Abstract
Candidemia has been considered a persistent public health problem with great impact on hospital costs and high mortality. We aimed to evaluate the epidemiology and prognostic factors of candidemia in a tertiary hospital in Northeast Brazil from January 2011 to December 2016. Demographic and clinical data of patients were retrospectively obtained from medical records and antifungal susceptibility profiling was performed using the broth microdilution method. A total of 68 episodes of candidemia were evaluated. We found an average incidence of 2.23 episodes /1000 admissions and a 30-day mortality rate of 55.9%. The most prevalent species were Candida albicans (35.3%), Candida tropicalis (27.4%), Candida parapsilosis (21.6%) and Candida glabrata (11.8%). Higher mortality rates were observed in cases of candidemia due to C. albicans (61.1%) and C. glabrata (100%), especially when compared to C. parapsilosis (27.3%). Univariate analysis revealed some variables which significantly increased the probability of death: older age (P = 0.022; odds ratio [OR] = 1.041), severe sepsis (P < 0.001; OR = 8.571), septic shock (P = 0.035; OR = 3.792), hypotension (P = 0.003; OR = 9.120), neutrophilia (P = 0.046; OR = 3.080), thrombocytopenia (P = 0.002; OR = 6.800), mechanical ventilation (P = 0.009; OR = 8.167) and greater number of surgeries (P = 0.037; OR = 1.920). Multivariate analysis showed that older age (P = 0.040; OR = 1.055), severe sepsis (P = 0.009; OR = 9.872) and hypotension (P = 0.031; OR = 21.042) were independently associated with worse prognosis. There was no resistance to amphotericin B, micafungin or itraconazole and a low rate of resistance to fluconazole (5.1%). However, 20.5% of the Candida isolates were susceptible dose-dependent (SDD) to fluconazole and 7.7% to itraconazole. In conclusion, our results could assist in the adoption of strategies to stratify patients at higher risk for developing candidemia and worse prognosis, in addition to improve antifungal management.
Introduction
Candidemia, or the bloodstream infection (BSI) caused by Candida species, is a subset of invasive candidiasis (IC) with increased incidence over the last few decades, considered a persistent public health problem with great impact on health care-associated costs and high crude (35% to 75%) and attributable mortality, despite advances achieved in diagnosis and treatment [1–6].
Candida species are generally referred as the fourth leading cause of nosocomial BSI in the United States (US), accounting for 8 to 10% of all hospital-acquired BSIs [1–3]. Recently, a study encompassing several US states reported Candida spp. as the most prevalent pathogens obtained from nosocomial BSIs, even overcoming some common bacterial species [7].
At least 15 different Candida spp. have been reported to cause human invasive infections. Nevertheless, more than 90% of them are caused by five main species, as follows: Candida albicans, Candida glabrata, Candida parapsilosis, Candida tropicalis and Candida krusei [2, 3, 8, 9]. The distribution of Candida spp. causing candidemia presents temporal and geographic variation, alongside the considerable influence of patient characteristics, antifungal stewardship and clinical practices [2, 3, 8]. Although C. albicans remains the most frequently isolated species from Candida BSI episodes, its incidence has recently decreased [2, 3, 8, 10].
Most candidemia predisposing factors are very common among critically ill patients in the ICU. This fact, together with the delay and lack of sensitivity of diagnostic tools, impair the prompt recognition and treatment of this infection [2, 6]. Moreover, antifungal susceptibility profiling may vary according to each Candida species and even within strains of the same species, whilst the development of microbial resistance may occur to any class of antifungal agents, making the management of candidemia even more difficult [2, 11].
Since the indiscriminate use of antifungals can generate great economic and ecological impact, antifungal prophylaxis and empirical treatment should be considered only in high-risk patients selected through strategies such as the colonization index, Candida score, and predictive rules based on combinations of risk factors [2, 5, 6, 12, 13, 14].
Considering the scarcity of Candida BSIs studies conducted in Northeast Brazil (Brazil’s lowest income region) and the relevance of the knowledge of local peculiarities to assist the optimization of strategies for prevention and treatment of infections, we aimed to evaluate the epidemiology of candidemia and risk factors associated with mortality in a tertiary hospital in this Brazilian region over 6 years.
Materials and methods
Study design
This is a retrospective, single-center, observational cohort study conducted at Onofre Lopes Hospital (Natal city, Brazil), a tertiary University Hospital with 248 beds. All patients who developed candidemia during a 6-year period (from January 2011 to December 2016) were included in the study. Candidemia or Candida BSI was defined as at least one positive blood culture for Candida spp. in patients hospitalized for more than 48 h. Only the first episode of candidemia was recorded for each patient. Therefore, Candida BSI episodes which occurred before 48 hours of hospitalization or represented relapses were excluded. Demographic and clinical data were collected from medical records within the preceding 30 days from the onset of Candida BSI (defined as the day of first Candida spp. positive blood culture) up to a 30-day follow-up period, except for data on surgery (collected up to 3 months before the onset of candidemia). Clinical data included vital signs, blood count, other infections/positive cultures, underlying conditions, predisposing factors for candidemia, previous exposure to antifungals, clinical management and outcome (survival or death). Vital signs were classified according to the parameters established in the literature [15, 16] together with the medical interpretation in the patients' records. Classification of blood cell counts were based on the reference ranges defined locally by the hospital laboratory. Sepsis, severe sepsis and septic shock were defined according to Angus and van der Poll [17]. Crude mortality rate was calculated at 7 and 30 days from candidemia onset. The following antifungal dosages were considered adequate: fluconazole (FLU) 400 mg/day, amphotericin B deoxycholate (AMB) 0.5–1.0 mg/kg/day, amphotericin B lipid complex (ABLC) 3.0–5.0 mg/kg/day, caspofungin (CPF) 50 mg/day, micafungin (MCF) 100 mg/day, anidulafungin (ADF) 100 mg/day [18].
Ethics
This study was approved by the Local Research Ethics Committee (“Comitê de Ética em Pesquisa da Liga Norte Riograndense Contra o Câncer”) under the protocol number 042/042/2012. Written patient consent was not required because of the observational nature of the study.
