Abstract
BACKGROUND
We previously reported that a median of 5.6 years of intensive as compared with standard glucose lowering in 1791 military veterans with type 2 diabetes resulted in a risk of major cardiovascular events that was significantly lower (by 17%) after a total of 10 years of combined intervention and observational follow-up. We now report the full 15-year follow-up.
METHODS
We observationally followed enrolled participants (complete cohort) after the conclusion of the original clinical trial by using central databases to identify cardiovascular events, hospitalizations, and deaths. Participants were asked whether they would be willing to provide additional data by means of surveys and chart reviews (survey cohort). The prespecified primary outcome was a composite of major cardiovascular events, including nonfatal myocardial infarction, nonfatal stroke, new or worsening congestive heart failure, amputation for ischemic gangrene, and death from cardiovascular causes. Death from any cause was a prespecified secondary outcome.
RESULTS
There were 1655 participants in the complete cohort and 1391 in the survey cohort. During the trial (which originally enrolled 1791 participants), the separation of the glycated hemoglobin curves between the intensive-therapy group (892 participants) and the standard-therapy group (899 participants) averaged 1.5 percentage points, and this difference declined to 0.2 to 0.3 percentage points by 3 years after the trial ended. Over a period of 15 years of follow-up (active treatment plus post-trial observation), the risks of major cardiovascular events or death were not lower in the intensive-therapy group than in the standard-therapy group (hazard ratio for primary outcome, 0.91; 95% confidence interval [CI], 0.78 to 1.06; P = 0.23; hazard ratio for death, 1.02; 95% CI, 0.88 to 1.18). The risk of major cardiovascular disease outcomes was reduced, however, during an extended interval of separation of the glycated hemoglobin curves (hazard ratio, 0.83; 95% CI, 0.70 to 0.99), but this benefit did not continue after equalization of the glycated hemoglobin levels (hazard ratio, 1.26; 95% CI, 0.90 to 1.75).
CONCLUSIONS
Participants with type 2 diabetes who had been randomly assigned to intensive glucose control for 5.6 years had a lower risk of cardiovascular events than those who received standard therapy only during the prolonged period in which the glycated hemoglobin curves were separated. There was no evidence of a legacy effect or a mortality benefit with intensive glucose control. (Funded by the VA Cooperative Studies Program; VADT ClinicalTrials.gov number, .)
OBSERVATIONAL COHORT STUDIES HAVE shown increasing risks of both macro-vascular and micro-vascular events with increasing average blood glucose levels.1–7 Trials involving patients with type 1 diabetes and patients with new-onset type 2 diabetes showed that improving glucose control reduced the incidence of micro-vascular complications of diabe-tes.8,9 Although these trials did not show significant reductions in the incidence of cardiovascular disease, their observational follow-up reports indicated a reduced risk of cardiovascular out-comes and reduced mortality.10,11 In contrast, trials involving patients with advanced type 2 diabetes (such as ACCORD [Action to Control Cardiovascular Risk in Diabetes], ADVANCE [Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation], and our own VADT [Veterans Affairs Diabetes Trial]) showed that improved glucose control over a median of 3 to 6 years provided modest and nonsignificant reductions in the incidence of cardiovascular events and did not reduce cardiovascular disease–related mortality or total mortality.12–15 However, a 10-year follow-up of the VADT showed an emerging benefit from the original intensive glucose lowering with regard to cardiovascular events.16
Longer-term follow-up of glucose lowering in patients with type 2 diabetes may help to clarify the duration of the potential benefit with regard to cardiovascular disease. Unlike in the United Kingdom Prospective Diabetes Study (UKPDS),17 observational follow-up of these three aforementioned trials involving patients with type 2 diabetes has yet to yield evidence of a “legacy effect.” However, with greater separation of the glycated hemoglobin curves between the treatment groups and with longer observational follow-up, the VADT was well suited to examine whether a reduction in the risk of new cardiovascular events with improved glucose control in the past was pre-served or expanded after the glucose levels equalized between the treatment groups. Extended follow-up may also be necessary to reveal the effects of intensive glucose lowering on the out-come of total mortality.
The VADT follow-up study (VADT-F) was de-signed to examine long-term consequences of intensive glycemic control on cardiovascular disease outcomes, quality of life, and mortality and, as previously reported,16 provides an opportunity for assessing legacy effects.18 We now present the prespecified 15-year results of our trial, which included nearly 10 years of observational follow-up after almost 6 years of intensive glucose lowering as compared with standard glucose lowering.
