Abstract
Stimulant abuse is disproportionately common in HIV-positive persons. Both HIV and stimulants are independently associated with deficits in reward-based decision making, but their interactive and/or additive effects are poorly understood despite their prevalent comorbidity. Here, we examined the effects of cocaine dependence and HIV infection in 69 adults who underwent functional magnetic resonance imaging (fMRI) while completing an economic loss aversion task. We identified two neural networks that correlated with the evaluation of the favorable characteristics of the gamble (i.e., higher gains/lower losses; ventromedial prefrontal cortex, anterior cingulate, anterior and posterior precuneus, and visual cortex) versus unfavorable characteristics of the gamble (i.e., lower gains/higher losses; dorsal prefrontal, lateral orbitofrontal, and posterior parietal cortex, anterior insula, and dorsal caudate). Behaviorally, cocaine and HIV had additive effects on loss aversion scores, with HIV-positive cocaine users being the least loss averse. Cocaine users had greater activation in brain regions that tracked the favorability of gamble characteristics (i.e., increased activation to gains, but decreased activation to losses). In contrast, HIV infection was independently associated with lesser activation in regions that tracked the unfavorability of gamble characteristics. These results suggest that cocaine is associated with an overactive reward-seeking system, while HIV is associated with an underactive cognitive control system. Together, these alterations may leave HIV-positive cocaine users particularly vulnerable to making unfavorable decisions when outcomes are uncertain.
Keywords: cocaine dependence, decision making, drug addiction, functional magnetic resonance imaging (fMRI), gambling, HIV/AIDS
Introduction
The use of illicit stimulants like cocaine is a major driver of HIV infections worldwide (El-Bassel et al., 2014; Strathdee and Stockman, 2010). Stimulant users tend to engage in high rates of risky behaviors, such as unprotected intercourse with multiple partners, that contribute to the acquisition and transmission of HIV (Daskalopoulou et al., 2014; Gamarel et al., 2015; Khan et al., 2013). The prevalence of stimulant use is disproportionately high among HIV-positive persons. In a recent study of >3,000 patients receiving HIV care in the United States, 9.0% used amphetamines and 8.5% used crack-cocaine in the past 3 months (Mimiaga et al., 2013), compared to 0.6% for amphetamines and 0.7% for cocaine in the general population (Substance Abuse and Mental Health Services Administration, 2016).
Chronic cocaine use is associated with deficits in reward-based decision making that result in risky choices (Spronk et al., 2013). Cocaine users demonstrate impairments on component decision-making processes, such as risk-taking propensity, inhibitory control, and preference for small, immediate over large, delayed rewards (MacKillop et al., 2011; Smith et al., 2014; Wittwer et al., 2016). These impairments are thought to be related to dysfunction within the corticostriatal system. Compared to healthy controls, cocaine users have diminished endogenous dopamine function, attenuated sensitivity of the reward circuit to non-drug cues, and decreased baseline activity within prefrontal regions implicated in decision-making processes (Volkow et al., 2011). These deficits may be further exacerbated by the effects of HIV on the brain (Buch et al., 2012).
HIV also appears to affect decision-making processes. On gambling tasks, HIV-positive persons, compared to HIV-negative persons, have been found to make riskier choices, which may be driven by neurocognitive impairment (e.g. Fujiwara et al., 2015; Iudicello et al., 2013; Martin et al., 2016). HIV-associated neurocognitive disorders remain prevalent, including prominent deficits in executive function (Heaton et al., 2010; Sacktor et al., 2016). During cognitively demanding tasks, HIV is generally associated with hyperactivity in brain regions implicated in executive control, including the prefrontal cortex (PFC), posterior parietal cortex (PPC), and striatum (Plessis et al., 2014). This pattern of hyperactivity has been interpreted as compensation for decreased neural efficiency and capacity following neuronal injury, in order to preserve behavioral function (Barulli and Stern, 2013). In MRI studies, HIV has been associated with reduced gray and white matter volume, lower fractional anisotropy, and metabolic changes indicative of neuronal loss (Bairwa et al., 2016; Jernigan et al., 2011; Wright et al., 2015). When choosing risky gambles, HIV-positive (compared to HIV-negative) individuals showed greater activation in the dorsolateral PFC (DLPFC), anterior cingulate cortex (ACC), caudate, basal nuclei, and thalamus; in contrast, when choosing safe gambles, they had lesser activation in the DLPFC and ACC (Connolly et al., 2014).
Many decisions – including those related to health behaviors – are made in the context of uncertainty. For instance, when deciding whether to take a dose of antiretroviral medication, one must weigh the potential costs (e.g., side effects, disclosure of HIV-positive status) and benefits (e.g., suppressed viral load, reduced HIV transmission risk), before deciding. When outcomes are uncertain, people tend to over-weigh losses relative to gains, a phenomenon known as loss aversion (Tversky and Kahneman, 1992). Healthy adults typically avoid gambles until the potential gain is 1.5–2 times greater than the potential loss (Tversky and Kahneman, 1992). During the valuation of equal probability gambles, the magnitude of gains and losses have been found to be independently coded in a similar set of regions, which includes the ventromedial PFC (VMPFC), DLPFC, ACC, and striatum (Tom et al., 2007).
While past research has demonstrated that drug addiction and HIV infection are each associated with disrupted decision-making processes, their effects are typically studied independently. Given that drug abuse is highly comorbid in HIV-positive persons, it is critical to determine if these conditions have synergistic effects on decision-making processes. The current study investigated the independent and combined effects of HIV infection and cocaine dependence on neural activity during the valuation of potential gains and losses in the context of uncertain outcomes. We used the same loss aversion paradigm as Tom et al. (2007) in their seminal study. We hypothesized that cocaine users would be less loss averse (i.e., over-weigh gains relative to losses) compared to non-cocaine users, and that they would exhibit greater activation in PFC and striatal regions in response to increasing potential gains and lesser activation in these regions in response to increasing losses. We hypothesized that HIV infection would also be associated with reduced loss aversion, but greater activity in PFC, PPC, and striatal regions in response to the valuation of both increasing gains and losses. Given our hypothesis that cocaine and HIV would each be associated with gain-related hyperactivations, we also tested for potential interactive effects.
Material and Methods
Participants
The sample included adults aged 18–55 years who differed on cocaine and HIV status. Exclusion criteria were: <8th grade education; severe learning disability; illiteracy; non-fluency in English; serious neurological disorders; acute opportunistic brain infections or a history of such infections without return to normal cognition; severe head trauma with loss of consciousness >30 minutes and persistent functional decline; severe mental illness; MRI contraindications; and impaired mental status.
Cocaine (COC) status.
COC+ was defined as: ≥3 days of cocaine use in the past month or a positive urine drug screen for cocaine; ≥1 year of regular cocaine use; and lifetime cocaine dependence. For COC+, current alcohol or marijuana dependence were permitted if cocaine dependence was the principal diagnosis. Exclusions for COC+ were: any other drug use disorder within the past 20 years; regular use of other drugs in the past year; any other drug use in the past 30 days (other than alcohol and marijuana); and positive urine screen for non-prescribed opioids or amphetamines. COC-was defined as: no lifetime cocaine use disorder; no history of regular cocaine use; no cocaine use in the past year; and a cocaine negative drug screen. Exclusions for COC-were: current alcohol or marijuana dependence; lifetime abuse or dependence on any other drug; history of regular drug use; any drug use in the past 30 days (other than alcohol and marijuana); and positive urine screen for any other drug.
HIV status.
For individuals with diagnosed HIV infection, HIV+ status was verified by medical record review. For others, an OraQuick© rapid test was conducted to verify HIV-status.