Laboratory procedures
Blood samples were processed using the Bact/Alert system (BioMérieux, France). All positive cultures were inoculated onto the surface of Sheep Blood Agar and incubated at 30°C for 48–96 h. Yeast growth was confirmed by Gram staining and the initial identification was performed at the referred hospital with the Vitek 2 Compact YST system (BioMérieux, France), according with manufacturer’s instructions. The strains were sent to the Laboratory of Medical and Molecular Mycology, Department of Clinical and Toxicological Analysis, Federal University of Rio Grande do Norte and a confirmation in identification was performed according to classical methods [19]. Of note, accurate identification was also performed using MALDI-TOF [20] when necessary. Unfortunately, some strains were not identified at the species level due to limitations of the initial screening performed at the hospital microbiology laboratory and lack of viability/or availability of some strains for further analysis. Antifungal susceptibility to amphotericin B (Sigma Chemical Corporation, St. Louis, MO, USA), fluconazole (Pfizer Incorporated, New York, NY, USA), itraconazole (Pfizer Incorporated, New York, NY, USA) and micafungin (Merck, Rahway, NJ, USA) was performed using the broth microdilution method according to the Clinical and Laboratory Standards Institute (CLSI) document M27-A3 [21]. The reference strains C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were used as quality controls. Minimum inhibitory concentration (MIC) values were interpreted according to the current clinical breakpoints suggested by CLSI for the most common species of Candida [22, 23].
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) and compared using Student t test or Mann-Whitney test. Categorical variables were expressed as frequencies and percentages and compared using Chi- square (X2) test or Fisher’s exact test, as appropriate. Logistic regression analysis was performed with variables that presented P ≤ 0.1 in the comparisons of groups to identify possible risk factors associated with mortality at 30 days after candidemia. Variables of clinical relevance and with sample size ≥60 found to be significant in the univariate analysis were included in a multivariate logistic model. All tests were 2-tailed, and a P-value <0.05 was determined to represent statistical significance. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software, version 20 (IBM SPSS, Chicago, IL, USA).
Results
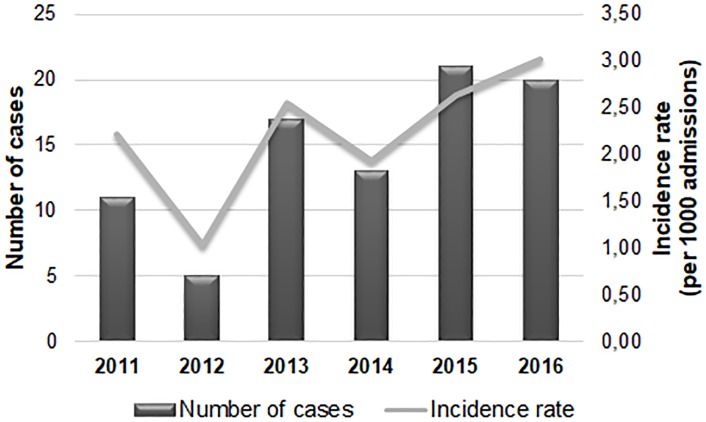
A total of 87 patients out of 37,768 admitted to the study hospital between 2011 and 2016 had at least one episode of candidemia. However, 19 individuals were excluded (16 patients who had candidemia before 48 h of hospitalization and 3 patients with no medical records). The mean incidence of candidemia cases was 2.23/1000 admissions, ranging from 1.03 to 3.02 throughout each different year of study, with a trend to increase from 2014–2016 (Fig 1).
Fig 1. Number of cases and incidence rate of candidemia observed during a 6-year period in a tertiary hospital in Northeast Brazil.
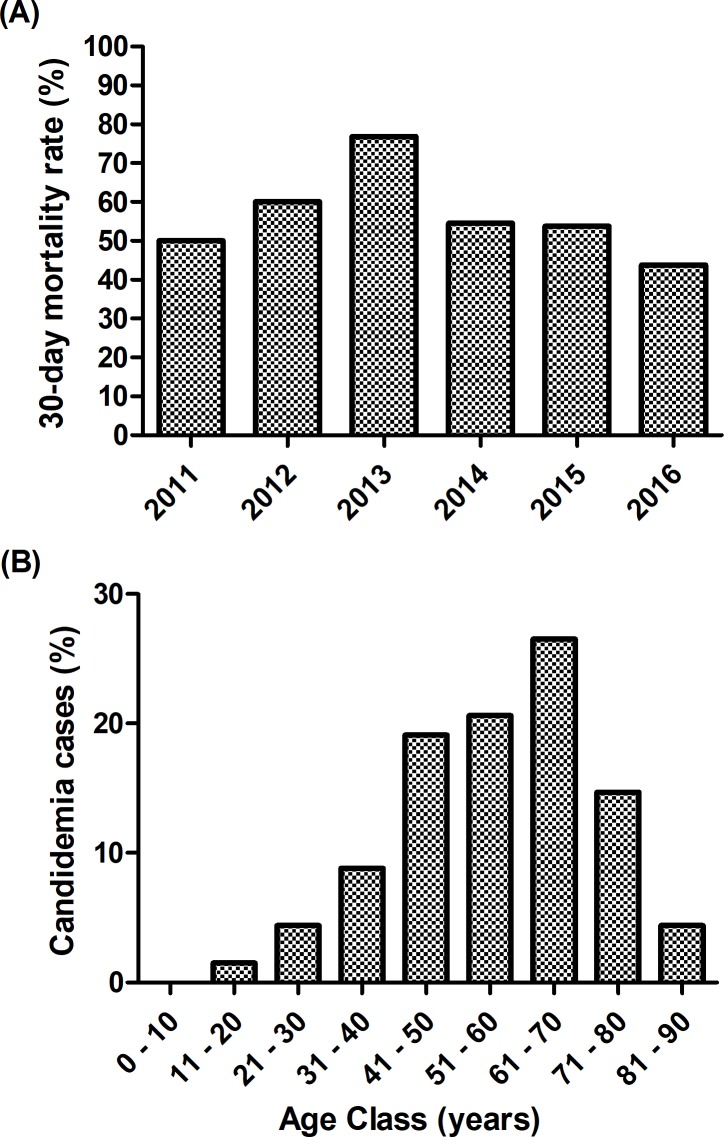
The 7-day and 30-day mortality rates were 33.8% (23/68) and 55.9% (38/68), respectively. The 30-day mortality rate was much higher in the ICU (70.8%, 17/24) compared to other sectors of the hospital (20/40; 50%). Over the 6 years of the study, the 30-day mortality rate ranged from 43.8% (7/16) in 2016 to 76.9% (10/13) in 2013, with a trend to increase between 2011 and 2013; and decrease between 2013 and 2016 (Fig 2A).
