Abstract
Objective
Detection of vascular endothelial growth factor (VEGF) levels in ocular tissue may perhaps provide insight into the role of VEGF in the pathogenesis and progression of diabetic retinopathy (DR). The aim of this study was to evaluate the levels of VEGF in tears and serum amongst type 2 diabetes mellitus (DM) patients.
Methods
A comparative cross-sectional study was conducted between August 2016 and May 2018 involving type 2 DM patients with no DR, non-proliferative DR (NPDR), and proliferative DR (PDR). Tear samples were collected using no.41 Whatman filter paper (Schirmer strips) and 5 mL blood samples were drawn by venous puncture. VEGF levels in tears and serum were measured by enzyme-linked immunosorbent assay.
Results
A total of 88 type 2 DM patients (no DR: 30 patients, NPDR: 28 patients, PDR: 30 patients) were included in the study. Mean tear VEGF levels were significantly higher in the NPDR and PDR groups (114.4 SD 52.5 pg/mL and 150.8 SD 49.7 pg/mL, respectively) compared to the no DR group (40.4 SD 26.5 pg/mL, p < 0.001). There was no significant difference in the mean serum VEGF levels between the three groups. There was a fair correlation between serum and tear VEGF levels (p = 0.015, r = 0.263).
Conclusion
VEGF levels in tears were significantly higher amongst diabetic patients with DR compared to those without DR and were significantly associated with the severity of DR. There was a fair correlation between serum and tear VEGF levels. Detection of VEGF in tears is a good non-invasive predictor test for the severity of DR. A large cohort study is needed for further evaluation.
Introduction
Diabetic retinopathy (DR) is the leading cause of blindness in the working-age population and has a considerable economic burden on society, especially on healthcare systems [1,2]. In Malaysia, the 2015 National Health and Morbidity Survey (NHMS) estimated that 3.5 million (17.5%) Malaysians were diabetic and projected that number to increase to 21.6% by the year 2020 [3].
Microvasculopathy and inflammation are the main pathways involved in the pathogenesis of DR [4]. Studies have shown that the breakdown of the blood-retinal barrier is due to high levels of inflammatory mediators and cytokines [5,6–8]. Vascular endothelial growth factor (VEGF) is involved in the angiogenesis process of DR. VEGF has been associated with many other neovascular retinopathies, such as ischemic retinal vein occlusion and retinopathy of prematurity [9]. Levels of VEGF in the vitreous and aqueous humours are found to be higher during active proliferation of DR [9,10]. VEGF levels are reduced during the quiescent phase of proliferative diabetic retinopathy (PDR) and diminish after successful laser therapy [10].
The quantification of VEGF levels in the aqueous and vitreous humours in DR is reflecting the pathophysiological process due to closer proximity to the retina [10]. However, the measurement of aqueous and vitreous VEGF levels is rather invasive. It may cause complications, such as endophthalmitis or retinal detachment, and it is only ethically possible if performed during surgical intervention, such as cataract or vitreoretinal surgery. Quantification of VEGF levels is also done through blood sampling. Several studies found an inconsistent result of serum VEGF levels in DR patients [11–13]. Serum VEGF levels may be affected by systemic illnesses, such as hypertension [14] and dyslipidaemia, which are common comorbidities in diabetics [15], and also by smoking [16].
In recent years, tear analysis has become an increasing interest in ophthalmology. Detection of VEGF levels in tears is less invasive, safe and an acceptable method of screening for DR [17]. VEGF is found throughout the ocular epithelial structures, including in the cornea, ciliary body, lens and retinal pigment epithelium [18,19]. Moreover, the cornea epithelium has regenerative capability and is constantly shed with tears. In addition, tear fluid secreted by the lacrimal glands may also contain VEGF. Furthermore, VEGF which is found in the blood can permeate through the conjunctival vessels [20]. Detection of VEGF levels in tears may represent the pathophysiological changes of DR. Like serum, the level of VEGF in tears may differ according to the severity of DR, from non-proliferative diabetic retinopathy (NPDR) to PDR. The aim of this study was to evaluate and compare the levels of VEGF in serum and tears among type 2 diabetes mellitus (DM) patients.
Methods
Participants and selection criteria
A comparative cross-sectional study was conducted in the eye clinic of a tertiary centre from August 2016 to May 2018. The sample size was calculated using G Power 3.1.9. Type 2 DM patients aged 30 years and above were recruited using a systematic random sampling method. Exclusion criteria were patients: (a) with a history of ocular surface disease; (b) receiving systemic or topical steroid therapy within three months prior to recruitment; (c) receiving intravitreal therapy; (d) receiving laser photocoagulation therapy; (e) with a history of ocular trauma; (f) recovering from post-intraocular surgery (cataract, vitreous or retinal detachment) within six months prior to recruitment; and (g) with a history of any ocular inflammatory disease, such as uveitis. DR patients who presented with diffuse diabetic macular oedema and any patients who were being treated with anti-VEGF therapy were also excluded. Patients with systemic conditions that upregulate VEGF, such as bronchial asthma, chronic obstructive pulmonary disease, immunological disorder, haematological disease, malignancies or any condition associated with hypoxia or inflammation were excluded.
