Abstract
Background:
The most common immediate hypersensitivity to macrogols is associated with PEG 3350, however the epidemiology, mechanisms and cross-reactivity are poorly understood. Thousands of medications contain either PEGs or structurally similar polysorbates.
Objective:
Our objective was to better understand the mechanism, cross-reactivity and scope of PEG hypersensitivity.
Methods:
Two cases with a past history of immediate hypersensitivity to PEG-containing medications were used to study potential mechanisms and cross-reactivity of immediate reactions to PEG 3350. Skin testing and oral challenges with PEG and polysorbate-containing agents were employed to determine clinical reactivity and cross-reactivity between the two allergens. Enzyme-linked immunosorbent assay (ELISA) and electrochemiluminescent immunoassay were used to detect anti-PEG specific IgG and IgE respectively, using PEGylated protein or PEG alone as antigens in two cases and six PEG 3350 tolerant controls. We searched FDA adverse event reports for immediate reactions to PEG 3350 to determine the potential scope of this problem in the United States.
Results:
Skin and provocation testing demonstrated symptomatic reactivity in both cases to PEG 3350 and polysorbate 80. Plasma samples were positive for anti-PEG specific IgE and IgG antibodies only in cases and binding increased directly proportional to the molecular weight of PEG tested. FDA adverse event reports revealed 53 additional cases of possible PEG 3350 anaphylaxis.
Conclusions:
Immediate hypersensitivity to PEG 3350 with cross-reactive polysorbate 80 hypersensitivity may be under recognized in clinical practice and can be detected with clinical skin testing. Our studies raise the possibility of an IgE mediated Type I hypersensitivity mechanism in some cases.
Keywords: polyethylene glycol, PEG, immediate hypersensitivity, allergy, polysorbate
Background:
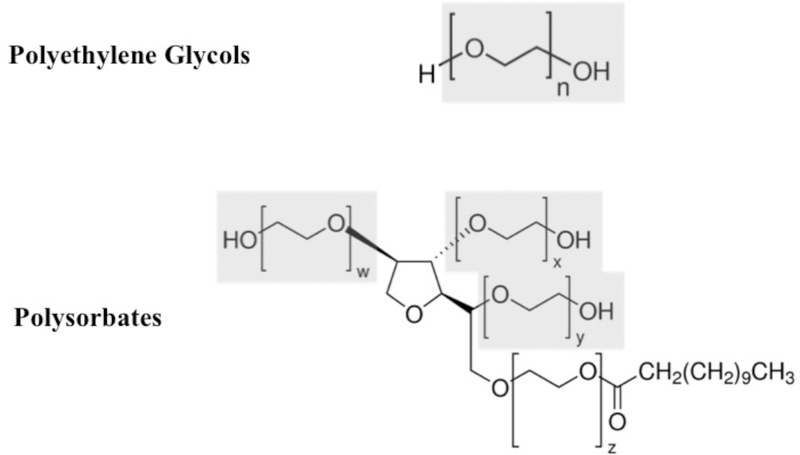
Macrogols, including polyethylene glycols (PEG) and the structurally related polysorbates (Figure 1), are compounds whose primary feature includes polyether groups. They have wide ranging use in medical and commercial settings, with molecular weights (MW) that range from 200 to 35,000g/mol.1 PEG of MW between 3350 and 6000 are frequently used as excipients in many liquid and solid formulations of medications.2, 3 PEG of MW 5000 is used in conjugated enzyme therapeutics, such as PEG-asparaginase and PEG-adenosine deaminase, to improve drug pharmacokinetics and lower immunogenicity. PEG of MW 3350 is the primary ingredient in commonly used oral bowel preparations for colonoscopy procedures in the United States.1, 4 Recently, PEGs of this MW range have been receiving attention as a cause of anaphylaxis to preparations used for colonoscopies,5 and as an immunogenic epitope in PEGylated asparaginase (Oncaspar and Pegcrisantaspase).6, 7 There is only limited awareness of their role in reactions to medications where they are present as an excipient.4, 8–10 Many patients report repeated cutaneous exposures11–14 or local reactions to PEG-containing topical items15 prior to the onset of systemic reactions to high molecular weight PEG containing medications, suggesting a cutaneous mode of sensitization. Gastrointestinal sensitization has been theorized in PEG allergic patients with an impaired epithelial barrier.16–18 However, the scope to which macrogol hypersensitivity might be a problem in the United States and the mechanism for PEG and polysorbate reactions are not well understood.8, 19, 20 After encountering two cases of life threatening immediate hypersensitivity to macrogols in our clinic, we sought to further understand the mechanism and scope of immediate hypersensitivity to PEG.
Figure 1:
Chemical structure of polyethylene glycols and polysorbates. Polysorbate 20 shown. Note the repeating polyether domains contained in both molecules, highlighted in gray. Source of chemical structure images: sigmaaldrich.com, accessed 5-15-2018. Highlights and labels added by authors to demonstrate similarity.
Methods:
Clinical Surveillance:
Cases were recruited through a dedicated drug allergy clinic at Vanderbilt University Medical Center. A detailed clinical case description was obtained from patients whose history suggested an immediate reaction to PEG 3350 containing colonoscopy preparations, laxatives, or injected corticosteroids during a 3 year period.
Skin Testing and Challenges:
To determine clinical reactivity to macrogols, including polyethylene glycols and polysorbate containing products, we used a combination of skin prick, intradermal and challenge testing with standard methodologies.21
Controls:
Two healthy adult volunteers served as negative controls for the skin testing protocol. Six additional healthy adult volunteers with previous exposure to PEG 3350 during colonoscopy preparation or use of laxatives during the last 5 years provided blood samples used as controls during laboratory assays.