Fig 2.
30-day mortality rate during a 6-year period (A) and age class distribution (B) of patients with candidemia in a tertiary hospital in Northeast Brazil.
Positive cultures for bacteria were obtained from 73.5% (50/68) of the patients, including blood cultures (32/68; 47.1%). Mixed bacterial and yeast bloodstream infection occurred on the day of candidemia onset in 8 cases (8/68; 11.8%). Other yeast-positive cultures were obtained from 47.1% (32/68) of the patients, comprising sterile (24/68; 35.3%) and non-sterile body sites (16/68; 23.5%).
At the onset of candidemia, 37.5% (24/64) of the patients were in the ICU, 23.4% (15/64) in internal medicine wards, 18.8% (12/64) in surgical wards, 7.8% (5/64) in cardiovascular wards (Table 1), 7.8% (5/64) in isolation wards, 3.1% (2/64) in transplantation wards and 1.6% (1/64) in oncohematology wards.
Table 1. Demographic characteristics and underlying conditions of patients with candidemia, including comparison between subgroups according to the outcome.
| Characteristics of patients, N (%) | All patients (N = 68) |
30-day outcome | P-value* | |
|---|---|---|---|---|
| Survival (N = 30) |
Death (N = 38) |
|||
| Gender (male) | 27 (39.7) | 10 (33.3) | 17 (44.7) | 0.340 |
| Age (years; mean ± SD) | 56.0 ± 15.5 | 51.0 ± 17.0 | 60 ± 13.2 | 0.017 |
| Underlying Conditions | ||||
| Cancer | 28 (42.4) | 10 (33.3) | 18 (50.0) | 0.173 |
| Cardiovascular Disease | 49 (72.1) | 21 (70.0) | 28 (73.7) | 0.737 |
| Gastrointestinal Disease | 25 (36.8) | 9 (30.0) | 16 (42.1) | 0.304 |
| Renal Failure | 35 (51.5) | 12 (40.0) | 23 (60.5) | 0.093 |
| Chronic Renal Failure | 15 (23.1) | 7 (58.3) | 8 (40.0) | 0.964 |
| Acute Renal Failure | 17 (26.2) | 5 (41.7) | 12 (60.0) | 0.107 |
| Lung Disease | 5 (7.4) | 1 (3.3) | 4 (10.5) | 0.374 |
| Diabetes Mellitus | 22 (59.5) | 11 (55.0) | 11 (64.7) | 0.549 |
| Obesity | 4 (5.9) | 1 (3.3) | 3 (7.9) | 0.624 |
Some information was missing in patients' records; therefore the valid N varies according to the variable.
* Student t Test/Mann-Whitney Test (continuous data) or Chi-Square Test/Fisher Exact Test (categorical data).
Candida albicans was the most prevalent species obtained from blood cultures, accounting for 35.3% (18/51) of candidemia episodes, followed by Candida tropicalis (14/51; 27.4%), Candida parapsilosis (11/51; 21.6%), Candida glabrata (6/51; 11.8%) and other less common species (2/51; 3.9%), including one episode caused by Candida lusitaniae (2%) and another by Kodamaea ohmeri (2%).
Tables 1 to 4 present the main demographic and clinical characteristics of all the patients included in the present study, classified according to the outcome of candidemia after 30 days (survival or death).
Table 4. Antifungal stewardship in patients with candidemia, including comparison between subgroups according to the outcome.
| Characteristics of patients, N (%) | All patients (N = 68) |
30-day outcome | P-value* | |
|---|---|---|---|---|
| Survival (N = 30) |
Death (N = 38) |
|||
| Previous exposure to antifungals | 11 (16.2) | 4 (13.3) | 7 (18.4) | 0.572 |
| Antifungal Treatment | 41 (61.2) | 20 (69.0) | 21 (55.3) | 0.254 |
| Timing of antifungal administration** (days; mean ± SD) | 5.0 ± 6.0 | 5.8 ± 7.3 | 4.2 ± 4.3 | 0.423 |
| Adequate antifungal dosage | 28 (68.3) | 13 (65.0) | 15 (71.4) | 0.658 |
Previous exposure to antifungals was collected from medical records within the preceding 30 days from the candidemia onset (defined as the day of first positive blood culture for Candida species). Some information was missing in patients' records; therefore the valid N varies according to the variable.
* Student t Test/Mann-Whitney Test (continuous data) or Chi-Square Test/Fisher Exact Test (categorical data).
**Timing of antifungal administration: Interval between candidemia onset and initiation of antifungal treatment.
The main predisposing factors found were the previous use of antibacterial agents (66/68; 97.1%), the presence of CVC (54/68; 79.4%), corticosteroid therapy (38/68; 55.9%) and surgery (38/68; 55.9%; Table 3).
Table 3. Predisposing factors for Candida bloodstream infection and other characteristics of patients with candidemia, including comparison between subgroups according to the outcome.