Data collection
Demographic data (age, race and gender), acknowledgment of previous ocular surgery or treatment, systemic comorbidities (hypertension and dyslipidaemia) and smoking status (active, non-smoker and ex-smoker) was obtained through history-taking and medical records. Those who fulfilled the selection criteria were explained the nature of study and written consent was obtained.
All patients underwent a comprehensive ophthalmologic examination that included best-corrected visual acuity, slit-lamp examination, dilated fundus examination and applanation tonometry. Based on the International Clinical DR Disease Severity Scale (ICDRS) [21], patients were divided into three groups: no DR, NPDR and PDR. For DM patients with NPDR and PDR, only one eye of the worst severity was selected. In patients with PDR in one eye, tears were only collected from the PDR eye. DM patients with bilateral no DR were considered as a no DR group. Blood and tear samples were collected and measured for VEGF levels.
Tear and blood sample collection
After a detailed explanation of the procedure to the patients, venepuncture was conducted and 5 mL of blood was collected. The blood samples were sent to a physiology laboratory. The blood samples were centrifugated at 1,000 rpm for 10 minutes to obtain the serum, which was then stored at −80°C prior to future analysis for VEGF.
Tears were collected using No.41 Whatman filter paper (Schirmer strips). Patients were asked to sit in an upright position with their backs well supported. A topical anaesthetic eye drop (Alcain-Alcon, Belgium) was instilled into the patient’s eye before the procedure. After 5 minutes, a Schirmer strip was inserted at the outer third of the lower eyelid. The patient was then asked to keep the eye gently closed until the tear wet the paper up to 15 mm. Then, forceps were used to remove the paper. The tear sample was kept in a clear 0.5 mL micro plastic test tube and sent immediately to a central research laboratory for storage. All samples were then kept at −80°C during the recruitment process.
Protein extraction from the Schirmer strips was performed by elution with triple-distilled water [22]. Each strip was cut into 3–5 mm pieces and placed in the same micro plastic test tubes. Then, 500 μL of ultrapure distilled water was added to this tube, which was centrifuged for 30 minutes at 10,000 rpm. The supernatant was transferred to another polypropylene tube and stored at −20°C until the following day, when the samples were thawed and centrifuged for 5 minutes at 14,000 rpm. The samples from the micro plastic test tubes were processed directly for analysis.
VEGF quantification
A human VEGF immunoassay kit by Quantikine (R&D Systems, Minneapolis, MN, USA) was used for the determination of VEGF levels in serum and tears. The quantitative sandwich enzyme immunoassay technique was used according to the manufacturer’s instructions. For the initial step, 100 μL of RDW1 assay solution was placed onto the microplate. Then, 100 μL of the sample (serum and tear fluid) was added to the microplate and incubated for 2 hours at room temperature. Next, 200 μL of conjugate was added after three washings, followed by re-incubation at room temperature. Subsequently, it was subjected to three more washings before being centrifuged at 25 rpm, together with 200 μL of substrate, and incubated. The last solution that was added was 50 μL of stop solution. Finally, the plates were read using a microplate reader at 450–570 nm wavelength.
Ethics approval
The study followed the tenets of the declaration of Helsinki and was approved by the local Human Research Ethics Committee (Universiti Sains Malaysia [USM]/Jawatankuasa Etika Penyelidikan Manusia [JEPeM]; Registration number: 16060224). Written and informed consent of participants was obtained for each patient prior to the study.
Statistical analysis
The statistical analysis was carried out using Statistical Package for Social Sciences (SPSS) Version 24. A Chi-square test was used to compare gender and systemic illnesses (such as hypertension and dyslipidaemia) between the severities of DR. A Fisher’s exact test was used to compare race and smoking status between severities of DR. A one-way ANOVA test was used to compare mean age and duration of DM. An ANCOVA test was used to compare VEGF levels in tears and serum between severities of DR among type 2 DM patients, adjusted for age, duration of DM, smoking status, and systemic illness (hypertension and dyslipidaemia). For the comparison of the tears, a Bonferroni post-hoc test was used to compare VEGF among the three groups of DR patients. Simple linear regression and multiple linear regression were used to identify the associated factors for tear and serum VEGF. A Pearson correlation was used for the correlation of serum and tear VEGF. A p-value of < 0.05 was considered significant.
Results
Demographic data
A total of 88 type 2 DM patients (30 patients with NPDR, 28 patients with PDR, 30 patients with no DR) were recruited in this study. There were equal percentages of both males and females among the type 2 DM patients (n = 44, 50% for each group). Racial distribution showed that 84 patients (95.5%) were Malay and 4 patients (4.5%) were Chinese. The mean age of the enrolled patients was 58.5 SD 10.2 years and ranged from 52.3 to 64.6 years. There was significant difference in the mean age between the three groups (p = 0.021), where the PDR group had the youngest mean age (52.3 SD 9.6 years).