METHODS
STUDY DESIGN
The original and follow-up designs of our trial have been published previously.13,16,19 In brief, the original trial included 1791 military veterans with type 2 diabetes who were randomly assigned to receive either intensive or standard glucose control. The goal for the intensive-therapy group was normal glycated hemoglobin levels, with a median level that was more than 1.5 percentage points lower than that in the standard-therapy group. The goal in the standard-therapy group was a glycated hemoglobin level between 8% and 9%. Participants who were alive and had not withdrawn at the conclusion of the trial (median follow-up, 5.6 years) were followed through national data registries (the complete cohort). Some participants (the survey cohort) consented to additional data collection, including yearly survey and chart reviews to detect outcomes and quality-of-life assessments20 that were not obtained from central data registries. Health-related quality of life was assessed on a scale from 0 to 100, with higher scores indicating better quality of life; a difference of 5 points was considered to be clinically meaningful. Detailed descriptions of these cohorts and assessments, as previously report-ed,13,16 are provided in Methods Sections A through I in the Supplementary Appendix (available with the full text of this article at NEJM.org).
Two authors who are statisticians vouch for the completeness and accuracy of the data and analyses. The authors vouch for the fidelity of the study to the protocol (available at NEJM.org). The first author wrote the manuscript, with all the coauthors approving the final version for submission. The statistical analysis plan was prespecified by the lead investigators before data access or knowledge of results. The protocol was approved by the Hines and Ann Arbor Veterans Affairs (VA) health system institutional review boards and was reviewed annually by a data and safety monitoring committee.
DATA SOURCES
As previously described,16 four national data registries — the central VA medical files, the Centers for Medicare and Medicaid Services (CMS) claims files, the VA death files, and the National Death Index (NDI) — were used to collect data and information about outcomes. Participants in the survey cohort also received annual self-administered surveys that queried for major events (e.g., myocardial infarctions and strokes) during the previous year. Medical records were reviewed and adjudicated as previously described16 (see the Methods Sections and Table S1 in the Supplementary Appendix) for participants younger than 65 years of age (CMS ineligible) reporting an event outside the VA system. Because of delays in the availability of registry data for some out-comes, we decided a priori that our primary analysis of the first major cardiovascular event (in a time-to-event analysis) would be limited to the period with complete capture of data about primary-outcome events (median follow-up, 13.6 years). Analyses of total mortality (in the complete cohort) used available outcome data at a median follow-up of nearly 15 years.
PRIMARY AND SECONDARY OUTCOMES
The prespecified primary outcome was the first major cardiovascular event (composite of nonfatal myocardial infarction, nonfatal stroke, new or worsening congestive heart failure, amputation for ischemic gangrene, or death from cardiovascular causes) in a time-to-event analysis. Prespecified secondary outcomes included death from cardiovascular causes, death from any cause, any major diabetes outcome (primary composite out-come plus end-stage renal disease [defined as a glomerular filtration rate of <15 ml per minute per 1.73 m2 of body-surface area, dialysis, or kidney transplantation] or nontraumatic amputation), and health-related quality of life. Hospitalizations were captured as part of protocol-specified goals to monitor health costs and were considered to be a prespecified tertiary outcome. During observational follow-up, outcomes were identified by means of the primary discharge diagnosis in VA or CMS registry files (as previously validated)21,22 or by review of outside records, as previously de-scribed (see Methods Section B in the Supplementary Appendix).16
STATISTICAL ANALYSIS
Data were censored when participants had a primary-outcome event, died, or withdrew from the study. The main intention-to-treat analyses used Kaplan–Meier survival curves generated by the product-limit method. Cox proportional-hazards modeling was used to estimate hazard ratios. In prespecified mediation analyses, Cox proportional-hazards models were used to examine the effects of the glycated hemoglobin level on the primary cardiovascular disease outcome and on the observed treatment effects. Specifically, the log-linear association of the cumulative glycated hemoglobin level (modeled as a time-varying covariate) with the primary cardio-vascular disease outcome was assessed during the period of separation of the glycated hemoglobin curves and after convergence (see Methods Section G in the Supplementary Appendix). Models examined the effect of treatment group (intensive therapy or standard therapy) on the primary outcome in an unadjusted analysis (model 1) or while accounting for baseline, most recent, or cumulative mean glycated hemoglobin level (models 2, 3, and 4, respectively).
We also conducted post hoc sensitivity analyses (not included in the protocol; with time intervals prespecified by the lead investigators) to compare the role of glucose control in different intervals of the cumulative follow-up period. The last observation of the glycated hemoglobin level was carried forward in these analyses owing to slow changes in glycated hemoglobin values over time and the low frequency of missing data. Proportionality assumptions held for 10 years but not thereafter, but relaxing the assumptions did not substantially affect any results.
We prespecified three variables (duration of diabetes, baseline cardiovascular risk [according to the baseline UKPDS score23], and history of cardiovascular disease) to examine heterogeneity within the treatment effects with regard to the primary outcome and any major diabetes out-come.24 Negative binomial regression was used to examine the number of hospitalizations, with adjustment for follow-up time. Sample-selection bias in persons who consented to participate in the survey cohort in the follow-up study was assessed with the use of primary and secondary outcomes in the complete cohort. We estimated that data collection in the larger, complete cohort missed less than 6% of the outcomes owing to delays in the availability of CMS and NDI data (see Methods Section B in the Supplementary Appendix). The frequency of missing data for key covariates, such as for the glycated hemoglobin level (missing for <5% of visits), was modest and was handled by carrying the most recent value forward.
