Abstract
Pain is regulated endogenously through both opioid and non-opioid mechanisms. We hypothesized that two novel pain modulation tasks, one drawing on context/expectations and one using voluntary reappraisal, would show differing levels of opioid dependence. Specifically, we expected that naloxone would block context-related analgesia, whereas mental imagery-based pain reappraisal would be opioid-independent.
A double-blind, placebo-controlled intravenous naloxone versus saline crossover design was used. Twenty healthy volunteers completed the two modulation tasks with acute heat stimuli calibrated to induce moderate pain. In the mental imagery task, participants imagined either a “pleasant” or a “comparison” scenario during painful heat. In the relative relief task, moderate heat stimuli coincided with visual cues eliciting relief from the expectation of intense pain, and were compared with moderate heat stimuli delivered under the expectation of non-painful warmth. Both “pleasant imagery” and “relative relief” conditions significantly improved ratings of pain intensity and pleasantness during saline treatment. Indeed, the target stimuli in both tasks, which had been calibrated to induce moderate pain, were rated as mildly pleasant. Furthermore, consistently with the main hypothesis, blocking endogenous opioid signaling with naloxone did not significantly affect imagery-induced regulation of pain intensity or pleasantness. In contrast, the relative relief-induced pain regulation (i.e., context/expectation) was blocked by naloxone. We conclude that endogenous opioid signaling is necessary for expectation-related relative relief analgesia, but not for pain reappraisal through mental imagery. These results support mental imagery as a powerful and clinically relevant strategy for regulating pain affect also in patients where endogenous opioid mechanisms might be compromised.
SIGNIFICANCE STATEMENT Neurotransmitter systems in the human brain can be probed through antagonist drugs. Studies using the opioid antagonist naloxone have demonstrated that the brain relies on both opioid and non-opioid mechanisms to downregulate pain. This holds clinical relevance given altered endogenous opioid processes in many chronic pain conditions. The present study used a double-blinded, placebo-controlled naloxone blockage of endogenous opioids in healthy humans to show differential opioid involvement in two pain modulation tasks. Context/expectation-driven (relative relief-related) analgesia was blocked by naloxone. In contrast, pain reappraisal through mental imagery was intact despite opioid receptor blockade, suggesting opioid independence. These results support mental imagery as a powerful, clinically relevant strategy for regulating pain as it does not rely on a functioning opioidergic system.
Keywords: descending pain modulation, expectation, mental imagery, naloxone, pain modulation, pain relief
Introduction
Pain perception is shaped by expectations and context (Tracey, 2010; Koban et al., 2017), physiological state (Schrimpf et al., 2015), and by voluntary regulatory techniques such as reappraisal (Johnstone et al., 2007; Phillips et al., 2008; Wiech et al., 2008a). In a seminal paper, Levine et al. (1978) used high-dose naloxone to show that placebo analgesia depends on endogenous opioids. Naloxone at high dose provides complete blockade of μ-opioid receptors (Mayberg and Frost, 1990). This opioid antagonist would be expected to block pain modulations mediated by endogenous opioid signaling in the descending pain inhibitory system (Tracey and Mantyh, 2007; Wiech et al., 2008a). Indeed, the finding has been replicated in several (Amanzio and Benedetti, 1999; Benedetti et al., 1999, 2007; Eippert et al., 2009; Rütgen et al., 2015, 2018), but not all subsequent placebo studies (Vase et al., 2005; Benedetti et al., 2011) using naloxone or naltrexone in humans. Converging evidence now points to the existence of both opioid-dependent and opioid-independent modes of endogenous analgesia in humans (Amanzio and Benedetti, 1999; Eippert et al., 2009; Sprenger et al., 2012).
Initial studies indicate that hypnosis (Moret et al., 1991), mindfulness meditation (Zeidan et al., 2016), and religious prayer (Elmholdt et al., 2017) can induce analgesia largely independently of μ-opioid blockade. A putative shared mechanism of these pain modulatory techniques is voluntary, conscious reappraisal, which can alter the meaning of pain without targeting the sensory aspects of the percept (Woo et al., 2015). In contrast, opioid signaling appears to be more important for modulations that alter pain perception without relying on voluntary reappraisal, such as placebo (Amanzio and Benedetti, 1999; Eippert et al., 2009), distraction (Sprenger et al., 2012), stress-induced analgesia, and rTMS (Werner et al., 2015). For many types of pain modulations, notably pain-induced analgesia [diffuse noxious inhibitory control (DNIC)/conditioned pain modulation (CPM)], mixed results of opioid blockade studies in humans highlight the need for further research (Werner et al., 2015).
Here, we used high-dose naloxone in a double-blind, randomized, placebo-controlled design with two novel pain modulation tasks: mental imagery and relative relief. Mental imagery involves the imagination of a more pleasant context or meaning for the painful stimulus. It has been used to help patients cope with pain in a variety of clinical contexts (Syrjala et al., 1995; Fors et al., 2002; Winterowd et al., 2003; Pincus and Sheikh, 2009; Posadzki and Ernst, 2011; Posadzki et al., 2012). The mechanisms behind this modulation have been little studied (Berna et al., 2012; Jensen et al., 2012), but may share some features with those of hypnosis and mindfulness meditation. Accordingly, we hypothesized that pain relief induced by mental imagery would be unaltered by naloxone. Importantly, we contrasted the effect of naloxone on this task with its effects on a novel, non-voluntary pain modulation task hypothesized to recruit endogenous opioids for pain relief. We previously reported reduced pain, increased positive affect and activity in the periaqueductal gray (PAG) when a context-related expectation of intense pain was interrupted by a visual cue indicating that intense pain has been avoided (Relative Relief cue; Leknes et al., 2013a). Thus, we make a direct comparison of two novel pain modulation tasks with the aim to reveal the opioid-independence of mental imagery (reappraisal) and the opioid dependence of relative relief (context/expectation) analgesia in healthy humans.
Materials and Methods
Participants
Participants were recruited through advertisements on posting boards and mailing lists at the University of Oxford, UK. They underwent a preliminary health screening over the phone to exclude any medical condition, chronic drug intake, or contraindications to MRI and naloxone. Recreational drug users and volunteers with psychiatric conditions were excluded. Opioid intake was tested for by urine analysis on each day of testing (none positive). Written informed consent was obtained on the first test day. The study was approved by the Milton Keynes Research Ethics Committee (09/H0603/17) and conformed to the guidelines of the Declaration of Helsinki (1996). Participants were reimbursed 35 GBP per session. The first four participants of the N = 27 recruited only completed one session because their fMRI data were corrupted by an fMRI coil dysfunction. Three further subjects did not return for a second session after experiencing side effects attributed to naloxone. Therefore, 20 participants completed the two runs of the study (mean age: 27; range: 20–38, 11 female), and were included in the analysis.
Procedure
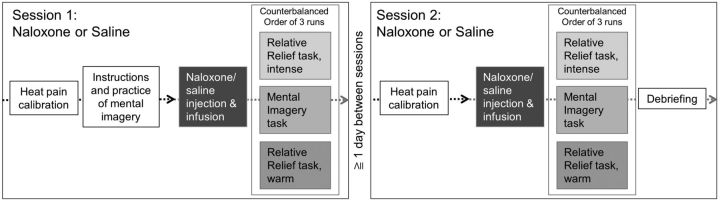
The study used a within-subjects, double-blind, placebo-controlled design with two sessions separated by at least 1 d (Fig. 1). The order of the sessions was randomized and counterbalanced across participants. The unblinded study physicians (C.B., R.N.M.) were responsible for preparing and administering the infusions. They did not communicate with participants until debriefing at the end of the second session, or in case of significant side effects (N = 3, participant discontinuation).
Figure 1.
Overview of study design. Participants were pseudorandomized into starting either with the naloxone or saline (placebo) session. After a brief standardized instruction, participants practiced the mental imagery tasks. Following that, the two sessions contained the same sequence: a heat pain calibration preceded the start of the infusion, which was followed by the three task runs presented in counterbalanced order. At the end of the second session, a debriefing took place.
After written consent, participants were given precise, standardized instructions via a PowerPoint presentation outside of the scanner. In both sessions, once in the scanner, yet before drug administration, heat-pain calibration was conducted to identify two levels of pain intensity for each participant: intense pain and moderate pain (Fig. 1). A brief practice run took place, including the mental imagery task with heat pain and the visual analog scale (VAS) rating system. Approximately 15 min after drug administration started, participants completed the mental imagery task (1 run) and the relative relief task (2 runs). Each run consisted of fourteen 5 s heat stimuli and lasted 12 min. The order of tasks and the within-run order of stimuli were pseudorandomized and counterbalanced. Each heat stimulus was followed by two VAS rating scales assessing stimulus pleasantness and pain intensity (fixed order of rating scales). Temperatures calibrated to yield a rating of 5/10 were used for all the moderately painful stimuli during each session, allowing for between-task comparisons. Participants were blinded to this fact. Two thermodes were used to allow shorter interstimulus intervals without representing a threat to the skin integrity (Details in the section on heat pain stimuli).
Testing occurred in a 3T Varian scanner; EPI images were collected during tasks (data to be reported elsewhere).
Pain modulation tasks
Mental imagery task.