Procedures
Participants were recruited via advertisements in local newspapers, websites, community-based organizations, and infectious diseases clinics. After completing a telephone pre-screen, potential participants completed an in-person screening. Eligible participants returned on another day for an MRI scan. Participants gave informed consent, and all procedures were approved by the Institutional Review Board at Duke University Health System.
Screening measures
Participants completed demographics and smoking history surveys and provided a urine sample for drug and pregnancy testing. The screening measures included: Wechsler Test of Adult Reading for premorbid verbal IQ (Wechsler, 2001); Structured Clinical Interview for DSM-IV-TR for substance use disorders (First et al., 1996); Addiction Severity Index-Lite for substance use (McLellan et al., 1992); and timeline follow-back for frequency of substance use in the past 90 days (Robinson et al., 2014). Participants provided a release for their medical records, which were used to obtain HIV disease indicators (e.g., CD4 cell counts). HIV+ participants estimated adherence to their antiretroviral medications using a visual analogue scale (Giordano et al., 2004).
fMRI task
The loss aversion task presents participants with gambles that have an equal probability of gaining or losing a variable amount of money Gambles were sampled from a matrix that ranged from $0 to $40 in $4 increments for gains and $0 to -$20 in -$2 increments for losses (Figure 1). Participants were extensively trained on the task prior to scanning.
Figure 1.
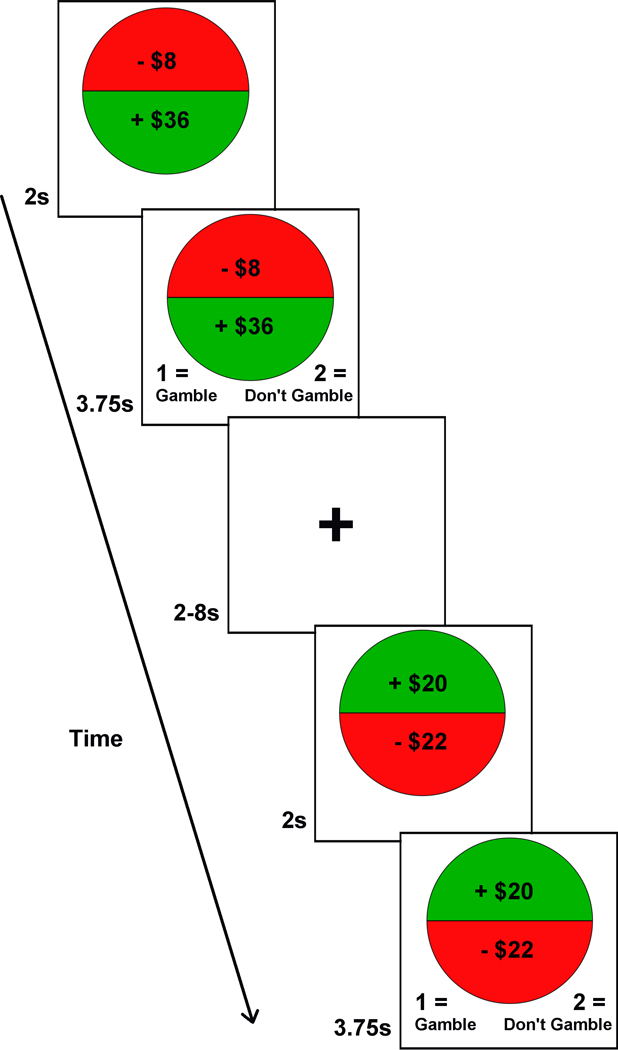
Illustration of the coin flip task. For each trial, there was a 2s presentation period, followed by a 3.75s response period. To signal the beginning of the response period, the options appeared on the bottom of the screen as 1= gamble (i.e., accept) or 2= don’t gamble (i.e., reject). Inter-trial intervals (ITI) ranged 2–8s (M= 3.31).
All 120 combinations of gambles (excluding $0/$0) were randomized once and divided across three functional runs (each 6 min). The order of the runs was randomized across participants. Participants chose whether to accept or reject each gamble. They were instructed to consider each gamble independently, like a coin flip, and the gambles were not played out during the scan. Each trial was presented for 5.75s, during which participants made their choice, followed by a variable inter-stimulus interval of 2–8s (M= 3.2s). This task was programed in MATLAB R2012a (Mathworks, Natick, MA) using the Psychtoolbox extension (www.psychtoolbox.org). Visual stimuli were displayed via projector onto a screen within the scanner bore, and responses were recorded using an MR-compatible response box held in the right hand.
Participants were told that, after the scan, one trial would be randomly selected. If they had rejected that gamble, the gamble was not played. If they had accepted that gamble, the outcome was determined with a coin flip. Rewards were scaled: $0 for losses of $11–20, $1 for losses of $0–10, $2 for gain of $1–10, $3 for gains of $11–13, $4 for gains of $21–30, and $5 for gains of $31–40.
To estimate behavioral loss aversion (lambda), a logistic regression predicting participants’ choice to accept each gamble was used to generate unstandardized regression coefficients (β values) for gains and losses. Trials with a $0 gain or $0 loss option were excluded from this analysis. As in prior studies, lambda was computed as -βloss/ βgain; higher scores indicate greater loss aversion (Tom et al., 2007).
MRI data acquisition
Imaging was performed on a 3T General Electric Signa EXCITE HD scanner (Milwaukee, WI) equipped with 40 mT/m gradients and an 8-channel head coil. Whole-brain BOLD images were collected using high-throughput T2*-weighted echo-planar imaging with the following parameters: TR= 2000ms; TE= 25ms; FOV= 24.0cm; flip angle= 90°; in-plane matrix size= 64 × 64; and slice thickness= 3.8mm, resulting in functional data from 35 axial slices with voxels of 3.75×3.75×3.8mm. High-resolution T1-weighted structural images were acquired with the following parameters: TR= 8.156ms; TE= 3.18ms; FOV= 25.6cm; flip angle= 12°; in-plane matrix size= 256 × 256; slice thickness= 1mm; and number of slices= 166. These images were collected as part of a 90-minute scanning session.
Quality control
Of the 81 participants who completed the experiment, one was excluded because of a procedural error during the scan. Ten participants were excluded because their behavioral performance on the loss aversion task indicated inattention or poor comprehension. Specifically, they were excluded for: a negative βgain value (i.e., they were less likely to accept gambles as potential gains increased); a positive βloss value (i.e., they were more likely to accept gambles as potential loss increased); and/or very small regression coefficients that indicated random or heuristic responding (βgain <.03 and βloss values >−.015). Visual inspection of heatmaps converged with the initial quality control check (see Supplementary Figure S1 for examples). One additional participant was excluded for skipping >30% of trials. Among the 69 participants in the final analysis, 63 (91%) missed ≤10% of trials (M= 2.58, SD= 4.69). The heatmaps of the 6 participants who missed >10% showed clear response patterns consistent with their loss aversion scores.
FSL’s Motion Outliers tool was used to calculate motion metrics during scanning. Framewise displacement (FD) ranged from 0.06 to 1.30 (M= 0.15, SD= 0.15). An analysis of variance (ANOVA) revealed a main effect of cocaine on FD [F(1,65)= 4.62, p= .035], such that COC+ had higher values than COC-. There was no significant effect of HIV (both p >.20). No participants were excluded for motion. To control for the effects of motion, six motion parameters calculated with MCFLIRT were included as regressors in the first level analyses. We also added mean FD as a covariate in the group comparison model.
Data analysis
Behavioral data.
Participants were characterized in terms of demographics, substance use, and HIV infection using descriptive statistics. Group differences were identified using chi-square, ANOVA, and Mann-Whitney tests. Loss aversion scores were examined using a 2 (COC+/COC-) x 2 (HIV+/HIV-) between-subjects ANOVA. All behavioral analyses were conducted in SPSS 22.0.
fMRI data.