Laboratory Methods:
To better understand the mechanism of macrogol hypersensitivity in the two cases, we next sought to detect the presence of polyethylene glycol specific antibodies. Enzyme-linked immunosorbent assay (ELISA) was used for the detection of anti-PEG antibodies. Briefly, Corning 96-well EIA/RIA assay microplates were coated with 5,000g/mol methoxy-PEG-E.coli asparaginase (Oncaspar) at 10 µg/ml. For anti-PEG IgG detection, plasma obtained from the aforementioned 2 cases 2~3 months after their last anaphylaxis episodes were incubated at 1:400 dilution. For anti-IgE detection, the same plasma samples were pretreated with Protein G Plus Agarose (Thermo Fisher Scientific) at 1:1 ratio to remove IgG, then incubated at 1:10 dilution. HRP-conjugated goat anti-human IgG (Sigma) or anti-human IgE (BioRad) antibodies were added at 1:1000 and 1:10,000 dilution respectively. Plates were read at dual wavelengths of 490 nm and 630 nm on an ELx808 microplate reader (BioTek). Plasma samples from 6 patients with similar exposure to colonoscopy preparations containing macrogols were used as controls.
To better determine the presence or absence of PEG specific IgE, we next used an electrochemiluminescent method with greater sensitivity for detection. Standard MULTI-ARRAY 96-well SECTOR plates were coated with Oncaspar and 5,000g/mol methoxy-PEG-bovine catalase at 10 µg/ml. Samples were processed with Protein G Plus Agarose as described above, then incubated at 1:10 dilution. Biotin-conjugated goat anti-human IgE (BioRad) antibody was added at 1:10,000 dilution. SULFO-TAG labeled Streptavidin was used as the detection reagent. Plates were read with a Sector Imager 6000 Analyzer (Meso Scale Discovery).
Furthermore, to investigate the effect of the molecular size of unconjugated PEG on anti-PEG specific IgG binding, we coated Nunc Maxisorp 96-well microplates (Thermo Fisher Scientific) with 5µg/ml HO-PEG-NH2 of MW ranging from 1kDa to 10 kDa (Creative PEGWorks). Case and control samples were incubated at 1:100 dilution. Other steps were the same as the anti-Oncaspar IgG detection ELISA aforementioned.
Public Data Review:
To evaluate the scope to which polyethylene glycol 3350 might be associated with anaphylaxis in the United States, we next undertook a review of the publicly available FDA Adverse Event Reporting System (FAERS) database from 1989 through 2017. Using the search terms “polyethylene glycol” and “anaphylactic shock” or “anaphylactic reaction” we reviewed the number of these complaints for polyethylene glycol containing colonoscopy preparations and laxative products. We evaluated cases associated with branded and generic colonoscopy and laxative products whose primary ingredient was PEG 3350, including colonoscopy products both with and without electrolytes.
Medication Excipient Review:
To evaluate the degree to which immediate hypersensitivity to PEG 3350 or polysorbate 80 might affect medication or vaccine safety for affected patients, we next reviewed publicly available data in the searchable “DailyMed” database provided by the National Library of Medicine,4 which allows for search queries targeting both active and inactive ingredients of all FDA approved and over-the-counter (OTC) medications in the United States. Searches conducted on the advanced search feature of this database will return reviewable information on the first 1000 hits. Using this database, we searched with the terms “polyethylene glycol 3350” and “polysorbate 80”, selecting that these ingredients must be either an “active” or “inactive” ingredient. We then classified the first 1000 hits by route of administration and indication for the medication. We also reviewed vaccine excipient summaries provided by the CDC for vaccines containing either of the two ingredients.22
Results:
Description of Cases:
During our 3 year period of surveillance, we encountered two patients with a history of anaphylaxis during preparation for colonoscopy and after methylprednisolone acetate injections.
The first such patient was a 57 year old white male with an occupational history as a mechanic and electrician, who presented to our clinic for evaluation of suspected medication allergies causing anaphylaxis. 5 years prior to presentation, he noted that while preparing for a colonoscopy, taking oral Colyte® brand colonoscopy preparation (active ingredient PEG 335023) he developed severe itching of his palate and throat, which was alleviated by diphenhydramine. Two years prior to presentation, he underwent injection of methylprednisolone acetate (excipient PEG 335024) into his neck as treatment of radicular pain from a bulging disk. Within seconds of receiving this medication, he developed urticaria, burning all over the body, throat tightness, wheezing, and hypotension. He was immediately given epinephrine, and transferred via emergency medical services to the emergency department, where he received additional epinephrine and IV fluid therapy. One year prior to presentation, he was scheduled for routine follow up of his initial colonoscopy. During his first few sips of Moviprep® brand colonoscopy preparation (active ingredient PEG 335025) he developed severe itching of his palate and throat, along with diffuse urticaria. Symptoms resolved over a couple of hours with immediate cessation of the bowel preparation and diphenhydramine. Three months prior to presentation, he attempted once again to undergo colonoscopy, using oral Gavilyte™-G generic preparation (active ingredient PEG 335026). He consumed approximately 10–12 ounces and subsequently developed itching, burning urticarial rash along with the urge to defecate. He went to the bathroom where he experienced syncope and fell, knocking a hole in the drywall with his head. Upon hearing the fall, his son, a nurse, arrived and checked his father’s blood pressure, which was 60/20, and administered 0.3mg of 1:1000 concentration intramuscular epinephrine. EMS was called, and administered additional intramuscular epinephrine on arrival, taking the patient to the emergency department where he received diphenhydramine, famotidine, and intravenous fluids. He was observed overnight and discharged the next day.