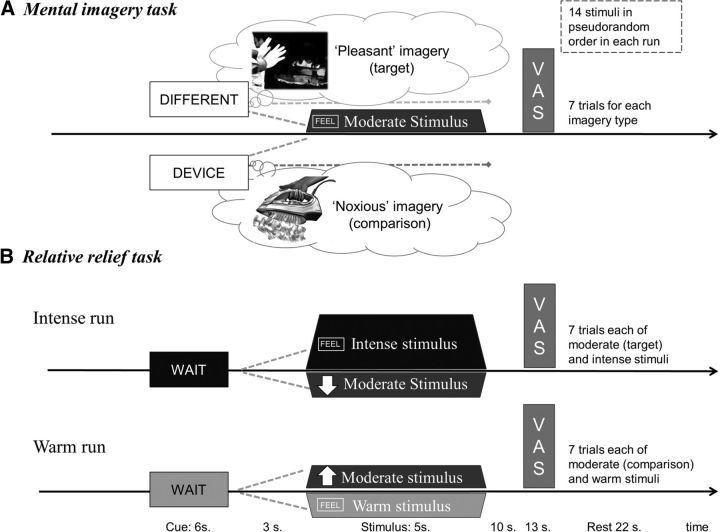
Participants were asked to use their imagination to hold a mental image (i.e., retrieve perceptual information from memory to create a new experience, allowing them to see with their mind's eye or feel with their mind's skin) of a context in which heat was actually pleasant. During the training phase outside of the scanner, participants were encouraged to find a suitable scenario, and provided with examples, such as “You are coming back to the cabin after a day out in the snow, and are cold. You sit by the fire. Your arm stretches out and gets close to the heat.” All participants were able to conjure up such a scenario and practiced it first without thermal stimulation and then again inside the scanner with thermal stimuli. Indeed, most were able to recount episodes in which feelings of cold had rendered intense heat pleasant. Because testing occurred in England during autumn/winter, many in fact imagined scenarios that occurred when they were freezing within their own (typically under-heated) lodgings. The comparison task required participants to hold a mental image of the thermode, a clothes iron, or another noxious heat-dissipating device being close to their skin. During the experiment, a visual cue appearing 9 s before the heat stimulus onset signaled the condition (cued with the words: DIFFERENT/DEVICE) and instructed participants to start imagining the specific context (pseudorandomized order, 50% chance of “pleasant” vs comparison condition; Fig. 2a). At the start of the thermal stimulus, the screen instructed participants to feel the sensation while continuing to hold their mental image: “FEEL (DIFFERENT/DEVICE)”. The visual cues consisted of black text within a purple or yellow frame (the colors were pseudorandomized between the two conditions). The moderate stimuli were delivered in pseudorandom order on either thermode, to avoid risk of skin sensitization.
Figure 2.
Overview of the two pain modulation tasks. The temperature, number and duration of moderately painful stimuli, as well as the timing of cues, stimuli and VASs were kept the same across tasks to facilitate comparison. To avoid skin sensitization while keeping experiments brief, two thermodes were used to deliver stimuli. A, In the mental imagery task, participants imagined scenarios in which the moderate heat-pain stimuli would either be pleasant (i.e., useful to alleviate hypothermia) or noxious (i.e., a standard, potentially harmful source of heat such as a pain device or a clothes iron). Visual cues displaying the words “different” (target: pleasant imagery) or “device” (comparison imagery) were presented 9 s before the onset of each heat stimulus, instructing participants to start imagining. B, In the relative relief task, visual cues displaying the word “Wait” were presented 9 s before each heat stimulus to induce expectation of intense pain (Intense run, text on red background) or non-painful warm stimuli (Warm run, text displayed on a blue background). Before task onset, participants were informed that the heat stimulus following the initial visual cue would “most likely” be the cued stimulus, the onset of which was then indicated by a cue reading “Feel” (same color background). However, when the second cue was an arrow pointing downward or upward, it indicated the delivery of a modified stimulus. Unbeknownst to participants, the temperature used then was always the one calibrated to induce moderate pain. In the Intense run, the downward pointing arrow indicated a lower temperature stimulus than expected, thus constituting a relative relief cue. In the comparison run, the upward pointing arrow indicated a higher temperature stimulus. The target and comparison stimuli were thus matched for expected and actual likelihood as well as for temperature, duration, and order of appearance.
Relative relief task.
The relative relief task was a modified version of the task reported by Leknes et al., 2013a (Fig. 2b). In the “Intense” run, visual cues indicating an upcoming intensely painful stimulus were presented 9 s before heat pain (“Wait” on a red background). In 50% of cases, the cue was followed by heat pain calibrated to induce intense pain (associated to a cue reading “Feel” on a red background). In the remaining 50% of cases, a downward pointing arrow cue (relative relief cue) indicated the occurrence of a lower temperature stimulus. The stimulus presented with the relative relief cue was always at a temperature precalibrated to induce moderate pain in the participant. In the Warm run, the first visual cue indicated an upcoming warm stimulus (Wait on a blue background), which was followed by a warm stimulus in 50% of cases (together with a cue reading Feel on a blue background). In 50% of cases however, the warm cue was followed by an upward pointing arrow (comparison cue), which indicated the occurrence of a higher temperature stimulus. This comparison moderate stimulus was identical to the relative relief stimulus with regard to temperature, duration, probability, and timing within the run.
To enhance the potency of this task, participants were led to believe that the heat stimuli in the Intense run would be intensely painful most of the time (and conversely, that the warm stimuli were most frequent in the Warm run). Specifically, they were told that “In this run, the stimuli will be mostly (intense/warm), but sometimes, the stimulus temperature will be (lower/higher)”. Intense and warm stimuli were delivered on one thermode, and moderate stimuli on another, to avoid risk of skin sensitization.
Stimuli and outcome measures
Heat pain stimuli
Two in-house built thermal heat pain devices (thermodes) were used to deliver 5-s-long thermal stimuli to the ventral aspect of the left forearm of the participants, with at least 6 cm distance between them (Leknes et al., 2013a). Two thermodes were used to alternate the delivery of the stimuli between the two sites, allowing shorter interstimulus intervals without representing a threat to the skin integrity. Both devices were used to deliver moderate painful stimuli in the imagery task. To avoid sensitization effects due to the use of a high-temperature stimulus in the relative relief task, moderate stimuli were delivered by one thermode, whereas the other one was used for the intense pain and warm stimuli (Leknes et al., 2013b).
Warm stimuli were set to 40°C. The individual temperatures identified during calibration to elicit ratings of “moderate pain” [∼5 on an 11-point numerical rating scale (NRS)] were 48.1 ± 2.3 (mean ± SD) across thermodes and sessions. For intense pain (∼8 on the 11-point NRS) an average temperature of 50.9 ± 2.0 was used. Potential effects of session and drug condition on temperatures used were addressed in a regression analysis and significant effects led to the inclusion of temperature information in subsequent analyses.
Poststimulus ratings
During testing, each heat stimulus was rated for pleasantness and pain intensity on a respective VAS: “How PLEASANT was the stimulus?” with anchors “very pleasant” to “very unpleasant”; and “How PAINFUL was the stimulus?” anchored “not painful” and “very painful” (always in this order).
State and trait measures
Current mood state was further assessed with six mood VASs, presented at the beginning and the end of each run. Participants rated four separate statements: “At this moment I feel …. sad/happy/calm/anxious” each on a VAS with anchors “not at all” and “very much so”.
A state version (Eikemo et al., 2016) of the Snaith–Hamilton Pleasure Scale was presented after completion of the Intense run to assess hedonic capacity. Participants also filled in trait questionnaires including BIS/BAS (Carver and White, 1994), STAI State and Trait Anxiety (Spielberger, 1983), LOT-R (Herzberg et al., 2006), EPQ12 (Eysenck et al., 1985), and imagery ability with the SUIS (Nelis et al., 2014; data not reported).
Debriefing
At the completion of each run, participants responded to a series of post-run questions from the experimenter, assessing anxiety induced by each of the visual cues preceding heat stimuli (0–10 NRS, both tasks); effort and perceived success of mental imagery performance for each imagery condition (0–10 NRS); disappointment about the up pointing arrow (Warm run) or relief elicited by the down pointing arrow (Intense run; 0–10 NRS); and positive/negative affect (−5 to +5 NRS) elicited by each of the possible outcomes. These ratings were collected to supplement the ratings of pleasantness and pain intensity collected during the task.
After each test session, but before participants were removed from the scanner, they were asked to guess whether they had received naloxone or saline.
During final debriefing, participants were asked to estimate the frequency of the intense versus moderate and warm versus moderate stimuli in the relative relief task. At the end of the debriefing session, participants were informed about the fact that the moderate painful stimulus was always the same, and were informed about the order of the drug conditions.
Urine test for opiates
Participants were screened for opiates using a urine test kit (Instalert, Innovacon) before the start of both sessions to prevent precipitating withdrawal (exclusion criterion). None of the tests were positive.
Drug administration
Approximately 15 min before the start of the testing, a 0.15 mg/kg bolus of naloxone (naloxone hydrochloride, 400 μg/ml vials; non-proprietary, UK) was administered intravenously through an intravenous line placed on the right forearm. This was immediately followed by an infusion at a rate of 0.2 mg/kg/h to keep the plasma level constant and ensure continuous complete blockade of the opioid receptors (Mangold et al., 2000; Eippert et al., 2009; Schoell et al., 2010). A matching quantity of saline (NaCl 0.9%) was administered in the control (placebo) condition.
