Abstract
Background
The ripening process of olive fruits is associated with chemical and/or enzymatic specific transformations, making them particularly attractive to animals and humans. In olive drupes, including ‘Cassanese’ ones, ripening is usually accompanied by progressive chromatic change, resulting in a final red-brown colourization of both epidermis and mesocarp. This event has an exception in the ‘Leucocarpa’, in which we observed the destabilization in the equilibrium between the chlorophyll metabolism and that of the other pigments, particularly the anthocyanins, whose switch-off during maturation promotes the white colouration of the fruits. Recently, transcription profiling of ‘Leucocarpa’ and ‘Cassanese’ olives along ripening, performed through an Illumina RNA-seq approach, has provided useful insights on genes functions involved in fruit maturation such as those related to the biosynthesis of flavonoids and anthocyanins.
Methodology
To assess expression alterations of genes involved in flavonoids and anthocyanins biosynthetic pathways during ripening, possibly caused by small nuclear RNA (snRNA) in olive drupes, snRNA libraries from ‘Leucocarpa’ and ‘Cassanese’ were constructed with RNAs extracted at 100 and 130 Days After Flowering (DAF) and sequenced by an Illumina approach. 130 conserved microRNAs (miRNA) in the Viridiplantae belonging to 14 miRNA families were identified. Regarding the 130 conserved miRNAs, approximately the 48% were identified in all libraries, 5 and 18 miRNAs were shared between the “Cassanese” (C100, C130) and “Leucocarpa” (L100, L130) libraries, respectively.
Conclusion
For the remaining reads not-matching with known miRNAs in the Viridiplantae, we combined secondary structure and minimum free energy to discover novel olive miRNAs. Based on these analyses, 492 sequences were considered as putative novel miRNAs. The putative target genes of identified miRNA were computationally predicted by alignment with the olive drupe transcripts obtained from the same samples. A total of 218 transcripts were predicted as targets of 130 known and 492 putative novel miRNAs. Interestingly, some identified target genes are involved in negative regulation of anthocyanin metabolic process. Quantification of the expression pattern of three miRNA and their target transcripts by qRT-PCR assay confirmed the results of Illumina sequencing.
Introduction
The snRNAs are a class of non-coding RNAs, very abundant in the eukaryotic genome, and include the small interfering RNAs (siRNAs) and microRNAs (miRNAs). The miRNA when expressed, match with the 3’untranslated region (UTR) of target mRNAs determining gene silencing through the transcript cleavage or the translational inhibition [1–5]. In plants RNAs molecules of ~ 25 nt in length have been associated with posttranscriptional gene silencing [6–9].
The RNA silencing has been proposed as a viral and tranposons defense mechanism [9], but since the discover of miRNAs in the worm Caenorhabditis elegans, as molecules controlling the timing of the larval development [10–11], it was suggested that they can be used by organisms for the regulation of endogenous genes. Currently, an increased number of studies established their role as key regulators of genome expression and their involvement in the modulation of pivotal events in development and response to environmental cues in most eukariotes. In plants, the importance of miRNAs for development is supported by the abnormalities observed in mutants and transgenic lines showing defects in miRNA accumulation or activity [12–17].
So far, plant miRNAs have been experimentally analysed and bioinformatically predicted in many relevant crop species, including Pyrus bretschneideri [18], Citrus sinensis [19], Solanum lycopersicum [20] and Olea europaea [21, 22]. Such studies revealed miRNAs to be master regulators, targeting TFs involved in diverse morpho-physiological processes including fruit development, ripening, and fruit alternate bearing [20, 21, 23, 24]. Accordingly, miR166 and miR168 are highly expressed in cotton ovules [25], miR168 is involved in developmental process of tomato fleshy fruits and seeds [26, 27] and miR169, suppresses C class MADS box genes in relation to carpel development suggesting that miR169 is involved in fruit development [28]. Moreover, in tomato fruit, miR157 controls Colourless Non-Ripening (CNR) expression which in turn modulate ripening-related gene expression [29] and carotenoid biosynthesis [24, 30]. miR159 negatively regulates MYB TFs which are important in seed germination and flower development [31]. Interestingly, in recent years, various reports suggested a significant role of miRNAs in regulating the biosynthesis and accumulation of secondary metabolites in plants [32, 33].
Anthocyanins are among the most relevant classes of secondary metabolites which commonly accumulate in flowers and fruits, but are also present in leaves, stems, and storage organs [34–37]. In addition, they can be used as dietary nutraceutics, exerting benefits for human health [37–40].
The anthocyanins are synthesized through the phenylpropanoid pathway, one of the most extensively studied pathways of secondary metabolites for transcriptional regulation in plants [41–43].
Recently, scientific efforts have been directed toward understanding the involvement of miRNAs in the post-transcriptional regulation of the phenylpropanoid pathway. It has been demonstrated that the miR156 targeted the SQUAMOSA PROMOTER BINDING PROTEIN- LIKE 9 (SPL9) TF, involved in both the dihydroflavonols and leucoanthocyanidin synthesis [33]. This occurs through the destabilization by SPL9 of the regulatory complex MYB-bHLH-WD40, that controls polyphenolic biosynthetic steps [44–50] and interferes with the DFR expression. Besides, in Arabidopsis SPL9 inhibits the expression of anthocyanin biosynthetic genes [51, 52]. Accordingly, higher levels of miR156 reduce the SPL activity and enhance the expression of F3H, DFR, and other anthocyanin biosynthetic-related genes [33, 51]. On the other hands, high levels of SPL reduce the accumulation of anthocyanins and increases flavonols production [33, 51].
Anthocyanin compounds are among the secondary metabolites which accumulate in olive fruit, together with other polyphenolic compounds [53]. Therefore olive (Olea europaea L. subsp. europaea var. europaea) drupes and olive oil represent an interesting source of phytochemicals, that have beneficial effects on health. Interestingly, several epidemiological studies suggest that the intake of olives and olive oils are responsible for the reduction of the incidence of cardiovascular diseases, certain types of cancer, and aging-related pathologies [54]. Anyway, the relative concentration of the biologically active compounds with health attributions in these food matrices is closely dependent on several factors, varying in relation to environmental growth conditions, the genetic traits, the varieties as well as the ripeness stage [55, 56].
Recently, transcription profiling performed through an Illumina RNA-seq approach on ‘Leucocarpa’ and ‘Cassanese’ drupes collected at different ripening stage, allowed us to obtain some information about genes involved in maturation such as genes involved in the biosynthetic pathways of flavonoid and anthocyanin [57]. In particular, we identified some TF members with similarity to MYB, MIC and WD40 family directly related to anthocyanin accumulation. Interestingly the transcript levels of these TFs were higher in Cassanese cv than in ‘Leucocarpa’, which is an olive variety characterized by a switch-off in skin colour at full ripeness.
Based on the participation of multiple miRNAs in controlling fruit ripening, in the present study we aimed to identify specific miRNAs involved in flavonoid and anthocyanin metabolism in olive drupes using high-throughput sequencing combined with bioinformatics analysis and molecular experiments. Then, four snRNA libraries covering two developmental stages of fruit ripening of ‘Leucocarpa’ and ‘Cassanese’ were constructed with RNAs from drupes collected at 100 and 130 Days After Flowering (DAF) and sequenced by an Illumina approach.
Material and methods
Plant material
Olives (Olea europaea L.) of two varieties Cassanese and Leucocarpa (S1 Fig), were harvested from trees grown under natural conditions in the field germplasm collection of the CREA—Research Centre for Olive, Citrus and Tree Fruit (Rende, Cosenza, Italy, latitude 39°21′57′′N, longitude 16°13′44′′ E and altitude 210 masl). Ripening drupes (n = 30) with 100 and 130 DAF were randomly collected from 20-years-old plants, fixed in liquid nitrogen and stored at -80°C. For quantitative real-time PCR (qRT-PCR) we used the same samples managed in a similar way but collected four years later at the same DAF.
Small RNA library preparation and sequencing
Total RNA was isolated from the epi-mesocarp tissues of drupes with the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) and treated with DNase I (Roche, Milan Italy), as previously described [57]. Agilent 2100 bioanalyzer (Agilent technologies) assay was carried out to evaluate RNA quality and quantity. We used for cDNA library preparation only the samples isolated and purified with a concentration of ≥ 400 ng/μl, OD260/280 = 1.8 ~ 2.2, RNA 28S:18S ≥ 1.0 and RNA Integrity Number (RIN) ≥ 7.0. Library preparation and sequencing was carried out by Technology Services of the Institute of Applied Genomics (IGA, Udine, Italy). Upon the quality control of the starting material libraries were generated and size was selected in a range maximizing the number of relevant reads. Sequencing was performed on HiSeq2500 in a 50 bp single-read mode.
miRNA analysis
Raw reads were pre-processed following pipeline illustrated as a flow diagram in S2 Fig. Briefly, pre-processing was based on miRExpress software [58] to merge the identical reads into a unique read, to count each unique read and to remove full and partial adaptor sequences. Sequences below 18 bp and above 32 bp were also discarded.