The second patient was a 51 year old with an occupational history as a mechanic exposed to glycol containing hydraulic fluids, presenting for evaluation due to concern for peri-operative anaphylaxis. Four months prior to presentation, he was to receive an outpatient c-spine epidural steroid injection for cervical spine degeneration. He received lidocaine followed by omnipaque and methylprednisolone acetate. Within 5 minutes after the procedure he became itchy, red, hypotensive and a code was called. He was given ondansetron and methylprednisolone sodium succinate in addition to IV fluids. He was taken to the emergency department where he noted swelling in his hand, itching, difficulty swallowing, and hoarseness. He was given epinephrine as well as IV diphenhydramine and famotidine. He was admitted to the ICU for observation. One month prior to presentation, he began to develop a reaction just prior to a scheduled colonoscopy after use of a polyethylene glycol 3350 colonoscopy preparation. He became hypotensive and flushed and was treated with diphenhydramine, epinephrine, and IV fluids.
Skin Testing and Challenges:
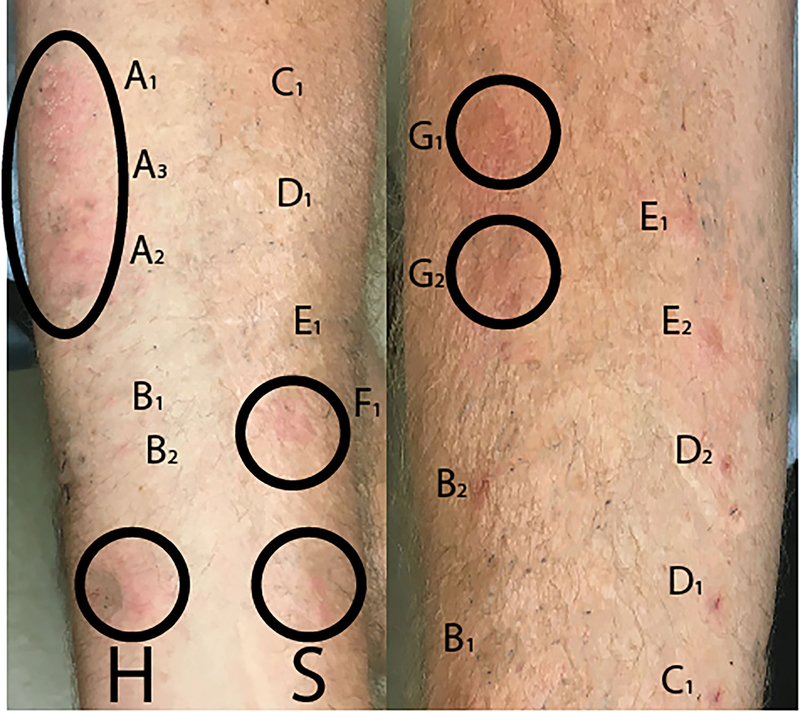
The three bowel preparations and methylprednisolone acetate to which the patients had experienced immediate hypersensitivity reactions all share the ingredient PEG 3350. Both patients subsequently underwent prick and intradermal skin tests with serial dilutions of common corticosteroids, including methylprednisolone acetate (containing PEG 3350), methylprednisolone succinate (containing neither PEG nor polysorbate 80), betamethasone (containing neither PEG nor polysorbate 80), dexamethasone (containing neither PEG nor polysorbate 80), and triamcinolone acetonide (containing polysorbate 80, which shares significant structural homology to PEG) (Table I). During intradermal testing to the steroid preparations, patient 1 developed a sensation of throat and body itching, with a visible urticarial rash expanding from testing sites which was alleviated with 10 mg of cetirizine and 300 mg of ranitidine, without necessitating further treatment with epinephrine (Figure 2). Patient 1 was subsequently demonstrated to have skin test positivity to other polysorbate 80 containing products, including eye drops and conjugated pneumococcal vaccine, but was able to asymptomatically tolerate a low molecular weight PEG oral challenge with PEG 300. While Patient 2 had negative prick testing to PEG 3350 containing products and negative intradermal skin testing to methylpredisolone acetate, he did have positive testing to triamcinolone acetonide containing polysorbate 80. Upon challenge with PEG 3350 he developed diffuse urticaria, respiratory distress and hypotension requiring epinephrine and emergency department transfer. Both patients were able to tolerate challenge with parenteral steroids that did not contain macrogols.