Participants were informed that naloxone is a drug that interacts with the opioid receptors, without clear effects on acute pain perception (Eippert et al., 2009). They were also informed about possible side effects, and that they may not be able to notice any differences between naloxone and saline infusion. The full information about the motivation to use naloxone during the study was presented during debriefing, in order not to bias expectations. The study physicians (C.B. and R.N.M.) preparing and administering the drugs did not participate in subject testing nor communicate with participants, unless significant side effects were declared, or during debriefing. The study staff conducting the testing (S.L. and A.H.A.) was blinded to the drug assignation.
Side effects were reported at the end of each testing session on a standardized scale (Eippert et al., 2009), where each adverse effect could be rated as “inexistent, very weak, weak, moderate, strong, very strong or extremely strong” (0–6).
Statistical analysis
The main outcomes were VASs for pain intensity and stimulus pleasantness of moderate heat stimuli. The stimulus pleasantness was recorded on a bivalent scale, which was converted to a numerical rating ranging from −5 (very unpleasant) to +5 (very pleasant), whereas pain intensity VAS ratings were converted to a number between 0 and 10.
Secondary outcomes included measurements of mood, stimulus temperatures, ratings of intense and warm stimuli from the Relative Relief task, as well as post-run and debriefing ratings.
Data were analyzed with linear mixed models (LMMs) using the mixed-effect model module (GENLINMIXED) in SPSS v24 (IBM). Mixed effects models include both fixed and random effects and offer superior flexibility compared with more traditional repeated measures analyses. These models are more robust with respect to unbalanced designs and missing data, allow different target distributions and the inclusion of all data points without aggregation across multiple trials of one type (Gueorguieva and Krystal, 2004).
The main analyses (one for each VAS rating type) assessed the effects of Stimulus type, Task type, and Drug on target stimuli (“pleasant” imagery and Intense run moderate) and the comparison moderate stimuli (“noxious” imagery and the Warm run moderate) across the naloxone and saline conditions. To test the hypothesis that pain modulation in the Relative Relief task would be opioid-dependent but not in the Mental Imagery task, we assessed the three-way interaction between Drug, Stimulus type, and Task type, followed by statistical assessment (planned pairwise contrasts) of target versus comparison stimuli for each task and each drug conditions. Any effects identified in the analysis of secondary outcomes, which could act as potential confounders (e.g., drug effects on mood, stimulus temperatures or intense/warm stimuli) were included as fixed effects in the main LMMs of moderate stimuli. To adjust for dependencies in the data related to individual characteristics of each participant, the main LMMs included by-subject random intercepts as well as by-subject random slopes for drug and task. The random effects variance-covariance matrix had a variance-components structure. LMMs of secondary outcomes all included a fixed effect of Drug and a random term for subject intercept. Overall, we aimed for parsimonious models. Starting with a model that included the key design factors (Drug, Stimulus type, and Task type) and their interactions, we then tested whether other relevant factors would explain significant variance (i.e., p < 0.05 and an improvement of the Bayesian information criterion >2). These additional factors were introduced to the model one by one in order of theoretical importance: Stimulus Temperature, pain intensity ratings of the intense heat stimulus (VASIntensity), Session, Trial Number, and Participant Gender. If a factor met the inclusion criteria, it was kept in the model when subsequent factors were tested. When a between-subjects factor (Gender) was kept in the model, relevant two-way interactions with design factors were also tested. Multicollinearity was assessed using collinearity diagnostics for linear regressions, and was found to be low (variance inflation factor scores <1.3). The LMMs of ratings of intense as well as warm stimuli used robust covariances because data and residuals did not meet criteria for normality.
Results
Control analyses
Mood
An average mood score was computed (inverse values were used for the items “anxious” and “sad”), and effects of Drug, Session, Run, and Rating Time (pre- or post-run) were assessed in an LMM. Self-reported mood was not significantly affected by naloxone treatment (saline, 2.14 ± 1.95; naloxone 2.24 ± 2.0; F(1,222) = 0.29, p = 0.59). Mood was marginally higher during the second session (F(1,222) = 3.8, p = 0.052). Further, self-reported mood was significantly lower before and after the intense pain task than before and after the two other tasks (mean mood reduction 0.4 on the 11-point VAS; F(2,222) = 3.3, p = 0.038). Naloxone treatment did not interact significantly with session, task, or rating time to affect mood.
Stimulus temperatures
Because stimulus temperatures were allowed to vary between sessions for each individual (calibration was conducted at the onset of each test session), effects of session and drug condition on stimulus temperatures were assessed in an LMM with the fixed factors Session, Drug, and Stimulus type (Moderate Thermode 1; Moderate Thermode 2; and intense pain).
The LMM on stimulus temperatures yielded significant main effects of Stimulus type, Session, and Drug. None of the interaction terms were significant (all F values <1.8, all p values >0.17). The effect of Stimulus (F(2,107) = 108.6, p < 0.001) reflected higher temperatures used to elicit intense pain (50.9°C ± 2.0), as well as slightly higher temperatures at thermode 2 (48.3°C ± 2.5) compared with Thermode 1 (47.8°C ± 2.0). A small but significant increase in temperatures used was also found for Session 2 (49.2°C ± 2.5) relative to Session 1 (48.8°C ± 2.6; F(1,107) = 6.0, p = 0.016) and for the naloxone condition (49.3°C ± 2.4) relative to saline (48.7°C ± 2.6; F(1,107) = 12.2, p = 0.001). The nonsignificant interaction between drug and session (F(1,107) = 0.96, p = 0.33) indicated no systematic difference in temperatures applied to the 10 participants randomized to receive saline in the first session compared with the remaining 10 who received saline in the second session. Nevertheless, stimulus temperature was entered as a control variable in the main LMMs of moderate stimuli.
Intense and warm stimuli
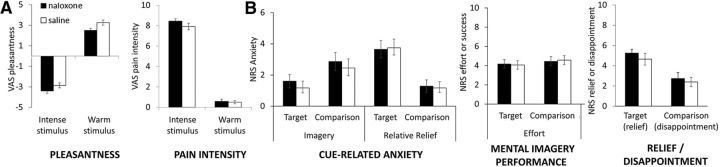
Separate LMMs were conducted to assess potential Drug and Session effects on VAS ratings of the non-moderate stimuli used in the Relative Relief task (intense and warm stimuli). LMMs of intense pain stimuli also included temperature as a fixed factor. Pain intensity ratings of the intense pain stimulus tended to be higher in the naloxone condition (saline, 8.0 ± 1.9; naloxone, 8.38 ± 1.0; F(1,277) = 2.44, p = 0.120; Fig. 4). Accordingly, VAS intensity ratings of the intense pain stimulus were included in the analysis of naloxone effects on pain downregulation of moderately painful stimuli. This was done to ensure that any naloxone-induced differences in relative relief could not be attributed to a putative naloxone effect on the intense stimulus. Pain intensity ratings also increased with higher temperature stimuli (F(1,277) = 9.35, p = 0.002). Conversely, pleasantness ratings of the intense stimulus were nonsignificantly lower after naloxone (saline, −2.93 ± 1.45; naloxone −3.33 ± 0.95; F(1,277) = 1.92, p = 0.168). Intense stimuli were also rated as more unpleasant with increasing temperatures (F(1,277) = 5.06, p = 0.025). Pleasantness and intensity ratings of the warm stimuli were not significantly altered by drug (intensity ratings: saline, 0.51 ± 0.77; naloxone, 0.58 ± 0.95; pleasantness ratings: saline, 3.21 ± 1.53; naloxone, 2.50 ± 1.92, F values <3.3.1, p values >0.072).
Figure 4.
Overview of secondary outcomes. A, VAS ratings of pleasantness and intensity for the intense heat and warm stimuli. A trend toward higher pain intensity ratings of the intense stimulus in the naloxone condition led to the inclusion of these ratings as a confound regressor in the analysis of the ratings of the target and comparison stimuli. B, Ratings of other relevant aspects of the tasks were collected via verbal, 11-point NRSs after each run. Post-run ratings of cue-related anxiety for moderate pain stimuli (target and comparison) in both tasks revealed a significant interaction between Task and Stimulus type, but no significant differences between Drug conditions. Post-run ratings of the effort that was exerted during mental imagery similarly did not reveal any effects of naloxone nor of type of imagery. Post-run ratings of relief (0–10 NRS scale after Intense run) or disappointment (0–10 NRS scale after Warm run) elicited by the delivery of the moderate stimulus revealed no significant effect of naloxone. Error bars are SEM.
Main analyses
Stimulus pleasantness ratings reveal differing opioid dependence across the pain modulation tasks
The LMM on pleasantness ratings of moderately painful stimuli was conducted with the fixed factors Drug, Session, Task type (Imagery or Relative Relief), Stimulus type (target or comparison moderate heat stimulus), two-way interactions between these, and the three-way interaction of Drug × Task type × Stimulus type. Gender and the interaction between Gender and Stimulus type were included in the final LMM (Table 1). Stimulus Temperature and VASIntensity did not reach significance and did not alter the pattern of results, with the exception that the main effect of Drug was no longer significant when VASIntensity was included.
Table 1.
