Abstract
Glyphosate is the most widely used broad-spectrum systemic herbicide in the world. Recent evaluations of the carcinogenic potential of glyphosate-based herbicides (GBHs) by various regional, national, and international agencies have engendered controversy. We investigated whether there was an association between high cumulative exposures to GBHs and increased risk of non-Hodgkin lymphoma (NHL) in humans. We conducted a new meta-analysis that included the most recent update of the Agricultural Health Study (AHS) cohort published in 2018 along with five case-control studies. Using the highest exposure groups when available in each study, we report the overall meta-relative risk (meta-RR) of NHL in GBH-exposed individuals was increased by 41% (meta-RR = 1.41, 95% CI, confidence interval: 1.13–1.75). For comparison, we also performed a secondary meta-analysis using high-exposure groups with the earlier AHS (2005), and we determined a meta-RR for NHL of 1.45 (95% CI: 1.11–1.91), which was higher than the meta-RRs reported previously. Multiple sensitivity tests conducted to assess the validity of our findings did not reveal meaningful differences from our primary estimated meta-RR. To contextualize our findings of an increased NHL risk in individuals with high GBH exposure, we reviewed available animal and mechanistic studies, which provided supporting evidence for the carcinogenic potential of GBH. We documented further support from studies of malignant lymphoma incidence in mice treated with pure glyphosate, as well as potential links between GBH exposure and immunosuppression, endocrine disruption, and genetic alterations that are commonly associated with NHL. Overall, in accordance with evidence from experimental animal and mechanistic studies, our current meta-analysis of human epidemiological studies suggests a compelling link between exposures to GBHs and increased risk for NHL.
Keywords: Glyphosate, pesticide, Roundup, Ranger Pro, carcinogenesis, meta-analysis
1. Background
1.1. Global Usage of Glyphosate-Based Herbicides
Glyphosate is a highly effective broad spectrum herbicide that is typically applied in mixtures known as glyphosate-based herbicides (GBHs) and commonly sold under the trade names of Roundup® and Ranger Pro®. Use of GBHs has increased dramatically worldwide in recent decades. In the United States alone, usage increased nearly sixteen-fold between 1992 and 2009 [1]. Most of this increase occurred after the introduction of genetically modified glyphosate-resistant “Roundup-ready” crops in 1996 [1]. In addition, there have been significant changes in usage. In particular, the practice of applying GBHs to crops shortly before harvest, so-called “green burndown,” began in the early 2000s to speed up their desiccation; as a consequence, crops are likely to have higher GBH residues [2]. By the mid-2000s, green burndown became widespread, and regulatory agencies responded by increasing the permissible residue levels for GBHs [3, 4].
1.2. Ubiquitous Exposure in Humans
Glyphosate and its metabolites persist in food [5–7], water [8], and dust [9], potentially indicating that everyone may be exposed ubiquitously. Non-occupational exposures occur primarily through consumption of contaminated food, but may also occur through contact with contaminated soil [9], dust [9] and by drinking or bathing in contaminated water [8]. In plants, glyphosate may be absorbed and transported to parts used for food; thus, it has been detected in fish [5], berries [6], vegetables, baby formula [7], and grains [10], and its use as a crop desiccant significantly increases residues. GBH residues in food persist long after initial treatment and are not lost during baking.
Limited data exist on internal glyphosate levels among GBH-exposed individuals [11]. Average urinary glyphosate levels among occupationally exposed subjects range from 0.26-73.5 μg/L, whereas levels in environmentally exposed subjects have been reported between 0.13-7.6 μg/L [11]. Two studies of secular trends have reported increasing proportions of individuals with glyphosate in their urine over time [12, 13]. Given that more than six billion kilograms of GBHs have been applied in the world in the last decade [2], glyphosate may be considered ubiquitous in the environment [14].
1.3. Controversy Surrounding the Carcinogenic Potential of GBHs
Exposure to GBHs is reportedly associated with several types of cancer, among which the most-well studied in humans is non-Hodgkin lymphoma (NHL). Some epidemiological studies have reported an increased risk of NHL in GBH-exposed individuals [15–17]; however, other studies have not confirmed this association [18, 19]. GBHs have recently undergone a number of regional, national, and international evaluations for carcinogenicity in humans [20–23], resulting in considerable controversy regarding glyphosate and GBHs’ overall carcinogenic potential. Hence, addressing the question of whether or not GBHs are associated with NHL has become even more critical. Here, we evaluated the all the published human studies on the carcinogenicity of GBHs and present the first meta-analysis to include the most recently updated Agricultural Health Study (AHS) cohort [24]. We also discuss the lymphoma-related results from studies of glyphosate-exposed animals as well as mechanistic considerations to provide supporting evidence for our analysis of the studies of human exposures to GBHs.
2. Current Meta-Analysis of GBHs and NHL
2.1. Meta-Analysis Objective
Epidemiological studies may vary in several ways, such as by study design, sample size, and exposure assessment methods. Results among individual studies vary and may appear to conflict, which poses challenges in drawing an overall conclusion. Meta-analysis is a quantitative statistical tool that is frequently applied to consolidate the results from similar but separate individual studies so that an overall conclusion about the effects of exposure can be drawn. Here, we conducted a meta-analysis using published human studies to better understand whether the epidemiological evidence supports an association between exposures to GBHs and increased NHL risk. Although three previously published meta-analyses have examined the same association and reported positive meta-risks for GBH-associated NHL [22, 25, 26], our analysis differs from earlier ones by focusing on an a priori hypothesis targeting biologically relevant exposure magnitude and by including the newly updated AHS study [24].
2.2. A Priori Hypothesis
Our a priori hypothesis is that the highest biologically relevant exposure to GBHs, i.e., higher levels, longer durations and/or with sufficient lag and latency, will lead to increased risk of NHL in humans. The hypothesis is based on the understanding that higher and longer cumulative exposures during a biologically relevant time window are likely to yield higher risk estimates, given the nature of cancer development [27]. Hence, when cumulative exposure is higher, either due to higher level or longer duration exposures, an elevated association with the cancer of interest is more likely to be revealed if a true association exists. This a priori approach has been employed to estimate meta-risks for benzene [28] and formaldehyde [29, 30], but not in any of the previous meta-analyses exploring the GBH-NHL association [22, 25, 26].
Risk estimates, including relative risks (RRs) and odd ratios (ORs), in high exposure groups are less likely to be dominated by confounding or other biases compared to RRs or ORs from groups experiencing average or low exposure [31]. Furthermore, including people with very low exposure in the exposed group can dilute risk estimates. Studying the most highly exposed group is also useful to ensure an adequate exposure contrast, given the potential that most people have been exposed either directly or indirectly to GBHs. Because our main goal is to determine whether there is an exposure effect and not to conduct a precise dose-response assessment or to evaluate risks in people with low exposures, we assert that this a priori hypothesis is appropriate for testing whether or not a GBH-NHL association exists.
2.3. Agricultural Health Study (AHS) Update
A recently published update [24] from the large AHS cohort of American pesticide applicators (N > 50,000) has been included for the first time in our primary meta-analysis. Although the original AHS report [19] was used in previous meta-analyses [22, 25, 26], the 2018 AHS update [24] contributes 11-12 additional years of follow-up with over five times as many NHL cases (N = 575 compared to N = 92 in the original study [19]), and >80% of the total cohort was estimated to be exposed to GBHs. As the largest and most recently published study, it adds substantial weight to the new meta-analysis [24]. We also performed a secondary comparison analysis using our a priori hypothesis with the original AHS report [19] for the purpose of comparing results with our primary meta-analysis (using the 2018 AHS update) and with meta-analyses published previously.
2.4. Identifying Relevant Human Studies
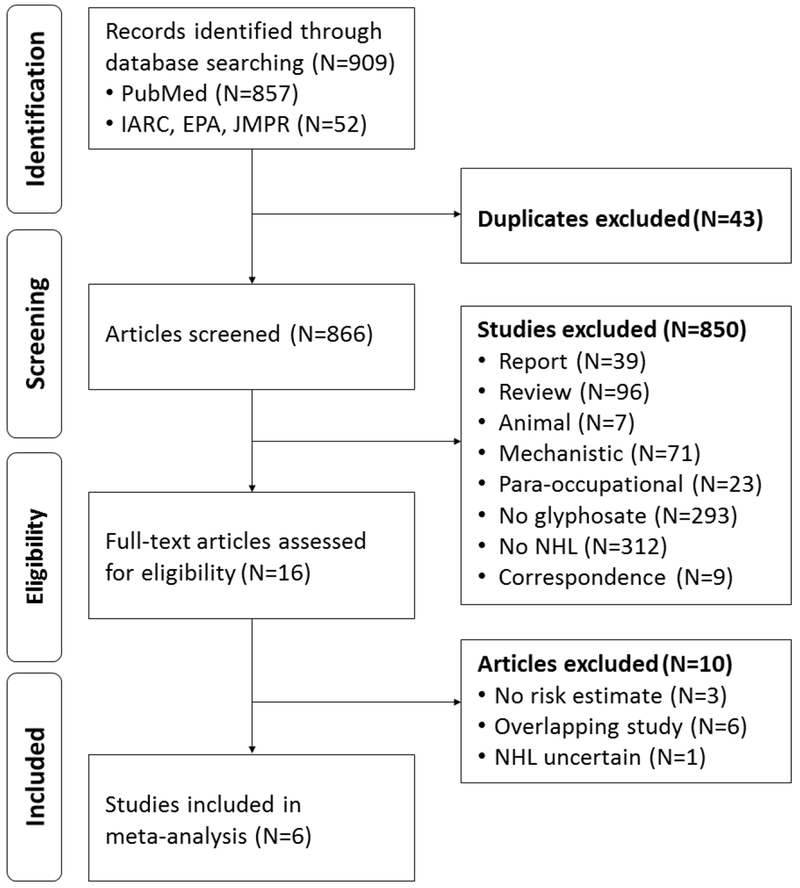
The literature search was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [32]. The screening process and results are shown in Figure 1. We conducted a systematic electronic literature review using PubMed in November 2017, and we updated it in March 2018 and again in August 2018. We used the following keywords: (glyphosat* OR pesticide [MeSH] or herbicides [MeSH]) AND (lymphoma, non-Hodgkin [MeSH] OR lymphoma [tiab] OR non–Hodgkin [tiab] OR non–hodgkins [tiab] OR lymphoma[tiab] OR lymphomas[tiab] OR NHL OR cancer OR cancers) AND (“occupational exposure”[MeSH] OR occupational exposure[tiab] OR occupational exposures[tiab] OR farmers [MeSH] OR farmer OR applicators OR applicator OR agricultural workers OR agricultural worker or workers or worker).
Figure 1.
Study Selection Process for Meta-Analysis using PRISMA Guidelines.
Searches included all cohort, case-control, and cross-sectional studies. No language restrictions were applied, although non-English language articles needed to be obtained in full and translated completely in order to be eligible for inclusion. From the PubMed search, we identified 857 studies. Additionally, we identified 52 studies from the IARC [22] evaluation of the carcinogenicity of glyphosate, the U.S. EPA [20] review of glyphosate, and the WHO JMPR [21] report on glyphosate, for a total of 909 studies.
After 43 duplicates were excluded, 866 studies were initially screened by title and abstract, of which 850 were excluded because they were reports, correspondence, reviews, irrelevant studies (animal, mechanistic, para-occupational), or did not include the exposure or outcome of interest (Figure 1). When the final 16 qualified epidemiological studies of GBHs and NHL were identified, 10 studies were further excluded because (1) they did not report RRs, ORs, or the data needed to calculate either [33–35], (2) the cohort overlapped with another study [19, 36–40], or (3) they did not specify whether the lymphomas were specifically NHL [41]. For studies including overlapping cohorts, we used results from the most complete and updated analysis with the greatest number of participants. Although overlapping, we kept the earlier AHS (2005) [19] for comparison with our primary meta-analysis (using the updated AHS 2018 publication) and with previous meta-analyses. The impact of selecting these studies was evaluated in sensitivity analyses (Section 3.5).
2.5 Review and Assessment of Selected Human Studies
2.5.1. Data Collection and Extraction
In total, six studies (one cohort [24] and five case-control control studies [15–18, 42]) with nearly 65,000 participants were eligible for inclusion in the meta-analysis. Two studies were conducted in the United States, one study was from Canada, two studies were from Sweden, and one study was from France. All six studies reported NHL risks (RRs or ORs) above or close to 1.0, three of which were statistically significant in the original analyses (Table 1). From each study, we abstracted information on study design, location, dates, sample size, participation rates, age, sex, case/control source, diagnosis, histologic verification, exposure assessment, results, and statistical adjustments. Table 1 summarizes key aspects of the design and exposure assessment, the results, strengths, and weaknesses of all the studies evaluated in this meta-analysis, including both versions of the AHS report (n = 6+1). As described above, the early AHS data [19] were also evaluated in Table 1 and in a comparison meta-analysis described later.
Table 1.