| Characteristics of patients, N (%) | All patients (N = 68) |
30-day outcome | P-value* | |
|---|---|---|---|---|
| Survival (N = 30) |
Death (N = 38) |
|||
| Medical Devices | ||||
| Central Venous Catheter (CVC) | 54 (79.4) | 26 (86.7) | 28 (73.7) | 0.189 |
| CVC removal within 48 hours | 12 (23.1) | 8 (34.8) | 4 (13.8) | 0.074 |
| Total Parenteral Nutrition | 23 (33.8) | 11 (36.7) | 12 (31.6) | 0.660 |
| Mechanical Ventilation (MV) | 22 (32.4) | 9 (30.0) | 13 (34.2) | 0.712 |
| MV on candidemia onset | 16 (23.5) | 2 (6.7) | 14 (36.8) | 0.004 |
| Other Features | ||||
| Previous Bacteremia | 19 (27.9) | 9 (30.0) | 10 (26.3) | 0.737 |
| Previous use of antibacterial agents | 66 (97.1) | 29 (96.7) | 37 (97.4) | 0.865 |
| Nº of antibacterial agents used previously (mean ± SD) | 3.4 ± 1.6 | 3.0 ± 1.2 | 3.7 ± 1.8 | 0.057 |
| Post use of antibacterial agents | 62 (92.5) | 25 (86.2) | 37 (97.4) | 0.158 |
| Corticosteroid Therapy | 38 (55.9) | 17 (56.7) | 21 (55.3) | 0.908 |
| Other immunosuppressants | 4 (5.9) | 3 (10.0) | 1 (2.6) | 0.314 |
| Chemotherapy | 5 (7.4) | 2 (6.7) | 3 (7.9) | 0.847 |
| Hemodialysis | 19 (27.9) | 10 (33.3) | 9 (23.7) | 0.379 |
| Surgery | 38 (55.9) | 20 (66.7) | 18 (47.4) | 0.112 |
| Number of surgeries (mean ± SD) | 2.1 ± 1.4 | 1.7 ± 0.7 | 2.7 ± 1.7 | 0.105 |
| Abdominal Surgery | 31 (45.6) | 16 (53.3) | 15 (39.5) | 0.255 |
| Kidney Transplantation | 3 (4.4) | 3 (10.0) | 0 | 0.081 |
Characteristics of patients with unspecified temporal relation or named as “previous” were collected from medical records only within the preceding 30 days from the candidemia onset (defined as the day of first positive blood culture for Candida species), except for data on surgery (collected up to 3 months before candidemia onset). Characteristics of patients named as “post” were collected from medical records up to a 30-day follow-up period from the candidemia onset. Some information was missing in patients' records; therefore the valid N varies according to the variable.
* Student t Test/Mann-Whitney Test (continuous data) or Chi-Square Test/Fisher Exact Test (categorical data).
Of the 68 patients included in the study, 19 came from another hospital at admission (27.9%), 41 were female (60.3%) with a mean age of 56.0 ± 15.5 years (Table 1), mean hospital length of stay (LOS) of 63.9 ± 50.5 days and mean time between admission and development of candidemia of 35.6 ± 32.2 days.
The predominant age class ranged from 61 to 70 years (18/68; 26.5%; Fig 2B). It is worth mentioning that 45.6% (31/68) of the patients were elderly (aged between 61 and 90 years); while only one 12-year-old child was enrolled in the study since our hospital did not have a pediatric ward (Fig 2B).
The most prevalent underlying conditions were cardiovascular disease (49/68; 72.1%), diabetes mellitus (22/37; 59.5%) and renal failure (35/68; 51.5%; Table 1). Other important comorbidities were cancer (28/66; 42.4%), including three cases of hematological malignancy (3/28; 10.7%); and gastrointestinal disease (25/68; 36.8%; Table 1).
Most of the patients presented sepsis (53/68; 77.9%) and, to a lesser extent, severe sepsis (29/68; 42.6%) and/or septic shock (18/68; 26.5%) at the time of candidemia (Table 2). Among the 53 patients who developed sepsis, 19 of them had only sepsis (19/68; 27.9%), 16 developed severe sepsis (16/68; 23.5%), five developed septic shock (5/68; 7.4%), while 13 patients had both severe sepsis and septic shock (13/68; 19.1%).
Table 2. Clinical condition of patients on candidemia onset, including comparison between subgroups according to the outcome.
| Characteristics of patients, N (%) | All patients (N = 68) |
30-day outcome | P-value* | ||
|---|---|---|---|---|---|
| Survival (N = 30) |
Death (N = 38) |
||||
| Sepsis | 53 (77.9) | 20 (66.7) | 33 (86.8) | 0.046 | |
| Severe Sepsis | 29 (42.6) | 5 (16.7) | 24 (63.2) | <0.001 | |
| Septic Shock | 18 (26.5) | 4 (13.3) | 14 (36.8) | 0.029 | |
| Vital Signs | |||||
| Fever | 29 (44.6) | 16 (53.3) | 13 (37.1) | 0.191 | |
| Heart Rate | Bradycardia | 2 (3.3) | 1 (3.8) | 1 (2.9) | 0.936 |
| Normocardia | 20 (32.8) | 9 (34.6) | 11 (31.4) | ||
| Tachycardia | 39 (63.9) | 16 (61.5) | 23 (65.7) | ||
| Respiratory Frequency | Bradypnea | 0 | 0 | 0 | 0.609 |
| Eupnea | 21 (32.3) | 10 (35.7) | 11 (29.7) | ||
| Tachypnea | 44 (67.7) | 18 (64.3) | 26 (70.3) | ||
| Blood Pressure | Hypotension | 24 (40.0) | 5 (19.2) | 19 (55.9) | 0.006 |
| Normotension | 19 (31.7) | 9 (34.6) | 10 (29.4) | ||
| Hypertension | 17 (28.3) | 12 (46.2) | 5 (14.7) | ||
| Blood Count | |||||
| Blood Leucocyte Count | Leukopenia | 5 (8.5) | 1 (4.2) | 4 (11.4) | 0.224 |
| Normal | 20 (33.9) | 11 (45.8) | 9 (25.7) | ||
| Leukocytosis | 34 (57.6) | 12 (50.0) | 22 (62.9) | ||
| Blood Neutrophil Count | Neutropenia | 3 (5.1) | 0 | 3 (8.6) | 0.042 |
| Normal | 24 (40.7) | 14 (58.3) | 10 (28.6) | ||
| Neutrophilia | 32 (54.2) | 10 (41.7) | 22 (62.9) | ||
| Blood Lymphocyte Count | Lymphopenia | 18 (30.5) | 7 (29.2) | 11 (31.4) | 0.175 |
| Normal | 33 (55.9) | 16 (66.7) | 17 (48.6) | ||
| Lymphocytosis | 8 (13.6) | 1 (4.2) | 7 (20.0) | ||
| Anemia | 55 (91.7) | 23 (92.0) | 32 (91.4) | 0.937 | |
| Blood Platelet Count | Thrombocytopenia | 27 (46.6) | 5 (21.7) | 22 (62.9) | |
| Normal | 28 (48.3) | 17 (73.9) | 11 (31.4) | 0.006 | |
| Thrombocytosis | 3 (5.2) | 1 (4.3) | 2 (5.7) | ||
Candidemia onset was defined as the day of first positive blood culture for Candida species. Some information was missing in patients' records; therefore the valid N varies according to the variable.