Basic and final LMMs for stimulus pleasantness
| VAS pleasantness | Model 1 |
Final model |
||||||
|---|---|---|---|---|---|---|---|---|
| Bayesian information criterion: 4102.75 | Bayesian information criterion: 4081.13 | |||||||
| Fixed effects | F | df1 | df2 | Sig | F | df1 | df2 | Sig |
| Corrected model | 80.744 | 7 | 1112 | 0.000 | 66.362 | 9 | 1110 | 0.000 |
| Task | 0.114 | 1 | 1112 | 0.736 | 0.128 | 1 | 1110 | 0.721 |
| Stimulus | 282.091 | 1 | 1112 | 0.000 | 269.806 | 1 | 1110 | 0.000 |
| Drug | 4.468 | 1 | 1112 | 0.035 | 5.016 | 1 | 1110 | 0.025 |
| Task × Stimulus | 262.400 | 1 | 1112 | 0.000 | 266.014 | 1 | 1110 | 0.000 |
| Task × Drug | 5.699 | 1 | 1112 | 0.017 | 5.777 | 1 | 1110 | 0.016 |
| Stimulus × Drug | 6.071 | 1 | 1112 | 0.014 | 6.155 | 1 | 1110 | 0.013 |
| Task × Stimulus × Drug | 4.363 | 1 | 1112 | 0.037 | 4.423 | 1 | 1110 | 0.036 |
| Gender | — | — | — | — | 7.740 | 1 | 1110 | 0.005 |
| Gender × Stimulus | — | — | — | — | 16.025 | 1 | 1110 | 0.000 |
| Estimates of covariance parameters | Estimate | SE | Z | Sig | Estimate | SE | Z | Sig |
|---|---|---|---|---|---|---|---|---|
| Residual | 1.965 | 0.086 | 22.969 | 0.000 | 1.939 | 0.084 | 22.947 | 0.000 |
| Intercept participant | 0.483 | 0.100 | 4.828 | 0.000 | 0.424 | 0.090 | 4.681 | 0.000 |
| Estimated means | Mean | SE | 95% CI | 95% CI | Mean | SE | 95% CI | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Saline | ||||||||
| Comparison | −0.914 | 0.294 | −1.491 | −0.337 | −0.956 | 0.279 | −1.504 | −0.409 |
| Target | 1.881 | 0.294 | 1.304 | 2.458 | 1.806 | 0.279 | 1.259 | 2.353 |
| Mental Imagery | ||||||||
| Comparison | 0.389 | 0.294 | −0.188 | 0.966 | 0.347 | 0.279 | −0.201 | 0.894 |
| Target | 0.820 | 0.294 | 0.243 | 1.397 | 0.745 | 0.279 | 0.197 | 1.292 |
| Relative Relief | ||||||||
| Comparison | −1.180 | 0.294 | −1.757 | −0.603 | −1.222 | 0.279 | −1.769 | −0.675 |
| Target | 1.553 | 0.294 | 0.976 | 2.130 | 1.477 | 0.279 | 0.930 | 2.025 |
| Mental Imagery | ||||||||
| Comparison | 0.073 | 0.294 | −0.504 | 0.650 | 0.031 | 0.279 | −0.517 | 0.578 |
| Target | −0.259 | 0.294 | −0.836 | 0.318 | −0.334 | 0.279 | −0.881 | 0.213 |
| Relative Relief | ||||||||
| Women | — | — | — | — | 0.824 | 0.283 | 0.268 | 1.379 |
| Men | — | — | — | — | −0.350 | 0.313 | −0.965 | 0.264 |
| Women | ||||||||
| Comparison | — | — | — | — | −0.031 | 0.289 | −0.597 | 0.536 |
| Target | — | — | — | — | 1.678 | 0.289 | 1.112 | 2.244 |
| Men | ||||||||
| Comparison | — | — | — | — | −0.870 | 0.319 | −1.496 | −0.244 |
| Target | — | — | — | — | 0.169 | 0.319 | −0.457 | 0.795 |
Sig, Significance.
Main effects and interactions.
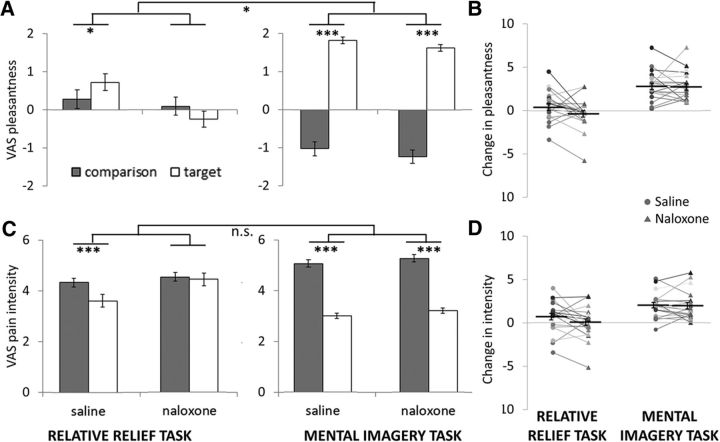
Significant main effects of Drug (F(1,1108) = 4.5, p = 0.035) and Stimulus type (F(1,1100) = 269.9, p < 0.001) on pleasantness ratings of heat stimuli calibrated to induce moderate pain, were qualified by significant interactions between Task type and Stimulus type (F(1,1100) = 266.1, p < 0.001), Task type, and Drug (F(1,1100) = 5.8, p = 0.016), Stimulus type and Drug (F(1,1100) = 6.2, p = 0.013) and between Drug, Task type, and Stimulus type (F(1,1100) = 4.4, p = 0.036). The basis for these statistics is described in more detail below. There was also a main effect of Gender (F(1,1100) = 10.2, p = 0.001) and a significant interaction between Gender and Stimulus type (F(1,1100) = 16.0, p < 0.001), reflecting greater pleasantness ratings during target stimuli in female participants.
Mental imagery task.
In the saline condition, participants were able to use pleasant imagery to shift pleasantness ratings of moderate heat stimuli from mildly unpleasant (−1.0 ± 1.1) to moderately pleasant (1.81 ± 1.49; t = −16.6, p < 0.001; Fig. 3A, right) on a scale where −5 indicated highly unpleasant and +5 indicated highly pleasant. As hypothesized, blocking endogenous opioid signaling with naloxone did not significantly reduce modulation of stimulus pleasantness in the imagery task (comparison stimulus: −1.2 ± 1.1; target stimulus: 1.57 ± 1.16; t = −16.2, p < 0.001). The modulation of stimulus pleasantness in the imagery task was robust (>2-point shift on 11-point VAS) and significantly stronger than the modulation elicited in the relative relief task, as indicated by the significant two-way interaction between Task and Stimulus type.
Figure 3.
Overview of pleasantness and pain intensity ratings for moderate heat stimuli in the two pain modulation tasks. A, As hypothesized, we observed a significant three-way interaction between Drug, Task type, and Stimulus type for pleasantness ratings. This reflected a significant reduction in pain pleasantness for the target stimulus in the relative relief task during naloxone. In contrast, participants' ability to use mental imagery to upregulate pain pleasantness was not reduced in the naloxone condition. We also observed significant two-way interactions between Drug, Task type and Stimulus type, as well as significant main effects of Drug and Stimulus type. B, Individual mean difference scores (comparison − target VAS pleasantness) for each task and each drug condition are included for illustration purposes. C, A similar pattern of results was found for ratings of pain intensity ratings. Although the three-way interaction did not reach significance, naloxone completely blocked the analgesic effect of the relative relief manipulation, yet did not significantly alter the analgesia induced by pleasant mental imagery. We also observed a significant two-way interaction between Task and Stimulus type, and a significant main effect of stimulus type. D, Individual mean difference scores (comparison − target VAS pain intensity) for each task and each drug condition are included for illustration purposes. Error bars are within-subject SEM. *p < 0.05, ***p < 0.001.
Relative relief task.
In the saline condition, the relative relief task nevertheless induced a small, but statistically significant positive modulation of stimulus pleasantness ratings (target: 0.75 ± 1.8; comparison stimulus: 0.35 ± 1.80; t = −2.4, p = 0.017; Fig. 3A, left). This modulation was blocked in the naloxone condition, where the target stimulus was rated as less pleasant (−0.33 ± 1.73) than the comparison stimulus (0.03 ± 1. 98; t = 2.2, p = 0.029). The average pleasantness ratings of the comparison stimulus in the Relative Relief task were unexpectedly positive (i.e., consistent with mild pleasure); an inspection of individual mean ratings indicated that half of the participants rated the comparison stimulus as unpleasant, and the other half as pleasant. Overall, the data from the saline condition replicates earlier findings (Leknes et al., 2013a) with a smaller effect size than in the original study. This could relate to inaccurate effect size estimation with modest sample sizes, or the use of different rating scales. Whereas Leknes et al. (2013a) showed a positive shift of 2.2 points on an 11-point scale from painful to pleasant, the present study used separate rating scales for pleasantness and pain intensity.
Comparison of modulations by task.
As indicated by the significant three-way interaction, naloxone treatment differentially affected the modulation of stimulus pleasantness induced by the two tasks. In other words, whereas successful upregulation of pleasantness was seen for stimuli calibrated to elicit moderate pain during saline in both tasks, this regulation effect was intact after naloxone for the imagery task but not for the relative relief task.