In order to identify known miRNAs in olive, the remaining unique sequences were aligned against known Viridiplantae miRNA (miRBase database version 21.0) [59] by using miRExpress software.
To identify putative novel miRNAs, sequences that no matched the miRBase database were aligned with genome sequence of the olive tree [60] using the SOAP 2.0 software [61]. The secondary structures of the matched sequences were analysed by the Mireap software (http://sourceforge.net/projects/mireap/) in order to identify new candidate miRNAs according to the following parameters: (1) miRNA sequence length 18–32 bp, (2) miRNA reference sequence length 18–32 bp, (3) maximal copy number of miRNAs on reference 600, (4) maximal free energy allowed for a miRNA precursor -30 kcal/mol, (5) maximal space between miRNA 450 bp, (6) minimal base pairs of miRNA 16bp, (7) maximal bulge of miRNA 3bp, (8) maximal asymmetry of duplex 4 bp, (9) flank sequence length of miRNA precursor 20 bp. The selected sequences were then folded into a secondary structure by Mfold program [62].
The frequency of both known and putative novel miRNA read count was normalized as transcript per million (TPM) and normalization of miRNA expression levels among four libraries was carried out as reported by [63].
miRNA target identification was carried out by alignment with the olive drupe transcripts obtained through an Illumina RNA-seq approach [57] by Bowtie program [64]. Target genes of both conserved and novel miRNAs were compared with Arabidopsis protein database and Gene Ontology (GO) and KEGG pathway enrichment analyses were performed using KOBAS 3.0 [65]. The enrichment analysis of each GO and KEGG term was performed using the hypergeometric test (Fisher's exact test), considering transcriptome obtained through an Illumina RNA-seq approach as background ‘genome’. Then, any GO and KEGG terms with an FDR adjusted p-value (q-value) less than of 0.05 were considered as the enriched ones.
Venn diagram was generated by a web-tool (http://bioinformatics.psb.ugent.be/webtools/Venn/) and part of miRNA expression data was visualized in a circular layout by Circos Table Viewer v0.63–9 [66].
The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus [67] and are accessible through GEO Series accession number GSE104763 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE104763).
Stem-loop quantitative real-time PCR
For stem-loop quantitative real-time PCR (qRT-PCR) reverse transcription reactions were performed as described previously with some modification [21]. Each reaction solution (final volume 10 μl) contained 1 μg of total RNA, 1 μM RT stem-loop primer (S1 Table) and Superscript III (Invitrogen), according to manufacturer’s instructions. The qRT-PCR was carried out using Applied Biosystems® 7500 fast instrument and a Thermo Scientific Maxima SYBR Green/ROX qPCR Master Mix (2X) according to manufacturer’s instructions. Each RT product was amplified with universal reverse primer and three specific miRNA forward primers (S1 Table). Three independent biological replicates were used for each cDNA and miRNA. PCR conditions were: 15ʹ at 95°C followed by 40 cycles of incubations at 95°C × 5ʹʹ, at 56°C × 10ʹʹ and at 72°C × 60ʹʹ. At the end of the PCR, the melting temperature of the amplicons was measured by denaturing the amplified DNA, in order to determine whether the amplified products are homogeneous and the correct product has been specifically amplified.
Quantitative real-time PCR of predicted target genes
First strand cDNA was synthesized from 1 μg total RNA with oligo-d(T) and Superscript III (Invitrogen), according to the manufacturer’s instructions. 50 ng cDNA treated with RNase was used as template for qRT-PCR assays, carried out using Applied Biosystems® 7500 fast instrument and a Thermo Scientific Maxima SYBR Green/ROX qPCR Master Mix (2X) according to manufacturer’s instructions and with primers for target transcripts of miR168, miR166 and miR159 and for elongation factor 1-alpha (ef-1a). Three independent biological replicates were used for each cDNA and transcript. PCR conditions were: 95°C × 5ʹʹ, followed by 45 cycles at 95°C × 15ʹʹ and at 58°C × 60ʹʹ. At the end of the PCR, the melting temperature of the amplicons was measured by denaturing the amplified DNA, in order to determine whether the amplified products are homogeneous and the correct product has been specifically amplified. Relative template abundance was quantified using the standard curve method, normalizing each data against the quantity of the actin transcript [68]. Serial 10-fold dilutions of each gene amplicon were used to calculate the amplification efficiency for each target and control transcript.
Results
To investigate the effects of small RNAs (sRNA) during fruit development in Olea europaea and identify potential novel miRNAs, we performed a deep sequencing of the sRNA libraries resulting from ripening fruits of two different olive cultivars. Drupes were collected at 100 and 130 DAF of both Leucocarpa (L) and Cassanese (C) cultivars and were used to generate four distinct sRNA libraries.
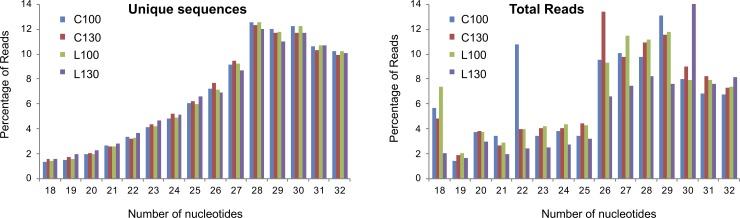
Illumina deep sequencing generated a total of 171,086,524 raw reads. After processing of primary reads by removing adaptors and discarding low quality reads, 2,488,061, 7,171,566, 3,106,156 and 3,901,395 total filtered reads were counted for C100, C130, L100 and L130 libraries, respectively (Table 1). The length of the sRNAs varied from 18 to 32 bp, being 28 bp the most abundant read length (Fig 1). Furthermore, these 16,667,178 clean reads were represented by 2,060,862 unique tags; 143 unique tags were sequenced more than 10k times, whereas 77.3% and 11.9% were present only one and two times, respectively.
Table 1. Summary of sequencing results from two varieties (ʹLeucocarpaʹ and ʹCassaneseʹ) at the two different ripening stages (100 and 130 DAF).
| C100 | C130 | L100 | L130 | |||||
|---|---|---|---|---|---|---|---|---|
| Total | Unique | Total | Unique | Total | Unique | Total | Unique | |
| Raw data | 26,513,964 | 7,048,949 | 70,190,746 | 13,462,730 | 34,379,362 | 8,167,774 | 40,002,452 | 5,480,276 |
| Adapter removed | 4,006,593 | 985,199 | 11,863,144 | 2,593,282 | 5,356,148 | 1,370,116 | 5,777,137 | 1,043,617 |
| Filtered data | 2,488,061 | 339,875 | 7,171,566 | 888,038 | 3,106,156 | 455,013 | 3,901,395 | 377,936 |
Fig 1. Small RNAs in each of the four libraries.
Length (bp) distribution of unique and total sequencing reads. Percentage of reads in specific length per library to the summed reads in corresponding library was shown.
Identification of conserved miRNAs
To identify miRNAs from ripening drupes, the sequenced sRNA fragments were aligned against miRBase database (version 21.0) that includes 8,496 known miRNAs belonging to 73 plant species.
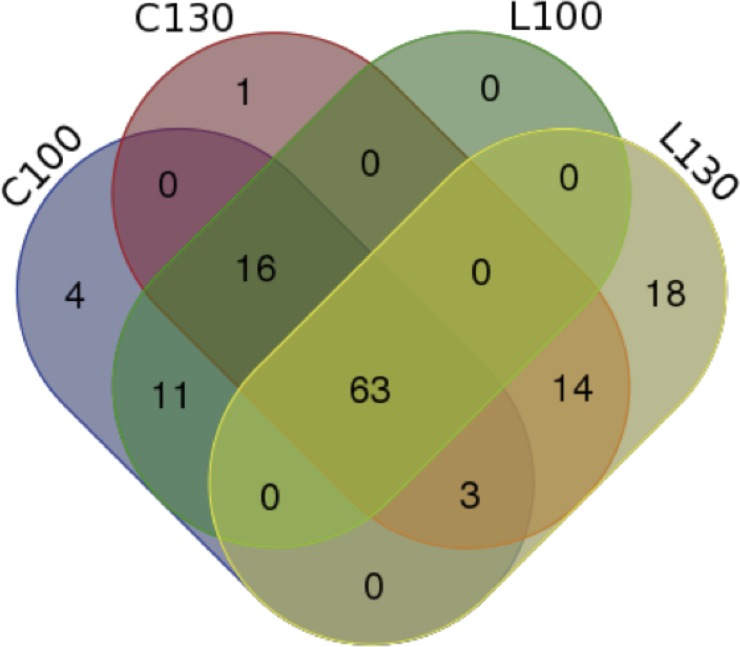
Comparison analyses revealed 130 conserved miRNAs (261,069 reads) clustered in 14 families; reduced to 11 and 10 mRNA families when limiting the dataset to Cassanese and Leucocarpa cv, respectively (S2 Table).