Epidemiologic studies of GBHs and cancer: studies used in the main meta-analysesa
| Author/location | Subject ascertainment | Participation rates | Exposure assessment | Exposure level | Results for NHLb | Weaknesses | Adjustments | Notes |
|---|---|---|---|---|---|---|---|---|
| Andreotti et al. [24] (Agricultural Health Study) Where: Iowa and North Carolina Design: Prospective cohort Years: 1993-97 to 2012-13 Percent exposed: 82.8% |
Who: 54,251 pesticide applicators recruited between 1993-97 Cases: NA Source of cases: Iowa and North Carolina Cancer Registries, state and national death registries Histologic verification: Not mentioned Controls: NA Source of controls: NA Similar demographics (exposed and unexposed): Similar age, sex, race, and smoking. Exposed higher education, alcohol consumption, and family history of cancer Final size: 575 NHL cancers, 53760 subjects without missing data Follow-up: from enrollment through December 31, 2013 in Iowa and December 31, 2012 in North Carolina (16-20 years) |
Exclusions: 3059 excluded (mostly missing data) Percent proxy interviews: None Missing Follow-Up Questionnaire: 37% (Pesticide use imputed) |
Collection: Self-administered and take home questionnaire at time of recruitment: 22 specific pesticides application methods, PPE, years of use, and days per use. Review: No Blinded: Prospective design Validation: Similar questions asked 1 year apart in 4,088 subjects, agreement on glyphosate ever use = 82%, days per year mixed = 52% [124] |
Exposed: Quartiles 1-4 calculated by multiplying lifetime exposure days by an intensity score. Intensity based on (mixing + application method + equipment repair) * PPE in Coble et al. 2011 [44] Unexposed: No glyphosate use Latency: 5, 10, 15, and 20 years |
Adjusted Intensity Weighted Cumulative Exposure (Days): RRs for Q1: 1–598.9; Q2: 599–1649.9; Q3: 1650–4339.9; and Q4: ≥4340.0, = 1.0 (Ref), 0.83 (0.59-1.18), 0.83 (0.61-1.12), 0.88 (0.65-1.19), 0.87 (0.64-1.20); p-trend = 0.95; n=54251 total, 111 cases in the high exposure group Similar results comparing highest to lowest quartile. Latency with Adjusted Intensity Weighted Cumulative Exposure: RRs for 20-year lag quartiles: Q1: 1–281.3; Q2: 281.4–895.9; Q3: 896–2609.9; Q4: ≥2610.0 = 1.00 (Ref), 1.22 (0.91-1.64), 1.15 (0.86-1.55), 0.98 (0.71-1.36), 1.12 (0.83-1.51) Latency with Adjusted Lifetime Cumulative Exposure: RRs for 20-year lag quartiles: Q1: 1-8.74; Q2: 8.75-21.24; Q3: 21.25-59.4; Q4: ≥59.5 = 1.00 (Ref), 1.52 (1.16-2.00), 0.95 (0.68-1.32), 0.97 (0.71-1.33), 1.13 (0.85-1.52) Change with adjustment: No |
1. Possible fairly short follow-up for some people 2. Somewhat weak validation 3. Imputed exposure data for participants who did not complete the follow-up questionnaire. |
Matched: NA Adjusted: adjusted for age, state of recruitment, education, cigarette smoking status, alcohol per month, family history of cancer, atrazine, alachlor, metolachlor, trifluralin, 2,4-D. Other: NA |
Censored on date the subjects left the state. Increased RR for AML (RR=2.6, 0.7-9.4); highest exposure group (RR = 2.44, 0.94 - 6.32). 79.3% of all cases ever used glyphosate Used updated Surveillance Epidemiology End Results (SEER) coding scheme for NHL which includes multiple myeloma [67] Update of De Roos et al. [19] |
| De Roos (2005) [19] (Agricultural Health Study) Where: Iowa and North Carolina Design: Prospective cohort Years: 1993-97 to 2001 Percent exposed: 75.5% |
Who: 54,315 pesticide applicators recruited between 1993-97 Cases: NA Source of cases: Iowa and North Carolina Cancer Registries, state and national death registries Histologic verification: Not mentioned Controls: NA Source of controls: NA Similar demographics (exposed and unexposed): Similar age, sex, smoking, alcohol. Exposed higher education and family history of cancer Final size: 92 NHL cancers, 36,509 subjects without missing data Follow-up: from enrollment through Dec. 2001 (5-8 years, median=6.7 years) |
Exclusions: 20,802 excluded (mostly missing data) (36.3%) 298 people lost to follow-up Percent proxy interviews: None |
Collection: Self-administered and take home questionnaire at time of recruitment: 22 specific pesticides application methods, PPE, years of use, and days per use. Review: No Blinded: Prospective design Validation: Similar questions asked 1 year apart in 4,088 subjects, agreement on glyphosate ever use = 82%, days per year mixed = 52% [124] |
Exposed: Ever or upper tertiles Intensity based on (mixing + application method + equipment repair) * PPE in Dosemeci et al. 2002 [45] Unexposed: Never or lower tertile Latency: Not mentioned |
Ever exposed: Age adjusted: RR=1.2 (0.7-1.9) Adjusted: RR=1.1 (0.7-1.9) Intensity-weighted exposure days: For exposures of 0.1-79.5, 79.6-337.1, and 337.2-18241, 1.0 (Ref), 0.6 (0.3-1.1), and 0.8 (0.5-1.4); p-trend = 0.99; n=36,823 total, 22 cases in the high exposure group Similar results comparing highest to lowest tertile. Adjusting for other pesticides did not change RR by more than 20% Change with adjustment: No |
1. Possible fairly short follow-up for some people 2. Large numbers of people excluded from dose-response analysis due to missing data 3. Somewhat weak validation |
Matched: NA Adjusted: Age, education, smoking, alcohol, family cancer history, state, 10 other pesticides most strongly correlated with glyphosate (if RR changes by >20%) Other: NA |
Censored on date the subjects left the state. Increased RR for multiple myeloma (OR=2.6, 0.7-9.4) but with large change after adjustment (unadjusted OR=1.1, 0.5-2.4) 75.5% ever used glyphosate |
| De Roos (2003) [15] Where: US Design: Case-control Years: 1979-86 Percent exposed: 5.5% |
Who: White men only. Combines data from three NCI case-control studies: Hoar et al. [33], Zahm et al. [34], and Cantor et al. [37]. Cases: Nebraska [34]: White subjects ≥ 21 years, 66 counties in eastern Nebraska, diagnosed 1983-86 Iowa/Minn [37]: White men ≥ 30 years, diagnosed 1980-83. Iowa: entire state. Minnesota: entire state except four large cities Kansas [33]: White men ≥ 21 years diagnosed 1979-81, entire state, 200 of 297 randomly selected Source of cases: Nebraska: Nebraska Lymphoma Study Group and area hospitals, Iowa/Minn: Iowa State Health Registry, surveillance system of Minnesota hospitals and pathology laboratories (not described) Kansas: State wide registry run by the University of Kansas Cancer Data Service, mandatory cancer reporting in the state Histologic verification: Yes, in all three studies (Kansas 90%) Controls: Randomly selected from the same areas Source of controls: Random digit dialing, Medicare, and state mortality records for deceased cases Similar demographics: Similar education; family history higher in cases; few other variables described Final size: 650 cases and 1933 controls |
Exclusions: >25% (missing data and worked on farm before 18 years old) Interview rates: Kansas Cases: Unclear but likely >90% Controls: 93% Iowa/Minn Cases: 89% Controls: 76-79% Nebraska: Cases: 91% Controls: 85% Percent proxy interviews: 30.9% in cases and 39.7% in controls |
Collection: Telephone (Kansas, Nebraska) or in-person (Iowa/Minn) interviews: SES, medical history, smoking, and family history Nebraska: Specific pesticides, number years used, average days used per year, PPE Iowa/Minn: Specific pesticides, first and last year used, method of use, personal application, and PPE Kansas: Open ended question about pesticides used, duration and days per year only for pesticide groups, and PPE Review: No Blinded: Partial (see individual studies) Validation: Kansas: Sought to confirm purchases in 110 subjects, suppliers usually reported fewer purchases, no consistent differences between cases and controls, few details given Iowa/Minn: No Nebraska: No |
Exposed: Any reported use, no further details Unexposed: No use of glyphosate Latency: Not mentioned |
Unadjusted (calculated): OR=1.80 (1.18-2.74) Adjusted: OR=2.1 (1.1-4.0), 36 exposed cases Hierarchical adjustment: OR=1.6 (0.9-2.8), 36 exposed cases Change with adjustment: Yes Other results: Iowa/Minn [37]: OR=1.1 (0.7-1.9), 26 exposed cases Kansas: No glyphosate data in Hoar et al. [33] Nebraska: No glyphosate data in Zahm et al. [34] In non-asthmatics OR=1.4 (0.98-2.1) in Lee et al. [36] |
1. Unknown whether there was full case ascertainment in some areas. For example, incidence rates in Nebraska were 77% of those in SEER [34]. Few details provided on Minnesota case surveillance system 2. Only includes White male subjects 3. Large number of proxy interviews 4. No details regarding timing of pesticide use in relation to disease onset |
Matched: Race, sex, age, and vital status Adjusted: Age study site, and 47 pesticides. Hierarchical models included pesticide class, and prior knowledge on carcinogenicity from IRIS and IARC Other: Family cancer history, education, and smoking had little impact on results |
Farmers: 59.9% among controls Exclusions: Subjects who did not work on farms after age 18; subjects with missing data on any of 47 pesticides (about 25% of subjects) In Nebraska, larger percentage of farmers reported no pesticide use Overlapping study with Cantor et al. [37]c and Lee et al. [36]d; both of which were evaluated in the sensitivity analysis. |
| Eriksson et al. [16] Where: Sweden Design: Case-control Years: 1999-2002 Percent exposed: 1.8% |
Who: Population based, men and women Cases: Age 18-74 diagnosed 1999-2002 Source of cases: 4 of 7 health service regions in Sweden associated with four University Hospitals, from physicians and pathologists Histologic verification: Yes Controls: Randomly selected from the same health regions, matched on age and sex Source of controls: Sweden population registry for the same health service regions Similar demographics: Data not provided Final size: 910 cases and 1016 controls |
Exclusions: 134 of 1163 (11.5%) with medical conditions or deceased Interview rates: Cases: 91% Controls: 92% Percent proxy interviews: Deceased cases were not included (n=88) |
Collection: Mailed questionnaire on work history, specific pesticides, number of years, days per year, hours per day, with follow-up telephone interviews as needed Review: No Blinded: Partial, interviewers blinded to case-control status Validation: No |
Exposed: One full day, or median number of days exposed in the controls. Unexposed: Unexposed to any included pesticide Latency: Exposures in the year of and the year before diagnosis disregarded |
Unadjusted: Not provided Adjusted: ≥1 day (univariate): OR=2.02 (1.10-3.71) ≤10 days: OR=1.69 (0.70-4.07) >10 days: OR=2.36 (1.04-5.37, n=17 exposed cases) ≥1 day adjusted for other pesticides (multivariate): OR=1.51 (0.77-2.94) Latency (≥1 day) 1-10 years: OR=1.11 (0.24-5.08) >10 years: OR=2.26 (1.16-4.40) Change with adjustment: Yes |
1. Deceased cases not included 2. True participation rates may be lower 3. Use of PPE not assessed 4. Population based: not a high exposure group 5. Adjustments for other pesticides not completely clear |
Matched: Age and sex Adjusted: Age, sex, and year of diagnosis. Adjusted for other pesticides in some analyses (MCPA, mercurial seed dressing…) Other: NA |
Authors state that all lymphoma treating clinics and all lymphoma pathologists in the study regions were covered by the study. Also gives RR by subtype Percent farmers unknown, but only 51 controls (5.0%) used herbicides |
| Hardell et al. [17] Where: Sweden Design: Case-control Years: 1987-90 Percent exposed: 0.7% |
Combines two published studies, one of NHL [38] and one of hairy cell leukemia (HCL) [39] Who: Population based, males ≥25 years old NHL cases (n=404): All male cases, living or deceased, diagnosed 1987-1990 from 7 Swedish counties HCL cases (n=121): All living male cases in whole country 1987-92 Source of cases: Regional cancer registries (compulsory reporting) Histologic verification: Yes Controls: 2-4 per case matched on age, county, and year of death (if deceased). Those closest in age of birth to case were selected Source of controls: National Population Registry and National Registry for Causes of Death Similar demographics: Data not provided Final size: 515 cases and 1141 controls |
Exclusions: Deceased HCL cases excluded, numbers unknown. No other obvious major exclusions Interview rates NHL Cases: 91% Controls: 84% HCL Cases: 91% Controls: 83% Percent proxy interviews: Approximately 43.4% of NHL cases and controls. HCL study only living subjects used. |
Collection: Mailed questionnaire: complete working history, exposure to specific chemicals (years and total number of days). Supplemented with phone interviews as needed. Review: No Blinded: Subjects blinded to hypothesis, interviewers blinded to case status Validation: None |
Exposed: Minimum exposure of 1 day (8 hours) Unexposed: No reported pesticide exposure Latency: At least one year |
NHL and HCL combined: Unadjusted: OR=3.04 (1.08-8.52) Adjusted: OR=1.85 (0.55-6.20), 8 exposed cases NHL only: [38] Unadjusted OR=2.3 (0.4-1.3), 4 exposed cases Adjusted OR=5.8 (0.6-54). Adjustment factors and sample sizes not given HCL only: [39] Unadjusted OR=3.1 (0.8-12), 4 exposed cases Adjusted results not given. Age adjustment decreases OR for “herbicides” (2.9 to 1.8) Change with adjustment: Yes |
1. Large change in ORs with adjustments, and changes were in opposite directions for NHL only vs. NHL and HCL combined 2. Adjustment factors not listed in some analyses 3. Cut-off for defining exposure is very low 4. Population based: not a high exposure group 5. Small numbers of exposed cases 6. Demographic data not provided 7. Large number of proxy interviews |
Matched: Age, county, and year of death (in deceased) Adjusted: Study (NHL vs. HCL), study area, vital status, (unclear, but it seems likely the multivariate analysis adjusted for 2-methyl-4-chlorophenoxyacetic acid, 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid, and other herbicides) |
Percent farmers unknown. 184/1141 controls (16.1%) used insecticides so probably low |
| McDuffie et al. [42] Where: Canada Design: Case-control Years: 1991-94 Percent exposed: 8.8% |
Who: Population based, males 19 years and older Cases: ICD9 200, 202 newly diagnosed 1991-94 Source of cases: Provincial cancer registries, except Quebec (hospitals) Histologic verification: Partial (84%) Controls: Men age 19 or older randomly selected matched on 2-year age groups Source of controls: Randomly selected from provincial health insurance records, telephone listings, or voter lists Similar demographics: Yes for age, farm, smoking, and missing data. Cases less likely to have mumps, measles, and allergy shots/tests; more likely to have previous cancer Final size: 517 cases and 1506 controls |
Exclusions: 68 cases in pilot study, all deceased cases (% unknown) Interview rates: Cases: 67.1% Controls: 48.0% Percent proxy interviews: Deceased cases excluded |
Collection: Mailed questionnaires: demographics, medical history, family cancer history, lifetime job history, exposure to specific substances, accidental spills, and protective equipment. Phone interviews in those with ≥10 hours/year of cumulative exposure to all pesticides combined. Asked about exposure to pesticides and number of days per year Review: No Blinded: Not mentioned Validation: In a pilot sample of 27 farmers, compared questionnaire data to pesticide purchases. “Excellent concordance” but no actual numbers given |
Exposed: Not provided Unexposed: No reported exposure to glyphosate Latency: Not mentioned |
Any exposure Unadjusted: OR=1.26 (0.87-1.80), n=51 exposed cases (age and province adjusted) Adjusted: OR=1.20 (0.83-1.74) Dose-response: ORs for 0, >0-≤2, and >2 days per year = 1.00 (Ref), 1.00 (0.63-1.57), and 2.12 (1.20-3.73; n=23 exposed cases) (adjusted only for age and province) Unadjusted in high exposure group (calculated) OR=1.88 (1.01-3.21) Change with adjustment: No Other: Glyphosate only: OR=0.92 (0.54-1.55) Glyphosate and malathion: OR=2.10 (1.31-3.37) Hohenadel et al. [40] |
1. Unclear if full case ascertainment in Quebec 2. Is registry compulsory 3. Fairly low participation rates, and difference seen between cases and controls 4. Average exposure in the highest group not given. Duration of exposure not given 5. Deceased cases excluded |
Matched: Age and province Adjusted: Age, province, measles, mumps, cancer allergy shots, and family cancer. Factors with p ≤0.05 retained in the models. Unclear what factors included in the high exposure analysis |
Farmers: 44.7% in controls had residence on farm Similar rural/urban make-up between respondents and non-respondents but other differences not assessed. See Kachuri et al. [35] for multiple myeloma data Overlapping study with Hohenadel et al. [40]e which was excluded from the main meta analysis. |
| Orsi et al. [18] Where: France (6 cities) Design: Case-control Years: 2000-04 Percent exposed: 5.5% |
Who: Population-based, males age 20-75 years old Cases: Diagnosed in one of the main hospitals in the 6 cities, ICD-0-3 codes (listed in their Table 1) Source of cases: Hospitals Histologic verification: Yes Controls: Hospital controls Source of controls: Men from the same hospitals, mostly orthopedic and rheumatology. Unclear if randomly selected. Similar demographics: Similar for SES, education, rural vs. urban Final size: 244 cases and 436 controls |
Exclusions: History of immuno-suppression (% unknown) Interview rates: Cases: 95.7% Controls: 91.2% Percent proxy interviews: Not mentioned |
Collection: Self-administered questionnaire: all jobs, years, tasks and products handled (open-ended); followed by structured personal interview including non-occupational and occupational use (in farmers) of pesticides, mixing or spraying, number and duration of applications Review: Questionnaires reviewed by occupational hygienist and agronomist Blinded: Interviewer and subject blind to hypothesis, and reviewer blind to case status. Unclear if interviewer was blinded Validation: Partial (see “Review”), pesticides compared to annual directories that list recommended pesticides by crop and pest |
Exposed: Any, possible or definite;duration greater than the median in the exposed Unexposed: Never exposed to glyphosate, similar results with “never used any pesticide” Latency: Not mentioned or assessed for glyphosate |
Unadjusted: OR=0.89 (0.44-1.81) Adjusted: OR=1.0 (0.5-2.2), 12 exposed cases for any exposure Change with adjustment: No |
1. Deceased cases probably not included 2. Private clinics not included 3. Unknown if control selection is population based 4. Population based: not a high exposure group or high risk group |
Matched: Center and age Adjusted: Age and center Other: Rural vs. urban, type of housing, education, infection, family history, skin characteristics, smoking, and alcohol had little impact on results |
Also has multiple myeloma results (OR=2.4; 0.8-7.3) Results for a few subtypes also given but with small numbers Farm, agriculture, or forestry work in 92 of 426 controls (21.1%) |
Abbreviations: HCL, Hairy Cell Leukemia; IARC, International Agency for Research on Cancer; ICD, International Classification of Disease; IRIS, US Environmental Protection Agency Integrated Risk Information System; Minn, Minnesota; NA, not applicable; NCI, National Cancer Institute; OR, odds ratio; PPE, personal protective equipment; Ref, reference; RR, relative risk; SEER, Surveillance Epidemiology End Results; SES, socioeconomic status
Although there is no overlapping study used in the main analysis, Cocco et al. [41] was excluded because only results for all B-cell lymphomas combined were reported (two cases of NHL, one case of multiple myeloma, and one unspecified B-cell lymphoma; n=4). It is evaluated in the sensitivity analysis.