Table I:
Skin Prick and Intradermal Testing with Corticosteroids and Polyethylene Glycols
| Skin Prick Test Results | ||||||
|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | |||||
| Agent (Concentration) | Wheal (mm) | Flare (mm) | Inter-pretation | Wheal (mm) | Flare (mm) | Interpretation |
| Histamine Control (0.1mg/ml) | 6 | 26 | Positive | 7 | 20 | Positive |
| Saline | 0 | 0 | Negative | 0 | 0 | Negative |
| PEG 3350 | 10 | 26 | Positive | 0 | 0 | Negative |
| PEG 3350 (1:10 dilution) | 11 | 22 | Positive | 0 | 0 | Negative |
| PEG 3350 (1:100 dilution) | 11 | 29 | Positive | 0 | 0 | Negative |
| PEG 300 (1:10 dilution) | 0 | 0 | Negative | |||
| PEG 300 (1:100 dilution) | 4 | 5 | Negative | |||
| Methylprednisolone Acetate | 5 | 12 | Positive | 0 | 0 | Negative |
| Methylprednisolone Sodium Succinate | 3 | 3 | Negative | 0 | 0 | Negative |
| Intradermal Skin Test Results | ||||||
| Patient 1 | Patient 2 | |||||
| Agent (Concentration) | Wheal (mm) | Flare (mm) | Inter-pretation | Wheal (mm) | Flare (mm) | Interpretation |
| Betamethasone (6 mg/ml) | 6 | 6 | Negative | 0 | 0 | Negative |
| Betamethasone (0.6mg/ml) | 5 | 5 | Negative | 0 | 0 | Negative |
| Dexamethasone (0.4mg/ml) | 5 | 0 | Negative | 0 | 0 | Negative |
| Dexamethasone (0.04mg/ml) | 7 | 0 | Negative | 0 | 0 | Negative |
| Methylprednisolone Sodium Succinate (5mg/ml) | 5 | 6 | Negative | 0 | 0 | Negative |
| Methylprednisolone Sodium Succinate (0.5mg/ml) | 0 | 0 | Negative | 0 | 0 | Negative |
| Methylprednisolone Acetate (4mg/ml) | 0 | 0 | Subacute response developed at 20 hours, with 14mm raised wheal | |||
| Methylprednisolone Acetate (0.4mg/ml) | 0 | 0 | Negative | |||
| Triamcinolone Acetonide (1mg/ml) | 10 | 19 | Positive | 10 | 30 | Positive |
| Triamcinolone Acetonide (0.1 mg/ml) | 15 | 24 | Positive | |||
| Conjugated pneumococcal vaccine (w/ polysorbate 80) | 20 | 35 | Positive | |||
| Conjugated pneumococcal vaccine (1:10 dilution) | 21 | 30 | Positive | |||
| Polysorbate 80 containing eye drop (1:10 dilution) | 15 | 30 | Positive | |||
Figure 2:
Selected skin testing images for patient 1: In the left panel is skin prick testing demonstrating positive responses to methylprednisolone acetate (MP acetate), and polyethylene glycol 3350 (PEG 3350). Other tested corticosteroids were negative. In the right panel is intradermal testing, which demonstrates a positive response to triamcinolone acetate (T) at 1mg and 0.1mg. Other tested corticosteroids were interpreted as negative. (Measurements recorded in TABLE I).
Two healthy adult controls underwent polyethylene glycol testing on the same day as Patient 2, with negative testing and no irritation at testing sites.
Laboratory Results:
Anti-PEG specific antibody concentrations were measured as optical density (OD) from the ELISA assay using methoxy-PEG-E.coli asparaginase as the antigen source. Anti-PEG specific IgG (sIgG) ODs in plasma samples from the 2 cases (0.50 for Patient 1 and 0.31 for Patient 2) were significantly higher than that of the 6 PEG-exposed controls (99% CI = 0.025 ± 0.019), indicating that both cases were positive for anti-PEG sIgG in these samples obtained 2~3 months after the last reaction (Table E1, Online Only). Anti-PEG specific IgE readings for the patients were negative by this method: ODs were 0.045 and 0.020 respectively for Patient 1 and Patient 2 compared to controls of 0.019 ± 0.0037, none of which were above the uncoated well background signal (99% CI = 0.050 ± 0.011).
Using the more sensitive Meso Scale Discovery electrochemiluminescence method we were then able to detect specific IgE directed against PEG in our two cases, but not our controls. Luminescence intensity from the two cases against Oncaspar (88 for Patient 1 and 77 for Patient 2) was significantly higher than that of the controls (99% CI = 55.9 ± 4.1). Similarly, luminescence intensity from the two cases against PEG-bovine catalase (246 for Patient 1 and 194 for Patient 2) was significantly higher than that of the controls (99% CI = 54.3 ± 9.3). The increase in luminescence intensity against both PEG containing reagents, when tested with sufficient sensitivity indicates that both cases were positive for anti-PEG sIgE (Table E1, Online Only).
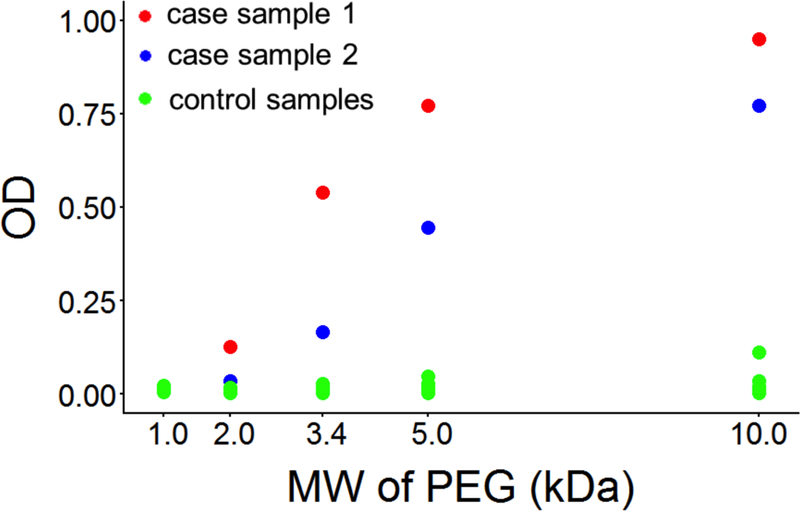
Using unconjugated PEG molecules of different sizes as the antigen source, samples from both cases showed strong preference towards PEGs of larger molecular weights (Figure 3). Although patients in both cases reacted clinically to PEG 3350, anti-PEG sIgG antibodies in their plasma samples displayed even higher binding for higher molecular weight PEG 5k and PEG 10k, and almost no binding towards the lowest molecular weight PEG 1k (ODs were 0.021 and 0.014 respectively) compared to controls (99% CI = 0.014 ± 0.006) who did not demonstrate binding at any molecular weight of PEG.