8 out of the 14 identified miRNA groups were already reported in olive [21, 69] while the remaining 6 are newly discovered (miR3630, miR5083, miR5538, miR6300, miR845 and miR894).
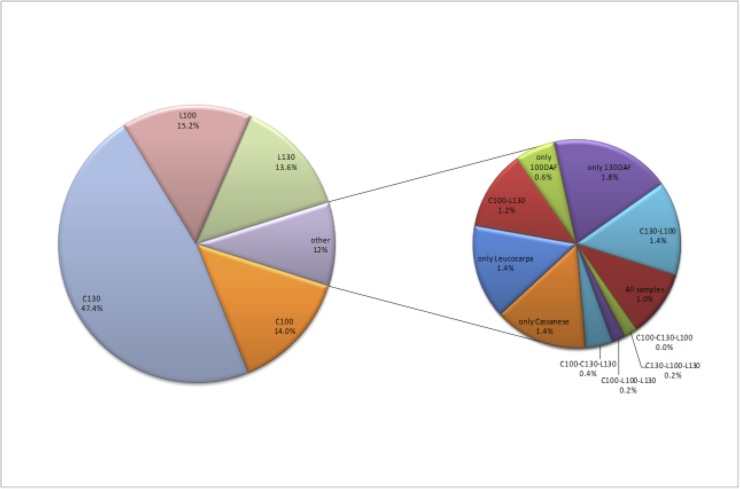
About half of the identified miRNAs (63) were identified in all libraries, but only 5 and 18 miRNAs are shared within ʹCassaneseʹ and ʹLeucocarpaʹ samples respectively, regardless to the ripening stage (Fig 2). Interestingly, a small but significant number of miRNAs, spanning around 8–11% of the total, are expressed at a specific ripening stage (100 DAF or 130 DAF), instead their expression is not subjected to relevant intra-species variability. Hence, they appear to be exclusively required at different times during the fruit ripening of olive (Fig 2).
Fig 2. Venn diagram of the conserved miRNA in the four libraries.
The intersecting portions of the Venn diagrams reports the number of common conserved miRNA families among the different comparisons between the two varieties (ʹLeucocarpaʹ and ʹCassaneseʹ) at the two different ripening stages (100 and 130 DAF).
The overall diversity of miRNA distribution and among the sequenced samples is quite constant in 3 out of the 4 libraries. ʹLeucocarpaʹ ripe library (L130), instead, contains the higher number of exclusively present (18) or absent (16) miRNA species (Fig 2).
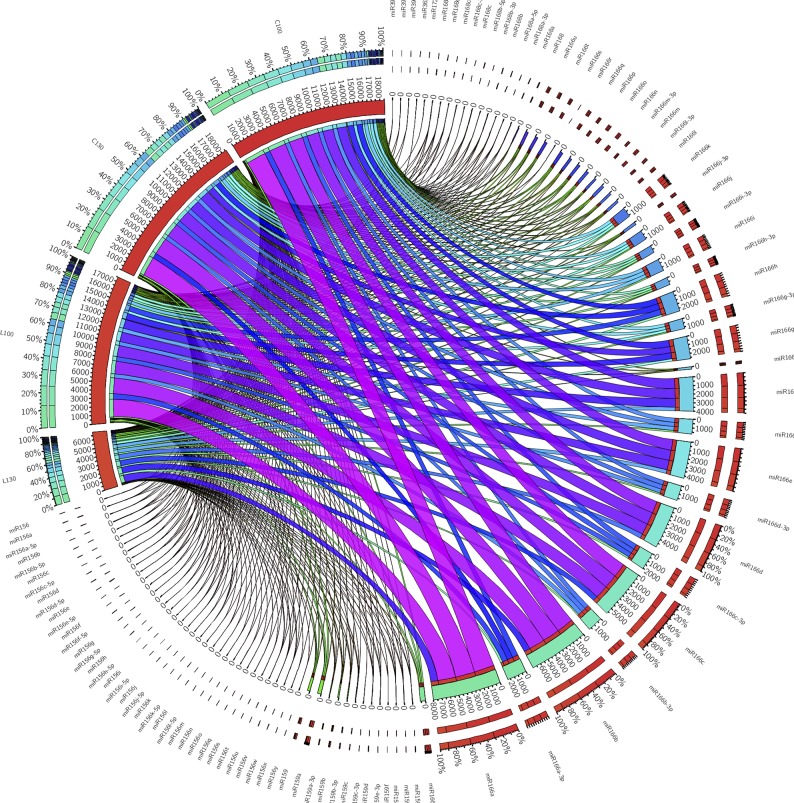
The abundance of defined miRNA was determined by measuring the normalized frequency of matching reads in the sequenced dataset. At a general level, this revealed that, most of the identified miRNA families were represented at low frequencies. On the other hand, higher redundancy was confined to only 4 families—miR166, miR159, miR168, miR156 - (S2 Table).
The miR166 family (59,825 reads and 34 variants) showed an expression 2.7-fold higher in ʹCassaneseʹ than in the Leucocarpa cv during 100–130 DAF transition (C130 vs L130). Furthermore, miRNA159 (1,204 reads) and miR156 (201 reads) families, with 13 and 36 variants in the dataset, were 2.7- and 2.3-fold higher in C130 with respect to L130. Finally, the miR168 family (322 reads and 11 variants), was more expressed in Cassanese cv, 4.5- and 1.5- fold in unripe and ripe drupes, respectively (Fig 3), suggesting a potential key role of these miRNAs in affecting gene expression of the white olive ʹLeucocarpaʹ.
Fig 3. Distribution of expression of the 130 conserved miRNAs belong the four libraries.
The data were visualized via Circos software [66]. The width of bars from each library and miRNA indicates their relative abundance.
Additional expression alterations were also revealed at the single miRNA species. In fact, L130 showed the lowest accumulation of miRNAs, with 63 downregulated miRNAs species, with respect to the other samples.
Prediction of novel olive miRNAs
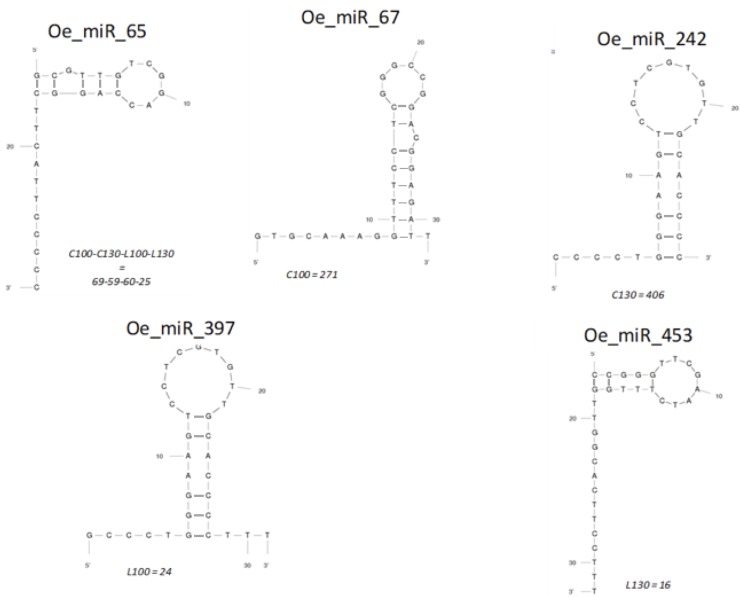
For the remaining reads not-matched with known Viridiplantae miRNAs, once mapped to genome sequence of the olive tree [60], we combined secondary structure and minimum free energy to discover novel miRNAs in olive. Based on these analyses, 11,519 novel miRNA-like reads were identified from the 4 sequenced libraries, representing 492 unique miRNAs (S3 Table). The folded secondary structures of the putative novel miRNAs are shown in Fig 4 and some of them showed peculiar expression profiles (S3 Table, Fig 5). Thus, 149 were expressed only in libraries created from ʹLeucocarpaʹ genotype. Some of them (Oe_mir_355, Oe_mir_368, Oe_mir_379, Oe_mir_380, Oe_mir_398, Oe_mir_408, Oe_mir_416) were detected in both 100 and 130 DAF libraries (L100, L130), while 75 and 67 were found only in the unripe fruit library (L100) and in the ripe fruit library (L130), respectively. On the contrary, 309 putative novel miRNAs were specifically detected in ʹCassaneseʹ libraries. Some of them (Oe_mir_5, Oe_mir_19, Oe_mir_23, Oe_mir_29, Oe_mir_33, Oe_mir_37, Oe_mir_43) were detected in both 100 and 130 DAF (C100, C130), while 69 and 233 were found only in the unripe fruit library (C100) and in the ripe fruit library (C130), respectively (S3 Table, Fig 5). Interestingly, of the putative novel miRNAs exclusively expressed in the Cassanese cv, Oe_mir_242 in ripe library and Oe_mir_67 showed an elevated read frequency in unripe library (406 and 271 reads) (Fig 4). Of the 147 putative novel miRNA expressed only in unripe libraries, only some of them (Oe_mir_54, Oe_mir_63, Oe_mir_70) were detected in both varieties (L100, C100), while 69 and 75 were specifically found in Cassanese and Leucocarpa varieties (C100, L100), respectively. On the contrary, 309 putative novel miRNA were detected only in ripe fruit libraries. 9 of them (Oe_mir_120, Oe_mir_184, Oe_mir_198, Oe_mir_226, Oe_mir_265, Oe_mir_303, Oe_mir_304, Oe_mir_329, Oe_mir_340) were identified in both genotype libraries (L130,C130), while 233 and 67 were found only in Cassanese and Leucocarpa varieties (C130, L130), respectively (S3 Table, Fig 5).