Pain intensity ratings consistent with differing opioid dependence across the pain modulation tasks
After model selection, the final LMM on pain intensity ratings of moderately painful stimuli included the fixed factors Drug, Session, Task type, and their interactions Participant Gender, Trial number, and VASIntensity (Table 2). Session and Stimulus Temperature were not found to contribute significantly to the model. A significant main effect of Stimulus type (F(1,1104) = 146.9, p < 0.001) was qualified by a significant interaction between Task type and Stimulus type (F(1,1104) = 63.9, p < 0.001). Trial number, pain intensity ratings of the intense heat stimulus, and Gender also significantly affected pain intensity ratings of the moderate heat stimuli. As with the pleasantness ratings, females showed significantly lower pain intensity ratings overall. Although the overall pattern of results was consistent with our hypothesis and the results for the pleasantness ratings, the three-way interaction between Drug, Task type, and Stimulus type was not significant for pain intensity ratings (F(1,1104) = 1.9, p = 0.167).
Table 2.
Basic and final LMMs for pain intensity
| VAS pain intensity | Model 1 |
Final model |
||||||
|---|---|---|---|---|---|---|---|---|
| Bayesian information criterion: 4479.39 | Bayesian information criterion: 4461.83 | |||||||
| Fixed effects | F | df1 | df2 | Sig | F | df1 | df2 | Sig |
| Corrected model | 31.399 | 7 | 1112 | 0.000 | 17.000 | 15 | 1104 | 0.000 |
| Corrected model | 0.220 | 1 | 1112 | 0.639 | 0.270 | 1 | 1104 | 0.603 |
| Task | 145.528 | 1 | 1112 | 0.000 | 146.987 | 1 | 1104 | 0.000 |
| Stimulus | 3.285 | 1 | 1112 | 0.070 | 1.114 | 1 | 1104 | 0.291 |
| Drug | 63.320 | 1 | 1112 | 0.000 | 63.955 | 1 | 1104 | 0.000 |
| Task × Stimulus | 2.620 | 1 | 1112 | 0.106 | 2.647 | 1 | 1104 | 0.104 |
| Task × Drug | 2.931 | 1 | 1112 | 0.087 | 2.961 | 1 | 1104 | 0.086 |
| Stimulus × Drug | 1.890 | 1 | 1112 | 0.170 | 1.909 | 1 | 1104 | 0.167 |
| Gender | — | — | — | — | 4.941 | 1 | 1104 | 0.026 |
| Trial | — | — | — | — | 2.877 | 6 | 1104 | 0.009 |
| VASintense | — | — | — | — | 7.883 | 1 | 1104 | 0.005 |
| Estimates of covariance parameters | Estimate | SE | Z | Sig | Estimate | SE | Z | Sig |
|---|---|---|---|---|---|---|---|---|
| Residual | 2.772 | 0.121 | 22.991 | 0.000 | 2.745 | 0.120 | 22.91 | 0.000 |
| Intercept participant | 0.608 | 0.125 | 4.865 | 0.000 | 0.477 | 0.104 | 4.59 | 0.000 |
| Estimated means | Mean | SE | 95% CI | 95% CI | Mean | SE | 95% CI | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Saline | ||||||||
| comparison | 4.810 | 0.333 | 4.156 | 5.464 | 4.969 | 0.305 | 4.370 | 5.567 |
| target | 2.784 | 0.333 | 2.131 | 3.438 | 2.943 | 0.305 | 2.345 | 3.541 |
| Mental Imagery | ||||||||
| comparison | 4.119 | 0.333 | 3.465 | 4.772 | 4.277 | 0.305 | 3.679 | 4.875 |
| target | 3.403 | 0.333 | 2.749 | 4.056 | 3.561 | 0.305 | 2.963 | 4.160 |
| Relative Relief | ||||||||
| Naloxone | ||||||||
| Mental Imagery | ||||||||
| comparison | 5.097 | 0.333 | 4.444 | 5.751 | 5.040 | 0.306 | 4.440 | 5.640 |
| target | 3.139 | 0.333 | 2.485 | 3.792 | 3.081 | 0.306 | 2.481 | 3.681 |
| Relative Relief | ||||||||
| comparison | 4.454 | 0.333 | 3.801 | 5.108 | 4.397 | 0.306 | 3.797 | 4.997 |
| arget | 4.353 | 0.333 | 3.699 | 5.006 | 4.296 | 0.306 | 3.696 | 4.895 |
| Women | — | — | — | — | 3.564 | 0.304 | 2.969 | 4.160 |
| Men | — | — | — | — | 4.576 | 0.336 | 3.917 | 5.236 |
| Trial 1 | — | — | — | — | 4.174 | 0.256 | 3.673 | 4.676 |
| Trial 2 | — | — | — | — | 4.201 | 0.256 | 3.699 | 4.702 |
| Trial 3 | — | — | — | — | 4.354 | 0.256 | 3.853 | 4.856 |
| Trial 4 | — | — | — | — | 4.073 | 0.256 | 3.571 | 4.575 |
| Trial 5 | — | — | — | — | 3.939 | 0.256 | 3.438 | 4.441 |
| Trial 6 | — | — | — | — | 3.658 | 0.256 | 3.156 | 4.160 |
| Trial 7 | — | — | — | — | 4.093 | 0.256 | 3.591 | 4.595 |
| Final Model: Continuous predictors are fixed at the following values: VASintense = 8.187 | ||||||||
Sig, Significance.
Mental imagery task.
In both the saline and naloxone conditions, participants were able to use pleasant imagery to significantly and robustly reduce pain intensity ratings (saline: comparison stimulus, 4.96 ± 1.7; target stimulus, 2.9 ± 1.5; t = 10.2, p < 0.001; naloxone: comparison stimulus, 5.1 ± 1.8; target, 3.1 ± 1.2; t = 9.9, p < 0.001; Fig. 3A, right). As with pleasantness ratings, imagery-induced regulation of pain intensity was robust (>2 points/30% reduction in pain scores) and significantly stronger than the effect elicited in the relative relief task.
Relative relief task.
Naloxone treatment significantly affected the relative relief-related downregulation of pain intensity. In the saline condition, mean pain intensity ratings decreased from 4.2 ± 1.86 to 3.5 ± 1.80 for the target stimulus (t = −3.6, p < 0.001). For naloxone, no such decrease was observed (saline, 4.3 ± 1.73; naloxone, 4.4 ± 1.98; t = 0.51, p = 0.61).
Comparison of modulations by task.
Contrary to the main hypothesis, we found no significant three-way interaction effect for pain intensity ratings, although the pattern of results was largely consistent with that observed for pleasantness ratings. Successful downregulation of pain intensity was seen for stimuli calibrated to elicit moderate pain during saline in both tasks. This regulation effect was intact after naloxone for the imagery task but not for the relative relief task.
Debriefing data
Post-run ratings
An LMM conducted on ratings of anxiety elicited by each pre-heat pain visual cue (collected at the end of each run) with the fixed factors Drug, Task, StimType, and their interactions, as well as Sex and Session, showed the expected interaction between Task and StimType (F(1,150) = 53.6, p < 0.001), reflecting higher mean stimulus-related anxiety for the comparison stimulus in the mental imagery task (noxious imagery, 2.6; pleasant imagery, 1.4), whereas the target stimulus was associated with higher anxiety in the relative relief task (Intense run moderate, 3.8; Warm run moderate, 1.3). There was no evidence for an interaction with naloxone on these effects (F(1,150) = 0.05, p = 0.8; Fig. 4B). Stimulus-related anxiety was somewhat higher in Session 1 (F(1,150) = 6.4, p = 0.013), but naloxone treatment did not significantly interact with session or other factors to influence these ratings.
The LMM on post-run ratings of relief intensity experienced during presentation of the relative relief cue (with Drug, Session, and Gender as fixed factors) revealed comparable relief across drug conditions (saline, 4.6; naloxone, 5.3; F(1,36) = 1.0, p = 0.32). Ratings of disappointment at the presentation of the comparison cue (upward pointing arrows in the warm run) also showed no significant modulation by drug condition, session, or participant gender (all F values <1.0; p values >0.32).
Analysis of self-reported success of mental imagery during pain revealed somewhat higher ratings for the pleasant compared with the noxious imagery condition (pleasant, 7.3; noxious, 6.8; F(1,74) = 5.5, p = 0.022). Self-reported effort during the imagery task was comparable across stimulus types and drug conditions (pleasant, 4.1; noxious, 4.5). No other factors or interactions significantly influenced self-reported imagery success or effort exerted during the mental imagery task (all F values <1.4; p values >0.24).
Manipulation check
The mean estimated probability of the intense stimulus was 66 ± 9% (i.e., vs 34% moderate) for the Intense run and at 64 ± 12% for the warm stimulus (i.e., vs 36% moderate) in the Warm run. In contrast, participants correctly estimated the probability of each of the conditions in the mental imagery task to 50% (± 6%). The target stimulus occurred in 50% of cases across all three runs. Hence, the deceptive suggestion used to boost the relative relief effect, i.e., that the intense and warm stimuli would be the most frequently occurring stimuli in the respective runs, was seen to influence the estimation of stimulus probability.
Naloxone side effects
Of note, three participants reported intense nausea after the first session and did not return for a second session. They attributed the symptoms to naloxone. Unblinding confirmed that they had indeed received naloxone. These participants were excluded from analysis given the incomplete data. Although the frequency of reported side effects in the study completers (N = 20) was somewhat higher for the naloxone condition, only the reports of nausea occurred significantly more frequently after naloxone (Table 3). When considering the severity of the symptoms (categorized on a numerical scale anchored at “very weak” to “extremely strong”), no symptom was categorized as >5 (strong). The comparison of the scores between groups revealed significantly higher ratings in the naloxone group for nausea and dry mouth (Table 3).