95% confidence intervals in parentheses
Cantor et al. [37] was excluded because it was combined with two other U.S. case-control studies in De Roos et al. [15].
Lee et al. [36] was excluded because it presents results comparing asthmatics to non-asthmatics and results are not adjusted for other pesticide use. It is evaluated in the sensitivity analysis.
Hohenadel etal. [40] was excluded because it presents results in subjects exposed and unexposed to malathion, which has not been consistently linked to NHL; the OR for glyphosate only was used in the sensitivity analysis
2.5.2. Study Quality Evaluation
The methodological quality of the cohort (Table 2) and case-control studies (Table 3) included in the meta-analyses was assessed independently by two co-authors using the Newcastle Ottawa Scale (NOS) [43]. Studies were evaluated based on selection, comparability, and outcome or exposure (in nine categories).
Table 2.
Quality assessment of the cohort studies in meta-analysis.*
| Study | Selection |
Comparability |
Outcome |
Overall Quality Scores | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of Exposed | Selection of Non-Exposed | Exposure Assessment | NHL Absent at Start | Controls for Other Pesticides | Controls for Age | Assessment of Outcome | Follow-up Length | Adequacy of Follow-up | ||
| Andreotti et al. [24] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 8 |
| De Roos (2005) [19] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 7 |
The study quality was assessed according to the Newcastle Ottawa Quality assessment scale for cohort studies [43]. One point was awarded for yes, and zero points were awarded for no, unable to determine, or inadequate.
Table 3.
Quality assessment of the case-control studies in meta-analysis.a
| Study | Selection |
Comparability |
Exposure |
Overall Quality Scores | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adequate Case Definition | Representativeness of Cases | Control Selection | Definition of Controls | Controls for Other Pesticides | Controls for Age | Exposure Assessment | Method Consistency | Non-response Rate | ||
| De Roos (2003) [16] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 6 |
| Eriksson et al. [17] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Hardell et al. [18] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 6 |
| McDuffie et al. [43] | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 6 |
| Orsi et al. [19] | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 |
The study quality was assessed according to the Newcastle Ottawa Quality assessment scale for case-control studies [44]. One point was awarded for yes, and zero points were awarded for no, unable to determine, or inadequate.
Cohort studies were evaluated based on (1) representativeness of the cohort, (2) selection of non-exposed, (3) ascertainment of exposure, (4) demonstration that outcome of interest was not present at the start of study, (5) comparability of cohort on the basis of controlling for other pesticide use and (6) age, (7) assessment of NHL outcome, and (8) sufficiency of follow-up length, and (9) response rate.
Case-control studies were evaluated on (1) the validation of cases, (2) representativeness of cases, (3) selection of controls, (4) absence of disease in the controls, (5) whether the study controlled for other pesticide use and (6) age, (7) exposure assessment, (8) concordance of method among cases and controls, and (9) similarity of response rate among both groups. Each study was awarded a maximum of one point for every item that was satisfied, with a total of 9 available points.
According to our quality assessment (Tables 2–3), the highest quality study in either design category was the AHS 2018 cohort [24]. The highest quality case-control study was Eriksson et al. [16], while the lowest quality studies were McDuffie et al. [42] and Orsi et al. [18].
2.6. Selection of the Most Highly Exposed Category
Based on our a priori hypothesis, when multiple RRs or ORs were given in the original studies, we selected estimates in the following order: (1) highest cumulative exposure and longest lag (the time period preceding NHL onset, which is excluded from the exposure estimate) or latency (time between first lifetime exposure and NHL diagnosis); (2) highest cumulative exposure; (3) longest exposure duration and longest lag or latency; (4) longest exposure duration; (5) longest lag or latency; and (6) ever-exposure. The definition of cumulative exposure includes duration and intensity. As we discuss in more detail in Section 5.2, in both AHS reports [19, 24] cumulative exposure was calculated as an intensity-weighted exposure (lifetime exposure days multiplied by an intensity score) [44, 45].
We prioritized highest cumulative exposure based on evidence of glyphosate’s persistence in the environment [46–48] and because chronic disease, including cancer, is usually the result of cumulative exposures [49]. We selected the longest lag or latency because decades may be needed for the health effects of many environmental toxicants to manifest as detectable cancers. If no high exposure data were available, we used the ever-exposure estimate. Given the relatively few human epidemiological studies published to date on the topic, we made this decision because we did not want to exclude any potentially relevant data, even though the inclusion of minimally exposed individuals in the “exposed” category could attenuate any potential association of interest.
Although there are different perspectives on the best way to account for other pesticide exposures, we selected RR estimates that adjusted for other pesticide use over their unadjusted counterparts to mitigate potentially substantial confounding by other pesticide use. Five of the seven studies adjusted for a combination of different pesticides [15–17, 19, 24], indicating they accounted for confounding by other pesticides. However, if these multiple pesticides acted synergistically or on different points along a pathway, this approach to adjustment may no longer be the appropriate, and alternatives such as interaction analysis should be considered. Reanalysis of the raw data, which is beyond the scope of this paper, would be helpful to address this possibility.
We evaluated the impact of our a priori exposure selection criteria in sensitivity analyses. We also conducted a separate meta-analysis of all ever-exposed individuals to assess the magnitude of potential bias caused by adding subjects with low exposures (ever-RR from De Roos et al. [19] was used; the ever-RR estimate from Andreotti et al. [24] was not available). In Table 4 we summarize the risk estimates selected from each original study and the study weights used in the meta-analyses.
Table 4:
Description and weight of studies selected for the current meta-analyses.
| Study (Author, Year) | Case No. (Exp/Tot) | Exposure Category | Risk Estimatea (95% CI) | Weightb |
|
|---|---|---|---|---|---|
| AHS 2018 | AHS 2005 | ||||
| AHS Cohort | |||||
| Andreotti et al. [24] | 55/575 | ≥2610 d/I c,d | 1.12 (0.83, 1.51)e | 54.04 | -- |
| De Roos (2005) [19] | 22/92 | ≥337.2 d/Ic | 0.8 (0.5, 1.4)f | -- | 28.43 |
| Case-Control | |||||
| De Roos (2003) [15] | 36/650 | Ever, log | 2.10 (1.10, 4.00) | 11.61 | 18.08 |
| Eriksson et al. [16] | 17/910 | >10 d/y | 2.36 (1.04, 5.37) | 7.18 | 11.18 |
| Hardell et al. [17] | 8/515 | Ever | 1.85 (0.55, 6.20) | 3.30 | 5.14 |
| McDuffie et al. [42] | 23/517 | >2 d/y | 2.12 (1.2, 3.73) | 15.05 | 23.43 |
| Orsi et al. [18] | 12/244 | Ever | 1.0 (0.5, 2.2) | 8.82 | 13.73 |
Abbreviations: AHS, Agricultural Health Study; d, days; exp, exposed; I, lifetime; log, logistic regression; tot, total; y, year.
Relative risk (RR) reported in both AHS analyses and odds ratio (OR) reported in all case-control studies.
Weight given to each study in the fixed effects model.
Intensity-weighted lifetime exposure days (cumulative exposure days multiplied by intensity score)
20 years or more lag (time between study recruitment and NHL onset).
Reference group is unexposed
Reference group is lowest exposed
2.7. Statistical Methods
We calculated the meta-analysis summary relative risk (meta-RR) and confidence intervals using both the fixed-effects inverse-variance method [31] and the random-effects method [50]. In the fixed-effects model, the weights assigned to each study are directly proportional to study precision, whereas in the random-effects model, weights are based on a complex mix of study precision, relative risk (RR), and meta-analysis size. One benefit of the random-effects model is the ability to incorporate between-study variance into the summary-variance estimate and confidence intervals, which may help prevent artificially narrow confidence intervals resulting from use of the fixed effects model in the presence of between-study heterogeneity [51]. However, a feature of the random-effects model is that study weighting is not directly proportional to study precision, and greater relative weight is given to smaller studies, which may result in summary estimates that are less conservative than the fixed-effects model [51]. For these reasons, our primary results focus on the fixed-effects model, although the random-effects model estimates are also reported. We also estimated between-study heterogeneity, defined as the X2-test statistic for heterogeneity being greater than its degrees of freedom (number of studies minus one), using the summary-variance method [51].
We evaluated publication bias through funnel plots, Egger’s test, and Begg’s test [52, 53]. All statistical analyses were conducted with Stata IC 15.1 [54] and Microsoft Excel 2013 [55].
3. Meta-Analysis Findings
3.1. Increased Meta-Relative Risk of NHL
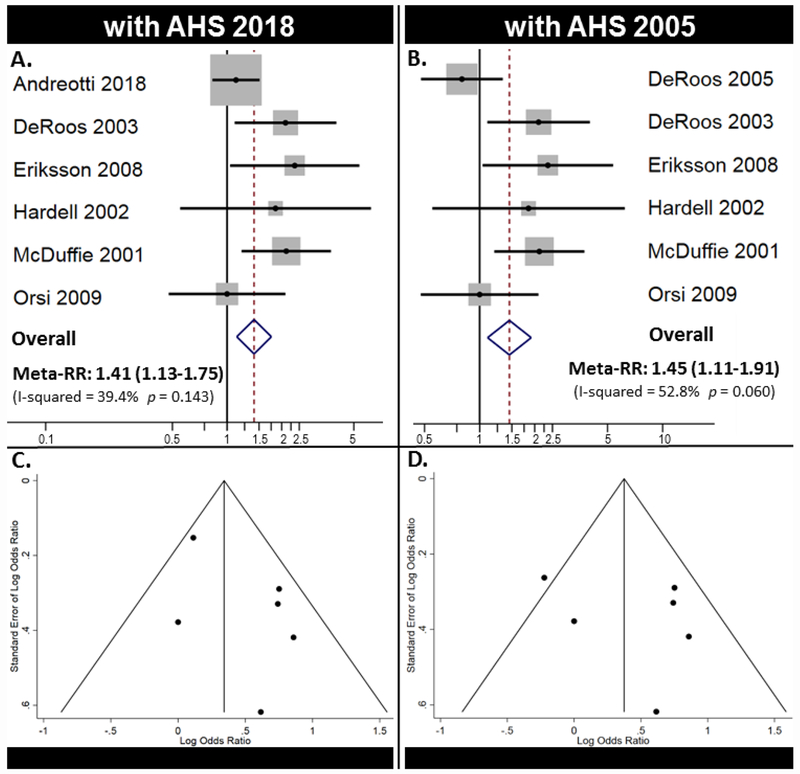
Table 5 includes the results from our two meta-analyses, which included the primary analysis using the most recently updated AHS cohort [24] and the secondary comparison analysis using the original report [19]. Using the updated AHS results [24], we observed a meta-RR of 1.41 (95% CI: 1.13-1.75), which indicates a statistically significant increased risk (41%) of NHL following high cumulative GBH exposure. With the original AHS 2005 cohort results, we observed a meta-RR of 1.45 (95% CI: 1.11–1.91) for NHL. The results did not change appreciably when comparing the fixed effects model to the random-effects model.
Table 5.
Major Findings from Current Meta-Analyses
| Analysis | N | Fixed Effects |
Random Effects |
Heterogeneitya |
|
|---|---|---|---|---|---|
| meta-RR (95% CI) | meta-RR (95% CI) | X2 | p | ||
| Highest cumulative exposure | |||||
| AHS (2018) [24] | 6 | 1.41 (1.13, 1.75) | 1.56 (1.12, 2.16) | 8.26 | 0.14 |
| AHS (2005) [19]b | 6 | 1.45 (1.11, 1.91) | 1.52 (1.00, 2.31) | 10.59 | 0.06 |
| Longest exposure duration | |||||
| AHS (2018) [24] | 6 | 1.41 (1.13, 1.74) | 1.56 (1.12, 2.16) | 8.21 | 0.15 |
| AHS (2005) [19]b | 6 | 1.56 (1.17, 2.06) | 1.57 (1.06, 2.26) | 7.81 | 0.17 |
| Study design | |||||
| Case-control [15–18, 42] | 5 | 1.84 (1.33, 2.55) | 1.86 (1.39, 2.48) | 3.36 | 0.50 |
| Cohort (AHS 2018) [24] | 1 | 1,12c (0.83, 1.51) | |||
Abbreviations: AHS, Agricultural Health Study; meta-RR, meta-relative risk; N, number of studies.
Heterogeneity is present when X2 heterogeneity statistic is greater than degrees of freedom (number of studies minus 1).
De Roos et al. [19] used instead of Andreotti et al. [24] for comparison. See Table 4 for clarifications about the risk estimates used.
Since there was only one cohort study, the RR is presented instead of a meta-RR.
Forest plots (Figure 2A–B) and Funnel plots (Figure 2C–D) from these two major meta-analyses are reported in Figure 2. We observed little evidence of publication bias in the Funnel plots (Figure 2C–D), Eggers (p = 0.185), and Beggs tests (p = 0.851).
Figure 2.
Major meta-analysis results. A) Forest plot for meta-analysis using AHS 2018 and B) using AHS 2005. C) Funnel plot for meta-analysis using AHS 2018 and D) using AHS 2005.
3.2. Sensitivity Analyses
We conducted several sensitivity analyses to evaluate the impact of excluding or including different studies as well as using different RRs/ORs from original studies (Tables 5 and 6). In general, results were similar across our sensitivity analyses, demonstrating the robustness of our findings.
Table 6.