Fig 4. Predicted secondary hairpin structures of some novel miRNAs identified in this study by mFold.
Fig 5. Summary of common and specific identified novel miRNAs among different libraries.
Finally, Oe_mir_32, Oe_mir_46, Oe_mir_49, Oe_mir_65, Oe_mir_91 were detected in all samples. Two of them (Oe_mir_49, Oe_mir_65) showed a similar expression pattern with a decrement of read frequencies during the ripening. Of the rest three, for Oe_mir_32 and Oe_mir_46 were observed an opposite pattern between genotypes with an increment and a decrement of read frequencies during 100–130 DAF transition in ʹCassaneseʹ and in ʹLeucocarpaʹ, respectively.
Target prediction of olive miRNAs
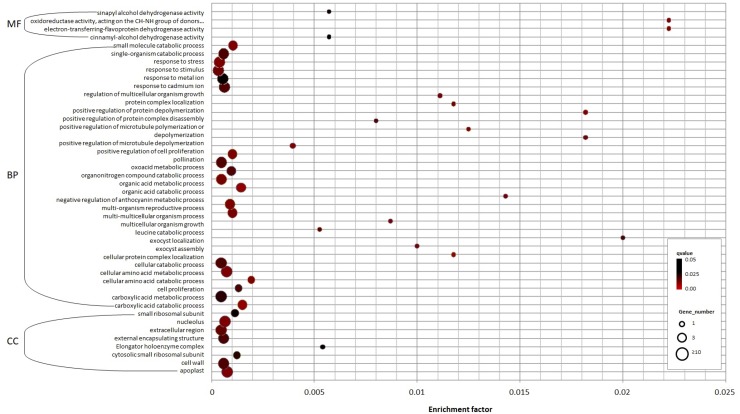
To increase insights into the functions of known and putative novel miRNAs in olive, putative target genes were computationally predicted by alignment with the olive drupe transcripts previously obtained from the same samples [57]. A total of 32 and 186 transcripts were predicted as targets of 130 known and 492 putative novel miRNA, respectively. All olive cDNAs predicted to be targets of known miRNAs were orthologues of miRNA target gene in Coffea canephora and other plant species (Table 2). The identified target genes are involved in a broad range of biological processes. In particular, GO enrichment analysis revealed that the target genes of olive miRNAs appeared to be significantly enriched in 44 GO terms (q-value < 0.05), the majority of which regrouped in biological process (72.7%) category followed by cellular component (18.2%) and molecular function (9.1%) (Fig 6, Table 2, S4 Table). Many target genes were related to ʹcatabolic processʹ (GO:0046395, GO:0016054, GO:0009063, GO:0044282, GO:1901565, GO:0044248, GO:0044712, GO:0006552) followed by process required for the high protein turnover existing in the ripening fruit as ʹribosomal proteinʹ, ʹprotein depolymerization and polymerizationʹ (GO:0031117, GO:1901881, GO:0031112, GO:0008284, GO:0034629, GO:0031503, GO:0008283, GO:0005618, GO:0022627, GO:0015935) and by ʹphenylpropanoid regulationʹ (GO:0031538, GO:0045551, GO:0052747). Among all, two molecular functions involved in oxidative phosphorylation (GO:0016649 and GO:0004174) shown a more enrichment factor (total number of the specific GO terms in the predicted miRNA targets vs. total number of the specific GO term in the olive transcriptome) (Fig 6, S4 Table).
Table 2. Predicted olive cDNA targets for known olive miRNAs.
| Functional Annotation | ||||
|---|---|---|---|---|
| miRNA | RNA_seq_ID | NCBI_ID | Gene ontology (GO) | KEGG pathway |
| miR156 | comp75091_c0_seq1 | GO:0045551 | cinnamyl-alcohol dehydrogenase activity | Biosynthesis of secondary metabolites |
| GO:0050896 | response to stimulus | Phenylpropanoid biosynthesis | ||
| GO:0052747 | sinapyl alcohol dehydrogenase activity | Metabolic pathways | ||
| comp75090_c0_seq1 | GO:0045551 | cinnamyl-alcohol dehydrogenase activity | Metabolic pathways | |
| GO:0050896 | response to stimulus | Phenylpropanoid biosynthesis | ||
| GO:0052747 | sinapyl alcohol dehydrogenase activity | Biosynthesis of secondary metabolites | ||
| comp42353_c0_seq1 | ||||
| miR159 | comp19510_c0_seq1 | GO:0004174 | electron-transferring-flavoprotein dehydrogenase activity | |
| GO:0006082 | organic acid metabolic process | |||
| GO:0006520 | cellular amino acid metabolic process | |||
| GO:0006552 | leucine catabolic process | |||
| GO:0009063 | cellular amino acid catabolic process | |||
| GO:0016054 | organic acid catabolic process | |||
| GO:0016649 | oxidoreductase activity, acting on the CH-NH group of donors, quinone or similar compound as acceptor | |||
| GO:0019752 | carboxylic acid metabolic process | |||
| GO:0043436 | oxoacid metabolic process | |||
| GO:0044248 | cellular catabolic process | |||
| GO:0044282 | small molecule catabolic process | |||
| GO:0044712 | single-organism catabolic process | |||
| GO:0046395 | carboxylic acid catabolic process | |||
| GO:0050896 | response to stimulus | |||
| GO:1901565 | organonitrogen compound catabolic process | |||
| comp13723_c0_seq1 | ||||
| comp33358_c0_seq1 | GO:0004174 | electron-transferring-flavoprotein dehydrogenase activity | ||
| GO:0006082 | organic acid metabolic process | |||
| GO:0006520 | cellular amino acid metabolic process | |||
| GO:0006552 | leucine catabolic process | |||
| GO:0009063 | cellular amino acid catabolic process | |||
| GO:0016054 | organic acid catabolic process | |||
| GO:0016649 | oxidoreductase activity, acting on the CH-NH group of donors, quinone or similar compound as acceptor | |||
| GO:0019752 | carboxylic acid metabolic process | |||
| GO:0043436 | oxoacid metabolic process | |||
| GO:0044248 | cellular catabolic process | |||
| GO:0044282 | small molecule catabolic process | |||
| GO:0044712 | single-organism catabolic process | |||
| GO:0046395 | carboxylic acid catabolic process | |||
| GO:0050896 | response to stimulus | |||
| GO:1901565 | organonitrogen compound catabolic process | |||
| comp46826_c0_seq1 | ||||
| comp68814_c0_seq1 | GO:0006950 | response to stress | ||
| GO:0050896 | response to stimulus | |||
| miR166 | comp97992_c0_seq1 | GO:0005576 | extracellular region | Sphingolipid metabolism |
| GO:0005618 | cell wall | Glycosphingolipid biosynthesis—globo series | ||
| GO:0030312 | external encapsulating structure | Galactose metabolism | ||
| GO:0044248 | cellular catabolic process | Glycerolipid metabolism | ||
| GO:0044712 | single-organism catabolic process | |||
| GO:0048046 | Apoplast | |||
| GO:1901565 | organonitrogen compound catabolic process | |||
| comp56340_c0_seq1 | GO:0005730 | Nucleolus | Spliceosome | |
| GO:0006950 | response to stress | |||
| GO:0050896 | response to stimulus | |||
| comp62505_c0_seq1 | GO:0008283 | cell proliferation | ||
| GO:0008284 | positive regulation of cell proliferation | |||
| GO:0050896 | response to stimulus | |||
| miR168 | comp9440_c0_seq1 | GO:0006950 | response to stress | Base excision repair |