* Student t Test/Mann-Whitney Test (continuous data) or Chi-Square Test/Fisher Exact Test (categorical data).
The use of antifungal agents before the onset of candidemia was observed in 16.2% (11/68) of the patients (Table 4), of which 63.6% (7/11) used fluconazole and 63.6% (7/11) used antifungal drugs for very short periods (1 to 5 days). The previous exposure to antifungals did not influence the isolation of NCAC species (P = 0.451).
Antifungal treatment was instituted in 61.2% of the patients (41/67; Table 4) and the most commonly used antifungal agent was fluconazole (38/41; 92.7%), alone (27/41; 65.9%) or in combination with other antifungal agents (11/41; 26.8%), mainly with amphotericin B deoxycholate (6/41; 14.6%). Echinocandins were used only in four patients among the group receiving treatment (9.8%).
Antifungal treatment did not influence the outcome of candidemia (P = 0.254; Table 4), and it is worth mentioning that 34.6% (9/26) of patients who were not treated survived.
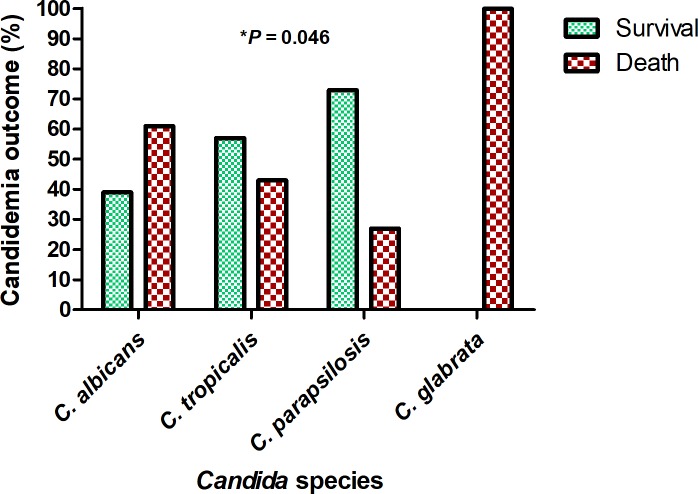
Compared to patients who survived, patients who died within 30 days after candidemia were older (P = 0.017), had a higher frequency of sepsis (P = 0.046), severe sepsis (P <0.001), septic shock (P = 0.029), hypotension (P = 0.006), neutrophilia (P = 0.042), thrombocytopenia (P = 0.006), the use of MV on candidemia onset (P = 0.004; Tables 1–3) and C. albicans and C. glabrata in blood cultures (P = 0.046; Fig 3).
Fig 3. Outcome of candidemia according to the Candida species in a tertiary hospital in Northeast Brazil.
* Chi- square (X2) test.
The relationship between these variables and the outcome was confirmed in the univariate logistic regression, except for sepsis and Candida species isolated (Table 5). Some characteristics of the patients significantly increased the probability of death: older patients (P = 0.022; OR = 1.041), severe sepsis (P < 0.001; OR = 8.571), septic shock (P = 0.035; OR = 3.792), hypotension vs. hypertension (P = 0.003; OR = 9.120), neutrophilia (P = 0.046; OR = 3.080), thrombocytopenia (P = 0.002; OR = 6.800), MV on candidemia onset (P = 0.009; OR = 8.167) and greater number of surgeries (P = 0.037; OR = 1.920; Table 5).
Table 5. Univariate logistic regression analysis of risk factors for 30-day mortality.
| Characteristics of patients | Univariate analysis | ||
|---|---|---|---|
| P-value | Odds ratio | 95% CI | |
| Age (years; mean ± SD) | 0.022 | 1.041 | 1.006–1.078 |
| Sepsis | 0.053 | 3.300 | 0.985–11.052 |
| Severe Sepsis | <0.001 | 8.571 | 2.675–27.470 |
| Septic Shock | 0.035 | 3.792 | 1.095–13.129 |
|
Blood Pressure (Hypotension vs. Hypertension) |
0.003 | 9.120 | 2.172–38.296 |
|
Blood Neutrophil Count (Neutrophilia vs. Normal count) |
0.046 | 3.080 | 1.022–9.284 |
|
Blood Platelet Count (Thrombocytopenia vs. Normal count) |
0.002 | 6.800 | 1.983–23.314 |
| C. tropicalis vs. C. albicans | 0.308 | 0.477 | 0.115–1.976 |
| C. parapsilosis vs. C. albicans | 0.085 | 0.239 | 0.047–1.219 |
| C. glabrata vs. C. albicans | 0.999 | - | - |
| CVC removal within 48 hours | 0.083 | 0.300 | 0.077–1.169 |
| MV on candidemia onset | 0.009 | 8.167 | 1.684–39.598 |
| Nº of antibacterial agents used previously (mean ± SD) | 0.064 | 1.393 | 0.981–1.978 |
| Surgery | 0.114 | 0.450 | 0.167–1.212 |
| Number of surgeries (mean ± SD) | 0.037 | 1.920 | 1.041–3.544 |
| Kidney Transplantation | 0.999 | - | - |
| Renal Failure | 0.095 | 2.300 | 0.865–6.117 |
| Acute Renal Failure | 0.113 | 2.609 | 0.796–8.550 |
CI: confidence interval; CVC: central venous catheter; MV: mechanical ventilation.
Multivariate analysis included age, severe sepsis, septic shock, use of MV and blood pressure on candidemia onset (Table 6). Age (P = 0.040; OR = 1.055), severe sepsis (P = 0.009; OR = 9.872) and hypotension vs. hypertension (P = 0.031; OR = 21.042) were independently associated with higher probability of death (Table 6). It is worth mentioning that the probability of death increased about 10-fold in patients who had severe sepsis and 21-fold in patients with hypotension compared to those who had hypertension at the onset of candidemia.
