Abstract
Objective:
To assess the cost effectiveness of newborn screening for congenital adrenal hyperplasia (CAH) in the U.S. newborn population.
Methods:
We constructed a decision model to estimate the incremental cost-effectiveness ratio (ICER) of CAH screening compared to a strategy of no screening. Two types of cost effectiveness analyses (CEA) were con-ducted to measure ICER as net cost per life year (LY): (1) traditional CEA with sensitivity and scenario analyses, and (2) probabilistic CEA.
Results:
ICERs for (1) base-case analysis in traditional CEA and (2) probabilistic CEA were USD 292,000 and USD 255,700 per LY saved in 2005 USD, respectively. ICERs were particularly sensitive to assumptions regarding the mortality rate for the salt wasting type of CAH, in a range from 2 to 9%. The ICERs for best-case and worst-case scenarios were USD 30,900 and USD 2.9 million per LY saved, respectively.
Conclusions:
Using common benchmarks for cost effectiveness, our results indicate that CAH screening would be unlikely to be considered cost effective unless assumptions favorable to screening were adopted, although it could meet economic criteria used to assess U.S. regulatory policies. A limitation is that the analysis excludes outcomes such as correct assignment of gender and quality of life.
Keywords: Congenital adrenal hyperplasia, Cost effectiveness, Newborn screening
Congenital adrenal hyperplasia (CAH) is a family of autosomal recessive disorders that affect endocrine function. Classical CAH is divided into salt wasting (SW) and simple virilizing (SV) subgroups. Female infants in both subgroups are born with ambiguous genitalia; the distinction is that infants with SW-CAH of both sexes are at risk of life-threatening adrenal crisis in the absence of early treatment [1]. The reported prevalence of classical CAH is 1 in 18,000 U.S. infants [2]. Of the 2 types of classical CAH, the proportions of SW and SV are approximately 65% and 35%, respectively [2]. Benefits of early identification of SW-CAH by newborn screening include reduced risk of death due to salt loss crises and reduced hospitalization [3]. Benefits of early identification for SV cases are correct sex assignment and the opportunity for surgical reconstruction of genitalia [3].
Screening for CAH is now almost universal in the U.S., following its inclusion in a core panel of 29 disorders recommended by an expert group of the American College of Medical Genetics in 2005 [4]. As of December 2007, CAH screening was implemented in 47 U.S. states [5]. Elsewhere, practice is mixed, with 12 European countries having adopted it as of 2004 [6].
The major goal of our study is to assess the cost effectiveness of CAH screening in terms of the prevention of early childhood death with a set of conservative, evidence-based assumptions. The cost effectiveness of screening for CAH is not well established [7], although costs are comparable to other newborn screening tests [8, 9]. A recent study by Carroll and Downs [9] estimated that the incremental cost-effectiveness ratio (ICER) of screening for CAH is USD 20,000 per quality adjusted life year (QALY) in 2004 USD, but this study used assumptions which tended to favor screening. Also, because no impact of CAH on quality of life was assumed, Carroll and Downs did not actually calculate QALYs, only life years (LYs) that were reported as QALYs. Insufficient information exists to assess improvements in quality of life from early detection of classical CAH or to quantify benefits of early detection of other forms of CAH in a cost effectiveness analysis (CEA) [7].
Methods
We conducted a CEA to compare 2 strategies, universal CAH screening and clinical recognition without screening, among the U.S. newborn population. Our analysis was implemented from the health care system perspective, which includes all health care costs regardless of payers but does not include opportunity costs experienced by families. The outcome variable is the ICER, which is the ratio of net health care costs per LY saved. Our analyses assumed that each infant death avoided would save 32.0 LYs, applying the 3% discount rate to 77.8 years of life expectancy at birth in the 2004 U.S. life table [10]. All analyses were conducted using the decision analysis software TreeAge Pro Health Care Module 2007.