The duration of DM was longer in the PDR group compared to the no DR and NPDR groups, and there was significant difference among the three groups (p = 0.043). Hypertension (75.0%) and dyslipidaemia (70.5%) were the commonest systemic illnesses among DM patients. However, there was no significant difference among the three groups. The demographic data and systemic profiles among the three groups is shown in Table 1.
Table 1. Demographic characteristics and systemic profile among type 2 diabetic patients.
| Variables | Total | No DR | NPDR | PDR | p-value |
|---|---|---|---|---|---|
| (n = 88) | (n = 30) | (n = 28) | (n = 30) | ||
| Age (years) | |||||
| Mean (SD) | 58.5 (10.2) | 64.6 (8.8) | 58.5 (8.2) | 52.3 (9.6) | 0.021a |
| Gender (n, %) | |||||
| Male | 44 (50.0) | 17 (56.7) | 11 (39.3) | 16 (53.3) | 0.377b |
| Female | 44 (50.0) | 13 (43.3) | 17 (60.7) | 14 (46.7) | |
| Race (n, %) | |||||
| Malay | 84 (95.5) | 28 (93.3) | 27 (96.4) | 29 (96.7) | 0.950c |
| Chinese | 4 (4.5) | 2 (6.7) | 1 (3.6) | 1 (3.3) | |
| Duration of DM (years) | |||||
| Mean (SD) | 9.2 (3.2) | 7.7 (2.6) | 9.9 (2.5) | 10.1 (3.9) | 0.043a |
| Smoking Status (n, %) | |||||
| Non-smoker | 37 (42.0) | 10 (33.3) | 15 (53.6) | 12 (40.0) | |
| Active smoker | 37 (42.0) | 14 (46.7) | 11 (39.3) | 12 (40.0) | 0.462c |
| Ex-Smoker | 14 (15.9) | 6 (20.0) | 2 (7.1) | 6 (20.0) | |
| Co-morbidity (n, %) | |||||
| Hypertension | 66 (75.0) | 22 (73.3) | 22 (78.6) | 22 (73.3) | 0.907b |
| Dyslipidaemia | 62 (70.5) | 21 (70.0) | 19 (67.9) | 22 (73.3) | 0.956b |
aANOVA,
bChi square test
cFisher exact test, p < 0.05, significant
Abbreviation: DM: diabetes mellitus, DR: diabetic retinopathy, NPDR: non-proliferative diabetic retinopathy, PDR: proliferative diabetic retinopathy
VEGF Levels in serum and tears
After adjusting for age, smoking status, duration of DM and systemic illness (hypertension and dyslipidaemia), the PDR group showed highest mean level of VEGF in serum among type 2 DM patients (342.4 SD 41.5 pg/mL) compared to NPDR (288.7 SD 34.8 pg/mL) and no DR (305.4 SD 43.0 pg/mL). However, there was no significant difference of mean serum VEGF levels between the three groups (p = 0.587) (Table 2).
Table 2. Comparison of mean VEGF level in serum and tears between the groups.
| VEGF (pg/mL) | No DR | NPDR | PDR | F statistics | p-value |
|---|---|---|---|---|---|
| (n = 30) | (n = 28) | (n = 30) | (df) | ||
| Serum VEGF | |||||
| Mean (SD) | 305.4 (43.0) | 288.7 (34.8) | 342.4 (41.5) | 0.54 (2, 77) | 0.587 |
| Tear VEGF | |||||
| Mean (SD) | 41.2 (11.3) | 114.9 (8.6) | 149.5 (10.4) | 18.75 (2, 80) | <0.001 |
ANCOVA test applied after adjustment for age, smoking status, duration of DM, hypertension and dyslipidemia, p < 0.05, significant
Abbreviation: DR: diabetic retinopathy, NPDR: non-proliferative diabetic retinopathy, PDR: proliferative diabetic retinopathy, VEGF: vascular endothelial growth factor
Among type 2 DM patients, the NPDR and PDR groups showed a higher mean level of VEGF in tears (114.9 SD 8.6 pg/mL and 149.5 SD 10.4 pg/mL) compared to the no DR group (41.2 SD 11.3 pg/mL). There was a significant difference of mean tear VEGF levels between the three groups (p < 0.001) after adjusting for age, smoking status, duration of DM and systemic illness (hypertension and dyslipidaemia) (Table 2).
After the Bonferroni post-hoc analysis, there was a significant mean difference of tear VEGF levels between the no DR and NPDR groups (p < 0.001) and between the no DR and PDR groups (p < 0.001) (Table 3).