Table 6. Multivariate logistic regression analysis of risk factors for 30-day mortality.
| Characteristics of patients | Multivariate analysis* | ||
|---|---|---|---|
| P-value | Odds ratio | 95% CI | |
| Age (mean ± SD) | 0.040 | 1.055 | 1.003–1.110 |
| Severe Sepsis | 0.009 | 9.872 | 1.776–54.880 |
| Septic Shock | 0.558 | 0.451 | 0.032–6.462 |
|
Blood Pressure (Hypotension vs. Hypertension) |
0.031 | 21.042 | 1.318–336.004 |
| MV on candidemia onset | 0.353 | 2.613 | 0.344–19.869 |
* X2(7) = 30.466; P < 0.001; R2 Nagelkerke = 0.540; Hosmer-Lemeshow Test P = 0.263; Specificity = 76.9%; Sensitivity = 84.8%; Accuracy = 81.4%. CI: confidence interval; MV: mechanical ventilation.
Table 7 shows the results of the in vitro activity of 4 systemically active antifungal agents against BSI isolates of Candida spp. All isolates tested were susceptible to amphotericin B and micafungin, while a few of them were resistant (2/39; 5.1%) and susceptible dose-dependent (SDD; 8/39; 20.5%) to fluconazole and SDD to itraconazole (3/39; 7.7%). There were two strains (an isolate of C. albicans and another of C. tropicalis) SDD to both fluconazole and itraconazole (2/39; 5.1%).
Table 7. Antifungal susceptibility test results for Candida spp. isolates.
| Species / Antifungal agent | MIC (μg/ml) | Resistance N (%) |
S-DD N (%) |
||
|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | |||
| All isolates tested (N = 39) | |||||
| Amphotericin B | 0.06–1.0 | 0.25 | 1.0 | 0 | - |
| Fluconazole | 0.125–64.0 | 1.0 | 2.0 | 2 (5.1) | 8 (20.5) |
| Itraconazole | <0.03–0.25 | 0.03 | 0.06 | 0 | 3 (7.7) |
| Micafungin | <0.015–1.0 | <0.015 | 0.03 | 0 | - |
| Candida albicans (N = 13 tested) | |||||
| Amphotericin B | 0.125–1.0 | 0.25 | 0.5 | 0 | - |
| Fluconazole | 0.125–4.0 | 0.5 | 4.0 | 0 | 2 (15.4) |
| Itraconazole | 0.03–0.25 | 0.06 | 0.125 | 0 | 2 (15.4) |
| Micafungin | <0.015–0.06 | <0.015 | 0.03 | 0 | - |
| Candida tropicalis (N = 12 tested) | |||||
| Amphotericin B | 0.06–1.0 | 0.25 | 1.0 | 0 | - |
| Fluconazole | 0.5–4.0 | 0.5 | 4.0 | 0 | 2 (16.7) |
| Itraconazole | 0.03–0.125 | 0.03 | 0.06 | 0 | 1 (8.3) |
| Micafungin | <0.015–0.06 | <0.015 | 0.03 | 0 | - |
| Candida parapsilosis (N = 9 tested) | |||||
| Amphotericin B | 0.125–1.0 | 0.5 | - | 0 | - |
| Fluconazole | 0.125–16.0 | 0.5 | - | 1 (11.1) | 0 |
| Itraconazole | <0.03–0.03 | <0.03 | - | 0 | 0 |
| Micafungin | <0.015–1.0 | 0.03 | - | 0 | - |
| Candida glabrata (N = 5 tested) | |||||
| Amphotericin B | 0.06–1.0 | 0.25 | - | 0 | - |
| Fluconazole | 0.5–64.0 | 1.0 | - | 1 (20) | 4 (80) |
| Itraconazole | 0.03–0.06 | 0.03 | - | 0 | 0 |
| Micafungin | <0.015–0.06 | <0.015 | - | 0 | - |
MIC: Minimum Inhibitory Concentration. MIC50 and MIC90: MIC required to inhibit 50% and 90% of the isolates, respectively. S-DD: Susceptible-Dose Dependent. Resistance breakpoints: fluconazole: MIC of ≥8 μg/ml; ≥64 μg/ml for C. glabrata; itraconazole: ≥1 μg/ml; amphotericin B: ≥2 μg/ml; micafungin: ≥1 μg/ml; ≥8 μg/ml for C. parapsilosis; ≥0.25 μg/ml for C. glabrata. S-DD breakpoints: fluconazole: MIC of 4 μg/ml; ≤32 μg/ml for C. glabrata; itraconazole: 0.25–0.5 μg/ml.
Discussion
The overall incidence rate of candidemia observed in our study (2.23 episodes per 1000 admissions) was close to the findings of Brazilian multicenter studies (2.42 to 2.49/1000 admissions) [24, 25] and also those reported in the US (1.9 to 2.4/1000 admissions) [2], but higher than the rates reported in a multicenter study in Latin America (1.18/1000 admissions) [26], in several European countries (0.23 to 1.5/1000 admissions) [27–34] and in a recent study conducted in Japan (0.056/1000 admissions) [35].
Compared to other studies around the world [32, 35, 36, 37], our patient’s mortality rate (55.9%) is higher, corroborating other Brazilian studies, ranging from 54 to 72.2% [24, 38, 39].
The distribution of Candida species observed in our study is consistent with other studies conducted in Brazil and Latin America, showing a relatively lower prevalence of C. albicans (although it is still the most prevalent species) and a higher prevalence of C. parapsilosis and C. tropicalis among the NCAC species (alternating between second and third places), and C. glabrata as the fourth most prevalent species [24, 26, 38, 39]; whereas in the US and several other European countries C. glabrata appears generally as the second most prevalent species [8].
C. albicans and C. glabrata were the species most associated with mortality, especially when compared to C. parapsilosis. Other studies have also found a correlation between C. albicans and C. glabrata with higher mortality, as well as lower mortality rates in cases of candidemia due to C. parapsilosis [11, 24, 31, 32, 35].
Another important finding of our study was the high frequency of fluconazole use, being the first choice in most cases. A recent guideline for the management of candidiasis recommended an echinocandin as initial therapy for candidemia [9], however this class of antifungal drugs is not yet very accessible due to its high cost [40]. Amphotericin B deoxycholate was the second most commonly used antifungal drug, however its lipid formulations are preferable because of its high toxicity [41], except in some specific cases [9].