Our analyses assume that screening for CAH would be based on collection of one routine specimen from each child. Nine states have a policy of routinely requesting a second specimen from each child for testing. Testing of second specimens has been shown to detect additional cases of SV-CAH, but not SW-CAH [11]. Because only detection of cases of SW-CAH is projected to yield benefits in screening for CEA, a policy of testing of second specimens for CAH would double costs of screening with no added benefits, making screening appear less cost effective.
Table 1 lists all parameters, including point estimates and ranges, for the CEA models. All costs were adjusted to year 2005 USD. Cost estimates were derived from a survey of 6 screening laboratories [8] and a detailed study conducted in Texas [12]. Specifically, the laboratory cost of screening one specimen was assumed to be in the range of USD 2.30–6.00 [8, 12], with a mid-point estimate of USD 4.15. The Employment Cost Index of the U.S. Bureau of Labor Statistics was used to adjust cost estimates from different years to 2005 USD, because screening costs were assumed to mainly consist of labor costs. Each screen positive infant was assumed to be associated with follow-up medical cost ranging from USD 130 (personal communication, William Young, May 6, 2008) to USD 637 [12], with a point estimate of USD 216 being a harmonic mean of the range.
Table 1.
Assumption in modelling newborn screening for CAH
| Parameter definition (notation in fig. 1) | Point estimatea | Range | Reference |
|---|---|---|---|
| Incidence of CAH | 13 | ||
| SW and SV combined (incidence) | 1 in 17,800 | 1 in 25,000, | |
| 1 in 12,000 | |||
| Proportion of SW among SW and SV (prop_sw) | 0.65 | ||
| Sensitivity of early clinical recognition | 11, 12, 14 | ||
| SW (sen_cl_sw) | 55% | 45–65% | |
| SV (sen_cl_sv) | 45% | 15–75% | |
| Screen false positive rate (false_pos) | 0.5% | 0.1–1.0% | 13 |
| Sensitivity of detection with screening | 11 | ||
| SW (sen_sw) | 1.0 | ||
| SV (sen_sv) | 0.40 | 0.3–0.9 | |
| Cost of screening per one specimen (c_test), USD | 4.15 | 2.30–6.00 | 8, 12 |
| Cost of follow-up and confirmatory test per screen positive (c_follow), USD | 216 | 130–637 | 12 |
| Reduced cost of hospitalization per male SW case (c_hosp), USD | 6,000 | 12, 17 | |
| SW mortality without screening (mr_sw) | 4.2% | 2.0–9.0% | 7, 16, 18, 26 |
| Reduction in SW mortality with screening (mr_reduce) | 80% | 74–86% |
Also used as a mode in a triangle distribution in probabilistic analysis. All cost values were in 2005 USD.
CAH = Congenital adrenal hyperplasia; SW = salt wasting; SV = simple virilizing.
The average cost of screening per child screened is the sum of the laboratory screening cost and the product of average follow-up costs and the probability that a specimen tests positive. During 2004–2006, an average of 0.5% of infants screened for CAH tested positive and required follow-up [13]. The sensitivity of screening for SW-CAH is assumed to be 100%, but based on data from the Texas program, which collects 2 specimens, the sensitivity of detection of SV-CAH from one specimen may only be 40% [11].
The alternative to newborn screening is reliance on early clinical recognition of CAH. The sensitivity of this strategy is the probability of a case being detected clinically based on symptoms or family history prior to the reporting of screening results. Based on the literature [11, 12, 14], the sensitivity of early clinical recognition for SW-CAH and SV-CAH is assumed to be 55% and 45%, respectively. We additionally assume that the specificity of clinical recognition is unity, i.e., no false positives.