Functional images were processed using FMRIB Software Library FSL v5.0 (Jenkinson et al., 2012). The first 5 volumes of each run were deleted to allow for magnetic resonance equilibrium. Functional images were temporally realigned to correct for interleaved slice acquisition, motion corrected using the MCFLIRT linear realignment tool, and high-pass temporal filtered (cutoff= 100s). Nonbrain voxels were removed from the anatomical images using the brain extraction tool (BET). Functional images were co-registered to participants’ anatomical image with a 12-degrees of freedom (DOF) linear transformation, and then normalized to the Montreal Neurological Institute 152 template (2×2×2mm) using FNIRT (Andersson et al., 2007). Functional images were spatially smoothed using a 5mm full-width half-maximum (FWHM) Gaussian kernel.
Data from individual runs for each participant were subjected to a general linear model using FILM prewhitening, a temporal derivative, and temporal filtering. Explanatory variables were modeled by convolving a delta function representing trial onset times with a canonical (double-gamma) hemodynamic response function. All trials were modeled using a single condition representing overall task-related activation, and two additional orthogonal regressors were included representing the magnitude of the potential gains (gain-related BOLD) and the magnitude of the potential losses (loss-related BOLD). Contrasts were defined for each of these three explanatory variables, and then were averaged across the three functional runs for each participant using a fixed-effects model (Beckmann et al., 2003).
In all analyses, gain and loss were analyzed separately. First, the overall pattern of gain-and loss-related activations in the sample was examined using one-sample t-tests. These whole-brain images were thresholded at z >3.1. Cluster correction was determined using 3DClustSim using FWHM and running 10,000 Monte Carlo simulations on a whole-brain mask composed of 258,298 voxels (https://afni.nimh.nih.gov/) (Ward, 2000). This produced an individual voxel threshold of p <0.001 and an extent threshold of ≥30 contiguous voxels for an overall corrected false positive rate of p <0.05. To ensure subsequent analyses were related to the main effects of the gain-and loss-related activations, a mask was created by adding both gain-and loss-related main effects using a more liberal threshold of p <0.01, extent threshold ≥80 voxels.
Second, gain-and loss-related activations were correlated with behavioral loss aversion scores, which were natural log transformed and mean-centered. The results of the correlations were masked with the main effects and thresholded at p <0.001, extent threshold ≥20 voxels for an overall corrected false positive detection rate of p <0.05.
Third, group differences in gain-and loss-related activations were examined using mixed-effects 2 (COC+/COC-) by 2 (HIV+/HIV-) ANOVAs controlling for motion. ANOVA results were masked with the main effects and thresholded at p <0.001, extent threshold ≥20 voxels for an overall corrected false positive detection rate of p <0.05. To reduce the likelihood of Type II errors, clusters that survived p <0.005, extent threshold ≥40 voxels are also reported. The Harvard-Oxford cortical and subcortical structural atlases were used to identify neuroanatomical locations of activation peaks (Desikan et al., 2006). Figures were made using MRIcron (www.mccauslandcenter.sc.edu/mricro/).
Results
Participant characteristics
The sample included 47 men and 22 women across four study groups: 21 COC-/HIV-, 16 COC+/HIV-, 17 COC-/HIV+, and 15 COC+/HIV+. Participants were primarily non-Hispanic (99%) and African-American (77%), and ranged in age from 24 to 55 years (M=44.13, SD=8.08). Table 1 compares the four groups. There were no group differences in demographics. On average, COC+ participants had been using cocaine regularly for 16.97 years (SD=8.57, range 1–33) and used cocaine on 11.19 (SD=7.99) of the past 30 days. The predominant route of cocaine administration was smoking (87%). Among COC+ participants, there was no difference between HIV+ and HIV-groups on these characteristics. Current use of alcohol to intoxication, marijuana, and nicotine was more common in COC+ compared to COC-. All HIV+ participants were currently in HIV care. They had been diagnosed with HIV for an average of 14.74 years (SD=8.05, range 0.33–29). All but one was on antiretroviral therapy, and 87% reported taking >90% of their medications over the past 4 weeks. Among HIV+ participants, the COC+ and COC-groups were comparable on current and nadir CD4 cell counts, AIDS diagnosis, and suppressed viral load (<50 copies/mL).
Table 1.
Sample characteristics by study group (N=69)
| COC+ / HIV+ N=15 |
COC+ / HIV− N=16 |
COC− / HIV+ N=17 |
COC− / HIV− N=21 |
Statistic | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Male, % | 80% | 63% | 65% | 67% | χ2(3)=1.32 |
| Age in years, M (SD) | 48.60 (6.89) | 44.38 (6.22) | 42.59 (8.69) | 42.00 (8.79) | F(3,65)=2.37 |
| Education in years, M (SD) | 12.47 (2.23) | 12.06 (2.82) | 14.18 (2.27) | 13.67 (2.50) | F(3,65)=2.71 |
| Ethnicity, % | χ2(6)=4.29 | ||||
| African American | 87% | 75% | 82% | 67% | |
| Caucasian | 7% | 19% | 18% | 19% | |
| Other/Mixed | 7% | 6% | 0% | 14% | |
| Premorbid verbal IQ, M (SD) | 87.67 (10.96) | 88.38 (14.90) | 90.35 (18.32) | 94.90 (15.58) | F(3,65)=.85 |
| Cocaine use characteristics | |||||
| Years of regular use, M (SD) | 18.87 (7.06) | 15.19 (9.66) | N/A | N/A | F(1,29)=1.45 |
| Days of use in past 90 days, M (SD) | 39.27 (28.67) | 34.25 (23.88) | N/A | N/A | F(1,29)=.28 |
| Current cocaine dependence, % | 100% | 88% | N/A | N/A | χ2(1)=2.00 |
| Days since last use at MRI, M (SD) | 2.13 (1.55) | 4.88 (5.99) | N/A | N/A | F(1,29)=2.95 |
| Other substance use in past 90 days | |||||
| Any alcohol to intoxication, % | 87% | 75% | 29% | 33% | χ2(3)=17.01*** |
| Days of use, M (SD) 1 | 25.62 (25.26) | 41.75 (29.61) | 9.40 (10.16) | 12.86 (10.30) | F(3,33)=3.33* |
| Any marijuana use, % | 67% | 44% | 6% | 5% | χ2(3)=23.07*** |
| Days of use, M (SD) 1 | 31.50 (35.74) | 43.00 (35.15) | 3.00 (N/A) | 14.00 (N/A) | F(3,15)=.52 |
| Any nicotine use, % | 67% | 69% | 29% | 15% | χ2(3)=15.46** |
| Days of use, M (SD) 1 | 90.00 (0.00) | 89.09 (3.02) | 74.20 (35.33) | 90.00 (0.00) | F(3,25)=1.61 |
| HIV characteristics 2 | |||||
| Years since HIV diagnosis, M (SD) | 17.00 (8.34) | N/A | 12.63 (7.39) | N/A | t(29)=1.55 |
| Nadir CD4 cell count, Mdn (IQR) | 100 (218) | N/A | 204 (244) | N/A | U=86.50 |
| Current CD4 cell count, Mdn (IQR) | 427 (449) | N/A | 573.5 (339) | N/A | U=96.00 |
| Suppressed HIV viral load, % | 53% | N/A | 69% | N/A | χ2(1)=.78 |
| AIDS diagnosis, % | 60% | N/A | 38% | N/A | χ2(1)=1.57 |
p < .05,
p < .01,
p < .001
Among persons who used the substance;
Medical record data was missing for one participant in the COC-/HIV+ group.