Figure 3:
IgG optical densities (ODs) of case and control plasma samples against HO-PEG-NH2 of different molecular sizes.
Public data review results:
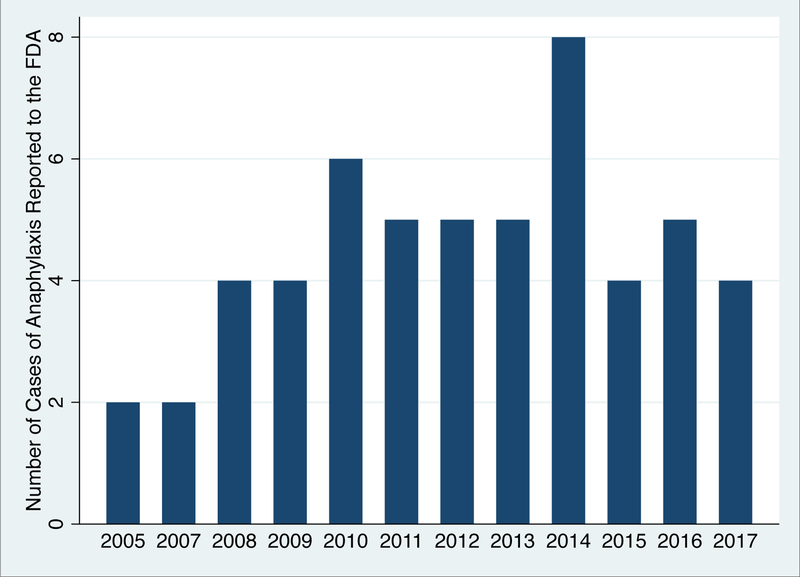
Using the preferred search term “anaphylactic” to capture both “anaphylactic shock” or “anaphylactic reaction”, we encountered 25,905 reports to the FDA between 1989 and the end of 2017. When the additional term “polyethylene glycol” was applied, we were left with 133 reports associating polyethylene glycol with anaphylaxis. Of these, we encountered 53 reports with unique case identifiers described as either anaphylactic shock or an anaphylactic reaction in which PEG containing bowel preparations or laxatives were the primary or sole agent suspected as causal. (Table II) The average age at reaction was 48.9 years (23% missing data), and 51% of those who reacted were male (15% missing data). At the time of reaction, 51% reported the PEG containing product was the sole agent they had ingested prior to anaphylaxis and were not using any other concomitant therapies. The other 49% were taking other concomitant therapies at the time of reaction, but their reports indicated primary suspicion was on PEG containing products. In terms of the clinical context, 72% of the reactions occurred prior to colonoscopy preparation, and 28% occurred during treatment of constipation. Reported reactions were distributed across the time period from 2005–2017, with an average of 4 cases reported per year during this time period. (Figure 4) We did not encounter any reports of PEG-related reactions prior to 2005.
Table II:
Cases of Anaphylaxis Reported to the FDA from 2005 to 2017 Where Polyethylene Glycol 3350 Containing Formulations of Colonoscopy Preparation or Laxatives Were the Primary Drug Suspected
| FAERS Report ID Number | Age | Sex | Year of Report | Formulation of PEG | Patient taking any other medications concomitantly | Indication (Colonoscopy Preparation vs. Constipation) |
|---|---|---|---|---|---|---|
| 4852819-0 | N/A | N/A | 2005 | Golytely | No | Preparation |
| 4885400-8 | 30 | Male | 2005 | Colyte | No | Preparation |
| 5347102-3 | 42 | Male | 2007 | Moviprep | No | Preparation |
| 5326935-3 | 33 | Female | 2007 | Polyethylene Glycol 3350- Brand not specified | No | Constipation |
| 5792732-8 | 68 | Male | 2008 | Golytely | No | Preparation |
| 5829663-0 | N/A | N/A | 2008 | Moviprep | No | Preparation |
| 5909593-6 | N/A | N/A | 2008 | Miralax | Yes | Constipation |
| 5923262-8 | 64 | Male | 2008 | Miralax | Yes | Constipation |
| 6187140-4 | 52 | Male | 2009 | Moviprep | Yes | Preparation |
| 6262262-8 | N/A | N/A | 2009 | Miralax | Yes | Preparation |
| 6301790-3 | 52 | Male | 2009 | Moviprep | Yes | Preparation |
| 6446535-1 | 30 | Female | 2009 | Moviprep | Yes | Preparation |
| 6567457-1 | N/A | N/A | 2010 | Polyethylene Glycol 3350- Brand not specified | Yes | Preparation |
| 6583005-4 | N/A | N/A | 2010 | Moviprep | No | Preparation |
| 6625930-1 | N/A | N/A | 2010 | Moviprep | No | Preparation |
| 6649325-X | 55 | Female | 2010 | Golytely | Yes | Preparation |
| 6681659-5 | 4 | Male | 2010 | Miralax | No | Constipation |
| 6784081-6 | 73 | Male | 2010 | Miralax | No | Constipation |
| 7610318-7 | 19 | Male | 2011 | Moviprep | Yes | Preparation |
| 7429359-8 | 59 | Female | 2011 | Polyethylene Glycol 3350- Brand not specified | Yes | Preparation |
| 7444601-5 | 55 | Male | 2011 | Miralax | No | Preparation |
| 7636123-3 | 64 | Female | 2011 | Moviprep | No | Preparation |
| 7759201-7 | 33 | Female | 2011 | Polyethylene Glycol 3350- Brand not specified | No | Preparation |
| 8274426-2 | 67 | Female | 2012 | Moviprep | Yes | Preparation |
| 8289679-4 | 57 | Female | 2012 | Polyethylene Glycol 3350- Brand not specified | Yes | Constipation |
| 8456637-6 | 46 | Female | 2012 | Polyethylene Glycol 3350- Brand not specified | Yes | Constipation |
| 8712178 | N/A | Female | 2012 | Miralax | No | Constipation |