The risk of death in SW-CAH in the absence of screening is not well-established. A previous CEA that relied on an expert opinion assumed that death would occur in 10% of all cases of classical CAH, or 13% of SW cases [9]. A recent review [15] cited a Hungarian study [16] as reporting an 11.3% mortality rate in SW-CAH, but that was based on historical data without reliable treatment; the mortality rate was 4.5% for the most recent period, 1983–1998 [16]. A systematic evidence review of mortality in un-screened CAH was conducted for the purpose of informing the present CEA [7]. Among population-based surveillance studies that compared deaths due to recognized CAH in screened and unscreened cohorts in high-income countries with similar prevalence rates of SW-CAH [17–19], the highest reported mortality rate was in a Swedish cohort with 2.2%. This is likely to be an underestimate because of deaths among infants with undiagnosed CAH. One study from Austria and the Czech Republic tested stored specimens for deaths attributed to sudden or unexplained causes and found 3 cases of CAH, equivalent to approximately 2% of infants born with SW-CAH in the 2 countries during the period of time covered by the study [20, 21]. The investigators did not report the number of deaths among children diagnosed with CAH and there could have been other deaths attributed to causes other than the true diagnosis of CAH. We took the sum of the two percentages, 4.2%, as a point estimate for the probabilistic and base-case analyses, with a range from 2 to 9%.
Similarly, the effectiveness of screening for CAH is not well-established, although it is known that screening does not prevent all deaths due to SW-CAH. A previous CEA cited an expert opinion for an assumption of 80% effectiveness in reducing mortality [9]. Published evidence suggests that effectiveness is likely to be higher. In a cohort of 88 infants with SW-CAH in Texas born over a 6-year period, one (1.1%) died prior to confirmation of an abnormal screening result [11]. However, no infant deaths were recorded among 56 Swedish and 13 Dutch infants with SW-CAH detected by newborn screening [14, 17], which means that the total death rate in published follow-up studies of screened cohorts with SW-CAH is 0.6%. Relative to the assumed 4.2% death rate in the absence of screening, 1.1% mortality in a screened cohort would imply a 74% reduction in deaths and 0.6% would mean an 86% reduction. We assumed an effectiveness of 80%, ranging be-tween 74% and 86%.
Two different approaches were used to take into account the inherent uncertainty in the values of various assumptions. First, we conducted a traditional CEA, with a base-case analysis using the point estimates in column 2 of table 1 and one-way sensitivity analyses to examine how the ICER is influenced by the range of each parameter. In addition, ‘best-case’ and ‘worst-case’ scenario analyses selected the most and least favorable values for each parameter, respectively. The most favorable values are exemplified by highest incidence, highest screening sensitivity and specificity, lowest screening cost, and highest reduction in mortality. These sets of assumptions for best/worst case scenarios are not considered to be likely. Second, we implemented a probabilistic CEA to capture the full range of available information using a triangular distribution for all parameters. The modal or most likely value for each parameter is the point estimate in the base-case analysis, using the ranges in column 3 to define the extremes of each distribution.
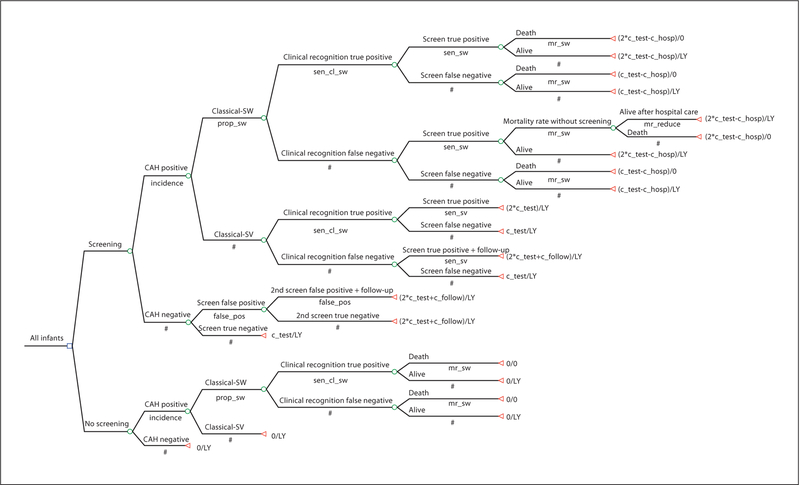
Figure 1 presents our decision model. The terminal nodes include the incremental cost, compared to the strategy of no screening and LY as effectiveness. Under the strategy of CAH screening, every infant has the incremental cost of a first CAH screening cost (point estimate of USD 4.15). We added the cost of a second screening test to all positive screens. The combined costs of follow-up medical care and the confirmatory diagnosis test (point estimate of USD 216) were assigned only to screen-positive infants who had not already presented clinically with CAH. These costs were not assigned for CAH infants who were recognized clinically because it was assumed that these children would incur the costs of follow-up medical care and confirmatory diagnosis regardless of screening results.