Behavioral performance on the loss aversion task
Figure 2A illustrates the pattern of gamble acceptance and rejection across the full sample. Participants were more likely to accept gambles as potential gains increased, and more likely to reject gambles as potential losses increased. As shown in Figure 2B, there was a step-wise pattern in loss aversion scores across groups. The COC-/HIV-group responded as expected, with a loss aversion score of 1.82 (SD= 0.88). This means that they tended to reject gambles until the potential gain was 1.8 times larger than the potential loss. Other groups had scores lower than is typical (Tversky and Kahneman, 1992). The ANOVA revealed a significant main effect for cocaine [F(1,65)= 5.45, p= .023] and a trend for HIV [F(1,65)= 3.68, p= .059], but no interaction effect [F(1,65)= 0.35, p= .555]. As a post-hoc analysis, we used the nonparametric Jonckheere-Terpstra test to assess whether the distribution of loss aversion scores differed based on the number of risk factors (COC-/HIV-=0, COC+/HIV-or COC-/HIV+ =1, COC+/HIV+ =2). This test was significant (J* statistic= −2.762, p= .006), indicating an additive effect in the presence of both factors.
Figure 2.
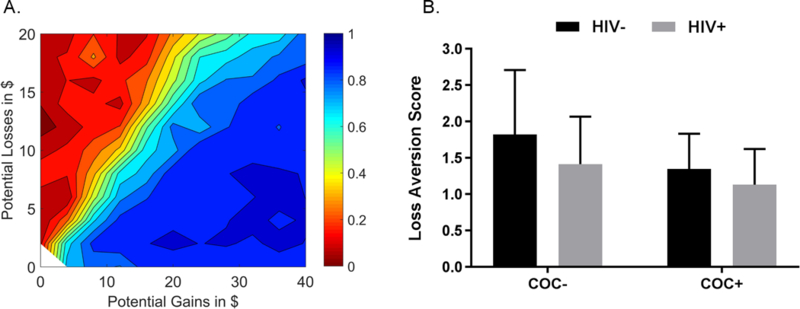
A. Contour map illustrating the pattern of choices on the coin flip task for the sample as a whole. The probability of accepting the gamble ranged from 0 (red) to 1 (blue). Note: N= 68, as one COC-/HIV-participant completed only two out of the three runs. B. Bar graph illustrating mean loss aversion scores for each group (error bars represent standard deviation from the mean). COC-/HIV-participants had the highest scores (i.e., the greatest loss aversion) and COC+/HIV+ participant had the lowest scores (i.e., the least loss aversion).
BOLD activation during the loss aversion task across the full sample
Gain-related activation (see Figure 3A, Table S1).
Figure 3.
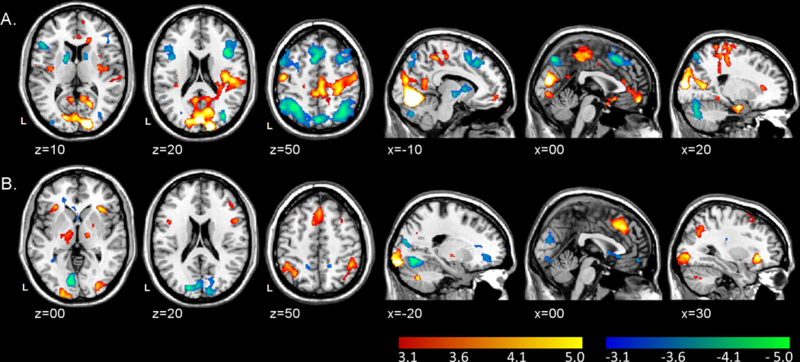
A. Clusters of significant activation (red-yellow scale) and deactivation (blue-green scale) associated with increasing monetary gain values. B. Clusters of significant activation (red-yellow scale) and deactivation (blue-green scale) associated with increasing monetary loss values. Clusters were thresholded at z > 3.1 (p < .001) with ≥30 contiguous voxels for an overall false positive detection rate of p < .05. Color bars represent z-values.
As potential gains increased, there was increasing activation bilaterally in the medial PFC [orbitofrontal cortex (mOFC) and VMPFC], cuneus, pre-and post-central gyri, limbic structures (hippocampus, parahippocampus, and amygdala), and superior temporal gyri. As potential gains increased, there was also decreasing activation (i.e., deactivation) bilaterally in the DLPFC, dorsomedial PFC (DMPFC), anterior insula, PPC (inferior and superior parietal lobules), dorsal striatum (putamen and caudate), pallidum, and cerebellum.
Behavioral loss aversion was positively correlated with gain-related activation in bilateral DLPFC and PPC, and negatively correlated with activation in bilateral occipital cortex, hippocampus, and amygdala, left cuneus, and right parietal operculum (see Table 2, Figure 4). As a post-hoc analysis, we examined the correlations between loss aversion scores and percent signal change extracted from these clusters by group. In gain-related clusters, the correlations were stronger in COC-compared to COC+. For example, in the right frontal pole, the correlations were strong and significant for COC-/HIV-(r= 0.57, p= .007) and COC−/HIV+ (r= 0.51, p= .037), but non-significant for COC+/HIV+ (r= −0.03, p= .924) and COC+/HIV-(r= 0.27, p= .313). In contrast, in loss-related clusters, the correlations were larger for HIV+ compared to HIV-. For example, in the left lingual gyrus, correlations were strong and significant for both COC+/HIV+ (r= −0.72, p=.003) and COC−/HIV+ (r= −0.66, p= .004), but non-significant for COC+/HIV-(r= −0.24, p= .376) and COC−/HIV− (r= −0.23, p= .315).
Table 2.
Correlations between BOLD activity in response to increasing gains and behavioral loss aversion score (lambda) in the full sample (N=69)
| Region of activation | MNI coordinates (x, y, z) at peak |
Vol (mm3) | Max Z-Value |
|---|---|---|---|
| Positive correlations | |||
| R posterior parietal cortex | 32, −68, 48 | 2,360 | 4.14 |
| L dorsolateral prefrontal cortex, precentral gyrus | −46, 8, 32 | 2,160 | 4.12 |
| R lateral frontal pole, dorsolateral prefrontal cortex | 42, 36, 18 | 1,072 | 4.10 |
| L posterior parietal cortex | −30, −66, 48 | 688 | 4.05 |
| R occipital pole | 32, −100, −14 | 672 | 4.35 |
| R posterior parietal cortex | 56, −44, 50 | 352 | 4.01 |
| R middle frontal gyrus | 40, 28, 28 | 168 | 3.38 |
| Negative correlations | |||
| L superior lateral occipital, cuneus, occipital pole | −18, −86, 20 | 2,256 | 4.49 |
| R hippocampus, amygdala | 32, −12, −24 | 1,560 | 4.05 |
| L hippocampus, amygdala | −30, −10, −26 | 896 | 4.06 |
| L lingual gyrus | −10, −60, 4 | 808 | 4.05 |
| R lingual gyrus | 10, −46, −4 | 400 | 3.89 |
| R parietal operculum cortex, planum temporale | 54, −34, 24 | 384 | 3.56 |
| L lingual gyrus | −24, −46, −8 | 384 | 4.02 |
| R postcentral gyrus | 10, −46, 70 | 296 | 3.65 |
Notes: R = right hemisphere; L = left hemisphere, B =bilateral. Clusters were Thresholded at an individual voxel threshold of p < 0.001 and an extent threshold of ≥ 20 contiguous voxels (160 mm3) for an overall corrected false positive detection rate of p < 0.05.
Figure 4.