RESULTS
CHARACTERISTICS OF THE PARTICIPANTS AT BASELINE
According to the baseline characteristics, most of the participants were men, were overweight or obese, and had a mean duration of diabetes of approximately 12 years (Table 1). No meaningful differences were observed between the baseline characteristics of the complete and survey cohorts or between the treatment groups (Table S2 in the Supplementary Appendix). The recruitment and follow-up flowchart has been published pre-viously16 and is provided in Figure S1 in the Supplementary Appendix.
Table 1.
Baseline Characteristics of the Participants in the Follow-up Study, According to Cohort.*
| Characteristic | Complete Cohort (N = 1655) |
Survey Cohort (N=1391) |
|---|---|---|
| Age — yr | 60.5±8.7 | 61.1±8.7 |
| Sex — no. (%) | ||
| Male | 1608 (97.2) | 1353 (97.3) |
| Female | 47 (2.8) | 38 (2.7) |
| Time since diabetes diagnosis — yr | 11.6±7.5 | 11.8±7.7 |
| Previous cardiovascular event — no. (%) | 673 (40.7) | 595 (42.8) |
| Hypertension — no./total no. (%)† | 1197/1652 (72.5) | 1014/1389 (73.0) |
| Race or ethnic group — no. (%)‡ | ||
| Non-Hispanic white | 1024 (61.9) | 886 (63.7) |
| Hispanic white | 265 (16.0) | 222 (16.0) |
| Black | 282 (17.0) | 220 (15.8) |
| Other | 84 (5.1) | 63 (4.5) |
| Tobacco smoking status — no./total no. (%) | ||
| Current | 270/1653 (16.3) | 210/1389 (15.1) |
| Past | 933/1653 (56.4) | 791/1389 (56.9) |
| Never | 450/1653 (27.2) | 388/1389 (27.9) |
| Body-mass index§ | 31.2±4.4 | 31.2±4.4 |
| Blood pressure — mm Hg | ||
| Systolic | 131.6±16.6 | 131.9±16.6 |
| Diastolic | 76.1±10.2 | 76.0±10.2 |
| Glycated hemoglobin level — % | 9.4±1.5 | 9.4±1.5 |
| Cholesterol — mg/dl | ||
| Total | 183.1±47.4 | 181.8±43.6 |
| Low-density lipoprotein | 107.7±32.5 | 106.4±31.5 |
| High-density lipoprotein | 36.0±10.2 | 35.7±9.8 |
| Triglycerides — mg/dl | ||
| Mean | 211.3±281.1 | 210.5±215.7 |
| Median (interquartile range) | 159 (112–238) | 162 (113–241) |
| Estimated GFR¶ | 82.0±22.2 | 81.1±22.1 |
| Creatinine — mg/dl | 1.0±0.2 | 1.0±0.2 |
| Ratio of albumin (in milligrams) to creatinine (in grams) | 113.0±338.1 | 118.9±352.7 |
| Estimated 10-yr cardiovascular risk — %‖ | 40±20 | 40±20 |
Plus–minus values are means ±SD. Baseline values were measured on entry into the original trial. The complete cohort included all the participants who were alive and enrolled at the conclusion of the trial and who had not specifically requested to withdraw from all additional follow-up. The survey cohort included a subgroup of participants who agreed to additional yearly survey follow-up. Percentages may not total 100 because of rounding. To convert values for glycated hemoglobin to millimoles per mole, multiply by 10.93 and then subtract 23.50. To convert values for cholesterol to millimoles per liter, multiply by 0.02586. To convert values for triglycerides to millimoles per liter, multiply by 0.01129. To convert values for creatinine to millimoles per liter, multiply by 88.4. The baseline characteristics according to trial group within each cohort are presented in Table S2 in the Supplementary Appendix.
Hypertension was defined as current treatment for hypertension or a blood pressure of 140/90 mm Hg or more.
Race and ethnic group were reported by the participant.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The estimated glomerular filtration rate (GFR) was calculated with the use of the Modification of Diet in Renal Disease Study equation.
The estimated 10-year cardiovascular risk was calculated with the use of the United Kingdom Prospective Diabetes Study Risk Engine, which takes into account weight and the levels of glycated hemoglobin, cholesterol, creatinine, and triglycerides (www.dtu.ox.ac.uk/riskengine).