Fig. 1.
Decision tree.
Only short-term medical costs associated with CAH are modeled, and hence there was no discounting of costs. We assumed that other long-term treatment costs would be equivalent between screened and unscreened children. The Dutch cohort study cited above reported that the average length of hospitalization in males was reduced from 13 to 7 days with screening [17]. Assuming that the cost of an inpatient day for a neonate is constant, the reduced hospitalization cost would be USD 6,000 based on an estimate from Texas [12] adjusted by the hospital provider price index to year 2005 USD. This reduced hospitalization cost is applied to male SW cases only, assuming the proportion of males is 50% as in the Dutch study [17].
For infants who would have died without screening but survived with screening, the difference in lifetime costs of care would be legitimate to include in the model. The problem is that we do not know the sign let alone the magnitude of the net cost. If the averted cost of a hospitalization associated with an infant death is lower than the long-term CAH treatment cost, the cost-effectiveness ratio would turn out to be less favorable for this screening. The relevant long-term treatment cost includes steroid therapy and, in the case of female infants, genital surgery. We followed the standard approach of not including long-term costs unrelated to CAH among infants who survive as a result of earlier detection [22]. Meltzer argued for the inclusion of ‘future costs’ [23], while the U.S. Panel on Cost-Effectiveness in Health and Medicine did not recommend their inclusion [22].
Results
The strategy of CAH screening is estimated to result in an incremental cost of approximately USD 9.3 million per infant death averted in 2005 USD compared to the alternative strategy of no screening. Dividing that figure by 32.0 discounted LY yields an ICER in the base-case analysis of USD 292,000 per LY saved in 2005 USD, as presented in the first row of table 2. With a set of assumptions favoring CAH screening, the best-case analysis yielded an ICER of USD 30,900, less than one ninth of the base-case ICER. The worst-case analysis showed an ICER of USD 2,866,200. The ICER of the probabilistic CEA was USD 255,700.
Table 2.
CEA for newborn screening for CAH: year 2005, discount rate 3%
| Range | ICER (USD/life year saved) | |
|---|---|---|
| Traditional CEA | ||
| Base-case analysis | 292,000 | |
| Best-case analysis | 30,900 | |
| Worst-case analysis | 2,866,200 | |
| One-way sensitivity analysis of base-case analysis | ||
| 1) Cost per screening infant without follow-up | USD 2.3 | 186,400 |
| USD 6.0 | 397,600 | |
| 2) Cost of follow-up and confirmatory test per positive screen | USD 130 | 267,600 |
| USD 637 | 411,600 | |
| 3) Screen false positive rate | 0.1% | 242,000 |
| 1.0% | 354,500 | |
| 4) Incidence of CAH | 1 in 25,000 | 411,300 |
| 1 in 12,000 | 194,300 | |
| 5) SW mortality without screening | 2.0% | 613,200 |
| 9.0% | 136,300 | |
| 6) Reduction in SW mortality with screening | 74% | 315,700 |
| 86% | 271,600 | |
| Probabilistic CEA | 255,700 |
CEA = Cost effectiveness analysis; CAH = congenital adrenal hyperplasia; SW = salt wasting; ICER = incremental cost-effectiveness ratio.
Out of the one-way sensitivity analyses presented in table 2, the SW-CAH mortality rate had the largest influence on the ICER. The mortality assumptions of 2 and 9% lead to ICERs of USD 613,200 and USD 136,300, respectively. These ICERs are the smallest and the largest values in 6 one-way sensitivity analyses in table 2. On the other hand, the changes in the reduction in SW mortality with screening (74–86%) had a relatively small effect on the ICER, ranging from USD 271,600 to USD 315,700.