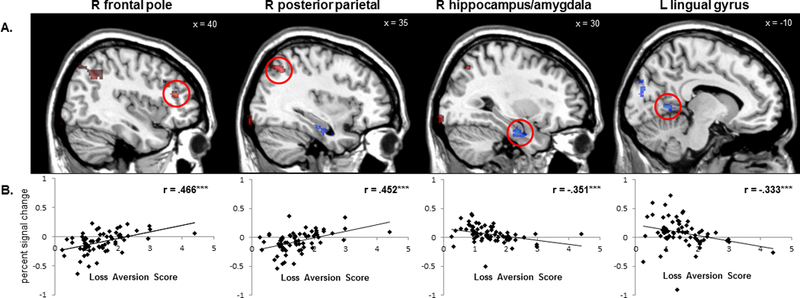
A. Significant clusters of positive (red) and negative (blue) correlations between gain-related BOLD activation and behavioral loss aversion scores. B. Scatter plots of significant positive and negative correlations between gain-related BOLD activation and loss aversion scores (from left to right) in the right frontal pole, right posterior parietal cortex, right hippocampus/amygdala, and left lingual gyrus. Clusters were thresholded at z > 3.1 (p < .001) with ≥20 contiguous voxels for an overall false positive detection rate of p < .05.
Loss-related activation (see Figure 3B, Table S1).
As potential losses increased, there was increasing activation bilaterally in the PPC, DMPFC, DLPFC, anterior insula, pallidum, thalamus, and inferior occipital pole. As potential losses increased, there was also decreasing activation bilaterally in the VMPFC, cuneus, anterior and posterior precuneus, and the right operculum and middle temporal gyrus. There were no regions where loss-related activation or de-activation correlated with loss aversion scores.
Discrete networks.
In the main analyses for gain-and loss-related activation, two discrete networks of activation emerged. The first, which had increased activity in response to increasingly favorable gamble characteristics (increasing gains/decreasing losses), consisted of bilateral mOFC/VMPFC, ACC, cuneus, supramarginal gyrus, superior occipital pole, pre-and post-central gyri, anterior and posterior precuneus, and parietal operculum. The second network, which had increased activity in response to increasingly unfavorable gambles characteristics (decreasing gains/increasing losses), included bilateral DMPFC, DLPFC, lateral OFC (lOFC), anterior insula, PPC, central precuneus, dorsal caudate, pallidum, inferior LOC, inferior occipital pole, and cerebellum. There were no regions of overlap between the gamble-favorability and gamble-unfavorability gamble networks. The combination of both networks was used to create the mask applied to the correlational and ANOVA analyses (see Figure S2).
Effects of cocaine and HIV on BOLD activation (Table 3)
Table 3.
Cocaine and HIV effects for gray matter activations in response to increasing gains and losses (N=69)
| Region of activation | MNI coordinates (x, y, z) at peak |
Vol (mm3) |
Max Z Value |
|---|---|---|---|
| Gain-related activations | |||
| COC+ > COC- | |||
| L posterior precuneus | −12, −56, 16 | 2,344 | 3.52* |
| L medial frontal pole/frontal medial cortex (VMPFC), paracingulate/anterior cingulate (ACC) | −10, 60, −4 | 1,688 | 3.53* |
| L superior temporal gyrus, planum temporale, central opercular cortex | −66, −24, 6 | 872 | 3.56 |
| R precentral gyrus | 54, −2, 52 | 592 | 3.32 |
| L parietal operculum cortex, planum temporale | 44, −28, 14 | 584 | 3.12 |
| R medial frontal pole/frontal medial cortex (VMPFC), paracingulate (ACC) | 10, 58, −10 | 328 | 3.23 |
| R medial frontal pole/frontal medial cortex (VMPFC), anterior cingulate (ACC) | 18, 38, −10 | 328 | 3.03 |
| COC+ < COC- | |||
| R angular gyrus/superior lateral occipital cortex (PPC) | 52, −62, 40 | 1904 | 3.72* |
| HIV+ < HIV− | |||
| R paracingulate (ACC) | 20, 40, 12 | 744 | 3.57 |
| Interaction | |||
| L posterior precuneus | −20, −42, 20 | 1,536 | 4.41* |
| B medial frontal pole (VMPFC) | 6, 68, 8 | 1,520 | 3.77* |
| Loss-related activations | |||
| COC+ < COC- | |||
| R medial frontal pole (VMPFC) | 24, 36, −4 | 784 | 3.82* |
| HIV+ < HIV- | |||
| R central precuneus, superior lateral occipital cortex/supramarginal gyrus (PPC) | 4, −76, 40 | 17296 | 4.57* |
| R lingual gyrus, occipital and temporal occipital fusiform gyrus, inferior temporal gyrus, cerebellum | 36, −48, −30 | 13192 | 4.44* |
| L inferior frontal gyrus (VLPFC), lateral frontal orbital cortex (OFC) | −56, 20, −8 | 6672 | 4.06* |
| L temporal occipital fusiform gyrus, inferior temporal gyrus, inferior lateral occipital cortex | −44, −60, −10 | 3640 | 4.14* |
| L thalamus, pallidum | −14, −22, 4 | 3256 | 4.13* |
| R inferior frontal gyrus/lateral frontal pole (VLPFC) | 52, 12, 20 | 2840 | 3.82* |
| L superior lateral occipital cortex (PPC) | −20, −68, 38 | 2552 | 3.83* |
| R lateral frontal pole (VLPFC) | 42, 40, −26 | 2352 | 3.78* |
| L supramarginal gyrus (PPC) | −34, −42, 36 | 1976 | 3.63* |
| R middle frontal gyrus (DLPFC) | 34, 12, 50 | 1192 | 3.32 |
| B posterior cingulate | 2, −32, 22 | 1120 | 3.58* |
| R thalamus | 8, −16, 0 | 1080 | 3.37 |
| L superior frontal gyrus (DMPFC) | 0, 6, 60 | 856 | 3.24 |
| R paracingulate gyrus (ACC) | 10, 24, 38 | 656 | 3.57* |
| R superior lateral occipital cortex (PPC) | 16, −74, 62 | 584 | 3.48 |
| R lateral frontal orbital cortex (OFC) | 36, 20, −12 | 576 | 3.53 |
| R cuneus | 4, −78, 36 | 368 | 3.65 |
| Interaction | |||
| L paracingulate (ACC) | −6, 48, −2 | 600 | 3.26 |
Notes: VMPFC = ventromedial prefrontal cortex; ACC = anterior cingulate cortex; PPC = posterior parietal cortex; VLPFC = ventrolateral prefrontal cortex; OFC = orbitofrontal cortex; DLPFC = dorsolateral prefrontal cortex; DMPFC = dorsomedial prefrontal cortex
Clusters were thresholded at an individual voxel threshold of p < .005 and an extent threshold of ≥40 contiguous voxels (320 mm3) for an overall corrected false positive rate of p < 0.05. Clusters that survived an individual voxel threshold of p < .001 and an extent threshold ≥20 contiguous voxels (160 mm3) are marked with an asterisk.
ANOVA of gain-related activation.
There was a main effect of cocaine in 8 clusters (Figure 5A). Seven of these were within the gamble-favorability network: Compared to COC-, COC+ had greater gain-weighted activation in the bilateral VMPFC and ACC; right precentral gyrus; and left posterior precuneus, superior temporal gyrus, and operculum. Conversely, COC+ had lesser gain-related activation in the right PPC of the gamble-unfavorability network. There was also a small main effect of HIV, such that HIV+ had lesser gain-related activation in the right ACC. Interaction effects were observed in the bilateral VMPFC and left posterior precuneus. For both clusters, COC+ was associated with larger activations than COC-in HIV-participants, but not in HIV+ participants.
Figure 5.
