Abstract
Mechanoreceptors mediate a wide variety of physiological processes, such as hearing, touch, proprioception, and blood flow regulation. It is generally believed that mechanoreceptors are force-gated ion channels. Now, Xu et al uncover a GPCR that is activated by shear force in endothelial cells of blood vessels.
Keywords: GPR68, Mechanosensation, Mechanotransduction, Piezo
In addition to neurons, many cell types, such as those in the bone, muscle, kidney, eye and blood vessels, respond to mechanical stimuli. However, the underlying mechanoreceptors are only beginning to be discovered, especially in mammals. Progress toward this goal in mammals has been hindered in part by the relatively small number of mechanosensory cells, the difficulty of performing electrophysiological experiments in vivo, and the challenge of reconstituting mechanoreceptor protein complexes in heterologous systems.
What we do know about mechanoreceptors largely comes from work in model organisms such as bacteria, C. elegans and Drosophila (Katta et al. 2015, Xiao and Xu 2010). This has led to the identification of a number of mechanoreceptors, including Msc channels in bacteria, TRPN channels in C. elegans and Drosophila, and ENaC/DEG channels in C. elegans (Katta et al. 2015, Xiao and Xu 2010, Kang et al. 2010). However, these mechanoreceptors either do not have homologues in the mammalian genome (e.g. Msc and TRPN) or their homologues have not yet been shown to be mechanosensitive in mammalian systems (e.g. ENaC/DEG). A major breakthrough was the discovery that Piezo channels are directly gated by mechanical force in mammalian cells and regulate touch, proprioception and blood vessel development (Coste et al. 2010). To date, all confirmed mechanosensitive proteins are force-gated ion channels that allow ions to pass through the cellular membrane in response to force (Katta et al. 2015).
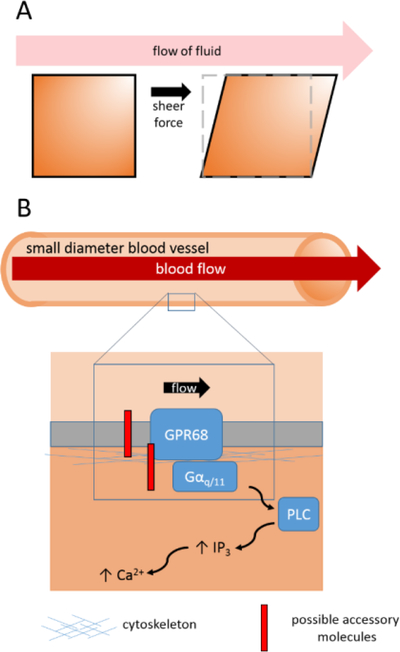
In this issue of Cell, Xu et al (2018) have now expanded the repertoire of mechanoreceptors from force-activated ion channels to GPCRs. The authors developed an elegant high throughput screening system in order to identify mechanoreceptors sensitive to shear stress (Fig. 1A). A relevant example of shear stress in the cardiovascular system is the frictional force applied by blood flowing along the surface of endothelial cells lining blood vessel walls (Fig. 1B). Their system applies shear stress to cultured cells by generating fluid flow parallel to the surface of the cell layer. The authors screened 25 human cell lines and found that MDA-MB-231 breast cancer cells exhibited robust calcium transients in response to shear stress.
Fig. 1.
(A) Shear stress arising from fluid flow across a surface parallel to the direction of flow. (B) GPR68 detects flow-mediated shear stress, which activates Gαq/11 signaling and increases intracellular Ca2+ levels.
Building on this system, the authors performed a focused RNAi screen in the newly discovered mechanosensitive cell line to identify mechanoreceptors underlying shear stress responses. Remarkably, knockdown of the G-protein coupled receptor (GPCR) GPR68 abolished the shear stress response. GPR68 is a Gαq/11-coupled receptor, and its activation leads to increased PLC-dependent intracellular Ca2+ levels. Consistent with GPR68 being activated by mechanical force, shear stress stimulates a PLC-dependent increase in calcium. In addition, heterologous expression of GPR68 in HEK293T cells induced robust shear stress-activated responses. Taken together, GPR68 is necessary and sufficient to act as a mechanoreceptor activated by shear stress.