Table 3. Post Hoc comparison of mean tears VEGF after adjustment.
| Variables | Group | Mean Difference (95%, CI) | p-value |
|---|---|---|---|
| No DR–NPDR | 68.9 (33.4, 104.6) | <0.001 | |
| Tear VEGF | No DR–PDR | 99.8 (56.9, 142.7) | <0.001 |
| NPDR–PDR | 30.8 (-0.9, 62.5) | 0.060 |
Bonferroni Post-Hoc comparison test, p < 0.05, significant
Abbreviation: DR: diabetic retinopathy, NPDR: non-proliferative
The associated factors of serum and tear VEGF levels are summarised in Table 4. Age, duration of DM, severity of DR and VEGF levels were the significant variables among the associated factors with p-value < 0.25 based on simple linear regression. The four significant variables (p < 0.25) among the associated factors were included in the multiple linear regression. Smoking status was included, as it was of clinical importance. The results show that the tear VEGF level was a significant associated factor for serum VEGF (p = 0.015), whereas for the tear VEGF level, the severity of DR was a significant associated factor (p < 0.001).
Table 4. Associated factors of serum and tears VEGF.
| Variables |
Regression coefficient (b) |
t-stats | p-value | Regression coefficient (b) |
t-stats | p-value |
|---|---|---|---|---|---|---|
| Simple Linear Regression* | Multiple Linear Regression** | |||||
| Serum VEGF | ||||||
| Age | -3.10 | -1.68 | 0.097 | -0.09 | -0.80 | 0.428 |
| Gender | -9.89 | -0.26 | 0.797 | |||
| Race | 186.31 | 2.11 | 0.380 | |||
| Smoking status | -11.64 | -0.63 | 0.533 | -0.07 | -0.66 | 0.514 |
| Duration of DM | 7.65 | 1.29 | 0.202 | 0.08 | 0.70 | 0.487 |
| Hypertension | -38.08 | -0.87 | 0.386 | |||
| Dyslipidaemia | 31.58 | 0.75 | 0.454 | |||
| Severity of DR | 54.16 | 2.43 | 0.017 | 0.14 | 0.91 | 0.364 |
| Tears VEGF | 0.71 | 2.49 | 0.015 | 0.71 | 2.49 | 0.015 |
| Tear VEGF | ||||||
| Age | -2.46 | -3.94 | <0.001 | -0.55 | -0.72 | 0.476 |
| Gender | 8.71 | 0.64 | 0.526 | |||
| Race | -18.31 | -0.56 | 0.579 | |||
| Smoking status | 0.47 | 0.07 | 0.944 | 1.63 | 0.34 | 0.737 |
| Duration of DM | 5.11 | 2.45 | 0.016 | 1.51 | 0.69 | 0.498 |
| Hypertension | -13.14 | -0.84 | 0.407 | |||
| Dyslipidaemia | -10.33 | -0.69 | 0.493 | |||
| Severity of DR | 55.23 | 9.54 | <0.001 | 49.67 | 5.48 | < 0.001 |
| Serum VEGF | 0.10 | 2.49 | 0.015 | 0.03 | 0.93 | 0.358 |
*Simple Linear Regression test, p < 0.25, significant
**Multiple Linear Regression test, p < 0.05, significant
Forward, Backward, Stepwise and Enter method were applied
Abbreviation: DM: diabetes mellitus, DR: diabetic retinopathy, VEGF: vascular endothelial growth factor
Correlation of VEGF levels in serum and tears
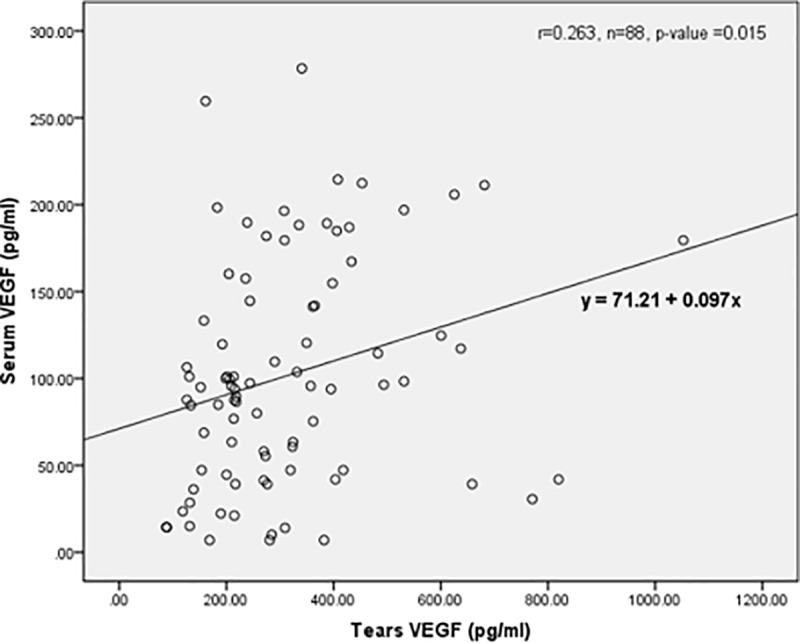
Fig 1 shows the correlation between serum and tear VEGF levels. There was a fair correlation between serum and tear VEGF levels (p = 0.015, r = 0.263).
Fig 1. Correlation between serum and tears of VEGF level.