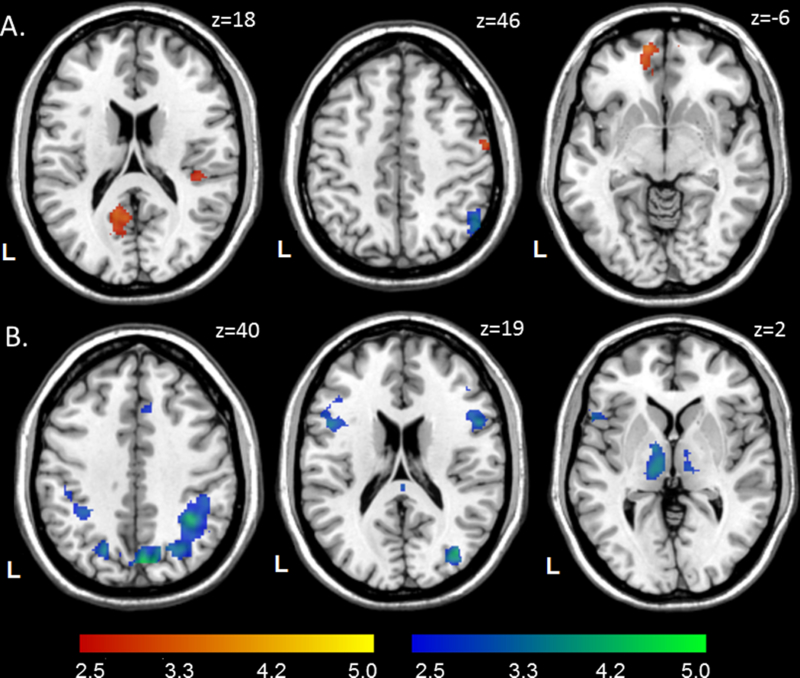
Main effects of cocaine and HIV on BOLD activation from the ANOVA. A. Effects of cocaine on gain-related BOLD activation. Red: COC+ > COC-, blue: COC-> COC+. B. Effects of HIV on loss-related BOLD activation. Blue: HIV-> HIV+. Clusters were thresholded at z > 2.58 (p < .005) with ≥ 40 contiguous voxels for an overall false positive detection rate of p < .05. Color bars represent z-values. The main effects of HIV for gain-related BOLD activation and cocaine for loss-related BOLD activation are not displayed.
ANOVA of loss-related activation.
There was a main effect of HIV in 17 clusters in the gamble-unfavorability network (Figure 5B): Compared to HIV-, HIV+ had lesser loss-related activation in the bilateral VLPFC/lOFC, posterior cingulate, PPC, inferior temporal gyrus, fusiform gyrus, and thalamus; right DLPFC, ACC, cuneus, central precuneus, and cerebellum; and left DMPFC and pallidum. There were no regions in which HIV+ participants had greater loss-related activation. The ANOVA also revealed a small main effect of cocaine, such that COC+ had lesser loss-related activation in the right VMPFC. There was one interaction in the left ACC. In HIV-, COC+ participants had mean deactivations, while COC-participants had mean activations; in HIV+, the pattern was reversed.
Discussion
Our primary finding is that cocaine and HIV had distinct effects on the neural substrates involved in the valuation of monetary gambles. As hypothesized, cocaine users were less loss averse than non-cocaine users, and there was an additive effect of HIV, such that HIV-positive cocaine users were the least loss averse. Cocaine use was associated with greater gain-related activation in the VMPFC, ACC, and posterior precuneus, while HIV was associated with lesser loss-related activation in several fronto-parietal regions. The effects of cocaine and HIV were mostly independent of one another, with interaction effects identified only in the VMPFC and posterior precuneus in response to gains and ACC in response to losses. These results suggest that the underlying neural mechanisms that contribute to reduced loss aversion in persons with cocaine use disorders and HIV infection may be different.
Our experimental paradigm was based on a study by Tom et al. (2007), which examined the neural substrates of loss aversion in 16 healthy young adults. They identified both gain-related activation and loss-related deactivation in the DLPFC, VMPFC, ACC, and striatum, which led to the conclusion that gains and losses are coded in the same brain regions (Tom et al., 2007). In another sample of 56 healthy adults, gain-related activation was similarly identified in the OFC and DLPFC, cingulate, thalamus, and right striatum (Canessa et al., 2013). This group also identified loss-related activation in the right amygdala and somatosensory cortex, and thus concluded that the valuation of potential gains and losses involves multiple neural systems (Canessa et al., 2013). Like both of these studies, we identified overlapping gain-related activations and loss-related deactivations in mOFC/VMPFC and ACC (i.e., gamble-favorability network), regions that are frequently linked to reward-based decision making (Rushworth et al., 2011). In this network we also identified limbic (i.e., amygdala and hippocampal) activation in response to gains, which is consistent with other studies supporting the role of the limbic system in loss aversion (De Martino et al., 2010; Sokol-Hessner et al., 2013). Behavioral loss aversion scores were negatively correlated with gain-related activation in the gamble-favorability network, meaning that individuals who were less loss averse had greater activations in response to increasing potential gains
In our sample, we also detected a network associated with the unfavorability of gambles (i.e., gain-related deactivation and loss-related activation) that encompassed regions typically linked with cognitive control (DLPFC/DMPFC, PPC, caudate, basal ganglia, thalamus) (Niendam et al., 2012; Venkatraman et al., 2009). Behavioral loss aversion scores correlated positively with gain-related activation in this network, meaning that individuals who were more loss averse had greater activation in response to increasing potential gains. The gamble-unfavorability network may represent the monitoring and oversight of incentive-based behavioral responses when multiple options need to be evaluated and compared (Haber and Knutson, 2010). Altogether, the reward-based/limbic network may mediate emotion-driven influences on gamble selection, while the cognitive control network is sensitive to factors that make gamble selection more difficult (e.g., increasing potential loss) and contributes to deliberate, conscious decisions to accept or reject each gamble. Both networks, working together, inform reward-based decision making.
As expected, we found that cocaine users were significantly less loss averse than non-cocaine users. Furthermore, cocaine users had greater gain-related activation than non-cocaine users in several regions in the gamble-favorability network. These differences were evidenced primarily in response to gains, not losses. While drug abuse is associated with striatal hypoactivation in tasks involving monetary reward anticipation (Luijten et al., 2017), there has been relatively less research on the role of the PFC in monetary decision making (Goldstein and Volkow, 2011). There is evidence that cocaine users may be less sensitive to increases in monetary rewards (Goldstein et al., 2007), but this may depend on the range of values. For example, Goldstein et al (2007) found that cocaine users overvalued small rewards and undervalued large rewards (up to $1,000), and that this reduced sensitivity to increasing reward value correlated with greater activations in the lOFC/VLPFC. The gambles in our study had gain values ranging from $0–40, which may help to explain the observed hyperactivation in the medial PFC and ACC. Our results support the hypothesis that risky choice in cocaine users may relate to an overactive reward-seeking system. HIV infection appeared to moderate this effect in the VMPFC and posterior precuneus, such that the cocaine-associated hyperactivity observed in HIV-negative individuals was reduced in HIV-positive individuals. While we speculate that the neurocognitive effects of HIV may diminish reward responsivity, more research is needed.