Table 3.
Side-effect reports
| Saline |
Naloxone |
Significance |
||||
|---|---|---|---|---|---|---|
| N | Rating M (SD) | N | Rating M (SD) | N | Ratings | |
| Dry mouth | 7 | 1.35 (0.49) | 9 | 1.90 (1.17) | 0.52 | 0.02* |
| Dry skin | 2 | 1.10 (0.31) | 3 | 1.30 (0.80) | 0.63 | 0.26 |
| Blurred vision | 3 | 1.25 (0.72) | 7 | 1.60 (0.88) | 0.14 | 0.07 |
| Sedation | 10 | 1.85 (0.99) | 14 | 2.35 (1.27) | 0.20 | 0.10 |
| Nausea | 1 | 1.10 (0.45) | 7 | 1.60 (0.94) | 0.02* | 0.02* |
| Dizziness | 9 | 1.75 (0.97) | 11 | 2.10 (1.25) | 0.53 | 0.17 |
| Headache | 6 | 1.45 (0.83) | 8 | 1.60 (0.88) | 0.51 | 0.33 |
Scores on the Bond–Lader scale did not show any significant differences between the naloxone and saline session on any of the four axes (mental sedation, tranquility, physical sedation, sociability; all p values >0.5).
Drug blinding
Despite these differences in side effects, blinding was maintained during the testing sessions. When asked to guess which drug they received immediately after each of the testing sessions, participants were at chance level, i.e., drug attribution was 50% correct.
Discussion
This study used a double-blind, placebo-controlled intravenous naloxone versus saline crossover design to test the opioid-reliance of two novel pain modulation tasks. In the saline condition, both the pleasant imagery (reappraisal) and relative relief (context/expectation) modulations significantly reduced pain intensity ratings and increased the reported pleasantness of a stimulus calibrated as moderately painful. Indeed, both target heat-pain stimuli in the imagery and relative relief tasks were rated as mildly pleasant. Furthermore, in accordance with the main hypothesis, blocking endogenous opioid signaling with naloxone did not significantly affect imagery-induced regulation of pain intensity or unpleasantness. Yet, as indicated by a significant three-way interaction between task, stimulus type, and drug, naloxone blocked the relative relief-induced pain modulation. Whereas prior naloxone studies have investigated pain modulations, a strength of the current approach lies in contrasting two novel techniques with an a priori hypothesis of opioid reliance versus independence.
In the saline condition, we replicate our previous finding that in a relative relief task, heat stimuli calibrated to induce moderate pain can elicit a positive affect (Leknes et al., 2013a) and show a corresponding decrease in pain intensity ratings. The mean improvement across these VASs was smaller than in our original paper, possibly reflecting minor differences in design and measurements. Self-reported relative relief-related effects have been supported by altered ventromedial prefrontal activity (Leknes et al., 2013a; Winston et al., 2014), and by monetary worth of avoiding moderately painful stimuli in the context of intense pain (compared with a low-pain context; Winston et al., 2014). The relative relief modulation relies on fully predictive visual cues informing participants that the stimulus will be less hot. In the absence of such cues, moderate stimuli are often perceived as more painful in a context of intense pain (Koyama et al., 2005; Keltner et al., 2006; Atlas et al., 2010). Perception of noisy or ambiguous stimuli such as experimental heat is heavily guided by the brain's prediction models (Johnston et al., 2012; Büchel et al., 2014; Tabor et al., 2017) and priors (Wiech et al., 2014b). For instance, expecting pain will increase the likelihood of interpreting an ambiguous sensory stimulus as painful (Taylor et al., 2017). Here, debriefing indeed confirmed that participants believed that the most likely outcome of the Intense run was intense pain. Hence, a fully predictive, unambiguous cue indicating the occurrence of a lower temperature stimulus interrupted the expectation of intense pain, updating the prediction about the actual stimulus.
Surprise enhances emotions (Mellers and Ritov, 2010). Inspired by our prior finding that dispositional pessimists reported more relief in response to an unambiguous, fully predictive relief cue (Leknes et al., 2011), we speculate that the relative relief effect relies on how predictive (i.e., certain) the intense pain and relative relief cues are perceived to be. Because the majority of placebo-induced expectation studies point to a major role for the μ-opioid system, and due to our prior finding that the relative relief manipulation increased positive affect and PAG activity, we hypothesized that the relative relief effect would rely on endogenous opioid signaling. The present observations that naloxone blocked the relative relief improvement in pain pleasantness and intensity ratings confirmed this hypothesis.
In the mental imagery task, participants successfully increased the perceived pleasantness and decreased the pain intensity of the stimulus compared with the control task, independently of naloxone treatment. The task was based on mental imagery used in clinical pain management (Winterowd et al., 2003; Pincus and Sheikh, 2009; Posadzki and Ernst, 2011; Posadzki et al., 2012). Notably, the task induced a large and clinically significant modulation (i.e., >30% reduction of pain intensity ratings; Farrar et al., 2001) of heat pain in healthy volunteers, regardless of drug condition. The modulation of pain pleasantness was equally robust and naloxone-independent, with a shift in perception into the positive affective domain. Participants quickly mastered the task, and reported exerting only moderate effort during both mental imagery conditions.
Our results showing the opioid independence of mental imagery-induced pain relief confirm prior untested hypotheses that such pain modulation may not rely on the descending inhibitory system. Ruscheweyh et al. (2011) reported that positive imagery left the RIII reflex (a measure of spinal nociception) unaltered. The neural underpinnings of mental imagery are little known (Jensen et al., 2012; Fardo et al., 2015). A likely underlying psychological mechanism is reappraisal. Reappraisal involves giving a new interpretation to an emotional or painful stimulus (Wiech et al., 2008b). Reappraisal is proposed to be driven by prefrontal cognitive control regions, followed by lateral temporal cortex activation and subsequent amygdala inhibition (Buhle et al., 2014). Repeated associations of a noxious input with a more positive meaning could also facilitate new associative learning. A heightened sense of control could also be at play, underpinned by increased ventrolateral prefrontal cortex and nucleus accumbens activity (Wiech et al., 2006, 2014a) and decreased amygdala activation (Salomons et al., 2015). Furthermore, positive imagery could raise other emotional processes such as enhanced mood, a more positive bias, or increased appetite for rewards (Linke and Wessa, 2017). At the neurochemical level, initial findings point to endocannabinoid signaling as the driving factor of both opioid-independent analgesia (Benedetti et al., 2011) and long-term associative learning (Schafer et al., 2018). Another study highlighted the contribution of endocannabinoids to reward processing in the ventral striatum (Horder et al., 2010). We therefore speculate that mental imagery analgesia may rely on activity in prefrontal-accumbens/amygdala circuits and could be endocannabinoid-dependent.
The present findings add to the literature on opioid-dependent and opioid-independent pain modulations. With the exception of placebo analgesia, which is reduced by naloxone and naltrexone in most published studies (Amanzio and Benedetti, 1999; Benedetti et al., 1999, 2007; Eippert et al., 2009; Rütgen et al., 2015, 2017), knowledge on the opioid dependence of pain modulations is limited by a lack of replication studies and inconsistent drug administration methods (Werner et al., 2015). In light of mounting evidence of endogenous opioid involvement in reward processing in rodents (Cooper and Turkish, 1989; Mahler and Berridge, 2009; Berridge and Kringelbach, 2015) and humans (Chelnokova et al., 2016; Eikemo et al., 2016, 2017; Weber et al., 2016), it is noteworthy that with the help of mental imagery, participants reported large shifts in their affective evaluation of pain (into the reward domain) even with their μ-opioid receptors blocked. Previous studies of the opioid reliance of reward-related analgesia in humans provided mixed results (Kut et al., 2011; Benedetti et al., 2013). Together, these findings may indicate that factors other than the affective value, such as expectations or the degree of voluntary regulation, could determine how much a pain modulation depend on opioid signaling.
Some potential limitations warrant consideration. Consistent with prior research, naloxone treatment at a sufficient dose for complete blockade of μ-opioid receptors (Mayberg and Frost, 1990) did not cause significant mood changes. Indeed, despite fewer side effects in the saline condition, drug blinding was full at the end of each experimental session. Three-way interaction tests assessed the main hypothesis of the study, i.e., that analgesia in the relative relief but not mental imagery task would be blocked by naloxone. Although previous studies using naloxone to block placebo analgesia indicated large effect sizes (Levine et al., 1978; Amanzio and Benedetti, 1999), our within-subjects sample size of 20 people limits the generalizability of the results. Indeed, while the pattern of results was consistent with the main hypothesis for both pleasantness and pain intensity ratings, the hypothesized interaction was statistically supported only for pleasantness ratings. Future studies should replicate, in larger samples, these and other key findings in the literature on the opioid dependence of pain modulations.
The pain modulation by mental imagery was significantly larger than by relative relief, consistent with prior findings of voluntary reappraisal profoundly altering responses to affective stimuli (Moret et al., 1991; Wager et al., 2008; Woo et al., 2015). The pain intensity and unpleasantness ratings of the comparison stimulus were significantly higher in the mental imagery than the relative relief task. This difference could be due to hyperalgesia induced by the noxious comparison imagery (Fardo et al., 2015), and/or to hypoalgesia given the reduced likelihood of pain in the Warm run (Taylor et al., 2017). Indeed, unexpectedly the relative relief task comparison stimulus was not consistently rated as unpleasant, despite a mean pain intensity score of 4/10. The moderate heat stimuli were delivered through two thermodes in the mental imagery task, compared with one in the relative relief task. This could also play a role. The contribution of these factors cannot be disentangled here, but have little bearing on the main results of the study as the target stimulus of each task had its own comparator.