Comparing our results using univariate and multivariate logistic regression analysis with the existing literature, we found other studies that have demonstrated an association between age, clinical condition (sepsis, septic shock, APACHE score) and mechanical ventilation with a higher mortality risk in patients with candidemia [30, 32, 35, 42, 43]. It is important to mention that hypotension is one of the criteria for the definition of septic shock [17] and it is also evaluated in the APACHE score, therefore its association with worse prognosis found in our study was expected; although we highlighted that its association as an independent risk factor had not yet been described.
Despite the low rate of antifungal resistance found in our study, there was a higher proportion of strains susceptible dose-dependent to fluconazole (20.5%), mainly among C. glabrata isolates (80%), consistent with the widely known lower susceptibility of C. glabrata to fluconazole [11]. These results indicate a greater probability of therapeutic failure if fluconazole is used, especially in cases of C. glabrata BSI.
Finally, our antifungal susceptibility profile corroborates with other studies conducted in Brazil and Latin America in general, where Candida spp. resistance to echinocandins and amphotericin B remains rare [39, 44].
In conclusion, we observed a high incidence of candidemia, displaying a tendency to increase over the 6 years of the study, as well as a high mortality rate, proving a nosocomial problem that deserves attention. We believe that our study contributed to the knowledge of the local epidemiology of candidemia and could be used to assist in the adoption of strategies to stratify patients at higher risk for developing candidemia and worse prognosis in low income regions of the globe, in addition to improve antifungal management (prophylaxis, empirical and definitive therapy) which has not been shown to be effective in the study hospital. We emphasize that this is the first study in Northeast Brazil that has made such a deep analysis in this regard, despite our limitations, mainly due to the nature of the study (retrospective and single center).
Supporting information
(XLSX)
Acknowledgments
We would like to thank to Professor Arnaldo Colombo for the donation of Candida spp. reference strains and Dr. Daniel Kacher, from the Department of Biophysics, Federal University of São Paulo, for the help with MALDI -TOF analysis. We are very grateful to Dr. Suzanne Hamilton for critically proofreading and editing the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37(9): 1172–1177. 10.1086/378745 [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20(1): 133–163. 10.1128/CMR.00029-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yapar N. Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag. 2014;10: 95–105. 10.2147/TCRM.S40160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duggan S, Leonhardt I, Hünniger K, Kurzai O. Host response to Candida albicans bloodstream infection and sepsis. Virulence. 2015;6(4): 316–326. 10.4161/21505594.2014.988096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eggimann P, Que YA, Revelly JP, Pagani JL. Preventing invasive candida infections. Where could we do better? J Hosp Infect. 2015;89(4): 302–308. 10.1016/j.jhin.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 6.Pfaller MA, Castanheira M. Nosocomial Candidiasis: Antifungal Stewardship and the Importance of Rapid Diagnosis. Med Mycol. 2016;54(1): 1–22. 10.1093/mmy/myv076 [DOI] [PubMed] [Google Scholar]
- 7.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13): 1198–208. 10.1056/NEJMoa1306801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect. 2014;20 Suppl 6: 5–10. [DOI] [PubMed] [Google Scholar]
- 9.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4): e1–50. 10.1093/cid/civ933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arendrup MC. Candida and candidaemia. Susceptibility and epidemiology. Dan Med J. 2013;60(11): B4698 [PubMed] [Google Scholar]
- 11.da Matta DA, Souza ACR, Colombo AL. Revisiting Species Distribution and Antifungal Susceptibility of Candida Bloodstream Isolates from Latin American Medical Centers. J Fungi (Basel). 2017;3(2). pii: E24 10.3390/jof3020024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pittet D, Monod M, Suter PM, Frenk E, Auckenthaler R. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg. 1994;220(6): 751–758. 10.1097/00000658-199412000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.León C, Ruiz-Santana S, Saavedra P, Almirante B, Nolla-Salas J, Alvarez-Lerma F, et al. A bedside scoring system ("Candida score") for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med. 2006;34(3): 730–737. 10.1097/01.CCM.0000202208.37364.7D [DOI] [PubMed] [Google Scholar]
- 14.Chaves GM, Santos FP, Colombo AL. The persistence of multifocal colonisation by a single ABC genotype of Candida albicans may predict the transition from commensalism to infection. Mem Inst Oswaldo Cruz. 2012;107(2): 198–204. 10.1590/s0074-02762012000200008 [DOI] [PubMed] [Google Scholar]
- 15.Guyton AC, Hall JE. Textbook of Medical Physiology. 11th ed. Philadelphia, PA: Saunders Elsevier; 2006. [Google Scholar]
- 16.Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson J, Loscalzo J. Harrison's Principles of Internal Medicine. 18th ed. New York: McGraw-Hill; 2012. [Google Scholar]
- 17.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9): 840–851. 10.1056/NEJMra1208623 [DOI] [PubMed] [Google Scholar]
- 18.Edwards JE Jr.. Candidiasis In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson J, Loscalzo J, editors. Harrison's Principles of Internal Medicine. New York: McGraw-Hill; 2012. pp. 1651–1655. [Google Scholar]
- 19.Yarrow D. Methods for the isolation, maintenance and identification of yeasts In: Kurtzman CP, Fell JW, editors. The Yeasts, a taxonomic study. Amsterdam: Elsevier Science; 1998. pp. 77–100. [Google Scholar]