In contrast to cocaine, the effect of HIV on behavioral loss aversion appears to be driven by alterations in neural activation in response to the magnitude of loss. Differences were found primarily in the gamble-unfavorability network, suggesting an underactive cognitive control system in the valuation of potential losses. Compared to HIV-negative participants, HIV-positive participants had lesser activation in the VLPFC, lOFC, dorsal PFC, central precuneus, PPC, and thalamus. This finding is consistent with a growing number of studies that have identified HIV-associated alterations in the fronto-parietal network. While a recent review concluded that HIV-positive persons demonstrate hyperactivity in task-relevant regions despite equivalent performance (Hakkers et al., 2017), a few studies have also reported hypoactivations. For example, Plessis et al. (2015) found that HIV was associated with hypoactivation in the ventral striatum during a task involving reward anticipation and reward outcome. Consistent with compensation theory (Barulli and Stern, 2013), two recent studies have shown that HIV-positive participants exhibit greater activation on easier tasks, but lesser activation as the task becomes more difficult (Caldwell et al., 2014; Meade et al., 2016). In another fMRI study that examined decision making in the context of uncertainty, HIV-positive individuals had greater activation than HIV-negative individuals in the ACC, DLPFC, basal ganglia, and thalamus when choosing risky gambles, but lesser activation in the ACC and DLPFC when choosing safe gambles (Connolly et al., 2014). The authors suggested that this may reflect overvaluation of larger rewards that are associated with uncertain outcomes (high gain/high risk) (Connolly et al., 2014). Thus, inconsistencies may reflect differences in the experimental paradigms utilized across studies. Collectively, these studies indicate that HIV is associated with dysregulation in fronto-parietal and sub-cortical regions during risky decision making. Future studies with experimental tasks that parse out the effects of risk probability and gain/loss magnitude are needed to understand the context in which compensatory activation emerges.
This is the first neuroimaging study on loss aversion conducted with a clinical sample characterized by drug abuse and HIV infection – two conditions that commonly co-occur. The strengths of our study include a robust sample size of 69, closely matched groups in terms of demographics, cocaine use, and HIV disease characteristics, and the use of a previously validated paradigm to examine neural activation in response to gain and loss valuation during economic risky choice. Since both cocaine and HIV are known to affect the neural systems involved in decision making, it is not surprising that we found a wider range of neural responsiveness to both gain and loss than previously reported. Nevertheless, comparisons to previous studies should be interpreted with caution, since subtle variations in data collection, processing, and statistical modeling would affect results. Our study is also not without limitations. Despite the relatively large sample, we were underpowered to detect small interaction effects between cocaine and HIV on the neural processing of gain and loss. Second, to simplify the interpretation of the main effects in this multi-group analysis, this study focused on identifying responses to gain and loss separately. This strategy allowed us to identify the unique effects of HIV and cocaine on the discrete networks associated in the valuation of risky monetary choices. Future studies may consider also examining the simultaneous evaluation of loss and gain, as was done as a follow-up analysis in the Tom et al. (2007) study. Furthermore, while we refer to discrete gain-and loss-related networks, we did not perform analyses of functional or effectivity connectivity of these networks. Such analyses could determine the extent to which these networks overlap in space or function. Finally, although participants were excluded for any history of “hard” drug use, we permitted the use of alcohol, marijuana, and nicotine due to their high prevalence in cocaine-using populations. Since cocaine users were indeed more likely than the non-cocaine users to use these substances, it is possible that the identified effects are not specific to cocaine, but rather to poly-drug use. Further research is necessary to characterize whether the neurobehavioral alterations arising from poly-drug use differ from those observed in single-drug use.
In conclusion, we speculate that the diminished loss aversion observed in HIV-positive cocaine users may be driven by the combined impact of an overactive reward-seeking system and an underactive cognitive control system. Specifically, this group evidenced distinct neural activation patterns related to cocaine dependence in the network associated with the evaluation of the favorable characteristics of the gamble (involving VMPFC, lOFC, ACC, and posterior precuneus) and HIV infection in the network associated with the evaluation of the unfavorable characteristics of the gamble (involving fronto-parietal regions). These neural alterations may help to explain the high rates of risk-taking in HIV-positive drug users, including sexual risk behaviors and poor medication adherence that contribute to the forward transmission of HIV to others. Treatment approaches that reward the inhibition of unhealthy behaviors (e.g., contingency management) may capitalize on the hyper-responsivity to gains observed in persons with cocaine use disorders. At the same time, cognitive training interventions that improve functioning of the cognitive control system in HIV-positive persons may improve decision making by increasing responsivity to unfavorable risks. Multi-pronged interventions that target both of these neural systems may have promise for improving decision making in this high-risk group.
Supplementary Material
Matrix maps of choices on the coin flip task from four example participants. Each trial was a combination of 11 potential gains and 11 potential losses (excluding the $0 gain/$0 loss option), resulting in 120 trials. Accepted gambles are shown as gray squares, rejected gambles are white squares, and missed trials are blue squares. The top row shows examples of two participants who responded consistently, with loss aversion scores of 2.02 and 1.31 in A and B, respectively. The bottom row shows examples of two participants who were excluded from subsequent analyses for random responding.
Separable networks of gain and loss-related BOLD activation. Green depicts regions of activation in response to increasingly favorable gambles (increasing gains and decreasing losses): ventromedial prefrontal cortex (VMPFC), cuneus (Cun), visual cortex (VC), precuneus (PC), parietal operculum (PO), occipital pole (OP), and lingual gyrus (LG). Red depicts regions of activation to increasingly unfavorable gambles (decreasing gains and increasing losses): dorsomedial prefrontal cortex (DMPFC), dorsolateral prefrontal cortex (DLPFC), lateral orbitofrontal cortex (OFC), posterior parietal cortex (PPC), anterior insula (AI), pallidum (PAL), and frontal pole (FP). Clusters were thresholded at z > 2.3 (p < .01) with ≥80 contiguous voxels for an overall false positive detection rate of p < .05. This image represents the mask applied to the correlation and ANOVA results; the combination of both networks was used to create the mask.