Discussion
In our study, we evaluate VEGF levels in serum and tears. In this study, a Schirmer strip was used for tear collection. According to Posa et al. [23], both a Schirmer strip and a capillary tube are highly suitable as gentle and non-invasive methods of sampling tear fluid from living subjects for efficient subsequent protein analysis. Furthermore, a Schirmer strip is easier to handle for the researcher and more pleasant to the patients.
We found that the mean serum VEGF of the no DR group was lower compared to the NPDR and PDR groups. Although the mean value of serum VEGF in the NPDR and PDR groups was higher than the mean value of VEGF in the no DR group, it was not statistically significant (p = 0.587). This is consistent with several studies done previously [11–13].
Serum levels of VEGF can be affected by the presence of other factors and systemic illness, which is common in diabetics. Some studies have suggested that hypertension [14], dyslipidaemia [15] and smoking [16] can influence serum VEGF levels. We also found a significant difference in the baseline characteristics, age (p = 0.021) and duration of DM (p = 0.043), of the three different groups (no DR, NPDR and PDR). The mean age of the patients was 58.5 SD 10.2 years and ranged from 52.3 to 64.6 years, which suggests that most of the type 2 DM patients are from the middle age groups, consistent with the common age of onset of type 2 DM. We found that the youngest age was observed in the PDR group. This group of patients may tend to present early when they are symptomatic due to complications of PDR, such as vitreous haemorrhage.
There is a strong relationship between duration of DM and stages of DR [24,25]. Although there are no pre-existing studies to link duration of DM and levels of VEGF, clinical data has shown that the longer the duration of diabetes, the more severe the stages of DR [26]. This is probably due to the longer duration of diabetic insult governed by various cytokines, such as interleukins and VEGF [27–29]. Therefore, adjustments to the possible confounding factors were made. After adjusting for age, smoking status, duration of DM and systemic illness (hypertension and dyslipidaemia), we found that there was no significance difference (p = 0.587) in serum VEGF levels. We speculate that the measurement of serum VEGF levels is non-specific and has limited use as biomarker for the prediction of DR. In addition, results of our study agree with other studies on different serum biomarkers of inflammation and haemostasis in association with DR after controlling for established risk factors [28–30]. Serum VEGF might not reflect the changes in the retina due to the relatively small size of affected tissue, and the amount of VEGF released in the large circulating blood volume [31].
On the other hand, we found that the mean tear VEGF levels were significantly higher (p < 0.001) in the PDR group and NPDR group compared with the no DR group after adjusting for age, smoking status, duration of DM and systemic illness (hypertension and dyslipidaemia). Interestingly, studies have demonstrated the presence of VEGF-like protein on the corneal epithelium [18,32]. Diabetic patients who are at a higher risk of developing diabetic keratopathy can easily shed corneal epithelium-containing VEGF proteins into the tear film, resulting in higher levels of VEGF [33,34]. Data on the role of the lacrimal gland in the secretion of VEGF via the tear aqueous layer is still lacking; nonetheless, studies on animals have shown us that significant VEGF is detected in the lacrimal glands of rat models [35]. This data could also explain the elevated tear film VEGF levels. A study done on central retinal vein occlusion patients found that eyes with retinal vein occlusion demonstrated higher levels of VEGF in tears compared to normal fellow eyes [36]. These findings support that there is an association between a hypoxic retina and the level of VEGF in tears [36]. Likewise, in our study, mean tear VEGF levels were higher in the patients with DR.
We are unable to compare our findings of tear VEGF levels, as thus far there have been no studies done to evaluate VEGF levels in tears among type 2 DM patients. Nevertheless, our results are comparable with a study done by Ermakova et al. on type 1 DM patients, which shows a relationship between the tear VEGF level and the degree of various manifestations of DR [37]. Tear VEGF levels may also be more representative of the ocular level of VEGF, as they are in closer proximity and share a similar circulation [38]. However, tear VEGF levels may be exposed to atmospheric changes. The influence of these changes on tear VEGF levels is not well studied yet. Possibly, to improve the reliability of our results of tear VEGF levels, an assessment of dry eye syndrome could be included in future studies. Dry eye syndrome is not only relatively common among DM patients, but it could influence the level of VEGF in the tears by reducing tear volume [39].
Our findings on tear VEGF is also in line with previous reports on other ocular fluid, such as aqueous and vitreous VEGF, although aqueous and vitreous samples are shown to yield a higher mean VEGF level in PDR patients [40–45]. This could be due to the proximity of the anterior chamber and vitreous cavity to the retina. Furthermore, aqueous and vitreous VEGF measurement is likely to eliminate any confounding factors on the ocular surface. The limitation of this study is we did not compare tears to aqueous humour and vitreous humour samples. This comparison might not only enhance our understanding of VEGF and its interactions in the eye but also solidify the association of VEGF in ocular fluids and DR. In view of that, we suggest a concurrent evaluation of VEGF levels in the aqueous humour or vitreous humour and ocular tear film VEGF levels in future studies. Aqueous and vitreous samples can probably be collected prior to cataract surgery and intravitreal anti-VEGF therapy, respectively. Another major drawback of our study is we did not include haemoglobin A1c (HbA1c) levels. Correlating HbA1c with serum and tear VEGF levels will give us a better overview of the control of DM and its relationship with systemic and ocular VEGF levels. We strongly recommend this in future studies.