Varying the incremental cost of laboratory screening in the range from USD 2.30 to USD 6.00 caused the ICER to vary from USD 186,400 to USD 397,600. A large variation in the follow-up cost for a positive test, from USD 130 to USD 637, was associated with variation in the ICER from USD 267,600 to USD 411,600. A similar range of the ICER (USD 242,000 to USD 354,500) was found for changes in the screen false positive rate (0.1–1.0%).
As expected, a higher CAH incidence (1 in 12,000) yielded an ICER of USD 194,300 that was more favorable to screening, compared to the lower incidence assumption (1 in 25,000) which was associated with an ICER of USD 411,300.
Discussion
Our analyses showed that the ICER of the CAH screening ranged from USD 255,700 to USD 292,000 per LY based on the base-case analysis and probabilistic CEA with plausible assumptions. This section discusses the policy implications of our estimated ICERs with various cost effectiveness benchmarks, reasons for discrepancies with findings of a previous CEA of CAH screening, and limitations of our study.
A standard approach in interpreting ICERs is to compare them to one or more benchmarks or thresholds. The most commonly mentioned benchmark among U.S. studies is USD 50,000 per LY or QALY, with USD 100,000 the second most widely cited value [24]. Such benchmarks are arbitrary and lack basis in both economic theory and practice [25]. As an alternative to a fixed benchmark, one can compare ICERs with estimated ICERs for other preventive services that are accepted as providing good value. According to a recent analysis, one fifth of all clinical preventive services recommended by the U.S. Preventive Services Task Force cost more than USD 165,000 per QALY in 2000 USD [26]. Finally, some economists have observed that U.S. regulatory policy decisions imply that society is willing to spend up to USD 300,000 per LY saved [25, 27, 28].
The ICER of both our base-case analysis (USD 292,000) and probabilistic analysis (USD 255,700) exceeds most commonly used benchmarks or thresholds. Although CAH screening would not be regarded as a cost effective strategy based on those benchmarks, it could be cost effective according to a benchmark of USD 300,000 per LY (‘value of a statistical life year’) used in regulatory analyses in the U.S. [27, 28]. CAH screening would be less likely to be regarded as cost effective in countries with lower thresholds for ICERs.
The ICER in our best-case analysis (USD 30,900) suggests that CAH screening could be cost effective even with a benchmark of USD 50,000 per QALY/LY, but only under a favorable set of assumptions. A previous CEA reported an ICER of USD 20,000 per LY in 2004 USD [9]. That study assumed a mortality rate of 13% in SW-CAH (based on expert opinion), compared with 9% in our best-case scenario. Also, the authors assumed USD 10,000 (based on expert opinion) as the present value of the cost of caring for someone with CAH, and USD 27,809 as hospitalization cost before death deriving from the ‘general’ child population [9]. This implies net cost savings from averted deaths, but we are not convinced that averting an infant death due to CAH will necessarily lower lifetime medical costs associated with CAH.
Our study has a number of limitations. One is the limited availability and quality of data regarding the parameters of our decision model, due in part to the low prevalence of CAH. To deal with uncertainty in variables, we conducted probabilistic CEAs as well as one-way sensitivity analyses. An important research priority should be obtaining better data on the risk of mortality in CAH in both screened and unscreened cohorts. For the latter, this requires testing stored blood spot specimens with infant and child deaths for CAH. This should be done for deaths in infancy or early childhood, because deaths might be incorrectly attributed to infectious causes.
Another limitation is that our decision models only addressed mortality, excluding other outcomes such as misassignment of gender, short stature due to virilization, and anxiety because of test results. No research has measured most of these outcomes in terms of either monetary value or utility to generate QALY estimates [7]. One study that examined quality of life in late-diagnosed individuals with CAH found no indication of any loss of quality of life [29]. Incorporating additional outcomes could potentially make CAH screening appear more favorable in terms of value for money.