| 8814458 | 24 | Male | 2012 | Polyethylene Glycol 3350- Brand not specified | Yes | Constipation |
| 9321913 | 16 | Female | 2013 | Miralax | No | Preparation |
| 9417033 | 56 | Female | 2013 | Golytely | Yes | Preparation |
| 9420162 | N/A | Female | 2013 | Miralax | Yes | Constipation |
| 9607762 | 50 | Male | 2013 | Golytely | No | Preparation |
| 9782506 | 70 | Female | 2013 | Moviprep | No | Preparation |
| 9828607 | 34 | Female | 2014 | Miralax | Yes | Preparation |
| 9894648 | N/A | Female | 2014 | Miralax | Yes | Constipation |
| 9934430 | 54 | Male | 2014 | Miralax | Yes | Constipation |
| 10235381 | 87 | Female | 2014 | Moviprep | Yes | Preparation |
| 10242352 | 13 | Male | 2014 | Miralax | No | Constipation |
| 10335513 | 54 | Female | 2014 | Glycolax | No | Preparation |
| 10428179 | 65 | Male | 2014 | Moviprep | Yes | Preparation |
| 10682474 | 59 | Male | 2014 | Moviprep | No | Preparation |
| 10710219 | 19 | Female | 2015 | Moviprep | Yes | Preparation |
| 11362693 | N/A | N/A | 2015 | Miralax | No | Preparation |
| 11573598 | N/A | Female | 2015 | Moviprep | No | Preparation |
| 11617696 | 74 | Male | 2015 | Moviprep | No | Preparation |
| 12787790 | 62 | Male | 2016 | Polyethylene Glycol 3350- Brand not specified | Yes | Preparation |
| 12849324 | 39 | Male | 2016 | Colyte | Yes | Preparation |
| 12865113 | 59 | Male | 2016 | Polyethylene Glycol 3350- Brand not specified | No | Preparation |
| 13243846 | 46 | Male | 2016 | Moviprep | Yes | Preparation |
| 13268930 | 64 | Male | 2016 | Polyethylene Glycol 3350- Brand not specified | No | Preparation |
| 13747359 | 68 | Female | 2017 | Miralax | Yes | Constipation |
| 13854981 | 73 | Female | 2017 | Golytely | No | Preparation |
| 13870252 | 61 | Female | 2017 | Moviprep | No | Preparation |
| 13896629 | 2 | Male | 2017 | Golytely | Yes | Constipation |
Data marked as N/A indicate that the information was not contained in the primary report to the FDA.
Figure 4:
Cases of anaphylaxis reported to the FDA (FAERS) implicating PEG containing bowel preparations or laxatives, by year.
Medication Excipient Review:
Using the search term “polyethylene glycol 3350” as an active or inactive ingredient returned 1155 FDA approved medications. A summary of the first 1000 hits can be found in Table E2 (Table E2, Online Only). This list demonstrates that polyethylene glycol 3350 can more commonly be found in film coated tablets, topical gels, and parenteral steroids. Using the search term “polysorbate 80” as an active or inactive ingredient returned 6821 FDA approved medications. A summary of the first 1000 hits can be found in Table E3 (Table E3, Online Only). This list demonstrates that polysorbate 80 can more commonly be found in film coated tablets, parenteral steroids, and vaccines.
Discussion:
The most commonly known clinical use of macrogols such as PEG 3350 is in colonoscopy preparation or constipation treatment.5, 23, 25, 26 However, a review of common products and the literature demonstrates that polyethylene glycol and structurally similar polysorbate compounds can be found in vascular graft materials10, surgical gels27, PEGylated medications,28–30 household and industrial compounds,1 and as an excipient in a multitude of other medications both injectable and oral,4, 31 In these settings, PEGs and polysorbates are not consistently described in ingredient lists.8 The NIH DailyMed online resource through the National Library of Medicine is a useful resource for determining an individual product’s excipient content of macrogols such as PEGs and polysorbates: https://dailymed.nlm.nih.gov/.4 Though cutaneous and systemic reactions to film coated tablets has been reported in patients with PEG hypersensitivity,8 both of our patients were otherwise healthy and taking no daily medications that contained PEG. Neither one is known to have reacted to any products other than what we have described in this report.