In the extension of our study, we also found that VEGF levels in serum and tears were associated with age, duration of DM and severity of DR. The VEGF level in serum was associated with the VEGF level in tears, and vice versa. Further, multiple linear regression analysis revealed that tear VEGF was the independent predictor of the VEGF level in serum of this cohort (p = 0.015). The analysis also showed that severity of DR was the only independent predictor of VEGF levels in tears of our patients. This indicates an association among severity of DR and tear VEGF in type 2 DM patients.
In our study, we also observed a fair correlation between serum and tear VEGF levels (p = 0.015 and r = 0.263). This suggests that an interaction may exist between serum and tear VEGF. However, the relationship between serum VEGF and tear VEGF remains to be investigated. Studies have shown that VEGF is known to increase microvascular permeability by raising calcium influx into the endothelial cells which form the vessel walls [46,47]. We postulate that VEGF in the blood circulation can get into the tear fluid through increased permeability of the conjunctival vessels. Despite this, we did not see a significant difference in mean serum VEGF levels amongst our patients. This could be explained by a study done by Burgos et al. [43]; in their study, they believe that ocular VEGF originates inside the eye. This could be due to the relatively small structure, such as the affected retina tissues, in comparison with the amount of VEGF released in the large circulating blood volume [31]. This correlation remains debatable. A larger sample size in future studies might be helpful.
Conclusion
Among type 2 DM patients, VEGF levels in tears were higher amongst patients with DR compared to those without DR. Tear VEGF levels were also significantly related to the severity of DR. There was a fair correlation between serum and tear VEGF levels. In the future, tear VEGF levels might be used as a non-invasive tool to expedite screening programs and to predict the severity of DR in patients with diabetes. A large cohort study is needed for further evaluation.
Abbreviations
- DM
Diabetes mellitus
- DR
Diabetic retinopathy
- HbA1c
Hemoglobin A1c
- ICDRS
International Clinical Diabetic Retinopathy Disease Severity Scale
- JEPeM
Jawatankuasa Etika Penyelidikan Manusia
- NHMS
National Health and Morbidity Survey
- NPDR
Non-proliferative diabetic retinopathy
- PDR
Proliferative diabetic retinopathy
- SD
Standard deviation
- SPSS
Statistical Package for Social Sciences
- USM
Universiti Sains Malaysia
- VEGF
Vascular endothelial growth factor
Data Availability
All relevant data are within the paper.
Funding Statement
This work is supported by University Bridging Grant Universiti Sains Malaysia (Account No: 304.PPSP.6316167). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Williams R, Airey M, Baxter H, Forrester J, Kennedy-Martin T, Girach A. Epidemiology of diabetic retinopathy and macular oedema: a systematic review. Eye (Lond). 2004;18(10):963–83. [DOI] [PubMed] [Google Scholar]
- 2.Javitt JC, Aiello LP. Cost-effectiveness of detecting and treating diabetic retinopathy. Ann Intern Med. 1996;124(1 Pt 2):164–9. [DOI] [PubMed] [Google Scholar]
- 3.Feisul MI, Azmi S. National diabetes registry report, Volume 1, 2009–2012. Kuala Lumpur: Ministry of Health, Malaysia; 2013. [Google Scholar]
- 4.Wu H, Hwang DK, Song X, Tao Y. Association between aqueous cytokines and diabetic retinopathy stage. J Ophthalmol. 2017;2017:9402198 10.1155/2017/9402198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2(14). pii: 93751 10.1172/jci.insight.93751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamis AP, Berman AJ. Immunological mechanisms in the pathogenesis of diabetic retinopathy. Semin Immunopatho. 2008;30(2):65–84. 10.1007/s00281-008-0111-x [DOI] [PubMed] [Google Scholar]
- 7.Abu El-Asrar AM, Struyf S, Kangave D, Geboes K, Van Damme J. Chemokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Eur Cytokine Netw. 2006;17(3):155–65. [PubMed] [Google Scholar]
- 8.Demircan N, Safran B, Soylu M, Ozcan A, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond). 2006;20(12):1366–9. [DOI] [PubMed] [Google Scholar]
- 9.Patz A. Studies on retinal neovascularization. Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1980;19(10):1133–8. [PubMed] [Google Scholar]
- 10.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–7. 10.1056/NEJM199412013312203 [DOI] [PubMed] [Google Scholar]