Conclusions
Using the most common benchmarks for cost effectiveness, our results indicate that CAH screening would be unlikely to be considered cost effective under assumptions based on available evidence. With optimistic assumptions favorable to screening, the cost-effectiveness ratio could meet the commonly used, albeit arbitrary figures of USD 50,000 or USD 100,000 per LY saved. CAH screening under fairly conservative assumptions could meet a criterion of USD 300,000 per LY saved that is consistent with values used in cost-benefit analyses of U.S. regulatory policies. However, under the least favorable set of assumptions, CAH screening would cost more than USD 2.8 million per LY saved and would not be considered cost effective under any criteria.
References
- 1.New MI: An update of congenital adrenal hyperplasia. Ann N Y Acad Sci 2004;1038:14–43. [DOI] [PubMed] [Google Scholar]
- 2.National Newborn Screening and Genetics Resource Center: National Newborn Screening Report – 2000 Austin, TX, The National Newborn Screening and Genetics Resource Center, February 2003. [Google Scholar]
- 3.van der Kamp HJ, Wit JM: Neonatal screening for congenital adrenal hyperplasia. Eur J Endocrinol 2004;151(suppl 3):U71–U75. [DOI] [PubMed] [Google Scholar]
- 4.American College of Medical Genetics New-born Screening Expert Group: Newborn screening: toward a uniform screening panel and system – executive summary. Pediatrics 2006;117:S296–S307. [DOI] [PubMed] [Google Scholar]
- 5.National Newborn Screening and Genetics Resource Center: U.S. National Screening Status Report. http://genes-r-us.uthscsa.edu/nbsdisorders.pdf (accessed January 8, 2008).
- 6.Loeber JG: Neonatal screening in Europe; the situation in 2004. J Inherit Metab Dis 2007;30:430–438. [DOI] [PubMed] [Google Scholar]
- 7.Grosse SD, Van Vliet G: How many deaths can be prevented by newborn screening for congenital adrenal hyperplasia? Horm Res 2007;67:284–291. [DOI] [PubMed] [Google Scholar]
- 8.Minutti CZ, Lacey JM, Magera MJ, Hahn SH, McCann M, Schulze A, Cheillan D, Dorche C, Chace DH, Lymp JF, Zimmerman D, Rinaldo P, Matern D: Steroid profiling by tandem mass spectrometry improves the positive predictive value of newborn screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab 2004;89:3687–3693. [DOI] [PubMed] [Google Scholar]
- 9.Carroll AE, Downs SM: Comprehensive cost-utility analysis of newborn screening strategies. Pediatrics 2006;117:S287–S295. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS). http://www.cdc.gov/nchs/fastats/lifexpec.htm (accessed December 6, 2007).
- 11.Therrell BL Jr, Berenbaum SA, Manter-Kapanke V, Simmank J, Korman K, Prentice L, Gonzalez J, Gunn S: Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics 1998;101:583–590. [DOI] [PubMed] [Google Scholar]
- 12.Brosnan CA, Brosnan P, Therrell BL, Slater CH, Swint JM, Annegers JF, Riley WJ: A comparative cost analysis of newborn screening for classic congenital adrenal hyperplasia in Texas. Public Health Rep 1998;113:170–178. [PMC free article] [PubMed] [Google Scholar]
- 13.National Newborn Screening Information System: Reports for Congenital Adrenal Hyperplasia (CAH). http://www2.uthscsa.edu/nnsis/ (accessed December 22, 2007).