In conclusion, this comparison of the effects of naloxone on two reward-oriented pain modulations suggests that relative relief is an opioid-dependent process, whereas mental imagery is opioid-independent. Mental imagery provides a useful therapeutic approach in the chronic pain state. Implemented here to modulate acute experimental pain, it had powerful effects and was easy to instruct to participants. Mental imagery might ultimately favor positive appraisals (Linke and Wessa, 2017), which are central to resilience (Kalisch et al., 2015). Clinically, tapping into opioid-independent pain modulatory mechanisms is very relevant as a complement to pharmacological therapy. It could be especially interesting in patients treated with long term opioids, as they might have alterations in emotion regulation processes (Garland et al., 2017a,b), or those where the opioidergic function may be disrupted, whether due to mood disorders (Hsu et al., 2015) or chronic pain itself (Harris et al., 2007; Martikainen et al., 2013).
Footnotes
This work was supported by a “Pépinière” Grant from the University of Lausanne Faculty of Biology and Medicine to C.B.; the Wellcome Trust and United Kingdom's Medical Research Council to I.T; and G.M.G. is a NIHR Senior Investigator, holds a Grant from Wellcome Trust, holds shares in P1vital. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
C.B., S.L., A.H.A., R.N.M., and I.T. declare no competing financial interests.
References
- Amanzio M, Benedetti F (1999) Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci 19:484–494. 10.1523/JNEUROSCI.19-01-00484.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD (2010) Brain mediators of predictive cue effects on perceived pain. J Neurosci 30:12964–12977. 10.1523/JNEUROSCI.0057-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Arduino C, Amanzio M (1999) Somatotopic activation of opioid systems by target-directed expectations of analgesia. J Neurosci 19:3639–3648. 10.1523/JNEUROSCI.19-09-03639.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Pollo A, Colloca L (2007) Opioid-mediated placebo responses boost pain endurance and physical performance: is it doping in sport competitions? J Neurosci 27:11934–11939. 10.1523/JNEUROSCI.3330-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M, Rosato R, Blanchard C (2011) Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nat Med 17:1228–1230. 10.1038/nm.2435 [DOI] [PubMed] [Google Scholar]
- Benedetti F, Thoen W, Blanchard C, Vighetti S, Arduino C (2013) Pain as a reward: changing the meaning of pain from negative to positive co-activates opioid and cannabinoid systems. Pain 154:361–367. 10.1016/j.pain.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Berna C, Tracey I, Holmes EA (2012) How a better understanding of spontaneous mental imagery linked to pain could enhance imagery-based therapy in chronic pain. J Exp Psychopathol 3:258–273. 10.5127/jep.017911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML (2015) Pleasure systems in the brain. Neuron 86:646–664. 10.1016/j.neuron.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Geuter S, Sprenger C, Eippert F (2014) Placebo analgesia: a predictive coding perspective. Neuron 81:1223–1239. 10.1016/j.neuron.2014.02.042 [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN (2014) Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex 24:2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL (1994) Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol 67:319–333. 10.1037/0022-3514.67.2.319 [DOI] [Google Scholar]
- Chelnokova O, Laeng B, Løseth G, Eikemo M, Willoch F, Leknes S (2016) The μ-opioid system promotes visual attention to faces and eyes. Soc Cogn Affect Neurosci 11:1902–1909. 10.1093/scan/nsw116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SJ, Turkish S (1989) Effects of naltrexone on food preference and concurrent behavioral responses in food-deprived rats. Pharmacol Biochem Behav 33:17–20. 10.1016/0091-3057(89)90422-X [DOI] [PubMed] [Google Scholar]
- Eikemo M, Løseth GE, Johnstone T, Gjerstad J, Willoch F, Leknes S (2016) Sweet taste pleasantness is modulated by morphine and naltrexone. Psychopharmacology (Berl) 233:3711–3723. 10.1007/s00213-016-4403-x [DOI] [PubMed] [Google Scholar]
- Eikemo M, Biele G, Willoch F, Thomsen L, Leknes S (2017) Opioid modulation of value-based decision-making in healthy humans. Neuropsychopharmacology 42:1833–1840. 10.1038/npp.2017.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C (2009) Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63:533–543. 10.1016/j.neuron.2009.07.014 [DOI] [PubMed] [Google Scholar]
- Elmholdt EM, Skewes J, Dietz M, Møller A, Jensen MS, Roepstorff A, Wiech K, Jensen TS (2017) Reduced pain sensation and reduced BOLD signal in parietofrontal networks during religious prayer. Front Hum Neurosci 11:337. 10.3389/fnhum.2017.00337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck S, Eysenck H, Barrett P (1985) A revised version of the psychoticism scale. Pers Individ Dif 6:21–29. 10.1016/0191-8869(85)90026-1 [DOI] [Google Scholar]
- Fardo F, Allen M, Jegindø EM, Angrilli A, Roepstorff A (2015) Neurocognitive evidence for mental imagery-driven hypoalgesic and hyperalgesic pain regulation. Neuroimage 120:350–361. 10.1016/j.neuroimage.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM (2001) Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94:149–158. 10.1016/S0304-3959(01)00349-9 [DOI] [PubMed] [Google Scholar]
- Fors EA, Sexton H, Götestam KG (2002) The effect of guided imagery and amitriptyline on daily fibromyalgia pain: a prospective, randomized, controlled trial. J Psychiatr Res 36:179–187. 10.1016/S0022-3956(02)00003-1 [DOI] [PubMed] [Google Scholar]
- Garland EL, Bryan CJ, Nakamura Y, Froeliger B, Howard MO (2017a) Deficits in autonomic indices of emotion regulation and reward processing associated with prescription opioid use and misuse. Psychopharmacology (Berl) 234:621–629. 10.1007/s00213-016-4494-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Bryan CJ, Finan PH, Thomas EA, Priddy SE, Riquino MR, Howard MO (2017b) Pain, hedonic regulation, and opioid misuse: modulation of momentary experience by mindfulness-oriented recovery enhancement in opioid-treated chronic pain patients. Drug Alcohol Depend 173:S65–S72. 10.1016/j.drugalcdep.2016.07.033 [DOI] [PubMed] [Google Scholar]
- Gueorguieva R, Krystal JH (2004) Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the archives of general psychiatry. Arch Gen Psychiatry 61:310–317. 10.1001/archpsyc.61.3.310 [DOI] [PubMed] [Google Scholar]
- Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK (2007) Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci 27:10000–10006. 10.1523/JNEUROSCI.2849-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg PY, Glaesmer H, Hoyer J (2006) Separating optimism and pessimism: a robust psychometric analysis of the revised life orientation test (LOT-R). Psychol Assess 18:433–438. 10.1037/1040-3590.18.4.433 [DOI] [PubMed] [Google Scholar]
- Horder J, Harmer CJ, Cowen PJ, McCabe C (2010) Reduced neural response to reward following 7 days treatment with the cannabinoid CB1 antagonist rimonabant in healthy volunteers. Int J Neuropsychopharmacol 13:1103–1113. 10.1017/S1461145710000453 [DOI] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Walker SJ, Mickey BJ, Koeppe RA, Langenecker SA, Zubieta JK (2015) It still hurts: altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol Psychiatry 20:193–200. 10.1038/mp.2014.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Berna C, Loggia ML, Wasan AD, Edwards RR, Gollub RL (2012) The use of functional neuroimaging to evaluate psychological and other non-pharmacological treatments for clinical pain. Neurosci Lett 520:156–164. 10.1016/j.neulet.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston NE, Atlas LY, Wager TD (2012) Opposing effects of expectancy and somatic focus on pain. PLoS One 7:e38854. 10.1371/journal.pone.0038854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ (2007) Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci 27:8877–8884. 10.1523/JNEUROSCI.2063-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Müller MB, Tüscher O (2015) A conceptual framework for the neurobiological study of resilience. Behav Brain Sci 38:e92. 10.1017/S0140525X1400082X [DOI] [PubMed] [Google Scholar]
- Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL (2006) Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J Neurosci 26:4437–4443. 10.1523/JNEUROSCI.4463-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koban L, Jepma M, Geuter S, Wager TD (2017) What's in a word? how instructions, suggestions, and social information change pain and emotion. Neurosci Biobehav Rev 81:29–42. 10.1016/j.neubiorev.2017.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ, Coghill RC (2005) The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci U S A 102:12950–12955. 10.1073/pnas.0408576102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kut E, Candia V, von Overbeck J, Pok J, Fink D, Folkers G (2011) Pleasure-related analgesia activates opioid-insensitive circuits. J Neurosci 31:4148–4153. 10.1523/JNEUROSCI.3736-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Lee M, Berna C, Andersson J, Tracey I (2011) Relief as a reward: hedonic and neural responses to safety from pain. PLoS One 6:e17870. 10.1371/journal.pone.0017870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Berna C, Lee MC, Snyder GD, Biele G, Tracey I (2013a) The importance of context: when relative relief renders pain pleasant. Pain 154:402–410. 10.1016/j.pain.2012.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Berna C, Lee MC, Snyder GD, Biele G, Tracey I (2013b) Response to the commentary “multiple potential mechanisms for context effects on pain”. Pain 154:1485–1486. 10.1016/j.pain.2013.04.002 [DOI] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, Fields HL (1978) The mechanism of placebo analgesia. Lancet 2:654–657. 10.1016/S0140-6736(78)92762-9 [DOI] [PubMed] [Google Scholar]