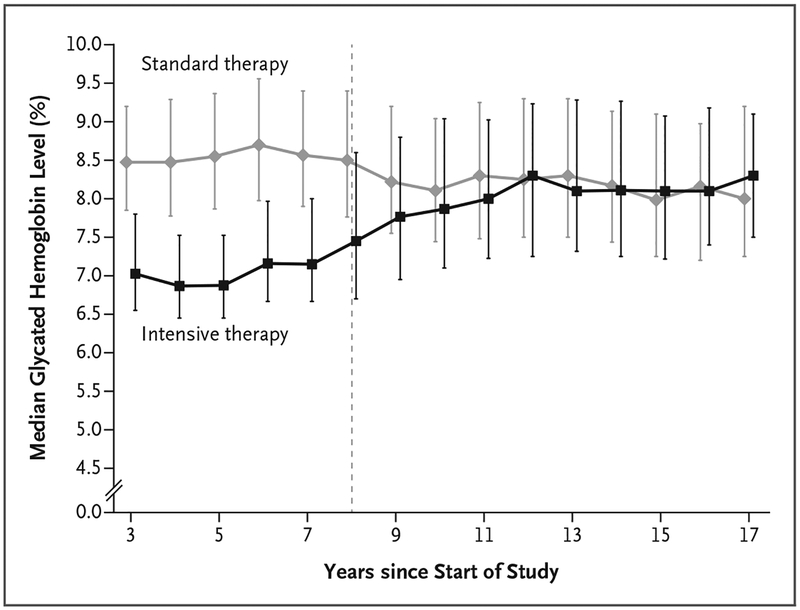
During the VADT, the median glycated hemoglobin level in the intensive-therapy group was 6.9%, and the median separation of the glycated hemoglobin curves between treatment groups was 1.5 percentage points over a median of 5.6 years (Fig. 1), as previously reported.13 The separation of the glycated hemoglobin curves gradually diminished to 0.2 to 0.3% in the 3 years after trial completion, and the glycated hemoglobin levels subsequently stabilized at a median of approximately 8% in both groups. The medications for treating diabetes that were used during the VADT to lower glucose levels were similar in the two groups, with only slightly higher doses of thia-zolidinediones, insulin, oral sulfonylureas, and acarbose used in the intensive-therapy group than in the standard-therapy group during the active treatment period (Table S3 in the Supplementary Appendix). Use of these medications was almost identical in the two treatment groups by the end of the VADT-F.
Figure 1. Median Glycated Hemoglobin Levels According to Year since Start of the Trial, Starting at Year 3.
Year 3 was a point at which all the participants had been enrolled and had been receiving treatment per protocol for at least 3 months. The I bars (slightly offset for better visibility) represent interquartile ranges. The dotted line represents the end of the interventional component of the trial and the beginning of the follow-up period. To convert values for glycated hemoglobin to millimoles per mole, multiply by 10.93 and then subtract 23.50.
Intensive glucose control was associated with weight gain during active treatment, which remained higher (by a mean body-mass index [the weight in kilograms divided by the square of the height in meters] of 1.3; 95% confidence interval [CI], 1.1 to 1.5) from year 3 (i.e., the time when all participants were enrolled) through year 17 (Fig. S2A in the Supplementary Appendix). Per protocol, other cardiovascular disease risk factors were aggressively treated in both treatment groups during the VADT, and the levels of blood pressure, high-density lipoprotein (HDL) cholesterol, non-HDL cholesterol, and triglycerides were similar in the two groups throughout the cumulative follow-up period (Fig. S2B through S2F in the Supplementary Appendix). The use of statins and medications for hypertension was similar during both the VADT and VADT-F (Tables S3 through S5 in the Supplementary Appendix).
PRIMARY AND SECONDARY OUTCOMES
At a median follow-up of 13.6 years, we found that the risk of the primary composite cardiovascular disease outcome was nonsignificantly lower, by 9%, in the intensive-therapy group than in the standard-therapy group (hazard ratio, 0.91; 95% CI, 0.78 to 1.06; P=0.23) (Table 2). Nonsignificantly lower risks of any major diabetes event (hazard ratio, 0.90; 95% CI, 0.78 to 1.04) and death from cardiovascular causes (hazard ratio, 0.94; 95% CI, 0.73 to 1.20) were also seen in the intensive-therapy group than in the standard therapy group. At a median follow-up of 15 years, there was no evidence of shorter time to death with intensive treatment than with standard therapy (hazard ratio, 1.02; 95% CI, 0.88 to 1.18), nor was there evidence of improved quality-of-life scores (mean scores, 63.8 and 62.2, respectively) (Table 2). Results for the individual components of the above composite outcomes are provided in Tables S7 through S9 in the Supplementary Appendix.
Table 2.