| GO:0050896 | response to stimulus | Purine metabolism | ||
| Pyrimidine metabolism | ||||
| Homologous recombination | ||||
| Metabolic pathways | ||||
| DNA replication | ||||
| Nucleotide excision repair | ||||
| comp55643_c0_seq1 | Alanine, aspartate and glutamate metabolism | |||
| comp60984_c0_seq1 | GO:0006950 | response to stress | ||
| GO:0008283 | cell proliferation | |||
| GO:0008284 | positive regulation of cell proliferation | |||
| GO:0031538 | negative regulation of anthocyanin metabolic process | |||
| GO:0033588 | Elongator holoenzyme complex | |||
| GO:0050896 | response to stimulus | |||
| comp9240_c0_seq1 | ||||
| comp17765_c0_seq1 | GO:0006950 | response to stress | ||
| GO:0008283 | cell proliferation | |||
| GO:0008284 | positive regulation of cell proliferation | |||
| GO:0031538 | negative regulation of anthocyanin metabolic process | |||
| GO:0033588 | Elongator holoenzyme complex | |||
| GO:0050896 | response to stimulus | |||
| comp55642_c0_seq1 | Alanine, aspartate and glutamate metabolism | |||
| miR172 | comp32954_c0_seq1 | GO:0009856 | Pollination | Spliceosome |
| GO:0044703 | multi-organism reproductive process | |||
| GO:0044706 | multi-multicellular organism process | |||
| miR3630 | comp701_c0_seq1 | |||
| miR390 | comp82582_c0_seq1 | GO:0015935 | small ribosomal subunit | Ribosome |
| GO:0022627 | cytosolic small ribosomal subunit | |||
| miR396 | comp19066_c0_seq1 | |||
| comp89993_c0_seq1 | ||||
| comp67855_c0_seq1 | ||||
| comp77260_c0_seq1 | ||||
| comp54983_c0_seq1 | ||||
| comp45923_c0_seq1 | ||||
| miR482 | comp6983_c0_seq1 | GO:0006082 | organic acid metabolic process | Vitamin B6 metabolism |
| GO:0006520 | cellular amino acid metabolic process | |||
| GO:0006950 | response to stress | |||
| GO:0019752 | carboxylic acid metabolic process | |||
| GO:0043436 | oxoacid metabolic process | |||
| GO:0050896 | response to stimulus | |||
| miR5083 | comp95403_c0_seq1 | |||
| miR5538 | comp67128_c0_seq1 | |||
| miR6300 | comp43487_c0_seq1 | |||
| miR845 | comp32983_c0_seq1 | |||
| miR894 | comp32058_c0_seq1 | GO:0006950 | response to stress | |
| GO:0044248 | cellular catabolic process | |||
Fig 6. Gene Ontology (GO) of unigenes targeted by conserved and predicted miRNAs.
The significantly enriched GO terms were categorized according to biological process, molecular function and cellular component. The enrichment factor (total number of the specific GO terms in the predicted miRNA targets vs. total number of the specific GO term in the reference olive trascriptome) is on the x-axis. The size of each point represents the number of genes enriched in a particular GO term. A larger enrichment factor value and lower Q-values indicates a greater degree of enrichment.
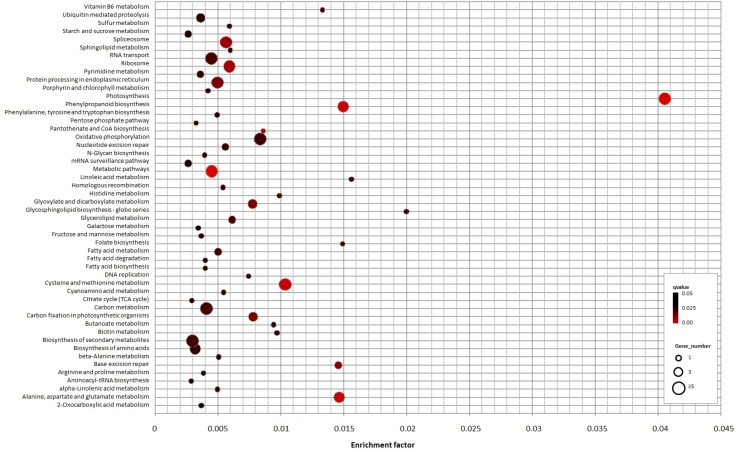
A total of 58 KEGG pathways were enriched of which 50 were detected at significantly high abundance level (q-value < 0.05) (Fig 7, Table 2, S5 Table). Notably, the most significantly enriched pathways were ʹphotosynthesisʹ (ko00195), ʹmetabolic pathwaysʹ (ko01100) and ʹphenylpropanoid biosynthesisʹ (ko00940). Because these metabolic categories are so broad, however, no pathway maps could be identified in the KEGG database. Nevertheless, several identified pathways have a role in the fruit ripening, like as ʹfatty acid degradation and biosynthesisʹ (Fig 7, Table 2, S5 Table).
Fig 7. The enriched KEGG pathways of the target genes.
The enrichment factor (total number of the specific pathways in the predicted miRNA targets vs. total number of the pathways in the reference olive trascriptome) is on the x-axis. The size of each point represents the number of genes enriched in a particular pathway. A larger enrichment factor value and lower Q-values indicates a greater degree of enrichment.
Interestingly, most of the annotated transcripts involved in phenylpropanoid regulation are target of miRNAs up-regulated in Cassanese cv (miR156 and miR168), suggesting a key role of this miRNA family in the regulation of colour transition in olive (Table 2).
Real-time quantification of miRNAs and their target genes
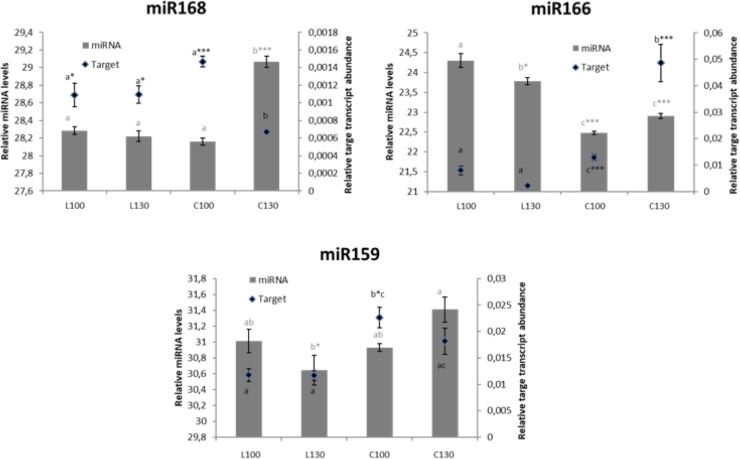
In this study, the expression levels of three miRNAs (miR159, miR166 and miR168) and their respective target genes (comp19510, comp97992 and com60984) were additionally analysed by Real-time qRT-PCR. In order to obtain a clear view of the miRNA regulation genotype and ripening-stage specific, we excluded potential environmental effects, as temperature, rainfall and age of plants, by collecting similar samples four years later those obtained for deep sequencing. Moreover, we preferred collect drupes at more late ripening stages to better appreciate differences between two genotypes. Notably, as expected, the results showed that the miRNAs were negatively correlated with the expression level of their targets (Fig 8). MiR168 was highly expressed at 130 DAF in Cassanese coloured cv, while its target gene was down regulated (Fig 8). On the other hand, miR166 was strongly expressed in white-coloured ʹLeucocarpaʹ in both ripening stages, while its target was down regulated (Fig 8). For the last analyzed miRNA (miR159), although less marked than the other analysed miRNAs, we observed a clear negative correlation between miRNA and its target expression levels, with a substantial down regulation of miR159 and up regulation of its target in Cassanese cv and an opposite trend in Leucocarpa cv (Fig 8).
Fig 8. qRT-PCR of selected miRNAs and their target transcripts.
The histograms show the relative values of miRNAs while the lines represent the relative abundance of target transcripts. The analyses were performed as triplicates and the error bars indicate the standard error of the mean (s.e.m.). For each value, different letters indicate significant differences among mean values (*p-value ≤ 0.05; ** p-value ≤ 0.01; *** p-value ≤ 0.001). For all variables with the same letter, the difference between the means is not statistically significant.