- 11.Simo R, Lecube A, Segura RM, Garcia Arumi J, Hernandez C. Free insulin growth factor-I and vascular endothelial growth factor in the vitreous fluid of patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2002;134(3):376–82. 10.1016/s0002-9394(02)01538-6 [DOI] [PubMed] [Google Scholar]
- 12.Hernandez C, Burgos R, Canton A, Garcia-Arumi J, Segura RM, Simo R. Vitreous levels of vascular cell adhesion molecule and vascular endothelial growth factor in patients with proliferative diabetic retinopathy: a case-control study. Diabetes Care. 2001;24(3):516–21. 10.2337/diacare.24.3.516 [DOI] [PubMed] [Google Scholar]
- 13.Hernandez C, Lecube A, Segura RM, Sararols L, Simo R. Nitric oxide and vascular endothelial growth factor concentrations are increased but not related in vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med. 2002;19(8):655–60. [DOI] [PubMed] [Google Scholar]
- 14.Boursiquot BC, Zabor EC, Glezerman IG, Jaimes EA. Hypertension and VEGF (Vascular Endothelial Growth Factor) receptor tyrosine kinase inhibition: Effects on renal function. Hypertension. 2017. pii: HYPERTENSIONAHA.117.09275. 10.1161/HYPERTENSIONAHA.117.09275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zechariah A, ElAli A, Hagemann N, Jin F, Doeppner TR, Helfrich I, et al. Hyperlipidemia attenuates vascular endothelial growth factor–induced angiogenesis, impairs cerebral blood flow, and disturbs stroke recovery via decreased pericyte coverage of brain endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(7):1561–7. 10.1161/ATVBAHA.112.300749 [DOI] [PubMed] [Google Scholar]
- 16.Ugur MG, Kutlu R, Kilinc I. The effects of smoking on vascular endothelial growth factor and inflammation markers: A case-control study. Clin Respir J. 2018;12(5):1912–1918. 10.1111/crj.12755 [DOI] [PubMed] [Google Scholar]
- 17.Quah JH, Tong L, Barbier S. Patient acceptability of tear collection in the primary healthcare setting. Optom Vis Sci. 2014;91(4):452–8. 10.1097/OPX.0000000000000188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van-Setten GB. Vascular endothelial growth factor (VEGF) in normal human corneal epithelium: detection and physiological importance. Acta Ophthalmol Scand. 1997;75(6):649–52. [DOI] [PubMed] [Google Scholar]
- 19.Ford KM, Saint-Geniez M, Walshe TE, D'Amore PA. Expression and role of VEGF-A in the ciliary body. Invest Ophthalmol Vis Sci. 2012;53(12):7520–7. 10.1167/iovs.12-10098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou L, Beuerman RW, Chan CM, Zhao SZ, Li XR, Yang H, et al. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res. 2009;8(11):4889–905. 10.1021/pr900686s [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmol. 2003;110(9):1677–82. [DOI] [PubMed] [Google Scholar]
- 22.Farias E, Yasunaga KL, Peixoto RVR, Fonseca MP, Fontes W, Galera PD. Comparison of two methods of tear sampling for protein quantification by Bradford method. Pesq Vet Bras. 2013;33:261–4. 10.1590/S0100-736X2013000200021 [DOI] [Google Scholar]
- 23.Posa A, Bräuer L, Schicht M, Garreis F, Beileke S, Paulsen F. Schirmer strip vs. capillary tube method: Non-invasive methods of obtaining proteins from tear fluid. Ann Anat. 2013;195:137–142. 10.1016/j.aanat.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 24.Nathan DM; DCCT/EDIC Research Group. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 Years: Overview. Diabetes Care. 2014;37(1):9–16. 10.2337/dc13-2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King P, Peacock I, Donnelly R. The UK Prospective Diabetes Study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48(5):643–8. 10.1046/j.1365-2125.1999.00092.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102(4):527–32. 10.1001/archopht.1984.01040030405011 [DOI] [PubMed] [Google Scholar]
- 27.Maier R, Weger M, Haller-Schober EM, El-Shabrawi Y, Wedrich A, Theisl A, et al. Multiplex bead analysis of vitreous and serum concentrations of inflammatory and proangiogenic factors in diabetic patients. Mol Vis. 2008;14:637–43. [PMC free article] [PubMed] [Google Scholar]
- 28.Doganay S, Evereklioglu C, Er H, Turkoz Y, Sevinc A, Mehmet N, et al. Comparison of serum NO, TNF-alpha, IL-1beta, sIL-2R, IL-6 and IL-8 levels with grades of retinopathy in patients with diabetes mellitus. Eye (Lond). 2002;16(2):163–70. [DOI] [PubMed] [Google Scholar]