Sensitivity tests for meta-analysis
| Analysis | N | Fixed Effects |
Random Effects |
Heterogeneity1 |
|
|---|---|---|---|---|---|
| meta-RR (95% Cl) | meta-RR (95% Cl) | X2 | p | ||
| Alternate Exposure Categories | |||||
| High level2 | 3 | 1.36 (1.06, 1.75) | 1.63 (0.97, 2.76) | 5.70 | 0.06 |
| Ever (AHS 2005) | 6 | 1.30 (1.03, 1.64) | 1.26 (1.07, 1.48) | 3.73 | 0.59 |
| Latency3 | 6 | 1.40 (1.13, 1.75) | 1.54 (1.12, 2.13) | 8.01 | 0.16 |
| Cell Type Specific | |||||
| Add Cocco et al. [41]4 | 7 | 1.43 (1.15, 1.78) | 1.59 (1.16, 2.18) | 9.10 | 0.17 |
| Exclude HCL [17]5 | 6 | 1.41 (1.13, 1.77) | 1.61 (1.11,2.34) | 9.58 | 0.09 |
| Only use HCL [17]6 | 6 | 1.43 (1.14, 1.78) | 1.62 (1.14, 2.31) | 9.36 | 0.10 |
| Study Location | |||||
| North America | 3 | 1.38 (1.08, 1.76) | 1.61 (0.99, 2.60) | 5.70 | 0.06 |
| Europe | 3 | 1.53 (0.93, 2.52) | 1.55 (0.88, 2.71) | 2.43 | 0.30 |
| Other pesticides7 | |||||
| Adjusted (AHS 2005) | 4 | 1.46 (1.05, 2.02) | 1.43 (1.06, 1.92 | 2.61 | 0.46 |
| Unadjusted (AHS 2005) | 4 | 1.69 (1.29, 2.23) | 1.70 (1.26, 2.30) | 3.47 | 0.33 |
| De Roos et al. [15] | |||||
| Hierarchal OR8 | 6 | 1.36 (1.09, 1.70) | 1.46 (1.08, 1.96) | 6.80 | 0.24 |
| Cantor et al. [37]9 | 6 | 1.29 (1.04, 1.59) | 1.36 (1.02, 1.80) | 7.07 | 0.22 |
| Lee et al. [36]10 | 6 | 1.35 (1.11, 1.65) | 1.41 (1.09, 1.82) | 6.63 | 0.25 |
| Other | |||||
| Hohenadel vs. McDuffie11 | 6 | 1.23 (0.99, 1.53) | 1.30 (0.96, 1.76) | 7.34 | 0.20 |
| Exclude one study12 | |||||
| Andreotti et al. [24] | 5 | 1.84 (1.33, 2.55) | 1.86 (1.39, 2.48) | 3.36 | 0.50 |
| De Roos et al. [15] | 5 | 1.34 (1.06, 1.69) | 1.47 (1.02, 2.11) | 6.59 | 0.16 |
| Eriksson et al. [16] | 5 | 1.35 (1.08, 1.70) | 1.47 (1.04, 2.07) | 6.62 | 0.16 |
| Hardell et al. [17] | 5 | 1.40 (1.12, 1.75) | 1.56 (1.08, 2.24) | 8.06 | 0.09 |
| McDuffie et al. [42] | 5 | 1.31 (1.03, 1.66) | 1.43 (1.01,2.03) | 5.90 | 0.21 |
| Orsi et al. [18] | 5 | 1.46 (1.16, 1.83) | 1.69 (1.16, 2.45) | 7.36 | 0.12 |
Abbreviations: Cl, confidence interval; HCL, hairy cell leukemia; meta-RR, meta-relative risk
Heterogeneity is present when X2 heterogeneity statistic is greater than degrees of freedom (number of studies minus 1).
Risk estimates for the most highly exposed group available in the three studies that stratify by exposure level.
Eriksson et al. [16] results for any glyphosate exposure >10 years latency was used instead of the higher exposure group used in the main analysis.
The study combined all B-cell lymphomas and is added to the analysis on highest cumulative exposure (AHS 2018).
Hairy cell leukemia cases excluded—results presented in Hardell and Eriksson [38].
NHL cases excluded; only HCL results used—results presented in Nordstrom et al. [39].
Studies that provided RRs that are both adjusted and not adjusted for other pesticide use for ever exposure, or reported that adjusting for pesticide use had little impact on the RR estimate. AHS (2018) did not report ever exposure, so AHS (2005) was used instead.
Hierarchical model RR used instead of the standard logistic regression model RR.
Cantor et al. [37] used instead of De Roos et al. [15]. Cantor et al. [37] was the only of the three studies combined by De Roos et al. [15] that presented data for glyphosate.
Lee et al. [36] used instead of De Roos et al. [15], Lee et al. [36] used same subjects as De Roos et al. [15] but did not adjust for other pesticide exposure, did not exclude those with missing data on other pesticide use, and used only non-asthmatics.
Hohenadel et al. [40] used same subjects as McDuffie et al. [42] but presented results in subjects exposed to glyphosate but not malathion (OR=0.92; 95% Cl: 0.54-1.55).
One study excluded at a time to evaluate the impact of each individual study on the overall meta-RR.
3.2.1. Alternative Exposure Criteria
As a sensitivity analysis, we also conducted a meta-analysis using the longest exposure duration results to compare with our primary analysis using the highest cumulative exposure results. When RRs corresponding to exposures with the longest duration were selected from the AHS 2018, the meta-RR remained the same at 1.41 (95% CI: 1.13-1.74). When the AHS 2005 report was included, the meta-RRs increased to 1.56 (95% CI: 1.17-2.06) (Table 5).
When evaluating studies with only the highest levels of exposure [16, 24, 42], the meta-RR was 1.36 (95% CI: 1.06-1.75, Table 6). In studies that combined all exposures as ever exposed [15–19, 42], the meta-RR was 1.30 (95% CI: 1.03-2.64). Although the higher exposure group was used in the main analysis, Eriksson et al. [16] also provided results for greater than 10 years latency, which contributed to a meta-RR of 1.40 (95% CI: 1.13-1.75). [Note: AHS 2018 did not provide ever-exposure, so AHS 2005 was used to calculate this statistic and ever exposure above].
3.2.2. Study Inclusion
When we limited our analysis to case-control studies (Table 5), there was little inter-study heterogeneity. We estimated a doubling of the NHL risk (meta-RR = 1.84, 95% CI: 1.33-2.55) from 41% to 84% compared to the estimate that included the cohort study.
To ensure that one individual study was not artificially inflating the meta-risk estimate, we excluded the case-control studies one at a time and found that they all nominally lowered the meta-RR, except for the exclusion of Orsi et al. [18], where the meta-RR increased to 1.46 (1.16-1.83) (Table 6).
3.2.2. NHL vs. Cell-type Specific Lymphomas
Although our primary meta-analysis included six studies, there was a possibility to include a seventh study [41]. We excluded this study from the primary analysis because it included all B-cell lymphomas (4 cases), which account for approximately 85% of all NHL [56]; however, not all four cases were confirmed to be NHL. When we added Cocco et al. [41] to the meta-analysis (n = 7, Table 6), the resulting RR remained fairly similar at 1.43 (95% CI: 1.15-1.78).
Similar to our inclusion of the Cocco et al. [41] study, another cell-type specific study evaluated all cases of hairy cell leukemia (HCL), a subtype of NHL [39]. It was one of two studies [38, 39] included in the Hardell et al. [17] analysis, with the other study examining NHL only [38]. Excluding HCL cases had no effect on the meta-RR (1.41, 95 % CI: 1.13-1.77, Table 6). Similarly, using only hairy cell leukemia cases from Hardell et al. [17] (reported in Nordstrom et al. [39]) did not impact the meta-RR (1.43, 95% CI: 1.14-1.78).
3.2.4. Study Location and Adjustment
Studies in North America [15, 24, 42] had a meta-RR of 1.38 (95% CI: 1.08-1.76), whereas European studies [16–18] had a meta-RR of 1.53 (95% CI: 0.93-2.52). On average, when studies were adjusted for other pesticide use [15–17, 19], the meta-RR for ever-exposure was lower than unadjusted risk estimates from the same studies (meta-RRadjusted = 1.46, 95% CI: 1.05-2.02; meta-RRunadjusted = 1.69, 95% CI: 1.29-2.23).
3.2.5. Logistic vs. Hierarchical Regressions
Consistent with the two previous meta-analyses by IARC [22] and Schinasi and Leon [25] discussed in Section 4.1 below, we selected the RR estimated using the more traditional logistic regression over the hierarchical regression estimate in the case-control study by De Roos et al. [15] and found that there was little impact of this selection (meta-RR = 1.36, 95% CI: 1.09-1.70). When Cantor et al. [37] or Lee et al. [36] were used instead of De Roos et al. [15], the meta-RR decreased to 1.29 (95% CI: 1.04-1.59) and 1.35 (95% CI: 1.11-1.65), respectively. Similarly, using Hohenadel et al. [40] instead of McDuffie et al. [42] caused the meta-RR to decrease to 1.23 (95% CI: 0.99-1.53).
4. Comparison with Previous Meta-Analyses
Three meta-analyses of NHL in relation to GBH exposure have been published [22, 25, 26], all of which report lower, albeit also positive, risk estimates. In contrast to our work, these analyses did not focus on the highest exposed groups. Table 7 summarizes the major results from all GBH-NHL meta-analyses conducted to date, including the current one.
Table 7.
Comparison of current meta-analysis to other published meta-analyses
| Studies | Current Meta-Analysis | ||||
|---|---|---|---|---|---|
| Schinasi and Leon [25]a |
IARC [22] |
Chang and Delzell [26]a, b |
with AHS 2005 [19] |
with AHS 2018 [24] |
|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Andreotti et al. [24] | N/A | N/A | N/A | N/A | 1.12 (0.83-1.51) |
| De Roos (2005) [19] | 1.1 (0.7, 1.9) | 1.1 (0.7, 1.9) | 1.1 (0.7, 1.9) | 0.8 (0.5, 1.4) | N/A |
| De Roos (2003) [15] | 2.1 (1.1,4.0) | 2.1 (1.1,4.0) | 1.6 (0.9, 2.8) | 2.1 (1.1,4.0) | 2.1 (1.1,4.0) |
| Eriksson et al. [16] | 2.0 (1.1,3.7) | 1.51 (0.77, 2.94) | 1.51 (0.77, 2.94) | 2.36 (1.04, 5.37) | 2.36 (1.04, 5.37) |
| Hardell et al. [17] | 3.0 (1.1, 8.5) | 1.85 (0.55, 6.20) | 1.85 (0.55, 6.20) | 1.85 (0.55, 6.20) | 1.85 (0.55, 6.20) |
| McDuffie et al. [42] | 1.2 (0.8, 1.7) | 1.20 (0.83, 1.74) | 1.20 (0.83, 1.74) | 2.12 (1.20, 3.73) | 2.12 (1.20, 3.73) |
| Orsi et al. [18] | 1.0 (0.5, 2.2) | 1.0 (0.5, 2.2) | 1.0 (0.5, 2.2) | 1.0 (0.5, 2.2) | 1.0 (0.5, 2.2) |
| meta-RR (95% CI) | 1.45 (1.08, 1.95)c | 1.30 (1.03, 1.64) | 1.27 (1.01, 1.59) | 1.45 (1.11, 1.91) | 1.41 (1.13, 1.75) |
Abbreviations: CI, confidence interval; meta-RR, meta-relative risk; RR, relative risk;
In their published reports, meta-RRs and their 95% confidence intervals were rounded to one digit right of the decimal point.
Findings from Model 1, the primary analysis, are reported here.
Random effects model.
Schinasi and Leon [25] first reported a meta-RR of 1.45 (95% CI: 1.08-1.95). Although their selection criteria stated that they used the most adjusted effect estimate for the dichotomously defined exposure with the greatest number of exposed cases, they did not use adjusted effect estimates in the two Swedish studies [16, 17]. The IARC Working Group subsequently corrected this discrepancy in an otherwise identical meta-analysis [22], resulting in a meta-RR of 1.30 (95% CI: 1.03 −1.65). Although both studies are listed in Table 7 for completeness, we consider IARC 2015 to be the most accurate and updated version of this meta-analysis.
Most recently, Chang and Delzell [26] reported a meta-RR of 1.27 (95% CI: 1.01-1.59) in their primary analysis (model one). For each included study, the authors selected the most fully adjusted RR from the publication with the most recent and complete study population with the largest number of exposed cases. (In their publication, the meta-RR was rounded to one digit to the right of the decimal point.)
Whereas the three previous meta-analyses focused on general exposure (ever versus never), our new meta-analysis differs primarily because of our a priori selection of risk estimates from the most highly exposed groups when available (from three studies [16, 19, 42]). In our secondary comparison meta-analysis with the same six studies (including AHS 2005), we document an additional 0.15-0.18 (or 15-18%) higher NHL RR than previous meta-RRs [22, 26] (not including Schinasi and Leon, because it was corrected in IARC 2015). Similarly, in our primary analysis with AHS 2018, our meta-RR estimate adds an additional 0.11-0.14 (11-14%) increase in NHL relative risk to the previous meta-RRs [22, 26]. Overall, the meta-RR obtained using our a priori hypothesis, while generally consistent with previous analyses, gave somewhat higher estimates and suggested increased risk of NHL in individuals highly exposed to GBHs.
5. Strengths and Limitations
In this section, we evaluate the strengths and limitations of our meta-analyses, as well as of the cohort study and the case-control studies utilized.
5.1. Current Meta-Analyses
The strengths of these meta-analyses are the inclusion of the updated AHS 2018 study and our novel a priori hypothesis. By using the highest exposure group in each study when it was reported, we maximized the ability to detect the presence of an exposure-disease association. The current meta-analysis is also the first study to include the newly updated AHS.
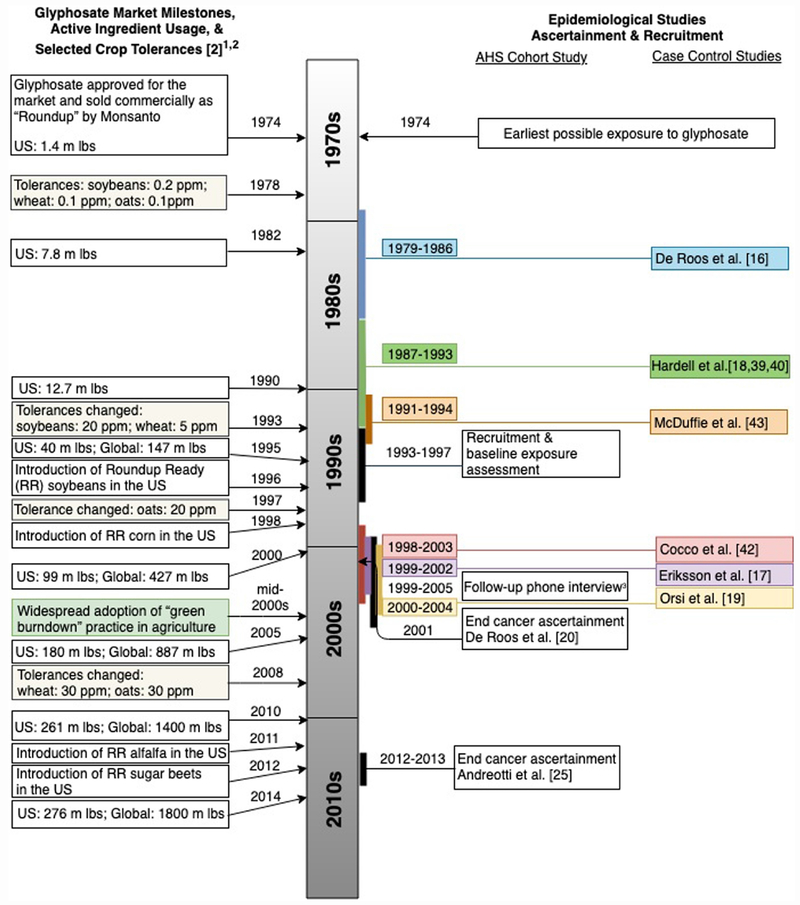
There are several weaknesses of our analysis that should be noted, however. First, there were only limited published data available for inclusion. Although meta-analysis prevents overemphasis on any single study [57], we cannot exclude the potential for publication bias, given the relatively few published studies to date. Second, there was imbalance in study design: among the only six included studies, five were case-control and one was a cohort. The collection of NHL findings from the cohort study was consistent with a wide range of risks [24], while, by contrast, most of the case-control studies did suggest an increased risk [15–17, 42]. There were also important differences in the comparison group utilized in the studies; some used the lowest exposure group as the reference, while others used the unexposed group. Because of this heterogeneity, and because no statistical tests can confirm elimination of publication bias or heterogeneity in a meta-analysis [58], our results should be interpreted with caution. Finally, as depicted in Figure 3 illustrating key milestones related to glyphosate use in society and in epidemiological studies, none of the available studies capture the effects of the significant increased usage of glyphosate that began with the introduction of “green-burn-down” in the mid-2000s.