Effect of Intensive Glucose Treatment on Major Cardiovascular Events, Mortality, and Health-Related Quality of Life.*
| Outcome | Intensive Therapy | Standard Therapy | Hazard Ratio or Difference (95% CI) |
P Value |
|---|---|---|---|---|
| Primary outcome: major cardiovascular event — no./total no. (rate per 1000 person-yr) | 325/703 (47.3) | 336/688 (51.8) | 0.91 (0.78 to 1.06) | 0.23 |
| Secondary outcomes | ||||
| Any major diabetes outcome — no./total no. (rate per 1000 person-yr) | 342/703 (50.4) | 355/688 (55.7) | 0.90 (0.78 to 1.04) | |
| Death from cardiovascular causes — no./total no. (rate per 1000 person-yr) | 118/837 (12.3) | 125/818 (13.1) | 0.94 (0.73 to 1.20) | |
| Death from any cause — no./total no. (rate per 1000 person-yr) | 376/837 (36.6) | 366/818 (35.9) | 1.02 (0.88 to 1.18) | |
| Health-related quality-of-life score† | 63.8±17.2 | 62.2±17.6 | 1.6 (−0.7 to 3.9) | |
The primary outcome was a composite of myocardial infarction, stroke, new or worsening congestive heart failure, amputation for ischemic gangrene, or death from cardiovascular disease causes and was analyzed in the survey cohort. Any major diabetes outcome included the primary composite outcome plus nontraumatic amputations and end-stage renal disease (defined as an estimated GFR of <15 during the original trial period or as an estimated GFR of <15 or dialysis or kidney transplantation during the follow-up study) and, along with healthrelated quality of life, was analyzed in the survey cohort. Mortality outcomes were analyzed in the complete cohort. All outcomes were prespecified. The confidence intervals were not adjusted for multiple testing, and inferences drawn from the intervals may not be reproducible.
Health-related quality of life was assessed on a scale from 0 to 100, with higher scores indicating better quality of life. The difference between the mean values is presented. A difference in the health-related quality-of-life score of 5 points was considered to be clinically meaningful.
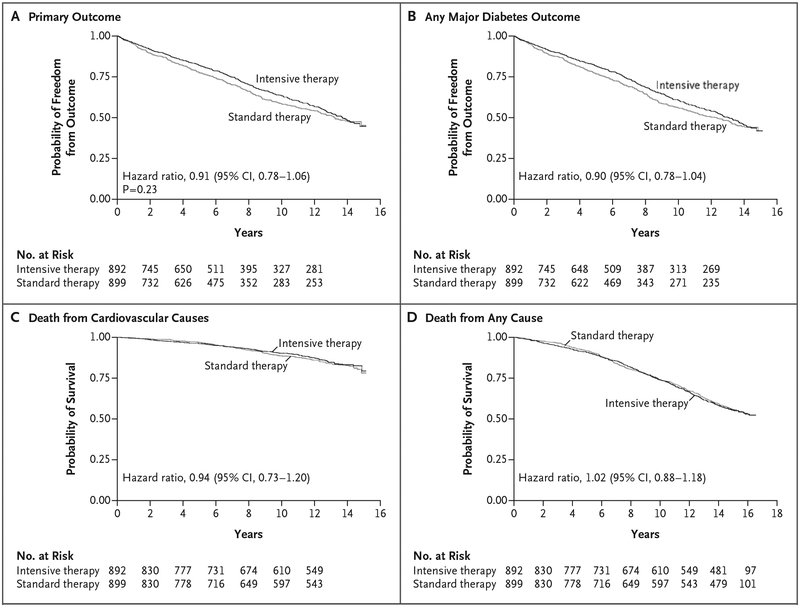
Kaplan–Meier survival curves for the primary and secondary outcomes are shown in Figure 2 and provide a more detailed overview of our time-to-event results. To examine the robustness of these findings, we repeated analyses for the primary outcome using the larger, complete cohort and found similar results (hazard ratio, 0.95; 95% CI, 0.83 to 1.09). We found no evidence that treatment effects varied across our three pre-specified risk factors of duration of diabetes, history of cardiovascular disease, or cardiovascular disease risk (Table S10 in the Supplementary Appendix). Rates of hospitalization were similar in the two treatment groups (160 events per 1000 person-years in the intensive-therapy group and 165 events per 1000 person-years in the standard-therapy group; hazard ratio, 0.98; 95% CI, 0.88 to 1.09), as was the median number of hospitalizations (3 hospitalizations in each group; incidence rate ratio, 1.02; 95% CI, 0.89 to 1.17) (Table S11 in the Supplementary Appendix).
Figure 2. Kaplan–Meier Curves for the Primary and Secondary Outcomes during the Trial and Follow-up Period.
The primary outcome was a major cardiovascular event (a composite of myocardial infarction, stroke, new or worsening congestive heart failure, amputation for ischemic gangrene, or death from cardiovascular causes). Secondary outcomes were any major diabetes event (primary composite outcome plus nontraumatic amputation or end-stage renal disease, defined as an estimated glomerular filtration rate [GFR] of <15 during the original trial period or as an estimated GFR of <15 or dialysis or kidney transplantation during the follow-up study), death from cardiovascular causes, and death from any cause.