- 20.Zuza-Alves DL, de Medeiros SS, de Souza LB, Silva-Rocha WP, Francisco EC, de Araújo MC, et al. Evaluation of Virulence Factors In vitro, Resistance to Osmotic Stress and Antifungal Susceptibility of Candida tropicalis Isolated from the Coastal Environment of Northeast Brazil. Front Microbiol. 2016;7: 1783 10.3389/fmicb.2016.01783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard 3rd ed CLSI document M27-A3. 2008; Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; third informational supplement CLSI document M27-S3. 2008; Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; fourth informational supplement CLSI document M27-S4. 2012; Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 24.Colombo AL, Nucci M, Park BJ, Nouér SA, Arthington-Skaggs B, da Matta DA, et al. Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. J Clin Microbiol. 2006;44(8): 2816–2823. 10.1128/JCM.00773-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colombo AL, Garnica M, Aranha Camargo LF, Da Cunha CA, Bandeira AC, Borghi D, et al. Candida glabrata: an emerging pathogen in Brazilian tertiary care hospitals. Med Mycol. 2013;51(1): 38–44. 10.3109/13693786.2012.698024 [DOI] [PubMed] [Google Scholar]
- 26.Nucci M, Queiroz-Telles F, Alvarado-Matute T, Tiraboschi IN, Cortes J, Zurita J, et al. Epidemiology of candidemia in Latin America: a laboratory-based survey. PLoS One. 2013;8(3): e59373 10.1371/journal.pone.0059373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cisterna R, Ezpeleta G, Tellaria O, Spanish Candidemia Surveillance Group. Nationwide sentinel surveillance of bloodstream Candida infections in 40 tertiary care hospital in Spain. J Clin Microbiol. 2010;48(11): 4200–4206. 10.1128/JCM.00920-10 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Pemán J, Cantón E, Quindós G, Eraso E, Alcoba J, Guinea J, et al. Epidemiology, species distribution and in vitro antifungal susceptibility of fungaemia in a Spanish multicentre prospective survey. J Antimicrob Chemother. 2012;67(5): 1181–1187. 10.1093/jac/dks019 [DOI] [PubMed] [Google Scholar]
- 29.Berdal JE, Haagensen R, Ranheim T, Bjørnholt JV. Nosocomial candidemia; risk factors and prognosis revisited; 11 years experience from a Norwegian secondary hospital. PLoS One. 2014;9(7): e103916 10.1371/journal.pone.0103916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassetti M, Merelli M, Ansaldi F, de Florentiis D, Sartor A, Scarparo C, et al. Clinical and therapeutic aspects of candidemia: a five year single centre study. PLoS One. 2015;10(5): e0127534 10.1371/journal.pone.0127534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caggiano G, Coretti C, Bartolomeo N, Lovero G, De Giglio O, Montagna MT. Candida Bloodstream Infections in Italy: Changing Epidemiology during 16 Years of Surveillance. Biomed Res Int. 2015;2015: 256580 10.1155/2015/256580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barchiesi F, Orsetti E, Gesuita R, Skrami E, Manso E; Candidemia Study Group. Epidemiology, clinical characteristics, and outcome of candidemia in a tertiary referral center in Italy from 2010 to 2014. Infection. 2016;44(2): 205–213. 10.1007/s15010-015-0845-z [DOI] [PubMed] [Google Scholar]
- 33.Trouvé C, Blot S, Hayette MP, Jonckheere S, Patteet S, Rodriguez-Villalobos H, et al. Epidemiology and reporting of candidaemia in Belgium: a multi-centre study. Eur J Clin Microbiol Infect Dis. 2017;36(4): 649–655. 10.1007/s10096-016-2841-3 [DOI] [PubMed] [Google Scholar]
- 34.Kocmanová I, Lysková P, Chrenkova V, Olišarová P, Dobiáš R, Janouškovcová H, et al. Nosocomial candidemia in the Czech Republic in 2012–2015: results of a microbiological multicentre study. Epidemiol Mikrobiol Imunol. 2018;67(1): 3–10. [PubMed] [Google Scholar]
- 35.Hirano R, Sakamoto Y, Kitazawa J, Yamamoto S, Kayaba H. Epidemiology, practice patterns, and prognostic factors for candidemia; and characteristics of fourteen patients with breakthrough Candida bloodstream infections: a single tertiary hospital experience in Japan. Infect Drug Resist. 2018;11: 821–833. 10.2147/IDR.S156633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez L, Bustamante B, Huaroto L, Agurto C, Illescas R, Ramirez R, et al. A multi-centric Study of Candida bloodstream infection in Lima-Callao, Peru: Species distribution, antifungal resistance and clinical outcomes. PLoS One. 2017;12(4): e0175172 10.1371/journal.pone.0175172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding X, Yan D, Sun W, Zeng Z, Su R, Su J. Epidemiology and risk factors for nosocomial Non-Candida albicans candidemia in adult patients at a tertiary care hospital in North China. Med Mycol. 2015;53(7): 684–690. 10.1093/mmy/myv060 [DOI] [PubMed] [Google Scholar]
- 38.Braga PR, Cruz IL, Ortiz I, Barreiros G, Nouér SA, Nucci M. Secular trends of candidemia at a Brazilian tertiary care teaching hospital. Braz J Infect Dis. 2018;22(4): 273–277. 10.1016/j.bjid.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doi AM, Pignatari AC, Edmond MB, Marra AR, Camargo LF, Siqueira RA, et al. Epidemiology and Microbiologic Characterization of Nosocomial Candidemia from a Brazilian National Surveillance Program. PLoS One. 2016;11(1): e0146909 10.1371/journal.pone.0146909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poulat C, Nivoix Y, Launoy A, Lutun P, Bachellier P, Rohr S, et al. Assessment of high-priced systemic antifungal prescriptions. Med Mal Infect. 2017;47(6): 382–388. 10.1016/j.medmal.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 41.Hamill RJ. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs. 2013;73(9): 919–934. 10.1007/s40265-013-0069-4 [DOI] [PubMed] [Google Scholar]
- 42.De Rosa FG, Corcione S, Filippini C, Raviolo S, Fossati L, Montrucchio C, et al. The Effect on mortality of fluconazole or echinocandins treatment in candidemia in internal medicine wards. PLoS One. 2015;10(5): e0125149 10.1371/journal.pone.0125149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sbrana F, Sozio E, Bassetti M, Ripoli A, Pieralli F, Azzini AM, et al. Independent risk factors for mortality in critically ill patients with candidemia on Italian Internal Medicine Wards. Intern Emerg Med. 2018;13(2): 199–204. 10.1007/s11739-017-1783-9 [DOI] [PubMed] [Google Scholar]
- 44.Nucci M, Queiroz-Telles F, Tobon AM, Restrepo A, Colombo AL. Epidemiology of opportunistic fungal infections in Latin America. Clin Infect Dis. 2010;51: 561–570 10.1086/655683 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.