- Linke J, Wessa M (2017) Mental imagery training increases wanting of rewards and reward sensitivity and reduces depressive symptoms. Behav Ther 48:695–706. 10.1016/j.beth.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC (2009) Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci 29:6500–6513. 10.1523/JNEUROSCI.3875-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold D, McCaul ME, Ali M, Wand GS (2000) Plasma adrenocorticotropin responses to opioid blockade with naloxone: generating a dose-response curve in a single session. Biol Psychiatry 48:310–314. 10.1016/S0006-3223(00)00885-4 [DOI] [PubMed] [Google Scholar]
- Martikainen IK, Peciña M, Love TM, Nuechterlein EB, Cummiford CM, Green CR, Harris RE, Stohler CS, Zubieta JK (2013) Alterations in endogenous opioid functional measures in chronic back pain. J Neurosci 33:14729–14737. 10.1523/JNEUROSCI.1400-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Frost JJ (1990) Opiate receptors. In: Quantitative imaging: neuroreceptors, neurotransmitters, and enzymes, pp 81–95. New York: Raven. [Google Scholar]
- Mellers BA, Ritov I (2010) How beliefs influence the relative magnitude of pleasure and pain. J Behav Decis Mak 23:369–382. 10.1002/bdm.662 [DOI] [Google Scholar]
- Moret V, Forster A, Laverrière MC, Lambert H, Gaillard RC, Bourgeois P, Haynal A, Gemperle M, Buchser E (1991) Mechanism of analgesia induced by hypnosis and acupuncture: is there a difference? Pain 45:135–140. 10.1016/0304-3959(91)90178-Z [DOI] [PubMed] [Google Scholar]
- Nelis S, Holmes EA, Griffith JW, Raes F (2014) Mental imagery during daily life: psychometric evaluation of the spontaneous use of imagery scale (SUIS). Psychologica Belgica 54:19–32. 10.5334/pb.ag [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC (2008) A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 13:829, 833–857. 10.1038/mp.2008.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus D, Sheikh AA (2009) Imagery for pain relief: a scientifically grounded guidebook for clinicians. New York: Routledge. [Google Scholar]
- Posadzki P, Ernst E (2011) Guided imagery for musculoskeletal pain: a systematic review. Clin J Pain 27:648–653. 10.1097/AJP.0b013e31821124a5 [DOI] [PubMed] [Google Scholar]
- Posadzki P, Lewandowski W, Terry R, Ernst E, Stearns A (2012) Guided imagery for non-musculoskeletal pain: a systematic review of randomized clinical trials. J Pain Symptom Manage 44:95–104. 10.1016/j.jpainsymman.2011.07.014 [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Kreusch A, Albers C, Sommer J, Marziniak M (2011) The effect of distraction strategies on pain perception and the nociceptive flexor reflex (RIII reflex). Pain 152:2662–2671. 10.1016/j.pain.2011.08.016 [DOI] [PubMed] [Google Scholar]
- Rütgen M, Seidel EM, Silani G, Riečanský I, Hummer A, Windischberger C, Petrovic P, Lamm C (2015) Placebo analgesia and its opioidergic regulation suggest that empathy for pain is grounded in self pain. Proc Natl Acad Sci U S A 112:E5638–5646. 10.1073/pnas.1511269112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rütgen M, Seidel EM, Pletti C, Riečanský I, Gartus A, Eisenegger C, Lamm C (2018) Psychopharmacological modulation of event-related potentials suggests that first-hand pain and empathy for pain rely on similar opioidergic processes. Neuropsychologia 116:5–14. 10.1016/j.neuropsychologia.2017.04.023 [DOI] [PubMed] [Google Scholar]
- Salomons TV, Nusslock R, Detloff A, Johnstone T, Davidson RJ (2015) Neural emotion regulation circuitry underlying anxiolytic effects of perceived control over pain. J Cogn Neurosci 27:222–233. 10.1162/jocn_a_00702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer SM, Geuter S, Wager TD (2018) Mechanisms of placebo analgesia: a dual-process model informed by insights from cross-species comparisons. Prog Neurobiol 160:101–122. 10.1016/j.pneurobio.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoell ED, Bingel U, Eippert F, Yacubian J, Christiansen K, Andresen H, May A, Buechel C (2010) The effect of opioid receptor blockade on the neural processing of thermal stimuli. PLoS One 5:e12344. 10.1371/journal.pone.0012344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrimpf M, Liegl G, Boeckle M, Leitner A, Geisler P, Pieh C (2015) The effect of sleep deprivation on pain perception in healthy subjects: a meta-analysis. Sleep Med 16:1313–1320. 10.1016/j.sleep.2015.07.022 [DOI] [PubMed] [Google Scholar]
- Spielberger CD. (1983) Manual for the state-trait anxiety inventory STAI (Form Y). Palo Alto, CA: Mind Garden. [Google Scholar]
- Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Büchel C (2012) Attention modulates spinal cord responses to pain. Curr Biol 22:1019–1022. 10.1016/j.cub.2012.04.006 [DOI] [PubMed] [Google Scholar]
- Syrjala KL, Donaldson GW, Davis MW, Kippes ME, Carr JE (1995) Relaxation and imagery and cognitive-behavioral training reduce pain during cancer treatment: a controlled clinical trial. Pain 63:189–198. 10.1016/0304-3959(95)00039-U [DOI] [PubMed] [Google Scholar]
- Tabor A, Thacker MA, Moseley GL, Körding KP (2017) Pain: a statistical account. PLoS Comput Biol 13:e1005142. 10.1371/journal.pcbi.1005142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VA, Chang L, Rainville P, Roy M (2017) Learned expectations and uncertainty facilitate pain during classical conditioning. Pain 158:1528–1537. 10.1097/j.pain.0000000000000948 [DOI] [PubMed] [Google Scholar]
- Tracey I. (2010) Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med 16:1277–1283. 10.1038/nm.2229 [DOI] [PubMed] [Google Scholar]
- Tracey I, Mantyh PW (2007) The cerebral signature for pain perception and its modulation. Neuron 55:377–391. 10.1016/j.neuron.2007.07.012 [DOI] [PubMed] [Google Scholar]
- Vase L, Robinson ME, Verne GN, Price DD (2005) Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain 115:338–347. 10.1016/j.pain.2005.03.014 [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN (2008) Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59:1037–1050. 10.1016/j.neuron.2008.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber SC, Beck-Schimmer B, Kajdi ME, Müller D, Tobler PN, Quednow BB (2016) Dopamine D2/3- and mu-opioid receptor antagonists reduce cue-induced responding and reward impulsivity in humans. Transl Psychiatry 6:e850. 10.1038/tp.2016.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner MU, Pereira MP, Andersen LP, Dahl JB (2015) Endogenous opioid antagonism in physiological experimental pain models: a systematic review. PLoS One 10:e0125887. 10.1371/journal.pone.0125887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K, Kalisch R, Weiskopf N, Pleger B, Stephan KE, Dolan RJ (2006) Anterolateral prefrontal cortex mediates the analgesic effect of expected and perceived control over pain. J Neurosci 26:11501–11509. 10.1523/JNEUROSCI.2568-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K, Ploner M, Tracey I (2008a) Neurocognitive aspects of pain perception. Trends Cogn Sci 12:306–313. 10.1016/j.tics.2008.05.005 [DOI] [PubMed] [Google Scholar]
- Wiech K, Farias M, Kahane G, Shackel N, Tiede W, Tracey I (2008b) An fMRI study measuring analgesia enhanced by religion as a belief system. Pain 139:467–476. 10.1016/j.pain.2008.07.030 [DOI] [PubMed] [Google Scholar]
- Wiech K, Edwards R, Moseley GL, Berna C, Ploner M, Tracey I (2014a) Dissociable neural mechanisms underlying the modulation of pain and anxiety? An FMRI pilot study. PLoS One 9:e110654. 10.1371/journal.pone.0110654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K, Vandekerckhove J, Zaman J, Tuerlinckx F, Vlaeyen JW, Tracey I (2014b) Influence of prior information on pain involves biased perceptual decision-making. Curr Biol 24:R679–R681. 10.1016/j.cub.2014.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, Vlaev I, Seymour B, Chater N, Dolan RJ (2014) Relative valuation of pain in human orbitofrontal cortex. J Neurosci 34:14526–14535. 10.1523/JNEUROSCI.1706-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterowd C, Beck AT, Gruener D (2003) Eliciting and modifying imagery. In: Cognitive therapy with chronic pain patients, pp 183–207. New York: Springer. [Google Scholar]
- Woo CW, Roy M, Buhle JT, Wager TD (2015) Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS Biol 13:e1002036. 10.1371/journal.pbio.1002036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F, Adler-Neal AL, Wells RE, Stagnaro E, May LM, Eisenach JC, McHaffie JG, Coghill RC (2016) Mindfulness-meditation-based pain relief is not mediated by endogenous opioids. J Neurosci 36:3391–3397. 10.1523/JNEUROSCI.4328-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]