Comparing PCR data with results of the deep sequencing, for two of the studied miRNAs (miR166, miR168) we observed a similar expression pattern during ripening in both genotypes.
Discussion
Fruit development and ripening is an effect of a fine harmonization of biochemical pathways leading to changes in colour, texture, nutrient accumulation and aroma. Some of these pathways are related to plant secondary metabolism and lead to the production of several compounds such as carotenoids and different phenol classes, including anthocyanins, which may also play an important role in the fruit postharvest performance. Namely, acting as antioxidants, they prevent lipid peroxidation, and maintain membrane integrity to decelerate cell senescence [70]. Accordingly, tomato, fruits enriched in anthocyanins in the epicarp, show less over-ripening and extend their shelf-life [71, 72]. Anthocyanins are also important phytonutrients in a healthy diet, exerting anti-tumour, pro-apoptotic, anti-oxidative, anti-inflammatory and anti-neurodegenerative properties [37, 39, 40, 73, 74].
In the last years many approaches were used to elucidate the molecular aspects of fruit development and ripening in a lot of plant species and recently thanks to easier access to the high-throughput sequencing technology, an enormous amount of genome data and gene expression profiles was produced. Some of these data deal specifically with the identification of several miRNAs which have been found to play key roles in the regulation of diverse biological processes and metabolic pathway [75, 76], including fruit development and ripening [77–79] as well as the biosynthesis and accumulation of secondary metabolites in plants such as flavonoids [32, 33]. In particular, it has been demonstrated that miRNAs interact with the major branches of phenylpropanoid pathway along which flavonoid backbone is synthesized [33].
Concerning olive, although two studies have been carried to identify and characterize miRNAs in Olea europaea [21, 69], in both cases no different varieties have been compared in order to highlights possible correlations between fruit pigmentation and ripening process genotype-dependent. Previously, we documented in two olive varieties, ʹLeucocarpaʹ, characterized by a switch-off in skin colour of ripe drupes, and ʹCassaneseʹ used as control plant, significant differences in anthocyanin transcript profiles both ripening and genotype dependent [57]. In this study, four drupe samples taken during 100–130 DAF transition and from Leucocarpa and Cassanese varieties were analysed, aiming to elucidate miRNA putative roles in the biosynthesis and accumulation of secondary metabolisms and particularly of phenylpropanoids.
This resulted in identification and characterization of 130 known conserved miRNAs across 14 families and 492 unique novel miRNAs. As shown in S2 Table and Fig 3, miR166 was most abundant among conserved families. In line with the presence of miR166 family in all analysed plant species and tissues, it was found in all our libraries confirming an its essential role across the plant kingdom with housekeeping functions [79, 80]. However, it was more than 2-fold down regulated in ripe drupes of Leucocarpa cv respect to the three other samples. miR166 was predicted to target three transcripts involved in cell proliferation, catabolic processes resulting in cell wall disassembly (Table 2). Moreover, in a previous study of its significant up regulation was observed in the unripe olive fruits, suggesting a potential inhibition of the fruit maturation by suppression of these target transcripts [21]. Our qRT-PCR assays confirmed this hypothesis showing an opposite trend, in the control variety (Cassanese), between miR166 and its target, up and down regulated in the late ripening stage, respectively (Fig 8). Interestingly, in the white coloured ʹLeucocarpaʹ, miR166 expression was found persistently high during the fruit ripening and the levels of its target transcript were down regulated in both ripening stages (Fig 8). It may be speculated that the induction of this miRNA may inhibit some typical ripening processes, like as fruit veraison, by suppressing its target gene. Illumina and qRT-PCR data are comparable within intravarietal ripening profiles while clear differences resulted within intervarietal comparisons. In fact, if on the one hand miR166 was significantly down regulated during ripening only in Leucocarpa cv on the other hand, the divergences in the intervarietal comparisons were probably due to a genotype-dependent responses.
The miR159, also detected in previous studies in olive [21, 69], was the second most abundant conserved miRNA family found in the olive fruit library. It was ubiquitously expressed in all the libraries but showed an opposite expression pattern between ʹLeucocarpaʹ and ʹCassaneseʹ (S2 Table, Table 2, Fig 3). Although qRT-PCR assays confirmed Illumina data highlighting a slight down regulation of miR159 in ‘Cassanese’ during ripening, the differences were not significant (Fig 8). Also, miRNA168 family, which is known to regulate target genes involved in plant development, signal transduction, metabolism, and defense response was detected in our libraries. Their transcripts were higher in ‘Cassanese’ fruit at 130 DAF than 100DAF while in ‘Leucocarpa’ they were unchanged during ripening transition and similar to those of ‘Cassanese’ 100 DAF. Also, the levels of its target gene were down regulated at the last ripening stages, suggesting a putative role in the biosynthesis and/or accumulation of anthocyanins metabolites.
In conclusion, miRNAs play a role in plant metabolism, development, and defense response. Accordingly, our results showed that miRNAs play a role in olive fruit development tightly related to the production of wealthy secondary metabolites, such as anthocyanins which exert benefits for human health. Interestingly miRNA168a and 159a, provided through diet, was able to pass through the mouse and human gastrointestinal track and decrease the LDL levels in the plasma [81, 82] showing that, exogenous miRNAs can regulate target gene expression and recipient cell function. Therefore, it is tempting to suggest that miRNA could represent potential molecules to promote the production of functional food.
Starting from these evidences, the development of the high-throughput sequencing technology and genome-scale approaches provides a powerful approach to investigate small RNA, including miRNAs and their target genes in organisms from model plant species to non-agricultural crops and agricultural species such as in olive.
Supporting information
Olive fruit branches of Olea europaea (A- Cassanese and B- Leucocarpa cultivars).
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(TXT)
(TXT)
Data Availability
All relevant data are available from the the NCBI Gene Expression Omnibus at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE104763/.
Funding Statement
Funded by Regione Calabria Award Number: POR Calabria FESR-FSE 2014-2020, Action 1.2.2, S3 strategies: "Extra virgin olive oil digital ID management - ODIN”. | Recipient: Prof. Adriana Chiappetta. Regione Calabria Award Number: POR Calabria FESR-FSE 2014-2020, Action 1.2.2, S3 strategies: "Extra virgin olive oil digital ID management - ODIN”. | Recipient: Dr. Innocenzo Muzzalupo, PhD. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Llave C, Xie Z, Kasschau KD, Carrington JC. Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science. 2002. 297:2053–2056. 10.1126/science.1076311 [DOI] [PubMed] [Google Scholar]
- 2.Baumberger N, Baulcombe DC. Arabidopsis Argonaute1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. Proc. Natl. Acad. Sci. USA. 2005; 102, 11928–11933. 10.1073/pnas.0505461102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAs and their regulatory roles in plant. Ann. Rev. Plant Biol. 2006; 57: 19–53. 10.1146/annurev.arplant.57.032905.105218 [DOI] [PubMed] [Google Scholar]
- 4.Gandikota M, Birkenbihl RP, Hohmann S, Cardon GH, Saedler H, Huijser P. The miRNA156/157 recognition element in the 3′ UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. Plant J. 2007; 49, 683–693. 10.1111/j.1365-313X.2006.02983.x [DOI] [PubMed] [Google Scholar]
- 5.Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L. et al. Widespread translational inhibition by plant miRNAs and siRNAs. Science. 2008; 320, 1185–1190. 10.1126/science.1159151 [DOI] [PubMed] [Google Scholar]
- 6.Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999; 286:5441, 950–952. 10.1126/science.286.5441.950 [DOI] [PubMed] [Google Scholar]