- 29.Yuuki T, Kanda T, Kimura Y, Kotajima N, Tamura J, Kobayashi I, et al. Inflammatory cytokines in vitreous fluid and serum of patients with diabetic vitreoretinopathy. J Diabetes Complications. 2001;15(5):257–9. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen TT, Alibrahim E, Islam FM, Klein R, Klein BE, Cotch MF, et al. Inflammatory, hemostatic, and other novel biomarkers for diabetic retinopathy: the multi-ethnic study of atherosclerosis. Diabetes Care. 2009;32(9):1704–9. 10.2337/dc09-0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nath M, Halder N, Velpandian T. Circulating biomarkers in glaucoma, age-related macular degeneration, and diabetic retinopathy. Indian J Ophthalmol. 2017;65(3):191–197. 10.4103/ijo.IJO_866_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bednarz J, Weich HA, Rodokanaki-von AS, Engelmann K. Expression of genes coding growth factors and growth factor receptors in differentiated and dedifferentiated human corneal endothelial cells. Cornea. 1995;14(4):372–81. [DOI] [PubMed] [Google Scholar]
- 33.Ljubimov AV, Huang ZS, Huang GH, Burgeson RE, Gullberg D, Miner JH, et al. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. J Histochem Cytochem.1998;46(9):1033–41. 10.1177/002215549804600907 [DOI] [PubMed] [Google Scholar]
- 34.Azar DT, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Altered epithelial-basement membrane interactions in diabetic corneas. Arch Ophthalmol. 1992;110(4):537–40. 10.1001/archopht.1992.01080160115045 [DOI] [PubMed] [Google Scholar]
- 35.Rossi S, Mantelli F, Lambiase A, Aloe L. Bevacizumab eye drop treatment stimulates tear secretion in rats through changes in VEGF and NGF lacrimal gland levels. Arch Ital Biol. 2012;150(1):15–21. 10.4449/aib.v150i1.1382 [DOI] [PubMed] [Google Scholar]
- 36.Kasza M, Balogh Z, Biro L, Ujhelyi B, Damjanovich J, Csutak A, et al. Vascular endothelial growth factor levels in tears of patients with retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2015;253(9):1581–6. 10.1007/s00417-015-3030-2 [DOI] [PubMed] [Google Scholar]
- 37.Ermakova NA, Syrodeeva ON, Antsiferov MB, Balatskaia NV, Krasnova LB. Role of vascular endothelial growth factor in the development of diabetic retinopathy in patients with type 1 diabetes mellitus. Vestn Oftalmol. 2008;124(1):25–8. [PubMed] [Google Scholar]
- 38.Matsumoto T, Saito Y, Itokawa T, Shiba T, Oba MS, Takahashi H, et al. Retinal VEGF levels correlate with ocular circulation measured by a laser speckle-micro system in an oxygen-induced retinopathy rat model. Graefes Arch Clin Exp Ophthalmol. 2017;255(10):1981–1990. 10.1007/s00417-017-3756-0 [DOI] [PubMed] [Google Scholar]
- 39.Rentka A, Hársfalvi J, Berta A, Köröskényi K, Szekanecz Z, Szücs G, et al. Vascular endothelial growth factor in tear samples of patients with systemic sclerosis. Mediators Inflamm. 2015;2015 573681 10.1155/2015/573681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baharivand N, Zarghami N, Panahi F, Dokht Ghafari MY, Fard AM, Mohajeri A. Relationship between vitreous and serum vascular endothelial growth factor levels, control of diabetes and microalbuminuria in proliferative diabetic retinopathy. Clin Ophthalmol. 2012;6:185–91. 10.2147/OPTH.S27423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawada O, Kawamura H, Kakinoki M, Sawada T, Ohji M. Vascular endothelial growth factor in aqueous humor before and after intravitreal injection of bevacizumab in eyes with diabetic retinopathy. Arch Ophthalmol. 2007;125(10):1363–6. 10.1001/archopht.125.10.1363 [DOI] [PubMed] [Google Scholar]
- 42.Funatsu H, Yamashita H, Noma H, Mimura T, Nakamura S, Sakata K, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005;243(1):3–8. 10.1007/s00417-004-0950-7 [DOI] [PubMed] [Google Scholar]
- 43.Burgos R, Simo R, Audi L, Mateo C, Mesa J, Garcia-Ramirez M, et al. Vitreous levels of vascular endothelial growth factor are not influenced by its serum concentrations in diabetic retinopathy. Diabetologia. 1997;40(9):1107–9. 10.1007/s001250050794 [DOI] [PubMed] [Google Scholar]
- 44.Hogeboom van Buggenum IM, Polak BC, Reichert-Thoen JW, de Vries-Knoppert WA, van Hinsbergh VW, Tangelder GJ. Angiotensin converting enzyme inhibiting therapy is associated with lower vitreous vascular endothelial growth factor concentrations in patients with proliferative diabetic retinopathy. Diabetologia. 2002;45(2):203–9. 10.1007/s00125-001-0747-8 [DOI] [PubMed] [Google Scholar]
- 45.Watanabe D, Suzuma K, Suzuma I, Ohashi H, Ojima T, Kurimoto M, et al. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2005;139(3):476–81. 10.1016/j.ajo.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 46.Bates DO, Curry FE. Vascular endothelial growth factor increases microvascular permeability via a Ca(2+)-dependent pathway. Am J Physiol. 1997;273(2 Pt 2):H687–94. [DOI] [PubMed] [Google Scholar]
- 47.Bates DO, Hillman NJ, Williams B, Neal CR, Pocock TM. Regulation of microvascular permeability by vascular endothelial growth factors. J Anat. 2002;200(6):581–97. 10.1046/j.1469-7580.2002.00066.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