GLUCOSE CONTROL AS A MEDIATOR OF RISK AND A LEGACY EFFECT
We first compared the event rates of major outcomes during and after the VADT (Table S12 in the Supplementary Appendix). There was no benefit with regard to any major outcome during the observational follow-up period for the trial. We then evaluated the treatment effect in the period before and after glycemic control nearly equalized in the two groups. We found that the risk of primary cardiovascular events was 17% lower in the intensive-therapy group than in the standard-therapy group during the approximate 10-year period when there was a separation of the glycated hemoglobin curves between the two groups (model 1: hazard ratio, 0.83; 95% CI, 0.70 to 0.99) (Table 3). In contrast, in the 5-year period after the glycated hemoglobin levels equalized in the treatment groups, the intensive-therapy group had a modestly higher risk of major cardiovascular events than the standard-therapy group (hazard ratio, 1.26; 95% CI, 0.90 to 1.75). In a sensitivity analysis that used the larger, complete cohort, similar results were seen. However, adjustment for the cumulative mean glycated hemoglobin level during the entire study (Table S13 in the Supplementary Appendix) or during the first 10-year period in which glucose control differed between groups abolished treatment-related differences in the risk of cardiovascular disease (model 4) (Table 3). This finding highlighted the importance of overall differences in glucose control between groups in mediating this outcome.
Table 3.
Effect of Intensive Glucose Therapy on Major Cardiovascular Events during Periods of Separation of Glycated Hemoglobin Curves (Years 0–10) and No Separation (Years 11–15).*
| Variable | Years 0–10 | Years 11–15 |
|---|---|---|
| No. of participants | 1791 | 633 |
| No. of events | 502 | 145 |
| Model and predictors — hazard ratio (95% CI) | ||
| Model 1 | ||
| Intensive therapy or standard therapy | 0.83 (0.70–0.99) | 1.26 (0.90–1.75) |
| Model 2 | ||
| Intensive therapy or standard therapy | 0.83 (0.70–0.99) | 1.27 (0.91–1.76) |
| Baseline glycated hemoglobin level | 1.06 (1.00–1.12) | 1.08 (0.96–1.21) |
| Model 3 | ||
| Intensive therapy or standard therapy | 0.86 (0.71–1.04) | 1.24 (0.89–1.74) |
| Most recent glycated hemoglobin level, as a time-varying covariate | 1.03 (0.97–1.09) | 0.93 (0.84–1.04) |
| Model 4 | ||
| Intensive therapy or standard therapy | 0.94 (0.77–1.15) | 1.37 (0.95–1.96) |
| Cumulative mean glycated hemoglobin level, as a time-varying covariate | 1.11 (1.02–1.20) | 1.09 (0.92–1.28) |
Shown are results from Cox proportional-hazards models for primary cardiovascular outcomes according to time intervals during the Veterans Affairs Diabetes Trial and the follow-up study. Years 0 through 10 reflect the interval of time for which there was evident separation of the glucose curves between the treatment groups. Years 11 through 15 reflect the initial interval when the glycated hemoglobin levels had merged between the two treatment groups and during which all data (from the Veterans Affairs, Centers for Medicare and Medicaid Services, and National Death Index databases) were available. Model 1 examined the effect of treatment group only on primary cardiovascular events during two time periods (years 0 through 10 and years 11 through 15). Models 2, 3, and 4 were applied to the same two time periods and included two predictors: the treatment group (intensive therapy or standard therapy) and the glycated hemoglobin level (baseline value, most recent value as a time-varying covariate, or cumulative mean as a time-varying covariate, respectively). In a sensitivity analysis, we found that only glycated hemoglobin levels from the most recent 3 years were needed to explain the treatment effect and that levels that were observed more than 3 years earlier had no evidence of either predictive value for cardiovascular events or evidence of mediation of the observed reduction in the risk of cardiovascular events in the clinical trial (see the Results Section in the Supplementary Appendix). The numbers of participants in each group reflect the number of participants enrolled in the trial who had not yet had a primary outcome at the beginning of the indicated intervals. The confidence intervals were not adjusted for multiple testing, and inferences drawn from the intervals may not be reproducible.
The lack of a glucose-control legacy effect was also supported in a sensitivity analysis that showed that glycated hemoglobin levels over the most recent 3 years, but not more distant levels, were associated with cardiovascular events (Table S14 in the Supplementary Appendix).
DISCUSSION
In this 15-year follow-up study, we found that 5.6 years of intensive glucose lowering that led to a median separation of 1.5 percentage points in the glycated hemoglobin curves did not result in a significantly lower risk of major cardiovascular events than standard therapy. These results did not vary according to baseline cardiovascular disease risk. Furthermore, we found no evidence of a mortality benefit, no decrease in the incidence of hospitalizations over the full follow-up period, and no long-term difference in quality of life.
The results show that during the approximate 10-year period of separation of the glycated hemoglobin curves (median duration, approximately 7.1 years), there was a significant reduction of nearly 17% (or roughly 10% per 1-point decline in the glycated hemoglobin level) in the risk of the primary cardiovascular disease outcome. These latter results suggest that there are modest long-term cardiovascular benefits of intensive glucose-lowering therapy in patients with more advanced diabetes. Results also show that long-term maintenance of a lower glycated hemoglobin level may be necessary to maintain these improvements. In contrast, there was no continued benefit with regard to the risk of cardiovascular disease after glycated hemoglobin levels equalized in the treatment groups. In fact, there was a decline in benefit with regard to the risk of cardiovascular dis-ease that coincided with equalization of glucose control within treatment groups and this continued during the 5 (or more) years of equal glycemic control that followed.