- 7.Fagard M, Boutet S, Morel JB, Bellini C, Vaucheret H. AGO1, QDE-2, and RDE-1 are related proteins required for post-tran- scriptional gene silencing in plants, quelling in fungi, and RNA interference in animals. Proc. Natl. Acad. Sci. 2000; 97, 11650–11654. 10.1073/pnas.200217597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matzke M, Matzke AJM, Kooter JM. RNA: guiding gene silencing. Science. 2001; 293:1080–1083. 10.1126/science.1063051 [DOI] [PubMed] [Google Scholar]
- 9.Vance V, Vaucheret H. RNA silencing in plants—defense and counterdefense. Science. 2001; 22:2277–2280. 10.1126/science.1061334 [DOI] [PubMed] [Google Scholar]
- 10.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993; 75:843–854. 10.1016/0092-8674(93)90529-y [DOI] [PubMed] [Google Scholar]
- 11.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000; 403:901–906. 10.1038/35002607 [DOI] [PubMed] [Google Scholar]
- 12.Allen RS, Li J, Stahle MI, Dubroue A, Gubler F, Millar AA, et al. Genetic analysis reveals functional redundancy and the major target genes of the Arabidopsis miR159 family. Proc Natl. Acad. Sci. USA 2007, 104, 16371–16376. 10.1073/pnas.0707653104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuck G, AM Cigan K, Saeteurn, Hake S. The heterochronic maize mutant Corngrass1 results from overexpression of a tandem microRNA. Nat. Genet. 2007; 39, 544–549. 10.1038/ng2001 [DOI] [PubMed] [Google Scholar]
- 14.Wang JW, Park MY, Wang LJ, Koo Y, Chen XY, Weigel D, et al. miRNA control of vegetative phase transition in trees. PLoS Genet. 2011; 7:e1002012 10.1371/journal.pgen.1002012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Xie X, Li J, Cui Y, Hou Y, Zhai L, et al. Conservation and diversification of the miR166 family in soybean and potential roles of newly identified miR166s. BMC Plant Biol. 2017; 17:32 10.1186/s12870-017-0983-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palatnik JF, Allen E, Wu X, Schommer C, Schwab R, Carrington JC, Weigel D. Control of leaf morphogenesis by microRNAs. Nature. 2003; 425:257–263. 10.1038/nature01958 [DOI] [PubMed] [Google Scholar]
- 17.Singh A, Roy S, Singh S, Das SS, Gautam V, Yadav S. Phytohormonal crosstalk modulates the expression of miR166/165s, target Class III HD-ZIPs, and KANADI genes during root growth in Arabidopsis thaliana. Sci Rep. 2017; 7:3408 10.1038/s41598-017-03632-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Wang Z, Shi Z, Zhang S, Ming R, Zhu S, et al. The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res. 2013; 23:396–408. 10.1101/gr.144311.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Wang L, Chen D, Wu X, Huang D, Chen L. Genome-wide comparison of microRNAs and their targeted transcripts among leaf, flower and fruit of sweet orange. BMC Genomics. 2014; 15:695 10.1186/1471-2164-15-695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohorianu I, Schwach F, Jing R, Lopez-Gomollon S, Moxon S, Szittya G. Profiling of short RNAs during fleshy fruit development reveals stage-specific sRNAome expression patterns. Plant J. 2011. 67: 232–246. 10.1111/j.1365-313X.2011.04586.x [DOI] [PubMed] [Google Scholar]
- 21.Yanik H, Turktas M, Dundar E, Hernandez P, Dorado G, Unver T. Genome-wide identification of alternate bearing-associated microRNAs (miRNAs) in olive (Olea europaea L.). BMC Plant Biol. 2013; 13: 10 10.1186/1471-2229-13-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minutolo A, Potestà M, Gismondi A, Pirrò S, Cirilli M, Gattabria F, et al. Olea europaea small RNA with functional homology to human miR34a in cross-kingdom interaction of anti-tumoral response. Sci. Rep. 2018; 8:12413 10.1038/s41598-018-30718-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira Silva GP Silva EM, da Silva Azevedo M, Guivin MAC, Ramiro Figueiredo CR, et al. microRNA156-targeted SPL/SBP box transcription factors regulate tomato ovary and fruit development. Plant J. 2014; 78, 604–618. 10.1111/tpj.12493 [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Kong J, Lai T, Manning K, Wu C, Wang Y, et al. Tuning LeSPL-CNR expression by SlymiR157 affects tomato fruit ripening. Sci. Rep. 2015; 5, 7852 10.1038/srep07852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang M, Woodward AW, Agarwal V, Guan X, Ha M, Ramachandran V, et al. Genome-wide analysis reveals rapid and dynamic changes in miRNA and siRNA sequence and expression during ovule and fiber development in allotetraploid cotton (Gossypium hirsutum L.). Genome Biol. 2009; 10, R122 10.1186/gb-2009-10-11-r122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin RC, Liu PP, Nonogaki H. MicroRNAs in seeds: modified detection techniques and potential applications. Can. J. Bot. 2006; 84:189–198. 10.1139/b05-141 [DOI] [Google Scholar]
- 27.Itaya A, Bundschuh R, Archual AJ, Joung JG, Fei Z, Dai X, et al. Small RNAs in tomato fruit and leaf development. Biochim. Biophys. Acta. 2008; 1779:99–107. 10.1016/j.bbagrm.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 28.Cartolano M, Castillo R, Efremova N, Kuckenberg M, Zethof J, Gerats T, et al. A conserved microRNA module exerts homeotic control over Petunia hybrida and Antirrhinum majus floral organ identity. Nat. Genet. 2007; 39, 901–905. 10.1038/ng2056 [DOI] [PubMed] [Google Scholar]
- 29.Eriksson EM, Bovy A, Manning K, Harrison L, Andrews J, De Silva J, et al. Effect of the colourless non-ripening mutation on cell wall biochemistry and gene expression during tomato fruit development and ripening. Plant Physiol. 2004; 136, 4184–4197. 10.1104/pp.104.045765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraser PD, Bramley P, Seymour GB. Effect of the Cnr mutation on carotenoid formation during tomato fruit ripening. Phytochem. 2001; 58, 75–79. 10.1016/S0031-9422(01)00175-3 [DOI] [PubMed] [Google Scholar]
- 31.Reyes JL, Chua NH. ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J. 2007; 49:592–606. 10.1111/j.1365-313X.2006.02980.x [DOI] [PubMed] [Google Scholar]
- 32.Bulgakov VP, Avramenko TV. New opportunities for the regulation of secondary metabolism in plants: focus on microRNAs. Biotechnol. Lett. 2015; 37, 1719–1727. 10.1007/s10529-015-1863-8 [DOI] [PubMed] [Google Scholar]
- 33.Gupta OP, Suhaset GK, Sagar B, Meena NL, Dahuja A. Contemporary understanding of miRNA-based regulation of secondary metabolites biosynthesis in plants. Front. Plant Sci. 2017; 8, 374 10.3389/fpls.2017.00374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delgado-Vargas F, Paredes-Lopez O. Anthocyanins and betalains In Natural Colourants for Food and Nutraceutical Uses, eds. Delgado-Vargas F, Paredes-Lopez O, 2003. pp. 167–219. Boca Raton, FL: CRC Press [Google Scholar]
- 35.Taylor LP, Grotewold E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005; 8:317–323. 10.1016/j.pbi.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 36.Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, et al. Genetics and biochemistry of seed flavonoids. Ann. Rev. Plant Biol. 2006; 57:405–430. 10.1146/annurev.arplant.57.032905.105252 [DOI] [PubMed] [Google Scholar]
- 37.Buer CS, Imin N, Djordjevic MA. Flavonoids: new roles for old molecules. J. Integr. Plant Biol. 2010; 52, 98–111. 10.1111/j.1744-7909.2010.00905.x [DOI] [PubMed] [Google Scholar]
- 38.Winkel-Shirley B. Flavonoid biosynthesis. a colourful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001; 126:485–493. 10.1104/pp.126.2.485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pojer E, Mattivi F, Johnson D, Stockley CS. The case for anthocyanin consumption to promote human health: a review. Comp. Rev. Food Sci. Food Saf. 2013; 12:483–508. 10.1111/1541-4337.12024 [DOI] [PubMed] [Google Scholar]
- 40.Ahmed NU, Park JI, Jung HJ, Yang TJ, Hur Y, Nou IS. Characterization of dihydroflavonol 4-reductase (DFR) genes and their association with cold and freezing stress in Brassica rapa. Gene. 2014; 550:46–55. 10.1016/j.gene.2014.08.013 [DOI] [PubMed] [Google Scholar]
- 41.Quattrocchio F, Verweij W, Kroon A, Spelt C, Mol J, Koes R. PH4 of Petunia is an R2R3 MYB protein that activates vacuolar acidification through interactions with basic-helix-loop-helix transcription factors of the anthocyanin pathway. Plant Cell. 2006; 18:1274–1291. 10.1105/tpc.105.034041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K., et al. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 2007; 50:660–677. 10.1111/j.1365-313X.2007.03078.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S. Transcriptional control of flavonoid biosynthesis: fine-tuning of the MYB-bHLH-WD40 (MBW) complex. Plant Signal. Behav. 2014; 9:e27522 10.4161/psb.27522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dixon RA, Xie DY, Sharma SB. Proanthocyanidins—a final frontier in flavonoid research? New Phytol. 2005; 165, 9–28. 10.1111/j.1469-8137.2004.01217.x [DOI] [PubMed] [Google Scholar]