A recent review of published case reports and case series in the literature by Garvey et al. found 37 cases of PEG hypersensitivity since 1977.8 Our review of the FDA data adds a large number of additional cases that may not have been noticed in the medical literature. Our data suggests an average of 4 cases per year of PEG-associated anaphylaxis during colonoscopy preparation or laxative use are reported to the FDA. However, it is clear that relying on patient or physician initiated reports to the FDA will understate the true volume of the problem. Our review of FDA adverse event data focused only on drugs that contained pure polyethylene glycol 3350 at concentrations of grams per dose. Therefore we can not currently offer much additional data on whether drugs containing PEG or polysorbate 80 as an excipient at milligram or microgram concentrations can precipitate reactions in sensitized patients. We can only report that both of our patients have had anaphylaxis upon parenteral exposure to methylprednisolone acetate, formulations of which typically contain around 29 mg/ml of PEG 3350.4
The mechanism for macrogol hypersensitivity has been poorly understood. Anti-PEG sIgG has been detected in patients receiving PEG-conjugated protein therapeutics6, but was not studied in unconjugated macrogol anaphylactic cases, while anti-PEG sIgE has not been directly measured in any human studies.32 Our findings of skin test reactivity and coexisting polyethylene glycol-directed sIgE and sIgG antibodies suggest an IgE mediated Type I hypersensitivity could be possible in clinical reactions to unconjugated macrogols. These cases may represent a separate phenotype of immediate hypersensitivity from what has been previously shown during reactions to PEG-asparaginase and other PEGylated compounds.7, 33 Of note, the absence of binding between patient IgG antibodies and lower MW PEGs also coincided with the tolerance of PEG 300 in both skin and oral challenges in vivo, supporting the involvement of antibodies specific for higher MW PEGs in the clinical reactions. The stronger reactivity of the patient samples against PEGs of higher molecular weight suggests that sensitization and risk of future reactions may depend partially on the molecular weight of PEG antigen exposures, and suggest that PEG may act as the primary antigen even when not conjugated to drug molecules. Detection of sIgE directed against PEG required use of the more sensitive Meso Scale Discovery electrochemiluminescence method and polysorbate-free testing reagents. Our results suggest that development of blood testing as a modality in diagnosis of macrogol hypersensitivity may be possible.
Conclusions:
High molecular weight polyethylene glycols are common excipients in a wide variety of medications, household products and industrial products which may provide a vehicle for sensitization in a subset of susceptible individuals. Allergists should be aware that cross-reactive immediate hypersensitivity to polyether containing compounds such as macrogols/PEGs and polysorbates can occur, that they may occur via a Type I hypersensitivity mechanism, and that they may be underrecognized.
Supplementary Material
Highlights:
What is already known about this topic?
The most common immediate hypersensitivity to macrogols is associated with PEG 3350, however the epidemiology, mechanisms and cross-reactivity are poorly understood. Thousands of medications contain either PEGs or structurally similar polysorbates.
What does this study add to our knowledge?
In vivo and ex vivo testing of two cases suggest an IgE mediated, Type I hypersensitivity mechanism to polyethylene glycol 3350 anaphylaxis. This hypersensitivity, while rare, may be more common than we recognize.
How does this study impact current management guidelines?
Immediate hypersensitivity to PEG 3350 with cross-reactive polysorbate 80 hypersensitivity may be under recognized in clinical practice and can be evaluated with clinical skin testing.
Acknowledgments
Sources of Funding Related to this Project:
Dr. Stone received funding support related to this project from NIH/NHLBI T32 HL87738 and NIH/NIGMS T32 GM007569.
Dr. Relling received funding related to this project from: NIH/NCI CA 142665, CA 21765, and NIH/NIGMS GM 115279
Dr. Phillips received funding related to this project from: National Institutes of Health (1P50GM115305-01, 1R01AI103348-01, 1P30AI110527-01A1), National Health and Medical Research Foundation of Australia and the Australian Centre for HIV and Hepatitis Virology Research.
IRB: This study was done under IRB approved protocols from Vanderbilt University Vanderbilt IRB #161455
Abbreviations:
- MW
molecular weight(s)
- PEG
polyethylene glycol
- OTC
over-the-counter
- OD
optical density
- ELISA
Enzyme-linked immunosorbent assay
- FDA
US Food and Drug Administration
- FAERS
FDA Adverse Event Reporting System
- CDC
Centers for Disease Control and Prevention
Footnotes
Conflicts of Interest: The authors declare that they have no conflicts of interest to disclose.
References:
- 1.CarbowaxTM Polyethylene Glycols: Industrial Brochure http://www.dow.com/polyglycols/polyethylene/products/carbowaxp.htm: Dow Chemical; 2016.].
- 2.Hyry H, Vuorio A, Varjonen E, Skyttä J, Mäkinen-Kiljunen S. Two cases of anaphylaxis to macrogol 6000 after ingestion of drug tablets. Allergy 2006; 61:1021. [DOI] [PubMed] [Google Scholar]
- 3.Wylon K, Dolle S, Worm M. Polyethylene glycol as a cause of anaphylaxis. Allergy Asthma Clin Immunol 2016; 12:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NIH Daily Med https://dailymed.nlm.nih.gov/dailymed/: National Library of Medicine; 2018.].