Interestingly, GPR68 is expressed in mammalian small-diameter blood vessels, which sense shear stress. Increased blood flow leads to acute vasodilation whereas long periods of increased blood flow cause structural remodeling, which causes outward growth and increased diameter of vessel walls (Davies 2009). The authors found that loss of GPR68 in knockout mice disrupts both vasodilatory responses to increased blood flow, arguing that GPR68 functions to mediate flow-mediated vasodilation and remodeling in vivo. Importantly, flow-mediated remodeling is an adaptive response to ischemic conditions and promotion of this remodeling is thought to counter the effects of ischemic diseases (Davies 2009). The authors showed that allosteric enhancement of GPR68 activity caused increased vasodilation in small-diameter blood vessels, raising the possibility that drugs targeting GPR68 could have potential therapeutic value for treating cardiovascular diseases.
GPCRs, such as AT1R and several others, were previously proposed to be mechanosensitive (Mederos y Schnitzler et al 2008; Sharif-Naeini et al 2009). However, direct in vivo evidence for mechanosensitive GPCRs has been lacking, as previous studies have relied on heterologous expression systems or isolated vessels in vitro. Interestingly, Xu et al (2018) showed that AT1R and other implicated mechanosensitive GPCRs were not activated by shear stress. Perhaps these GPCRs are merely indirectly involved in mechanosensation.
GPCRs already have roles in many sensory transduction mechanisms, such as sensing odorants, tastants, pheromones, and light. Now there is compelling evidence that GPCRs can also sense mechanical stimuli. Perhaps other GPCRs can detect different forms of mechanosensory stimuli. Given this exciting discovery, a new focus on determining the structure-function relationship of GPR68 will deepen our understanding of its role in mechanotransduction. For instance, what is the mechanism by which GPR68 is activated by shear force? GPR68 is also proton-sensitive and its ability to detect shear stress is pH-dependent. How exactly does GPR68 respond to both types of stimuli? Are there any differences in downstream signaling between stimulation types? Altogether, the current study opens the doors for new investigations into mechanoreceptor identities and mechanisms and future insights into how GPCRs function in various sensory systems.
Acknowledgements
A.J.I. is supported by an NRSA fellowship grant from the NIDCD. Research in the Xu lab is supported by grants from the NIH (X.Z.S.X.).
References
- 1.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, … Patapoutian A (2010). Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science (New York, N.Y.), 330(6000), 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies PF (2009). Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nature Clinical Practice Cardiovascular Medicine, 6(1), 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang L, Gao J, Schafer WR, Xie Z, Xu XZS. (2010) C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron..67,381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katta S, Krieg M, & Goodman MB (2015). Feeling Force: Physical and Physiological Principles Enabling Sensory Mechanotransduction. Annual Review of Cell and Developmental Biology, 31(1), 347–371. [DOI] [PubMed] [Google Scholar]
- 5.Sharif-Naeini R, Folgering JHA, Bichet D, Duprat F, Delmas P, Patel A, & Honoré E (2010). Sensing pressure in the cardiovascular system: Gq-coupled mechanoreceptors and TRP channels. Journal of Molecular and Cellular Cardiology, 48(1), 83–89. [DOI] [PubMed] [Google Scholar]
- 6.Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, and Gudermann T (2008). Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. The EMBO journal 27, 3092–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao R and Xu XZS (2010). Mechanosensitive Channels: In Touch with Piezo. Current Biology. 20(21), R936–R938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Mathur J, et al. (2018). GPR68 senses shear stress and regulates flow-mediated dilation and remodeling in arterioles. Cell, [Google Scholar]