- 14.Thil’en A, Nordenstrom A, Hagenfeldt L, von Dobeln U, Guthenberg C, Larsson A: Benefits of neonatal screening for congenital adrenal hyperplasia (21-hydroxylase deficiency) in Sweden: Pediatrics 1998;101:E11. [DOI] [PubMed] [Google Scholar]
- 15.Kaye CI, Committee on Genetics, Accurso F, La Franchi S, Lane PA, Hope N, Sonya P, Schaefer GB, Lloyd-Puryear MA: Newborn screening fact sheets. Pediatrics 2006;118:e934–e963. [DOI] [PubMed] [Google Scholar]
- 16.Kovács J, Votava F, Heinze G, Sólyom J, Lebl J, Pribilincová Z, Frisch H, Battelino T, Waldhauser F, Middle European Workshop on Paediatric Endocrinology-Congenital Adrenal Hyperplasia Study Group: Lessons from 30 years of clinical diagnosis and treatment of congenital adrenal hyperplasia in five middle European countries. J Clin Endocrinol Metab 2001;86:2958–2964. [DOI] [PubMed] [Google Scholar]
- 17.Van der Kamp HJ, Noordam K, Elvers B, Van Baarle M, Otten BJ, Verkerk PH: Newborn screening for congenital adrenal hyperplasia in the Netherlands. Pediatrics 2001;108:1320–1324. [DOI] [PubMed] [Google Scholar]
- 18.Thilen A, Larsson A: Congenital adrenal hyperplasia in Sweden 1969–1986. Prevalence, symptoms and age at diagnosis. Acta Paediatr Scand 1990;79:168–175. [DOI] [PubMed] [Google Scholar]
- 19.Brosnan PG, Brosnan CA, Kemp SF, Domek DB, Jelley DH, Blackett PR, Riley WJ: Effect of newborn screening for congenital adrenal hyperplasia. Arch Pediatr Adolesc Med 1999;153:1272–1278. [DOI] [PubMed] [Google Scholar]
- 20.Strnadova KA, Votava F, Lebl J, Mühl A, Item C, Bodamer OA, Torresani T, Bouska I, Waldhauser F, Sperl W: Prevalence of con-genital adrenal hyperplasia among sudden infant death in the Czech Republic and Austria. Eur J Pediatr 2007;166:1–4. [DOI] [PubMed] [Google Scholar]
- 21.Grosse SD, Van Vliet G: Outcomes in CAH: Need for evidence-based estimates. Horm Res 2007;68:203. [Google Scholar]
- 22.Luce BR, Manning WG, Siegel JE, Lipscomb J: Estimating costs in cost-effectiveness analysis; in Gold MR, Siegel JE, Russell LB, Weinstein MC (eds): Cost-Effectiveness in Health and Medicine. New York, Oxford University Press, 1996, pp 176–213. [Google Scholar]
- 23.Meltzer D: Accounting for future costs in medical cost-effectiveness analysis. J Health Econ 1997;16:33–64. [DOI] [PubMed] [Google Scholar]
- 24.Neumann PJ, Sandberg EA, Bell CM, Stone PW, Chapman RH: Are pharmaceuticals cost-effective? A review of the evidence. Health Aff (Millwood) 2000;19:92–109. [DOI] [PubMed] [Google Scholar]
- 25.Grosse SD: Assessing cost effectiveness in health care: the history of the USD 50,000 per QALY threshold. Exp Rev Pharmacoecon Outcomes Res 2008;8:165–178. [DOI] [PubMed] [Google Scholar]
- 26.Maciosek MV, Coffield AB, Edwards NM, Flottemesch TJ, Goodman MJ, Solberg LI: Priorities among effective clinical preventive services: results of a systematic review and analysis. Am J Prev Med 2006;31:52–61. [DOI] [PubMed] [Google Scholar]
- 27.Ubel PA, Hirth RA, Chernew ME, Fendrick AM: What is the price of life and why doesn’t it increase at the rate of inflation? Arch In-tern Med 2003;163:1637–1641. [DOI] [PubMed] [Google Scholar]
- 28.Aldy JE, Viscusi WK: Age differences in the value of statistical life: revealed preference evidence. Rev Environ Econ Policy 2007;1:241–260. [Google Scholar]
- 29.Jaaskelainen J, Voutilainen R: Long-term outcome of classical 21-hydroxylase deficiency: diagnosis, complications and quality of life. Acta Paediatr 2000;89:183–187. [DOI] [PubMed] [Google Scholar]