In addition, almost all the association between glucose control and major cardiovascular events was explained by the mean glycated hemoglobin level in the 3 most recent years, with no evidence that earlier years of glucose control had a carry-over effect. Several sensitivity analyses showed no evidence that 5.6 years of tight glucose control resulted in a legacy effect in our study population. Because the duration of separation of the glycated hemoglobin curves during our original trial and the post-trial observational follow-up was long, our findings of no legacy effect and no mortality benefit expand on similar results in the ACCORD and ADVANCE trials, which also involved older patients with well-established type 2 diabetes.12,13,25,26
Our results of an approximate 10% lower risk of major cardiovascular disease outcomes per 1-point reduction in the glycated hemoglobin level over a period of 4 to 6 years are consistent with the pooled results (9% lower risk of cardio-vascular disease) of the four major trials of tighter glucose control,15 which suggests this is a reasonable estimate of the glucose-lowering benefit in these recent moderate-duration trials in type 2 diabetes. Further support for this finding comes from the mediation analysis showing that cumulative separation of the glycated hemoglobin curves could largely explain the degree of difference in the risk of cardiovascular disease that was observed in the intensive-therapy group as compared with the standard-therapy group. However, these benefits must be weighed against adverse effects such as hypoglycemia and weight gain. Studies showing major reductions in the risk of cardiovascular outcomes with diabetes agents, such as sodium–glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists, that only achieve modest improvements in glycemic control27–29 high-light the importance of also considering non-glycemic approaches to reducing the risks of cardiovascular events and death among high-risk patients with type 2 diabetes.
Our results run counter to the mortality benefits regarding cardiovascular disease and to the legacy effects that have been observed in patients with recently diagnosed type 1 diabetes (in the Diabetes Control and Complications Trial [DCCT]) and in patients with new-onset type 2 diabetes (in the UKPDS).8,9 Several explanations have been proposed regarding a reduced benefit from glucose lowering in older patients with long-standing type 2 diabetes (such as the participants in the ACCORD and ADVANCE trials and in our original trial).30–32 It is possible that underlying atherosclerosis and cardiovascular injury were too advanced in these participants to be effectively altered by glucose lowering. Support for this notion comes from a substudy of our original trial, which showed that participants with high coronary-artery calcium scores (a validated measure of atherosclerosis) at baseline had less reduction in the risk of cardiovascular events with intensive glucose lowering than those with lower coronary-artery calcium scores.33 The DCCT and UKPDS were also conducted at a time before widespread statin use and tight blood-pressure control, whereas participants in the VADT and other recent trials have had aggressive treatment of all cardiovascular disease risk factors. It is plausible that the cardiovascular protective effects of tight glycemic control have diminishing re-turns once other cardiovascular disease risk factors are well controlled by medications that may also have vasoactive properties.
There are several limitations to the current study. The cohort in our original trial consisted largely of older men. However, our results are generally consistent with the findings of the ACCORD and ADVANCE follow-up studies, which had greater representation of women.25,26 Because the post-trial follow-up of events was conducted with the use of electronic records, by necessity the definitions of outcomes varied slightly from those during the active trial. However, patterns of outcomes between treatment groups did not vary notably during the transition from the active trial phase to the observation period, which suggests that these constraints did not alter the results. We also acknowledge that variables not well captured by medical records could have differed between groups and influenced outcomes during the observational follow-up. Our findings that the risk of cardiovascular disease was reduced during the period of separation of the glycated hemoglobin curves and not after equalization and that recent, but not distant, glycated hemoglobin levels mediated the reduction in risk of cardiovascular disease are observational and do not carry the same strength of a true experiment.34 This type of post-trial follow-up also prevented our collection of data regarding retinopathy and neuropathy, thus preventing our ability to address these microvascular complications. Finally, although the duration of glucose lowering in our original trial was substantial, it is possible that more sustained efforts may have led to greater long-term benefits.
In conclusion, in this group of participants with type 2 diabetes who were at high risk for cardiovascular disease, 5.6 years of intensive glucose lowering to a glycated hemoglobin level of 6.9% did not reduce the incidence of major cardiovascular events over a follow-up of 13.6 years or reduce total mortality or improve quality of life over a total follow-up of 15 years. Although there was a significantly lower risk of major cardiovascular events during the 7.1 years of separation of the glycated hemoglobin curves (during the trial and observation periods), there was no evidence of a beneficial legacy effect after this period of improved glucose control.
Supplementary Material
Acknowledgments
The views expressed in this article are those of the authors and do not represent the views of the Department of Veterans Affairs (VA) or the U.S. government.