- 5.Pizzimenti S, Heffler E, Gentilcore E, Raie A, Bussolino C, Nebiolo F, et al. Macrogol hypersensitivity reactions during cleansing preparation for colon endoscopy. J Allergy Clin Immunol Pract 2014; 2:353–4. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong J, Hempel G, Koling S, Chan L, Fisher T, Meiselman H, et al. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer 2007; 110:103–11. [DOI] [PubMed] [Google Scholar]
- 7.Rau R, Dreyer Z, Choi M, Liang W, Skowronski R, Allamneni K, et al. Outcome of pediatric patients with acute lymphoblastic leukemia/lymphoblastic lymphoma with hypersensitivity to pegaspargase treated with PEGylated Erwinia asparaginase, pegcrisantaspase: A report from the Children’s Oncology Group. Pediatr Blood Cancer 2018; 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenande E, Garvey L. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy 2016; 46:907–22. [DOI] [PubMed] [Google Scholar]
- 9.Borderé A, Stockman A, Boone B, Franki A, Coppens M, Lapeere H, et al. A case of anaphylaxis caused by macrogol 3350 after injection of a corticosteroid. Contact Dermatitis 2012; 67:376–8. [DOI] [PubMed] [Google Scholar]
- 10.Siani A, Accrocca F, De Vivo G, Mounyaergi F, Siani L, Antonelli R. Anaphylactic Reaction during Implantation of Ovation Abdominal Stent Graft in Patients with Abdominal Aortic Aneurysm. Ann Vasc Surg 2016; Epub ahead of print. [DOI] [PubMed]
- 11.Antolin-Amerigo D, Sánchez-González M, Barbarroja-Escudero J, Rodríguez-Rodríguez M, Álvarez-Perea A, Alvarez-Mon M. Allergic Reaction to polyethylene glycol in a painter. Occup Med (London) 2015; 65:502–4. [DOI] [PubMed] [Google Scholar]
- 12.Corazza M, Virgili A, Ricci M, Bianchi A, Borghi A. Contact sensitization to Emulsifying Agents: An Underrated Issue? Dermatitis 2016; 27:276–81. [DOI] [PubMed] [Google Scholar]
- 13.Wolf G, Höger P. Hypoallergenic and non-toxic emollient therapies for children. J Dtsch Dermatol Ges 2009; 7:50–60. [DOI] [PubMed] [Google Scholar]
- 14.Co-Minh H, Demoly P, Guillot B, Raison-Peyron N. Anaphylactic shock after oral intake and contact urticaria due to polyethylene glycols. Allergy 2007; 62:92–3. [DOI] [PubMed] [Google Scholar]
- 15.Rowe R, Sheskey P, Quinn M, editors. Handbook of Pharmaceutical Excipients 6th ed. London, England: Pharmaceutical Press; 2009. [Google Scholar]
- 16.Lee S, Hwang S, Park J, Park H, Shin Y. Anaphylaxis to Polyethylene Glycol (Colyte®) in a Patient with Diverticulitis. J Korean Med Sci 2016; 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Henry W, Chen L, Khasab M. Urticaria due to polyethylene glycol-3350 and electrolytes for oral solution in a patient with jejunal nodular lymphoid hyperplasia. Ann Gastroenterol 2015; 28:148–50. [PMC free article] [PubMed] [Google Scholar]
- 18.Shah S, Prematta T, Adkinson N, Ishmael F. Hypersensitivity to polyethylene glycols. J Clin Pharmacol 2013; 53:352–5. [DOI] [PubMed] [Google Scholar]
- 19.Badiu I, Geuna M, Heffler E, Rolla G. Hypersensitivity reaction to human papillomavirus vaccine due to polysorbate 80. BMJ Case Rep 2012:Online Pub. [DOI] [PMC free article] [PubMed]
- 20.Palacios Castano M, Venturini Diaz M, Lobera Labairu T, Gonzalez Mahave I, Del Pozo Gil M, Blasco Sarramian A. Anaphylaxis Due to the Excipient Polysorbate 80. J Investig Allergol Clin Immunol 2016; 26:394–6. [DOI] [PubMed] [Google Scholar]
- 21.Joint Task Force on Practice P, American Academy of Allergy A, Immunology, American College of Allergy A, Immunology, Joint Council of Allergy A, et al. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010; 105:259–73. [DOI] [PubMed] [Google Scholar]
- 22.Vaccine Excipient & Media Summary. Centers for Disease Control and Prevention; 2015] Available from https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/excipient-table-2.pdf.
- 23.Package Insert: Colyte® with Flavor Packs (peg-3350 & electrolytes for oral solution)] Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/018983s047lbl.pdf.
- 24.Package Insert: Depo-Medrol (methylprednisolone acetate injectable suspension, USP)] Available from http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/011757s103lbl.pdf.
- 25.Package Insert: Moviprep] Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021881s016lbl.pdf.
- 26.Gavilyte Product Information] Available from http://gavilyte.com.
- 27.Yamasuji Y, Higashi Y, Sakanoue M, Katsue H, Kawai K, Arai N, et al. A case of anaphylaxis caused by polyethylene glycol analogues. Contact Dermatitis 2013; 69:183–5. [DOI] [PubMed] [Google Scholar]
- 28.Ganson N, Povsic T, Sullenberger B, Alexander J, Zelenkofske S, Sailstad J, et al. Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer J Allergy Clin Immunol 2016; 137:1610–3.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henrikson L, Harila-Saari A, Ruud E, Abrahamsson J, Pruunsild K, Vaitkeviciene G, et al. PEG-asparaginase allergy in children with acute lymphoblastic leukemia in th eNOPHO ALL2008 protocol. Pediatr Blood Cancer 2015; 62:427–33. [DOI] [PubMed] [Google Scholar]
- 30.Meller S, Gerber P, Kislat A, Hevezi P, Göbel T, Wiesner U, et al. Allergic sensitization to pegylated interferon-α results in drug eruptions. Allergy 2015; 70:775–83. [DOI] [PubMed] [Google Scholar]
- 31.Wenande E, Kroigaard M, Mosbech H, Garvey L. Polyethylene Glycols (PEG) and Related Structures: Overlooked Allergens in the Perioperative Setting. A &A Case Reports 2015; 4:61–4. [DOI] [PubMed] [Google Scholar]
- 32.Povsic T, Lawrence M, Lincoff A, Mehran R, Rusconi C, Zelenkofske S, et al. Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer. J Allergy Clin Immunol 2016; 138:1712–5. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez C, Stewart E, Panetta J, Wilkinson M, Morrison A, Finkelman F, et al. Successful challenges using native E. coli asparaginase after hypersensitivity reactions to PEGylated E. coli asparaginase. Cancer Chemother Pharmacol 2014; 73:1307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.