Figure 3.
Timeline of glyphosate use milestones in relation to cohort and case-control study events.
1 Glyphosate active ingredient usage includes agricultural and non-agricultural applications
2 m = millions; Ibs = pounds
3 Completed by 63% of AHS participants
5.2. AHS Cohort Study
In general, cohort studies are considered the gold standard among observational studies because of their ability to estimate exposure before disease occurrence (which allows for clarity of temporality and can minimize recall bias), to estimate incidence, to examine multiple outcomes, and for some target populations, to study a large number of exposed subjects. Our new meta-analysis is the first to include the AHS 2018 update, which is the largest, newest, and most heavily weighted study (>50%, Table 4). Given its importance and because it was the only cohort study in our analyses, we discuss below several aspects of the AHS 2018 study and its comparison with the AHS results reported in 2005. Key differences between the AHS 2018 and AHS 2005 are summarized in Table 8.
Table 8:
Key differences between AHS 2005 and AHS 2018, with an emphasis on exposure quantification
| AHS 2005 [19] | AHS 2018 [24] | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposure assessment | Self-report at baseline | Self-report at baseline and follow-up questionnaire with exposure simulation1 | ||||||
| Exposure quantification | Ever/never | Cumulative exposure days | Intensity-weighted exposure days2 | Ever/never3 | Cumulative exposure days3 | Intensity-weighted exposure days2 | ||
| Lag period | Unlagged | Unlagged | 5-year lag | 20-year lag4 | ||||
| Exposure groups among exposed (days)5 | Ever exposed | T1: 1-20; T2: 21-56; T3: 57-2678 |
T1: 0.1-79.5; T2: 79.6-337.1; T3: 337.2-18,241 |
Ever exposed | T1: 1-19.9; T2: 20.0-61.9; T3: >62.0 |
Q1: 1–598.9; Q2: 599–1649.9; Q3: 1650–4339.9; Q4: >4340.0 |
Q1: 1–530.9; Q2: 531.0–1511.9; Q3: 1512.0–4063.4; Q4: >4063.5 |
Q1: 1–281.3; Q2: 281.4–895.9; Q3: 896–2609.9; Q4: >2610.0 |
| Exposure duration (years)6 | Maximum possible range= 20-24;7 Actual maximum: 7.38 Median = not provided; IQR = not provided |
Maximum possible range = 26–32;9 Actual maximum: not provided Median = 8.5; IQR = 5-14 |
Max. possible range = 21-27;9 Actual max: not provided Median = 4.1 ;10 IQR = not provided |
Max. possible range = 6-12;9 Actual max: not provided Median = 2.5;10 IQR = not provided |
||||
| Reference group | Unexposed | Lowest exposure tertile | Lowest exposure tertile | Unexposed | ||||
| Potential Exposure misclassification | Differential misclassification unlikely; Non-differential misclassification likely |
Differential misclassification possible; Non-differential misclassification likely |
||||||
| Follow-up (years) | Median = 6.7; Maximum possible = 911 |
Median = not provided Maximum possible = 2011 |
||||||
| Outcome inclusion | Multiple myeloma (MM) not included in NHL cases | Multiple myeloma (MM) included in NHL cases | ||||||
This was referred to as “multiple imputation” by study authors; see manuscript text for further details
The algorithm for calculating “intensity-weighted exposure days” was updated between 2005 and 2018. Key differences include rescaling of scores by a factor of 10 and altering the weights for mixing, certain pesticide application techniques, and the use of chemically resistant gloves [44]. Therefore, these metrics cannot be directly compared.
Ever/never and cumulative exposure days were only presented in the AHS 2018 supplement but are presented here to facilitate comparisons with AHS 2005
Results and quartiles for 10- and 15-year lags are presented in the AHS 2018 supplement
Exposure group abbreviations are as follows: Tertiles = “T;” Quartiles = “Q.”
The values provided in this row are based on the subset of individuals who reported using glyphosate
This theoretical maximum duration value was calculated based on the year that glyphosate entered the market (1974) and the end of AHS enrollment (1993-1997), since AHS 2005 used only baseline exposure information
This value was calculated based on the upper bound of the cumulative exposure days tertiles
These theoretical maximum duration values were calculated based on the year that glyphosate entered the market (1974) and the end of AHS follow-up exposure questionnaire (1999-2005), with the appropriate adjustments for the lag times as indicated.
These medians were calculated using the information provided in the footnote in Table 3 of the AHS 2018 publication
These follow-up times were calculated based on timing of study enrollment and follow-up
5.2.1. Exposure Assessment and Quantification
Exposures were self-reported using questionnaires. AHS 2005 used the exposures reported at baseline only, whereas AHS 2018 supplemented this information with responses to a follow-up questionnaire returned by 63% of AHS participants.
The risk estimates generated from the follow-up AHS 2018 report depended on a “multiple imputation” approach with multiple steps to generate GBH exposure information for the 37% of participants who did not complete the follow-up questionnaire [24]. A standard imputation model captures the full distribution of the exposure by relying on two parts of a model: the regression or predictable part and the residual error part. The validity of the imputed exposures and the resulting risk estimates relies on the validity of both parts of the imputation model. The AHS imputation method for ever/never pesticide use conditioned on the reported pesticide use and other data, including demographics, medical history at baseline, and farming characteristics at enrollment, with some covariates chosen by stepwise regression (see Table 2 in Heltshe et al. [59]). Based on their analysis of a 20% holdout dataset, the prevalence of glyphosate use was underreported by 7.31%, suggesting some lack of validity in the predictable part of the imputation model that may in turn affect the NHL risk estimates. The imputations of days of use per year and most recent year of farming activity relied upon a stratified sampling with replacement approach, with values sampled from Phase 2 respondents based on strata defined using Phase 1 information.
The imputations did not use the NHL or any other cancer outcome information reported by Andreotti et al. [24]. This approach is problematic because of how the residual error part of the imputation model is handled. It is known that multiple imputation of a covariate (i.e., glyphosate exposure) in a model that omits the outcome variable to be used in the inference leads to attenuation of the effect estimate for that covariate due to lack of correlation with the outcome in the residual error part of the imputed exposures [60]. As we discuss further in the next paragraph, this approach effectively “bakes into the results” the null hypothesis of no increased risk of NHL exposure due to glyphosate risk.
Because the NHL outcome information was not used in the imputation procedure, the exposure “imputation” method used in the AHS 2018 report can be better named “exposure simulation” as described by Gryparis et al. [61]. This term gives a much more accurate understanding of the impact of the imputation of the data on the risk estimates because when exposure is simulated in a model that does not take the NHL outcome into account, the uncertainty in the “imputed” exposure behaves like classical measurement error and, thus, will bias the effect estimate towards the null [62].
AHS 2018 authors argue that their imputation approach “likely did not materially impact risk estimates” [63]. However, their argument has to do with the impact on the average change in the number of predicted events in an outcome-augmented imputation model and not the role of classical measurement error in the imputed exposure estimates.
There was also a subtle yet important difference in the categorization and quantification of exposure data between AHS 2005 and 2018. As depicted in Table 8, both studies classified exposure based on (1) ever/never, (2) cumulative exposure days, and (3) intensity-weighted exposure days. However, the algorithm utilized to calculate intensity-weighted exposure days was updated between 2005 and 2018. Key differences include rescaling of scores by a factor of 10 and altering the weights for mixing, certain pesticide application techniques, and the use of chemically resistant gloves [44]. Therefore, these metrics cannot be directly compared.
Additionally, it is crucial to highlight the difference in reference groups between these two studies, which further limits the comparability of their estimates. AHS 2005 utilized the lowest exposed tertile as the comparison group for risk estimation. They justified this decision as an attempt to control residual confounding, because of the presence of significant differences in key characteristics between the never-exposed and lowest-exposed groups. By contrast, AHS 2018 utilized the unexposed group as the reference group even though our comparison of the demographics reported in each paper’s Table 1 does not suggest there is substantially better comparability between groups in AHS 2018. Furthermore, because the exposure information by which these groups were classified was based on their imputation procedure, the limitations of which are highlighted above, the actual comparability between groups may differ from the values reported. Not only would it be helpful to be able to compare directly the risk estimates across the two papers, it would be useful to investigate whether there was residual confounding introduced into the AHS 2018 analysis by the use of the “unexposed” group as the reference.
5.2.2. Exposure Misclassification
Differential misclassification is unlikely in a cohort study when exposure is assessed prior to the disease occurrence. In AHS 2018, however, we believe there is some potential for differential misclassification. Sixty-three percent of the original cohort provided updated exposure information by questionnaire one time between the years of 1999 and 2005. Although details are not provided, it is likely that some of the cases reported their exposure after disease occurrence, allowing for potential differential misclassification in the self-reported exposures in this cohort similar to general concerns with case-control studies. Furthermore, noting large societal trends in GBH exposure between initial exposure ascertainment and the follow-up questionnaire, and the 7.3% under-prediction of glyphosate exposures in the holdout dataset [59], the prediction part of the imputation modeling was likely differentially under-predicting exposures.
Non-differential misclassification occurs when exposure status is equally misclassified among exposed cases and unexposed controls[64]. The approach in AHS 2018 to exposure imputation is one theoretically well-understood source of non-differential misclassification. In addition, it may be more problematic in the context of a ubiquitous exposure because it is hard for participants to know to what extent or how long they have been exposed. Glyphosate’s ubiquity in the environment leads to profound concerns that even “unexposed” individuals in the cohort are likely to have been exposed to GBHs; consequently, the magnitude of any potential association relative to the unexposed group may be attenuated due to this misclassification. This problem is encountered with other environmental exposures such as environmental tobacco smoke (ETS): never smokers with ETS exposure carry some cancer risk and are not the ideal true reference group in studies of smoking and tobacco-related cancers [65]. As we noted above, non-differential misclassification is likely to attenuate measures of association, biasing the RR toward the null of 1.0 [66]. Although it is difficult to ascertain exactly, the extent of this source of non-differential misclassification can be estimated through smaller-scale validation studies [66].
5.2.3. Disease Classification & Latency
The updated AHS 2018 included multiple myeloma (MM) in their NHL cases, but the previous AHS 2005 did not. Although MM traditionally did not belong to NHL, WHO recently revised the classification of lymphoid neoplasms and suggested some types of MM (e.g., IgM mutation-related MM) are related more closely to lymphomas, including NHL, than to myelomas [67].
There is much uncertainty surrounding the latency period for NHL. The latency period for short-term high-dose exposures to carcinogens may be as short as two years, but it may also be as long as 15 years or more. Low-dose long-term exposures are expected to have longer median latencies between 15 to 20 years for NHL [68, 69]. It is possible that different NHL subtypes may also have different latencies. Given the uncertainty surrounding NHL latency, it is possible that the follow-up period (median = 6.7 years) in the 2005 AHS study [19], which was unlagged, may have been too short for a sufficient number of exposure-related cancer events to manifest. Given that participants had been exposed to GBHs prior to enrolling in the study (median = 8 years; mean = 7.5 years; SD = 5.3 years), participants could have had an exposure duration ranging from as low as 0 years to as high as 18 years at the time of enrollment, assuming a normal distribution. Hence, although some AHS members may have had sufficient exposure durations to develop NHL, many fell short of the median 15-20 years of expected NHL latency.
The 2018 AHS publication added 11-12 further years of follow-up for all study participants, an additional 483 cases of NHL, and considered five, ten, fifteen, and twenty year exposure lags, which was not possible in AHS 2005 due to its short duration. Epidemiologic studies often lag exposures to account for disease latency under the assumption that recent exposures have little impact on disease development. Theoretically, longer exposure durations and/or lags would present more biologically plausible associations with NHL. For AHS 2018 specifically, not only are the risk estimates associated with longer lag times more plausible that unlagged risk estimates in AHS 2005 and 2018, but the twenty-year exposure lag, specifically, may also be free of the bias caused by exposure imputation described above, given that at this lag exposure information may have been derived exclusively from the baseline questionnaire.
5.2.4. Summary
Overall, the study features highlighted above related to exposure assessment and quantification; misclassification; and latency and lag suggest caution in direct comparisons between AHS 2005 and 2018. Additionally, the limitations with AHS 2018 with regard to exposure simulation, potential residual confounding, and misclassification may have accounted for the weaker meta-RR estimate that we obtained when incorporating this study into the meta-analysis.
5.3. Case-Control Studies
Although cohort studies are the gold standard in observational epidemiology, they are often challenging to conduct due to the small number of incident cases for rare diseases such as NHL. Case-control studies can be more efficient for evaluation of rare diseases. For example, the AHS had to recruit tens of thousands of participants (N = 53,760) and follow them for more than a decade in order to gather 575 new cases of NHL, whereas the 5 case-control studies assembled 2,836 NHL cases among all participants (N = 8,868) in a much shorter period of time (Tables 1 and 4). Though the case-control studies are smaller and carry less weight than the large cohort study, it is worth noting that results from multiple case-control studies displayed little heterogeneity (Table 5) and reported similar findings pointing away from null (Table 4).
However, there are other challenges and concerns relevant to the case-control studies utilized in our meta-analysis, which we briefly discuss below.
5.3.1. Control Selection and Exposure Quantification
Four of the five case-control studies utilized here are population-based, while one is hospital-based. There may be important differences between hospital-based controls and population-based controls that could impact the interpretability and comparability of the resulting risk estimates. Of relevance to this concern is that, as noted above in our sensitivity analyses, exclusion of Orsi et al. [18] (the hospital-based case-control study) resulted in an increased meta-RR of 1.46 (95% CI: 1.16-1.83), while sequential exclusion of each of the population-based case control studies produced decreased meta-RRs.
Exposure was also quantified differently between the selected case-control studies, further impacting their comparability. While all the studies considered in our meta-analysis conducted exposure assessment based on self-reported questionnaire data, some studies considered ever/never exposure, while others evaluated exposure based on number of days per year (see Tables 1 and 4). Some studies also relied on proxy respondents such as next of kin.
5.3.2. Exposure Misclassification
It is always possible for the internal validity of case-control studies to be threatened by recall bias, a form of differential exposure misclassification that occurs when exposures are remembered differently by cases (or their proxies) and controls. Cases may have been more motivated to recall GBH exposure, and the exposures may be more vivid or meaningful due to awareness of the risk factors for their disease. While differential misclassification can bias the OR in either direction, differential misclassification due to cases being more likely to report exposure tends to artificially inflate the OR.