Supported by the Office of Research and Development of the VA Cooperative Studies Program. The primary VA Diabetes Trial (VADT; CSP 465) received support from the VA Cooperative Studies Program, the American Diabetes Association, and the National Eye Institute. Pharmaceutical and other supplies and financial assistance for the VADT were provided by GlaxoSmithKline, Novo Nordisk, Roche Diagnostics, Sanofi–Aventis, Amylin Pharmaceuticals, and Kos Pharmaceuticals.
Dr. Reaven reports receiving grant support from AstraZeneca, Bristol-Myers Squibb, and Novo Nordisk, fees for serving on an advisory board from Sanofi and Boston Heart Diagnostics, and lecture fees from Takeda Pharmaceutical; and Dr. Emanuele, receiving lecture fees from Merck. No other potential conflict of interest relevant to this article was reported.
We thank Carlos Abraira, M.D., William G. Henderson, M.D., Ph.D., and Grant D. Huang, M.P.H., Ph.D., for assistance in initiating and conducting the VADT; Hertzel Gerstein, M.D., and Juraj Koska, M.D., for suggestions on an earlier version of the manuscript; and Ling Ge, M.S., for statistical programming.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular dis-ease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004;141:413–20. [DOI] [PubMed] [Google Scholar]
- 2.Kirkman MS, McCarren M, Shah J, Duckworth W, Abraira C. The association between metabolic control and prevalent macrovascular disease in Type 2 diabetes: the VA Cooperative Study in diabetes. J Diabetes Complications 2006;20:75–80. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004;141: 421–31. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 1984;102:527–32. [DOI] [PubMed] [Google Scholar]
- 5.Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia 2001;44:156–63. [DOI] [PubMed] [Google Scholar]
- 6.Adler AI, Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Smith DG. Risk factors for diabetic peripheral sensory neuropa-thy: results of the Seattle Prospective Diabetic Foot Study. Diabetes Care 1997;20: 1162–7. [DOI] [PubMed] [Google Scholar]
- 7.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovas-cular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Diabetes Control and Complica-tions Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–53. [PubMed] [Google Scholar]
- 10.Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC Study 30-year follow-up. Diabetes Care 2016;39: 686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89. [DOI] [PubMed] [Google Scholar]
- 12.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–39. [DOI] [PubMed] [Google Scholar]
- 14.The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–72. [DOI] [PubMed] [Google Scholar]
- 15.Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macro-vascular outcomes in type 2 diabetes. Dia-betologia 2009;52:2288–98. [DOI] [PubMed] [Google Scholar]
- 16.Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–206. [DOI] [PubMed] [Google Scholar]
- 17.Murray P, Chune GW, Raghavan VA. Legacy effects from DCCT and UKPDS: what they mean and implications for future diabetes trials. Curr Atheroscler Rep 2010;12:432–9. [DOI] [PubMed] [Google Scholar]
- 18.Chalmers J, Cooper ME. UKPDS and the legacy effect. N Engl J Med 2008;359: 1618–20. [DOI] [PubMed] [Google Scholar]
- 19.Abraira C, Duckworth W, McCarren M, et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications 2003;17:314–22. [DOI] [PubMed] [Google Scholar]
- 20.The DCCT Research Group. Reliability and validity of a diabetes quality-of-life measure for the Diabetes Control and Complications Trial (DCCT). Diabetes Care 1988;11:725–32. [DOI] [PubMed] [Google Scholar]
- 21.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Ac-curacy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J 2004;148:99–104. [DOI] [PubMed] [Google Scholar]
- 22.Petersen LA, Wright S, Normand SL, Daley J. Positive predictive value of the diagnosis of acute myocardial infarction in an administrative database. J Gen Intern Med 1999;14:555–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.UKPDS risk engine. Oxford, United Kingdom: Oxford Centre for Diabetes, Endocrinology and Metabolism, 2017. (www.dtu.ox.ac.uk/riskengine). [Google Scholar]
- 24.Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials 2010;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The ACCORD Study Group. Nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Dia-betes Care 2016;39:701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zoungas S, Chalmers J, Neal B, et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med 2014;371:1392–406. [DOI] [PubMed] [Google Scholar]
- 27.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44. [DOI] [PubMed] [Google Scholar]
- 28.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 29.Marso SP, Daniels GH, Brown-Frand-sen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazzone T Intensive glucose lowering and cardiovascular disease prevention in diabetes: reconciling the recent clinical trial data. Circulation 2010;122:2201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riddle MC. Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation 2010; 122:844–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bianchi C, Miccoli R, Del Prato S. Hy-perglycemia and vascular metabolic memory: truth or fiction? Curr Diab Rep 2013; 13:403–10. [DOI] [PubMed] [Google Scholar]
- 33.Reaven PD, Moritz TE, Schwenke DC, et al. Intensive glucose-lowering therapy reduces cardiovascular disease events in veterans affairs diabetes trial participants with lower calcified coronary atherosclerosis. Diabetes 2009;58:2642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med 2006;145:520–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.