increase in venous pressure or decrease in the venous oncotic pressure will result in impaired CSF reabsorption and consequent hydrocephalus. Future studies should investigate whether treating the latent venous pathology is an effective therapeutic approach for these types of hydrocephalus.
Second, the close connections between breathing patterns and increased intracranial pressure (ICP), a consequence of hydrocephalus, are well known to physicians. Increased ICP leads to abnormal breathing patterns (such as Cheyne–Stokes breathing). On the other hand, the use of both mechanical ventilation in intensive care units and continuous positive airway pressure (CPAP) for the treatment of obstructive sleep apnea syndrome, two therapies that revert the negative intrathoracic venous pressure, has been linked to increased ICP (Duncan et al., 1986; Nyquist et al., 2008). The findings by Dreha-Kulaczewski et al. (2017) indicate that impaired CSF reabsorption due to increased venous pressure may be an important component for the pathogenesis of these complications. Future research should clarify the safety of mechanical ventilation and CPAP in patients who are at risk of hydrocephalus.
Finally, recent work suggests that CSF circulation plays a critical role in clearing waste metabolites from the brain (Xie et al., 2013), a process that might be particularly relevant for pathologies such as Alzheimer's disease (AD) and subarachnoid hemorrhage (SAH). Accumulation of amyloid-β in the brain is considered to be pathogenetic in AD. In SAH, blood clots are thought to impair CSF reabsorption, often leading to hydrocephalus (Chen et al., 2017). In addition, recent MRI-based work has shown that chronic hydrocephalus after SAH is not due to blood occlusion of the aqueduct or of the fourth ventricle (Saliou et al., 2012). A better understanding of CSF circulation, with particular relevance to the site and mechanisms of reabsorption, would be critical for the development of novel therapeutic approaches for pathologies characterized by impaired brain metabolic clearance.
In conclusion, Dreha-Kulaczewski et al. (2017) identify the negative venous pressure occurring during inspiration as the main force driving CSF movement from the spine into the head. This work is crucial for our understanding of human CSF dynamics and has important clinical implications. Future studies should aim to unravel the role of respiration and venous pressure in the pathogenesis of different types of hydrocephalus and other pathologies.
Footnotes
Editor's Note: These short reviews of recent JNeurosci articles, written exclusively by students or postdoctoral fellows, summarize the important findings of the paper and provide additional insight and commentary. If the authors of the highlighted article have written a response to the Journal Club, the response can be found by viewing the Journal Club at www.jneurosci.org. For more information on the format, review process, and purpose of Journal Club articles, please see http://jneurosci.org/content/preparing-manuscript#journalclub.
We thank Sandra Gerlich for helpful discussion and manuscript review. This review article is meant to exclusively express the authors' personal ideas. The ideas summarized are not intended to represent the opinions of the authors' institutions.
The authors declare no competing interests.
References
- Caplan LR. (2006) Cardiac encephalopathy and congestive heart failure: a hypothesis about the relationship. Neurology 66:99–101. 10.1212/01.wnl.0000191327.62136.b1 [DOI] [PubMed] [Google Scholar]
- Chen Q, Feng Z, Tan Q, Guo J, Tang J, Tan L, Feng H, Chen Z (2017) Post-hemorrhagic hydrocephalus: recent advances and new therapeutic insights. J Neurol Sci 375:220–230. 10.1016/j.jns.2017.01.072 [DOI] [PubMed] [Google Scholar]
- Dreha-Kulaczewski S, Joseph AA, Merboldt KD, Ludwig HC, Gärtner J, Frahm J (2015) Inspiration is the major regulator of human CSF flow. J Neurosci 35:2485–2491. 10.1523/JNEUROSCI.3246-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreha-Kulaczewski S, Joseph AA, Merboldt KD, Ludwig HC, Gärtner J, Frahm J (2017) Identification of the upward movement of human CSF in vivo and its relation to the brain venous system. J Neurosci 37:2395–2402. 10.1523/JNEUROSCI.2754-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW, Oh TE, Hillman DR (1986) PEEP and CPAP. Anaesth Intensive Care 14:236–250. [DOI] [PubMed] [Google Scholar]
- Geibprasert S, Pereira V, Krings T, Jiarakongmun P, Lasjaunias P, Pongpech S (2009) Hydrocephalus in unruptured brain arteriovenous malformations: pathomechanical considerations, therapeutic implications, and clinical course. J Neurosurg 110:500–507. 10.3171/2008.7.JNS0815 [DOI] [PubMed] [Google Scholar]
- Greitz D, Wirestam R, Franck A, Nordell B, Thomsen C, Ståhlberg F (1992) Pulsatile brain movement and associated hydrodynamics studied by magnetic resonance phase imaging. The Monro-Kellie doctrine revisited. Neuroradiology 34:370–380. 10.1007/BF00596493 [DOI] [PubMed] [Google Scholar]
- Herbowski L. (2013) The maze of the cerebrospinal fluid discovery. Anat Res Int 2013:596027. 10.1155/2013/596027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4:147ra111. 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato RD, Lamas E, Cordobes F, Munoz MJ, Roger R (1980) Chronic adult hydrocephalus due to uncommon causes. Acta Neurochir (Wien) 55:85–97. 10.1007/BF01808923 [DOI] [PubMed] [Google Scholar]
- Meila D, Grieb D, Melber K, Jacobs C, Maslehaty H, Petridis A, Brassel F (2016) Hydrocephalus in vein of Galen malformation: etiologies and therapeutic management implications. Acta Neurochir (Wien) 158:1279–1284. 10.1007/s00701-016-2836-y [DOI] [PubMed] [Google Scholar]
- Mokri B. (2001) The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology 56:1746–1748. 10.1212/WNL.56.12.1746 [DOI] [PubMed] [Google Scholar]
- Nyquist P, Stevens RD, Mirski MA (2008) Neurologic injury and mechanical ventilation. Neurocrit Care 9:400–408. 10.1007/s12028-008-9130-7 [DOI] [PubMed] [Google Scholar]
- O'Connell JEA. (1943) The vascular factor in intracranial pressure and maintenance of cerebrospinal fluid circulation. Brain 66:204–228. 10.1093/brain/66.3.204 [DOI] [Google Scholar]
- Saliou G, Paradot G, Gondry C, Bouzerar R, Lehmann P, Meyers ME, Gars DL, Deramond H, Balédent O (2012) A phase-contrast MRI study of acute and chronic hydrodynamic alterations after hydrocephalus induced by subarachnoid hemorrhage. J Neuroimaging 22:343–350. 10.1111/j.1552-6569.2011.00594.x [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M (2013) Sleep drives metabolite clearance from the adult brain. Science 342:373–377. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]