5.3.3. Latency and Lag
As discussed in Section 5.2.3, the latency for NHL is uncertain and could be anywhere from 2 years to greater than 15 years. There were differences in how the case-control studies considered and incorporated latency and lag into their analyses. For example, De Roos et al. [15] and McDuffie et al. [42] do not mention these considerations; by contrast, Hardell et al. [17], Orsi et al. [18], and Eriksson et al. [16] each incorporate latency and lag, albeit differently. These differences suggest caution in the integration of these results.
6. Summary of the GBH and NHL Association in Humans
Overall, the results from our new meta-analysis employing the a priori hypothesis and including the updated AHS 2018 study (1) demonstrated a significantly increased NHL risk in highly GBH-exposed individuals (meta-RR = 1.41, 95% CI: 1.13-1.75; Table 5 and Figure 2A), (2) are aligned with findings (Table 7) from previous meta-analyses [22, 26], and (3) revealed an additional 11-14% and 15-18% increase in NHL relative risk due to high levels of GBH exposure (Table 7) when using the AHS 2018 and the AHS 2005 cohort, respectively.
Together, all of the meta-analyses conducted to date, including our own, consistently report the same key finding: exposure to GBHs are associated with an increased risk of NHL.
Because most people in these epidemiological studies were not exposed to pure glyphosate, but rather glyphosate-based formulations (e.g. Roundup® or Ranger Pro ®) with a number of adjuvants, it could be argued that the NHL manifested as a result of exposure to the mixture or an ingredient other than glyphosate in the formulation. To investigate causal inference regarding the association between glyphosate exposure and NHL, we discuss briefly whether or not the association identified from epidemiological studies could be supported further by experimental animal and mechanistic studies.
7. Animal Data: Lymphoma Prevalence in Glyphosate-Exposed Mice
The animal study outcome most closely linked to human NHL is malignant lymphoma. We identified six unpublished glyphosate and lymphoma studies in mice that are in the public domain from two sources: a presentation by the European Food Safety Authority [70] at the EPA FIFRA Scientific Advisory Panel on Carcinogenic Potential of Glyphosate and a report by The Food and Agriculture Organization of the United Nations and World Health Organization Joint Meeting on Pesticide Residues [21]. EFSA [70] reported results from five unpublished studies: four in CD-1 [71–74] and one in Swiss albino mice [75], whereas JMPR [21] also reported data from a study in female CD-1 mice [76]. Each study reported four glyphosate doses and corresponding lymphoma incidence in males and females except for Takahashi [76], where the only data available in the public domain was for female mice [21 ].
7.1. Results of Murine Lymphoma Studies
Results from all studies (n = 6) of malignant lymphomas in mice available in the public domain are presented in Table 9. Study durations ranged from 1.5 to 2 years. All studies administered glyphosate through the diet [71–76], and the concentrations tested ranged from 100 ppm to 50,000 ppm [21]. EFSA [70] and JMPR [21] reported slightly different doses, with JMPR [21] further stratifying by sex. Lymphoma incidence was abstracted from EFSA [70], with slightly different numbers for one study [71]. Table 9 provides the dietary concentration of glyphosate (reported in ppm), the doses (reported in mg/kg/day) provided by EFSA [70] and JMPR [21], and lymphoma incidence in males and females. One study [73] reported food consumption, which was recorded for each treatment group, and weekly mean achieved-dose levels were averaged to calculate actual doses for males and females. Information on how doses were calculated for the other studies [71, 72, 74–76] was not available.
Table 9.
Data from Publically Available Studies of Malignant Lymphomas in Mice Exposed to Glyphosatea
| Study | Strains | Study Duration | Concentration in Diet (ppm) | Dose (mg/kg/day) |
Incidenceb(%) |
||
|---|---|---|---|---|---|---|---|
| EFSA [70] | JMPR [21]c | Male | Female | ||||
| Wood et al. [73] | CD-1 | 1.52 years (79 weeks) | 0 | 0 | 0, 0 | 0/51 (0) | 11/51 (22) |
| 500 | 71 | 71.4, 97.9 | 1/51 (2) | 8/51 (16) | |||
| 1500 | 234 | 234.2, 299.5 | 2/51 (4) | 10/51 (20) | |||
| 5000 | 810 | 810, 1081.2 | 5/51(10)* | 11/51 (22) | |||
| Kumar [75] | Swiss Albino | 1.5 years | 0 | 0 | 0, 0 | 10/50 (20) | 18/50 (36) |
| 100 | 15 | 14.5, 15.0 | 15/50 (30) | 20/50 (40) | |||
| 1000 | 151 | 149.7, 151.2 | 16/50 (32) | 19/50 (38) | |||
| 10000 | 1460 | 1453, 1466.8 | 19/50 (38)* | 25/50 (50)* | |||
| Sugimoto [72] | CD-1 | 1.5 years | 0 | 0 | 0, 0 | 2/50 (4) | 6/50 (12) |
| 1600 | 153 | 165, 153.2 | 2/50 (4) | 4/50 (8) | |||
| 8000 | 787 | 838.1,786.8 | 0/50 (0) | 8/50 (16) | |||
| 40000 | 4116 | 4348, 4116 | 6/50 (12)* | 7/50 (14) | |||
| Atkinson et al. [74] | CD-1 | 2 years | N/A | 0 | 0 | 4/50 (8) | 14/50 (28) |
| N/A | 100 | 100 | 2/50 (4) | 12/50 (24) | |||
| N/A | 300 | 300 | 1/50 (2) | 9/50 (18) | |||
| N/A | 1000 | 1000 | 6/50 (12) | 13/50 (26) | |||
| Knezevich and Hogan [71] | CD-1 | 2 years | 0 | 0 | 0, 0 | 2/48 (4) | 6d/50 (12) |
| 1000 | 157 | 157, 190 | 5d/49 (10) | 6/48 (13) | |||
| 5000 | 814 | 814,955 | 4/50 (8) | 7d/49 (14) | |||
| 30000 | 4841 | 4841,5874 | 2/49 (4) | 11d/49 (22) | |||
| Takahashi [76] | CD-1 | 1.5 years | 0 | N/A | 0, 0 | N/A | 3/50 (6) |
| 500 | 67.6, 93.2 | 1/50 (2) | |||||
| 5000 | 685, 909 | 4/50 (8) | |||||
| 50000 | 7470,8690 | 6/50 (12)* | |||||
Abbreviations: N/A, not available.
Number of lymphomas / total mice in group.
Data for male, female mice.
Reported slightly differently in JMPR [21] (N ± 1).
In summarizing these studies, EFSA [70] noted that Sugimoto [72] and Wood et al. [73] showed statistically significant dose-response in males according to the Cochran-Armitage test for linear trend, whereas Kumar [75] showed a statistically significant Z-test for both males and females. In agreement, JMPR [21 ] noted that Sugimoto [72] and Wood et al. [73] showed a statistically significant trend in males and that Kumar [75] reported statistically significant increases in malignant lymphoma in high-dose groups of both males and females. JMPR [21] further reported Takahashi [76] had a statistically significant increased incidence in lymphoma among females by their trend test. The remaining two studies did not report evidence of a statistically significant dose-response effect.
7.2. Additional Considerations and Recommendations
One challenge with these studies is that at face value they appear to be inconsistent because some show statistically significant findings whereas others do not. However, based on EPA’s Cancer Guidelines, evidence of increased lymphoma incidence should not be discounted due to lack of statistical significance in trend and/or pairwise comparison tests. Additional factors that should not be used to exclude study findings are the use of high doses and/or incidence rates that are consistent with levels seen in historical controls [77].
Another consideration is that the study lengths in these animal experiments may have been insufficient for development of NHL. There are proposals that the standard timeframe of two years for a cancer bioassay to approximate long-term cancer incidence in humans should be extended to account for potentially longer latencies. Eighty percent of all human cancers occur after the age of sixty. A two-year-old rat approximates a human of 60-65 years, indicating a traditional two-year bioassay may not be sufficient for late-developing tumors [78].
Future work should combine the results from these six studies into an overall pooled analysis to give a more robust assessment of the evidence. A pooled analysis would take into account the varying study durations (of 18 or 24 months) as well as other between-study differences in dose regimens and mouse strains.
These studies, in which mice were exposed to only glyphosate, may have underreported incidence of malignant lymphoma given evidence of increased toxicity of GBHs compared to glyphosate alone [79–81]. GBH mixtures, which contain a number of adjuvants, have been reported to exert synergistic toxic effects in mechanistic studies (Section 6). Therefore, we also recommend the evaluation of GBHs in chronic animal carcinogenicity studies to better capture representative exposure of humans.
8. Potential Mechanistic Context
There are several possible mechanistic explanations for the increased NHL risk in humans and lymphomas in animals. The etiology of NHL remains largely unknown; however, potential risk factors include autoimmune diseases, infection with viruses and/or bacteria, immunosuppressant medications, and exposures to some pesticides [82, 83]. Although not a formally recognized risk factor for NHL, endocrine disruptors have been associated recently with risk of B-cell neoplasms [84], most of which are NHL [56]. Furthermore, a genetic hallmark of NHL is the recurrence of chromosomal translocations, such as t(14;18), involving the immunoglobulin heavy chain gene fusion (BCL2-IGH), which are frequently detected in subgroups of NHL patients [85] and in pesticide-exposed farmers [86, 87]. Hence, immunosuppression, viral/bacterial infections, endocrine disruption, and genetic alterations have been suspected as key underlying mechanisms in the development of lymphoma (lymphomagenesis). Although not specifically linked to NHL, oxidative stress is a general mechanism of carcinogenesis that could contribute to lymphomagenesis.
8.1. Immunosuppression/Inflammation
The strongest factors known to increase NHL risk are congenital and acquired states of immunosuppression [88]. Several studies suggest that glyphosate alters the gut microbiome [79, 89] and cytokine IFN-γ and IL-2 production [90]. These changes could impact the immune system, promote chronic inflammation [91], and contribute to susceptibility of invading pathogens, such as H. pylori [92].
8.2. Endocrine Disruption
Disruption of sex hormones may contribute to lymphomagenesis/NHL [93]. Glyphosate may act as an endocrine disrupting chemical (EDC) because it has been found recently to alter sex hormone production. Several in vivo studies of male rats exposed to glyphosate have reported significantly lower testosterone levels [94–96], spermatid numbers [94], altered sperm and testicular morphology [94, 95], greater development of the mammary gland [97], and a surge in mast cell infiltration and proliferation accompanied by increased estrogen receptor (ESR1)[97]. In ovarian granulosa cells, glyphosate exposure resulted in decreased cell proliferation and estradiol production [98], which may contribute to lymphomagenesis [93].
8.3. Genetic Alterations
Several studies report that glyphosate can induce single- and double-strand DNA breaks [99–102], purine and pyrimidine oxidation [100], increased comet tail moment [103], and activation of the canonical non-homologous end-joining pathway (c-NHEJ) [101] that stimulates DNA repair. Glyphosate was also reported to induce micronuclei [104–110], sister chromatid exchanges [109], and chromosomal aberrations [111], but other studies found no change in these parameters [112–116]. Conclusions on the genotoxicity of glyphosate remain controversial in the debate on its carcinogenic potential [117]. A recent review reported that this discrepancy could be attributed to differences in the literature analyzed (published versus unpublished), exposure type (glyphosate versus GBHs), and exposure magnitude (low everyday exposures versus higher exposure groups) [118].
8.4. Oxidative Stress
Numerous studies indicate glyphosate causes oxidative stress [119–122]. Biomarkers of oxidative stress have been reported in a number of tissues in rats and mice, including liver, skin, kidney, brain, and plasma. In a study of albino male rats, levels of hepatic reduced glutathione were significantly decreased in GBH-exposed animals (1.64 mmol/g) compared to controls (2.64 mmol/g) [80]. A different study in glyphosate-exposed Wistar rats reported increased lipid peroxidation across all tissues studied and reactive nitrogen species in the brain and plasma [119]. A proteomic analysis of Swiss albino mice reported overexpression of carbonic anhydrase 3, a cytoplasmic protein that plays a role in cellular response to oxidative stress [123]. These mechanisms, among others, provide evidence of biological plausibility for the observed link between glyphosate exposure and human NHL, though further work is needed to better understand these pathways.
9. Conclusions and Future Directions
The rise of glyphosate as the most widely used herbicide raises serious health concerns, given its potential links with NHL. Using our high-exposure a priori hypothesis and including the recently updated AHS cohort in a meta-analysis for the first time, we report that GBH exposure is associated with increased risk of NHL in humans. Our findings are consistent with results reported from prior meta-analyses but show higher risk for NHL because of our focus on the highest exposure groups. However, given the heterogeneity between the studies included, the numerical risk estimates should be interpreted with caution. Additionally, as noted above and depicted in Figure 3, the available studies do not capture the possible effects of increased population exposures due to secular increases in use where “green burn-down” practices introduced in the mid-2000s may be a particularly important source of population exposures. The totality of the evidence from six studies of glyphosate-exposed mice support this association in humans. Although the underlying mechanisms remain unknown, mechanistic studies of glyphosate-induced immunosuppression/inflammation, endocrine disruption, genetic alterations, and oxidative stress suggest plausible links between GBH exposure and NHL development. The overall evidence from human, animal, and mechanistic studies presented here supports a compelling link between exposures to GBHs and increased risk for NHL.
Acknowledgements:
The authors thank Christina Gillezeau, MPH from Icahn School of Medicine at Mount Sinai, New York for carefully checking epidemiological data and Phum Tachachartvanich, PhD for intellectual review and discussion on mechanisms of endocrine disruption. We also thank the anonymous reviewers for their helpful comments. R.M.S. was supported by National Institutes of Environmental Health Sciences (NIEHS) award T32ES015459 and the University of Washington Retirement Association Aging Fellowship.
Abbreviations:
- AHS
Agricultural Health Study
- c-NHEJ
canonical non-homologous end joining pathway
- CI
confidence interval
- EDC
endocrine disrupting chemical
- EFSA
European Food Safety Authority
- EPA
Environmental Protection Agency
- ETS
environmental tobacco smoke
- GBHs
glyphosate-based herbicides
- IARC
International Agency for Research on Cancer
- IFN-γ
interferon gamma
- IL-2
Interleukin-2
- JMPR
Joint Meeting on Pesticide Residues by the Food and Agriculture Organization of the United Nations and World Health Organization
- meta-RR
meta-analysis relative risk
- mg/kg/day
milligrams per kilogram per day
- MM
multiple myeloma
- NHL
non-Hodgkin lymphoma
- OR
odds ratio
- ppm
parts per million
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- RR
relative risk
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest: All authors have no financial conflicts of interest to declare. We disclose Drs. Zhang, Taioli, and Sheppard served as Science Review Board Members of the US EPA FIFRA Scientific Advisory Panel (SAP) Meeting that evaluated glyphosate in December 2016.