References
- Andersson JLR, Jenkinson M, Smith S (2007) Non-linear registration, aka spatial normalisation In: FMIRB Analysis Group Technical Reports. Oxford Centre for Functional MRI of the Brain: Oxford, United Kingdom. [Google Scholar]
- Bairwa D, Kumar V, Vyas S, Das BK, Srivastava AK, Pandey RM, Sharma SK, Jagannathan NR, Sinha S (2016) Case control study: magnetic resonance spectroscopy of brain in HIV infected patients. BMC Neurol 16:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barulli D, Stern Y (2013) Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci 17:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM (2003) General multilevel linear modeling for group analysis in FMRI. NeuroImage 20:1052–1063. [DOI] [PubMed] [Google Scholar]
- Buch S, Yao H, Guo M, Mori T, Seth P, Wang J, Su TP (2012) Cocaine and HIV-1 interplay in CNS: cellular and molecular mechanisms. Curr HIV Res 10:425–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JZK, Gongvatana A, Navia BA, Sweet LH, Tashima K, Ding M, Cohen RA (2014) Neural dysregulation during a working memory task in Human Immunodeficiency Virus-seropositive and Hepatitis C coinfected individuals. J Neurovirol 20:398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa N, Crespi C, Motterlini M, Baud-Bovy G, Chierchia G, Pantaleo G, Tettamanti M, Cappa SF (2013) The functional and structural neural basis of individual differences in loss aversion. J Neurosci 33:14307–14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Bischoff-Grethe A, Jordan SJ, Woods SP, Ellis RJ, Paulus MP, Grant I, Translational Methamphetamine Aids Research Center (TMARC) Group (2014) Altered functional response to risky choice in HIV infection. PLoS ONE 9:e111583. [Google Scholar]
- Daskalopoulou M, Rodger A, Phillips AN, Sherr L, Speakman A, Collins S, Elford J, Johnson MA, Gilson R, Fisher M, Wilkins E, Anderson J, McDonnell J, Edwards S, Perry N, O’Connell R, Lascar M, Jones M, Johnson AM, Hart G, Miners A, Geretti AM, Burman WJ, Lampe FC (2014) Recreational drug use, polydrug use, and sexual behaviour in HIV-diagnosed men who have sex with men in the UK: results from the cross-sectional ASTRA study. Lancet HIV 1:e22–31. [DOI] [PubMed] [Google Scholar]
- De Martino B, Camerer CF, Adolphs R (2010) Amygdala damage eliminates monetary loss aversion. Proc Natl Acad Sci U S A 107:3788–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31:968–980. [DOI] [PubMed] [Google Scholar]
- El-Bassel N, Shaw SA, Dasgupta A, Strathdee SA (2014) Drug use as a driver of HIV risks: re-emerging and emerging issues. Curr Opin HIV AIDS 9:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (1996) Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient/Non-patient Edition Biometrics Research, New York State Psychiatric Institute: New York. [Google Scholar]
- Fujiwara E, Tomlinson SE, Purdon SE, Gill MJ, Power C (2015) Decision making under explicit risk is impaired in individuals with human immunodeficiency virus (HIV). J Clin Exp Neuropsychol 37:733–750. [DOI] [PubMed] [Google Scholar]
- Gamarel KE, Woolf-King SE, Carrico AW, Neilands TB, Johnson MO (2015) Stimulant use patterns and HIV transmission risk among HIV-serodiscordant male couples. J Acquir Immune Defic Syndr 68:147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR (2004) Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials 5:74–79. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Cottone LA, Zhang L, Telang F, Volkow ND (2007) Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug Alcohol Depend 87:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B (2010) The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkers CS, Arends JE, Barth RE, Du Plessis S, Hoepelman AIM, Vink M (2017) Review of functional MRI in HIV: Effects of aging and medication. J Neurovirol 23:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Cattie JE, Doyle K, Grant I, The HIV. Neurobehavioral Research Program Group (2013) Risky decision-making in HIV-associated neurocognitive disorders (HAND). Clin Neuropsychol 2:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) FSL. NeuroImage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Wolfson T, Letendre SL, Ellis RJ, Heaton RK, Gamst AC, Franklin DR Jr., Clifford DB, Collier AC, Gelman BB, Marra, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I(2011) Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol 17:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MR, Berger A, Hemberg J, O’Neill A, Dyer TP, Smyrk K (2013) Non-injection and injection drug use and STI/HIV risk in the United States: the degree to which sexual risk behaviors versus sex with an STI-infected partner account for infection transmission among drug users. AIDS Behav 17:1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Schellekens AF, Kuhn S, Machielse MW, Sescousse G (2017) Disruption of reward processing in addiction: An image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR (2011) Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology 216:305–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, Gonzalez R, Vassileva J, Maki PM, Bechara A, Brand M (2016) Sex and HIV serostatus differences in decision making under risk among substance-dependent individuals. J Clin Exp Neuropsychol 38:404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M (1992) The fifth edition of the Addiction Severity Index. J Subst Abuse Treat 9:199–213. [DOI] [PubMed] [Google Scholar]
- Meade CS, Cordero DM, Hobkirk AL, Metra BM, Chen N-K, Huettel SA (2016) Compensatory activation in fronto-parietal cortices among HIV-infected persons during a monetary decision-making task. Hum Brain Mapp 37:2455–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimiaga MJ, Reisner SL, Grasso C, Crane HM, Safren SA, Kitahata MM, Schumacher JE, Mathews WC, Mayer KH (2013) Substance use among HIV-infected patients engaged in primary care in the United States: findings from the Centers for AIDS Research Network of Integrated Clinical Systems cohort. Am J Public Health 103:1457–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS (2012) Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci 12:241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessis SD, Vink M, Joska JA, Koutsilieri E, Bagadia A, Stein DJ, Emsley R (2015) HIV infection results in ventral-striatal reward system hypo-activation during cue processing. AIDS 29:1335–1343. [DOI] [PubMed] [Google Scholar]
- Plessis SD, Vink M, Joska JA, Koutsilieri E, Stein DJ, Emsley R (2014) HIV infection and the fronto-striatal system: a systematic review and meta-analysis of fMRI studies. AIDS 28:803–811. [DOI] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, Leo GI (2014) Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav 28:154–162. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Noonan MP, Boorman ED, Walton ME, Behrens TE (2011) Frontal cortex and reward-guided learning and decision-making. Neuron 70:1054–1069. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, Ragin A, Levine A, Miller E (2016) Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 86:334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Mattick RP, Jamadar SD, Iredale JM (2014) Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend 145:1–33. [DOI] [PubMed] [Google Scholar]
- Sokol-Hessner P, Camerer CF, Phelps EA (2013) Emotion regulation reduces loss aversion and decreases amygdala responses to losses. Soc Cogn Affect Neurosci 8:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk DB, van Wel JHP, Ramaekers JG, Verkes RJ (2013) Characterizing the cognitive effects of cocaine: A comprehensive review. Neurosci Biobehav Rev 37:1838–1859. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Stockman JK (2010) Epidemiology of HIV among injecting and non-injecting drug users: current trends and implications for interventions. Curr HIV/AIDS Rep 7:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration CfBHSaQ (2016) Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health Substance Abuse and Mental Health Services Administration: Rockville, MD. [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA (2007) The neural basis of loss aversion in decision-making under risk. Science 315:515–518. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D (1992) Advances in prospect theory: Cumulative representation of uncertainty. J Risk Uncertainty 5:297–323. [Google Scholar]
- Venkatraman V, Payne JW, Bettman JR, Luce MF, Huettel SA (2009) Separate neural mechanisms underlie choices and strategic preferences in risky decision making. Neuron 62:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F (2011) Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A 108:15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD (2000) Simultaneous inference for fMRI data. Biophysics Research Institute: Medical College of Wisconsin. [Google Scholar]
- Wechsler D (2001) Wechsler Test of Adult Reading (WTAR) Manual. Harcourt Assessment: San Antonio, TX. [Google Scholar]
- Wittwer A, Hulka LM, Heinimann HR, Vonmoos M, Quednow BB (2016) Risky decisions in a lottery task are associated with an increase of cocaine use. Front Psychol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PW, Vaida FF, Fernández RJ, Rutlin J, Price RW, Lee E, Peterson J, Fuchs D, Shimony JS, Robertson KR, Walter R, Meyerhoff DJ, Spudich S, Ances BM (2015) Cerebral white matter integrity during primary HIV infection. AIDS 29:433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Matrix maps of choices on the coin flip task from four example participants. Each trial was a combination of 11 potential gains and 11 potential losses (excluding the $0 gain/$0 loss option), resulting in 120 trials. Accepted gambles are shown as gray squares, rejected gambles are white squares, and missed trials are blue squares. The top row shows examples of two participants who responded consistently, with loss aversion scores of 2.02 and 1.31 in A and B, respectively. The bottom row shows examples of two participants who were excluded from subsequent analyses for random responding.
Separable networks of gain and loss-related BOLD activation. Green depicts regions of activation in response to increasingly favorable gambles (increasing gains and decreasing losses): ventromedial prefrontal cortex (VMPFC), cuneus (Cun), visual cortex (VC), precuneus (PC), parietal operculum (PO), occipital pole (OP), and lingual gyrus (LG). Red depicts regions of activation to increasingly unfavorable gambles (decreasing gains and increasing losses): dorsomedial prefrontal cortex (DMPFC), dorsolateral prefrontal cortex (DLPFC), lateral orbitofrontal cortex (OFC), posterior parietal cortex (PPC), anterior insula (AI), pallidum (PAL), and frontal pole (FP). Clusters were thresholded at z > 2.3 (p < .01) with ≥80 contiguous voxels for an overall false positive detection rate of p < .05. This image represents the mask applied to the correlation and ANOVA results; the combination of both networks was used to create the mask.