- 45.Ramsay NA, Glover BJ. MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 2005; 10:63–70 10.1016/j.tplants.2004.12.011 [DOI] [PubMed] [Google Scholar]
- 46.He F, Pan QH, Shi Y, Duan CQ. Biosynthesis and genetic regulation of proanthocyanidins in plants. Molecules. 2008; 13:2674–2703. 10.3390/molecules13102674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian L, Pang Y, Dixon RA. Biosynthesis and genetic engineering of proanthocyanidins and (iso)flavonoids. Phytochem. Rev. 2008; 7:445–465. 10.1007/s11101-007-9076-y [DOI] [Google Scholar]
- 48.Alagna F, D'Agostino N, Torchia L, Servili M, Rao R., Pietrella, et al. Comparative 454 pyrosequencing of transcripts from two olive genotypes during fruit development. BMC Genomics. 2009; 10, 399 10.1186/1471-2164-10-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galla G, Barcaccia G, Ramina A, Collani S, Alagna F, Baldoni L, et al. Computational annotation of genes differentially expressed along olive fruit development. BMC Plant Biol. 2009; 9,128 10.1186/1471-2229-9-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ravaglia D, Espley RV, Henry-Kirk RA, Andreotti C, Ziosi V, Hellens RP, et al. Transcriptional regulation of flavonoid biosynthesis in nectarine (Prunus persica) by a set of R2R3 MYB transcription factors. BMC Plant Biol. 2013; 13:68 10.1186/1471-2229-13-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gou JY, Felippes FF, Liu CJ, Weigel D, Wang JW. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell. 2011; 23, 1512–1522. 10.1105/tpc.111.084525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui LG, Shan JX, Shi M, Gao JP, Lin HX. The miR156- SPL9-DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J. 2014; 80, 1108–1117. 10.1111/tpj.12712 [DOI] [PubMed] [Google Scholar]
- 53.Servili M, Taticchi A, Esposto S, Sordini B, Urbani S. Technological aspects of olive oil production Chapter 8. In: The olive cultivation, table olive and olive oil industry in Italy ed. Muzzalupo I. 2012. pp.151–172. IntechOpen, London, United Kingdom: 10.5772/3314 [DOI] [Google Scholar]
- 54.Vauzour D, Rodriguez-Mateos A, Corona G, Oruna-Concha MJ, Spencer JPE. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients. 2010; 2:1106–1131. 10.3390/nu2111106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loumou A, Giourga C. Olive groves: the life and identity of the Mediterranean. Agric. Hum. Values. 2003; 20:87–95. 10.1023/A:1022444005336 [DOI] [Google Scholar]
- 56.Conde C, Delrot S, Gerós H. Physiological, biochemical and molecular changes occurring during olive development and ripening. J. Plant Physiol. 2008; 165, 1545–1562. 10.1016/j.jplph.2008.04.018 [DOI] [PubMed] [Google Scholar]
- 57.Iaria DL, Chiappetta A, Muzzalupo I. A de novo transcriptomic approach to identify flavonoids and anthocyanins “switch-off” in olive (Olea europaea L.) drupes at different stages of maturation. Front. Plant Sci. 2016; 6:1246 10.3389/fpls.2015.01246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang WC, Lin FM, Chang WC, Lin KY, Huang HD, Lin NS. miRExpress: Analyzing high-throughput sequencing data for profiling microRNA expression. BMC Bioinform. 2009; 10:328 10.1186/1471-2105-10-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008; 36:D154 10.1093/nar/gkm952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cruz F, Julca I, Gómez-Garrido J, Loska D, Marcet-Houben M, Cano M. et al. Genome sequence of the olive tree, Olea europaea. Gigascience. 2016; 27:29 10.1186/s13742-016-0134-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li R, Yu C, Li Y, Lam TW, Yiu SM, Kristiansen K, Wang J. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics. 2009; 25:1966 10.1093/bioinformatics/btp336 [DOI] [PubMed] [Google Scholar]
- 62.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003; 31:3406 10.1093/nar/gkg595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eldem V, Akcay CU, Ozhuner E, Bakir Y, Uranbey S, Unver T. Genome-wide identification of miRNAs responsive to drought in peach (Prunus persica) by high-throughput deep sequencing. PLoS One. 2012; 7:e50298 10.1371/journal.pone.0050298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009; 10:R25 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mao X, Cai T, Olyarchuk JG, Wei L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinf. 2005; 21:3787 10.1093/bioinformatics/bti430 [DOI] [PubMed] [Google Scholar]
- 66.Krzywinski MI, Schein JE, Birol I, Connors J, Gascoyne R, Horsman D, et al. Circos: An information aesthetic for comparative genomics. Genome Res. 2009; 19:1639 10.1101/gr.092759.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002; 30, 207 10.1093/nar/30.1.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCT method. Methods. 2001; 25:402 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 69.Donaire L, Pedrola L, de la Rosa R, Llave C. High-throughput sequencing of RNA silencing-associated small RNAs in olive (Olea europaea L.). PLoS One. 2011; 6:e27916 10.1371/journal.pone.0027916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiao Y. Studies on antioxidant capacity of anthocyanin extract from purple sweet potato (Ipomoea batatas L.). Afr. J. Biotech. 2012;11 10.5897/AJB11.3859 [DOI] [Google Scholar]
- 71.Bassolino L, Zhang Y, Schoonbeek H, Kiferle C, Perata P, Martin C. Accumulation of anthocyanins in tomato skin extends shelf life. New Phytol. 2013; 200, 650–655. 10.1111/nph.12524 [DOI] [PubMed] [Google Scholar]
- 72.Zhang H, Chen X, Wang C, Xu Z, Wang Y, Liu X, et al. Long non- coding genes implicated in response to stripe rust pathogen stress in wheat (Triticum aestivum L.). Mol. Biol. Rep. 2013; 40:6245–6253. 10.1007/s11033-013-2736-7 [DOI] [PubMed] [Google Scholar]
- 73.De Pascual-Teresa S, Moreno DA, Garcıa-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int. J. Mol. Sci. 2010; 11, 1679–1703. 10.3390/ijms11041679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spencer JP. The impact of fruit flavonoids on memory and cognition. British J. Nutr. 2010; 104:S40–S47. 10.1017/S0007114510003934 [DOI] [PubMed] [Google Scholar]
- 75.Giacomelli JI, Weigel D, Chan RL, Manavella PA. Role of recently evolved miRNA regulation of sunflower HaWRKY6 in response to temperature damage. New Phytol. 2012; 195:766 10.1111/j.1469-8137.2012.04259.x [DOI] [PubMed] [Google Scholar]
- 76.Gao F, Nan F, Feng J, Lv J, Liu Q, Xie S. Identification and characterization of microRNAs in Eucheuma denticulatum by high-throughput sequencing and bioinformatics analysis. RNA Biolog. 2016; 13:343 10.1080/15476286.2015.1125075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeng S, Liu Y, Pan L, Hayward A, Wang Y. Identification and characterization of miRNAs in ripening fruit of Lycium barbarum L. using high-throughput sequencing. Front. Plant Sci. 2015; 6:778 10.3389/fpls.2015.00778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Šurbanovski N, Brilli M, Moser M, Si-Ammour A. A highly specific microRNA-mediated mechanism silences LTR retrotransposons of strawberry. Plant J. 2016; 85:70 10.1111/tpj.13090 [DOI] [PubMed] [Google Scholar]
- 79.Yue J, Lu X, Zhang H, Ge J, Gao X, Liu Y. Identification of conserved and novel microRNAs in blueberry. Front. Plant Sci. 2017; 8:1155 10.3389/fpls.2017.01155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barik S, Sarkar Das S, Singh A, Gautam V, Kumar P, Majee M, et al. Phylogenetic analysis reveals conservation and diversification of micro RNA166 genes among diverse plant species. Genomics. 2014; 103, 114 10.1016/j.ygeno.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 81.Zhang J, Pan L, Xu Y, Wu C, Wang C, Cheng Z, Zhao R. Total cholesterol content of erythrocyte membranes in acute coronary syndrome: correlation with apolipoprotein A-I and lipoprotein (a). Coron Artery Dis. 2011; 22:145–152 10.1097/MCA.0b013e328343fbbb [DOI] [PubMed] [Google Scholar]
- 82.Zhang L, Hou D, Chen X, Li D, Zhu L, Zhang Y. Exogenous plantMIR168a specifically targets mammalian LDLRAP1: Evidence of cross-kingdom regulation by microRNA. Cell Res. 2012; 22:107–126. 10.1038/cr.2011.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Olive fruit branches of Olea europaea (A- Cassanese and B- Leucocarpa cultivars).
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(TXT)
(TXT)
Data Availability Statement
All relevant data are available from the the NCBI Gene Expression Omnibus at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE104763/.