References
- [1].Thelin G, Stone W, Estimation of annual agricultural pesticide use for counties of the conterminous United States, 1992–2009: U.S Geological Survey Scientific Investigations Report 2013–5009, 2013. [Google Scholar]
- [2].Benbrook CM, Trends in glyphosate herbicide use in the United States and globally, Environmental sciences Europe, 28 (2016) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].EPA, Glyphosate Reregistration Eligibility Document (RED). Glyphosate. EPA-738-R-93-014, U.S. Environmental Protection Agency, Washington, DC, 1993. [Google Scholar]
- [4].EPA, Glyphosate; pesticide tolerances, in: Regul FRR. (Ed.), U.S. Environmental Protection Agency, Washington, DC, 2013, pp. 25396–25401. [Google Scholar]
- [5].Wang Y, Jaw C, Chen Y, Accumulation of 2,4-D glyphosate in fish and water hyacinth, Water, Air and Soil Pollution, 74 (1994) 397–403. [Google Scholar]
- [6].Roy D, Konar S, Banerjee S, Charles D, Thompson D, Prasad R, Uptake and persistence of the herbicide glyphosate (Vision®) in fruit of wild blueberry and red raspberry, Canadian Journal of Forest Research, 19 (1989) 842–847. [Google Scholar]
- [7].Rodrigues NR, de Souza APF, Occurrence of glyphosate and AMPA residues in soy-based infant formula sold in Brazil, Food additives & contaminants. Part A, Chemistry, analysis, control, exposure & risk assessment, 35 (2018) 723–730. [DOI] [PubMed] [Google Scholar]
- [8].Alferness P, Iwata Y, Determination of Glyphosate and (Aminomethyl)phosphonic Acid in Soil, Plant and Animal Matrices, and Water by Capillary Gas Chromatography with Mass-Selective Detection, J. Agric. Food. Chem, 42 (1994) 2751–2759. [Google Scholar]
- [9].Bento CPM, Goossens D, Rezaei M, Riksen M, Mol HGJ, Ritsema CJ, Geissen V, Glyphosate and AMPA distribution in wind-eroded sediment derived from loess soil, Environmental pollution, 220 (2017) 1079–1089. [DOI] [PubMed] [Google Scholar]
- [10].Granby K, Johannesen S, Vahl M, Analysis of glyphosate residues in cereals using liquid chromatography-mass spectrometry (LC-MS/MS), Food additives and contaminants, 20 (2003) 692–698. [DOI] [PubMed] [Google Scholar]
- [11].Gillezeau C, van Gerwen M, Shaffer RM, Rana I, Zhang L, Sheppard L, Taioli E, The evidence of human exposure to glyphosate: a review, Environmental health : a global access science source, 18 (2019) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Conrad A, Schroter-Kermani C, Hoppe HW, Ruther M, Pieper S, Kolossa-Gehring M, Glyphosate in German adults - Time trend (2001 to 2015) of human exposure to a widely used herbicide, International journal of hygiene and environmental health, 220 (2017) 8–16. [DOI] [PubMed] [Google Scholar]
- [13].Mills PJ, Kania-Korwel I, Fagan J, McEvoy LK, Laughlin GA, Barrett-Connor E, Excretion of the Herbicide Glyphosate in Older Adults Between 1993 and 2016, Jama, 318 (2017) 1610–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Villarreal-Chiu JF, Acosta-Cortés AG, Kumar S, Kaushik G, Biological Limitations on Glyphosate Biodegradation, in: Singh R, Kumar S (Eds.) Green Technologies and Environmental Sustainability, Springer International Publishing, Cham, 2017, pp. 179–201. [Google Scholar]
- [15].De Roos AJ, Zahm SH, Cantor KP, Weisenburger DD, Holmes FF, Burmeister LF, Blair A, Integrative assessment of multiple pesticides as risk factors for non-Hodgkin’s lymphoma among men, Occupational and environmental medicine, 60 (2003) E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eriksson M, Hardell L, Carlberg M, Akerman M, Pesticide exposure as risk factor for non-Hodgkin lymphoma including histopathological subgroup analysis, International journal of cancer, 123 (2008) 1657–1663. [DOI] [PubMed] [Google Scholar]
- [17].Hardell L, Eriksson M, Nordstrom M, Exposure to pesticides as risk factor for non-Hodgkin’s lymphoma and hairy cell leukemia: pooled analysis of two Swedish case control studies, Leukemia & lymphoma, 43 (2002) 1043–1049. [DOI] [PubMed] [Google Scholar]
- [18].Orsi L, Delabre L, Monnereau A, Delval P, Berthou C, Fenaux P, Marit G, Soubeyran P, Huguet F, Milpied N, Leporrier M, Hemon D, Troussard X, Clavel J, Occupational exposure to pesticides and lymphoid neoplasms among men: results of a French case-control study, Occupational and environmental medicine, 66 (2009) 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].De Roos AJ, Blair A, Rusiecki JA, Hoppin JA, Svec M, Dosemeci M, Sandler DP, Alavanja MC, Cancer incidence among glyphosate-exposed pesticide applicators in the Agricultural Health Study, Environmental health perspectives, 113 (2005) 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].EPA, Revised Glyphosate Issue Paper: Evaluation of Carcinogenic Potential, EPA’s Office of Pesticide Programs, United States Environmental Protection Agency; 2017. [Google Scholar]
- [21].JMPR, World Health Organization: Pesticide residues in food-2016: toxicological evaluations Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Geneva, Switzerland, 9–13 May 2016, World Health Organization; 2017. [Google Scholar]
- [22].IARC, Some Organophosphate Insecticides and Herbicides: Diazinon, Glyphosate, Malathion, Parathion, and Tetrachlorvinphos, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 112 , International Agency for Research on Cancer (IARC), Lyon, France, 2015. [Google Scholar]
- [23].California Office of Environmental Health Hazard Assessment, Safe Drinking Water and Toxic Enforcement Act of 1986: The Proposition 65 List, 1985. [Google Scholar]
- [24].Andreotti G, Koutros S, Hofmann JN, Sandler DP, Lubin JH, Lynch CF, Lerro CC, De Roos AJ, Parks CG, Alavanja MC, Silverman DT, Beane Freeman LE, Glyphosate Use and Cancer Incidence in the Agricultural Health Study, Journal of the National Cancer Institute, 110 (2018) 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schinasi L, Leon ME, Non-Hodgkin lymphoma and occupational exposure to agricultural pesticide chemical groups and active ingredients: a systematic review and meta-analysis, International journal of environmental research and public health, 11 (2014) 4449–4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chang ET, Delzell E, Systematic review and meta-analysis of glyphosate exposure and risk of lymphohematopoietic cancers, Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes, 51 (2016) 402–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rom WN, Markowitz S, Environmental and occupational medicine, 4th ed, Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, 2007. [Google Scholar]
- [28].Steinmaus C, Smith AH, Jones RM, Smith MT, Meta-analysis of benzene exposure and non-Hodgkin lymphoma: biases could mask an important association, Occupational and environmental medicine, 65 (2008) 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang L, Steinmaus C, Eastmond DA, Xin XK, Smith MT, Formaldehyde exposure and leukemia: a new meta-analysis and potential mechanisms, Mutation research, 681 (2009) 150–168. [DOI] [PubMed] [Google Scholar]
- [30].Duong A, Steinmaus C, McHale CM, Vaughan CP, Zhang L, Reproductive and developmental toxicity of formaldehyde: a systematic review, Mutation research, 728 (2011) 118–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Greenland S, Meta-analysis, in: Rothman K, Greenland S (Ed.) Modern Epidemiology, Lippincott Raven, Philadelphia, 1998, pp. 643–673. [Google Scholar]
- [32].Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement, PLoS medicine, 6 (2009) e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hoar SK, Blair A, Holmes FF, Boysen CD, Robel RJ, Hoover R, Fraumeni JF Jr., Agricultural herbicide use and risk of lymphoma and soft-tissue sarcoma, Jama, 256 (1986) 1141–1147. [PubMed] [Google Scholar]
- [34].Zahm SH, Weisenburger DD, Babbitt PA, Saal RC, Vaught JB, Cantor KP, Blair A, A case-control study of non-Hodgkin’s lymphoma and the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) in eastern Nebraska, Epidemiology, 1 (1990) 349–356. [DOI] [PubMed] [Google Scholar]
- [35].Kachuri L, Demers PA, Blair A, Spinelli JJ, Pahwa M, McLaughlin JR, Pahwa P, Dosman JA, Harris SA, Multiple pesticide exposures and the risk of multiple myeloma in Canadian men, International journal of cancer, 133 (2013) 1846–1858. [DOI] [PubMed] [Google Scholar]
- [36].Lee WJ, Cantor KP, Berzofsky JA, Zahm SH, Blair A, Non-Hodgkin’s lymphoma among asthmatics exposed to pesticides, International journal of cancer, 111 (2004) 298–302. [DOI] [PubMed] [Google Scholar]
- [37].Cantor KP, Blair A, Everett G, Gibson R, Burmeister LF, Brown LM, Schuman L, Dick FR, Pesticides and other agricultural risk factors for non-Hodgkin’s lymphoma among men in Iowa and Minnesota, Cancer research, 52 (1992) 2447–2455. [PubMed] [Google Scholar]
- [38].Hardell L, Eriksson M, A case-control study of non-Hodgkin lymphoma and exposure to pesticides, Cancer, 85 (1999) 1353–1360. [DOI] [PubMed] [Google Scholar]
- [39].Nordstrom M, Hardell L, Magnuson A, Hagberg H, Rask-Andersen A, Occupational exposures, animal exposure and smoking as risk factors for hairy cell leukaemia evaluated in a case-control study, British journal of cancer, 77 (1998) 2048–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hohenadel K, Harris SA, McLaughlin JR, Spinelli JJ, Pahwa P, Dosman JA, Demers PA, Blair A, Exposure to multiple pesticides and risk of non-Hodgkin lymphoma in men from six Canadian provinces, International journal of environmental research and public health, 8 (2011) 2320–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cocco P, Satta G, Dubois S, Pili C, Pilleri M, Zucca M, t Mannetje AM, Becker N, Benavente Y, de Sanjose S, Foretova L, Staines A, Maynadie M, Nieters A, Brennan P, Miligi L, Ennas MG, Boffetta P, Lymphoma risk and occupational exposure to pesticides: results of the Epilymph study, Occupational and environmental medicine, 70 (2013) 91–98. [DOI] [PubMed] [Google Scholar]
- [42].McDuffie HH, Pahwa P, McLaughlin JR, Spinelli JJ, Fincham S, Dosman JA, Robson D, Skinnider LF, Choi NW, Non-Hodgkin’s lymphoma and specific pesticide exposures in men: cross-Canada study of pesticides and health, Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 10 (2001) 1155–1163. [PubMed] [Google Scholar]
- [43].Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P, The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa (ON): Ottawa Hospital Research Institute; 2009, Available in March, (2016). [Google Scholar]
- [44].Coble J, Thomas KW, Hines CJ, Hoppin JA, Dosemeci M, Curwin B, Lubin JH, Beane Freeman LE, Blair A, Sandler DP, Alavanja MC, An updated algorithm for estimation of pesticide exposure intensity in the agricultural health study, International journal of environmental research and public health, 8 (2011) 4608–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dosemeci M, Alavanja MC, Rowland AS, Mage D, Zahm SH, Rothman N, Lubin JH, Hoppin JA, Sandler DP, Blair A, A quantitative approach for estimating exposure to pesticides in the Agricultural Health Study, The Annals of occupational hygiene, 46 (2002) 245–260. [DOI] [PubMed] [Google Scholar]
- [46].Torstensson NTL, Lundgren LN, Stenstrom J, Influence of Climatic and Edaphic Factors on Persistence of Glyphosate and 2,4-D in Forest Soils, Ecotox Environ Safe, 18 (1989) 230–239. [DOI] [PubMed] [Google Scholar]
- [47].Honer L, Dissipation of Glyphosate and Aminomethylphosphonic Acid in Forestry Sites: Lab Project Number: MSL-9940; 993. Unpublished study prepared by Monsanto Agricultural Co. 555 pp. in 1992, (Unpublished results). [Google Scholar]
- [48].de Andrea MM, Peres TB, Luchini LC, Bazarin S, Papini S, Matallo MB, Savoy VLT, Influence of repeated applications of glyphosate on its persistence and soil bioactivity, Pesqui Agropecu Bras, 38 (2003) 1329–1335. [Google Scholar]
- [49].McHale CM, Osborne G, Morello-Frosch R, Salmon AG, Sandy MS, Solomon G, Zhang L, Smith MT, Zeise L, Assessing health risks from multiple environmental stressors: Moving from GxE to IxE, Mutation research, 775 (2018) 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].DerSimonian R, Laird N, Meta-analysis in clinical trials, Control Clin Trials, 7 (1986) 177–188. [DOI] [PubMed] [Google Scholar]
- [51].Petitti D, Statistical Methods in Meta-analysis, Meta-Analysis, Decision Analysis, and Cost-Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine, Oxford University Press, New York, NY, 1994, pp. 94–118. [Google Scholar]
- [52].Begg CB, Mazumdar M, Operating characteristics of a rank correlation test for publication bias, Biometrics, 50 (1994) 1088–1101. [PubMed] [Google Scholar]
- [53].Egger M, Davey Smith G, Schneider M, Minder C, Bias in meta-analysis detected by a simple, graphical test, BMJ, 315 (1997) 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].L. StataCorp, Stata Statistical Software: Release 15, College Station, TX, 2017. [Google Scholar]
- [55].Microsoft Corporation, Microsoft Excel Version 3, Redmond, Washington, 2013. [Google Scholar]
- [56].Dotan E, Aggarwal C, Smith MR, Impact of Rituximab (Rituxan) on the Treatment of B-Cell Non-Hodgkin’s Lymphoma, P & T : a peer-reviewed journal for formulary management, 35 (2010) 148–157. [PMC free article] [PubMed] [Google Scholar]
- [57].Ioannidis JP, Why most published research findings are false, PLoS medicine, 2 (2005) e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ioannidis JP, Interpretation of tests of heterogeneity and bias in meta-analysis, Journal of evaluation in clinical practice, 14 (2008) 951–957. [DOI] [PubMed] [Google Scholar]
- [59].Heltshe SL, Lubin JH, Koutros S, Coble JB, Ji BT, Alavanja MC, Blair A, Sandler DP, Hines CJ, Thomas KW, Barker J, Andreotti G, Hoppin JA, Beane Freeman LE, Using multiple imputation to assign pesticide use for non-responders in the follow-up questionnaire in the Agricultural Health Study, Journal of exposure science & environmental epidemiology, 22 (2012) 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Little R, Regression with Missing X’s: A Review, Journal of the American Statistical Association, 87 (1992) 1227–1237. [Google Scholar]
- [61].Gryparis A, Paciorek CJ, Zeka A, Schwartz J, Coull BA, Measurement error caused by spatial misalignment in environmental epidemiology, Biostatistics, 10 (2009) 258–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sheppard L, Shaffer RM, Re: Glyphosate Use and Cancer Incidence in the Agricultural Health Study, Journal of the National Cancer Institute, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Andreotti G, Lubin JH, Koutros S, Hofmann JN, Sandler DP, Lerro CC, Parks CG, Silverman DT, Beane Freeman LE, Response to Sheppard and Shaffer, Journal of the National Cancer Institute, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Porta M, A dictionary of epidemiology, Oxford university press; 2008. [Google Scholar]
- [65].Eberth B, Olajide D, Craig P, Ludbrook A, Smoking-related disease risk, area deprivation and health behaviours, Journal of public health, 36 (2014) 72–80. [DOI] [PubMed] [Google Scholar]
- [66].Pearce N, Checkoway H, Kriebel D, Bias in occupational epidemiology studies, Occupational and environmental medicine, 64 (2007) 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES, The 2016 revision of the World Health Organization classification of lymphoid neoplasms, Blood, 127 (2016) 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Weisenburger DD, Pathological classification of non-Hodgkin’s lymphoma for epidemiological studies, Cancer research, 52 (1992) 5456s–5462s; discussion 5462s-5464s. [PubMed] [Google Scholar]
- [69].Weisenburger DD, Epidemiology of non-Hodgkin’s lymphoma: recent findings regarding an emerging epidemic, Annals of oncology : official journal of the European Society for Medical Oncology, 5 Suppl 1 (1994) 19–24. [DOI] [PubMed] [Google Scholar]
- [70].EFSA, European Food Safety Authority: EU assessment of the carcinogenic potential of glyphosate at FIFRA Scientific Advisory Panel on Carcinogenic Potential of Glyphosate, 13-16 December 2016, 2016.
- [71].Knezevich A, Hogan G, A chronic feeding study of glyphosate (Roundup Technical) in mice: Project no. 77-2061: Monsanto report BDN-77-420. 1983, (Unpublished results).
- [72].Sugimoto K, HR-001: 18-month oral oncogenicity study in mice. The Institute of Environmental Toxicology, Tokyo, Japan: Laboratory project ID IET 94–0151, Sankyo Co., Ltd., Tokyo, Japan. 1997, (Unpublished results). [Google Scholar]
- [73].Wood E, Dunster J, Watson P, Brooks P, Dietary carcinogenicity study in the mouse Harlan Laboratories Limited, Shardlow, Derbyshire, England, UK: SPL project no.: 2060-0011. 2009, Unpublished study, (Unpublished results). [Google Scholar]
- [74].Atkinson C, Martin T, Hudson P, Robb D, Glyphosate: 104 week dietary carcinogenicity study in mice. Unpublished report No. 7793. IRI project No. 438618, dated 12 April 1991. Inveresk Research International, Tranent, Scotland, UK: Submitted to WHO by Cheminova A/S, Lemvig, Denmark, (Unpublished results). [Google Scholar]
- [75].Kumar D, Carcinogenicity study with glyphosate technical in swiss albino mice Toxicology Department, Rallis Research Centre, Rallis India Limited, Bangalore, India: Data owner: Feinchemie Schwebda GmbH. Study no.: Toxi:1559.CARCI-M. 2001, (Unpublished results). [Google Scholar]
- [76].Takahashi M, Oral feeding carcinogenicity study in mice with AK-01, Nippon Experimental Medical Research Institute Co. Ltd, Agatsuma, Gunma, Japan: Technical project no. H-95056, 3303–58, (1999). [Google Scholar]
- [77].EPA, Guidelines for Carcinogen Risk Assessment, EPA’s Office of Pesticide Programs, United States Environmental Protection Agency, Washington, DC, 2005. [Google Scholar]
- [78].Huff J, Jacobson MF, Davis DL, The limits of two-year bioassay exposure regimens for identifying chemical carcinogens, Environmental health perspectives, 116 (2008) 1439–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mao Q, Manservisi F, Panzacchi S, Mandrioli D, Menghetti I, Vornoli A, Bua L, Falcioni L, Lesseur C, Chen J, Belpoggi F, Hu J, The Ramazzini Institute 13-week pilot study on glyphosate and Roundup administered at human-equivalent dose to Sprague Dawley rats: effects on the microbiome, Environmental health : a global access science source, 17 (2018) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].El-Shenawy NS, Oxidative stress responses of rats exposed to Roundup and its active ingredient glyphosate, Environmental toxicology and pharmacology, 28 (2009) 379–385. [DOI] [PubMed] [Google Scholar]
- [81].Richard S, Moslemi S, Sipahutar H, Benachour N, Seralini GE, Differential effects of glyphosate and roundup on human placental cells and aromatase, Environmental health perspectives, 113 (2005) 716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hu L, Luo D, Zhou T, Tao Y, Feng J, Mei S, The association between non-Hodgkin lymphoma and organophosphate pesticides exposure: A meta-analysis, Environmental pollution, 231 (2017) 319–328. [DOI] [PubMed] [Google Scholar]
- [83].Kato I, Koenig KL, Shore RE, Baptiste MS, Lillquist PP, Frizzera G, Burke JS, Watanabe H, Use of anti-inflammatory and non-narcotic analgesic drugs and risk of non-Hodgkin’s lymphoma (NHL) (United States), Cancer causes & control : CCC, 13 (2002) 965–974. [DOI] [PubMed] [Google Scholar]
- [84].Costas L, Infante-Rivard C, Zock JP, Van Tongeren M, Boffetta P, Cusson A, Robles C, Casabonne D, Benavente Y, Becker N, Brennan P, Foretova L, Maynadie M, Staines A, Nieters A, Cocco P, de Sanjose S, Occupational exposure to endocrine disruptors and lymphoma risk in a multi-centric European study, British journal of cancer, 112 (2015) 1251–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Shankland KR, Armitage JO, Hancock BW, Non-Hodgkin lymphoma, Lancet, 380 (2012) 848–857. [DOI] [PubMed] [Google Scholar]
- [86].Chiu BC, Blair A, Pesticides, chromosomal aberrations, and non-Hodgkin’s lymphoma, Journal of agromedicine, 14 (2009) 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Roulland S, Lebailly P, Lecluse Y, Briand M, Pottier D, Gauduchon P, Characterization of the t(14;18) BCL2-IGH translocation in farmers occupationally exposed to pesticides, Cancer research, 64 (2004) 2264–2269. [DOI] [PubMed] [Google Scholar]
- [88].Evens AM, Blum KA, Non-hodgkin lymphoma, Springer; 2015. [Google Scholar]
- [89].Aitbali Y, Ba-M’hamed S, Elhidar N, Nafis A, Soraa N, Bennis M, Glyphosate based- herbicide exposure affects gut microbiota, anxiety and depression-like behaviors in mice, Neurotoxicology and teratology, 67 (2018) 44–49. [DOI] [PubMed] [Google Scholar]
- [90].Nakashima K, Yoshimura T, Mori H, Kawaguchi M, Adachi S, Nakao T, Yamazaki F, [Effects of pesticides on cytokines production by human peripheral blood mononuclear cells--fenitrothion and glyphosate], Chudoku kenkyu : Chudoku Kenkyukai jun kikanshi = The Japanese journal of toxicology, 15 (2002) 159–165. [PubMed] [Google Scholar]
- [91].Donaldson GP, Lee SM, Mazmanian SK, Gut biogeography of the bacterial microbiota, Nature reviews. Microbiology, 14 (2016) 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Schwabe RF, Jobin C, The microbiome and cancer, Nature reviews. Cancer, 13 (2013) 800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hosgood HD, Gunter MJ, Murphy N, Rohan TE, Strickler HD, The Relation of Obesity-Related Hormonal and Cytokine Levels With Multiple Myeloma and Non-Hodgkin Lymphoma, Frontiers in oncology, 8 (2018) 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Nardi J, Moras PB, Koeppe C, Dallegrave E, Leal MB, Rossato-Grando LG, Prepubertal subchronic exposure to soy milk and glyphosate leads to endocrine disruption, Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 100 (2017) 247–252. [DOI] [PubMed] [Google Scholar]
- [95].Romano RM, Romano MA, Bernardi MM, Furtado PV, Oliveira CA, Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology, Archives of toxicology, 84 (2010) 309–317. [DOI] [PubMed] [Google Scholar]
- [96].Pandey A, Rudraiah M, Analysis of endocrine disruption effect of Roundup((R)) in adrenal gland of male rats, Toxicology reports, 2 (2015) 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Altamirano GA, Delconte MB, Gomez AL, Ingaramo PI, Bosquiazzo VL, Luque EH, Munoz-de-Toro M, Kass L, Postnatal exposure to a glyphosate-based herbicide modifies mammary gland growth and development in Wistar male rats, Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 118 (2018) 111–118. [DOI] [PubMed] [Google Scholar]
- [98].Perego MC, Schutz LF, Caloni F, Cortinovis C, Albonico M, Spicer LJ, Evidence for direct effects of glyphosate on ovarian function: glyphosate influences steroidogenesis and proliferation of bovine granulosa but not theca cells in vitro, Journal of applied toxicology : JAT, 37 (2017) 692–698. [DOI] [PubMed] [Google Scholar]
- [99].Bolognesi C, Bonatti S, Degan P, Gallerani E, Peluso M, Rabboni R, Roggieri P, Abbondandolo A, Genotoxic activity of glyphosate and its technical formulation roundup, J Agr Food Chem, 45 (1997) 1957–1962. [Google Scholar]
- [100].Wozniak E, Sicinska P, Michalowicz J, Wozniak K, Reszka E, Huras B, Zakrzewski J, Bukowska B, The mechanism of DNA damage induced by Roundup 360 PLUS, glyphosate and AMPA in human peripheral blood mononuclear cells – genotoxic risk assessement, Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, (2018). [DOI] [PubMed] [Google Scholar]
- [101].Suarez-Larios K, Salazar-Martinez AM, Montero-Montoya R, Screening of Pesticides with the Potential of Inducing DSB and Successive Recombinational Repair, Journal of toxicology, 2017 (2017) 3574840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Paz-y-Mino C, Sanchez ME, Arevalo M, Munoz MJ, Witte T, De-La-Carrera GO, Leone PE, Evaluation of DNA damage in an Ecuadorian population exposed to glyphosate, Genetics and Molecular Biology, 30 (2007) 456–460. [Google Scholar]
- [103].Milic M, Zunec S, Micek V, Kasuba V, Mikolic A, Lovakovic BT, Semren TZ, Pavicic I, Cermak AMM, Pizent A, Vrdoljak AL, Valencia-Quintana R, Sanchez-Alarcon J, Zeljezic D, Oxidative stress, cholinesterase activity, and DNA damage in the liver, whole blood, and plasma of Wistar rats following a 28-day exposure to glyphosate, Arhiv za higijenu rada i toksikologiju, 69 (2018) 154–168. [DOI] [PubMed] [Google Scholar]
- [104].Bolognesi C, Carrasquilla G, Volpi S, Solomon KR, Marshall EJ, Biomonitoring of genotoxic risk in agricultural workers from five colombian regions: association to occupational exposure to glyphosate, Journal of toxicology and environmental health. Part A, 72 (2009) 986–997. [DOI] [PubMed] [Google Scholar]
- [105].Koller VJ, Furhacker M, Nersesyan A, Misik M, Eisenbauer M, Knasmueller S, Cytotoxic and DNA-damaging properties of glyphosate and Roundup in human-derived buccal epithelial cells, Archives of toxicology, 86 (2012) 805–813. [DOI] [PubMed] [Google Scholar]
- [106].Suresh T, Mutagenicity – Micronucleus test in Swiss Albino mice. Rallis India Limited, Banglore, India: Study no. TOXI:889-MUT-CH.MN, dated 6 May 1993. Sponsor: M/s Feinchemie Schwebda GmbH, Schwebda, Germany., (Unpublished results). [Google Scholar]
- [107].Rodrigues H, Penha-Silva N, deAraujo M, Nishijo H, Aversi-Ferreira TA, Effects of Roundup® Pesticide on the Stability of Human Erythrocyte Membranes and Micronuclei Frequency in Bone Marrow Cells of Swiss Mice, The Open Biology Journal, 4 (2011) 54–59. [Google Scholar]
- [108].Prasad S, Srivastava S, Singh M, Shukla Y, Clastogenic effects of glyphosate in bone marrow cells of swiss albino mice, Journal of toxicology, 2009 (2009) 308985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Amer SM, Aly FAE, Farghaly AA, I. AAE, In vitro and in vivo evaluation of the genotoxicity of the herbicide glyphosate in mice, Bulletin of the National Research Centre, 31 (2006) 427–446. [Google Scholar]
- [110].Ghisi Nde C, de Oliveira EC, Prioli AJ, Does exposure to glyphosate lead to an increase in the micronuclei frequency? A systematic and meta-analytic review, Chemosphere, 145 (2016) 42–54. [DOI] [PubMed] [Google Scholar]
- [111].Lioi MB, Scarfi MR, Santoro A, Barbieri R, Zeni O, Di Berardino D, Ursini MV, Genotoxicity and oxidative stress induced by pesticide exposure in bovine lymphocyte cultures in vitro, Mutation research, 403 (1998) 13–20. [DOI] [PubMed] [Google Scholar]
- [112].Paz-y-Mino C, Munoz MJ, Maldonado A, Valladares C, Cumbal N, Herrera C, Robles P, Sanchez ME, Lopez-Cortes A, Baseline determination in social, health, and genetic areas in communities affected by glyphosate aerial spraying on the northeastern Ecuadorian border, Reviews on environmental health, 26 (2011) 45–51. [DOI] [PubMed] [Google Scholar]
- [113].Sivikova K, Dianovsky J, Cytogenetic effect of technical glyphosate on cultivated bovine peripheral lymphocytes, International journal of hygiene and environmental health, 209 (2006) 15–20. [DOI] [PubMed] [Google Scholar]
- [114].Dimitrov BD, Gadeva PG, Benova DK, Bineva MV, Comparative genotoxicity of the herbicides Roundup, Stomp and Reglone in plant and mammalian test systems, Mutagenesis, 21 (2006) 375–382. [DOI] [PubMed] [Google Scholar]
- [115].Rank J, Jensen AG, Skov B, Pedersen LH, Jensen K, Genotoxicity testing of the herbicide Roundup and its active ingredient glyphosate isopropylamine using the mouse bone marrow micronucleus test, Salmonella mutagenicity test, and Allium anaphase-telophase test, Mutation research, 300 (1993) 29–36. [DOI] [PubMed] [Google Scholar]
- [116].Grisolia CK, A comparison between mouse and fish micronucleus test using cyclophosphamide, mitomycin C and various pesticides, Mutation research, 518 (2002) 145–150. [DOI] [PubMed] [Google Scholar]
- [117].Hollert H, Beckhaus T, Some food for thought: a short comment on Charles Benbrook′ paper “How did the US EPA and IARC reach diametrically opposed conclusions on the genotoxicity of glyphosate-based herbicides?” and its implications, Environmental sciences Europe, In press (2019). [Google Scholar]
- [118].Benbrook CM, How did the US EPA and IARC reach diametrically opposed conclusions on the genotoxicity of glyphosate-based herbicides?, Environ Sci Eur, In press (2019). [Google Scholar]
- [119].Astiz M, de Alaniz MJ, Marra CA, Antioxidant defense system in rats simultaneously intoxicated with agrochemicals, Environmental toxicology and pharmacology, 28 (2009) 465–473. [DOI] [PubMed] [Google Scholar]
- [120].Elie-Caille C, Heu C, Guyon C, Nicod L, Morphological damages of a glyphosate-treated human keratinocyte cell line revealed by a micro- to nanoscale microscopic investigation, Cell biology and toxicology, 26 (2010) 331–339. [DOI] [PubMed] [Google Scholar]
- [121].Gehin A, Guillaume YC, Millet J, Guyon C, Nicod L, Vitamins C and E reverse effect of herbicide-induced toxicity on human epidermal cells HaCaT: a biochemometric approach, International journal of pharmaceutics, 288 (2005) 219–226. [DOI] [PubMed] [Google Scholar]
- [122].George J, Shukla Y, Emptying of Intracellular Calcium Pool and Oxidative Stress Imbalance Are Associated with the Glyphosate-Induced Proliferation in Human Skin Keratinocytes HaCaT Cells, ISRN dermatology, 2013 (2013) 825180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].George J, Prasad S, Mahmood Z, Shukla Y, Studies on glyphosate-induced carcinogenicity in mouse skin: a proteomic approach, Journal of proteomics, 73 (2010) 951–964. [DOI] [PubMed] [Google Scholar]
- [124].Blair A, Tarone R, Sandler D, Lynch CF, Rowland A, Wintersteen W, Steen WC, Samanic C, Dosemeci M, Alavanja MC, Reliability of reporting on life-style and agricultural factors by a sample of participants in the Agricultural Health Study from Iowa, Epidemiology, 13 (2002) 94–99. [DOI] [PubMed] [Google Scholar]